Introduction
Based on the strategy of targeting both
proliferating tumor cells and endothelial cells in our previous
studies, we constructed a fusion protein of the human IgG3 upper
hinge region and 2 tumstatin-derived specific sequences, which we
named vascular basement membrane-derived multifunctional peptide
(VBMDMP) (1). Recombinant VBMDMP
(rVBMDMP) was found to exhibit anti-proliferation and
anti-angiogenic activities and to significantly inhibit tumor
growth and metastasis in a mouse lung carcinoma model (2). Moreover, rVBMDMP selectively inhibited
endothelial cell and human colon cancer cell proliferation, induced
endothelial cell apoptosis in vitro and suppressed human
colon cancer xenograft growth in Balb/c-nu mice (3). We determined that the interaction of
rVBMDMP with αVβ3 integrin is critical for rVBMDMP binding to cells
and mediates the rVBMDMP-induced inhibition of proliferation
(4).
Integrins are a family of heterodimeric
transmembrane proteins comprising unrelated α and β subunits that
serve as receptors for extracellular matrix (ECM) proteins such as
fibronectin (FN), laminins and collagens. In mammals, 18 types of α
subunits and 8 types of β subunits assemble to form 24 different
receptors. Integrins initiate a variety of downstream signaling
events including survival or death pathways in response to ECM
ligation (5). The integrin αVβ3
receptor is implicated in cardiovascular and bone function and
recognizes glycoprotein ligands such as vitronectin and FN. Upon
activation of the integrin αVβ3 receptor, downstream molecules,
including phosphatidylinositol 3 kinase (PI3K)/Akt, are
phosphorylated, which increases cell tolerance to chemotherapy,
resulting in secondary resistance in a variety of ways (6). In our previous study, we demonstrated
that rVBMDMP binds αVβ3 integrins and enhances the growth
inhibitory activity of cisplatin in A549 cells (7). We also found that the expression of
the multidrug resistance protein 2 (MRP-2) showed a downward trend
in A549 cells following treatment with rVBMDMP (unpublished
data).
MRP-2 is a member of the ATP-binding cassette (ABC)
transporter superfamily. ABC genes are divided into 7 distinct
subfamilies (ABC1, MDR/TAP, MRP, ALD, OABP and GCN20) and encode
proteins that transport various molecules across extracellular and
intracellular membranes (8). MRP-2
is a member of the MRP subfamily, which is involved in multi-drug
resistance (9). Its substrates
include anticancer drugs, such as vinblastine and thus MRP-2
contributes to drug resistance in mammalian cancer cells.
Therefore, we speculated that the rVBMDMP-mediated inhibition of
MRP-2 has the potential to reverse tumor cell resistance to
chemotherapeutic drugs.
In the present study, we demonstrated that rVBMDMP
inhibited cisplatin-resistant A549/DDP human lung carcinoma cell
proliferation using in vitro and in vivo models of
tumor growth. We also demonstrated that rVBMDMP potently reversed
A549/DDP cisplatin resistance by inhibiting MRP-2 expression, which
may occur via the PI3K/Akt pathway. These data suggest that rVBMDMP
could be a potentially useful therapeutic molecule targeting human
lung cancer.
Materials and methods
rVBMDMP
rVBMDMP (6.4 kDa) was produced in BL-21 E.
coli using the pGEX-4T-1-VBMDMP expression plasmid and purified
as previously described (1).
Cell culture
Human lung carcinoma cells (A549) and
cisplatin-resistant human lung carcinoma cells (A549/DDP) were
obtained from the China Center for Type Culture Collection (CCTCC,
Wuhan, China) and maintained in RPMI-1640 medium (Gibco-BRL, Grand
Island, NY, USA), supplemented with 10% (v/v) dialyzed
heat-inactivated bovine serum (BS) (Gibco), 100 U/ml penicillin and
100 μg/ml streptomycin at 37°C in 5% CO2.
Cell viability assay
Cell viability was determined using the MTS assay.
In brief, ~1.0×104 A549 and A549/DDP cells/well were
plated in 96-well plates and incubated overnight. Cells were
treated with various concentrations of rVBMDMP and cisplatin for 48
h, and 20 μl of
3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyltetrazolium bromide [MTS,
5 g/l in phosphate-buffered saline (PBS)] (Promega, Madison, WI,
USA) was added. The plates were incubated for 6 h, and the formed
formazan dye was dissolved in 100 μl of DMSO (Sigma-Aldrich, St.
Louis, MO, USA). Absorbance was recorded at 570 nm using a Biotek
Synergy2 microplate reader (Biotek Instruments, Winooski, VT, USA).
All experiments were repeated 3 times. Cell viability was
calculated as: Cell viability rate (%) = (T − B)/(U − B) × 100%;
where T is the treated cell absorbance, U is the untreated cell
absorbance and B is the background absorbance when neither drug nor
MTS was added.
Signal transduction antibody array
Serum-starved A549 cells were treated with 10 μmol/l
rVBMDMP for 30 min, which was optimal for inhibiting endothelial
cell proliferation and were lysed in 0.5% Triton X-100 buffer. This
rVBMDMP concentration was determined to be optimal at inhibiting
A549 cell proliferation in this study. The antibody array membrane
(HM3000 signal transduction antibody array; Hypromatrix Inc.,
Worcester, MA, USA) was treated in blocking buffer containing 0.01%
Tween-20 followed by incubation with sample diluted in 1% dry
milk/PBS for 2 h at room temperature with slow shaking at 40 rpm.
After the antibody filters were incubated with the supernatant
protein solution at room temperature for 2 h, the antibody array
filter was washed with TBST and blotted with HRP-conjugated
anti-phospho-tyrosine monoclonal antibodies for 2 h.
Anti-phospho-tyrosine reactivity was visualized by enhanced
chemiluminescence (ECL; Amersham Biosciences) and exposed to X-ray
film. The gray-scale chip scanogram was analyzed with chip image
analysis software (QuantArray, Packard Biochip Technologies Inc.
USA) to correct for protein signals. Immunoreactivity on the chip
that had been incubated with control cell lysate was set to 1 for
each spotted antibody. Phosphorylation ratios >2 or <0.5 were
considered to indicate increased or decreased phosphorylation,
respectively.
Western blot analysis
The anti-integrin αV, anti-integrin β3, anti-MRP-2,
anti-NF κB, anti-caspase 3, anti-PARP, anti-bcl2, anti-Akt,
anti-p-Akt, anti-PI3K, anti-pPI3K and anti-β-actin antibodies were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). An
anti-GAPDH antibody (Upstate Biotechnology, Lake Placid, NY, USA)
was used as a loading control. After the treatments, the cells were
collected and lysed. Approximately 100 ng of total protein was
electrophoresed on a 10% SDS-PAGE gel and then transferred to a
PVDF membrane. After blocking the membrane with 5% nonfat milk in
PBS + 0.1% Tween-20 overnight at 4°C, the blot was incubated with
the primary antibody for 1 h, washed with PBS + 0.1% Tween-20 3
times (15 min each time), incubated with the secondary antibody
(IgG) conjugated with horseradish peroxidase for 1 h and washed
with PBS + 0.1% Tween-20 3 times. The signal was visualized with a
chemiluminescence kit (SuperSignal, Pierce).
In vivo tumor growth inhibition
studies
Female 6-week old Balb/c-nu mice weighing ~16 g were
implanted with 2×106 A549/DDP human lung cancer cells
into the subcutis on the back. Tumor length and width were measured
using a vernier caliper, and the tumor volume was calculated using
the standard formula of length × width2 × 0.52 (10). When the tumors were ~100
mm3, the animals were divided into groups of 5 mice.
rVBMDMP (5 mg/kg), the angiogenesis inhibitor TNP-470 (20 mg/kg),
cisplatin (10 mg/kg), a combination of rVBMDMP (5 mg/kg) and
cisplatin (10 mg/kg) and vehicle control were administered via
intravenous injection twice daily for 16 days. Mice were weighed
twice weekly. Tumor volume was calculated every 3 days. Tumor
volume ± SD was plotted vs. time over the treatment period. Upon
treatment termination, the mice were weighed and sacrificed and
their tumors were excised, weighed and photographed. The mean tumor
weight per group was calculated. The mean ratio of the treated
tumor weight to the mean vehicle control tumor weight × 100 was
subtracted from 100% to provide the tumor growth inhibition for
each group. All images were captured with a Canon digital camera
and developed with Kodak 400 DK-coated TMAM. The experiments were
performed using 5 mice per group and all animal procedures were
performed in accordance with institutional guidelines. The study
protocol was approved by the Ethics Committee of Guangzhou Medical
University.
Statistical analysis
Continuous data are expressed as the mean ± SD.
Comparisons between groups were performed using the Student’s
t-test. Analysis of variance was used to examine differences in
response to treatments and between groups. P-values <0.05 were
considered to indicate statistically significant results.
Results
rVBMDMP treatment alters the
phosphorylation of signaling proteins
To explore the molecular mechanism of
rVBMDMP-mediated lung cancer inhibition, we examined the effects of
rVBMDMP on the phosphorylation of 400 signaling proteins using a
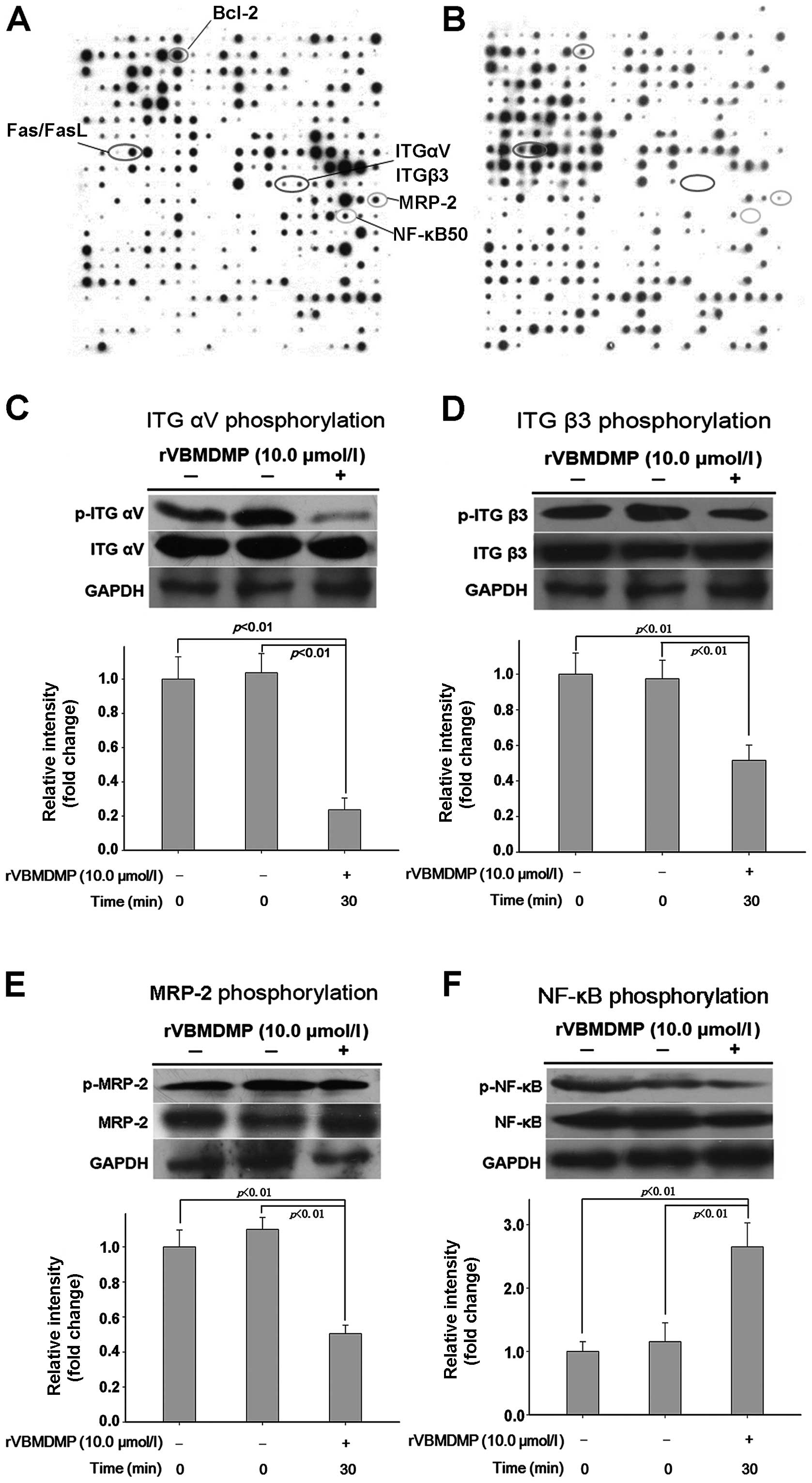
protein phosphorylation chip (Fig. 1A
and B). rVBMDMP treatment increased phosphorylation (defined as
a 2-fold or higher increase compared with the controls) of focal
adhesion kinase (FAK), caspase-6, Fas, FasL and FAF1. rVBMDMP
treatment decreased phosphorylation (defined as a 0.5-fold or more
decrease compared with the controls) of integrin αV, integrin β3,
PI3K/Akt, NF-κB and MRP-2 (Table
I). Western blot analysis confirmed that treatment with 10
μg/ml rVBMDMP for 30 min was sufficient to inhibit integrin αV,
integrin β3, MRP-2 and NF-κB phosphorylation (Fig. 1C–F). These results were concordant
with the antibody microarray data.
 | Table IRatios of cell signaling protein
phosphorylation levels in A549 cells after 1.0 μmol/l VBMDMP
treatment for 30 min. |
Table I
Ratios of cell signaling protein
phosphorylation levels in A549 cells after 1.0 μmol/l VBMDMP
treatment for 30 min.
| Position | Symbol | Ratio | Description and
function |
|---|
| 1 | 14-3-3 | 3.90 | Critical for cell
transformation and mitotic signaling |
| 2 | c-Abl | 2.44 | Abelson murine
leukemia virus; a 120-kDa protein with tyrosine kinase activity and
an SH2 domain |
| 42 | Brk | 2.31 | Human homolog of
Sik (Src-related intestinal kinase) |
| 43 | Brm | 2.03 | Similar to the
brahma protein of Drosophila; helicase and ATPase
activities |
| 61 | Caspase-6 | 2.30 | Cysteine-aspartic
acid protease 6 |
| 72 | CD27 | 2.04 | Homodimeric
lymphocyte-specific surface antigen, belongs to the TNF receptor
superfamily |
| 82 | Cdk2 | 2.66 | Cyclin-dependent
protein kinase |
| 89 | CIDE-B | 2.39 | A DNAse that is
responsible for DNA degradation during apoptosis |
| 90 | Clathrin | 2.12 | Clathrin |
| 102 | Cyclin B | 3.56 | Cyclin protein
B |
| 103 | Cyclin D3 | 3.52 | Cyclin protein
D3 |
| 104 | Cyclin E | 2.87 | Cyclin protein
E |
| 109 | Desmoglein | 2.33 | A member of the
cadherin family of adhesion molecules |
| 112 | DMBT1 | 2.05 | Deleted in
malignant brain tumors 1; a candidate tumor-suppressor gene |
| 121 | E2F1 | 2.28 | E2F transcription
factor 1 |
| 122 | EGFR | 4.13 | Epidermal growth
factor receptor |
| 123 | p-EGFR | 2.43 | Phosphorylated
epidermal growth factor receptor |
| 124 | Egr-1 | 3.93 | EGR family of
C2H2-type zinc-finger proteins, is a cancer suppressor gene |
| 125 | Egr-2 | 4.25 | EGR family of
C2H2-type zinc-finger proteins, is a cancer suppressor gene |
| 126 | Egr-3 | 4.52 | EGR family of
C2H2-type zinc-finger proteins, is a cancer suppressor gene |
| 129 | EphA4 | 2.56 | Ephrin receptor
A4 |
| 130 | EphB1 | 5.24 | Ephrin receptor
B1 |
| 131 | Eps8 | 3.65 | Epidermal growth
factor receptor substrate 8 |
| 141 | FAF-1 | 6.64 | FAS interacting
protein |
| 142 | FAK | 5.50 | Focal adhesion
associated protein-tyrosine kinase |
| 143 | Fas | 5.08 | A member of the
tumor necrosis factor family of cell surface receptors |
| 144 | FasL | 2.27 | Fas ligand |
| 146 | FGFR1 | 7.32 | Fibroblast growth
factor receptor 1 |
| 147 | FGFR2 | 2.70 | Fibroblast growth
factor receptor 2 |
| 148 | FGFR3 | 2.11 | Fibroblast growth
factor receptor 3 |
| 149 | FGFR4 | 3.23 | Fibroblast growth
factor receptor 4 |
| 150 | FHIT | 5.25 | A member of the
histidine triad protein family; a candidate tumor suppressor |
| 161 | GATA-1 | 4.05 | Transcription
factor |
| 162 | GATA-2 | 4.96 | Transcription
factor |
| 163 | GATA-3 | 7.17 | Transcription
factor |
| 164 | G-CSFR | 2.12 | Colony stimulating
factor receptor |
| 165 | gp130 | 2.30 | Glycoprotein
130 |
| 166 | Granzyme B | 4.50 | Cytotoxic
T-lymphocyte-associated serine esterase 1 |
| 167 | GRB2 | 2.27 | Growth factor
receptor-bound protein 2 |
| 169 | GRB14 | 3.56 | Growth factor
receptor-bound protein 14 |
| 170 | GRK2 | 4.44 | G protein-coupled
receptor kinase 2 |
| 182 | IFN-aRa | 2.82 | Type I interferon α
receptor α |
| 183 | IFN-gRa | 7.28 | Type II interferon
γ receptor α |
| 184 | IL1R1 | 4.64 | Interleukin-1
receptor 1 |
| 186 | IL2Rβ | 5.43 | Interleukin-2
receptor β |
| 187 | IL2Rγ | 3.77 | Interleukin-2
receptor γ |
| 188 | IL3 | 2.86 | Interleukin-3 |
| 189 | IL4Ra | 2.06 | Interleukin-4
receptor α |
| 202 | Jak1 | 2.65 | Janus kinase 1 |
| 203 | Jak2 | 2.49 | Janus kinase 2 |
| 206 | p-JNK1,2,3 | 2.13 | Phosphorylated
c-Jun N-terminal kinases 1,2,3 |
| 209 | KAP | 2.18 | A dual specificity
phosphatase that interacts with cyclin-dependent kinases |
| 222 | MEK1 | 2.08 | Mitogen-activated
protein kinase kinase 1 |
| 252 | Nip3 | 2.03 | A member of the
BCL2/adenovirus E1B 19 kDa-interacting protein (BNIP) family, Nip3
preferentially binds to Bcl-xL and induces apoptosis by suppressing
the anti-apoptosis activity of Bcl-xL |
| 282 | PDGFRβ | 2.39 | Platelet-derived
growth factor receptor β |
| 291 | PTEN | 2.06 | Phosphatase and
tensin homolog; the PTEN gene is a tumor suppressor gene |
| 292 | SH-PTP | 2.33 | SH-protein tyrosine
phosphatase 1 |
| 307 | RalA | 2.46 | Small GTPase
superfamily; Ras family of proteins |
| 308 | RanBP-1 | 2.31 | Ras-related nuclear
protein BP-1 |
| 311 | RARr | 2.18 | Retinoic acid
receptors |
| 312 | RXR a,b, r | 2.30 | Retinoid X
receptors a, b, r |
| 325 | RIP | 2.39 | Receptor
interacting protein |
| 331 | P-Selectin | 3.12 | Cell adhesion
molecule |
| 332 | SHC | 2.56 | Src homology 2
domain containing |
| 343 | Blk | 2.27 | Proto-oncogenic
non-receptor tyrosine kinase |
| 346 | Lck | 2.72 | Leukocyte-specific
protein tyrosine kinase |
| 347 | Lyn | 3.54 | A member of the Src
family of protein tyrosine kinases |
| 351 | STAM | 3.59 | Signal transducing
adaptor molecule |
| 364 | TANK | 2.68 | TRAF-associated
NF-κB activator |
| 365 | TCRα | 3.92 | T-cell receptor
α |
| 366 | TCRβ | 4.14 | T-cell receptor
β |
| 367 | TDAG51 | 3.32 | T-cell death
associated gene 51 |
| 370 | Thyroid Rα1 | 3.56 | Thyroid hormone
nuclear receptor α 1 |
| 371 | TIA-1 | 5.52 | A member of an
RNA-binding protein family; a mediator of apoptotic cell death |
| 372 | TIAR | 4.06 | TIA receptor |
| 375 | TOSO | 2.57 | |
| 392 | VDR | 2.99 | Vitamin D
receptor |
| 394 | VEGFR2 | 2.35 | VEGF receptor
2 |
| 397 | XRCC4 | 2.38 | X-ray repair
cross-complementing protein 4 |
| 14 | APC | 0.43 | Adhesion
protein |
| 27 | Bcl-2 | 0.42 | B-cell lymphoma
2 |
| 39 | BARD1 | 0.36 | BRCA1-associated
RING domain gene 1 is a major cellular binding partner of
BRCA1 |
| 40 | BRCA1 | 0.42 | Breast cancer
1 |
| 60 | Caspase-5 | 0.49 | Cysteine-aspartic
acid protease 5 |
| 100 | CUL-1 | 0.43 | A member of the
cullin protein family |
| 118 | DR5 | 0.32 | Death receptor
5 |
| 139 | Ezrin | 0.48 | Cytoplasmic
protein; a major cytoplasmic substrate of various protein-tyrosine
kinases |
| 158 | GADD45 | 0.30 | Growth arrest and
DNA damage 45 |
| 174 | Ne-dlg | 0.49 | Neuronal and
endocrine dlg (Discs large) |
| 175 | hIL | 0.50 | IAP family
member |
| 179 | ICSBP | 0.49 | Interferon
consensus sequence-binding protein |
| 180 | ID1 | 0.38 | A member of the Id
family of basic helix-loop-helix (bHLH) proteins |
| 194 | ITG αV | 0.12 | Integrin α
subunit |
| 195 | ITG β1 | 0.12 | Integrin β subunit
(CD29) |
| 196 | ITG β3 | 0.14 | Integrin β
subunit |
| 197 | IRAK | 0.25 | IL-1
receptor-associated kinase |
| 198 | IRF1 | 0.41 | Interferon
regulatory factor-1 |
| 213 | LIFR | 0.41 | Leukemia inhibitory
factor receptor |
| 215 | MAD2 | 0.16 | Mitotic
arrest-deficient 2 |
| 216 | Maspin | 0.12 | A serpin and tumor
suppressor gene |
| 217 | Max | 0.45 | Transcription
factor |
| 218 | MDA-7 | 0.40 | Melanoma
differentiation-associated protein-7 |
| 220 | MRP-2 | 0.47 | Multiple drug
resistance protein |
| 235 | NF1GRP | 0.27 | Neurofibromin
protein |
| 237 | NFATC | 0.46 | Nuclear factor of
activated T-cells, cytoplasmic, calcineurin-dependent |
| 238 | NF-κB-p50 | 0.40 | Nuclear factor-κB
50 |
| 256 | Notch | 0.46 | A human gene
encoding a single-pass transmembrane receptor |
| 278 | Pax-5 | 0.48 | Nuclear
transcription factor |
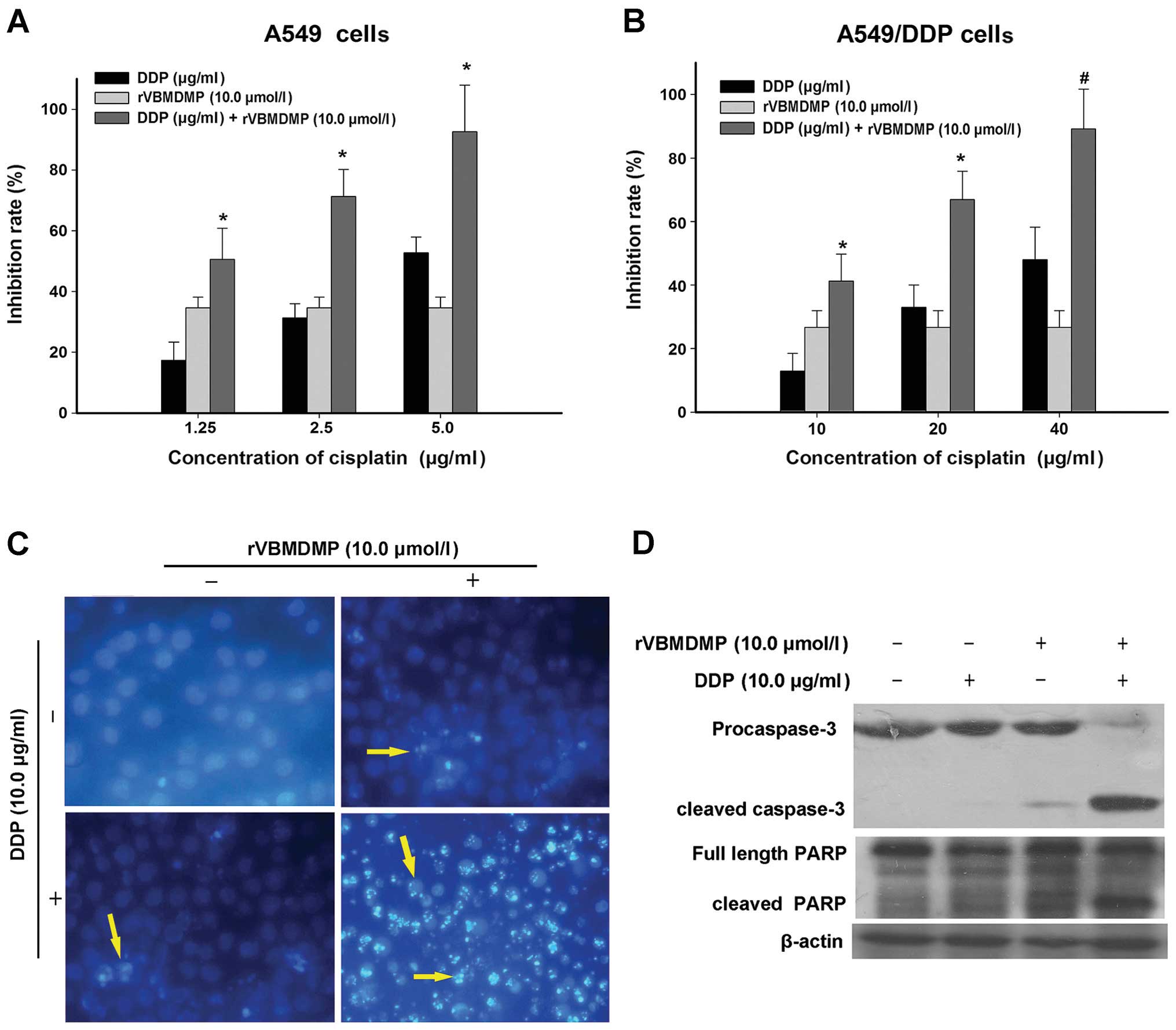
rVBMDMP promotes A549/DDP cell cisplatin
sensitivity and apoptosis
We treated A549/DDP cells with cisplatin
concentrations of 5, 10, 20 and 40 μg/ml and determined that higher
cisplatin concentrations inhibited A549/DDP cell proliferation. The
concentrations of cisplatin required for A549/DDP cell growth
inhibition were significantly higher than those required to inhibit
A549 cell proliferation (Fig. 2A).
After treating A549/DDP cells with 20 μg/ml cisplatin for 48 h, the
growth inhibition rate was 32.9±7.1%, which increased with
increasing cisplatin concentration (Fig. 2B). According to the formula
IC50 = lg−1 [Xm-i (P-0.5)], the
IC50 in A549/DDP cells treated with cisplatin for 48 h
was 31.19 μg/ml. The resistance index of A549/DDP cells to
cisplatin was the IC50 of A549/DDP cells divided by
IC50 of the A549 cells or 31.19/4.614 μg/ml=6.759. This
result demonstrates that the A549/DDP cell line has a certain
resistance to cisplatin, which was suitable for this drug
resistance study.
Next, A549/DDP cells were treated with 10 μM rVBMDMP
along with 10, 20 or 40 μg/ml cisplatin for 48 h (Fig. 2B, Table
II). When A549/DDP cells were treated with 10 μg/ml cisplatin
alone, the growth inhibition rate was 12.8±5.6% and the inhibitory
rate increased to 45.2±8.5% when combined with 10 μM rVBMDMP. The Q
value was 0.88. When A549/DDP cells were treated with 20 μg/ml
cisplatin alone, the growth inhibition rate was 32.9±7.1% and the
inhibitory rate increased to 66.9±8.9% when combined with 10 μM
rVBMDMP. The Q value was 1.15. When A549/DDP cells were treated
with 40 μg/ml cisplatin alone, the growth inhibition rate was
52±10.2% and the inhibitory rate increased to 89.1±12.3% when
combined with 10 μM rVBMDMP. The Q value was 1.22. According to the
formula IC50 = lg−1 [Xm-i (P-0.5)], the
IC50 was 11.82 μg/ml when combined with 10 μM rVBMDMP in
A549/DDP cells. Thus, the multidrug resistance reversal index (RI)
was 2.639. Together, these results demonstrated that the combined
application of rVBMDMP with cisplatin produced an additive
inhibition to significantly reduce A549/DDP cell survival.
 | Table IIInhibition rate and Q values of
cisplatin in combination with rVBMDMP on A549/DDP cell growth. |
Table II
Inhibition rate and Q values of
cisplatin in combination with rVBMDMP on A549/DDP cell growth.
| Group | Growth inhibition
rate (%) | Q value | Cisplatin
IC50 | Reversal index
(RI) |
|---|
| DDP (10 μg/ml) | 12.8±5.6 | | 31.19 | |
| DDP (20 μg/ml) | 32.9±7.1 | | | |
| DDP (40 μg/ml) | 52.0±10.2 | | | |
| DDP (10 μg/ml) +
rVBMDMP (10.0 μM) | 45.2±8.5 | 0.88 | 11.82 | 2.639 |
| DDP (20 μg/ml) +
rVBMDMP (10.0 μM) | 66.9±8.9 | 1.15 | | |
| DDP (40 μg/ml) +
rVBMDMP (10.0 μM) | 89.1±12.3 | 1.22 | | |
We next evaluated A549/DDP cell apoptosis after
cisplatin and rVBMDMP treatment using Hoechst 33258 staining
(Fig. 2C). Apoptotic cells were
observed after treatment with 10 μM rVBMDMP or 10 μg/ml cisplatin
alone for 48 h. However, the combination of these 2 drugs markedly
enhanced A549/DDP cell apoptosis compared with the control group,
which underwent little apoptosis. The nuclei as observed by normal
fluorescence microscopy were large and evenly stained; pyknotic
nuclei appeared smaller and the nuclear chromatin was densely
stained towards the edge or showed chunky dense staining in
apoptotic cells.
As shown in Fig. 2D,
caspase-3 was mildly activated (cleaved) in the A549/DDP cells
following treatment with rVBMDMP alone and was nearly completely
cleaved when combined with cisplatin. PARP cleavage showed a
similar trend. This suggests that combined treatment causes
caspase-3 activation, thereby inducing apoptosis and PARP cleavage.
This may be coupled with MRP-2 downregulation, which then blocks
cisplatin cellular efflux. This may be one of the mechanisms
involved in the reversal of A549/DDP cell chemotherapeutic
resistance by rVBMDMP.
rVBMDMP and cisplatin treatment in
combination significantly inhibit survival
We next investigated the molecular mechanism by
which the combination treatment of rVBMDMP and cisplatin mediates
anti-survival effects in A549/DDP cells.
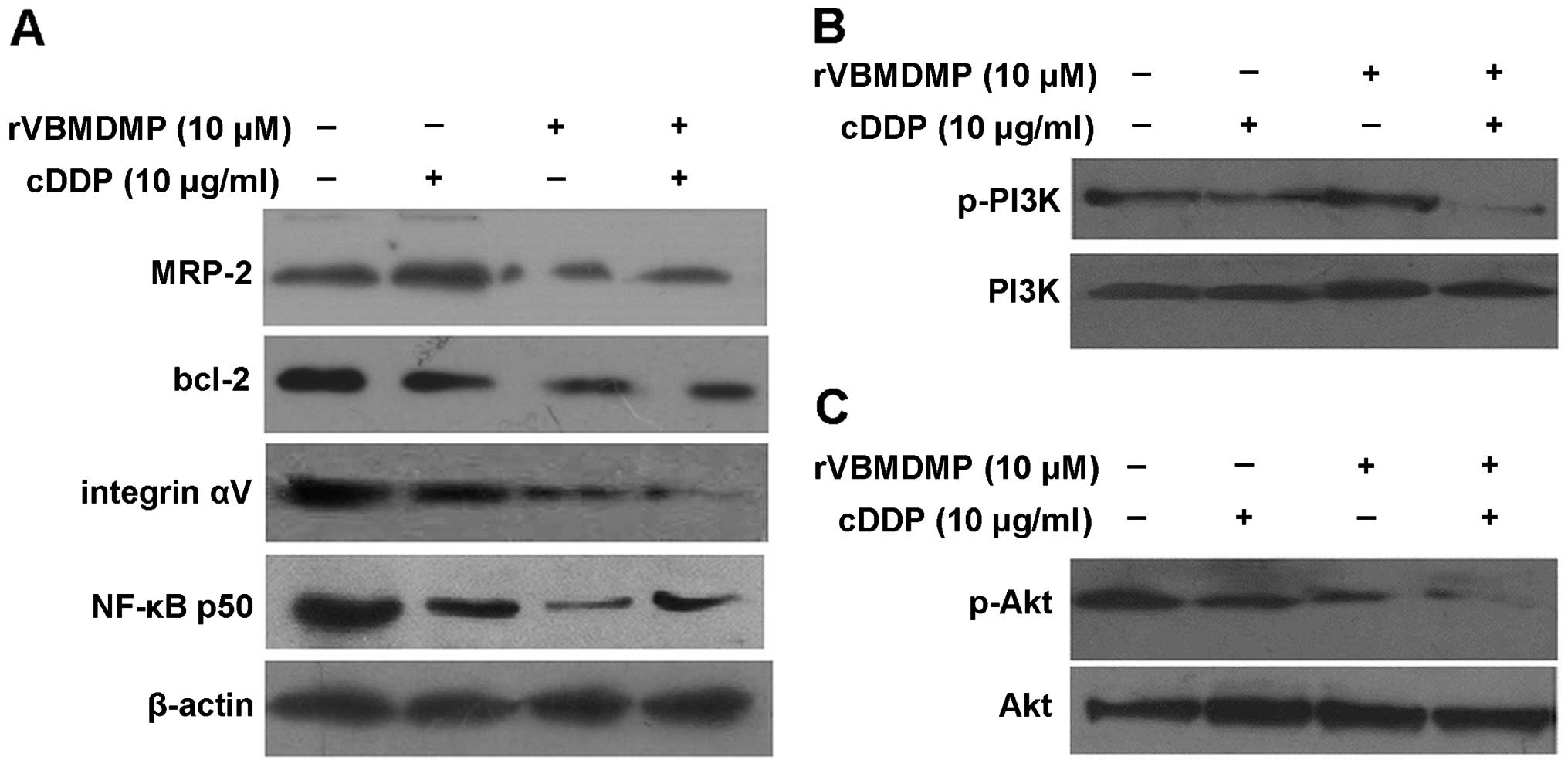
After treatment with 10 μM rVBMDMP alone, A549/DDP
cells showed downregulation of MRP-2, integrin αV and NF-κB p50
protein expression, while cisplatin alone had no effect on MRP-2,
Bcl-2, integrin αV or NF-κB p50 protein expression (Fig. 3A). However, upon the combination
treatment of cisplatin and rVBMDMP, levels of the above proteins
were significantly reduced compared with the controls. rVBMDMP
downregulation of MRP-2, integrin αV and NF-κB p50 protein
expression may be related to the reversal of A549/DDP cell drug
resistance.
Total PI3K and Akt protein levels were not altered
in all 4 A549/DDP cell treatment groups, while PI3K/Akt protein
phosphorylation was markedly decreased following 10 μM rVBMDMP
treatment, indicating that rVBMDMP inhibited PI3K and Akt
phosphorylation in the A549/DDP cells (Fig. 3B and C). Phosphorylated PI3K/Akt
levels were not altered in the cisplatin-treated group. These data
suggest that the PI3K/Akt signal transduction pathway may be
associated with the rVBMDMP-mediated reversal of multidrug
resistance.
rVBMDMP-mediated human lung carcinoma
xenograft growth inhibition in BALB/c nude mice
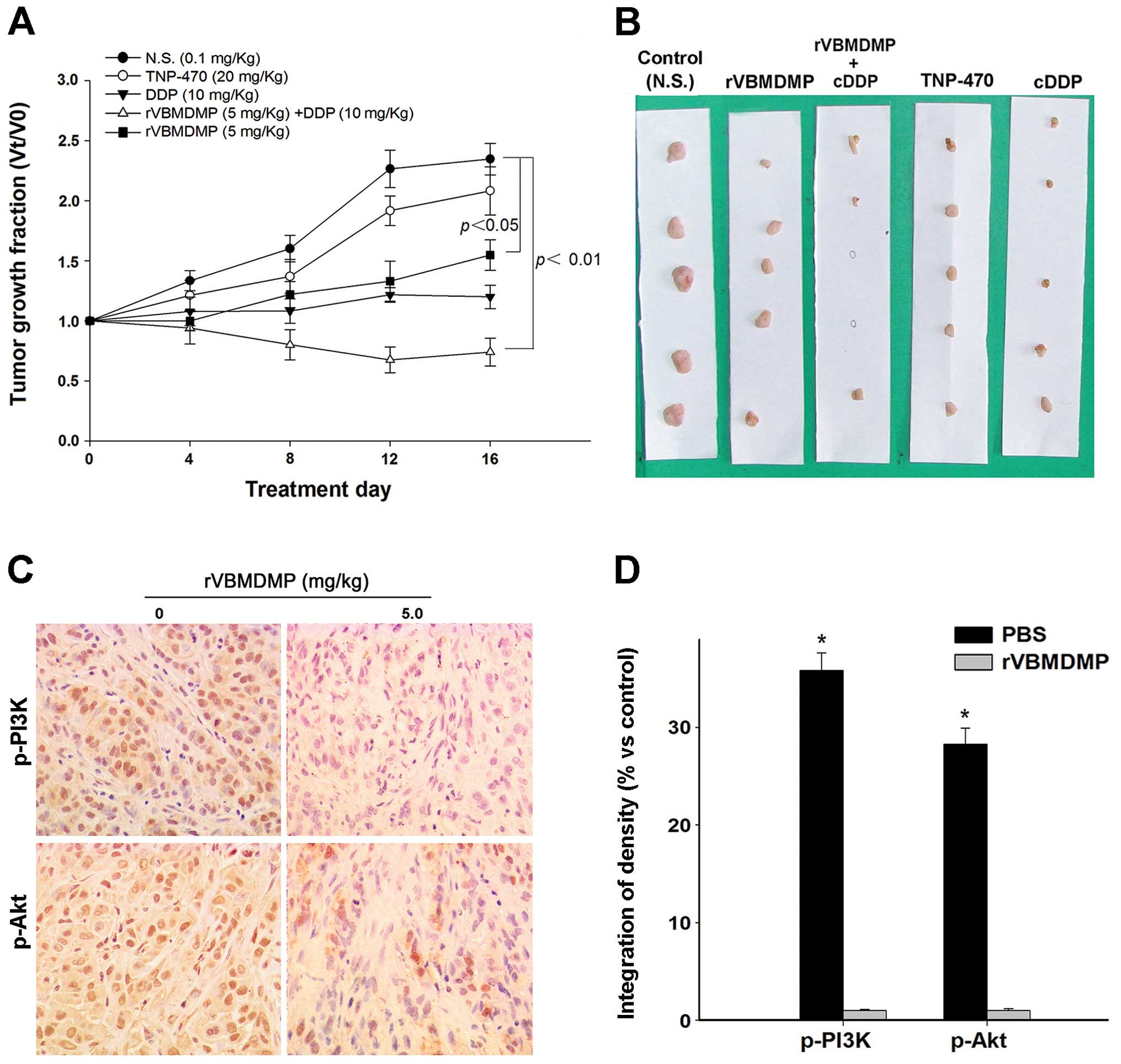
To investigate the inhibition of rVBMDMP on A549/DDP
cell growth in vivo, we examined the effects of rVBMDMP on
established primary human lung carcinoma xenograft models in nude
mice. Our results showed that rVBMDMP significantly inhibited human
lung carcinoma xenograft growth (Fig.
4). rVBMDMP treatment decreased the tumor growth rate as
evaluated by measurement of the tumor volume at regular intervals
(Fig. 4A). Administration of 5 mg/
kg rVBMDMP combined with 10 mg/kg cisplatin resulted in 37 and 74%
reduction in tumor volume, respectively (Fig. 4B). After 16 days of treatment, the
final wet tumor weight in the 5 mg/kg rVBMDMP-treated group was
reduced by 77% (P<0.05), whereas the tumor weight was reduced by
4% after TNP 470 (20 mg/kg) treatment and 42% after cisplatin (10
mg/ kg) treatment.
Discussion
Our previous research determined that rVBMDMP and
the tumstatin 197–215 amino acid peptide can significantly inhibit
tumor cell (A549 and SW480 cells) proliferation and growth in a
dose-dependent manner, (3,7) while there were no significant effects
on normal human embryo lung (KMB-17) and Chinese hamster ovary
(CHO-K1) cell proliferation and growth. These results indicate that
rVBMDMP not only preserves the anti-tumor activity of the tumstatin
197–215 amino acid peptide but also has relative selectivity to
cancer cells compared with normal cell lines. rVBMDMP significantly
inhibited human lung and colon cancer xenograft growth in nude mice
in a dose-dependent manner (3).
Therefore, the tumstatin 197–215 amino acid peptide as part of
rVBMDMP may be responsible for its inhibition of tumor cell
proliferation and growth. Shahan et al confirmed that the
tumstatin N-terminal 197–215 peptide exhibits biological function
by binding integrin αVβ3 on the tumor cell surface (11). However, the underlying detailed
mechanism is not clear. Our previous study confirmed that rVBMDMP
also binds integrin αVβ3 (4).
Integrin αVβ3 expression is highly cell specific,
with nearly no expression on the surface of resting endothelial
cells and some normal cells, such as hepatic stellate cells
(12). However, αVβ3 expression is
significantly higher in lung adenocarcinoma A549 cells (13), prostate cancer (14) and breast cancer (15). Integrin has a bidirectional signal
transmission function: its outward intracellular signal
transduction regulates cell adhesion and migration, while integrin
ligand binding triggers signals to regulate cell growth,
differentiation and apoptosis (16,17).
In the present study, the antibody array results revealed that
rVBMDMP treatment can down-regulate integrin αVβ3 subunit
phosphorylation in A549 cells, suggesting effects on its downstream
pleiotropic and complex signal transduction.
Integrin signal transduction is closely related to
FAK activation (18,19). FAK is a cytoplasmic non-receptor
tyrosine kinase and its activation is accompanied by the
accumulation of focal adhesion. The FAK signal transduction pathway
can be activated substantially by integrins. Activated FAK binds
multiple intracellular proteins that contain SH2 domains, thus
activating several signaling pathways. Among these is the PI3K/ Akt
pathway, on which we previously focused our studies (20). PI3K regulates signaling pathways
that are involved in multiple cellular functions including
survival, proliferation, apoptosis, cell differentiation and
cytoskeleton structure. PI3K is a phosphoinositide-dependent kinase
family member that specifically catalyzes PI-4,5-P2 and Ptdlns-4-P
to generate Ptdlns-3, 4, 5-P3 and Ptdlns-3, 4-P2, respectively. The
last 2 multi-phosphatidylinositol derivatives have biological roles
as messenger molecules by binding and activating Akt, thus causing
tumor cell proliferation and inhibiting apoptosis (21), which is an important cause of tumor
drug resistance (22). Thus, the
PI3K pathway may play a role in multidrug resistance (23). FAK can recruit and directly activate
PI3K, which activates its downstream target Akt.
Akt is a main target of PI3K and is closely related
to a variety of cell biological behaviors such as metabolism
regulation, cell survival and particularly apoptosis (24,25).
Activation of key survival signaling molecules such as PI3K/Akt,
especially increasing Akt phosphorylation levels, is not only
closely related to cancer cell development and apoptosis inhibition
but is also a main step leading to multidrug resistance (26,27).
Activated Akt can promote cell growth and proliferation by
phosphorylating downstream molecules such as mammalian target of
rapamycin (mTOR), p27WAF1/Cipl, GSK3 and tuberous
sclerosis complex 2 (TSC2) (28,29).
It also inhibits apoptosis via NF-κB and 14-3-3
phosphorylation-mediated down-regulation of FasL-induced apoptosis
protein (30,31) as well as phosphorylation of several
apoptosis-related molecules including Bcl-2 family members such as
Bcl-2, Bcl-xL and Bcl-xs (32,33),
inhibitor of apoptosis protein family members (IAPs) (34) and caspase-8, -9 and -3 (35), which inhibit apoptosis, thus
inducing cancer cell chemotherapeutic drug resistance (36). Our results demonstrated that rVBMDMP
treatment of lung cancer cells also affected integrin-FAK pathway
signal transduction by downregulating FAK, PI3K, Akt and NF-κB
survival signaling molecule phosphorylation and further affecting
A549/DDP lung cancer survival cell signaling, weakening its cell
survival ability and even directly inducing apoptosis.
Here, we determined that rVBMDMP treatment when
combined with cisplatin can reverse A549/DDP cell multi-drug
resistance. This result was displayed by i) a significantly
decreased cisplatin IC50 in A549/DDP cells and (2) significantly decreased MRP expression
in A549/DDP cells. The results obtained from the animal experiments
also demonstrated that rVBMDMP treatment combined with cisplatin
can effectively inhibit A549/DDP cell growth in nude mice. These
data suggest that rVBMDMP is not only an effective antitumor drug,
but it can also reverse the resistance of A549/DDP cells to
cisplatin.
Chemotherapy resistance is a major cause of
non-small cell lung carcinoma (NSCLC) chemotherapy failure and
disease progression, and chemotherapy tolerance-induced tumor cell
apoptosis is an important mechanism of tumor resistance. Cisplatin
is a commonly used drug with a high curative effect on lung cancer.
Cisplatin resistance is often indicative of multidrug resistance,
the phenomenon in which cells exhibit insensitivity to many types
of chemotherapy drugs. Therefore, clinical follow-up treatment for
patients with cisplatin resistance is often difficult.
Multidrug resistance consists of a complex
mechanism, in which MRP-2 plays a major role. MRP-2, also called
multispecific organic anion transporter (cMOAT), functions as a
transport protein for organic anions and a variety of drugs
(37). MRP-2 is considered as the
mediator of cisplatin resistance, as neither P-gp nor MRP1, related
multidrug resistance proteins, recognize cisplatin as substrate.
Ishikawa et al first demonstrated that the MRP-2/GS-X pump
could transport glutathione-cisplatin conjugates from the cells,
which mediates tumor cell resistance to cisplatin (38). The authors determined that the
glutathione S efflux pump activity in tumor cells with high MRP-2
expression was enhanced, suggesting that MRP-2 can identify and
transport glutathione-drug conjugates and promote tumor drug or
modified product efflux to produce multidrug resistance (39). The present study also observed
downregulation of PI3K/ Akt phosphorylation in human lung cancer
A549/DDP cells following rVBMDMP treatment, weakening survival
signaling. Expression of anti-apoptotic proteins Bcl-2 and MRP-2
were also reduced, thus weakening the anti-apoptotic ability and
the drug pumping effect of cisplatin-resistant cells. It has been
suggested that rVBMDMP can weaken A549/DDP cell tolerance to
cisplatin, enhance cisplatin sensitivity, facilitate endogenous
cisplatin-induced apoptosis signals and even reverse the drug
resistance traits of A549/DDP cells. Above are some of the
molecular mechanisms by which rVBMDMP increases chemotherapy
sensitivity and reverses the effects of multidrug resistance. These
results suggest that the antitumor activity of rVBMDMP on A549 lung
cancer cells was not related to A549/DDP cell drug resistance,
indicating there is no cross resistance to cisplatin and rVBMDMP.
Conversely, these results also suggest that cisplatin and rVBMDMP
affect different pathways. Therefore, rVBMDMP treatment can still
have favorable effects for patients suffering from
cisplatin-resistant lung adenocarcinoma.
In conclusion, the results of this study provide a
theoretical and experimental basis for further evaluation of the
molecular mechanisms of rVBMDMP in regulating tumor cell signaling
networks and reversing drug resistance in lung cancer.
Acknowledgements
This study was supported by the Natural Science
Foundation of Guangdong Province (grant no. S2012010008995) and the
Doctoral Fund of the Education Ministry of China (grant no.
20124423110003.
References
|
1
|
Peng SP, Fang WY, Dai WJ, Zou XQ, Liu HY
and Cao JG: Cloning expression and space conformation analysis of
vascular basement membrane-derived multifunctional peptide. Chinese
J Cancer Biother. 10:185–189. 2003.
|
|
2
|
Peng SP, Fang WY, Jiang RC, Zhou JG and
Cao JG: Prokaryotic expression of vascular basement
membrane-derived multi-functional peptide and its anti-tumor
activity assay. Zhongguo Yaolixue Tongbao. 19:678–682. 2003.
|
|
3
|
Cao JG, Peng SP, Sun L, Li H, Wang L and
Deng HW: Vascular basement membrane-derived multifunctional
peptide, a novel inhibitor of angiogenesis and tumor growth. Acta
Biochim Biophys Sin (Shanghai). 38:514–521. 2006. View Article : Google Scholar
|
|
4
|
Wang C, Cao J, Qu J, Li Y, Peng B, Gu Y
and He Z: Recombinant vascular basement membrane derived
multifunctional peptide blocks endothelial cell angiogenesis and
neovascularization. J Cell Biochem. 111:453–460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View
Article : Google Scholar
|
|
6
|
Long QZ, Zhou M, Liu XG, Du YF, Fan JH, Li
X and He DL: Interaction of CCN1 with αvβ3 integrin induces
P-glycoprotein and confers vinblastine resistance in renal cell
carcinoma cells. Anticancer Drugs. 24:810–817. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang CK, Cao JG, Peng B, Gu YX, Zheng GP
and He ZM: Inhibition of growth and motility of human A549 lung
carcinoma cells by a recombined vascular basement membrane derived
peptide. Cancer Lett. 292:261–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scheer N, Balimane P, Hayward MD, Buechel
S, Kauselmann G and Wolf CR: Generation and characterization of a
novel multidrug resistance protein 2 humanized mouse line. Drug
Metab Dispos. 40:2212–2218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tiwari AK, Sodani K, Dai CL, et al:
Nilotinib potentiates anticancer drug sensitivity in murine ABCB1-,
ABCG2- and ABCC10-multidrug resistance xenograft models. Cancer
Lett. 328:307–317. 2013. View Article : Google Scholar
|
|
10
|
Laitinen EM, Vaaralahti K, Tommiska J,
Eklund E, Tervaniemi M, Valanne L and Raivio T: Incidence,
phenotypic features and molecular genetics of Kallmann syndrome in
Finland. Orphanet J Rare Dis. 6:412011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shahan TA, Ziaie Z, Pasco S, Fawzi A,
Bellon G, Monboisse JC and Kefalides NA: Identification of
CD47/integrin-associated protein and alpha(v)beta3 as two receptors
for the alpha3(IV) chain of type IV collagen on tumor cells. Cancer
Res. 59:4584–4590. 1999.PubMed/NCBI
|
|
12
|
Huang XW, Wang JY, Li F, Song ZJ, Xie C
and Lu WY: Biochemical characterization of the binding of cyclic
RGDyK to hepatic stellate cells. Biochem Pharmacol. 80:136–143.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang CY, Fong YC, Lee CY, Chen MY, Tsai
HC, Hsu HC and Tang CH: CCL5 increases lung cancer migration via
PI3K, Akt and NF-kappaB pathways. Biochem Pharmacol. 77:794–803.
2009. View Article : Google Scholar
|
|
14
|
Ummanni R, Teller S, Junker H, et al:
Altered expression of tumor protein D52 regulates apoptosis and
migration of prostate cancer cells. FEBS J. 275:5703–5713. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang P, Enomoto A and Takahashi M: Cell
biology of the movement of breast cancer cells: intracellular
signalling and the actin cytoskeleton. Cancer Lett. 284:122–130.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Winograd-Katz SE, Fassler R, Geiger B and
Legate KR: The integrin adhesome: from genes and proteins to human
disease. Nat Rev Mol Cell Biol. 15:273–288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bouvard D, Pouwels J, De Franceschi N and
Ivaska J: Integrin inactivators: balancing cellular functions in
vitro and in vivo. Nat Rev Mol Cell Biol. 14:430–442. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu P and Luo BH: Integrin bi-directional
signaling across the plasma membrane. J Cell Physiol. 228:306–312.
2013. View Article : Google Scholar
|
|
19
|
Yin B: Focal adhesion kinase as a target
in the treatment of hematological malignancies. Leuk Res.
35:1416–1418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Riaz A, Ilan N, Vlodavsky I, Li JP and
Johansson S: Characterization of heparanase-induced
phosphatidylinositol 3-kinase-AKT activation and its integrin
dependence. J Biol Chem. 288:12366–12375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guenther MK, Graab U and Fulda S:
Synthetic lethal interaction between PI3K/Akt/mTOR and Ras/MEK/ERK
pathway inhibition in rhabdomyosarcoma. Cancer Lett. 337:200–209.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi BH, Kim CG, Lim Y, Shin SY and Lee
YH: Curcumin down-regulates the multidrug-resistance mdr1b gene by
inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett.
259:111–118. 2008. View Article : Google Scholar
|
|
23
|
Goler-Baron V, Sladkevich I and Assaraf
YG: Inhibition of the PI3K-Akt signaling pathway disrupts
ABCG2-rich extracellular vesicles and overcomes multidrug
resistance in breast cancer cells. Biochem Pharmacol. 83:1340–1348.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Konopleva MY, Walter RB, Faderl SH, et al:
Preclinical and early clinical evaluation of the oral AKT
inhibitor, MK-2206, for the treatment of acute myelogenous
leukemia. Clin Cancer Res. 20:2226–2235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neri LM, Cani A, Martelli AM, et al:
Targeting the PI3K/ Akt/mTOR signaling pathway in B-precursor acute
lymphoblastic leukemia and its therapeutic potential. Leukemia.
4:739–748. 2014. View Article : Google Scholar
|
|
26
|
Chen KC, Yang TY, Wu CC, et al: Pemetrexed
induces S-phase arrest and apoptosis via a deregulated activation
of Akt signaling pathway. PLoS One. 9:e978882014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang F, Li T, Zhang B, et al:
MicroRNA-19a/b regulates multidrug resistance in human gastric
cancer cells by targeting PTEN. Biochem Biophys Res Commun.
434:688–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ning J and Clemmons DR: AMP-activated
protein kinase inhibits IGF-I signaling and protein synthesis in
vascular smooth muscle cells via stimulation of insulin receptor
substrate 1 S794 and tuberous sclerosis 2 S1345 phosphorylation.
Mol Endocrinol. 24:1218–1229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu L, Dai Y, Chen J, et al: Maelstrom
promotes hepatocellular carcinoma metastasis by inducing
epithelial-mesenchymal transition by way of Akt/GSK-3β/Snail
signaling. Hepatology. 59:531–543. 2014. View Article : Google Scholar
|
|
30
|
Bak Y, Kim H, Kang JW, et al: A synthetic
naringenin derivative, 5-hydroxy-7,4′-diacetyloxyflavanone-N-phenyl
hydrazone (N101–43), induces apoptosis through up-regulation of
Fas/FasL expression and inhibition of PI3K/Akt signaling pathways
in non-small-cell lung cancer cells. J Agric Food Chem.
59:10286–10297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krzyzowska M, Shestakov A, Eriksson K and
Chiodi F: Role of Fas/FasL in regulation of inflammation in vaginal
tissue during HSV-2 infection. Cell Death Dis. 2:e1322011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shenoy AR, Kirschnek S and Hacker G: IL-15
regulates Bcl-2 family members Bim and Mcl-1 through JAK/STAT and
PI3K/AKT pathways in T cells. Eur J Immunol. 2500–2507. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bogdal MN, Hat B, Kochanczyk M and
Lipniacki T: Levels of pro-apoptotic regulator Bad and
anti-apoptotic regulator Bcl-xL determine the type of the apoptotic
logic gate. BMC Syst Biol. 7:672013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gravina GL, Marampon F, Giusti I, et al:
Differential effects of PXD101 (belinostat) on androgen-dependent
and androgen-independent prostate cancer models. Int J Oncol.
40:711–720. 2012.
|
|
35
|
Wang TE, Wang YK, Jin J, Xu BL and Chen
XG: A novel derivative of quinazoline, WYK431 induces G2/M phase
arrest and apoptosis in human gastric cancer BGC823 cells through
the PI3K/Akt pathway. Int J Oncol. 45:771–781. 2014.PubMed/NCBI
|
|
36
|
Liu Z, Sun C, Zhang Y, Ji Z and Yang G:
Phosphatidylinositol 3-kinase-C2β inhibits cisplatin-mediated
apoptosis via the Akt pathway in oesophageal squamous cell
carcinoma. J Int Med Res. 39:1319–1332. 2011. View Article : Google Scholar
|
|
37
|
Le Vee M, Jouan E, Stieger B, Lecureur V
and Fardel O: Regulation of drug transporter expression by
oncostatin M in human hepatocytes. Biochem Pharmacol. 82:304–311.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ishikawa T, Wright CD and Ishizuka H: GS-X
pump is functionally overexpressed in cis-diamminedichloroplatinum
(II)-resistant human leukemia HL-60 cells and down-regulated by
cell differentiation. J Biol Chem. 269:29085–29093. 1994.PubMed/NCBI
|
|
39
|
Kibria G, Hatakeyama H and Harashima H:
Cancer multidrug resistance: mechanisms involved and strategies for
circumvention using a drug delivery system. Arch Pharm Res.
37:4–15. 2014. View Article : Google Scholar
|