Introduction
Lung cancer is the leading cause of
cancer-associated human mortality (1). Non-small cell lung cancer (NSCLC) is
the most commonly diagnosed form of the disease accounting for
>85% of the cases (2). In 2004,
66.7/100,000 individuals succumbed to lung cancer in China
(3,4). Chemotherapy and radiotherapy are the
main approaches for cancer therapy in addition to surgery. However,
due to the lack of tumor specificity, these therapies kill cancer
and healthy cells, eliciting severely toxic side effects (5).
Tumor necrosis factor (TNF) was first described for
its ability to induce hemorrhagic necrosis in mouse tumor (6,7). Owing
to its notable ability to kill tumor cells in vitro or in
vivo, TNF-α has potential application value as an antitumor
biological preparation (5).
Nevertheless, a major obstacle to TNF-α in clinical application is
a series of side effects such as fever, nausea and vomiting
following frequent injections thereof, which are necessary to
achieve the maximum blood drug concentration, particularly the
tumor local drug concentration, and the narrow therapeutic window.
The proinflammatory activities of TNF-α can provoke a potentially
lethal systemic inflammatory response syndrome (SIRS) characterized
by hypotension and severe hepatitis (8). Currently, TNF-α is administered in
patients only through locoregional drug delivery systems such as
isolated limb perfusion (ILP) and isolated hepatic perfusion (IHP)
(9–11). Matrix metalloproteinases (MMPs) play
a central role in the breakdown of the extracellular matrix and are
typically upregulated in cancer cells (12–14).
MMPs are overexpressed in various types of human cancer including
NSCLC (15,16). Of all the MMPs, MMP-2 and MMP-9 are
important in the process of angiogenic progression. MMP-2 and MMP-9
have been associated with increased tumor spread and poor prognosis
in lung cancer (17). A high
expression of MMP-2 and MMP-9 in NSCLC was previously identified,
demonstrating a potential prognostic role of MMP-9 and MMP-2 in
stage 1A NSCLC patients (18).
Findings of a meta-analysis support the fact that MMP-2 may be a
prognostic factor in further prospective trials studying NSCLC
(19). Results of previous studies
showed that MMP-1, MMP-2 and MMP-9 had a high expression in A549
lung cancer cell lines (20–22).
Consequently, the A549 lung adenocarcinoma transplantation tumor
model was selected for use in the present study. Considering that
MMP-2 was overexpressed in cancer cells, we constructed the fold
on-MMP-2-rhTNF-α fusion protein, whose mutant hTNF-α linked a MMP-2
substrate sequence and a fold-on sequence (the fold on the domain
of T4 phage fibrous super-helix protein). Theoretically, when a
fusion protein reaches tumor tissues, MMPs that are centered in the
tissue hydrolyze their substrates. Thus, the receptor binding sites
of hTNF-α are exposed, hTNF-α block is disengaged and the active
form, or trimer form, of hTNF-α is concentrated in tumor tissue,
playing an antitumor targeting role.
In this study, we specifically utilized the MMP-2
substrate sequence to construct a mutant hTNF-α concentrate in
tumor tissues. Subsequently, we developed hTNF-α ramification and
assessed its efficacy and safety.
Materials and methods
Cell line and cell culture
A549 lung adenocarcinoma cells were provided by the
laboratory of the Guangdong Medical College Institute (Guangdong,
China). A549 cancer cells were routinely grown in RPMI-1640
(Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal
calf serum (Zhejiang Tianhang Biological Technology Co., Ltd.,
Hangzhou, China) and 1% antibiotics (100 U/ml penicillin, 100 μg/ml
streptomycin) (Life Technologies) at 37°C in a humidified
atmosphere with 5% CO2 and cultured to logarithmic
phase.
Animals
Kunming mice (n=35 male and n=35 female) and
specified pathogen-free (SPF) nude mice (n=25 male and n=25 female)
with an average weight of 20±2 g/mouse, were provided by the
Experimental Animal Center of Guangdong Medical College. Animal
experiments were followed and approved by the institutional
guidelines of the Guangdong Medical College.
Drugs and primary instruments
The von Willebrand factor (vWF) antibody was
purchased from Novus Biologicals (Littleton, CO, USA). The
biochemical indicators kit was purchased from Hefei TianYi
Institute of Biological Technology (Anhui, China). A biological
microscope (SZ-8D) was purchased from Wuzhou Optical Instrument
Factory (Guangxi, China). An optical microscope was purchased from
Olympus (Tokyo, Japan). The LX20 automatic biochemical analyzer was
purchased from Beckman Coulter (Brea, CA, USA). Other common
reagents and instruments were provided by the Institute of
Biochemistry and Molecular Biology, Guangdong Medical College.
Acute toxicity experiment in mice
Male and female adult Kunming mice were randomly
divided into seven groups (10 mice/group, 5 females and 5 males).
The mice were injected fusion protein (extracted by our research
group) intravenously at doses of 1,000; 800; 600; 500; 400; 250 and
125 μg/kg, respectively. The injection volume of each mouse was 0.2
ml. Subsequent to administration, the mice were observed closely
during the day, for any toxicity manifestation, such as increased
motor activity, salivation, convulsion, coma and death.
Subsequently, observations were made twice a day every 12 h. The
animals were under constant observation up to a period of 14 days,
after which the live mice were euthanized and laparotomy was
performed. Heart, liver and kidney were extracted from the mice for
pathological examination, and specimens were evaluated
histologically.
Establishment of animal model, grouping
and injection
The logarithmic phase A549 cells were suspended in a
moderate amount of RPMI-1640 medium without serum and antibiotics
(100 U/ml penicillin, 100 μg/ml streptomycin). The cell suspension
was required to be evenly mixed and with uniform density. The cell
density was adjusted to 1×107/ml following cell count.
Under aseptic conditions, 0.2 ml cell suspension was withdrawn by
using a 1 ml syringe (Shifeng Medical Apparatus and Instrument,
Chengdu, China) and administered to SPF nude mice on their right
flank with a slow axillary subcutaneous injection. The long
diameters a) and short diameters b) of the
subcutaneously-transplanted tumor of each SPF nude mouse was
measured using a vernier caliper (Guilin Guanglu Measuring
Instrument Co., Ltd., Guilin, China). Tumor volumes were calculated
using the formula V=1/2ab2. Drug administration was
initiated when the tumor volume ranged 100–150 mm3.
After the tumor-bearing SPF nude mice model was
successfully established, the mice were randomized into the saline
group, fusion proteins with high-dose group (200 μg/kg), fusion
protein with middle-dose group (100 μg/kg), fusion proteins with
low-dose group (50 μg/kg), and standard substance group (2 μg/kg)
according to the weight (n=10 mice/group. In each group, mice were
administered the drug once on alternate days, with the intratumor
injection volume of the standard substance group being 0.1 ml and
that of the fusion protein being 0.2 ml, consecutively for 18 days.
The volume of the subcutaneously transplanted tumor of the SPF nude
mice was measured every 3 days. The SPF nude mouse diet, mental
state and growth of the transplanted tumor were observed during the
period of drug delivery on a daily basis. A growth curve was
subsequently drawn based on the tumor volume results.
Treatment of SPF nude mice and tumor
After 24 h of drug administration, blood was drawn
from orbital venous in mice to detect their biochemical indicators,
subcutaneous tumor tissue was selected, and hearts, livers and
kidneys were extracted. The organs were sliced and stained with
hematoxylin and eosin (H&E). The cell morphology of the viscera
was observed to evaluate the side effects of transformed fusion
proteins. Tumor tissues of each group were detected by
immunohistochemistry. Measurements of the tumor inhibition rate
were made using the formula:
R=(1-V1)x100%/V2, where R is the tumor
inhibition rate, V1 the average tumor volume of the
experimental group, and V2 the tumor volume of the
control group.
Immunohistochemical detection of tumor
angiogenesis and microvascular density
Tumor tissues were assessed using the
immunohistochemical streptavidin-peroxidase (SP) technique, using
the Weidner capillary counting method (23) to calculate the microvessel quantity:
a low power lens (x100) was used to select three areas with the
highest density of blood vessels. At high power (x400), each area
was divided four visual fields and the average microvascular number
was counted.
In situ detection of apoptosis by TUNEL
(apoptosis detection assays)
Using the In situ cell death detection kit (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China), sections were examined
under a light microscope in randomly selected five high-power
fields. The apoptotic index was calculated using in situ
labeling of terminal deoxynucleotidyl transferase-mediated nick
end-labeling (TUNEL).
Statistical analysis
Data were presented as mean ± SD and analyzed using
SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA) using
the formula: Probit (P)=−2.617+4.291X. The Chi-square test was used
to determine the goodness-of-fit. Comparisons of the mean of two
samples were performed using the t-test, while comparisons of
numerous samples was performed using one-way analysis of variance
(one-way ANOVA). To determine homogeneity of variances when the
population variance was equal the least significant difference
(LSD) was employed. By contrast, Tamhane’s T2 test was utilized for
the unequal variances. P<0.05 was considered statistically
significant.
Results
Acute toxicity experiment results of
fusion protein fold on-MMP-2-rhTNF-α to mice
Mice in the high-dose groups (1,000, 800 and 600
μg/kg) became tachypneic, systemic cyanotic and costive, whereas
mice in the low-dose groups (500, 400, 250 and 125 μg/kg) had no
obvious abnormalities. The death time was ~1–5 days following
treatment. The dead animals were dissected and the main organs
(heart, liver and kidney) were observed with the naked eye. No
obvious pathological changes were identified.
The probit method (Table
I) was utilized to analyze data through SPSS 17.0 statistical
software using the formula: Probit (P)=−2.617+4.291X. The
Chi-square test result showed that Probit (P)=0.911 yielded an
excellent goodness-of-fit.
 | Table ILD50 concerning fusion
protein fold on-MMP-2-hTNF-α intravenous drug delivery in Kunming
mice (probit method). |
Table I
LD50 concerning fusion
protein fold on-MMP-2-hTNF-α intravenous drug delivery in Kunming
mice (probit method).
| Dose (μg/kg) | Sample (n) | Mortality (n) | Distribution of death
(days) | Death rate (%) | LD50
(μg/kg) | 95% confidence limit
(μg/kg) |
|---|
|
|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|
| 1,000 | 10 | 10 | 2 | 4 | 2 | 1 | 1 | 0 | 0 | 100 | | |
| 800 | 10 | 7 | 1 | 3 | 2 | 1 | 0 | 0 | 0 | 70 | | 522.0 |
| 600 | 10 | 5 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 50 | | |
| 500 | 10 | 3 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 30 | 609.8 | |
| 400 | 10 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 20 | | 718.1 |
| 250 | 10 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 10 | | |
| 125 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | | |
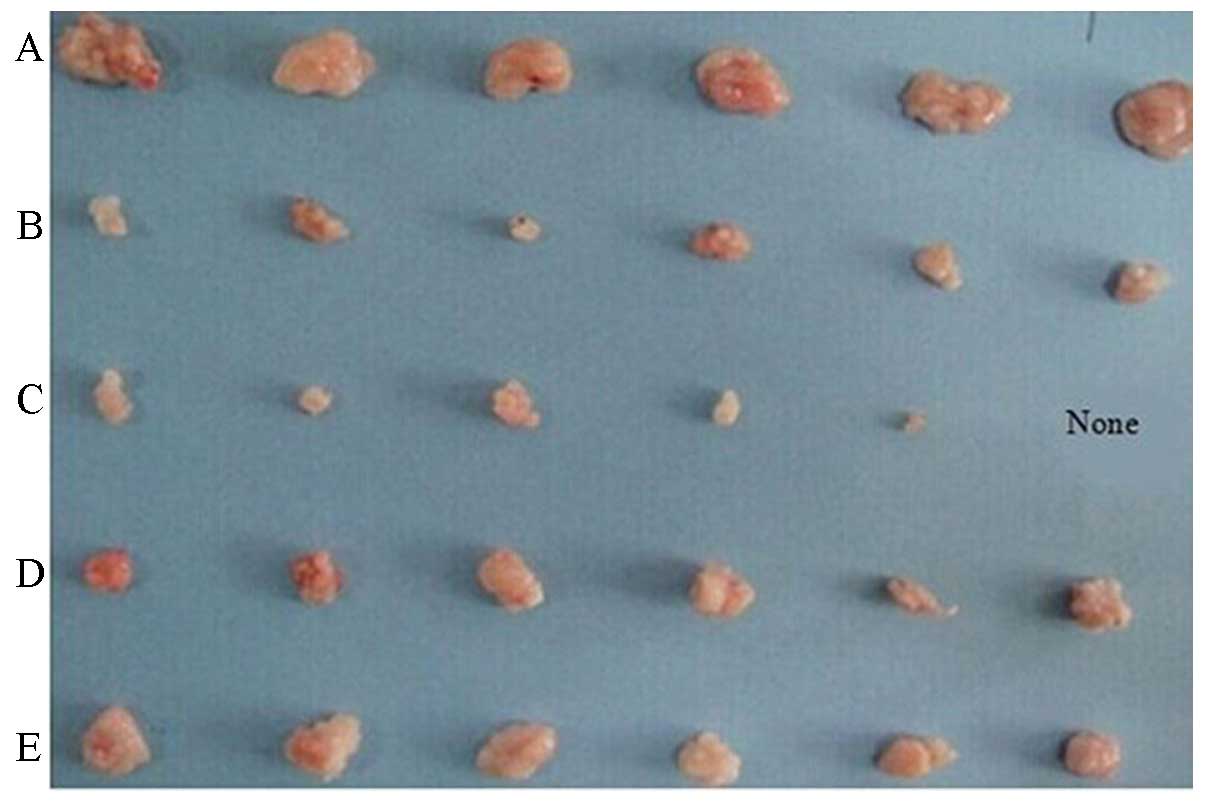
Growth inhibitory effect of fusion
protein on A549 lung cancer-transplanted solid tumor
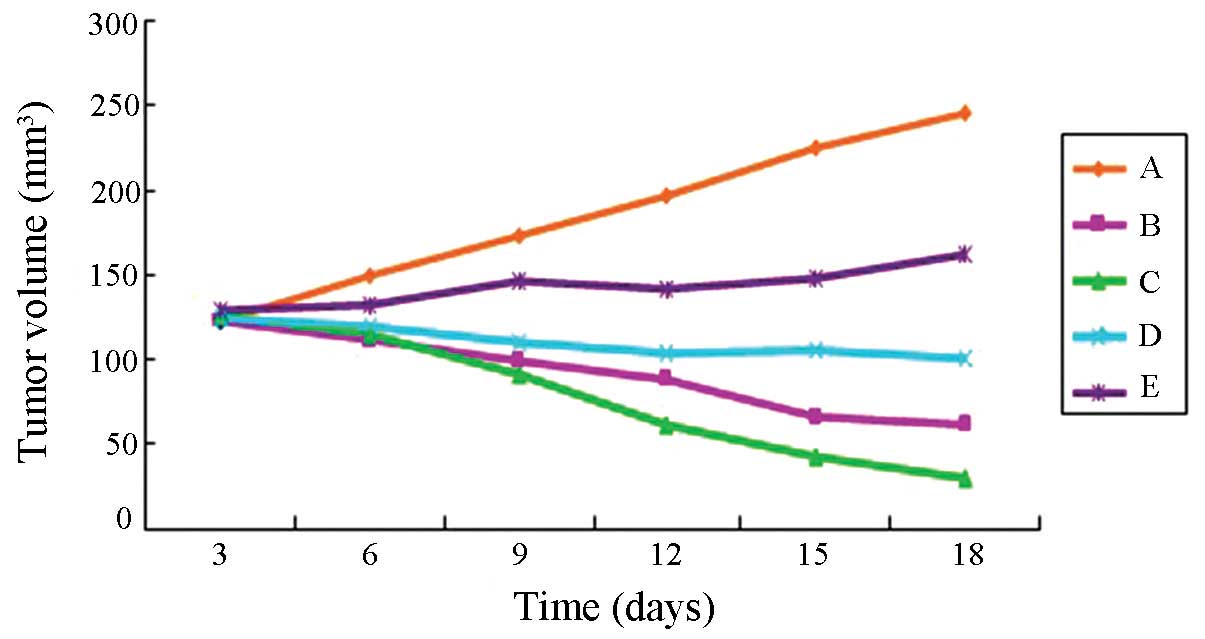
The tumor growth curve was drawn based on the marked
changes in transplanted tumor volume (Fig. 1). Statistic analysis revealed that
the inhibition ratio of the high-dose experimental, standard hTNF-α
and middle-dose experimental groups, respectively, was 85.91, 72.25
and 55.66%. Tumor growth was markedly inhibited in the high-dose
experimental, standard hTNF-α and middle-dose experimental groups
(Table II). Tumor volume and
weight were lower than the control group (P<0.01). In Fig. 2, the result of the tumor volume for
all the groups was consistent with the abovementioned results. A
significant dose-dependent association was identified. In the
experiments, the mice in the standard hTNF-α group exhibited
pupillary dilation and were lethargic. Mice in the fusion protein
groups were vigorous and flexibile in action. This result indicated
that the toxicity of the fusion protein on mice in the fusion
protein groups was decreased as compared to that in the standard
hTNF-α group, indirectly proving that fold on-MMP-2-rhTNF-α serves
as a good target for the selection of tumor cells.
 | Table IIThe role of fusion protein fold
on-MMP-2-hTNF-α to A549 lung cancer solid tumors of nude mice. |
Table II
The role of fusion protein fold
on-MMP-2-hTNF-α to A549 lung cancer solid tumors of nude mice.
| Groups (g) | Tumor weight
(mm3) | Tumor volume rate
(%) | Antitumor |
|---|
| A | 0.32±0.07 | 245.32±39.08 | - |
| B | 0.09±0.32a | 60.88±22.10a | 72.25 |
| C | 0.05±0.35a | 30.40±22.97a | 85.91 |
| D | 0.14±0.30a |
100.01±22.26a | 55.66 |
| E | 0.23±0.04 | 162.27±29.14 | 29.76 |
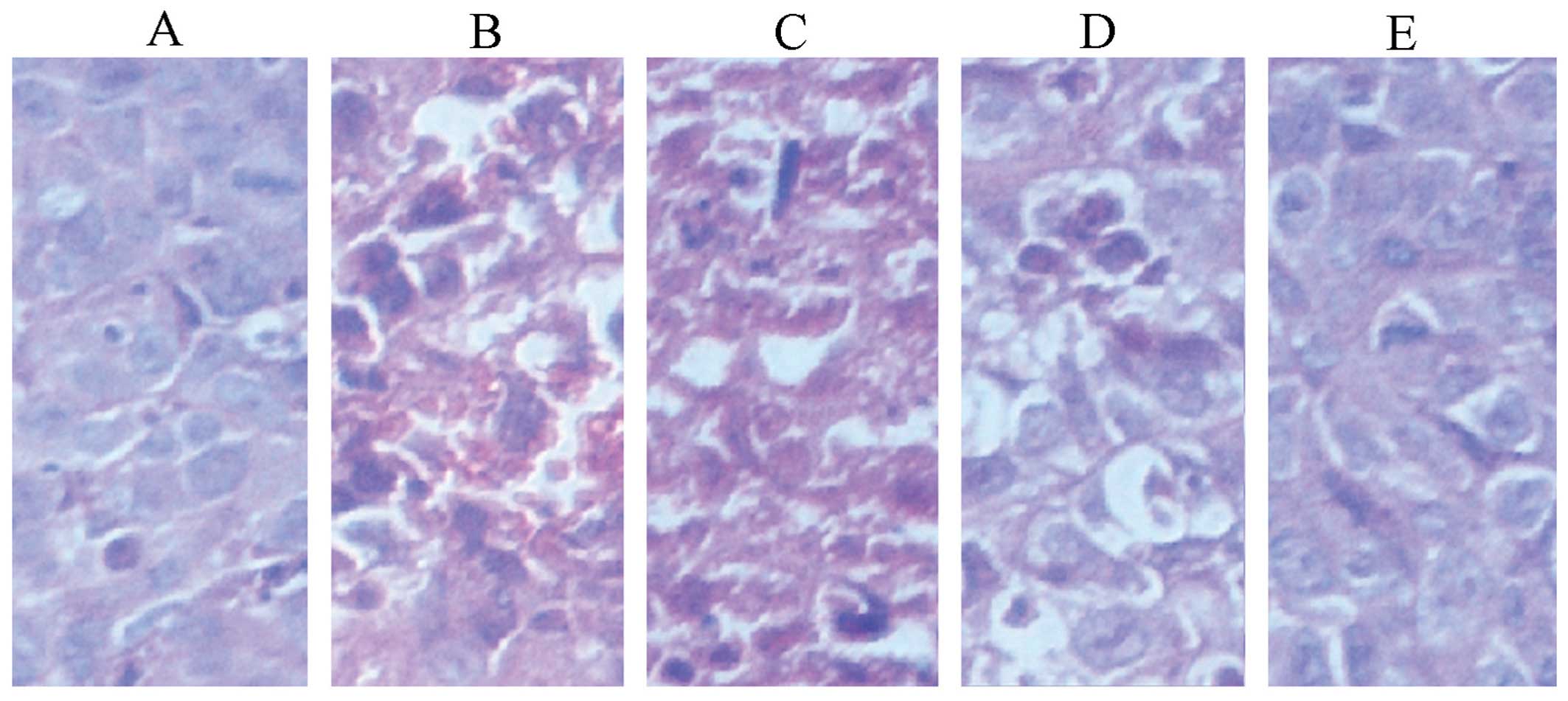
Effect of fusion protein on tumor
tissues
Tumor tissue sections stained with H&E had a
large area of necrosis cells, incomplete cell membranes and
karyopyknosis in the standard hTNF-α group (Fig. 3). In the high-dose experimental
group, almost all the cells became necrotic. The cell structure
disappeared and the necrotic area was red. In the saline and
low-dose experimental groups marked tumor cell growth was
identified. The cells had coincident size, abundant cytoplasm, and
obvious mitotic figure. However, in the middle-dose experimental
group, the cells had a disorderly arrangement and had vacuolar
degeneration, and an uneven size.
Effect of fusion protein on SPF nude
mouse viscera
The perspective of biological enzyme levels and
histomorphology concerning the effects of fusion protein on
tumor-bearing mouse viscera are analyzed below.
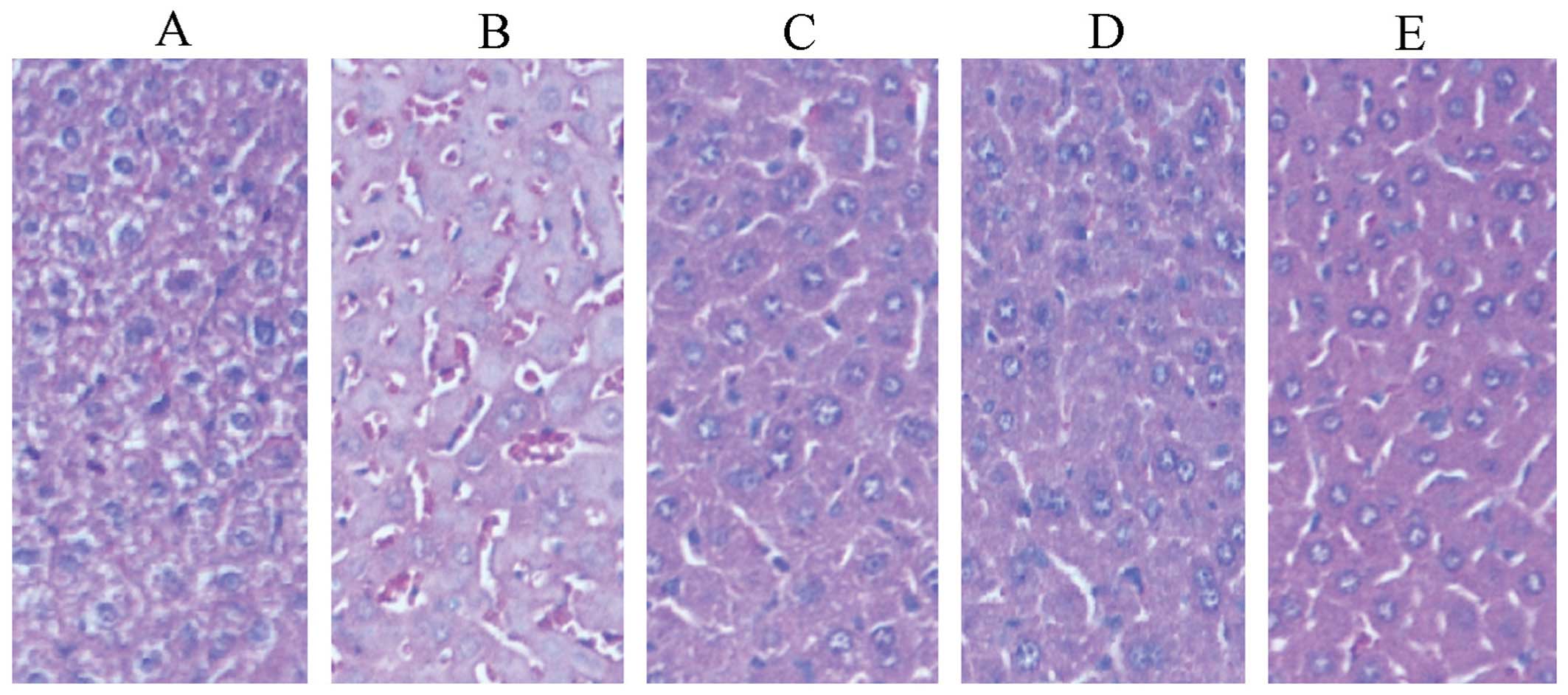
Effects on livers
Fig. 4 shows the
pathological sections of livers. In the fusion protein groups, the
number of liver cells decreased, the liver plate and liver blood
sinus were clear and a radiating arrangement was identified at low
magnification, as compared to the saline group.
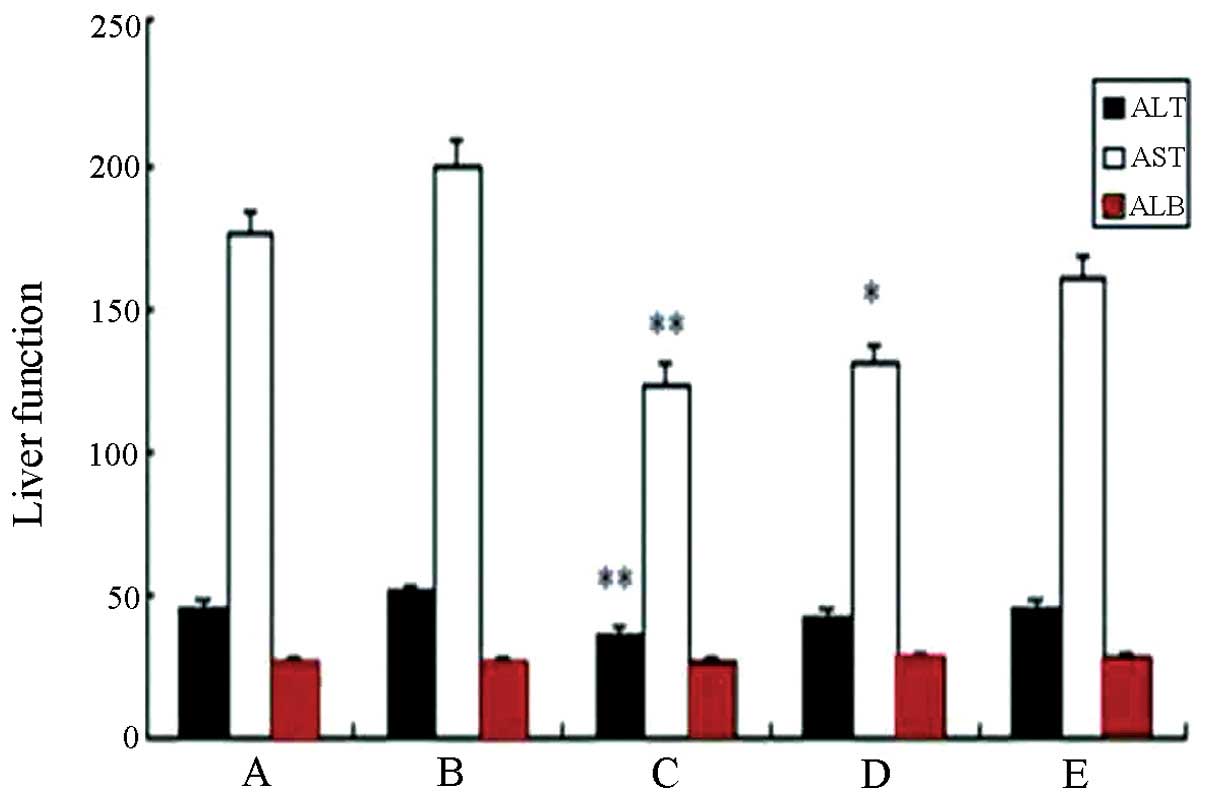
Serum concentrations of glutamic-pyruvic
transaminase (ALT) and aspartate aminotransferase (AST) in the
high-dose experimental group were significantly lower than that in
the standard hTNF-α group (P<0.01) (Table III and Fig. 5). The results showed that the effect
of fusion protein on the liver injury of tumor-bearing mice in the
high-dose experimental group was significantly smaller than of the
standard substance of TNF. Compared with the saline group, no
significant difference was identified in any of the groups
(P>0.05). Similarly, no significant difference was found in the
albumin (ALB) levels, suggesting that the protein synthesis
function of liver was not significantly affected by different doses
of medication.
 | Table IIIMeasured values of hepatic function
of fusion protein fold on-MMP-2-hTNF-α on A549 lung cancer solid
tumors of nude mice. |
Table III
Measured values of hepatic function
of fusion protein fold on-MMP-2-hTNF-α on A549 lung cancer solid
tumors of nude mice.
| Group | ALT (IU/l) | AST (IU/l) | ALB (g/l) |
|---|
| A | 45.17±15.32 | 175.37±69.40 | 27.00±1.38 |
| B | 51.12±10.41 | 198.93±49.93 | 26.93±1.30 |
| C | 36.54±4.93a |
123.67±20.89a | 27.13±2.33 |
| D | 43.31±12.30 |
130.30±32.45b | 27.34±1.28 |
| E | 45.40±8.16 | 160.77±72.95 | 27.89±1.95 |
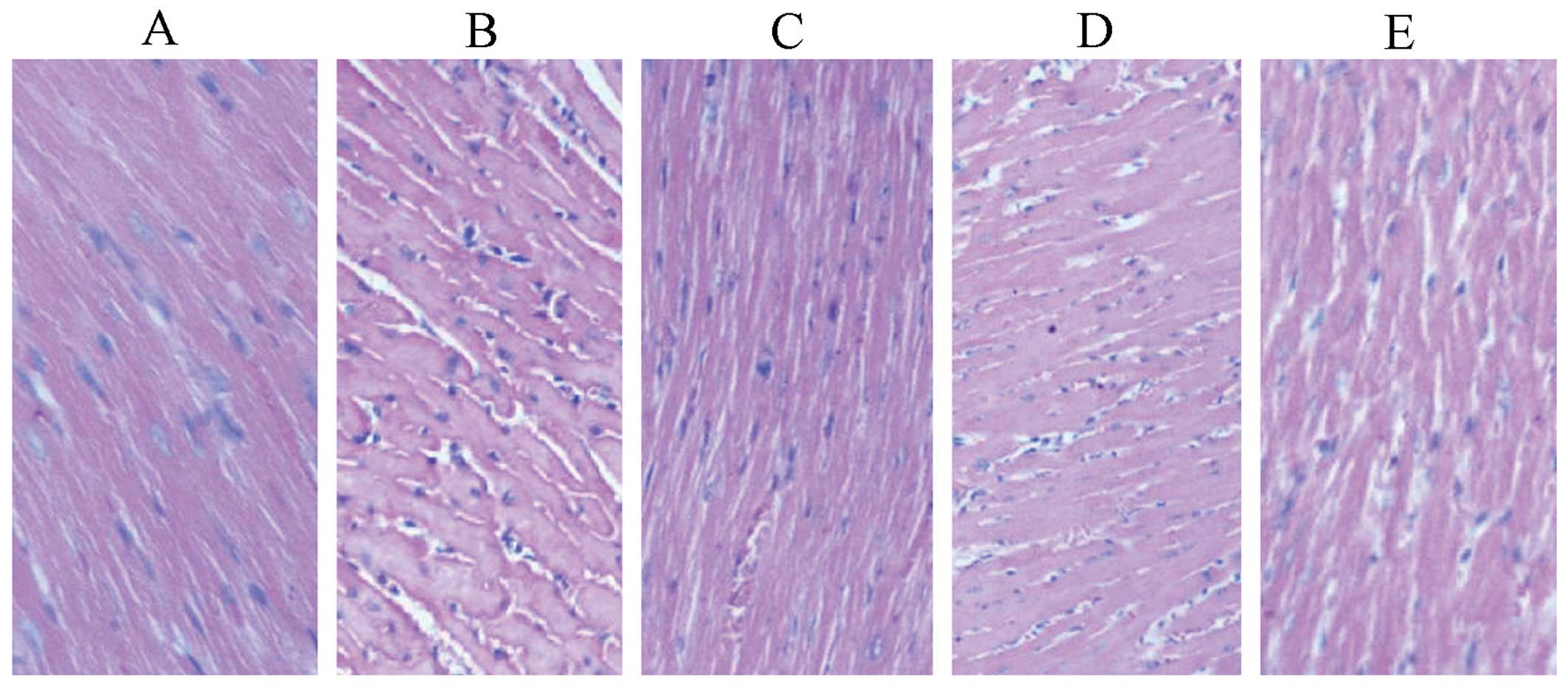
Effects on hearts
In the standard hTNF-α group, there was a little
congestion between muscle bundles and inflammatory cell
infiltration, whereas in the other treatment groups, the
myofilaments were dense, ranked neatly and the nucleus was
centrally located. No obvious abnormality was found (Fig. 6).
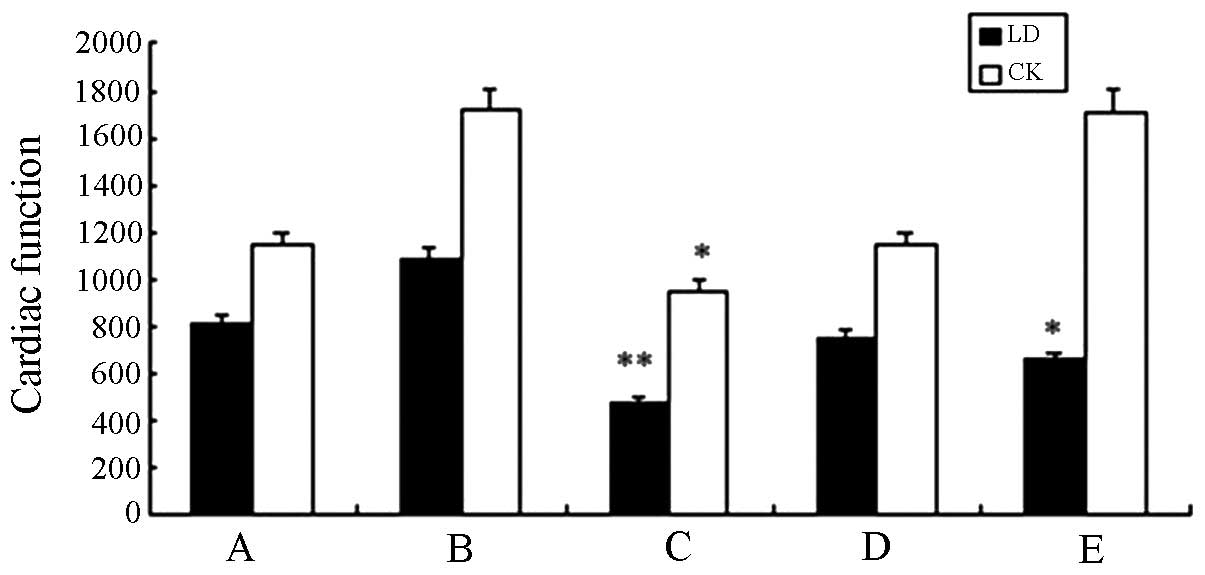
Compared with the saline group, no significant
difference (P>0.05) was identified with any of the remaining
groups (Table IV). Serum
concentrations of lactic dehydrogenase (LD) and creatine kinase
(CK) in the high-dose experimental group were significantly lower
than those in the standard hTNF-α group (P<0.05), thus, high
doses of fusion protein may play a protective role in myocardial
cells. A histogram that visually demonstrated the changing trends
of LD and CK among the groups was created (Fig. 7).
 | Table IVMeasured values of LD and CK of
fusion protein fold on-MMP-2-hTNF-α on A549 lung cancer solid
tumors of nude mice. |
Table IV
Measured values of LD and CK of
fusion protein fold on-MMP-2-hTNF-α on A549 lung cancer solid
tumors of nude mice.
| Group | LD (IU/l) | CK (IU/l) |
|---|
| A | 809.56±441.68 | 1145.87±466.23 |
| B | 1080.31±448.03 | 1719.54±425.56 |
| C |
475.46±107.54a |
951.51±171.32b |
| D | 746.61±260.35 | 1140.10±460.50 |
| E |
656.02±334.65b |
1715.04±1100.62 |
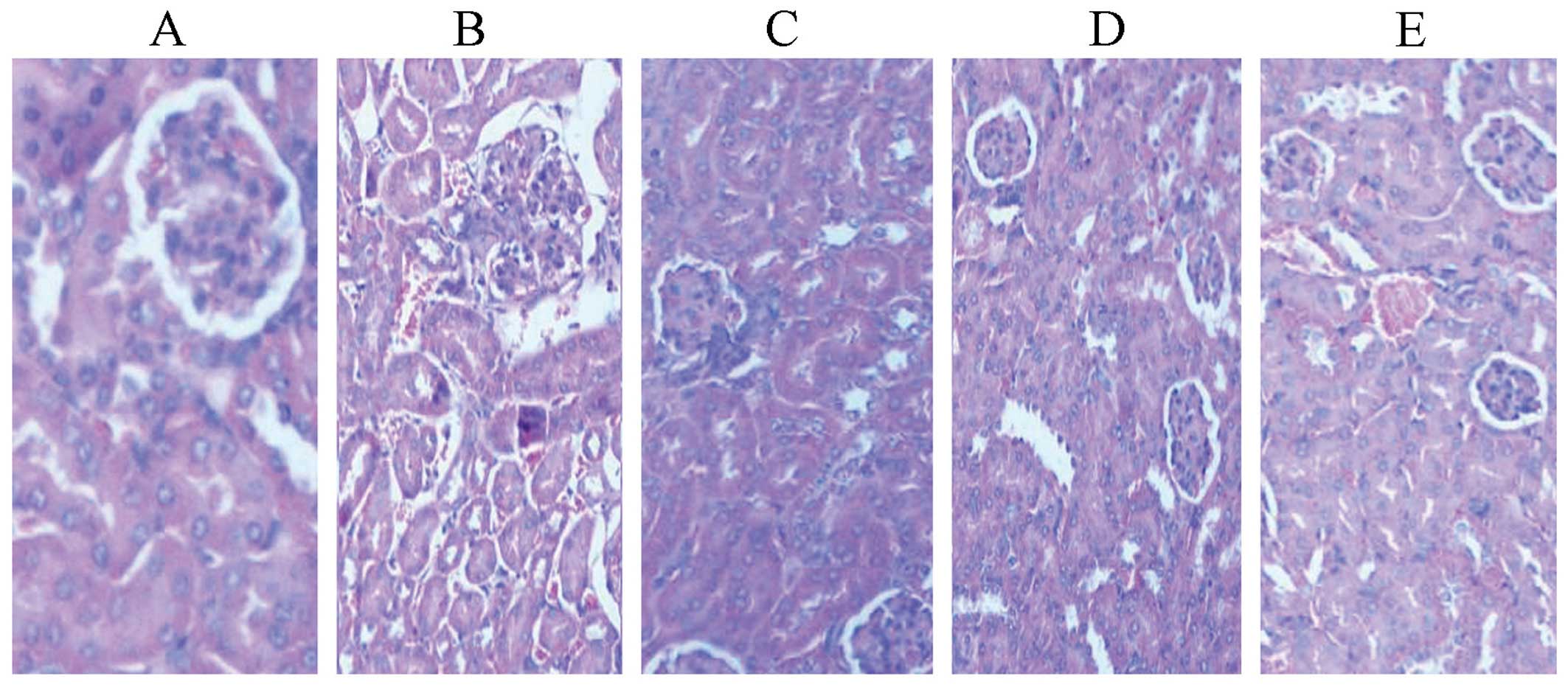
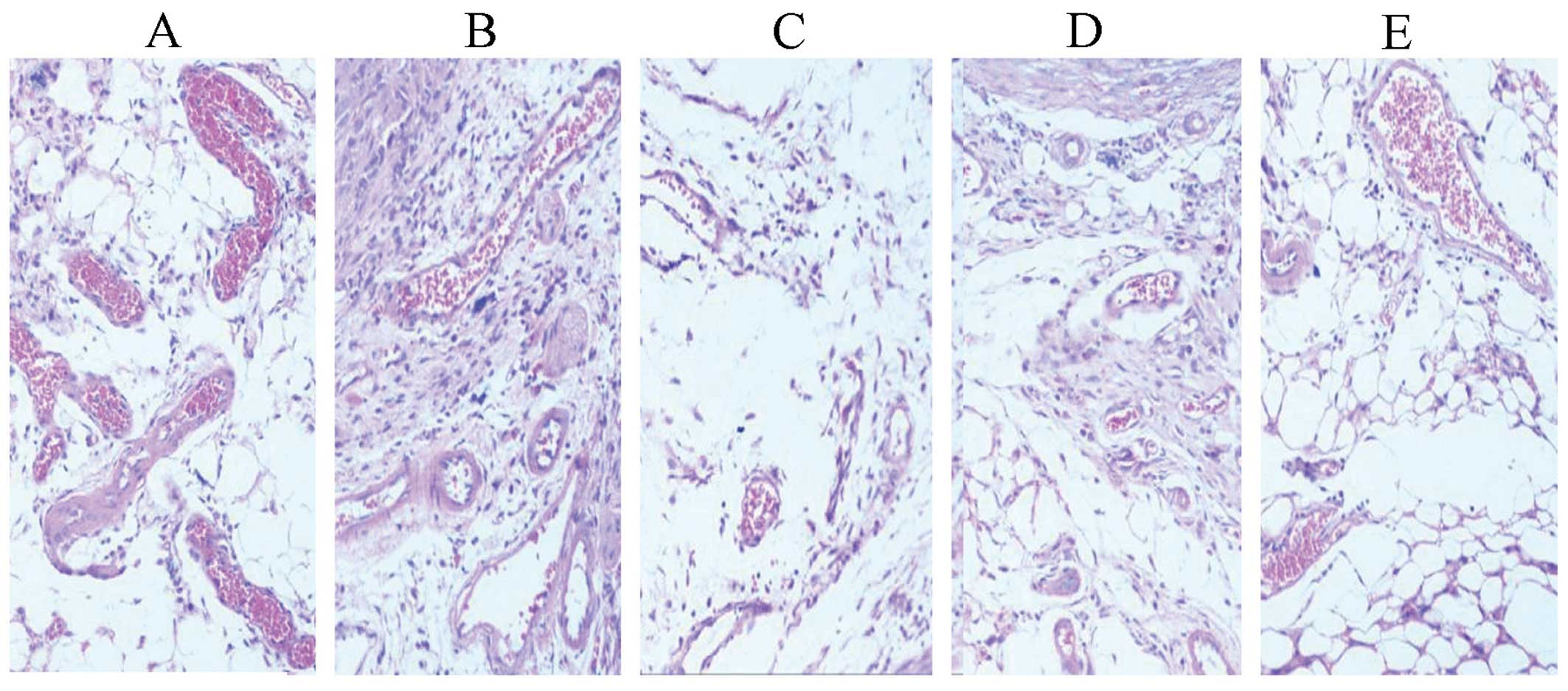
Effects on kidneys
In the standard hTNF-α group, cuboidal surface cells
of proximal convoluted tubules (PCT) and distal convoluted tubules
(DCT) were fused. The nucleus was reduced, while the nephrons were
unsound (Fig. 8).
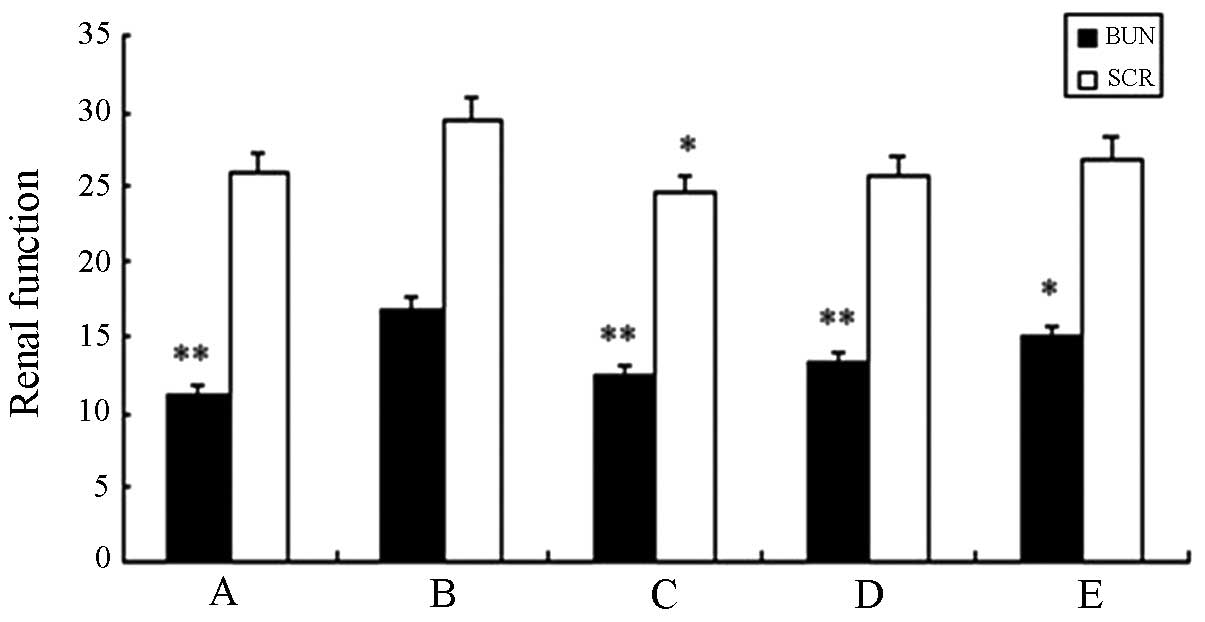
Table V and Fig. 9 show that compared with the saline
group, no significant difference (P>0.05) was identified in any
of the remaining groups. The level of blood urea nitrogen (BUN) and
serum creatinine (SCR) in the high-dose experimental group showed a
statistical significant difference when compared with the standard
hTNF-α group (P<0.01). This result suggested that the fusion
protein did not sufficiently affect the nephritic filtration
function and no obvious renal damage was identified.
 | Table VMeasured values of renal function for
fusion protein fold on-MMP-2-hTNF-α on A549 lung cancer solid
tumors of nude mice. |
Table V
Measured values of renal function for
fusion protein fold on-MMP-2-hTNF-α on A549 lung cancer solid
tumors of nude mice.
| Group | BUN (mmol/l) | SCR (μmol/l) |
|---|
| A | 11.17±1.06a | 25.90±2.88 |
| B | 16.84±2.67 | 29.50±3.70 |
| C | 12.60±2.17a | 24.53±3.04b |
| D | 13.27±1.71a | 25.80±7.58 |
| E | 14.92±1.57b | 26.92±4.20 |
Detection of the fusion protein effect on
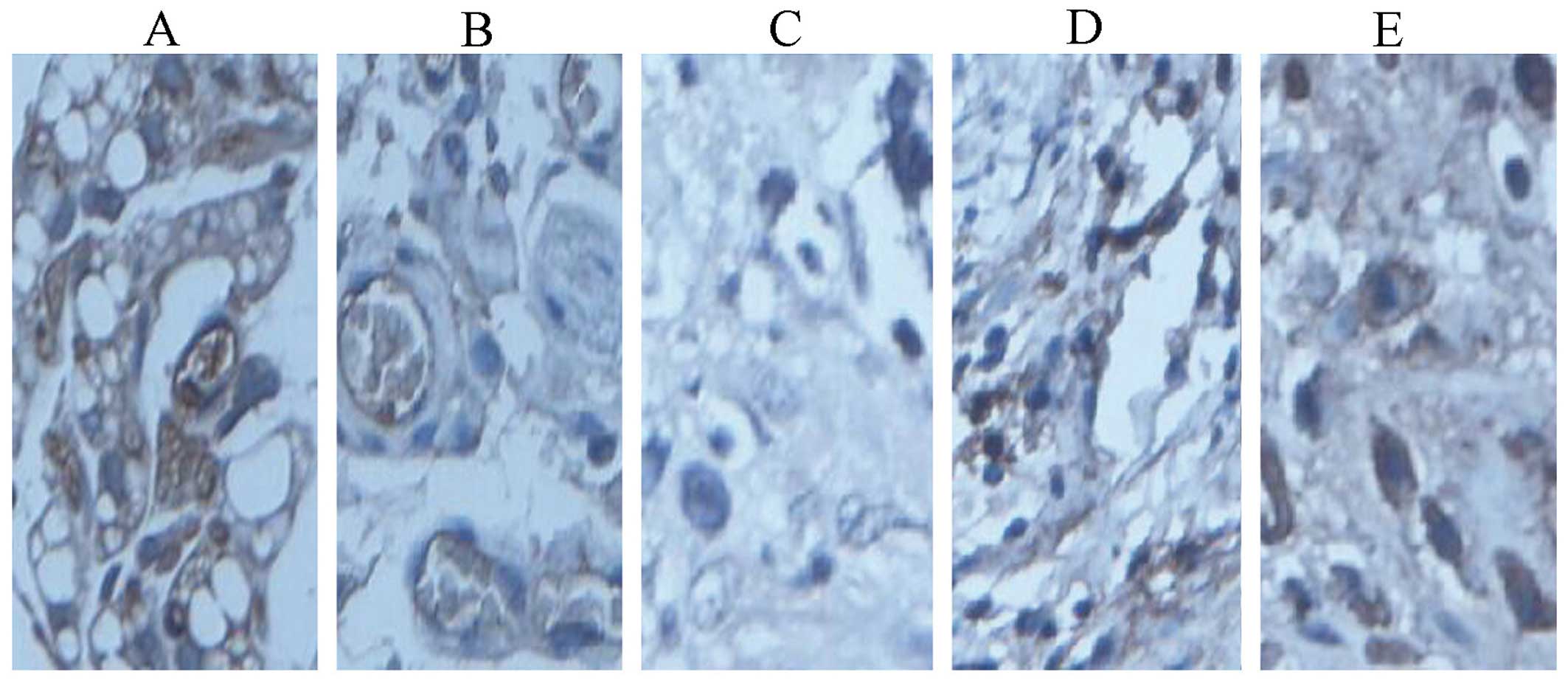
tumor angiogenesis by immunohistochemistry
We observed the richness and angiogenesis of
interstitial blood vessel in all the groups (Fig. 10).
vWF was closely associated with the multiplication
of vascular endothelial cells. The adhesive protein, which was
synthesized in endothelial cells was considered to be the most
reliable marker of endothelial cells in culture (24–27).
Fig. 11 shows the
immunohistochemical result of tumor tissue identified by the
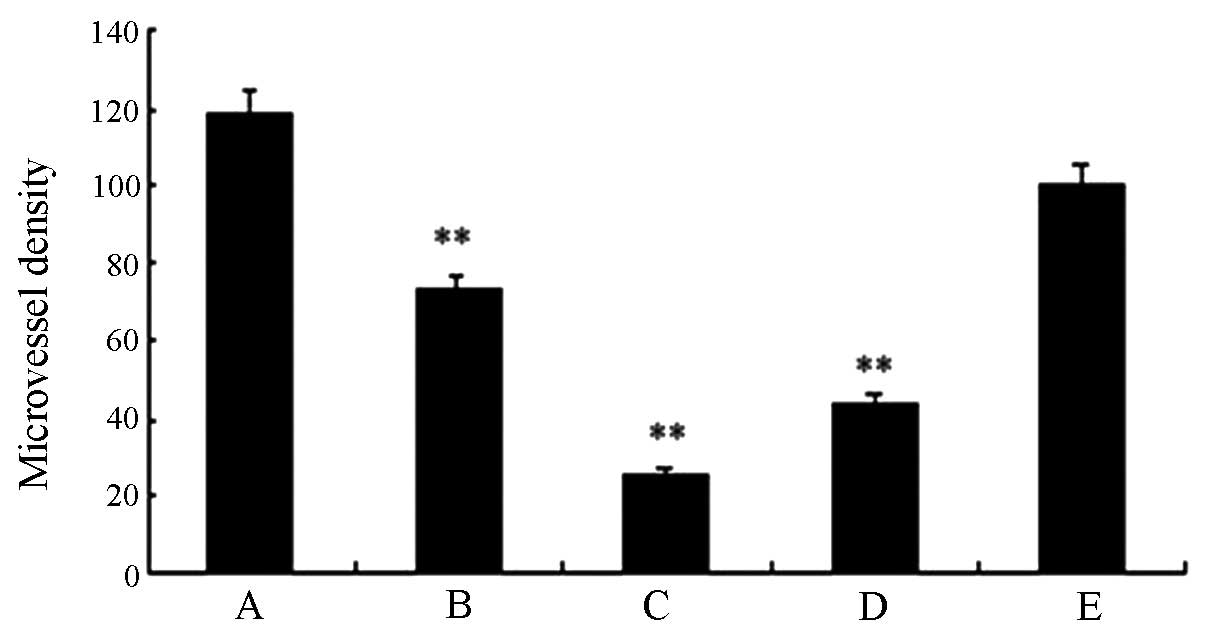
immunofluorescent detection of vWF. Fig. 10 indicates that the fusion protein
inhibited angiogenesis. The microvessel density (MVD) of the high-
and middle-dose experimental groups and the standard hTNF-α group
exhibited statistical significance (P<0.01) compared with the
saline group (Fig. 12). This
finding was consistent with the results obtained from
immunohistochemical and H&E staining. Thus the fusion protein
was able to inhibit angiogenesis.
In situ detection of apoptosis by
TUNEL
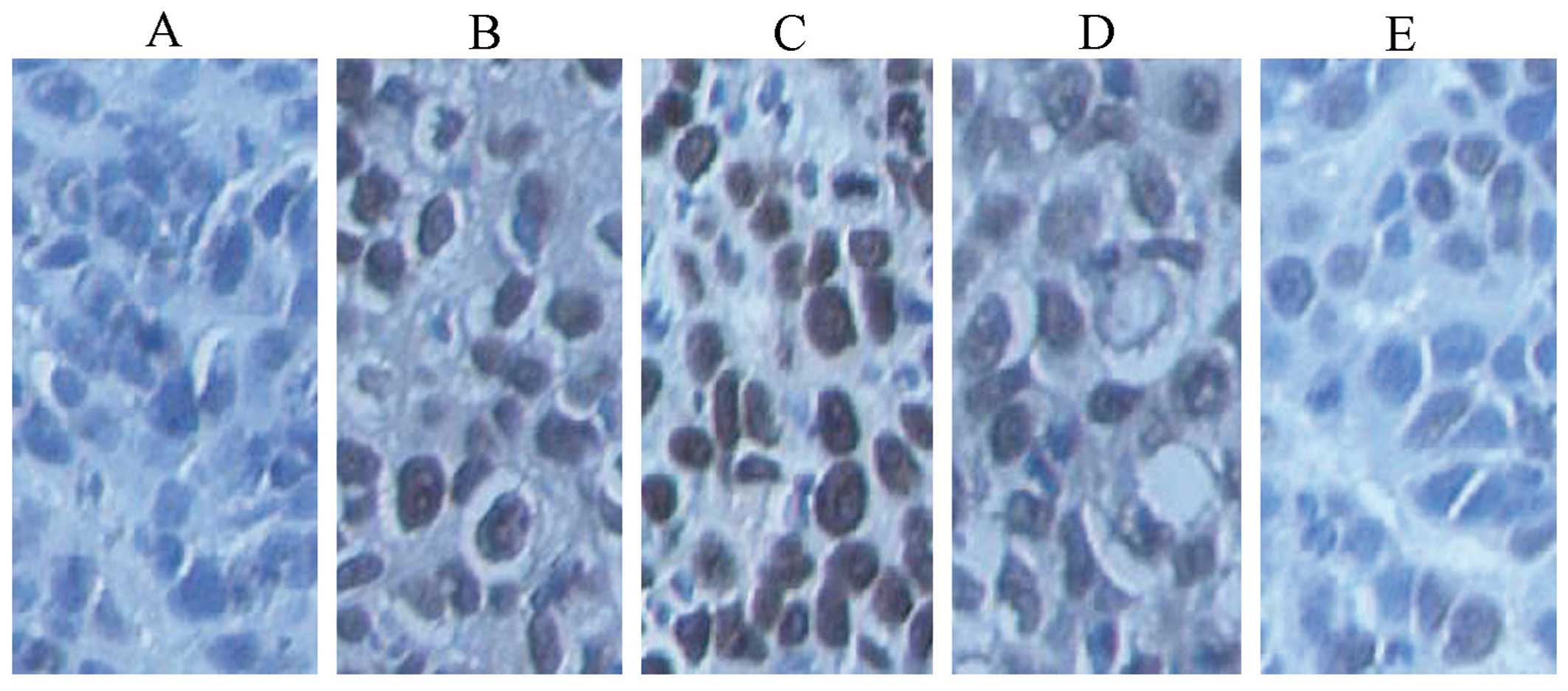
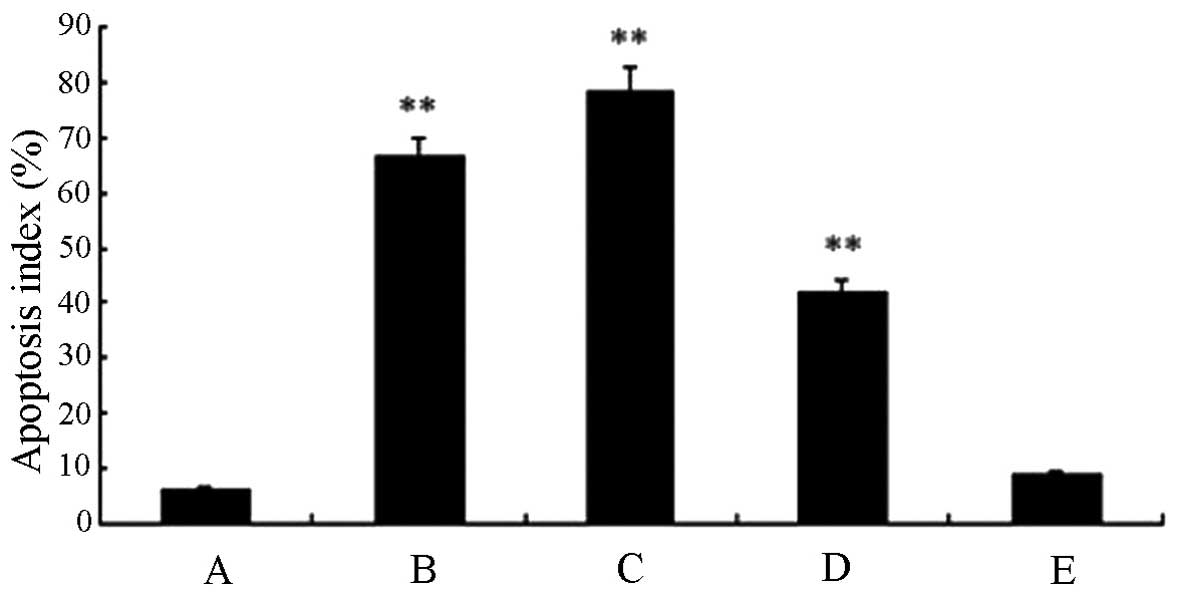
Fig. 13 shows the
microscopic area of apoptotic cells in transplanted tumor tissue by
TUNEL assay. The data in Fig. 14
were statistically processed. The differences in the high-dose
experimental and standard hTNF-α groups were particularly evident.
The apoptotic index reached 78.78 and 66.65%, respectively, and
indicated a significant statistical difference (P<0.01). The
apoptotic index of the middle-dose group was 42.31%
(P<0.01).
Discussion
Various strategies have been explored for the
tumor-targeted delivery of TNF-α over the last two decades, such as
the use of inducible or tissue-specific viral vectors for cancer
gene therapy and the generation of fusion proteins specific for
certain molecular cancer markers (28). Traditional chemotherapeutic drugs
have high toxicity and poor tumor targeting. Molecular-targeted
drugs possess advantages such as targeting, safety and durability,
which are crucial in alleviating the disease. The development of
medical molecular biology has led to an increase in focus on
molecular-targeted treatment, a new method for treating malignant
tumors (29). In a previous study,
Cooke et al (30) used a
genetic fusion of human recombinant TNF-α with MFE-23, a
single-chain Fv antibody fragment directed against the
carcinoembryonic antigen. Radiolabeled MFE-23::TNF-α fusion protein
bound both mouse and human TNF receptor 1 in vitro and was
able to localize effectively in nude mouse-bearing human LS174T
xenografts. As results of that study showed, following intravenous
injection the tumor/tissue ratios achieved 24 and 48 h,
respectively, were 21:1 and 60:1. Findings of those studies showed
that MFE-23::TNF-α provides an efficient means for systemically
administered cancer therapy.
Our preliminary experiments confirm that the fusion
protein has the function of killing tumor cells at the cellular
level. In this experiment, the result of H&E staining of tumor
tissues, tumor weights and volume of each dose group indicated that
the fusion protein exerted an inhibitory effect on tumor tissue.
Further contrasting the results of the pathological section and
biochemical indicator of SPF nude mouse liver, heart and kidney,
the modified fusion protein was confirmed to specifically target
tumor cells, inhibiting cell growth. However, damage to the liver,
heart and kidney was significantly reduced. The result of tumor
tissue immunohistochemical staining using vWF as the antibody
showed that fusion protein fold on-MMP-2-rhTNF-α exerted a certain
inhibitory effect on angiogenesis. Detection of apoptotic cells via
the TUNEL method demonstrated that the apoptotic index of the high-
and middle-dose experimental group was statistically significant
(P<0.01). It was also identified that tumor growth was markedly
inhibited in the high- and middle-dose experimental groups.
Recent studies have focused on the use of different
doses of fusion protein to target antitumor effect and reduce the
functional damage to organs. However, large doses of fusion protein
that are tolerant to the body have not been previously
experimentally validated. Future studies should focus on whether
modified fusion protein exerts the same inhibitory effect on other
tumors besides lung adenocarcinoma. Investigation of the cellular
level and the present experimental approach at an animal level,
confirms that the antitumor effect of modified fold
on-MMP-2-rhTNF-α was improved, and that the side effects were
reduced.
Acknowledgements
The present study was supported by the First Batch
of Science and Technology Plan in Guangdong Province (no.
2008B030301023) and the Dongguan Science and Technology Projects
for higher education institutions (nos. 200910815264 and
2012108102016).
References
|
1
|
Jo SK, Hong JY, Park HJ and Lee SK:
Anticancer activity of novel daphnane diterpenoids from Daphne
genkwa through cell-cycle arrest and suppression of Akt/STAT/Src
signalings in human lung cancer cells. Biomol Ther (Seoul).
20:513–519. 2012. View Article : Google Scholar
|
|
2
|
Wangari-Talbot J and Hopper-Borge E: Drug
resistance mechanisms in non-small cell lung carcinoma. J Can Res
Updates. 2:265–282. 2013.
|
|
3
|
Zhao P, Dai M, Chen W and Li N: Cancer
trends in China. Jpn J Clin Oncol. 40:281–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y, Wu JZ, Zhang JY, et al: Detection
of circulating vascular endothelial growth factor and matrix
metalloproteinase-9 in non-small cell lung cancer using Luminex
multiplex technology. Oncol Lett. 7:499–506. 2014.PubMed/NCBI
|
|
5
|
Wang S: The promise of cancer therapeutics
targeting the TNF-related apoptosis-inducing ligand and TRAIL
receptor pathway. Oncogene. 27:6207–6215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carswell EA, Old LJ, Kassel RL, Green S,
Fiore N and Williamson B: An endotoxin-induced serum factor that
causes necrosis of tumors. Proc Natl Acad Sci USA. 72:3666–3670.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Old LJ: Tumor necrosis factor (TNF).
Science. 230:630–632. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van Roy M, Wielockx B, Baker A and Libert
C: The use of tissue inhibitors of matrix metalloproteinases to
increase the efficacy of a tumor necrosis factor/interferon gamma
antitumor therapy. Cancer Gene Ther. 14:372–379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alexander HR Jr, Bartlett DL and Libutti
SK: Current status of isolated hepatic perfusion with or without
tumor necrosis factor for the treatment of unresectable cancers
confined to liver. Oncologist. 5:416–424. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grunhagen DJ, Brunstein F, ten Hagen TL,
van Geel AN, de Wilt JH and Eggermont AM: TNF-based isolated limb
perfusion: a decade of experience with antivascular therapy in the
management of locally advanced extremity soft tissue sarcomas.
Cancer Treat Res. 120:65–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grunhagen DJ, de Wilt JH, ten Hagen TL and
Eggermont AM: Technology insight: Utility of TNF-alpha-based
isolated limb perfusion to avoid amputation of irresectable tumors
of the extremities. Nat Clin Pract Oncol. 3:94–103. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Son KJ, Shin DS, Kwa T, Gao Y and Revzin
A: Micropatterned sensing hydrogels integrated with reconfigurable
microfluidics for detecting protease release from cells. Anal Chem.
85:11893–11901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weng Y, Cai M, Zhu J, et al: Matrix
metalloproteinase activity in early-stage lung cancer. Onkologie.
36:256–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kao SJ, Su JL, Chen CK, et al: Osthole
inhibits the invasive ability of human lung adenocarcinoma cells
via suppression of NF-κB-mediated matrix metalloproteinase-9
expression. Toxicol Appl Pharmacol. 261:105–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leinonen T, Pirinen R, Bohm J, Johansson
R, Ropponen K and Kosma VM: Expression of matrix metalloproteinases
7 and 9 in non-small cell lung cancer. Relation to
clinicopathological factors, beta-catenin and prognosis. Lung
Cancer. 51:313–321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Siejka A, Barabutis N and Schally AV: GHRH
antagonist inhibits focal adhesion kinase (FAK) and decreases
expression of vascular endothelial growth factor (VEGF) in human
lung cancer cells in vitro. Peptides. 37:63–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shao W, Wang W, Xiong XG, et al:
Prognostic impact of MMP-2 and MMP-9 expression in pathologic stage
IA non-small cell lung cancer. J Surg Oncol. 104:841–846. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qian Q, Wang Q, Zhan P, et al: The role of
matrix metalloproteinase 2 on the survival of patients with
non-small cell lung cancer: a systematic review with meta-analysis.
Cancer Invest. 28:661–669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Desmard M, Amara N, Lanone S, Motterlini R
and Boczkowski J: Carbon monoxide reduces the expression and
activity of matrix metalloproteinases 1 and 2 in alveolar
epithelial cells. Cell Mol Biol (Noisy-le-grand). 51:403–408.
2005.
|
|
21
|
Chauhan V, Breznan D, Thomson E,
Karthikeyan S and Vincent R: Effects of ambient air particles on
the endothelin system in human pulmonary epithelial cells (A549).
Cell Biol Toxicol. 21:191–205. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pardo A, Gibson K, Cisneros J, et al:
Up-regulation and profibrotic role of osteopontin in human
idiopathic pulmonary fibrosis. PLoS Med. 2:e2512005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weidner N: Current pathologic methods for
measuring intratumoral microvessel density within breast carcinoma
and other solid tumors. Breast Cancer Res Treat. 36:169–180. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mendolicchio GL and Ruggeri ZM: New
perspectives on von Willebrand factor functions in hemostasis and
thrombosis. Semin Hematol. 42:5–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao C, Qi Y, Chen W, Zhu Y and Chen X:
Effects of IKKɛ on oxidised low-density lipoprotein-induced injury
in vascular endothelial cells. Heart Lung Circ. 22:366–372. 2013.
View Article : Google Scholar
|
|
26
|
Ulger H, Karabulut AK and Pratten MK:
Labelling of rat endothelial cells with antibodies to vWF, RECA-1,
PECAM-1, ICAM-1, OX-43 and ZO-1. Anat Histol Embryol. 31:31–35.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ruggeri ZM: Structure of von Willebrand
factor and its function in platelet adhesion and thrombus
formation. Best Pract Res Clin Haematol. 14:257–279. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai W, Kerner ZJ, Hong H and Sun J:
Targeted cancer therapy with tumor necrosis factor-alpha. Biochem
Insights. 2008:15–21. 2008.PubMed/NCBI
|
|
29
|
Gaughan EM and Costa DB: Genotype-driven
therapies for non-small cell lung cancer: focus on EGFR, KRAS and
ALK gene abnormalities. Ther Adv Med Oncol. 3:113–125. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cooke SP, Pedley RB, Boden R, Begent RH
and Chester KA: In vivo tumor delivery of a recombinant single
chain Fv::tumor necrosis factor-alpha fusion [correction of factor:
a fusion] protein. Bioconjug Chem. 13:7–15. 2002. View Article : Google Scholar : PubMed/NCBI
|