Introduction
Oral squamous cell carcinoma (OSCC) is one of the
most common types of malignant tumors in the world. Approximately
275,000 new cases of oral cancer occur each year, and OSCC accounts
for more than 90% of the diagnosed cases of oral cancer (1–4). Oral
cancer is the eighth leading cause of cancer-related mortality in
men. The causes of oral cancer are tobacco, alcohol and ultraviolet
light (5). Although conservative
treatments for oral cancer, including surgery, radiation, and
chemotherapy are well advanced, the 5-year survival rate remains at
<50% (6). Surgical resection,
radiotherapy and combination therapy with chemotherapy are typical
OSCC therapeutic methods (7).
Failure of treatment is often due to local and regional recurrence.
However, due to improvements in local disease control, treatment
failure of oral cancer occurs most frequently as metastasis
(8). Therefore, new anticancer
agents are urgently required to improve the therapeutic effect.
7,8-Dihydroxyflavone (7,8-DHF) is a flavonoid that
exerts beneficial pharmacological and biochemical activities.
7,8-DHF has high selectivity and binding affinity to the
tropomyosin-receptor kinase B (TrkB) receptor and activates
downstream signaling (9,10). 7,8-DHF is a powerful synthetic
analog to brain-derived neurotrophic factor leading to robust
activation of TrkB in the mouse brain (10). Flavonoids offer neuronal protection
against oxidative stress due to glutamate toxicity (11) and show a spectrum of biological
activities, including antiinflammatory, antioxidant, antimutagenic
and anticarcinogenic effects (12–16).
Therefore, the development of anticancer agents from flavonoids and
other natural products is an important topic. However, little is
known concerning the other biological effects of 7,8-DHF. In the
present study, we investigated whether 7,8-DHF could modulate cell
cycle progression and specificity protein (Sp) repression; thus,
leading to the apoptotic death of OSCC.
Sp is a transcription factor that is generally
expressed in all mammalian cells (17), and protein expression levels of Sp1
are often greater in cancer cells than those in normal cells
(18). Sp1 is a recently defined
transcription factor (19)
including Sp/Krüppel. These factors are involved in controlling the
cell cycle, apoptosis, and angiogenesis and play an important role
in other physiological processes (20–23).
For example, Sp1 levels are higher in lung, breast, gastric,
thyroid and colorectal cancers (17,21,24).
Moreover, Sp1 plays important roles in the carcinogenesis and
metastasis of human tumors by regulating growth-related signal
transduction, apoptosis, tumor-suppressor genes, cell cycle control
molecules, oncogenes and angiogenesis-related factors (25,26).
Therefore, inhibiting Sp1 is an effective therapeutic strategy for
preventing cancer.
We specifically examined the anticancer effect of
7,8-DHF on cell viability against the OSCC cell lines HN22 and HSC4
and identified proteins regulated by 7,8-DHF in the cells. We
investigated whether expression of Sp1 and its downstream proteins
and other important apoptotic proteins were altered by 7,8-DHF
treatment. Our results provide evidence for the chemotherapeutic
efficacy of 7,8-DHF in oral squamous cells. Our results suggest
that 7,8-DHF has chemotherapeutic efficacy.
Materials and methods
Cell culture
The HN22 and HSC4 human OSCC lines were obtained
from Dankook University (Cheonan, Korea) and Hokkaido University
(Hokkaido, Japan), respectively, and were cultured in Hyclone
Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific,
Logan, UT, USA) containing 10% heat-inactivated fetal bovine serum,
and 100 U/ml each of penicillin and streptomycin (Thermo Fisher
Scientific) at 37°C with 5% CO2 in humidified air.
MTS cell viability assay
Cell viability was measured using the CellTiter 96™
AQueous assay kit (Promega, Madison, WI, USA) according to the
manufacturer’s protocol. HN22 and HSC4 cells were seeded in a
96-well plate for 24 h and then treated with 7,8-DHF (5, 10, 20 and
40 μM) for 24 and 48 h. Cell viability was measured by adding the
3-(4,5-dimethyl-thiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) dehydrogenase enzyme substrate and the electron coupling
reagent phenazine methosulfate. The plates were incubated at 37°C
in 5% CO2 for 2 h after a 24 h or 48 h post-treatment
with 7,8-DHF. Absorbance at 490 nm was recorded using a
GloMax-Multi Microplate Multimode reader (Promega).
DAPI staining
The levels of nuclear condensation and fragmentation
were observed by nucleic acid staining with DAPI. HN22 and HSC4
cells treated with 7,8-DHF were harvested by trypsinization, and
fixed in 100% methanol at room temperature for 20 min. The cells
were seeded on slides, stained with DAPI (2 μg/ml), and monitored
by FluoView confocal laser microscopy (Fluoview FV10i; Olympus
Corp., Tokyo, Japan).
Propidium iodide (PI) staining
After 48 h of 7,8-DHF treatment, the HN22 and HSC4
cells were collected by centrifugation and combined with adherent
cells. The cells were washed with cold phosphate-buffered saline
(PBS), pooled, and centrifuged before being fixed in 70% ice-cold
ethanol overnight at −20°C, and then treated with 150 μg/ml RNase A
and 20 μg/ml PI (Sigma-Aldrich, St. Louis, MO, USA). The stained
cells were analyzed, and the distribution of the cells in different
phases of the cell cycle was calculated using flow cytometry with a
MACSQuant Analyzer (Miltenyi Biotec GmbH, Bergisch Gladbach,
Germany).
Annexin V/7-AAD assay
The cells were seeded on a 100-mm dish containing
5.2×105 cells/well for HN22 cells and 8.8×105
cells/well for HSC4 cells and treated with various concentrations
of 7,8-DHF (10, 20 and 40 μM) for 48 h. Both adherent and floating
cells were harvested and washed once with PBS. The cells were
incubated with Annexin V/7-AAD for 20 min at room temperature in
the dark to detect apoptosis, followed by a 6 h incubation at 37°C.
Apoptotic and necrotic cells were analyzed by flow cytometry (Muse
Cell Analyzer; Merck Millipore, Billerica, MA, USA) using the Muse
Annexin V/7-AAD & Dead Cell kit (MCH100105; Merck Millipore).
The experiment was performed in triplicate.
Reverse transcription-polymerase chain
(RT-PCR) reaction
Total RNA was extracted from the cells using the
TRIzol® reagent (Life Technologies, Carlsbad, CA, USA),
and 2.5 μg of RNA was used to synthesize cDNA using the HelixCript™
first-strand cDNA synthesis kit (NanoHelix, Seoul, Korea). cDNA was
obtained by PCR using β-actin-specific and Sp1-specific primers as
described below under the following PCR conditions (35 cycles: 1
min at 95°C, 1 min at 56°C and 1 min at 72°C). The β-actin primers
were: forward, 5′-GTG GGG CGC CCC AGG CAC CA-3′ and reverse, 5′-CTC
CTT AAT GTC ACG CAC GAT TTC-3′; and the Sp1 primers were: forward,
5′-ATG CCT AAT ATT CAG TAT CAA GTA-3′ and reverse, 5′-CCC TGA GGT
GAC AGG CTG TGA-3′. PCR products were analyzed by 1% agarose gel
electrophoresis.
Immunocytochemistry
HN22 and HSC4 cells were seeded onto sterilized
glass coverslips on 6-well tissue culture plates for 24 h and
incubated with 7,8-DHF for 48 h. The cells were fixed and
permeabilized with Cytofix/Cytoperm solution for 30 min. The cells
were blocked with 1% bovine serum albumin and then incubated with
monoclonal Sp1 and cleaved caspase-3 antibody at 4°C overnight to
express Sp1 and cleaved caspase-3. After washing with PBS
containing 0.05% Tween-20 (PBST), Sp1 and cleaved caspase-3
antibodies were reacted with a Jackson 488-conjugated anti-mouse
and Jackson 647-conjugated anti-rabbit secondary antibody at room
temperature for 1 h and mounted with Vectashield mounting medium to
assess DAPI fluorescence (Vector Laboratories, Inc., Burlingame,
CA, USA). The cells were visualized using a FluoView confocal laser
microscope.
Pull-down assay
This method was previously described (27). Briefly, HN22 and HSC4 cell lysate
(500 μg) was reacted with Sepharose 4B or 7,8-DHF-Sepharose 4B (GE
Healthcare Bio-Sciences, Piscataway, NJ, USA) matrix beads (0.2 g)
in reaction buffer [50 mM Tris, pH 7.5, 5 mM
ethylenediamine-tetraacetic acid (EDTA), 150 mM NaCl, 1 mM
dithiothreitol (DTT), 0.01% Nonidet P-40, 2 μ/ml bovine serum
albumin, 0.02 mM phenylmethylsulfonyl fluoride (PMSF) and 1X
proteinase inhibitor cocktail]. After overnight incubation with
gentle rocking at 4°C, the beads were washed five times with
washing buffer (50 mM Tris, pH 7.5, 5 mM EDTA, 150 mM NaCl, 1 mM
DTT, 0.01% Nonidet P-40 and 0.02 mM PMSF) and proteins bound to the
beads were analyzed by western blot analysis.
Western blot analysis
HN22 and HSC4 cells were treated with 7,8-DHF (10,
20 and 40 μM) for 48 h, washed with PBS, and harvested in ice-cold
PRO-PREP™ protein extraction solution (Intron Biotechnology, Inc.,
Daejeon, Korea) containing a protease inhibitor. The extracted
proteins were measured using a Pierce® BCA protein assay
kit (Thermo Fisher Scientific). Equal amounts of protein were
separated via 10% or 15% (v/v) SDS-polyacrylamide gel
electrophoresis and then transferred onto a polyvinylidene
difluoride membrane. After blocking for 2 h at room temperature
with 5% non-fat dried milk in PBST containing 0.1% Tween-20, the
membrane was incubated overnight at 4°C with the specific
antibodies. Enhanced chemiluminescence western blotting was
performed according to the manufacturer’s instructions (Thermo
Fisher Scientific).
Statistical analysis
Statistical significance was assessed using the
Student’s t-test. P<0.05 relative to the control was considered
significant.
Results
7,8-DHF inhibits cell viability and
increases apoptosis in OSCC cells
The aim of the present study was to investigate the
efficiency of 7,8-DHF to inhibit the growth of OSCC cells. The
chemical structure of 7,8-DHF is shown in Fig. 1A. To confirm the growth inhibitory
effect of 7,8-DHF on OSCC, HN22 and HSC4 cells were treated with
7,8-DHF, and cell viability was determined by the MTS assay. As
shown in Fig. 1B, the MTS assay was
performed after treatment with 7,8-DHF at various concentrations
(5, 10, 20, 30 and 40 μM) for 24 and 48 h. Fig. 1B shows that 7,8-DHF inhibited the
viability of OSCC cells in a dose-dependent manner. The changes in
the appearance of OSCC cells were observed with an optical
microscope after 48 h (Fig. 1C).
The apoptotic phenotype was a rounded cell, with cytoplasmic
bleeding and an abnormal shape. These results indicate that 7,8-DHF
inhibited the growth of human OSCC.
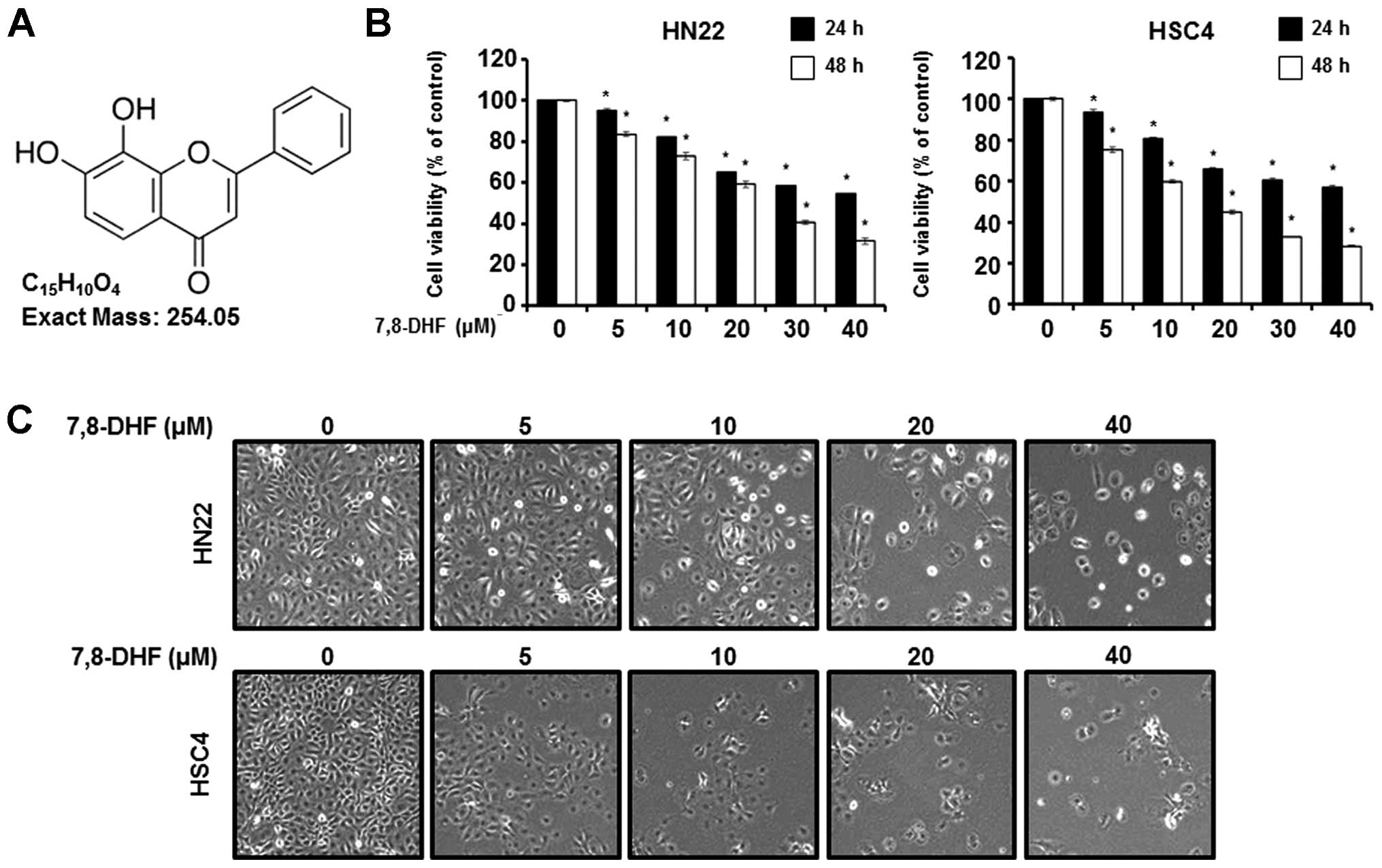 | Figure 1Effect of 7,8-DHF on the viability of
oral squamous carcinoma cells. (A) Chemical structure of 7,8-DHF.
(B) Cell viability in 7,8-DHF (5, 10, 20, 30, and 40 μM)-treated
HN22 and HSC4 cells was detected using an MTS assay kit. Data
represent mean percentage levels ± standard deviations.
Significantly different compared with DMSO-treated control by the
paired t-test (n=3; *P<0.05). (C) Morphological
changes observed in the 7,8-DHF treated (5, 10, 20, 30, and 40 μM)
or untreated HN22 and HSC4 cells 48 h post-treatment. |
7,8-DHF causes G1 phase cell
cycle arrest of OSCC cells
Cancer cell proliferation can be suppressed by
apoptosis, inducing cell cycle arrest or both. DAPI staining was
performed to confirm the induction of apoptosis by 7,8-DHF in HN22
and HSC4 cells, as DAPI specifically stains nuclei. The results
showed fragmented and condensed nuclei in cells treated with
7,8-DHF (10, 20 and 40 μM) for 48 h compared to that in the control
(Fig. 2A). The cell cycle
distribution was analyzed by FACS analysis. As shown in Fig. 2C, a significant increase in the
number of sub-G1 phase HN22 cells was observed
(11.2±1.4% in the presence of 10 μM 7,8-DHF, 31.0±2.3% in the
presence of 20 μM 7,8-DHF, and 44.0±2.6% in the presence of 40 μM
7,8-DHF) compared with that of untreated control cells. An increase
in the number of sub-G1 phase HSC4 cells was also
observed (5.3±0.3% in the presence of 10 μM 7,8-DHF, 11.5±1.2% in
the presence of 20 μM 7,8-DHF and 58.8±1.2% in the presence of 40
μM 7,8-DHF) compared with that in the untreated control cells.
Cells stained with Annexin V only were defined as early apoptotic
and Annexin V (lower right) and 7-AAD double-stained cells were
defined as late apoptotic (upper right). As shown in Fig. 2D, 7,8-DHF displayed marked effects
to induce apoptosis of HN22 and HSC4 cells in a dose-dependent
manner. Treatment of the HN22 cells with 10, 20 and 40 μM of
7,8-DHF for 48 h resulted in 6.2±1.5, 26.3±2.5 and 19.3±0.9% of
early apoptotic cells and 6.1±0.8, 7.1±0.2 and 36.3±0.4% of late
apoptotic cells, respectively. Similarly, treatment of HSC4 cells
with 7,8-DHF also led to 18.5±2.3, 33.7±0.3 and 22.9±2.2% of early
apoptotic cells and 4.2±0.8, 6.9±0.2 and 26.4±1.0% of late
apoptotic cells at the same three concentrations as above,
respectively. Apparently, 7,8-DHF-mediated apoptosis of HN22 and
HSC4 cells, at least in part, contributed to its antiproliferative
effects.
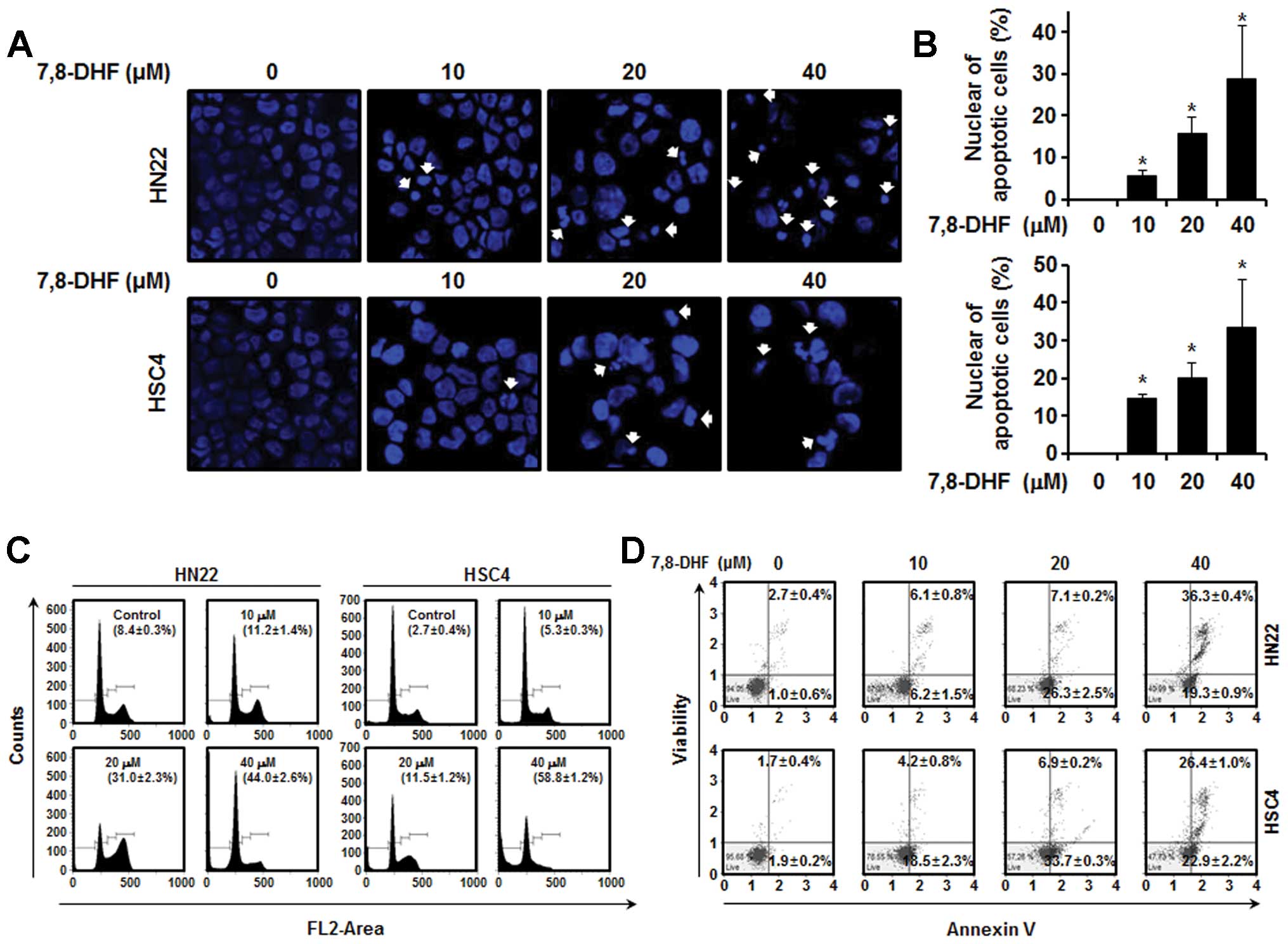 | Figure 2Apoptotic effect induced by 7,8-DHF in
oral squamous cell carcinoma. HN22 and HSC4 cells were cultured
without 7,8-DHF (control) or with 7,8-DHF (10, 20 and 40 μM) for 48
h. (A) Fluorescence microscopic (magnification, ×200) images of
DAPI-stained cells. White arrows indicate DNA fragmentation and
nuclear condensation. (B) DNA fragmentation and nuclear
condensation were quantified, and data represent the mean
percentage levels ± standard deviations (n=3;
*P<0.05). (C) HN22 and HSC4 cells were treated with
10, 20 and 40 μM 7,8-DHF or DMSO (vehicle), and the cells were
washed, fixed, stained with propidium iodide (PI) and analyzed for
DNA content by flow cytometry 48 h after treatment. The ratio of
apoptotic cells was measured by flow cytometry after PI staining.
(D) Quantitative detection of Annexin V/7-AAD positive cells was
performed with the Muse Cell Analyzer. Cells stained with Annexin V
only were defined as early apoptotic (lower right); Annexin V and
7-AAD double-stained cells were defined as late apoptotic (upper
right). |
7,8-DHF suppresses Sp1 expression and
binds with Sp1 in OSCC cells
As the Sp1 protein plays an important role in
oncogenesis, a therapeutic agent that can effectively modulate the
Sp1 protein may be a suitable anticancer drug to suppress tumor
progression (28). Both HN22 and
HSC4 cells were treated with various concentrations of 7,8-DHF (10,
20 and 40 μM) for 48 h to observe Sp1 protein expression levels.
Fig. 3A and B shows a significant
decrease in Sp1 protein expression levels in the HN22 and HSC4
cells in a dose-dependent manner. We also observed downregulation
of the Sp1 protein in HN22 and HSC4 cells at 40 μM 7,8-DHF after
different periods of time (0, 12, 24, 36 and 48 h). Additionally,
Sp1 mRNA was suppressed by 7,8-DHF in both HN22 and HSC4 cells
(Fig. 3C). Consistent with these
observations, the immunocytochemistry results revealed decreased
Sp1 and increased cleaved caspase-3 levels in a dose-dependent
manner in both the HN22 and HSC4 cell lines (Fig. 3D). We conclude that suppression of
Sp1 by 7,8-DHF treatment leads to apoptotic cell death. Next, a
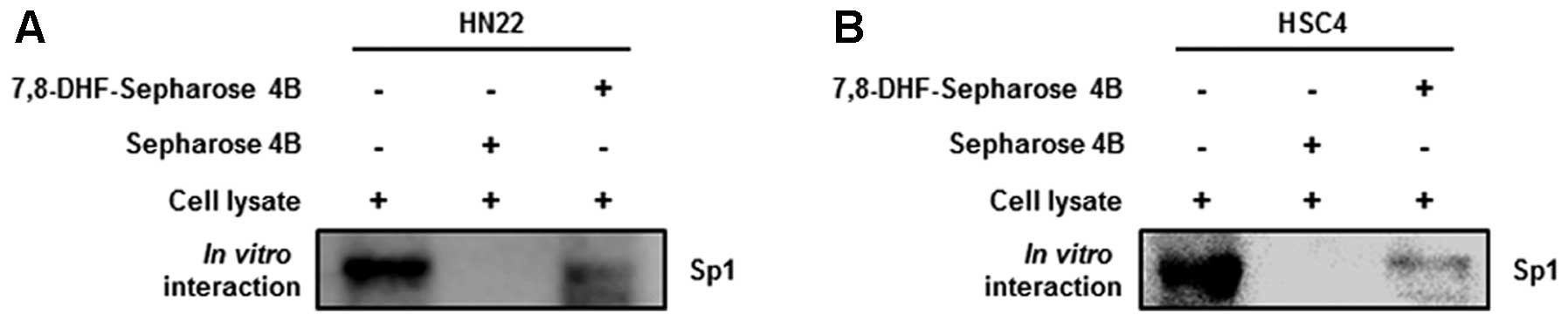
pull down assay was performed to determine whether 7,8-DHF directly
binds to Sp1. Fig. 4A and B showed
that 7,8-DHF strongly suppressed Sp1 activity by directly binding
to Sp1.
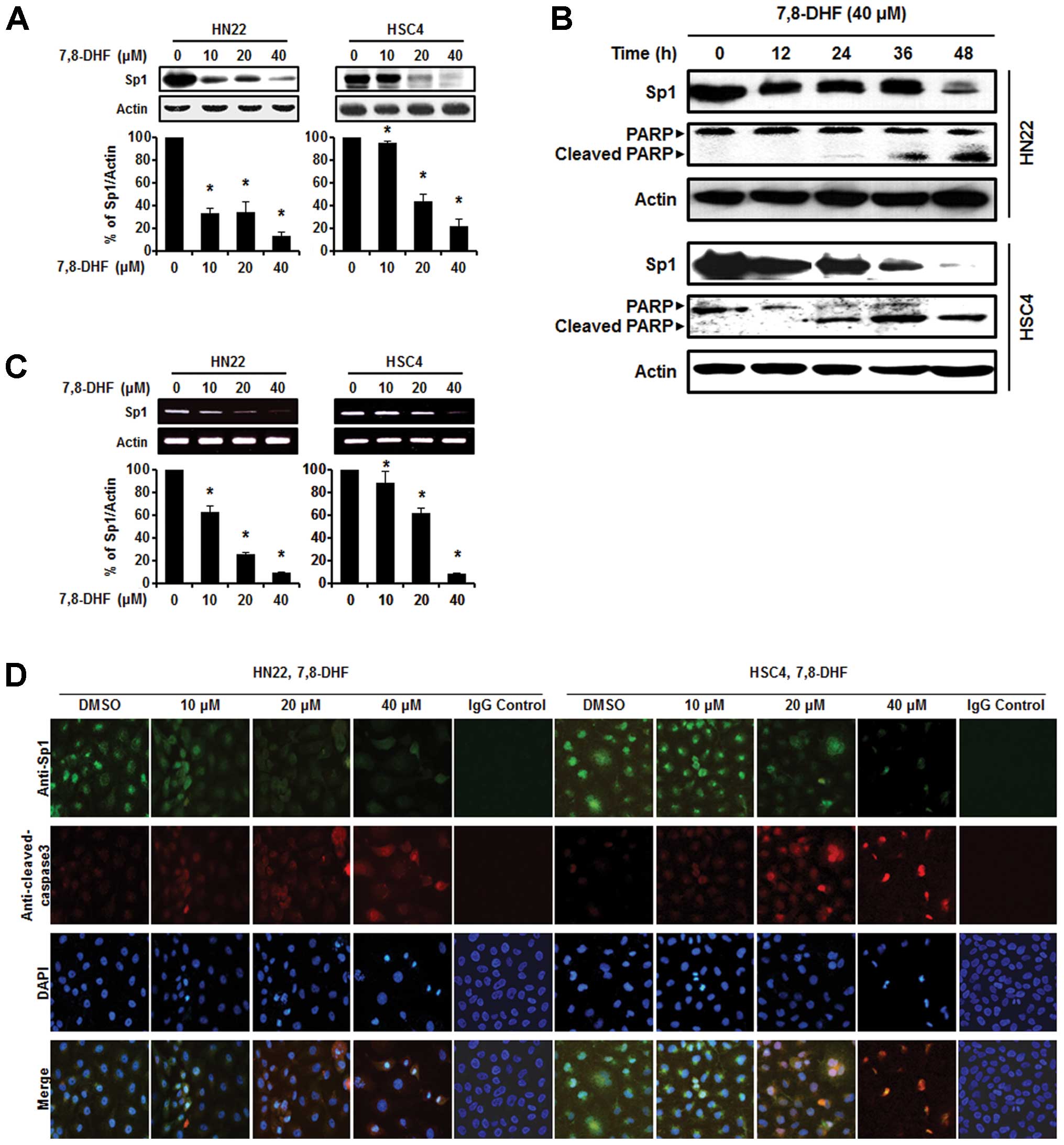 | Figure 37,8-DHF suppresses the Sp1 protein via
apoptosis in oral squamous cell carcinoma. (A) HN22 and HSC4 cells
were treated with 10, 20 and 40 μM 7,8-DHF for 48 h, whole-cell
extracts were prepared, separated by SDS-PAGE gel electrophoresis,
and subjected to western blotting with the Sp1 antibody. Actin was
employed as the loading control. The histograms indicate the ratio
of Sp1 to actin expression. (B) Experiments to assess
time-dependent effects of 7,8-DHF on Sp1 and PARP were performed
using HN22 and HSC4 cells treated with 40 μM 7,8-DHF for 0, 12, 24,
36 and 48 h. (C) The effect of 7,8-DHF (10, 20 and 40 μM) on Sp1
mRNA after 48 h. (D) Immunofluorescence microscopy was performed in
7,8-DHF-treated HN22 and HSC4 cells. HN22 and HSC4 cells were
treated with different concentrations of 7,8-DHF (10, 20 and 40 μM)
for 48 h and the cells were immunostained with anti-Sp1 and
anti-cleaved caspase-3. The signals were detected with Jackson
488-conjugated anti-mouse and Jackson 647-conjugated anti-rabbit
secondary antibody. DAPI was used for nuclear staining. |
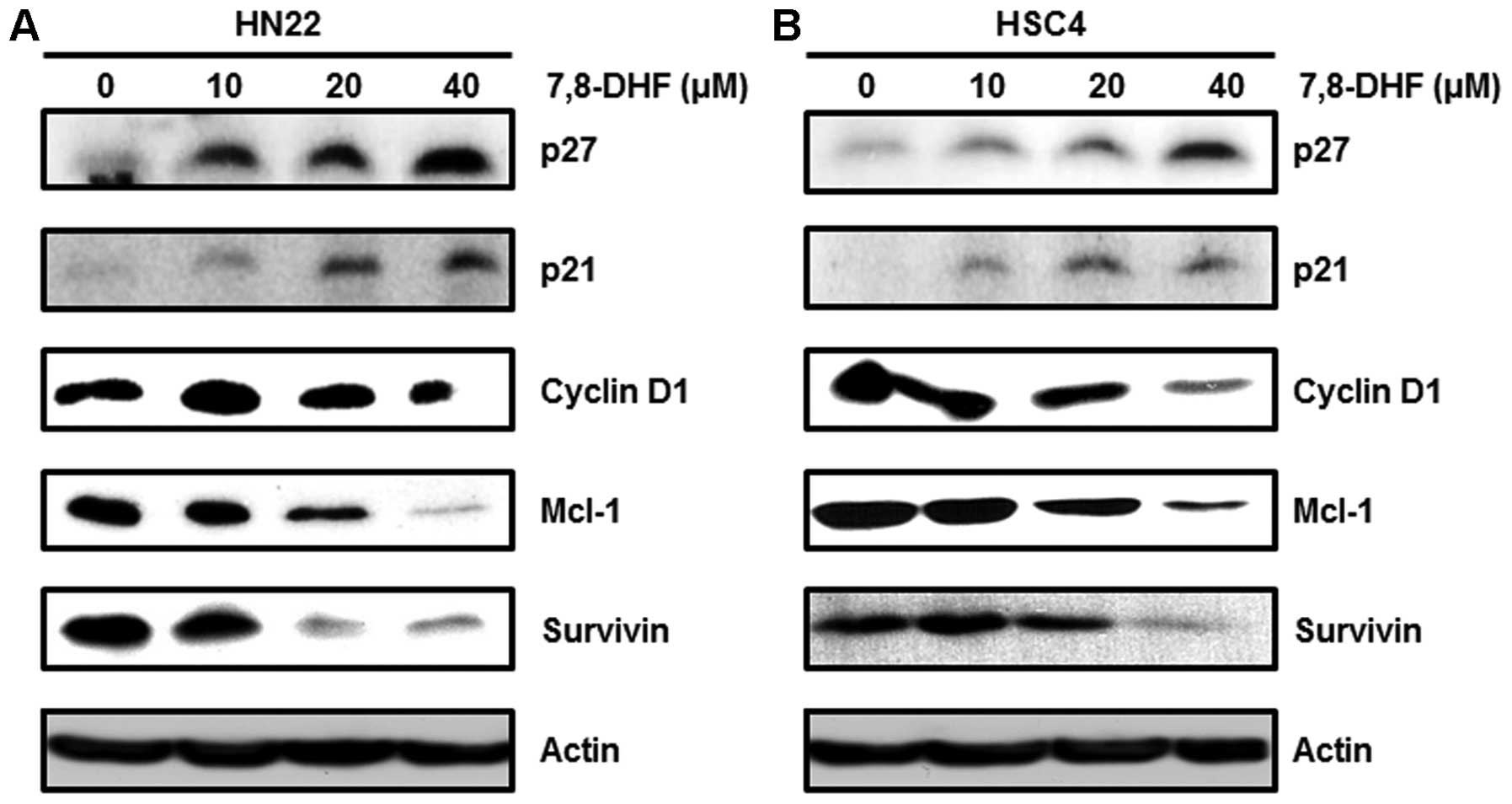
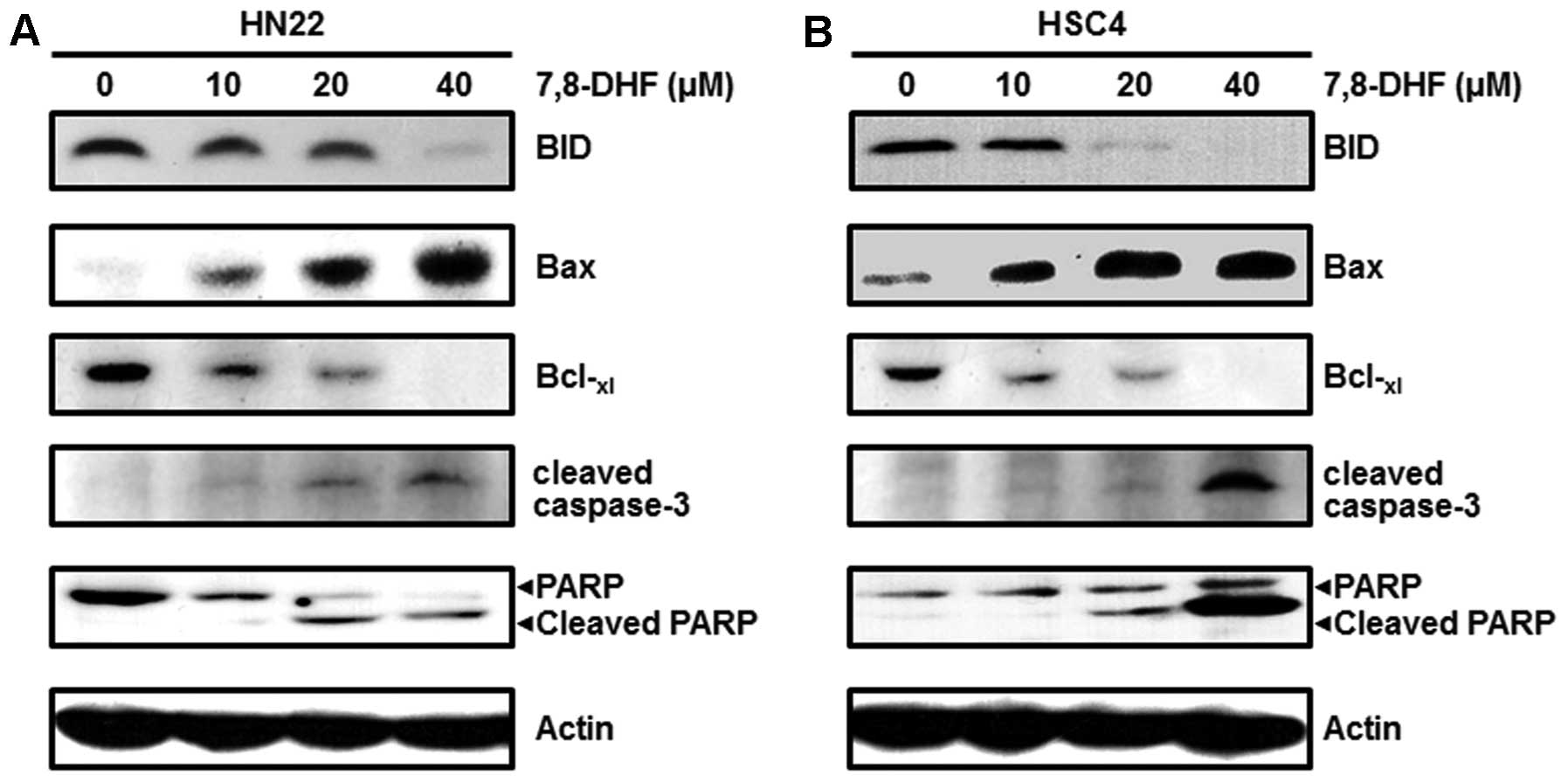
7,8-DHF modulates the factors concerned
with cell cycle arrest or apoptosis of OSCC cells
In many previous studies, the downregulation of Sp1
was found to induce apoptosis, and Sp1 was confirmed to be
associated with cell apoptosis (28–30).
We used western blot analysis to clarify the association between
7,8-DHF and Sp1-mediated apoptosis. We investigated the cell-cycle
arrest proteins such as p27 and p21 and found increased levels
following 7,8-DHF treatment. We also investigated cell
proliferation and survival-related proteins such as cyclin D1,
Mcl-1 and survivin, which were decreased in a dose-dependent manner
following 7,8-DHF treatment (Fig.
5). We found a decrease in BID and Bcl-xl and an
increase in Bax expression. These proteins were associated with the
apoptotic cell death induced by 7,8-DHF. Finally, cleaved caspase-3
and cleaved PARP were induced by 7,8-DHF in a dose-dependent manner
(Fig. 6). These results revealed
that 7,8-DHF treatment of OSCC decreases Sp1, resulting in growth
arrest and apoptotic cell death.
Discussion
Flavonoids demonstrate antiallergic,
antiinflammatory, antioxidant, and anticancer effects. They also
regulate enzyme activities and the immune system (12,31–34).
Among them, 7,8-DHF is a novel compound isolated from the flavone
family that appears to inhibit the proliferation of cancer cells
(11), but its mechanism of action
has not yet been investigated in detail.
In the present study, we examined the apoptotic
effect of 7,8-DHF in OSCC. The effect of 7,8-DHF treatment on
initiating apoptosis and cell cycle arrest in HN22 and HSC4 cells
was determined by flow cytometry and DAPI staining. The percentage
of sub-G1 phase cells increased in 7,8-DHF-treated cells
compared with that in untreated control cells. Furthermore, the
Annexin V assay revealed that 7,8-DHF induced early apoptosis
(Fig. 2C). To determine the level
of protein Sp1 expression due to 7,8-DHF treatment, HN22 and HSC4
cells were treated with various concentrations (10, 20 and 40 μM)
of 7,8-DHF for 48 h and different periods of time (12, 24, 36 and
48 h). As shown in Fig. 3A and B,
treatment with 7,8-DHF induced a significant decrease in Sp1
protein expression levels in HN22 and HSC4 cells in a dose- and
time-dependent manner. Furthermore, Sp1 mRNA was suppressed by
7,8-DHF in both HN22 and HSC4 cells (Fig. 3C). The immunocytochemistry results
revealed decreased Sp1 levels and increased levels of cleaved
caspase-3 in a dose-dependent manner in the HN22 and HSC4 cell
lines (Fig. 3D). These results
suggest that 7,8-DHF plays an important role as an anti-tumor agent
by downregulation of Sp1, leading to apoptotic cell death in
OSCC.
We examined a 7,8-DHF pull-down assay to identify
7,8-DHF molecular targets in tumorigenesis and confirmed that
7,8-DHF specifically binds with Sp1 (Fig. 4). To further characterize the
effects of 7,8-DHF on Sp1, we analyzed the effect of p27, p21,
cyclin D1, Mcl-1, and the survivin protein on Sp1 protein levels by
western blot analysis (35–37). The results showed that levels of the
Sp1 target proteins such as p27, p21, cyclin D1, Mcl-1, and
survivin were inhibited by 7,8-DHF in a dose-dependent manner
(Fig. 5). 7,8-DHF reduced BID and
Bcl-xl, increased Bax, and cleaved caspase-3 and PARP,
suggesting that 7,8-DHF regulates Sp1, ultimately leading to
apoptotic cell death (Fig. 6). Our
results indicate that 7,8-DHF may be capable of effectively
treating cancer. Sp1 expression increases during cancer
transformation and plays an important role in the maintenance and
development of tumors. Downregulation of Sp1 is useful for treating
tumor cells, and Sp1 overexpression induces the proliferation of
cancer or transformed cells (22).
7,8-DHF clinical studies are necessary to describe
the clinical applications and potential unexpected toxicities.
Acknowledgements
This study was supported by a grant from the
Next-Generation BioGreen 21 Program (No. PJ00811604), Rural
Development Administration, Republic of Korea.
References
|
1
|
Hamada T, Wakamatsu T, Miyahara M, et al:
MUC4: a novel prognostic factor of oral squamous cell carcinoma.
Int J Cancer. 130:1768–1776. 2012. View Article : Google Scholar
|
|
2
|
Rapidis AD, Gullane P, Langdon JD,
Lefebvre JL, Scully C and Shah JP: Major advances in the knowledge
and understanding of the epidemiology, aetiopathogenesis,
diagnosis, management and prognosis of oral cancer. Oral Oncol.
45:299–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scully C and Bagan J: Oral squamous cell
carcinoma overview. Oral Oncol. 45:301–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neville BW and Day TA: Oral cancer and
precancerous lesions. CA Cancer J Clin. 52:195–215. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mashberg A, Boffetta P, Winkelman R and
Garfinkel L: Tobacco smoking, alcohol drinking, and cancer of the
oral cavity and oropharynx among U.S. veterans. Cancer.
72:1369–1375. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar
|
|
7
|
Sher DJ, Thotakura V, Balboni TA, et al:
Treatment of oral cavity squamous cell carcinoma with adjuvant or
definitive intensity-modulated radiation therapy. Int J Radiat
Oncol Biol Phys. 81:e215–e222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sano D and Myers JN: Metastasis of
squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev.
26:645–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang R, Kang KA, Piao MJ, et al:
Preventive effect of 7,8-dihydroxyflavone against oxidative stress
induced genotoxicity. Biol Pharm Bull. 32:166–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jang SW, Liu X, Yepes M, et al: A
selective TrkB agonist with potent neurotrophic activities by
7,8-dihydroxyflavone. Proc Natl Acad Sci USA. 107:2687–2692. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen J, Chua KW, Chua CC, et al:
Antioxidant activity of 7,8-dihydroxyflavone provides
neuroprotection against glutamate-induced toxicity. Neurosci Lett.
499:181–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chahar MK, Sharma N, Dobhal MP and Joshi
YC: Flavonoids: a versatile source of anticancer drugs. Pharmacogn
Rev. 5:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Galati G, Teng S, Moridani MY, Chan TS and
O’Brien PJ: Cancer chemoprevention and apoptosis mechanisms induced
by dietary polyphenolics. Drug Metabol Drug Interact. 17:311–349.
2000. View Article : Google Scholar
|
|
14
|
Yang CS, Landau JM, Huang MT and Newmark
HL: Inhibition of carcinogenesis by dietary polyphenolic compounds.
Annu Rev Nutr. 21:381–406. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang WY, Cai YZ and Zhang Y: Natural
phenolic compounds from medicinal herbs and dietary plants:
potential use for cancer prevention. Nutr Cancer. 62:1–20. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kilani-Jaziri S, Frachet V, Bhouri W,
Ghedira K, Chekir-Ghedira L and Ronot X: Flavones inhibit the
proliferation of human tumor cancer cell lines by inducing
apoptosis. Drug Chem Toxicol. 35:1–10. 2012. View Article : Google Scholar
|
|
17
|
Davie JR, He S, Li L, et al: Nuclear
organization and chromatin dynamics - Sp1, Sp3 and histone
deacetylases. Adv Enzyme Regul. 48:189–208. 2008. View Article : Google Scholar
|
|
18
|
Li L and Davie JR: The role of Sp1 and Sp3
in normal and cancer cell biology. Ann Anat. 192:275–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wan J, Carr BA, Cutler NS, Lanza DL, Hines
RN and Yost GS: Sp1 and Sp3 regulate basal transcription of the
human CYP2F1 gene. Drug Metab Dispos. 33:1244–1253. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chu S and Ferro TJ: Sp1: regulation of
gene expression by phosphorylation. Gene. 348:1–11. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chuang JY, Wu CH, Lai MD, Chang WC and
Hung JJ: Overexpression of Sp1 leads to p53-dependent apoptosis in
cancer cells. Int J Cancer. 125:2066–2076. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deniaud E, Baguet J, Mathieu AL, Pages G,
Marvel J and Leverrier Y: Overexpression of Sp1 transcription
factor induces apoptosis. Oncogene. 25:7096–7105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L, Liu Q, Qin R, et al: Amplification
and functional characterization of MUC1 promoter and
gene-virotherapy via a targeting adenoviral vector expressing
hSSTR2 gene in MUC1-positive Panc-1 pancreatic cancer cells in
vitro. Int J Mol Med. 15:617–626. 2005.PubMed/NCBI
|
|
24
|
Kong LM, Liao CG, Fei F, Guo X, Xing JL
and Chen ZN: Transcription factor Sp1 regulates expression of
cancer-associated molecule CD147 in human lung cancer. Cancer Sci.
101:1463–1470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garcia A, Zheng Y, Zhao C, et al: Honokiol
suppresses survival signals mediated by Ras-dependent phospholipase
D activity in human cancer cells. Clin Cancer Res. 14:4267–4274.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng J, Qian Y, Geng L, et al: Involvement
of p38 mitogen-activated protein kinase pathway in honokiol-induced
apoptosis in a human hepatoma cell line (hepG2). Liver Int.
28:1458–1464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Urusova DV, Shim JH, Kim DJ, et al:
Epigallocatechin-gallate suppresses tumorigenesis by directly
targeting Pin1. Cancer Prev Res. 4:1366–1377. 2011. View Article : Google Scholar
|
|
28
|
Shin JA, Kim JJ, Choi ES, et al: In vitro
apoptotic effects of methanol extracts of Dianthus chinensis and
Acalypha australis L. targeting specificity protein 1 in human oral
cancer cells. Head Neck. 35:992–998. 2013. View Article : Google Scholar
|
|
29
|
Dasari A, Bartholomew JN, Volonte D and
Galbiati F: Oxidative stress induces premature senescence by
stimulating caveolin-1 gene transcription through p38
mitogen-activated protein kinase/Sp1-mediated activation of two
GC-rich promoter elements. Cancer Res. 66:10805–10814. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jutooru I, Chadalapaka G, Sreevalsan S, et
al: Arsenic trioxide downregulates specificity protein (Sp)
transcription factors and inhibits bladder cancer cell and tumor
growth. Exp Cell Res. 316:2174–2188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Curin Y and Andriantsitohaina R:
Polyphenols as potential therapeutical agents against
cardiovascular diseases. Pharmacol Rep. 57(Suppl): 97–107.
2005.
|
|
32
|
Pan MH and Ho CT: Chemopreventive effects
of natural dietary compounds on cancer development. Chem Soc Rev.
37:2558–2574. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Prasad S, Phromnoi K, Yadav VR, Chaturvedi
MM and Aggarwal BB: Targeting inflammatory pathways by flavonoids
for prevention and treatment of cancer. Planta Med. 76:1044–1063.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo W, Kong E and Meydani M: Dietary
polyphenols, inflammation, and cancer. Nutr Cancer. 61:807–810.
2009. View Article : Google Scholar
|
|
35
|
Blume SW, Snyder RC, Ray R, Thomas S,
Koller CA and Miller DM: Mithramycin inhibits SP1 binding and
selectively inhibits transcriptional activity of the dihydrofolate
reductase gene in vitro and in vivo. J Clin Invest. 88:1613–1621.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chintharlapalli S, Papineni S, Lei P,
Pathi S and Safe S: Betulinic acid inhibits colon cancer cell and
tumor growth and induces proteasome-dependent and -independent
downregulation of specificity proteins (Sp) transcription factors.
BMC Cancer. 11:3712011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pietrzak M and Puzianowska-Kuznicka M:
p53-dependent repression of the human MCL-1 gene encoding an
anti-apoptotic member of the BCL-2 family: the role of Sp1 and of
basic transcription factor binding sites in the MCL-1 promoter.
Biol Chem. 389:383–393. 2008. View Article : Google Scholar : PubMed/NCBI
|