Introduction
Oral squamous cell carcinoma (OSCC) represents 1–3%
of all human malignancies, among which, tongue squamous cell
carcinoma represents 25–50% of all cases of OSCC (1–3).
Tongue squamous cell carcinoma is characterized by its high rate of
proliferation and nodal metastasis. Although it is visibly located
in the oral cavity, ~50% of patients are in advanced stage III and
IV upon presentation (4,5). The understanding of the molecular
pathways of carcinogenesis or progression would be helpful in
improving diagnosis, therapy and prevention of this disease.
MicroRNAs (miRNAs) are small (~20–22 nucleotides),
endogenous, noncoding RNAs, functioning as negative regulators of
gene expression through antisense complimentarity to their target
messenger RNAs (6). Evidence
suggests that disordered expression of miRNAs contributes to the
initiation and progression of human cancer (7,8). A
previous study conducted by Wong et al evaluated the miRNA
expression patterns in human tongue squamous cell carcinoma and
revealed that miR-26b, an miRNA known to have tumor-suppressive
properties, was downregulated in human tongue squamous cell
carcinoma tissues (9). Reduced
miR-26b expression has been observed in several types of tumors,
including breast, colorectal and pancreatic cancer, and it may play
an important role in the regulation of carcinogenesis and tumor
progression via targeting specific signaling pathways (10–15).
In the present study, we revealed that reduced
miR-26b expression was correlated with advanced clinical stage,
lymph node metastasis, and poor prognosis in patients with tongue
squamous cell carcinoma. More importantly, we illustrated that
miR-26b could regulate the malignant phenotype of human tongue
squamous cell carcinoma cells by directly targeting PTGS2
(prostaglandin-endoperoxide synthase-2, encoding COX-2), a known
mediator of cell proliferation and metastasis (16,17).
Thus, our findings provide valuable clues towards understanding the
specific tumor-suppressive function and the regulatory mechanisms
of miR-26b in human tongue squamous cell carcinoma. Further
investigation may validate miR-26b as an effective therapeutic
target in the future.
Materials and methods
Clinical samples
Tissues of tongue squamous cell carcinoma and the
matched normal counterparts were obtained from surgical specimens
collected immediately after resection from patients undergoing
primary surgical treatment of oral tongue carcinoma at the
Department of Stomatology, The First Affiliated Hospital of the PLA
General Hospital, and Department of Stomatology, Wuhan General
Hospital of Guangzhou Command. The samples were flash frozen in
liquid nitrogen and stored at −80°C. Histology of the tissues was
evaluated by the hospital pathologist. Written consent of tissue
donation for research purposes was obtained from patients before
tissue collection, and the protocol was approved by the Ethics
Committees of The First Affiliated Hospital of the PLA General
Hospital, and Wuhan General Hospital of Guangzhou Command.
Cell culture and cell transfection
Human tongue squamous cell carcinoma cell lines
HSC-3, SCC-4 and Cal27, and human normal oral keratinocytes (hNOKs)
were routinely cultured in RPMI-1640 medium (Hyclone Laboratories,
Logan, UT, USA) supplemented with 10% fetal bovine serum (Hyclone)
at 37°C in a 5% CO2 incubator. For transient
transfection, the cells were transfected with miR-26b
mimics/inhibitor and their respective negative control duplexes
(Ambion, Austin, TX, USA) using Lipofectamine 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA). After a 24-h transfection, the
cells were collected for quantitative real-time RT-PCR (qRT-PCR)
analysis or further processing.
qRT-PCR
The TaqMan stem-loop RT-PCR method was used to
assess the expression of mature miR-26b with kits from Applied
Biosystems (Foster City, CA, USA). The real-time PCR results,
recorded as threshold cycle numbers (Ct), were normalized against
an internal control (U6). For relative expression levels, the
2−ΔCt method was used as previously described (18). Experiments were carried out in
triplicate for each data point, and analysis was carried out by
using Bio-Rad IQ software.
Cell cycle analysis
Cells transfected with miR-26b mimics/inhibitor were
harvested 24 h later by trypsinization, washed with ice-cold PBS,
fixed in 70% ethanol and stored at 4°C. Following overnight
incubation, cells were washed and resuspended in propidium iodide
(PI) staining buffer. DNA content was evaluated by flow cytometry
(XL-MCL; Coulter Epics, Miami, FL, USA).
Apoptosis analysis
Detection of apoptotic cells by flow cytometry was
performed as described previously (19). Cells transfected with miR-26b
mimics/inhibitor were harvested 24 h later and then Annexin V/PI
analysis by flow cytometry (XL-MCL; Coulter Epics) was
performed.
Migration and invasion analysis
Transwell chambers (8-μm pore size, Corning, Inc.,
Corning, NY, USA) were used in the migration and invasion analysis.
For migration assays, 1×105 cells were plated in the top
chamber lined with a non-coated membrane. For invasion assays, the
chamber inserts were coated with 200 μg/ml of Matrigel and dried
overnight under sterile conditions. Then, 1×105 cells
were plated in the top chamber. In both assays, cells were
suspended in medium without serum or growth factors, and medium
supplemented with serum was used as a chemoattractant in the lower
chamber. After incubation at 37°C for 48 h, the top chambers were
wiped with cotton wool to remove the non-migrating or non-invasive
cells. The invasive cells on the underside of the membrane were
fixed in 100% methanol for 10 min, air-dried, stained with 0.1%
crystal violet and counted under a microscope. The mean of
triplicate assays for each experimental condition was used.
Western blot analysis
Total cell lysate was prepared in 1× SDS buffer.
Equal amounts of protein were analyzed by western blotting, using
antibodies against COX-2, VEGF-C, cyclin D1 and β-actin (Santa Cruz
Biotechnology, Santa Cruz, CA, USA).
Luciferase assay
The 3′UTR segments of PTGS2 mRNA containing the
miR-26b binding sites were amplified by PCR from human genomic DNA
and inserted into the pMIR-Report luciferase reporter vector
(Ambion) and named pMIR-PTGS2-3′UTR. A mutant version from the site
of perfect complementarity was also generated and named
pMIR-mut-PTGS2-3′UTR. The recombinant reporter vectors with
wild-type or mutant PTGS2 3′UTR were then cotransfected with
miR-26b mimics or control into SCC-4 cells, respectively, using
Lipofectamine™ 2000. The luciferase assay was performed according
to the manufacturer’s instructions.
Statistical analysis
The data are expressed as means ± SEM. Differences
were compared by one-way ANOVA analysis followed by the LSD t-test.
Survival curves were plotted by the Kaplan-Meier’s method, and the
log-rank test was carried out to compare differences in survival.
All statistical analyses were performed using SPSS 19.0 software
(SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Expression of miR-26b is downregulated in
human tongue squamous cell carcinoma and is associated with poor
patient survival
To investigate the role of miR-26b in human tongue
squamous cell carcinoma, we first compared the expression levels of
miR-26b between carcinoma tissue samples and paired adjacent
non-neoplastic mucosal tissues from 30 cases of tongue squamous
cell carcinoma patients. Consistent with the microarray results
presented by Wong et al (9),
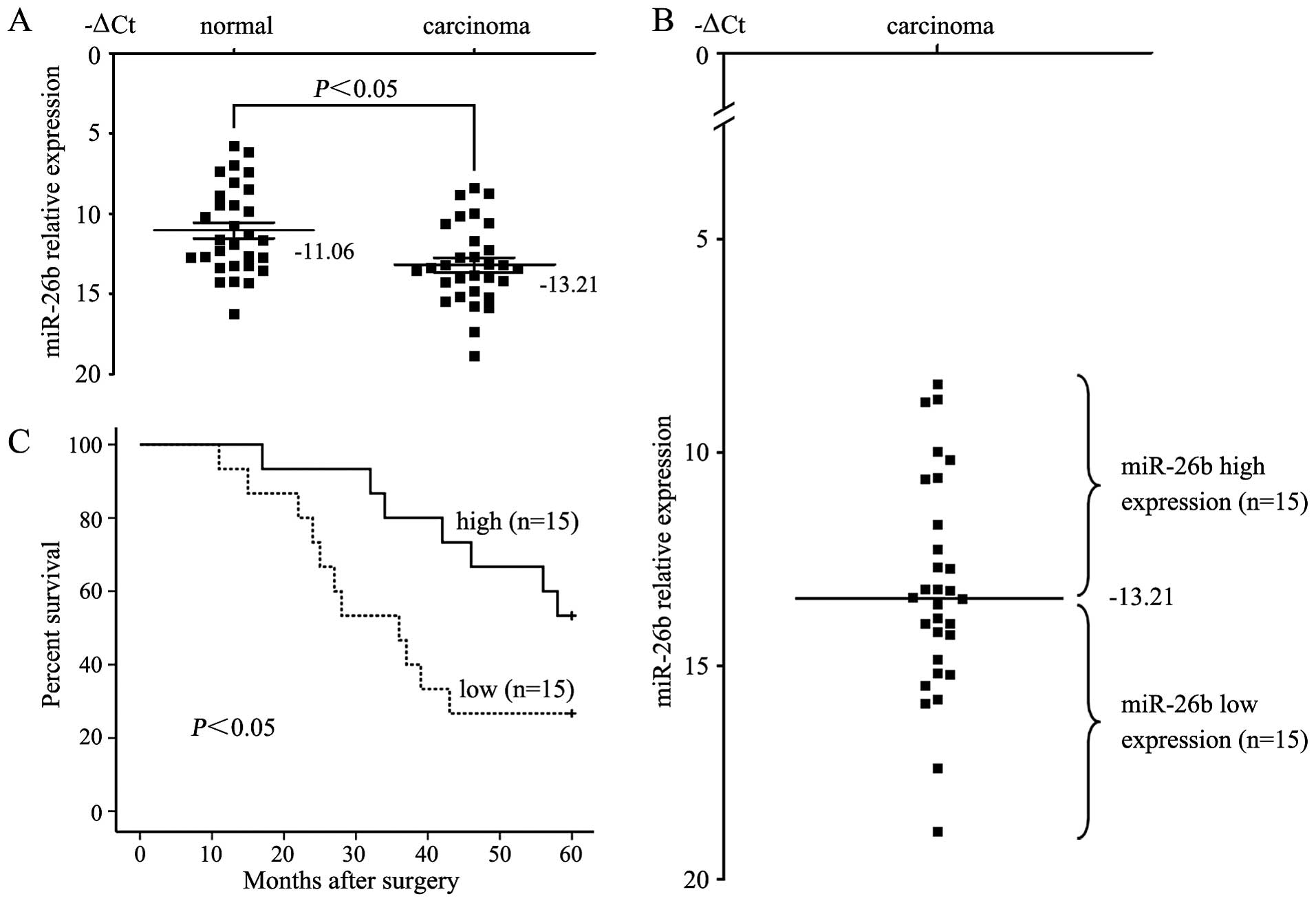
the qRT-PCR results verified that the miR-26b expression level in
tongue squamous cell carcinoma tissues (−13.21±0.46) was
significantly lower than that in the non-neoplastic mucosal tissues
(−11.06±0.50) (P<0.05, t=8.355, paired t-test) (Fig. 1A). Correlations between the miR-26b
expression level and clinicopathologic characteristics of the
tongue squamous cell carcinoma cases are summarized in Table I. Statistically significant
associations between miR-26b expression and clinical stage and
between miR-26b expression and metastasis were observed in the
present study. The median expression of miR-26b was −14.18±0.58 in
the 15 cases with advanced stage (stage III and IV) disease,
whereas the median expression was −12.24±0.63 (P<0.05,
Mann-Whitney U test) in the other 15 cases with early-stage (stages
I and II) disease. In the 14 cases with lymph node metastasis, the
median expression of miR-26b was −14.28±0.62, which was
significantly lower than the median expression (−12.24±0.63) in the
16 non-metastatic cases (P<0.05). The expression of miR-26b in
the tongue squamous cell carcinoma patients did not correlate with
age, gender, tumor size, or cell differentiation. Moreover, we
examined whether the level of miR-26b expression was associated
with survival in the patients with tongue squamous cell carcinoma.
Patients were subsequently divided into low expression (n=15) and
high expression groups (n=15) based on miR-26b levels greater or
less than the mean (−13.21) (Fig.
1B). Kaplan-Meier’s survival analysis revealed that patients
whose primary tumors displayed low expression of miR-26b had a
shorter median survival time. The 5-year survival rate of patients
with low miR-26 expression was 26.7%, which was significantly lower
than the survival rate in patients with high miR-26b expression
(53.3%, P<0.05, log-rank test, Fig.
1C).
 | Table IRelationship between the
clinicopathological parameters and miR-26b expression in the human
tongue squamous cell carcinoma cases. |
Table I
Relationship between the
clinicopathological parameters and miR-26b expression in the human
tongue squamous cell carcinoma cases.
| Variable | No. of cases (%) | miR-26b
expression | P-value |
|---|
| Age (years) |
| ≥60 | 17 (56.7) | −13.35±0.59 | 0.739 |
| <60 | 13 (43.3) | −13.03±0.74 | |
| Gender |
| Male | 19 (63.3) | −13.47±0.63 | 0.459 |
| Female | 11 (36.7) | −12.76±0.62 | |
| Tumor size |
| ≥4 | 8 (26.7) | −12.72±0.76 | 0.532 |
| <4 | 22 (73.3) | −13.39±0.56 | |
| Differentiation |
| Well/moderately | 20 (66.7) | −13.50±0.60 | 0.377 |
|
Poor/undifferentiated | 10 (33.3) | −12.63±0.67 | |
| TNM stage |
| I and II | 15 (50) | −12.24±0.63 | 0.032 |
| III and IV | 15 (50) | −14.18±0.58 | |
| Lymph node
status |
| No metastasis | 16 (53.3) | −12.27±0.59 | 0.026 |
| Metastasis | 14 (46.7) | −14.28±0.62 | |
Association between miR-26b expression
and tumorigenesis of human tongue squamous cell carcinoma
cells
We further detected the expression of miR-26b in
hNOKs and 3 human tongue squamous cell carcinoma cell lines, HSC-3,
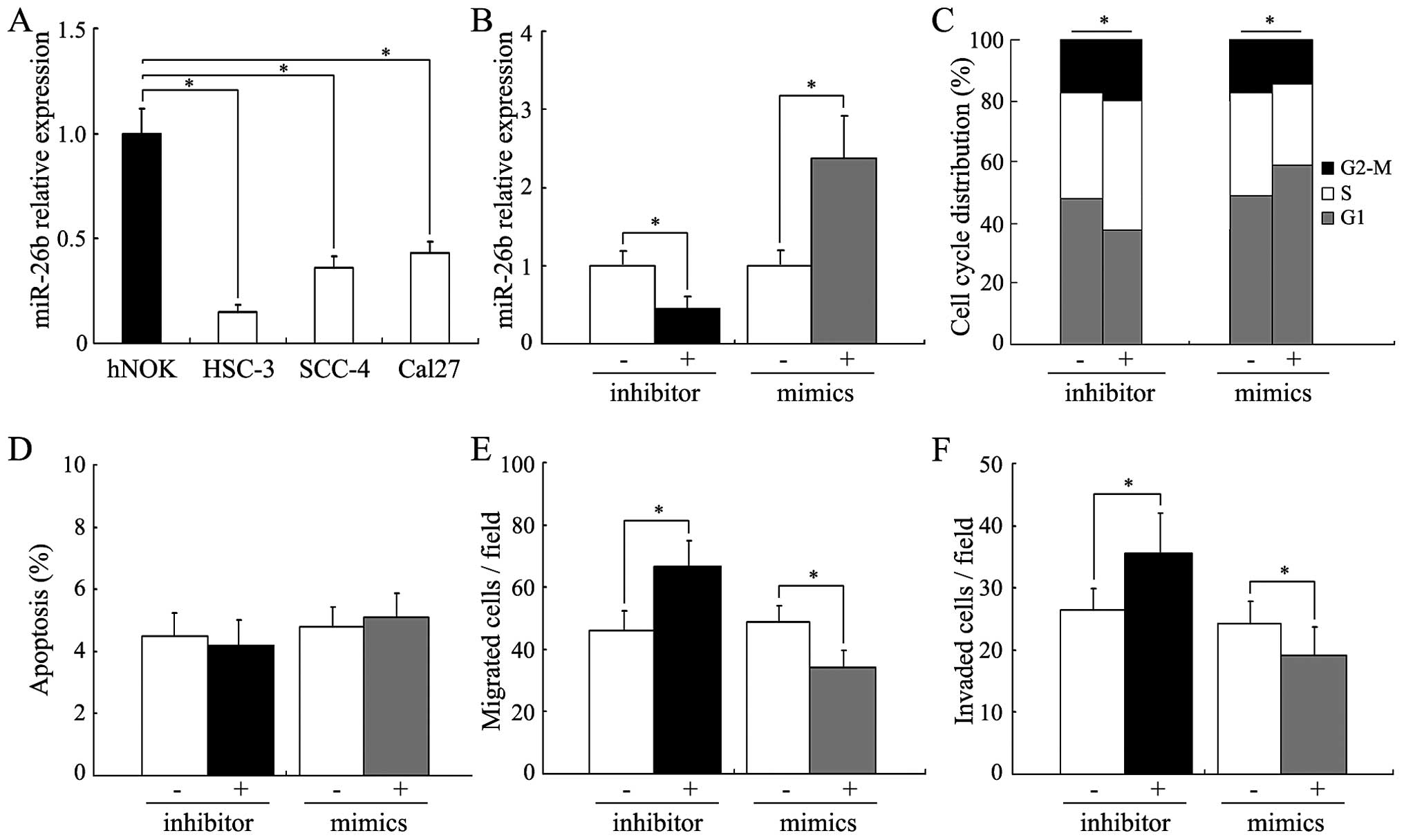
SCC-4 and Cal27. As shown in Fig.
2A, all of the 3 carcinoma cell lines had a relatively low
miR-26b expression compared to that of the hNOKs. Among the 3
carcinoma cell lines, SCC-4 cells had a moderate expression of
miR-26b. Therefore, the SCC-4 cells were chosen to evaluate the
effects of miR-26b expression on the oncogenicity of human tongue
squamous cell carcinoma cells. Fig.
2B shows that miR-26b expression was strongly reduced or
increased in the SCC-4 cells after transfection of the
miR-26b-specific inhibitor or mimics. By flow cytometry, a
significant accumulation of cells in the G1 phase was observed in
the miR-26b-upregulated SCC-4 cells, while inhibition of miR-26b
expression led to increased cell proliferation (Fig. 2C, P<0.05). No significant
influence on apoptosis was observed in the SCC-4 cells after
treatment with the miR-26b specific inhibitor or mimics (Fig. 2D). We further performed Transwell
assays to determine whether altered miR-26b expression could
influence the metastatic phenotype of the SCC-4 cells. As shown in
Fig. 2E and F, miR-26b inhibition
significantly increased the ability of the SCC-4 cells to migrate
and invade, while miR-26b upregulation suppressed the migration and
invasion (P<0.05).
COX-2 is the direct functional target of
miR-26b
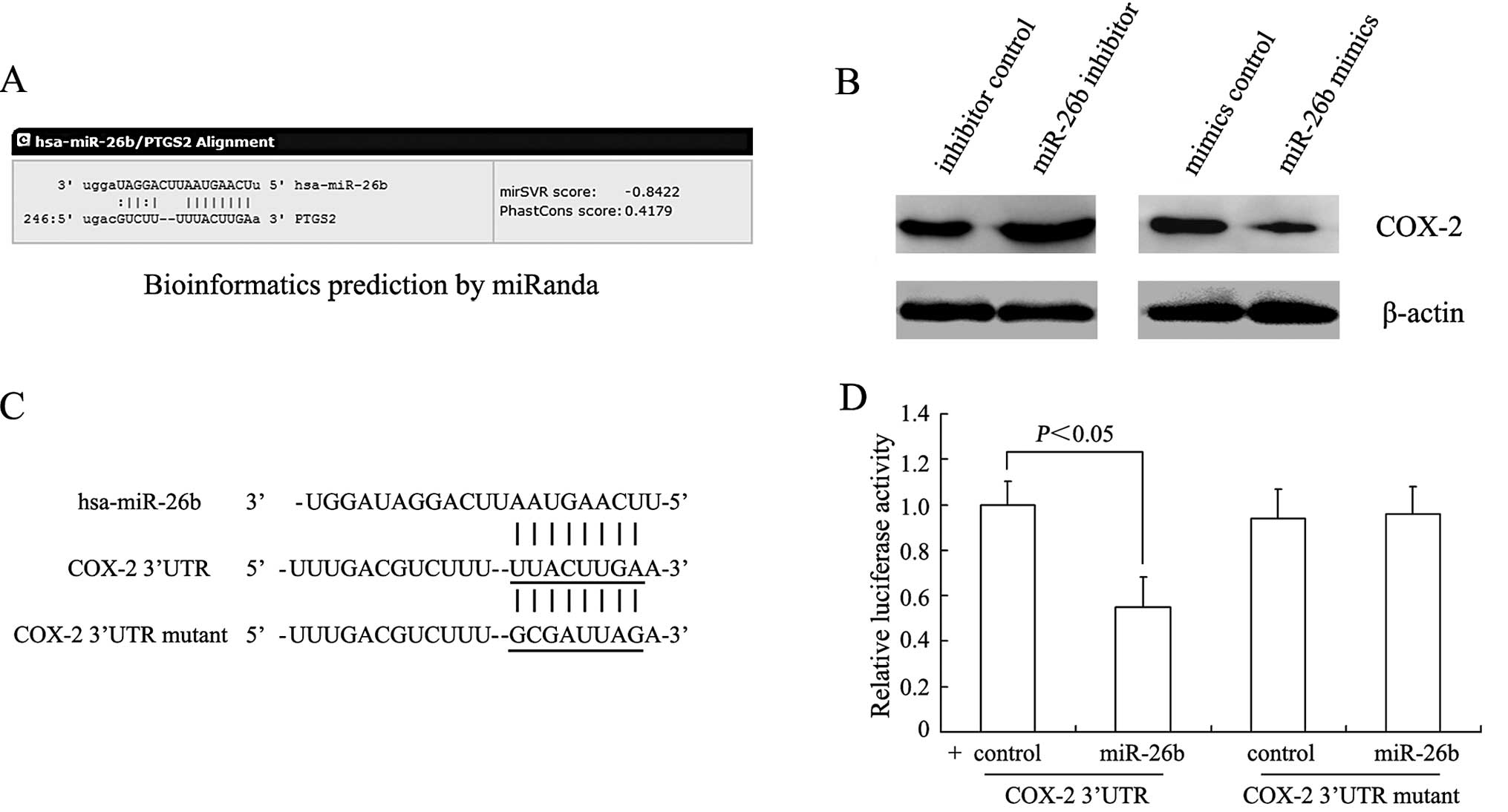
Using bioinformatics analysis, such as miRanda and
TargetScan, we found that miR-26b contained specific binding
sequences for the 3′UTR region of the COX-2 gene (PTGS2) (Fig. 3A). In agreement with the computer
prediction, COX-2 protein levels were significantly suppressed in
the miR-26b mimic group whereas these levels were increased in the
miR-26b inhibitor group (Fig. 3B).
In order to further validate the relationship between miR-26b and
COX-2, a dual luciferase reporter assay was performed in the SCC-4
cells. The sequences of the 3′UTR of wild-type and mutated COX-2
are shown in Fig. 3C. No reduction
in luciferase activity was observed in the SCC-4 cells transfected
with miR-26b mimics and mutated COX-2, but an ~45% reduction in
luciferase activity was observed in wild-type COX-2 (P<0.05,
Fig. 3D).
A COX-2-dependent mechanism is involved
in the regulation of oncogenesis induced by miR-26b
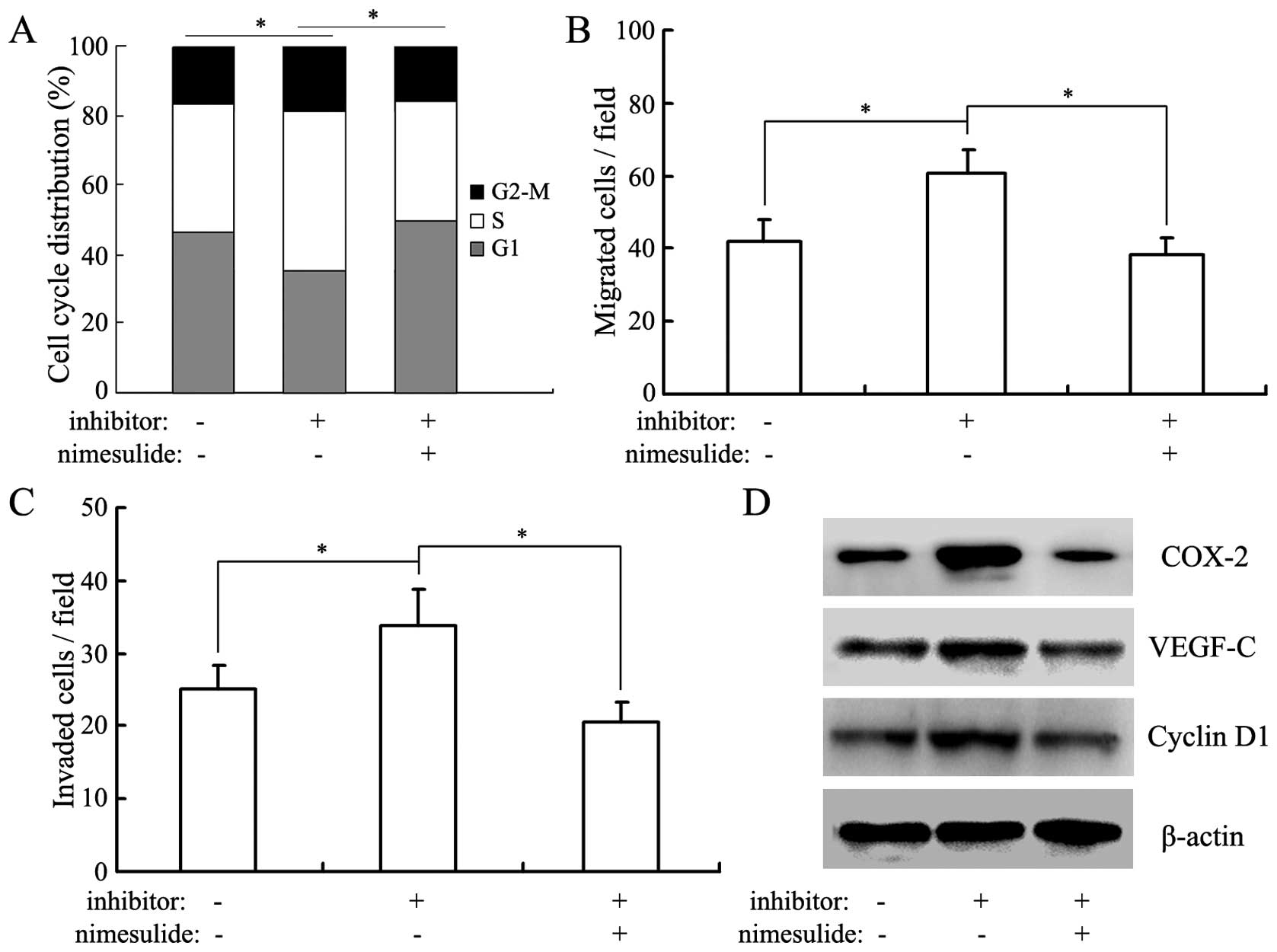
We further investigated the influence of nimesulide,
a COX-2-specific inhibitor, on the proliferation, migration and
invasion of SCC-4 cells (20). As
shown in Fig. 4A–C, nimesulide
abolished the promoting effects of miR-26b inhibition on the
proliferation, migration and invasion in SCC-4 cells, suggested the
critical role of COX-2 in miR-26b-regulated oncogenesis
(P<0.05). Previous studies revealed that COX-2 could promote the
proliferation and metastasis of human tongue squamous cell
carcinoma cells through the VEGF-C pathway (21–25).
In the present study, we investigated the expression changes of
VEGF-C in SCC-4 cells by western blot analysis. As shown in
Fig. 4D, the protein level of
VEGF-C was significantly increased in the miR-26b-silenced SCC-4
cells, however, this was reversed by treatment of nimesulide. We
also investigated the expression changes in cyclin D1, a key cell
cycle-regulatory protein. Western blot analysis results indicated
that cyclin D1 was also significantly upregulated in the
miR-26b-silenced SCC-4 cells and this upregulation was reversed by
nimesulide.
Discussion
Dysregulation of miRNAs is common in various
cancers. Dysregulated miRNAs play a role in carcinogenesis or tumor
progression by altering the normal gene expression patterns.
miR-26b has been found to be downregulated in several types of
human tumors and has been suggested to be a tumor-suppressor gene
(10–15). In the present study, we confirmed
the decrease in miR-26b expression in human tongue squamous cell
carcinoma and defined the clinical importance of miR-26b in
predicting lymph node metastasis and survival. Furthermore, we
identified that COX-2 is the functional target of miR-26b in human
tongue squamous cell carcinoma cells.
miR-26b (hsa) is classified as a member of the
miR-26 family (miR-26a/b) and has been reported as a critical
regulator in carcinogenesis and tumor progression by acting as a
tumor-suppressor gene in various cancers (10–15).
miR-26b was first revealed to be downregulated in human breast
cancer tissues and to mediate the proliferation and apoptosis of
human breast cancer cells through targeting SLC7A11, PTGS2
(encoding COX-2) and CDK8 (10,26,27).
Subsequently, miR-26b was found to be downregulated in human
colorectal, pancreatic and parathyroid cancer, glioma and
hepatocellular carcinoma (11–15),
further indicating the tumor suppressive property of this miRNA.
Wong et al and Hsu et al (28) consistently reported that miR-26b
expression was decreased in human head and neck cancer tissues
compared with that in paired normal tissues. In human tongue
squamous cell carcinoma, Wong et al revealed that miR-26b
had a ~4-fold reduction in expression level in laser capture
microdissection-procured carcinoma cells compared with the matched
normal cells (9). In the present
study, we confirmed the reduced expression of miR-26b in 30 cases
of tongue squamous cell carcinoma patients and revealed that its
expression was associated with clinical stage, lymph node
metastasis, and survival prognosis.
The PTGS2 gene encodes the COX-2 enzyme, which
catalyzes the conversion of arachidonic acid to prostaglandins and
other eicosanoids. COX-2 expression is undetectable in most normal
tissues but is induced in response to hypoxia, inflammatory
cytokines, tumor promoters, growth factors and other stressors
(17). Mounting evidence has
revealed that COX-2 expression is overexpressed in a variety of
human tumors, including human tongue squamous cell carcinoma
(29–31). COX-2 overexpression may contribute
to carcinogenesis by modulating metabolism, proliferation,
apoptosis, metastasis, angiogenesis and immune surveillance.
Several clinical studies have established the potent anticancer
activity of COX-2 inhibition by using COX-2 specific inhibitors
(such as celecoxib) (32,33). Targeting COX-2 with specific miRNAs
may be another reasonable approach to inhibit COX-2 activity. At
present, many miRNA-based cancer therapeutics is either in the
preclinical or clinical trial phase. In the present study, we
observed that ectopic expression of miR-26b could inhibit the
proliferation and metastasis of human tongue squamous cell
carcinoma cells and revealed that miR-26b could decrease the
protein level of COX-2 by directly binding to its 3′UTR.
Considering the reduced expression of miR-26b and the critical role
of COX-2 expression in carcinogenesis, we suggest that the
replacement therapy of miR-26b, for example lentiviral-mediated
delivery of mimic miR-26b, may be a promising therapeutic approach
for human tongue squamous cell carcinoma.
Taken together, we revealed that reduced miR-26b
expression is correlated with advanced clinical stage, lymph node
metastasis, and poor prognosis in patients with tongue squamous
cell carcinoma. In addition, we provide evidence that COX-2 is the
direct functional target of miR-26b. These results indicate that
miR-26b may serve as a tumor-suppressor gene involved in human
tongue squamous cell carcinoma.
References
|
1
|
Albuquerque R, López-López J, Marí-Roig A,
Jané-Salas E, Roselló-Llabrés X and Santos JR: Oral tongue squamous
cell carcinoma (OTSCC): alcohol and tobacco consumption versus
non-consumption. A study in a Portuguese population. Braz Dent J.
22:517–521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marocchio LS, Lima J, Sperandio FF, Corrêa
L and de Sousa SO: Oral squamous cell carcinoma: an analysis of
1,564 cases showing advances in early detection. J Oral Sci.
52:267–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pettus JR, Johnson JJ, Shi Z, Davis JW,
Koblinski J, et al: Multiple kallikrein (KLK 5, 7, 8, and 10)
expression in squamous cell carcinoma of the oral cavity. Histol
Histopathol. 24:197–207. 2009.
|
|
4
|
Yuen PW, Lam KY, Chan AC, Wei WI and Lam
LK: Clinicopathological analysis of local spread of carcinoma of
the tongue. Am J Surg. 175:242–244. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Po Wing Yuen A, Lam KY, et al: Prognostic
factors of clinically stage I and II oral tongue carcinoma - a
comparative study of stage, thickness, shape, growth pattern,
invasive front malignancy grading, Martinez-Gimeno score, and
pathologic features. Head Neck. 24:513–520. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kasinski AL and Slack FJ: Epigenetics and
genetics. MicroRNAs en route to the clinic: progress in validating
and targeting microRNAs for cancer therapy. Nat Rev Cancer.
11:849–864. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wong TS, Liu XB, Wong BY, Ng RW, Yuen AP
and Wei WI: Mature miR-184 as potential oncogenic microRNA of
squamous cell carcinoma of tongue. Clin Cancer Res. 14:2588–2592.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu XX, Li XJ, Zhang B, et al:
MicroRNA-26b is underexpressed in human breast cancer and induces
cell apoptosis by targeting SLC7A11. FEBS Lett. 585:1363–1367.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang C, Tong J and Huang G: Nicotinamide
phosphoribosyl transferase (Nampt) is a target of microRNA-26b in
colorectal cancer cells. PLoS One. 8:e699632013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schultz NA, Dehlendorff C, Jensen BV, et
al: MicroRNA biomarkers in whole blood for detection of pancreatic
cancer. JAMA. 311:392–404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rahbari R, Holloway AK, He M, Khanafshar
E, Clark OH and Kebebew E: Identification of differentially
expressed microRNA in parathyroid tumors. Ann Surg Oncol.
18:1158–1165. 2011. View Article : Google Scholar
|
|
14
|
Wu N, Zhao X, Liu M, et al: Role of
microRNA-26b in glioma development and its mediated regulation on
EphA2. PLoS One. 6:e162642011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ji J, Shi J, Budhu A, Yu Z, et al:
MicroRNA expression, survival, and response to interferon in liver
cancer. N Engl J Med. 361:1437–1447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Speed N and Blair IA: Cyclooxygenase- and
lipoxygenase-mediated DNA damage. Cancer Metastasis Rev.
30:437–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khan Z, Khan N, Tiwari RP, et al: Biology
of Cox-2: an application in cancer therapeutics. Curr Drug Targets.
12:1082–1093. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Zhang YF, Zhang AR, Zhang BC, Rao ZG, et
al: MiR-26a regulates cell cycle and anoikis of human esophageal
adenocarcinoma cells through Rb1-E2F1 signaling pathway. Mol Biol
Rep. 40:1711–1720. 2013. View Article : Google Scholar
|
|
20
|
Suleyman H, Cadirci E, Albayrak A and
Halici Z: Nimesulide is a selective COX-2 inhibitory, atypical
non-steroidal anti-inflammatory drug. Curr Med Chem. 15:278–283.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morita Y, Morita N, Hata K, et al:
Cyclooxygenase-2 expression is associated with vascular endothelial
growth factor-c and lymph node metastasis in human oral tongue
cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 117:502–510.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kono M, Watanabe M, Abukawa H, Hasegawa O,
Satomi T and Chikazu D: Cyclo-oxygenase-2 expression is associated
with vascular endothelial growth factor C expression and lymph node
metastasis in oral squamous cell carcinoma. J Oral Maxillofac Surg.
71:1694–1702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morita Y, Hata K, Nakanishi M, Nishisho T,
Yura Y and Yoneda T: Cyclooxygenase-2 promotes tumor
lymphangiogenesis and lymph node metastasis in oral squamous cell
carcinoma. Int J Oncol. 41:885–892. 2012.PubMed/NCBI
|
|
24
|
Wang YH, Wu MW, Yang AK, et al: COX-2 gene
increases tongue cancer cell proliferation and invasion through
VEGF-C pathway. Med Oncol. 28:S360–S366. 2011. View Article : Google Scholar
|
|
25
|
Kyzas PA, Stefanou D and Agnantis NJ:
COX-2 expression correlates with VEGF-C and lymph node metastases
in patients with head and neck squamous cell carcinoma. Mod Pathol.
18:153–160. 2005. View Article : Google Scholar
|
|
26
|
Li J, Kong X, Zhang J, Luo Q, Li X and
Fang L: MiRNA-26b inhibits proliferation by targeting PTGS2 in
breast cancer. Cancer Cell Int. 13:72013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Li X, Kong X, Luo Q, Zhang J and
Fang L: MiRNA-26b inhibits cellular proliferation by targeting CDK8
in breast cancer. Int J Clin Exp Med. 7:558–565. 2014.PubMed/NCBI
|
|
28
|
Hsu CM, Lin PM, Wang YM, Chen ZJ, Lin SF
and Yang MY: Circulating miRNA is a novel marker for head and neck
squamous cell carcinoma. Tumour Biol. 33:1933–1942. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rizzo MT: Cyclooxygenase-2 in oncogenesis.
Clin Chim Acta. 412:671–687. 2011. View Article : Google Scholar
|
|
30
|
Ghosh N, Chaki R, Mandal V and Mandal SC:
COX-2 as a target for cancer chemotherapy. Pharmacol Rep.
62:233–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin DT, Subbaramaiah K, Shah JP,
Dannenberg AJ and Boyle JO: Cyclooxygenase-2: a novel molecular
target for the prevention and treatment of head and neck cancer.
Head Neck. 24:792–799. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghosh N, Chaki R, Mandal V and Mandal SC:
COX-2 as a target for cancer chemotherapy. Pharmacol Rep.
62:233–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kraus S, Naumov I and Arber N: COX-2
active agents in the chemoprevention of colorectal cancer. Recent
Results Cancer Res. 191:95–103. 2013. View Article : Google Scholar
|