Introduction
Lung cancer still remains one of the most prevalent
types of cancer and the leading cause of cancer-related deaths in
the world. Metastasis is a common feature of lung cancer.
Approximately 90% of lung cancer patient deaths are due to
metastasis, rather than to the primary tumor (1). However, currently the processes and
steps involved in metastasis are too complex to understand at a
sufficient level. These facts highlight the need for investigating
and clarifying the molecular mechanisms underlying and modulating
lung cancer metastasis, in order to find novel therapeutic
targets.
The aberrant activation of the Wnt and Notch
signaling pathways, evolutionarily conserved pathways governing
embryonic development, have been reported to contribute to lung
cancer metastasis. Wnt ligands are a family of secreted
glycoproteins. Through the binding of Wnt ligands to Frizzled and
the low-density lipoprotein receptor-related protein-5/6 (LRP5/6)
receptor, the canonical Wnt signaling pathway is initially
activated and prevents the degradation of β-catenin in the
cytoplasm causing it to accumulate in the nucleus and bind to the
transcription factor of lymphoid enhancer-binding factor and the
T-cell factor (LEF/TCF) to regulate expression of various genes.
The aberrant activation of the Wnt signaling pathway promotes the
colony formation and invasion of non-small cell lung cancer (NSCLC)
cells, while antagonists to the Wnt signaling pathway inhibit
epithelial-mesenchymal transition (EMT), cell migration and
invasion (2–7). In mammals, the notch family consists
of four Notch receptors (Notch1-4) and five ligands (Jagged1-2,
delta-like 1, 3 and 4). Upon the interaction of notch ligands
expressed on one cell with the receptors on an adjacent cell, the
intracellular portion of the receptor is released and translocates
to the nucleus where it interacts with recombining binding protein
for immunoglobulin κ J region (RBP-J) and leads to the release and
activation of the transcriptional co-repressors. Notch1-4 are
upregulated in NSCLC tissues and Notch1 and 2 are positively
correlated with lymph node metastasis (8). The inhibition of the Notch signaling
pathway was found to decrease the invasive ability of NSCLC cells
(9).
Although the role of the Wnt and Notch signaling
pathways in NSCLC metastasis has been highlighted, it is not fully
understood. In our previous study, we observed that the Wnt and
Notch signaling pathways synergistically promoted NSCLC cell
proliferation (10). As known,
tumor metastasis is largely affected by cell proliferation in
vivo (11,12). This raises a question of whether
there is some relationship between the Wnt and Notch signaling
pathways in the modulation of metastasis. In the present study, we
investigated the effects of Wnt3a, a Wnt signaling agonist, or
Notch3 shRNA, or the combined application of Wnt3a and Notch3 shRNA
on the metastatic abilities of NSCLC cells, such as cell invasion,
anchorage-independent growth in soft agar and EMT in
vitro.
Materials and methods
Cell culture and treatment
Three human lung cancer cell lines (A549, H157 and
H460) were cultured in a DMEM/F12 medium supplemented with 10%
fetal calf serum (FCS) (both from HyClone, Beijing, China).
Recombinant Wnt3a (5036-WNP-010; R&D Systems, Minneapolis, MN,
USA) was reconstituted at 200 μg/ml in sterile PBS containing at
least 0.1% bovine serum albumin and stored at −20°C. The cells were
placed in a fresh medium plus 50 or 100 ng/ml Wnt3a and were
cultivated for 24 h before cell analysis. PBS was used as
control.
The plasmid encoding the shRNA targeting the Notch3
gene (sc-37136-SH; Santa Cruz Biotechnology, USA) and the
non-target shRNA control plasmid were transfected into cells with
TranSmarter (Abmart, Shanghai, China). After 48 h of transfection,
the cells were selected with puromycin (Sigma-Aldrich, St. Louis,
MO, USA) at a final concentration of 1 μg/ml for 2 weeks.
Puromycin-resistant clones were isolated and subsequently cultured
for the following experiment. Validation of the Notch3 silencing in
the transfected cells was carried out by western blotting.
Immunofluorescence
The cells seeded in 35-mm dishes were stained
according to the following protocol. The medium mixture was
discarded and 4% paraformaldehyde was added to fix the cells at
room temperature (RT) for 10 min, and cells were washed with PBS
for 5 min in a shaker and 0.2% Triton X-100 was added for 10 min.
The cells were washed with PBS twice and TRITC-conjugated
phalloidin (Sigma-Aldrich) was added at RT for 40 min, and the
cells were washed with PBS containing 0.02% Triton X-100 for three
times. Nuclei were counterstained with Hoechst 33342 (5 μg/ml) for
30 min at RT, cells were washed with PBS containing 0.02% Triton
X-100 for three times and finally, 200 μl PBS was added. The
staining was examined under a laser scanning confocal microscope
(FV1000; Olympus, Tokyo, Japan).
Reverse transcription (RT)-polymerase
chain reaction (PCR)
The total RNA was prepared from the cultured cells
using TRIzol reagent (Invitrogen Inc., Carlsbad, CA, USA) according
to the manufacturer’s instructions. The cDNA was synthesized using
oligo(dT) as a primer. Primers (Table
I) were designed with Primer3 on line. The PCR was carried out
using a PCR kit (RT-PCR AMV 3.0 kit; Takara, Tokyo, Japan). The PCR
products were electrophoresed on a 2% agarose gel and visualized by
staining with GeneFinder (Baiweixin, Xiamen, China). The bands were
quantified by densitometry to obtain the integrated density values
(IDV). The relative amounts of each protein to
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are represented as
the ratio of their IDV in the histograms.
 | Table IOligonucleotide primers used for
RT-PCR analyses. |
Table I
Oligonucleotide primers used for
RT-PCR analyses.
| Gene | Primer sequence
(strand) | Product size
(bp) |
|---|
| Notch3 |
5′-caacccggtgtacgagaagt-3′ (+)
5′-gaacgcagtagctcctctgg-3′ (−) | 180 |
| HES1 |
5′-ctctcttccctccggactct-3′ (+)
5′-aggcgcaatccaatatgaac-3′ (−) | 186 |
| HEYL |
5′-caagcatgcaactccaaaga-3′ (+)
5′-aggaaggcttggggatagaa-3′ (−) | 184 |
| GAPDH |
5′-tctgcccggagcctccttcc-3′ (+)
5′-gatgcacccgctgcgcacta-3′ (−) | 196 |
Cell invasion assay
The cells in the medium with 1% FCS were seeded into
the upper chambers of a 24-well Transwell plate (3422; Corning, NY,
USA) with BioCoat Matrigel (1:8; BD Bioscience, San Jose, CA, USA).
The medium with 10% FCS was added to the lower chambers as a
chemoattractant. After 24 h of incubation, cells that invaded
through the membrane filter were fixed with 75% ethanol and stained
with 0.1% crystal violet. The number of invading cells was counted
under an inverted microscope with a digital CCD imaging system
[IX70/SPOT RT-KE (color), Olympus/DI, Japan/USA] with a ×10
objective in five random fields.
Anchorage-independent colony formation
assay in soft agar
Soft agar plates were prepared using a Gene Med
soft-agar kit (GMS10024; Gene Med, Shanghai, China) according to
the manufacturer’s instructions. To prepare the base layer, reagent
A mixed with reagent B in equal volumes was added into the 12-well
plate and incubated at 50°C for 2 h to allow the agar to solidify.
Top layer agar was prepared by mixing reagent C with reagent A at a
3:1 ratio for semi-solidity, and the cells suspended in this top
layer of agar were plated over the base layer. Then, the plates
were incubated at 37°C overnight. The next day the liquid reagent D
was added over the top layer of agar. Moreover, the cultures were
fed with 0.25 ml of reagent D at 2-day intervals for 2 weeks. In
the Wnt3a treatment groups, Wnt3a was added to the semi-solidity
agar reagent and the reagent D was replenished to the concentration
of 100 ng/ml. Finally, colonies with diameter >120 μm were
scored under a light microscope at low magnification [IX70/SPOT
RT-KE (color), Olympus/DI, Japan/USA]. The numbers of the colonies
in the treatment groups were converted to a percentage of the
control (by considering the control as 100%).
Western blot analysis
Cells were lysed in a phospho-lysis buffer (50 mM
Tris-Cl, pH 7.5, 150 mM NaCl, 1 mM MgCl2, 0.5% NP-40, 1
mg/ml BSA, 0.1 mM PMSF). Samples were analyzed by 10%
SDS-polyacrylamide gel electrophoresis, followed by western
blotting using rabbit monoclonal anti-Notch3 (D11B8; Cell Signaling
Technology, Beverly, MA, USA), rabbit polyclonal N-cadherin (W745),
rabbit polyclonal E-cadherin (R868), (both from Bioworld
Technology, Nanjing, China), and vimentin (ab8069; Abcam,
Cambridge, MA, USA), respectively, and the secondary antibody of
goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP;
HuaAn Biotech, Hangzhou, China). The membrane was developed using
chemiluminescent reagents (SuperSignal West Pico Chemiluminescent
Substrate; Pierce, Rockford, IL, USA). The bands were quantified by
densitometry to obtain the IDV. The relative amounts of each
protein to actin are represented as the ratio of their IDV in the
histograms.
Statistical analysis
Data are presented as the mean ± SE of at least
three independent experiments. The one-way analysis of variance
(ANOVA) was used for statistical analysis. Statistical significance
was accepted at P<0.05.
Results
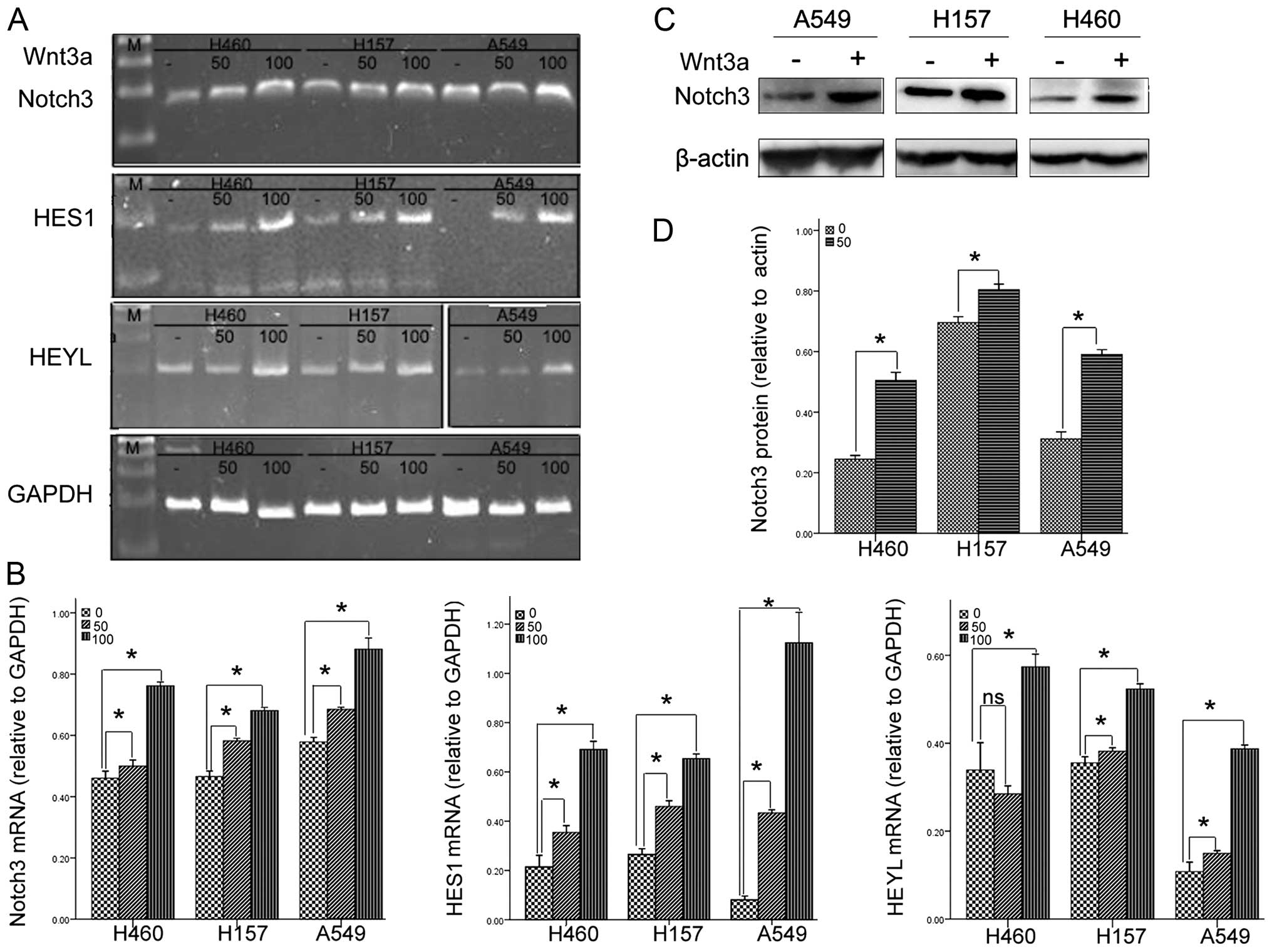
Wnt3a upregulates the expression of
Notch3 and its down stream genes in NSCLC cell lines
Wnt3a, a member of the secreted Wnt ligands, is
consistently used to mimic the biochemical effects of the canonical
Wnt signaling pathway (13,14). Notch3 is one of the four Notch
receptors identified in mammals. Previous studies concerned with
the pathogenesis of lung cancer have identified that Notch3 plays
an essential role in NSCLC (15,16).
Compared with the control, Wnt3a treatment increased the mRNA
expression of Notch3 and its downstream gene, HES1, in all the
three cell lines in a dose-dependent manner. As for HEYL, another
downstream gene of Notch3, Wnt3a treatment dose-dependently
increased its mRNA expression in the H157 and A549 cells. In the
H460 cells, only 100 ng/ml of Wnt3a increased the mRNA expression
of HEYL (Fig. 1A and B). To further
confirm the effects of Wnt3a on Notch3 expression, we assessed the
protein level of Notch3, using western blotting in the absence or
presence of 100 ng/ml of Wnt3a. Consistent with the data at the
mRNA level, Wnt3a treatment significantly increased the expression
of Notch3 at the protein level (Fig. 1C
and D). Altogether, these data strongly indicate that Wnt3a may
upregulate Notch3.
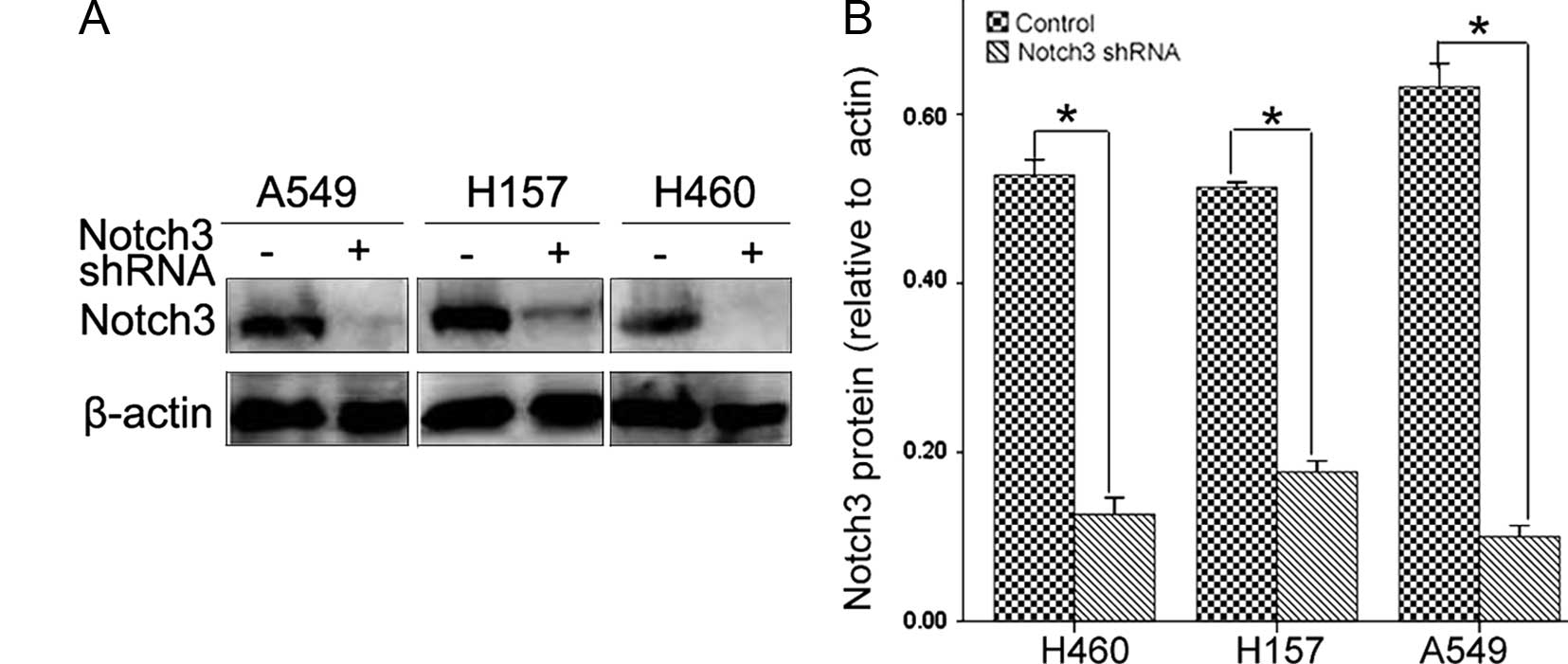
Notch3 shRNA specifically reduces Notch3
protein expression
Notch3 is one of the four Notch receptors identified
in mammals and its overexpression is frequently noted in NSCLC. In
the present study, the expression of Notch3 was confirmed by
western blotting. Three lung cancer cell lines transfected with
Notch3 shRNA showed a significant reduction in Notch3 protein
expression (Fig. 2A and B).
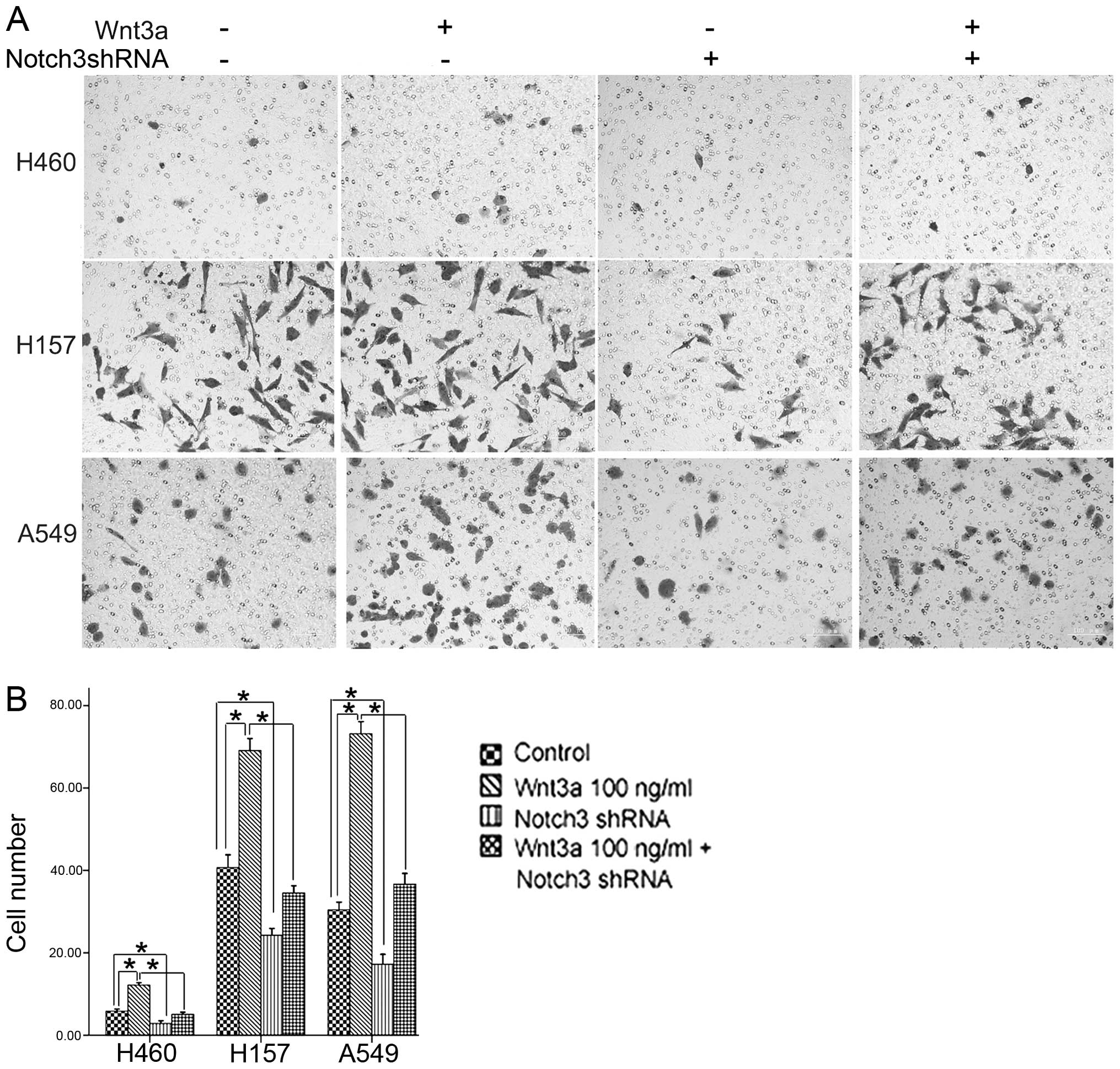
Roles of Wnt3a and Notch3 in cell
invasion
As in vitro invasion is one of the
characteristics of metastasis, the Transwell invasion assay was
carried out to assess the effects of Wnt3a and Notch3 on cell
invasion. Cell invasion was increased in the three cell lines
treated with 100 ng/ml Wnt3a. Notch3 reduction led to decreased
cell invasion in the three cell lines. Moreover, Notch3 reduction
significantly attenuated the effects of Wnt3a treatment on the
invasive ability of cells (Fig. 3A and
B). The data demonstrated that Wnt3a and Notch3 promoted in
vitro invasion of the NSCLC cells and indicate that the Wnt
pathway may promote cell invasion partially via Notch3.
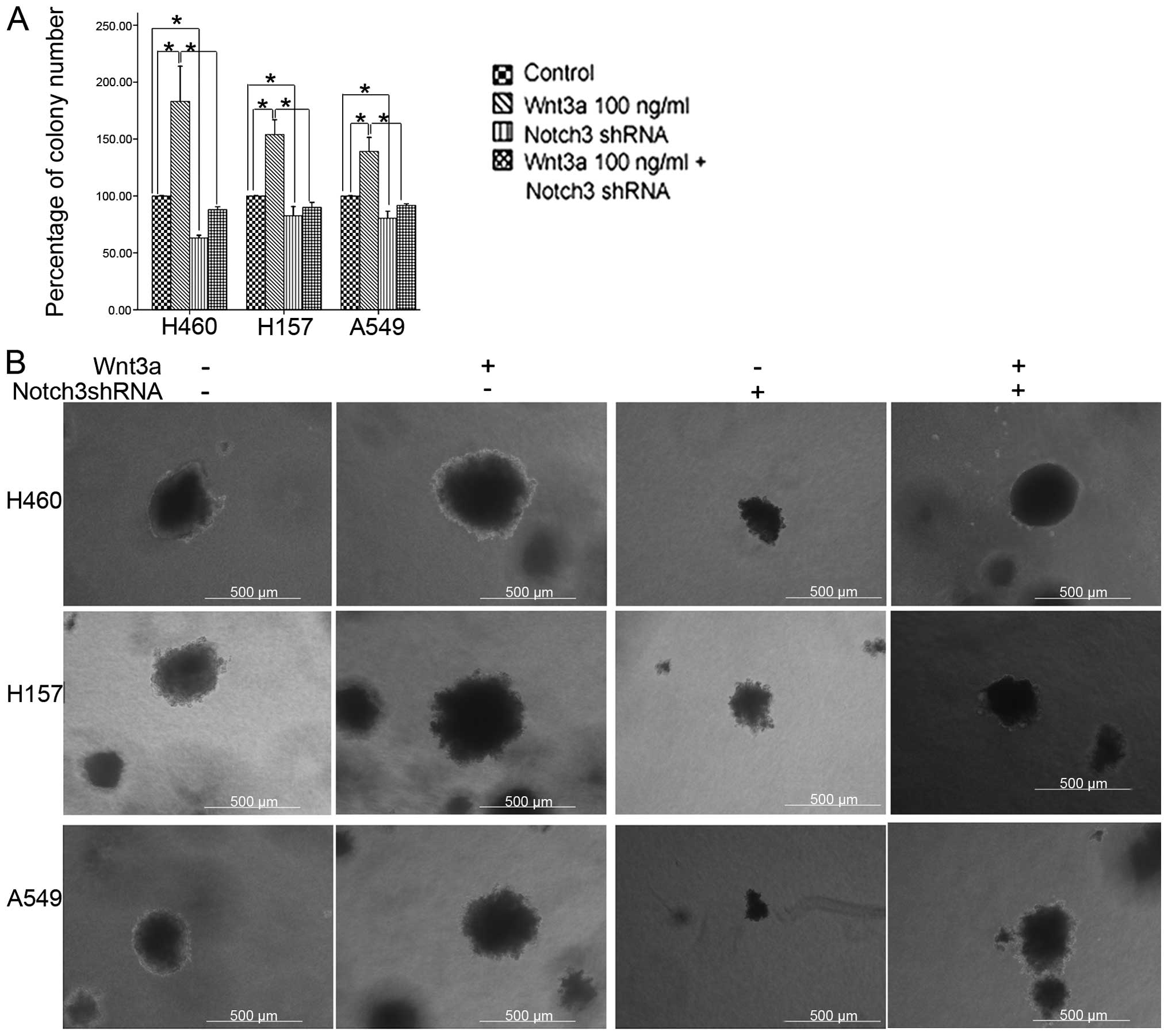
Roles of Wnt3a and Notch3 in the
anchorage-independent growth in soft agar
For cells to undergo metastasis, they must have the
ability to overcome anoikis and survive without cell-substratum
interaction (17). The
anchorage-independent growth in soft agar has been shown to be one
of the independent factors of the metastatic potential in cancer
(18,19). To further analyze the effects of
Wnt3a and Notch3 on the characteristics of metastatic outgrowth, a
soft-agar colony assay was used in the present study. When cultured
in the reagent with 100 ng/ml Wnt3a, the cells formed compact
spherical colonies that grew larger in size and in number, when
compared to the control cells. Meanwhile, Notch3 reduction
decreased the size and the number of the colonies. Consistent with
the results of the cell invasion assay, Notch3 reduction also
weakened the effects of Wnt3a on the size and number of colonies,
suggesting that Notch3 may be involved in the colony outgrowth
prompted by the Wnt signaling pathway in the NSCLC cells (Fig. 4).
Roles of Wnt3a and Notch3 in the
regulation of EMT
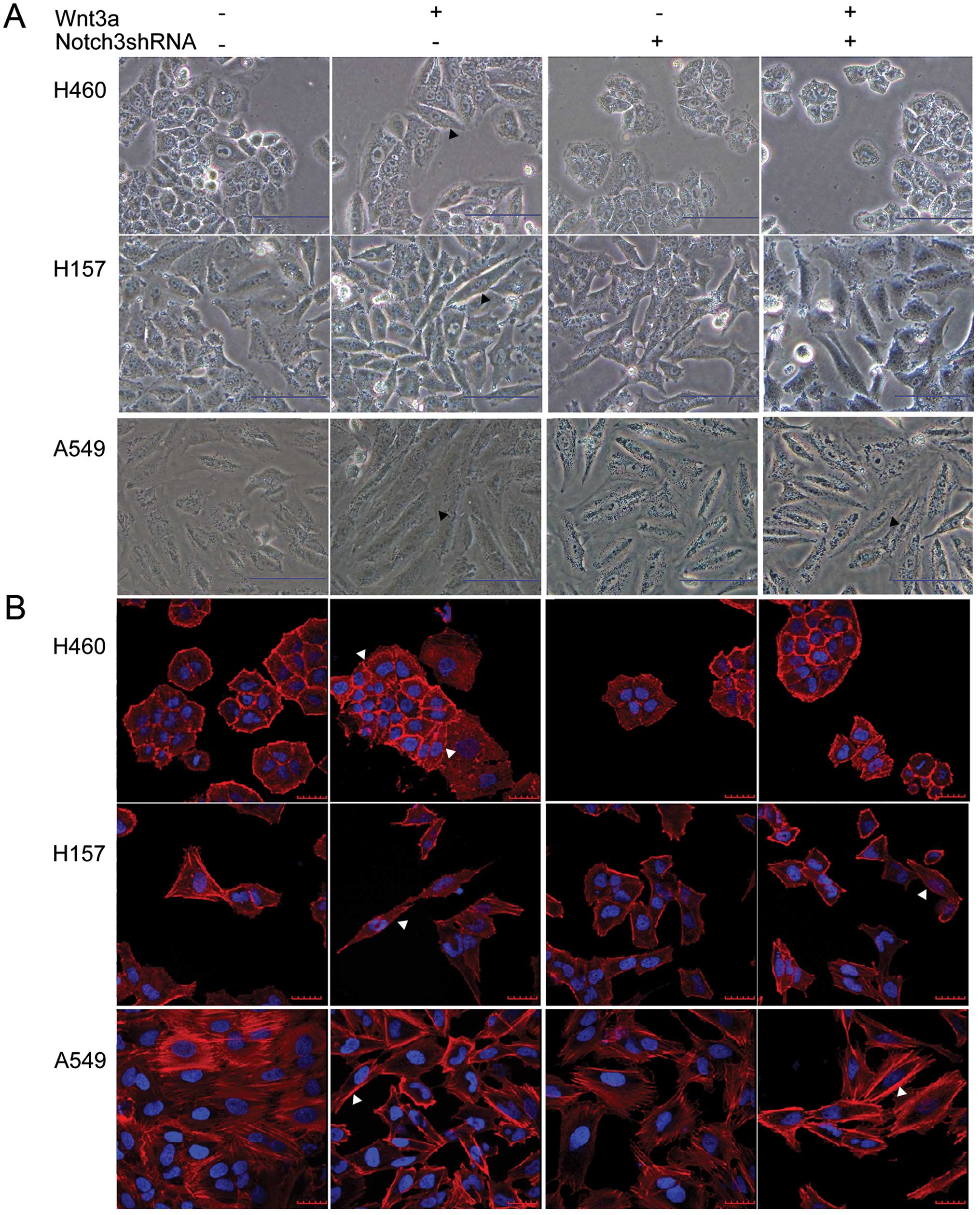
Accumulating evidence suggests that the EMT is a key
event in cancer metastasis. During EMT, cell morphological changes
and dynamic remodeling of the actin cytoskeleton consistently occur
(20,21). In the control cells, the majority of
cells maintained typical epithelial morphology, while some of the
Wnt3a-treated cells underwent elongation to become fibroblast-like
spindle-shaped cells (Fig. 5A,
black arrowhead). The F-actin staining also showed that some of the
Wnt3a-treated cells became longer (Fig.
5B, white arrowhead). Moreover, we observed that the F-actin
filaments of the control cells were mainly organized in cortical
thin bundles whereas the actin filaments of the Wnt3a-treated cells
appeared to be assembled into thick bundles at the cell surface in
the A549 cells (Fig. 5B, white
arrowhead). However, compared to the Wnt3a treatment, Notch3 shRNA
appeared to have opposite effects on the cell morphology and actin
reorganization (Fig. 5A and B).
These results indicate that Wnt3a and Notch3 may induce the EMT
process in the cultured cells.
To further explore the roles of Wnt3a and Notch3 on
EMT, we measured the protein expression levels of E-cadherin,
N-cadherin and vimentin, key protein markers involved in EMT. The
downregulation of the epithelial marker E-cadherin and the
upregulation of the mesenchymal markers N-cadherin and vimentin are
all hallmarks of EMT (20). Wnt3a
treatment significantly induced the downregulation of E-cadherin
and the upregulation of N-cadherin and vimentin at the protein
level. In contrast, Notch3 shRNA significantly induced the
downregulation of N-cadherin and vimentin and the upregulation of
E-cadherin. Moreover, Notch3 shRNA partly inhibited the regulation
of Wnt3a on the expression of three protein factors (Fig. 5C and D). Taken together, these
findings indicate that Wnt3a treatment is sufficient to induce EMT
in the NSCLC cells, in which Notch3 is involved.
Discussion
Metastasis occurs as a multi-step process during
which cancer cells in the primary tumor lose cell-cell adhesion and
break through the basement membrane with increased invasive
properties and intravasate the circulation to be transported to
distant tissues, extravasate out of the circulatory system and
subsequently colonize remote sites (22,23).
Both the Wnt and Notch signaling pathways have been reported to
transform cancer cells to an invasive and metastatic phenotype. The
canonical Wnt signaling pathway may contribute to the metastatic
progression of cancer by promoting EMT, regulating the expression
of matrix metalloproteinases (MMPs) and other factors that play a
role in the regulation of the extracellular matrix (24–28).
The notch signaling pathway may participate in cancer metastasis by
the establishment of stem cell populations that allow for the
creation of metastatic niches and its interaction with hypoxia can
accelerate the steps of invasion and metastasis in cancer
modulating EMT (29–31).
Wnt3a and Notch3 are important components of the Wnt
and Notch signaling pathways, respectively. In the present study,
Wnt3a-treated cells exhibited increased invasion ability and
anchorage-independent growth compared to the controls. Moreover,
Wnt3a introduced the EMT-like elongation of cell morphology,
F-actin reorganization as well as the downregulation of E-cadherin
and upregulation of N-cadherin and vimentin. In contrast, Notch3
shRNA transfection had the opposite effects on the invasion
ability, the anchorage-independent growth cell morphology, F-actin
reorganization and the expression of EMT markers, compared with
Wnt3a treatment. These observations are consistent with previous
studies that Wnt3a and Notch3 induce EMT and promote metastasis in
other types of cancer (32–34). Therefore, the data of the present
study strongly indicate that both Wnt3a and Notch3 may positively
regulate NSCLC metastasis.
The Wnt and Notch signaling pathways are closely
inter-connected in the development of various types of cancer and
their interaction is indispensable for tumorigenesis, such as
breast cancer or colon cancer (35,36).
However, the crosstalk between the Wnt and Notch signaling pathways
in NSCLC metastasis is poorly addressed. In our experiment, Wnt3a
upregulated the expression of Notch3 and downstream genes.
Moreover, Wnt3a induced EMT and the in vitro characteristics
of metastasis were partially reversed by the knockdown of Notch3.
Together, these findings indicate that the crosstalk between the
Wnt and the Notch signaling pathways may exist in NSCLC
metastasis.
Although further studies are still required to
clarify how Wnt3a and Notch3 cooperatively promote NSCLC
metastasis, our findings may support the existence of crosstalk
between the two pathways during the metastatic process in NSCLC
cells. The results of the present study may facilitate our
understanding of the molecular mechanisms modulating NSCLC
metastasis. This should be important for the identification of
novel targets to prevent NSCLC progression.
Acknowledgements
This research was sponsored by the Scientific
Research Foundation for the Returned Overseas Chinese Scholars,
State Education Ministry (to C.L., no. 20091001) and the Liaoning
Nature Science Project (to C.L., no. 201102258).
References
|
1
|
Perlikos F, Harrington KJ and Syrigos KN:
Key molecular mechanisms in lung cancer invasion and metastasis: A
comprehensive review. Crit Rev Oncol Hematol. 87:1–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie C, Jiang G, Fan C, et al: ARMC8α
promotes proliferation and invasion of non-small cell lung cancer
cells by activating the canonical Wnt signaling pathway. Tumour
Biol. 35:8903–8911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao Y, Song C, Hui L, et al:
Overexpression of RNF146 in non-small cell lung cancer enhances
proliferation and invasion of tumors through the Wnt/β-catenin
signaling pathway. PLoS One. 9:e853772014. View Article : Google Scholar
|
|
4
|
Su K, Huang L, Li W, et al: TC-1 (c8orf4)
enhances aggressive biologic behavior in lung cancer through the
Wnt/β-catenin pathway. J Surg Res. 185:255–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang S, Wang Y, Dai SD and Wang EH:
Down-regulation of NKD1 increases the invasive potential of
non-small-cell lung cancer and correlates with a poor prognosis.
BMC Cancer. 11:1862011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ren J, Wang R, Huang G, Song H, Chen Y and
Chen L: sFRP1 inhibits epithelial-mesenchymal transition in A549
human lung adenocarcinoma cell line. Cancer Biother Radiopharm.
28:565–571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh T and Katiyar SK: Honokiol inhibits
non-small cell lung cancer cell migration by targeting
PGE2-mediated activation of β-catenin signaling. PLoS
One. 8:e607492013. View Article : Google Scholar
|
|
8
|
Westhoff B, Colaluca IN, D’Ario G, et al:
Alterations of the Notch pathway in lung cancer. Proc Natl Acad Sci
USA. 106:22293–22298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu L, Chen X, Wang Y, et al: Notch3 is
important for TGF-β-induced epithelial-mesenchymal transition in
non-small cell lung cancer bone metastasis by regulating ZEB-1.
Cancer Gene Ther. 21:364–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li C, Zhang S, Lu Y, Zhang Y, Wang E and
Cui Z: The roles of Notch3 on the cell proliferation and apoptosis
induced by CHIR99021 in NSCLC cell lines: a functional link between
Wnt and Notch signaling pathways. PLoS One. 8:e846592013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao CF, Xie Q, Su YL, et al: Proliferation
and invasion: plasticity in tumor cells. Proc Natl Acad Sci USA.
102:10528–10533. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Al-Mehdi AB, Tozawa K, Fisher AB, Shientag
L, Lee A and Muschel RJ: Intravascular origin of metastasis from
the proliferation of endothelium-attached tumor cells: a new model
for metastasis. Nat Med. 6:100–102. 2000. View Article : Google Scholar
|
|
13
|
Kikuchi A, Yamamoto H and Kishida S:
Multiplicity of the interactions of Wnt proteins and their
receptors. Cell Signal. 19:659–671. 2007. View Article : Google Scholar
|
|
14
|
Stapp AD, Gómez BI, Gifford CA, Hallford
DM and Hernandez Gifford JA: Canonical WNT signaling inhibits
follicle stimulating hormone mediated steroidogenesis in primary
cultures of rat granulosa cells. PLoS One. 9:e864322014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yi F, Amarasinghe B and Dang TP: Manic
fringe inhibits tumor growth by suppressing Notch3 degradation in
lung cancer. Am J Cancer Res. 3:490–499. 2013.PubMed/NCBI
|
|
16
|
Zheng Y, de la Cruz CC, Sayles LC, et al:
A rare population of CD24(+)ITGB4(+)Notch(hi) cells drives tumor
propagation in NSCLC and requires Notch3 for self-renewal. Cancer
Cell. 24:59–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Frisch SM and Francis H: Disruption of
epithelial cell-matrix interactions induces apoptosis. J Cell
Biol1. 24:619–626. 1994. View Article : Google Scholar
|
|
18
|
Nomura Y, Tashiro H and Hisamatsu K: In
vitro clonogenic growth and metastatic potential of human operable
breast cancer. Cancer Res. 49:5288–5293. 1989.PubMed/NCBI
|
|
19
|
Nakanishi K, Sakamoto M, Yasuda J, et al:
Critical involvement of the phosphatidylinositol 3-kinase/Akt
pathway in anchorage-independent growth and hematogeneous
intrahepatic metastasis of liver cancer. Cancer Res. 62:2971–2975.
2002.PubMed/NCBI
|
|
20
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta GP and Massagué J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Poste G and Fidler IJ: The pathogenesis of
cancer metastasis. Nature. 283:139–146. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bo H, Zhang S, Gao L, Chen Y, Zhang J,
Chang X and Zhu M: Upregulation of Wnt5a promotes
epithelial-to-mesenchymal transition and metastasis of pancreatic
cancer cells. BMC Cancer. 13:4962013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ford CE, Jary E, Ma SS, Nixdorf S,
Heinzelmann-Schwarz VA and Ward RL: The Wnt gatekeeper SFRP4
modulates EMT, cell migration and downstream Wnt signalling in
serous ovarian cancer cells. PLoS One. 8:e543622013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kwon M, Lee SJ, Wang Y, et al: Filamin A
interacting protein 1-like inhibits WNT signaling and MMP
expression to suppress cancer cell invasion and metastasis. Int J
Cancer. 135:48–60. 2014. View Article : Google Scholar
|
|
27
|
Jiang W, Crossman DK, Mitchell EH, Sohn P,
Crowley MR and Serra R: WNT5A inhibits metastasis and alters
splicing of Cd44 in breast cancer cells. PLoS One. 8:e583292013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dey N, Barwick BG, Moreno CS, et al: Wnt
signaling in triple negative breast cancer is associated with
metastasis. BMC Cancer. 13:5372013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Espinoza I and Miele L: Deadly crosstalk:
Notch signaling at the intersection of EMT and cancer stem cells.
Cancer Lett. 341:41–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Espinoza I, Pochampally R, Xing F, Watabe
K and Miele L: Notch signaling: targeting cancer stem cells and
epithelial-to-mesenchymal transition. Onco Targets Ther.
6:1249–1259. 2013.PubMed/NCBI
|
|
31
|
Du R, Sun W, Xia L, et al: Hypoxia-induced
down-regulation of microRNA-34a promotes EMT by targeting the Notch
signaling pathway in tubular epithelial cells. PLoS One.
7:e307712012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Q, Bai X, Chen W, et al:
Wnt/β-catenin signaling enhances hypoxia-induced
epithelial-mesenchymal transition in hepatocellular carcinoma via
crosstalk with hif-1α signaling. Carcinogenesis. 34:962–973. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhai Y, Iura A, Yeasmin S, et al: MSX2 is
an oncogenic downstream target of activated WNT signaling in
ovarian endometrioid adenocarcinoma. Oncogene. 30:4152–4162. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gupta N, Xu Z, El-Sehemy A, Steed H and Fu
Y: Notch3 induces epithelial-mesenchymal transition and attenuates
carboplatin-induced apoptosis in ovarian cancer cells. Gynecol
Oncol. 130:200–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Katoh M and Katoh M: NUMB is a break of
WNT-Notch signaling cycle. Int J Mol Med. 18:517–521.
2006.PubMed/NCBI
|
|
36
|
Bertrand FE, Angus CW, Partis WJ and
Sigounas G: Developmental pathways in colon cancer: crosstalk
between WNT, BMP, Hedgehog and Notch. Cell Cycle. 11:4344–4351.
2012. View
Article : Google Scholar : PubMed/NCBI
|