Introduction
Malignant melanoma is highly immunogenic and
responds to immunotherapy (1). As
expected, promising results in metastatic melanoma have been
observed with anti-CTLA-4 (2,3),
anti-PD-1 (4), anti-PD-L1 (5) and combinations. However, as the
disease still progresses in many of the patients, additional
modalities are needed. Clinical and biologic evidence highlights
carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1)
as a possible target for immunotherapy (6). CEACAM1 is a transmembranal
glycoprotein belonging to the Ig superfamily, composed of several
extracellular Ig-like domains (7).
CEACAM1 has been shown to be involved in the development of
cancerous transformation, particularly in melanoma, yet also in
non-small cell lung cancer and gastric carcinoma (8–10).
Importantly, a strong correlation between CEACAM1 expression and
the development of metastatic disease with poor overall survival
was demonstrated in melanoma (11)
and in lung cancer (12,13). CEACAM1 operates by homophilic
intercellular interactions with another CEACAM1 molecule.
Engagement of CEACAM1 expressed by activated lymphocytes causes
downstream signaling resulting in dephosphorylation of tyrosine
residues, which consequently inhibits effector functions (14,15).
Indeed, CEACAM1 was shown to protect melanoma cells by inhibiting
the action of NK and activated T cells (6,16–18).
This may be part of a sophisticated method of metastatic melanoma
cells escaping the inherent immunogenic response which we see
prevalent in melanoma. It has also been found that serum levels of
circulating CEACAM1 correlate with response to established
immunotherapy treatment (19).
Most of the currently available information on
CEACAM1 expression in melanoma is derived from comparisons between
patients and healthy individuals, or between isolated samples from
various patients. There is to date, no data on CEACAM1 expression
in a cohort of patients followed up from the primary tumor,
lymphatic spread and subsequent distant metastasis. The present
study characterized the CEACAM1 expression profile in a
longitudinal study during melanoma progression, in lesions obtained
from the same patients: a primary skin lesion, a lymph node and a
distant metastasis. The present study is therefore expected to
increase our understanding of the expression patterns of CEACAM1 in
melanoma development. We also compared the expression of CEACAM1 in
different metastatic lesions obtained from the same patients. Since
CEACAM1 is a potential target for immunotherapy, this pilot study
will provide initial data on the variability of CEACAM1 expression
in potential target lesions.
Materials and methods
Patients
The Ella Institute for Melanoma, within the Chaim
Sheba Medical Center, maintains a prospective database of melanoma
patients and is the largest tertiary referral center for melanoma
in Israel. From our database we searched and identified 13 patients
with pathology paraffin block specimens available in our
institution which included a primary lesion, a subsequent lymph
node metastasis and finally a distant metastasis. In addition we
found 7 patients with a primary lesion and subsequent distant
metastasis, for a total of 20 patients who were analyzed for
CEACAM1 expression over the course of disease progression. In
addition, we analyzed 4 patients who had pathology specimens of
metastases from more than one site. We also identified 5 patients
who, in addition to a primary invasive lesion, had another lesion
with melanoma in situ for comparison. All studies were
conducted according to the approval of the Institutional Review
Board.
Pathology and immunohistochemistry
The pathology blocks were cut, and two slides were
generated for each specimen. One underwent standard hematoxylin and
eosin (H&E) staining and a corresponding slide underwent
immunohistochemical staining for the detection of CEACAM1, as
previously described in detail by Ortenberg et al (6). Briefly, the slides were prepared for
immunostaining using standard protocols and were incubated
overnight at 4°C with MRG1 mAb, which is a murine IgG1 monoclonal
antibody against human CEACAM1. It recognizes the CEACAM1-specific
N-domain with high selectivity and affinity (KD
~2 nmol/l). Detection was conducted using the Histostain-SP Broad
Spectrum kit (Invitrogen, Carlsbad, CA, USA) and visualized with
the substrate chromogen AEC. Sections were counter-stained with
hematoxylin (Ventana Medical Systems, Tucson, AZ, USA) and
coverslipped with an aqueous mounting fluid (glycergel). Stained
sections were reviewed by an expert pathologist, and suitable
digital images were captured with an Olympus BX51 microscope.
Slides were assessed for both membranal and cytoplasmic staining.
The percentage of cells stained and the intensity of staining on a
scale of 0–3 were noted and recorded. In addition, primary lesions
were assessed for Clark level, Breslow depth, presence of
ulceration and lymphocytic infiltration.
Statistical analysis
Statistical analyses using standard non-parametric
testing, including Kruskal-Wallis one-way analysis of variance,
Friedman’s two-way analysis of variance, Mann-Whitney-Wilcoxon
test, Spearman’s rank correlation coefficient, as well as Fisher’s
exact test, were performed on the data by an independent
biostatistician.
Results
After reviewing the database and pathology specimens
of all patients undergoing treatment for melanoma in the Chaim
Sheba Medical Center, 29 pathology specimens were identified which
were included in the present study.
We identified the following groups of patients and
i) 13 patients with pathology specimens including a primary
invasive lesion, a lymph node and a distant metastasis; ii) 7
patients with pathology specimens including a primary invasive
lesion and a distant metastasis; iii) 4 patients with pathology
specimens with multiple metastatic lesions; and iv) 5 patients with
melanoma in situ, who were also evaluated for CEACAM1
membranal and cytoplasmic staining, were included.
The pathological features of the primary lesions are
summarized in Table I.
 | Table IPathological features of the primary
melanoma lesions. |
Table I
Pathological features of the primary
melanoma lesions.
| Pts | Clark | Breslow | Ulceration | Vascular
invasion | Lymphocyte
infiltration |
|---|
| 1 | 4 | 3.8 | No | No | No |
| 2 | 5 | 3.1 | No | No | No |
| 3 | 2 | 1.2 | No | No | Yes |
| 4 | N/A | N/A | No | No | No |
| 5 | 3 | 1.84 | No | No | No |
| 6 | 2 | 0.5 | No | No | No |
| 7 | 3 | 1.8 | No | No | Yes |
| 8 | 5 | 4 | Yes | No | No |
| 9 | 5 | 30 | Yes | No | No |
| 10 | 5 | 6 | Yes | No | No |
| 11 | 5 | 5 | No | No | No |
| 12 | 5 | 3.5 | No | No | No |
| 13 | 5 | 5 | No | No | No |
| 14 | 5 | 3.5 | Yes | No | Yes |
| 15 | 5 | 5 | Yes | No | No |
| 16 | 5 | 14 | No | No | No |
| 17 | 4 | 2.75 | No | No | No |
| 18 | 5 | 15 | No | No | Yes |
| 19 | 5 | 6 | No | No | No |
| 20 | 2 | 1.4 | No | No | No |
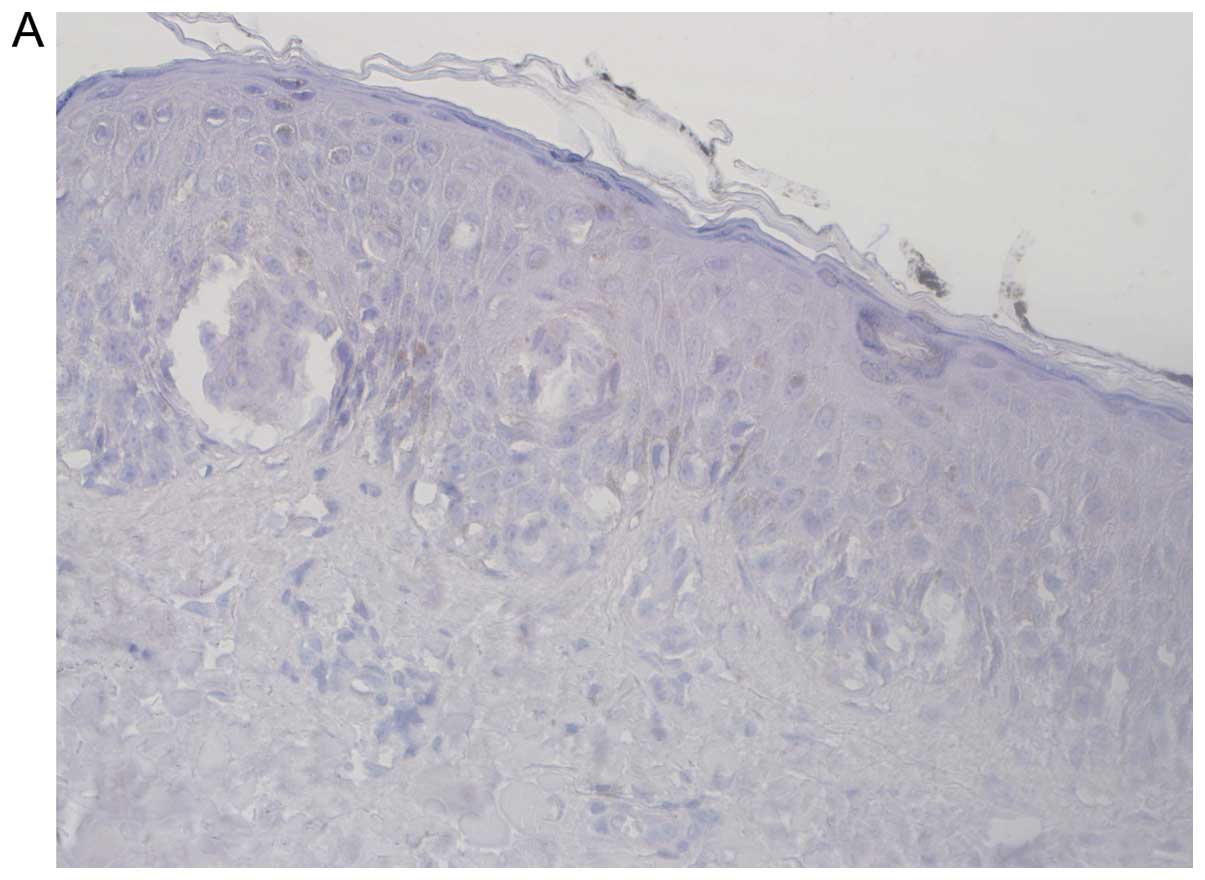
Melanoma in situ preparations had no evidence
of any membranal or cytoplasmic CEACAM1.
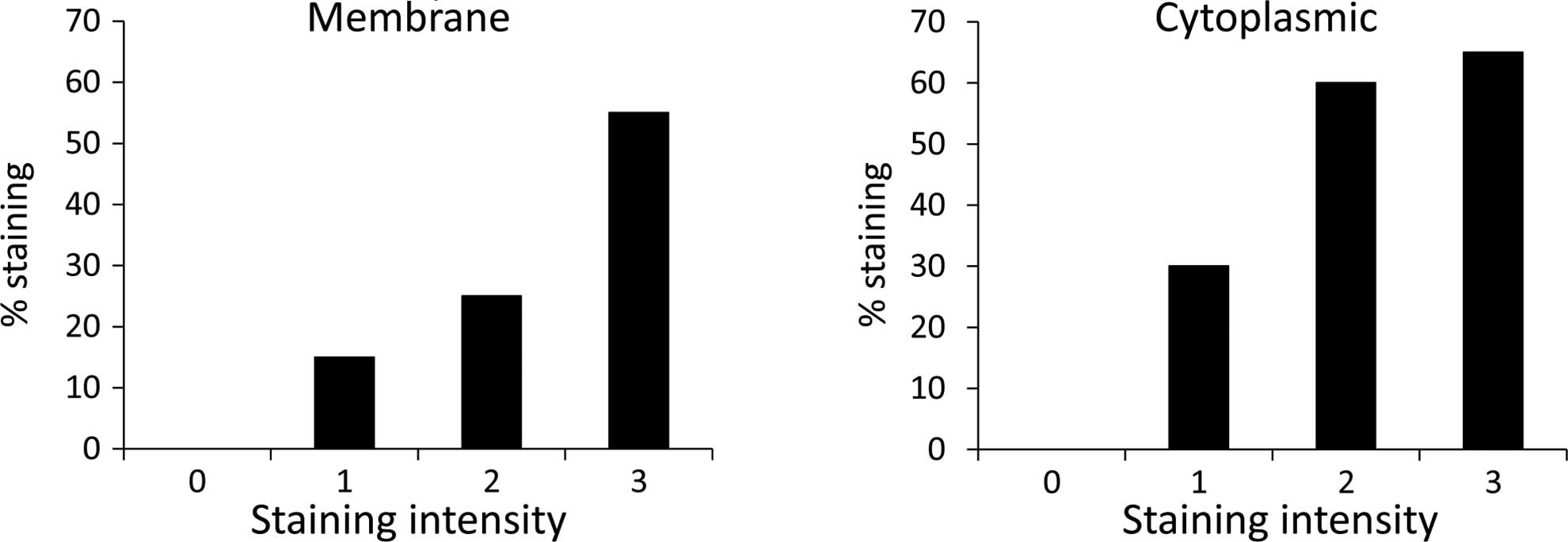
For the invasive primary lesions, staining intensity
was correlated directly and significantly with the percentage of
cells stained, in both membranal CEACAM1 (P<0.001) and
cytoplasmic CEACAM1 (P<0.02) (Fig.
1).
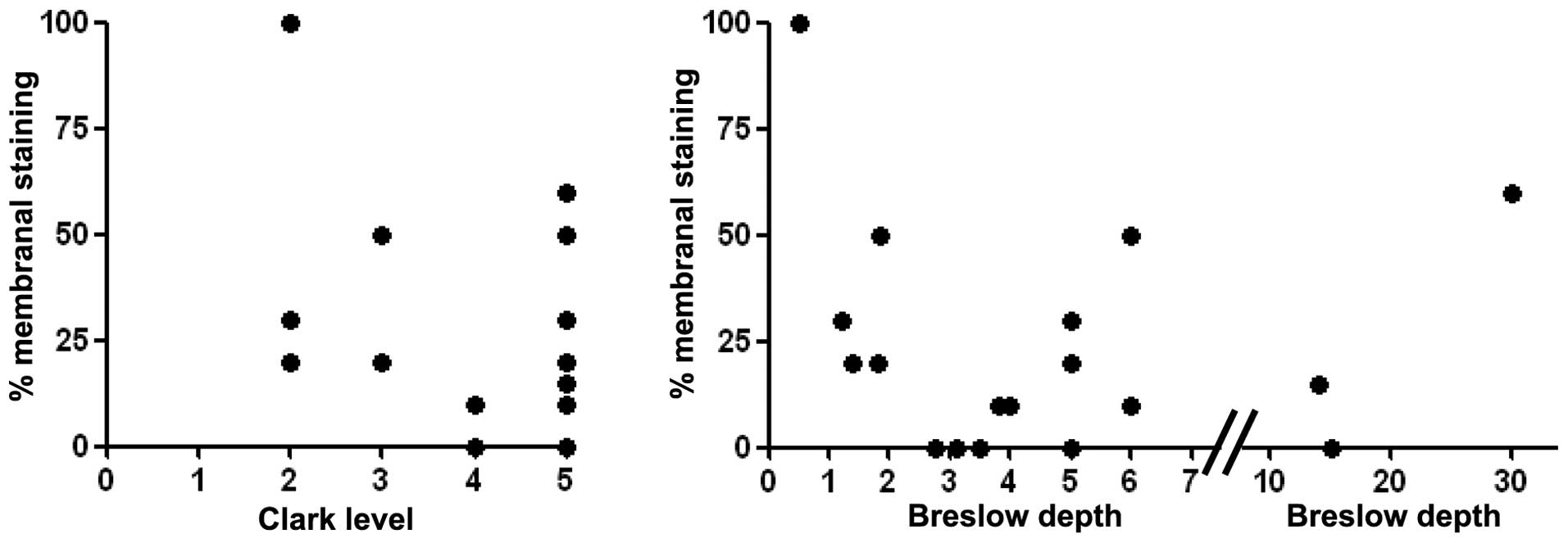
However, no correlation was found between the amount
of membranal CEACAM1 staining and Clark level or Breslow depth of
the primary lesions (Fig. 2). For
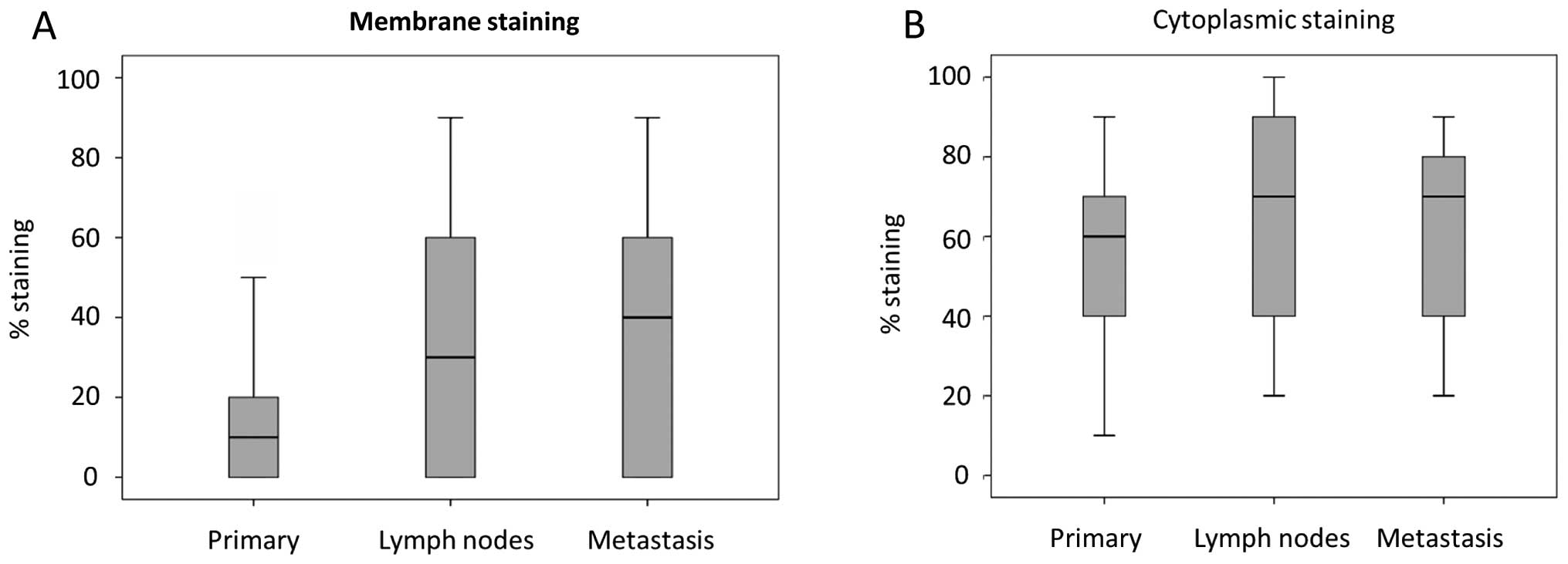
the 13 patients who were able to be followed up serially from
primary lesion, lymph node and distant metastasis, a borderline
significant increase in membranal staining was noted
(Kruskal-Wallis test, P=0.06). In contrast, there was no equivalent
increase in cytoplasmic CEACAM1 in the same group of patients
(Fig. 3).
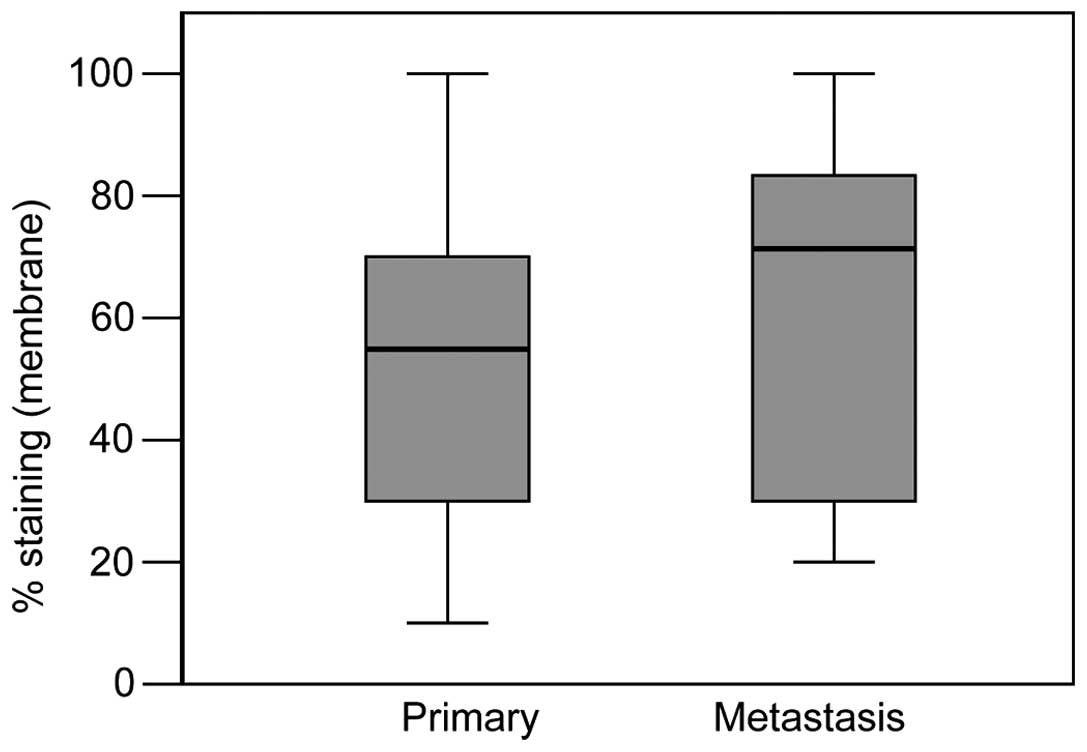
For the cohort of 20 patients with primary and
distant metastasis, a significant increase in membranal staining
was noted, (Mann-Whitney test, P=0.026) (Fig. 4) and again, no equivalent
significant increase in cytoplasmic was observed.
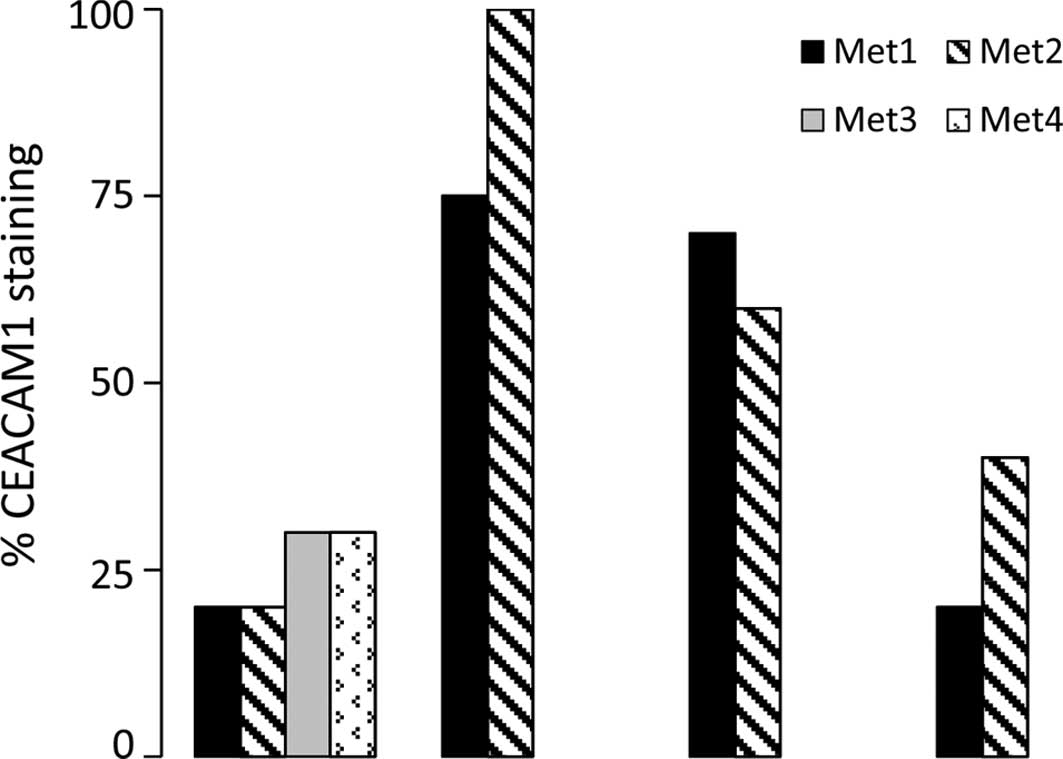
In the 4 patients who had more than one metastatic
lesion, no difference was found in the level of CEACAM1 membranal
staining between the individual metastatic lesions (Fig. 5).
Discussion
Several studies have shown that normal melanocytes
do not express CEACAM1 while most malignant melanoma lesions do
express CEACAM1 (10,20). We previously demonstrated that up to
89% of metastatic melanoma lesions are positive for CEACAM1
(6,10) and that its expression increases
concomitantly with tumor development and progression (10). Furthermore, in an observational
study by Thies et al that followed the outcome of 100
melanoma patients for 10 years, of the 40 patients with
CEACAM1-positive primary melanoma, 28 patients developed metastatic
disease as compared to only 6 of the 60 patients who were
CEACAM1-negative (11). Taken
together, this implies that CEACAM1 expression may be a crucial
factor in the development of melanoma metastasis and poor
prognosis. However, most of the currently available information on
CEACAM1 expression in melanoma is derived from comparisons between
patients and healthy individuals, or between isolated samples from
various patients.
What makes the present study unique is that we were
able to examine CEACAM1 expression in the same patient from primary
tumor, to lymph node involvement, to distant metastasis, on a
longitudinal axis. Importantly, as expected, CEACAM1 expression
increased along the course of an individual’s disease development
and progression (Fig. 6A–C) There
was a positive correlation between the percentage of cells stained
for CEACAM1 and the intensity of staining. Notably, melanoma in
situ specimens showed no CEACAM1 staining, implying that
CEACAM1 expression is associated with the ability of the primary
tumor to invade the basement membrane of the epidermis and
subsequently metastasize. This conforms with Gambichler et
al who found that CEACAM1 expression increases with progression
from benign nevi to dyplastic nevi to invasive melanoma (21). This finding may enable a more
accurate mapping of the timing of CEACAM1 expression during
neoplastic progression. However, we did not find a correlation
between CEACAM1 expression and the various prognostic indicators in
melanoma such as primary lesion thickness, ulceration and
lymphocytic infiltration.
We recently reported that CEACAM1 facilitates the
proliferation of melanoma cells in a Sox-2-dependent manner
(10). Additionally, melanoma cells
utilize CEACAM1 as an immune evasion mechanism (16,18,19).
Moreover, we previously demonstrated that higher CEACAM1 expression
levels improve the immune evasive phenotype (22). Therefore, it is conceivable that the
rise in CEACAM1 expression along the disease progression of a
patient contributes to its ability to proliferate and escape immune
destruction to facilitate metastasis.
We previously reported on a monoclonal antibody
blocking CEACAM1, which renders melanoma cells more vulnerable to
natural killer cells and cytotoxic T cells (6). This strategy is analogous to the
anti-PD-1 paradigm, and could be further developed as a novel drug.
Multiple clinical trials have proven the efficacy of various
immunooncology agents, such as anti-CTLA-4 (2,3) or
anti-PD-1 (4), and initial positive
data have even emerged in the adjuvant setting with anti-CTLA-4
(23). The present study may
provide pertinent data for future development of CEACAM1-guided
therapy. In addition, different metastatic lesions within the same
patient exhibited similar CEACAM1 expression pattern (Fig. 5). This suggests that the different
metastatic deposits benefit similarly by targeting CEACAM1.
However, this issue should be further studied in a larger
cohort.
There are many aspects of the function and
expression of CEACAM1 which remain unknown. However, there may be
valuable therapeutic consequences in understanding the mechanisms
contributing to overexpression of CEACAM1. Malignant melanoma
remains an extremely challenging problem for clinicians. Although
at an early stage, melanoma is treated primarily with surgical
extirpation, treatment of metastatic melanoma remains a profound
clinical problem, as melanoma is resistant to most standard
chemotherapy and radiation strategies. The use of immunotherapy in
disseminated melanoma has increased dramatically with the addition
of new immunotherapies to the physician’s armamentarium, such as
the anti-CTLA-4 agent ipilimumab, or adoptive cell transfer, in
addition to the use of the cytokine interferon α, all of which
attempt to optimize the immunogenic response of the host to the
disease. CEACAM1 represents a novel area of research which may have
profound influence in future methods of harnessing cellular
immunity to combat this disease.
There are limitations to the present study. The
study cohort was small, and biased towards more aggressive lesions,
and thus may not be representative of the entire spectrum of
melanoma. It was undertaken to confirm our previous findings
concerning the importance of CEACAM1 expression, within the same
individual patient with disease progression. As CEACAM1 expression
and potential blockade may offer a new horizon in immunotherapy,
the results of the present study, combined with our previous
studies, confirm that CEACAM1 is potentially an extremely useful
target for arresting melanoma progression.
References
|
1
|
Sapoznik S, Hammer O, Ortenberg R, et al:
Novel anti-melanoma immunotherapies: Disarming tumor escape
mechanisms. Clin Dev Immunol. 2012:8182142012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hodi FS, O’Day SJ, McDermott DF, et al:
Improved survival with ipilimumab in patients with metastatic
melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robert C, Thomas L, Bondarenko I, et al:
Ipilimumab plus dacarbazine for previously untreated metastatic
melanoma. N Engl J Med. 364:2517–2526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamid O, Robert C, Daud A, et al: Safety
and tumor response with lambrolizumab (anti-PD-1) in melanoma. N
Engl J Med. 369:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brahmer JR, Tykodi SS, Chow LQ, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ortenberg R, Sapir Y, Raz L, et al: Novel
immunotherapy for malignant melanoma with a monoclonal antibody
that block CEACAM1 homophillic interactions. Mol Cancer Ther.
6:1300–1310. 2012. View Article : Google Scholar
|
|
7
|
Gray-Owen SD and Blumberg RS:
CEACAM1:contact-dependent control of immunity. Nat Rev Immunol.
6:433–446. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dango S, Sienel W, Schreiber M, et al:
Elevated expression of carcinoembryonic antigen-related cell
adhesion molecule 1 (CEACAM1) is associated with increased
angiogenic potential in non small cell lung cancer. Lung Cancer.
60:426–433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou CJ, Liu B, Zhu KX, et al: The
different expression of carcinoembryonic antigen-related cell
adhesion molecule 1 (CEACAM1) and possible roles in gastric
carcinoma. Pathol Res Pract. 205:483–489. 2009. View Article : Google Scholar
|
|
10
|
Ortenberg R, Galore-Haskel G, Greenberg I,
et al: CEACAM1 promotes melanoma cell growth through Sox-2.
Neoplasia. 16:451–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thies A, Moll I, Berger J, et al: CEACAM1
expression in cutaneous malignant melanoma predicts the development
of metastatic disease. J Clin Oncol. 20:2530–2536. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laack E, Nikbakht H, Peters A, et al:
Expression of CEACAM1 in adenocarcinoma of the lung: a factor of
independent prognostic significance. J Clin Oncol. 20:4279–4284.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sienel W, Dango S, Woelfle U, et al:
Elevated expression of carcinoembryonic antigen-related cell
adhesion molecule 1 promotes progression of non-small cell lung
cancer. Clin Cancer Res. 9:2260–2266. 2003.PubMed/NCBI
|
|
14
|
Muller MM, Klaile E, Vorontsova O, et al:
Homophillic adhesion and CEACAM1-S regulate dimerization of
CEACAM1-L and recruitment of SHP-2 and c-SRC. J Cell Biol.
187:569–581. 2009. View Article : Google Scholar
|
|
15
|
Chen Z, Chen L, Qiao SW, et al:
Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits
proximal TCR signaling by targeting ZAP-70. J Immunol.
180:6085–6093. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Markel G, Leiberman N, Katz G, et al:
CD66a interactions between human melanoma and NK cells: a novel
class I MHC-independent inhibitory mechanism of cytotoxicity. J
Immunol. 168:2803–2810. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Markel G, Wolf D, Hanna J, Gazit R, et al:
Pivotal role of CEACAM1 protein in the inhibition of activated
decidual lymphocyte functions. J Clin Invest. 110:943–953. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Markel G, Seidman R, Stern N, et al:
Inhibition of human tumor-infiltrating lymphocyte effector
functions by the homophillic carcinoembryonic cell adhesion
molecules 1 interactions. J Immunol. 177:6062–6071. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Markel G, Ortenberg R, Seidman R, et al:
Systemic dysregulation of CEACAM1 in melanoma patients. Cancer
Immunol Immmunother. 59:215–230. 2010. View Article : Google Scholar
|
|
20
|
Ebrahimnejad A, Streichert T, Nollau P, et
al: CEACAM1 enhances invasion and migration of melanocytic and
melanoma cells. Am J Pathol. 165:1781–1787. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gamblicher T, Grothe S, Rotterdam S, et
al: Protein expression of carcinoembryonic antigen cell adhesion
molecules in benign and malignant melanocytic skin lesions. Am J
Clin Pathol. 131:782–787. 2009. View Article : Google Scholar
|
|
22
|
Markel G, Seidman R, Cohen Y, et al:
Dynamic expression of protective CEACAM1 on melanoma cells during
specific immune attack. Immunology. 126:186–200. 2009. View Article : Google Scholar :
|
|
23
|
Eggermont AM, Chiarion-Seleni V, Grob JJ,
et al: Ipilimumab versus placebo after complete resection of stage
III melanoma: initial efficacy and safety results from the EORTC
18071 phase III trial. In: Presented at the 2014 Annual Meeting of
the American Society of Clinical Oncology; May 30–June 2, 2014;
Chicago, IL. J Clin Oncol. 32(15 Suppl): pp. LBA90082014
|