Introduction
Human pancreatic carcinoma (PC) is a highly
aggressive malignant cancer with a poor prognosis. Numerous
treatment protocols have been applied to PC, however, the 5-year
survival rate remains <5%, partly due to PC cells being
resistant to chemotherapy and radiation (1,2).
Vascular invasion and distant metastasis are the critical features
in the aggressive phenotype of PC, and contribute to the principal
causes of PC deaths. Thus, biomarkers associated with the invasion
and metastasis and survival of PC are required to predict patient
prognosis and to aid in the design of effective target therapy.
Rho GTPases, including Rac1, Cdc42 and RhoC, are
involved in the regulation of cell migration, cell motility, cell
cycle progression and cytoskeleton organization (3,4).
Aberrant signaling of these proteins is commonly observed in many
types of human cancer and is associated with aggressive phenotype.
The biological activities of GTPases are regulated by guanine
nucleotide exchange factors (GEFs), GTPase-activating proteins
(GAPs) and Rho GDP dissociation inhibitors (RhoGDIs) (5). Rho GDP dissociation inhibitor 2
(RhoGDI2), which belongs to a family of RhoGDIs, is verified to be
differentially expressed in human cancers (6,7).
Accumulating evidence has shown that RhoGDI2 acts as a positive or
negative regulator of cancer progression depending on the tumor
type (8). In a previous study, we
showed that RhoGDI2 promoted PC cell invasion and metastasis in
vitro (9). However, the
expression of RhoGDI2 and its correlation with poor prognosis in PC
patients as well as the pathway of RhoGDI2 in tumor metastasis
remain to be examined.
Epithelial to mesenchymal transition (EMT) is a
critical morphologic conversion during tumor progression and
results in the promotion of cell motility, invasion and metastasis
(10). Increasing evidence suggests
that EMT occurs in several types of cancer, including colorectal
cancer (11), gastric cancer
(12), and breast cancer (13). Loss of expression of the epithelial
cell adhesion molecule E-cadherin is a prerequisite of EMT.
In this study, we examined the expression of RhoGDI2
in human PC tissues, and compared it with the clinicopathological
characteristics and prognosis. Moreover, we investigated the
association between RhoGDI2 and E-cadherin, a critical factor of
EMT, and determined the possible pathway that RhoGDI2 may be
involved in the aggressive phenotype of PC.
Materials and methods
Clinical samples
Tissue samples were collected from 77 PC patients
during surgical resections performed at the First Affiliated
Hospital of Soochow University between January, 2008 and December,
2010. Tumorous tissues and adjacent non-tumorous tissues (NT) were
frozen immediately after surgical removal in liquid nitrogen and
stored at −80°C. The patients had not received any preoperative
chemo-, radio- or immunotherapy. Grades of differentiation and
clinical stage were classified according to the World Health
Organization. All the samples were obtained following patient
consent and approval by the Ethics Committee of Soochow
University.
Immunohistochemistry (IHC)
The samples were fixed with formalin, embedded in
paraffin and sliced. Serial sections (4 μm) subjected to
immunohistological staining were fixed with freshly prepared 3%
H2O2 with 0.1% sodium azide to quench
endogenous peroxidase and then treated with antigen retrieval
solution for 15 min. After placing in blocking reagent for 15 min,
the sections were incubated in primary anti-RhoGDI2 or
anti-E-cadherin monoclonal antibody overnight at 4°C, followed by
incubation with the secondary antibody and Extravidin-conjugated
horseradish peroxidase. The staining intensity was scored as: 0,
negative; 1, weak; 2, medium and 3, strong. The extent of staining
was scored as: 0, 0%; 1, 1–25%; 2, 26–50%; 3, 51–75% and 4 >76%.
The final score was obtained by the sum of the intensity score and
the quantity score. A score of ≥3 was considered as positive
expression, while a score of ≥6 was considered as strong-positive
expression.
RT-PCR
Total RNA from samples was extracted by TRIzol
(Invitrogen, Carlsbad, CA, USA). Total RNA (10 μg) was used to
synthesize single-stranded cDNA for a PCR template by reacting with
random primers and M-MLV reverse transcriptase (Promega, Madison,
WI, USA). The relative expression of RhoGDI2 mRNA transcripts to
that of the control (β-actin) was determined by RT-PCR. The primers
used were: RhoGDI2 (606 bp) forward, 5′-ATGACTGAAAAAGCC CCA-3′ and
reverse, 5′-TCATTCTGTCCACTCCTT-3′; β-actin (308 bp) forward,
5′-AGCGGGAAATCGTGCGTG-3′ and reverse, 5′-CAGGGTACATGGTGGTGCTGCC-3′.
The PCR amplification was 40 cycles (95°C for 15 sec, 62°C for 45
sec and 72°C for 30 sec). The amplified segments were analyzed by
2.5% agarose gels.
Western blotting
Tissues were lysed in lysis buffer on ice. Total
proteins were separated by 5–12% SDS-PAGE and transferred onto PVDF
membrane. The membrane as placed in a TBST solution with 5% non-fat
milk powder for 1 h at room temperature and incubated at 4°C
overnight with primary antibodies: anti-RhoGDI2 antibody (1:200) ),
anti-E-cadherin antibody (1:400; both from Abcam, Cambridge, UK)
and anti-β-actin antibody (1:200), followed by incubation at room
temperature for 1 h with a goat anti-mouse IgG (1:2,000, both from
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), conjugated
with horseradish peroxidase. Reactive bands were detected using ECL
western blotting detection reagent.
Statistical analysis
SPSS version 17.0 was used for statistical analysis.
Data were presented as mean ± SD. The t-test and Chi-square test
were performed for inter-group comparison. The correlation between
RhoGDI2 and E-cadherin expression was determined by the Pearson
correlation analysis. Survival was assessed according to the
Kaplan-Meier method and compared using the log-rank test.
Multivariate analysis of prognostic markers was performed with the
Cox proportional hazards regression model. P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression of RhoGDI2 in PC tissues
To investigate the expression pattern of RhoGDI2 in
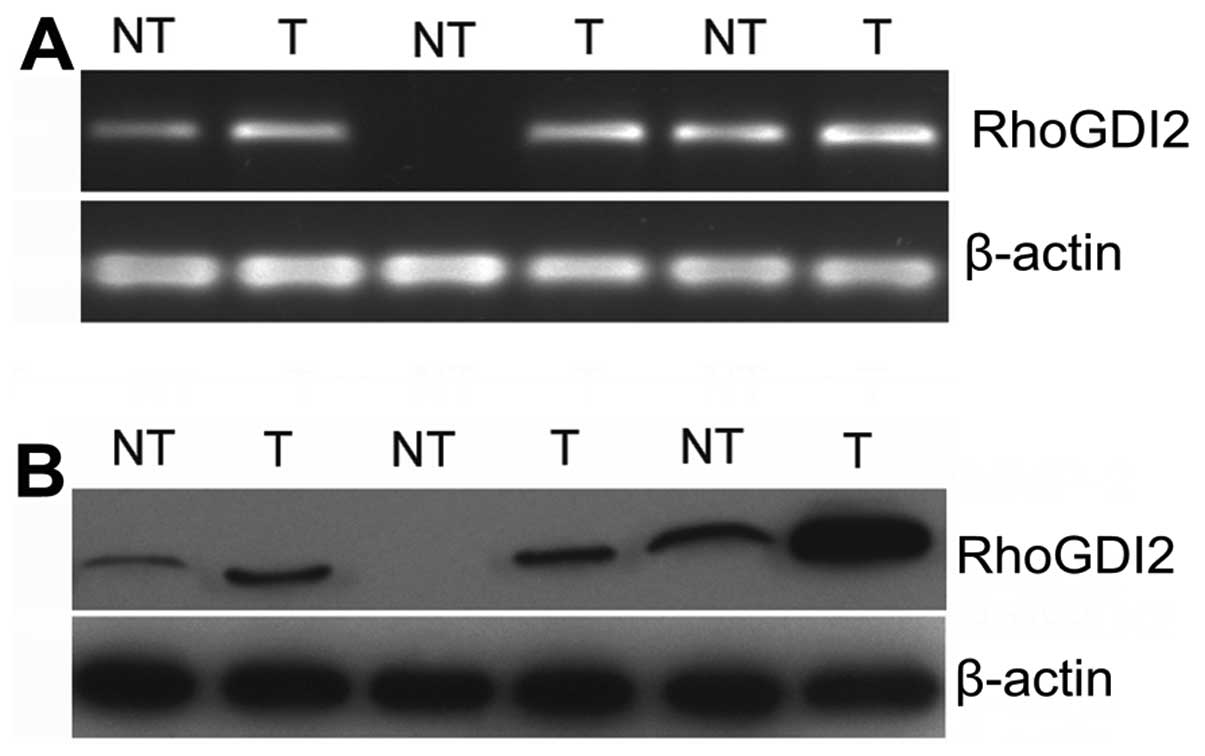
clinical fresh PC tissues, RT-PCR and western blotting were used in
20 paired PC tissues and adjacent non-tumorous tissues. The
expression of RhoGDI2 in PC tissues was higher than that in
non-tumorous tissues at the mRNA level (P<0.05) (Fig. 1). Similarly, western blotting
results revealed that the expression of RhoGDI2 protein was
upregulated in PC tissues compared with that in non-tumorous
tissues (Fig. 2, P<0.05). These
results indicated that RhoGDI2 was overexpressed in PC tissues.
Correlation between RhoGDI2 expression
and clinicopathological parameters
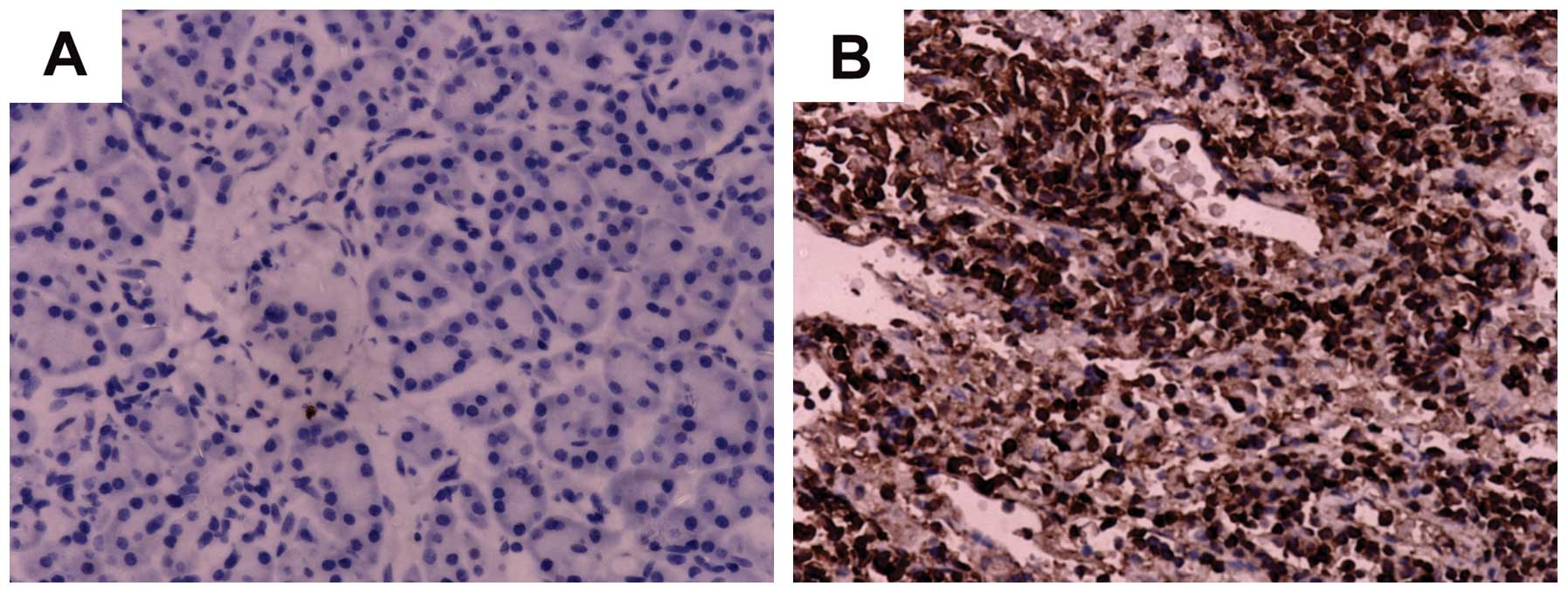
To elucidate the role of RhoGDI2 in the progression
of PC, we detected the expression of RhoGDI2 protein in PC tissues
by IHC staining. The subcellular location of RhoGDI2 protein was
observed mainly in the cytoplasm of cancer cells in PC tissues
(Fig. 2). Among 77 PC tissues, 49
cases (63.6%) exhibited a positive expression of RhoGDI2, including
31 strong-positive cases (40.3%) in tumor tissues. Among the
non-tumorous tissues, there were 66 RhoGDI2-negative expression
(85.7%) and 11 weak-positive expression (14.3%) cases, showing a
significant difference (χ2=39.428, P=0.001). The
association between RhoGDI2 expression and clinicopathological
parameters showed that RhoGDI2 expression was significantly
correlated with clinical stage (χ2=19.983, P=0.008) and
lymph-node metastasis (χ2=16.418, P=0.013), but did not
show a statistically significant association with gender, age,
tumor location, tumor size and differentiation (P>0.05, Table I) . These results indicated that the
overexpression of RhoGDI2 may be correlated with the progression of
PC.
 | Table IRelationship between RhoGDI2
expression and clinicopathological characteristics in PC. |
Table I
Relationship between RhoGDI2
expression and clinicopathological characteristics in PC.
| | RhoGDI2 | | |
|---|
| |
| | |
|---|
| Variables | Cases | Negative | Positive | χ2 | P-value |
|---|
| Gender | | | | 0.506 | 0.625 |
| Male | 48 | 16 | 32 | | |
| Female | 29 | 12 | 17 | | |
| Age (years) | | | | 0.430 | 0.632 |
| ≤65 | 45 | 15 | 30 | | |
| >65 | 32 | 13 | 19 | | |
| Tumor location | | | | 0.075 | 0.807 |
| Head | 51 | 18 | 33 | | |
| Body and tail | 26 | 10 | 16 | | |
| Tumor size (cm) | | | | 0.229 | 0.641 |
| ≤2 | 33 | 13 | 20 | | |
| >2 | 44 | 15 | 29 | | |
| Differentiation | | | | 0.003 | 0.999 |
| Well | 22 | 8 | 14 | | |
| Moderate | 25 | 9 | 16 | | |
| Poor | 30 | 11 | 19 | | |
| Clinical stage | | | | 19.983 | 0.008a |
| I | 23 | 17 | 6 | | |
| II | 54 | 11 | 43 | | |
| Lymph node
metastasis | | | | 16.418 | 0.013a |
| Yes | 40 | 6 | 34 | | |
| No | 37 | 22 | 15 | | |
Correlation between RhoGDI2 expression
and PC patient prognosis
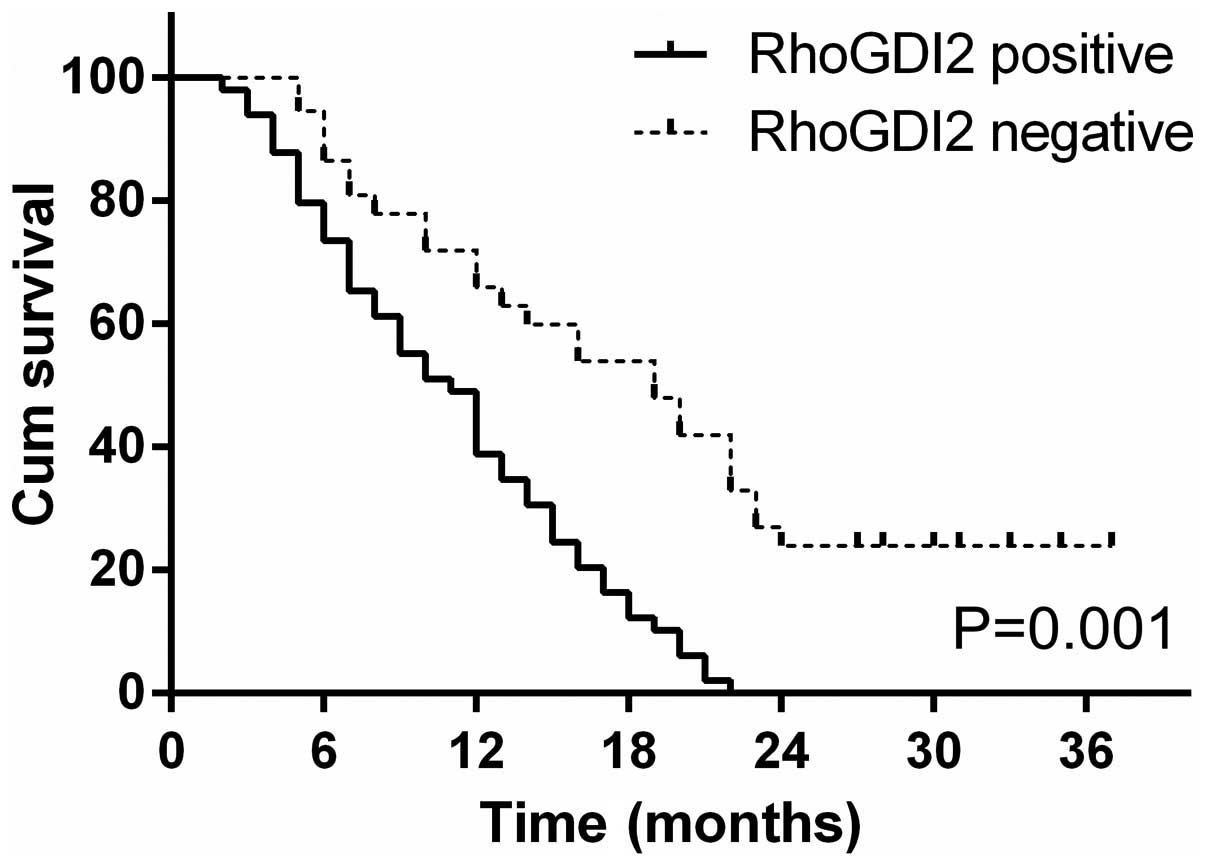
Among 77 PC patients, the follow-up success rate was
100%. After 3 years of follow up, only 8 of 77 (10.4%) patients
were alive and 69 patients (89.6%) were deceased. The median
survival time was 20 months (RhoGDI2-negative expression) and 11
months (RhoGDI2-positive expression), respectively. Kaplan-Meier
curve assessment showed that the patients with RhoGDI2-negative
expression had a significantly longer survival time than those with
a RhoGDI2-positive expression (log-rank test, P=0.001, Fig. 3).
The univariate analysis results revealed that
RhoGDI2 expression (P=0.003), clinical stage (P=0.007) and
lymph-node metastasis (P=0.006) were closely correlated with
patient survival time (Table II).
RhoGDI2 expression was closely associated with clinical stage and
lymph-node metastasis. Thus, we used RhoGDI2 expression as the
grouping variable, while clinical stage and lymph-node metastasis
were considered the subgrouping variables. Stratified analysis
showed that survival time of the RhoGDI2-positive expression group
was significantly shorter than that of the RhoGDI2-negative
expression group in the different subgroup levels (P<0.01,
Table III). The multivariate
analysis results revealed that RhoGDI2 expression is one of the
independent prognostic factors by Cox proportional hazards model
(P=0.008, Table IV). The results
indicated that the overexpression of RhoGDI2 was correlated with
poor prognosis.
 | Table IIUnivariate analysis of survival time
of PC patients (Kaplan-Meier). |
Table II
Univariate analysis of survival time
of PC patients (Kaplan-Meier).
| Variables | Cases | Average survival
period (months) | P-value |
|---|
| Gender | | | 0.887 |
| Male | 48 | 14.4±1.2 | |
| Female | 29 | 14.9±1.5 | |
| Age (years) | | | 0.495 |
| ≤65 | 45 | 14.2±1.1 | |
| >65 | 32 | 15.2±1.6 | |
| Tumor location | | | 0.379 |
| Head | 51 | 15.1±1.2 | |
| Body and tail | 26 | 13.6±1.4 | |
| Tumor size
(cm) | | | 0.131 |
| ≤2 | 33 | 13.0±1.3 | |
| >2 | 44 | 15.8±1.3 | |
|
Differentiation | | | 0.178 |
| Well | 22 | 12.5±1.5 | |
| Moderate | 25 | 14.2±1.5 | |
| Poor | 30 | 16.4±1.7 | |
| Clinical stage | | | 0.007a |
| I | 23 | 20.7±1.9 | |
| II | 54 | 12.0±0.8 | |
| Lymph node
metastasis | | | 0.006a |
| Yes | 40 | 11.1±1.0 | |
| No | 37 | 18.4±1.4 | |
| RhoGDI2
expression | | | 0.003a |
| Positive | 49 | 11.1±0.8 | |
| Negative | 28 | 20.7±1.6 | |
 | Table IIIStratified analysis of related
prognostic markers of PC patients. |
Table III
Stratified analysis of related
prognostic markers of PC patients.
| | Clinical stage | Lymph-node
metastasis |
|---|
| |
|
|
|---|
| Group | Cases | I | II | No | Yes |
|---|
| RhoGDI2
positive | 49 | 16.8±5.3 | 10.7±0.8 | 15.8±2.3 | 9.9±0.8 |
| RhoGDI2
negative | 28 | 22.1±1.8 | 17.3±2.3 | 20.2±1.7 | 17.7±3.4 |
| P-value | | 0.696 | 0.003a | 0.164 | 0.005a |
 | Table IVMultivariate analysis of prognostic
markers in PC patients. |
Table IV
Multivariate analysis of prognostic
markers in PC patients.
| Variables | HR | 95% CI | P-value |
|---|
| Gender | 0.966 | 0.591–1.581 | 0.892 |
| Age | 0.956 | 0.585–1.562 | 0.858 |
| Tumor location | 0.677 | 0.404–1.136 | 0.140 |
| Tumor size | 2.170 | 1.274–3.697 | 0.004 |
|
Differentiation | 1.174 | 0.680–2.028 | 0.565 |
| Clinical stage | 0.491 | 0.254–0.948 | 0.034a |
| Lymph-node
metastasis | 1.889 | 1.085–3.288 | 0.025a |
| RhoGDI2
expression | 3.344 | 1.718–6.505 | 0.008a |
RhoGDI2 expression correlated with
E-cadherin expression in PC tissues
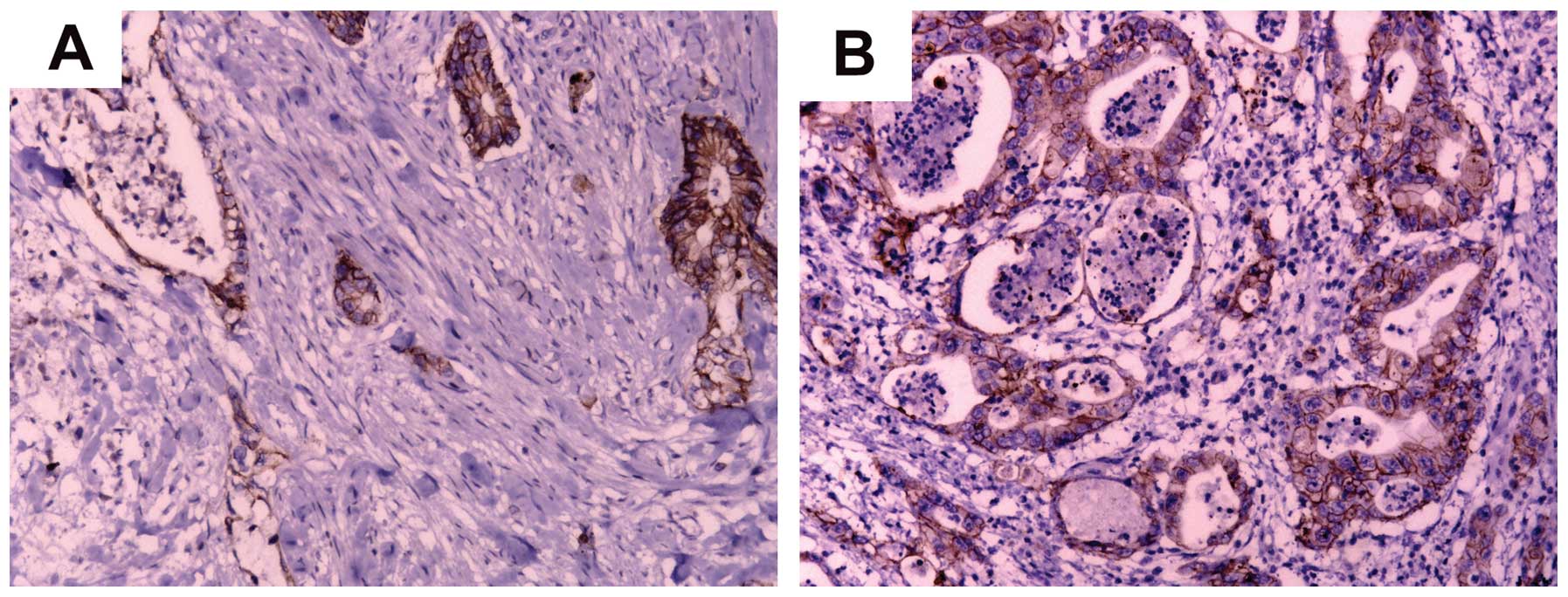
E-cadherin is involved in epithelial to mesenchymal
transition (EMT), which is involved in invasion and metastasis in
PC. To clarify the association between RhoGDI2 and E-cadherin, we
firstly examined the expression of E-cadherin protein in 77 PC
tissues by IHC (Fig 4). Data of the
statistical analysis suggested that the expression of RhoGDI2 was
negatively correlated with the expression of E-cadherin in PC
tissues (P=0.002, Table V).
 | Table VCorrelation between RhoGDI2 and
E-cadherin expression in PC. |
Table V
Correlation between RhoGDI2 and
E-cadherin expression in PC.
| E-cadherin
expression | RhoGDI2
expression | r | P-value |
|---|
|
|---|
| Positive (n) | Negative (n) |
|---|
| Positive (n) | 6 | 21 | | |
| Negative (n) | 43 | 7 | −0.633 | 0.002a |
Discussion
In this study, we examined the expression of RhoGDI2
in 30 matched clinical fresh tissues and 77 cases of
paraffin-embedded PC tissues. The results show that RhoGDI2 was
overexpressed in PC tissues at mRNA and protein levels, and that
RhoGDI2 expression was correlated with clinical stage, lymph-node
metastasis and vascular invasion. Additionally, RhoGDI2 was one of
the independent prognostic factors. We also found that the
expression of RhoGDI2 was negatively correlated with the expression
of E-cadherin in PC tissues. These findings suggest that the
upregulation of RhoGDI2 is involved in the progression and
prognosis of PC.
RhoGDI2, also known as D4-GDI or LyGDI, has been
identified as a regulator of Rho GTPases, which play important
roles in cell motility, invasion and metastasis (14, 15).
RhoGDI2 was preferentially expressed in hematopoietic tissues,
predominantly in B and T lymphocytes (16). However, accumulating evidence
reveals that RhoGDI2 is also aberrantly expressed in human cancers.
In the majority of studies, RhoGDI2 has been shown to promote tumor
cell invasion, angiogenesis and metastasis, such as in lung and
gastric cancer (17,18). However, it can function as a
metastasis-suppressor gene in bladder cancer and Hodgkin’s lymphoma
(19,20). Our results indicate that RhoGDI2 was
overexpressed in PC and associated with clinicopathological
characteristics of PC patients, including clinical stage and
lymph-node metastasis. The conflicting role of RhoGDI2 may result
from the dual roles of RhoGDI2 in the regulation of activities of
Rho GTPases during cancer progression. RhoGDI2 binds the majority
of Rho GTPases in the cytoplasm, maintaining Rho in an inactive
form and inducing the disruption of Rho-dependent cell motility
(21,22). On the other hand, RhoGDI2 acted as
an escort protein directing Rho GTPases to the membrane and is
associated with active forms of Rho, Rac and Cdc42, maintaining
them in an active form (23,24).
However, the exact mechanisms remain to be determined.
In this study, we have demonstrated that RhoGDI2
expression is one of the independent prognostic factors in PC, and
overexpression of RhoGDI2 was correlated with poor prognosis.
Stratified analysis of survival time showed that in lymph-node
positive patients, the prognosis of PC with RhoGDI2-positive
expression was worse than that of ones with RhoGDI2-negative
expression. Similar results were obtained in stage II of PC
patients with different RhoGDI2 expression. This finding indicated
that, for PC patients with lymph-node metastasis and clinical stage
II, we may draw up individualized gene therapy and evaluate
prognosis by detecting RhoGDI2 expression.
EMT is an essential cell mechanism during tumor
progression, which induces tumor cell migration, invasion and
metastasis (25). In all EMT
processes, cells lose the expression of a cell-to-cell adhesion
molecule known as E-cadherin, which functions as a molecular glue
that attaches cells to one another (26). To clarify the underlying mechanism
of RhoGDI2 in the progression of tumor invasion and metastasis, we
also investigated the relationship between and in PC tissues. Our
results (data not shown) indicated E-cadherin was down-regulated in
PC tissues and was negatively correlated with the expression of
RhoGDI2. In our previous study, RhoGDI2 was known to promote PC
cell invasion and migration in vitro, but to the best of our
knowledge, this is the first study showing that RhoGDI2 expression
was correlated with E-cadherin expression in PC tissues.
In conclusion, our study has demonstrated that the
overexpression of RhoGDI2 was associated with PC progression and
played an important role in predicting the prognosis of PC
patients. Moreover, upregulation of RhoGDI2 was associated with
reversal of E-cadherin expression in PC tissues. These findings
indicate that targeting RhoGDI2 may be a useful strategy for
inhibiting the invasion and metastasis of PC.
Acknowledgements
This study was supported by the Project of Nature
Science Foundation of China (81201905), Nature Science Research
Grants at the University of Jiangsu Province of P.R. China
(14KJB320019) as well as the Project of Medical Research of Jiangsu
Province (Q201402).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vega FM and Ridley AJ: Rho GTPases in
cancer cell biology. FEBS Lett. 582:2093–2101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reymond N, Riou P and Ridley AJ: Rho
GTPases and cancer cell transendothelial migration. Methods Mol
Biol. 827:123–142. 2012. View Article : Google Scholar
|
|
5
|
Garcia-Mata R, Boulter E and Burridge K:
The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat Rev
Mol Cell Biol. 12:493–504. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho HJ, Baek KE and Yoo J: RhoGDI2 as a
therapeutic target in cancer. Expert Opin Ther Targets. 14:67–75.
2010. View Article : Google Scholar
|
|
7
|
Agarwal NK, Chen CH, Cho H, Boulbes DR,
Spooner E and Sarbassov DD: Rictor regulates cell migration by
suppressing RhoGDI2. Oncogene. 32:2521–2526. 2013. View Article : Google Scholar
|
|
8
|
Griner EM and Theodorescu D: The faces and
friends of RhoGDI2. Cancer Metastasis Rev. 31:519–528. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yi B, Hu Y, Qin G, et al: Depletion of
RhoGDI2 expression inhibits the ability of invasion and migration
in pancreatic carcinoma. Int J Mol Med. 34:205–212. 2014.PubMed/NCBI
|
|
10
|
Rhim AD, Mirek ET, Aiello NM, et al: EMT
and dissemination precede pancreatic tumor formation. Cell.
148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kevans D, Wang LM, Sheahan K, et al:
Epithelial-mesenchymal transition (EMT) protein expression in a
cohort of stage II colorectal cancer patients with characterized
tumor budding and mismatch repair protein status. Int J Surg
Pathol. 19:751–760. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuoka J, Yashiro M, Doi Y, et al:
Hypoxia stimulates the EMT of gastric cancer cells through
autocrine TGFβ signaling. PLoS One. 8:e623102013. View Article : Google Scholar
|
|
13
|
Burgess DJ: Breast cancer: Circulating and
dynamic EMT. Nat Rev Cancer. 13:1482013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nitz MD, Harding MA and Theodorescu D:
Invasion and metastasis models for studying RhoGDI2 in bladder
cancer. Methods Enzymol. 439:219–233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Wang J, Zhang X, Zeng Y, Liang L and
Ding Y: Overexpression of RhoGDI2 correlates with tumor progression
and poor prognosis in colorectal carcinoma. Ann Surg Oncol.
19:145–153. 2012. View Article : Google Scholar
|
|
16
|
Scherle P, Behrens T and Staudt LM:
Ly-GDI, a GDP-dissociation inhibitor of the RhoA GTP-binding
protein, is expressed preferentially in lymphocytes. Proc Natl Acad
Sci USA. 90:7568–7572. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niu H, Li H, Xu C and He P: Expression
profile of RhoGDI2 in lung cancers and role of RhoGDI2 in lung
cancer metastasis. Oncol Rep. 24:465–471. 2010.PubMed/NCBI
|
|
18
|
Cho HJ, Baek KE, Kim IK, et al:
Proteomics-based strategy to delineate the molecular mechanisms of
RhoGDI2-induced metastasis and drug resistance in gastric cancer. J
Proteome Res. 11:2355–2364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Theodorescu D, Sapinoso LM, Conaway MR,
Oxford G, Hampton GM and Frierson HF Jr: Reduced expression of
metastasis suppressor RhoGDI2 is associated with decreased survival
for patients with bladder cancer. Clin Cancer Res. 10:3800–3806.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma L, Xu G, Sotnikova A, et al: Loss of
expression of LyGDI (ARHGDIB), a rho GDP-dissociation inhibitor, in
Hodgkin lymphoma. Br J Haematol. 139:217–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dovas A and Couchman JR: RhoGDI: multiple
functions in the regulation of Rho family GTPase activities.
Biochem J. 390:1–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
DerMardirossian C and Bokoch GM: GDIs:
central regulatory molecules in Rho GTPase activation. Trends Cell
Biol. 15:356–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hart MJ, Maru Y, Leonard D, Witte ON,
Evans T and Cerione RA: A GDP dissociation inhibitor that serves as
a GTPase inhibitor for the Ras-like protein CDC42Hs. Science.
258:812–815. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chuang TH, Xu X, Knaus UG, Hart MJ and
Bokoch GM: GDP dissociation inhibitor prevents intrinsic and GTPase
activating protein-stimulated GTP hydrolysis by the Rac GTP-binding
protein. J Biol Chem. 268:775–778. 1993.PubMed/NCBI
|
|
25
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Theys J, Jutten B, Habets R, et al:
E-Cadherin loss associated with EMT promotes radioresistance in
human tumor cells. Radiother Oncol. 99:392–397. 2011. View Article : Google Scholar : PubMed/NCBI
|