Introduction
Since the first embryonic stem cell (ESC) line
derived from human blastocysts was described by Thomson in 1998
(1), ESCs have received much
attention for their great potential in medicine. However, ethical
issues related to ESCs are a major obstacle to their application in
clinical practice (2), and
therefore, adult stem cells were suggested as an alternative
(3). Mesenchymal stem cells (MSCs)
are one such candidates, and have been widely investigated due to
their potential application in the treatment of chronic wounds,
metabolic bone disease, fractures and myocardial infarction
(4). Furthermore, the diverse
effects of these cells on cancer highlight their possible
application in cancer treatment (5–10).
However, there have been many contradictory reports concerning the
effects of MSCs in tumorigenesis, including the promotion (11–13)
and inhibition (14) of tumor
growth.
To date, most cancer-related studies have been
performed using bone marrow-derived mesenchymal stem cells (BMMSCs)
(6–8,15,16),
while studies using adipose tissue-derived ADMSCs are rare. This is
despite the many advantages of using ADMSCs, which include easier
harvest, higher yield and comparable differentiation potential
compared to BMMSCs (17). In
addition, most studies concerning the tumorigenesis of ADMSCs have
been performed after the intravenous injection of cells, i.e.,
systemic administration (7), and
have involved in vitro experiments (18). To the best of our knowledge, there
is no in vivo study dealing with the effect and behavior of
locally administered ADMSCs in early tumor formation.
Recent advances in bioluminescence imaging (BLI)
techniques have enabled us to assess the status of cells
administered to living animals without sacrifice during the
follow-up period. Furthermore, high resolution ultrasound (US) has
made it possible to precisely measure small objects by enabling the
volumetric calculation of the tumor mass in various shapes and
locations. In the present study, we aimed to evaluate the
proliferation and survival of local treatment with ADMSCs in early
tumor formation by monitoring the reporter ADMSCs using BLI. We
also evaluated the effect of these cells on tumor growth by the
comparison of serial BLI data and the tumor volume measured by
small animal-dedicated high resolution US.
Materials and methods
Tumor cells
UMR-106 osteosarcoma cells (UMRs), which are a
clonal derivative of a transplantable rat osteosarcoma induced by
the injection of radiophosphorus, were purchased from the American
Type Culture Collection (ATCC; Manassas, VA, USA). The cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing
10% fetal bovine serum (FBS) and 100 IU/ml penicillin, 100 IU/ml
streptomycin and 0.25 μg/ml amphotericin at 37°C in an atmosphere
of 5% CO2, and were passaged by standard methods of
trypsinization. Only cells at passages 2 and 3 were used in the
experiments.
Mesenchymal stem cell harvest and
culture
All experiments were performed following animal
protocols approved by the Institutional Animal Care and Use
Committee at Stanford University. ADMSCs with permanent expression
of a reporter gene signal during follow-up BLI were extracted from
the adipose tissues of transgenic mice expressing the β-actin
promoter and double reporter genes of GFP and firefly luciferase.
This was performed in order to minimize errors in BLI data caused
by the viral transfection of reporter genes into ADMSCs. The
transgenic mice used for the harvesting of ADMSCs were kindly
supplied by Dr Contag’s laboratory at Stanford University.
Subcutaneous fat tissues were collected from the lower anterior
abdominal wall to the inguinal area of the transgenic mice. The
tissue fragments were rinsed 3 times in phosphate-buffered saline
(PBS) before being finely minced for 5 min. These tissues were then
digested using 0.075% collagenase (Sigma-Aldrich, St. Louis, MO,
USA) at 37°C for 1 h. Neutralized cells were centrifuged and the
mature adipocytes and fibrovascular fraction were selected and
removed. Pelleted stromal cells were passed through a 100-μm cell
strainer before plating. Cells were cultured at 37°C in an
atmosphere of 5% CO2, in DMEM containing 10% FBS, 100
IU/ml penicillin, 100 IU/ml streptomycin and 0.25 μg/ml
amphotericin. Only the cells that adhered to plastic were used in
this experiment, and cells floating during culture were discarded.
Preliminary in vitro BLI of cultured ADMSCs was performed to
validate the performance of luciferase gene expression before
implantation in animals.
Local administration of ADMSCs in an
osteosarcoma xenograft model
Twenty nude mice (NU/NU) aged 8–10 weeks were
purchased from Charles River Laboratories (Wilmington, MA, USA) and
categorized into 4 groups. Each group was injected with ADMSC-UMR
mixtures containing 5, 10, 15, or 25% ADMSCs, defined as G1–G4,
respectively. This experiment was performed twice separately due to
limitations in stem cell harvest and culture, the preparation of
UMRs and BLI and US imaging capacity.
Different numbers of ADMSCs and UMRs were mixed with
30 μl of solubilized basement membrane preparation without growth
factor (Matrigel; BD Biosciences, San Jose, CA, USA) and
subcutaneously injected into the right flank of the mice. The tumor
xenograft was performed in 4 groups at 2 different time points. The
first 2 groups, G1 and G3, were injected with mixtures of
5×104 and 1.5×105 ADMSCs in 1×106
UMRs. The second two groups, G2 and G4, were injected with
2×105 and 5×105 ADMSCs in 2×106
UMRs. As a control, 1×106 and 2×106 UMRs in
basement membrane preparation were xenografted at the contralateral
area of mice in the first (G1 and G3) and second groups (G2 and
G4), respectively. The numbers of ADMSCs and UMRs and proportions
of ADMSCs in each group are summarized in Table I. These procedures were carried out
under general anesthesia with isoflurane inhalation.
 | Table INumber of UMR-106 cells and
ADMSCs. |
Table I
Number of UMR-106 cells and
ADMSCs.
| Group | UMR-106 cells | ADMSCs | Proportion (%) |
|---|
| G1 | 1,000,000 | 50,000 | 5 |
| G2 | 2,000,000 | 200,000 | 10 |
| G3 | 1,000,000 | 150,000 | 15 |
| G4 | 2,000,000 | 500,000 | 25 |
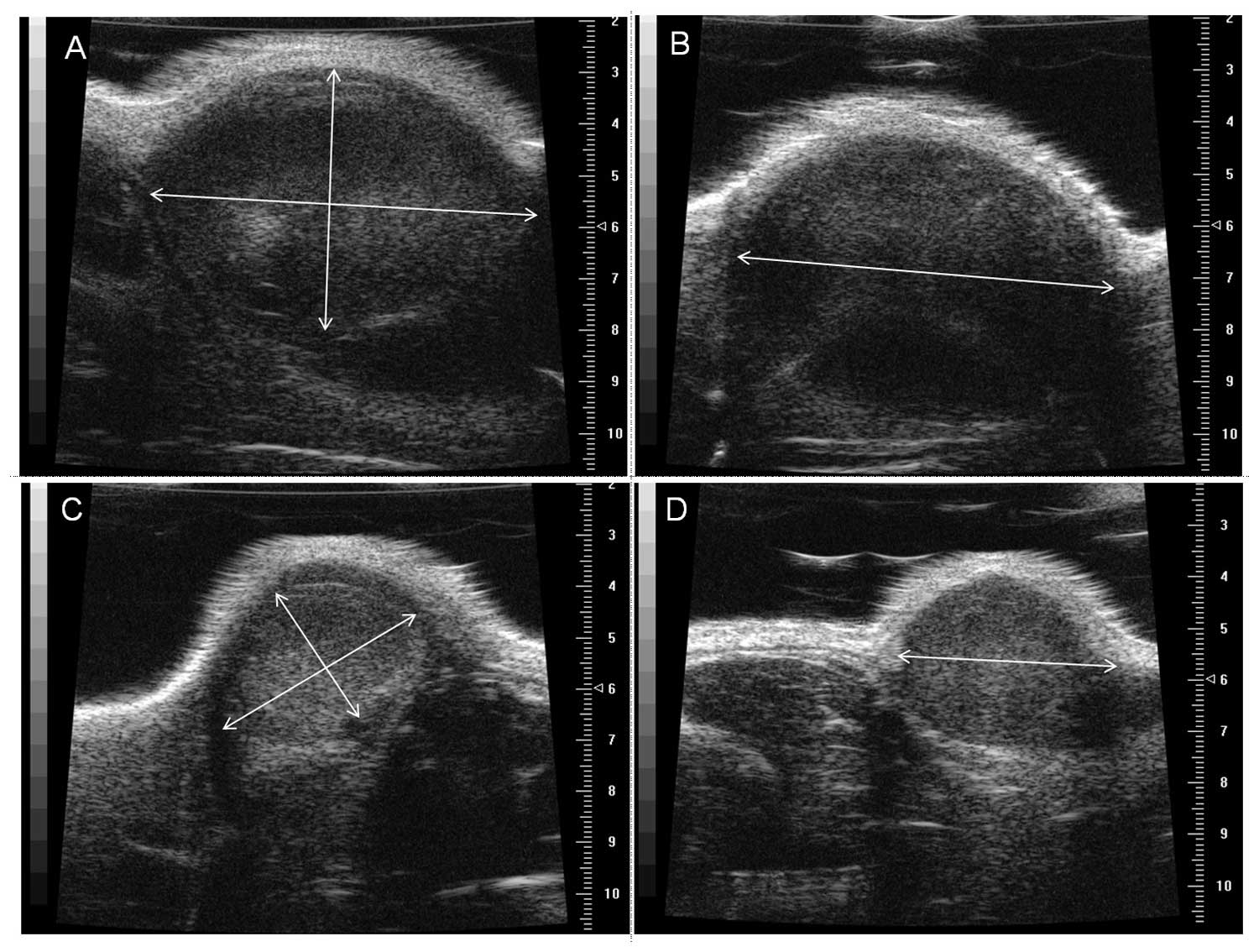
Tumor volume obtained by small
animal-dedicated high resolution ultrasound
A specially designed high-resolution micro-US system
for small animal with a 40 MHz transducer (Vevo 770; Visual Sonics,
Toronto, Ontario, Canada) was used to evaluate the characteristics
of the growing tumor implants with and without ADMSCs. The spatial
resolution and penetration depth of the 40 MHz transducer used in
this study was 30 μm and 6 mm, respectively. US imaging was
performed at 2- or 3-day intervals before sacrificing the animals
16 days after the xenograft. In order to obtain the exact tumor
volume taking into account various contours, we assumed each tumor
mass was an elliptical ball and obtained two tangential US images
along the longitudinal and transverse planes to calculate the exact
volume. The equation for the calculation of this mass is as
follows: Volume = 4/3πABC, where A, B and C are the measured radii
of each elliptical ball, and the unit of the calculated volume was
mm3 (Fig. 1).
The changes in the tumor volumes of the
ADMSC-treated and control sides were monitored and compared in each
group. The ratio of tumor volume (volume of ADMSC-treated site/
volume of control side) was also measured for each mouse, and these
results were compared between the groups to reduce the bias from
individual variance. The tumor-suppressive or tumor-promoting
effect of ADMSCs was determined by the comparison of the ratios of
tumor volume on the final day as well as the mean volumes.
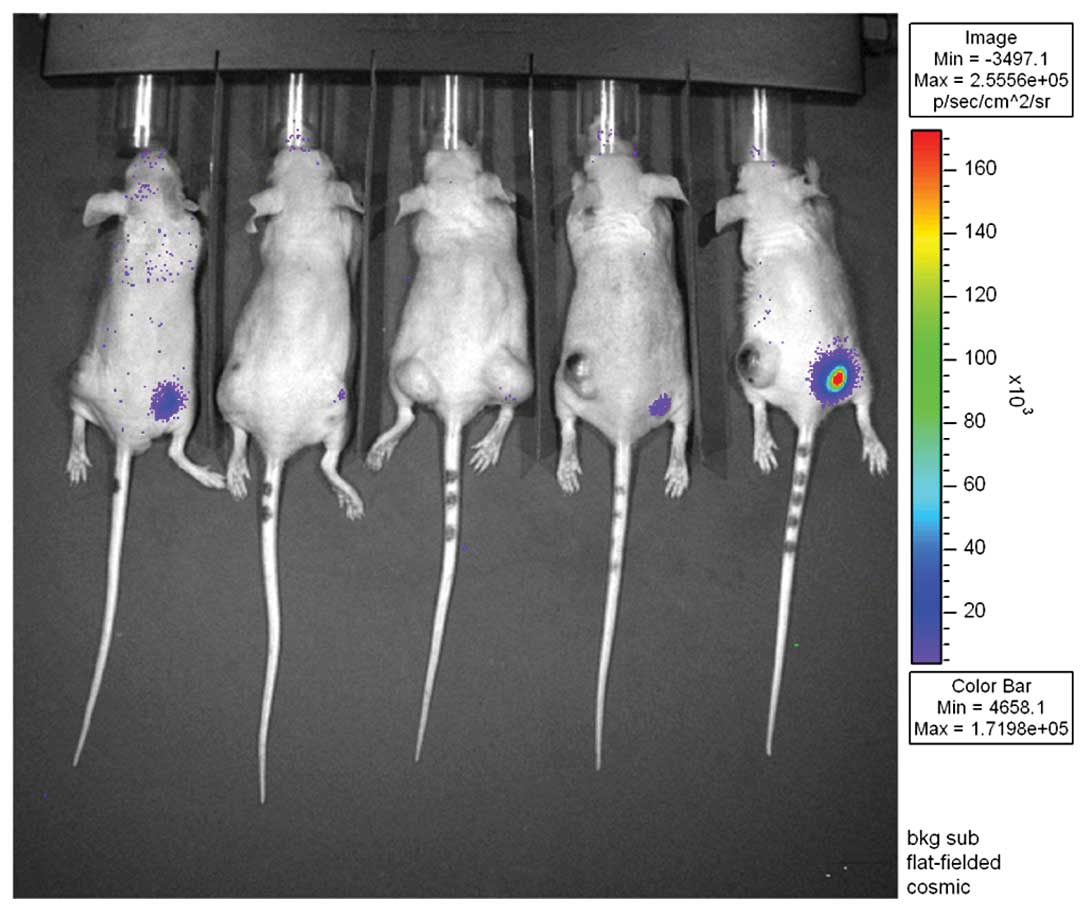
In vivo BLI
An ~30 mg/ml solution of luciferin was made by
dissolving 1 g D-luciferin firefly potassium salt (Biosynth
International, IL, USA) in 33.3 ml of PBS. Approximately 4.5 mg/150
μl of luciferin was injected into the intraperitoneal space of each
mouse. BLI was obtained 10 min after the administration of
luciferin using a cooled charge-coupled device camera (IVIS;
Xenogen). Mice were imaged at high resolution for 5 min, and five
mice were simultaneously imaged in the prone position. BLIs were
performed for 16 days with 2-day intervals for G1 and G3, and 3-day
intervals for G2 and G4. The acquired BLI data were analyzed by a
specialized program (Living Image Software; Xenogen USA). A round
region of interest with a 3.2-cm diameter was located in the
ADMSC-inoculated right flank to measure the luciferase activity
from the ADMSCs. The measured bioluminescence activity was
expressed as average radiance (photon/cm2/sec/
steradian).
Immunohistochemistry
The rabbit anti-firefly luciferase antibody was
obtained from Abcam (cat. no. ab21176, Cambridge, UK). Xenografts
were embedded in optimum cutting temperature compound (Sakura
Finetek, Torrance, CA, USA) using liquid nitrogen. Sections of
10-μm thickness were fixed in 2% paraformaldehyde for 10 min, and
then washed using PBS. Prior to incubation with the antibodies, the
sections were blocked using PBS containing 0.1% Triton X-100 and 5%
normal goat serum. The primary antibody was diluted (1:50) in
blocking solution and applied overnight at 4°C. Alexa
488-conjugated goat anti-rabbit IgG secondary antibody (Invitrogen,
Carlsbad, CA, USA) was then added at a 1:100 dilution for 30 min at
room temperature. Stained sections were mounted using antifade
reagent containing 4′,6-diamidino-2-phenylindole dihydrochloride
(DAPI; Vecta Shield, Burlingame, CA, USA) to visualize cell nuclei.
Fluorescent microscopic images were captured using the Nikon
Eclipse E800 (Nikon Imaging).
Statistical analysis
The mean volumes of the xenografted tumors on the
ADMSC-treated UMR side (right) and the UMR-only control side were
compared using the Wilcoxon matched-pairs signed ranks test to
determine the differences in tumor volumes in the same subjects.
Then the volume ratio of the ADMSC-treated UMR mass to the control
tumor was calculated in each mouse from the same group, and the
means of these ratios were compared across groups using the
Mann-Whitney U test.
The Pearson correlation coefficient was used to
evaluate the correlation between BLI activity and tumor volume in
G2 and G3. P<0.05 was taken to indicate a statistically
significant difference. All statistical evaluations were performed
using SAS statistical software (ver. 9.2; SAS Institute Inc., Cary,
NC, USA).
Results
Comparison of the changes in tumor
volumes and proportions of ADMSCs
The initial volume of injection on both sides of
each mouse was ~30 mm3 in all 20 mice in the 4 groups.
On post-operation date (POD)2, the volume of xenografts was
slightly decreased compared with the initial measurement time due
to the absorption of water from the basement membrane preparation
used. Thereafter, the volume of the tumors continuously increased
on both the ADMSC-treated and control sides, but the increment
pattern was different among all 4 groups. In the group containing
the lowest proportion of ADMSCs, G1 (5%), the ADMSC-treated
xenograft was smaller than the contralateral tumor-only side in 2/5
mice. The remaining 3 mice had a larger tumor volume on the
ADMSC-treated side. The injection of mice with higher proportions
of ADMSCs (G2 and G3, 10 and 15%, respectively) led to the
development of smaller tumors in 4/5 mice (Fig. 2). However, all mice in the group
with the highest proportion of ADMSCs, group G4 (25%), showed
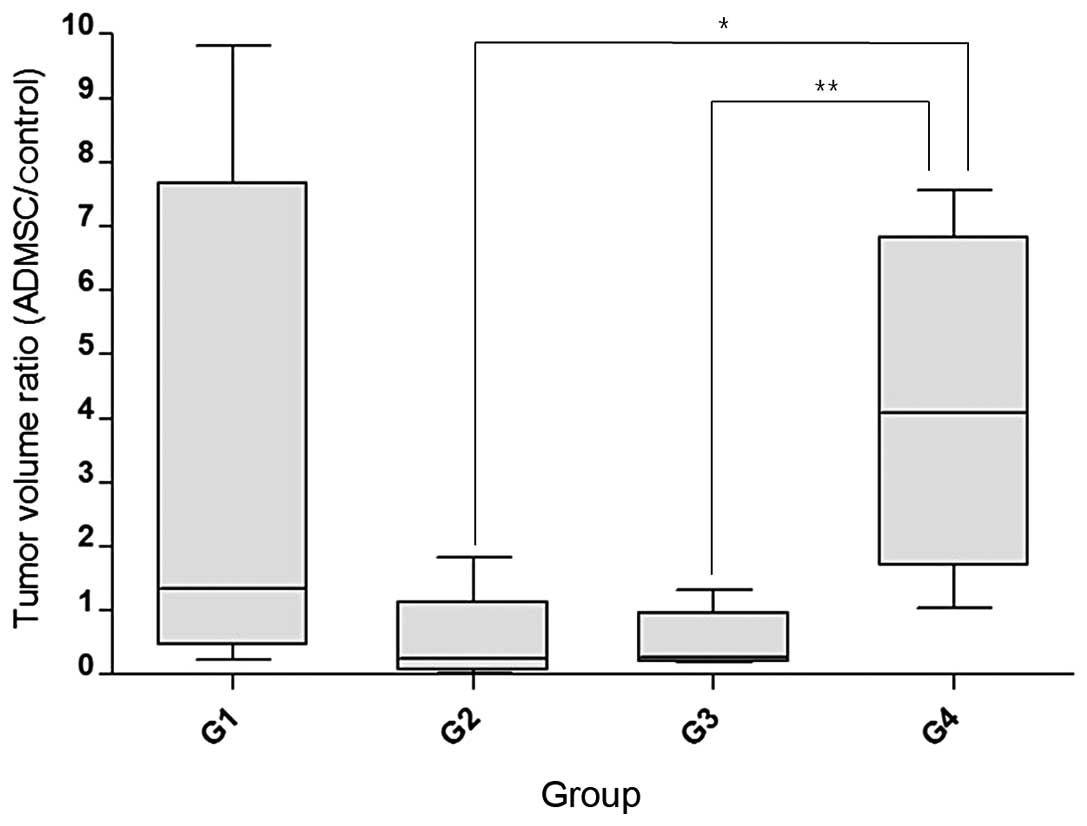
larger tumor volumes. These results are summarized in Table II. The Wilcoxon matched-pairs
signed-ranks test revealed no statistically significant difference
in the mean volume of xenografted tumors on the right and left
sides from all 4 groups because of the wide range of standard
deviations. Thus, the tumor volume ratio of the AMDSC-treated side
to the control side was introduced to reduce the bias from
individual variance and this value showed significant differences
between G2 and G4, and G3 and G4, by the Mann-Whitney U test
(Fig. 3). However, the Pearson
correlation coefficient between the last tumor volume and initial
BLI activity in G2 and G3 was 0.453, which showed no significant
correlation between these two factors (P=0.1915).
 | Table IIAverage volume of the xenografted
tumors in each group. |
Table II
Average volume of the xenografted
tumors in each group.
| | POD2 | POD5 | POD8 | POD11 | POD13 | POD16 |
|---|
| G1 | ADMSCs | 7.19 | 12.44 | 13.37 | 39.72 | 53.80 | 72.91 |
| Control | 7.93 | 4.84 | 6.01 | 10.35 | 19.14 | 38.06 |
| G2 | ADMSCs | 20.04 | 18.31 | 35.08 | 69.59 | 128.32 | 144.61 |
| Control | 30.06 | 58.14 | 110.55 | 170.42 | 234.18 | 308.23 |
| G3 | ADMSCs | 6.81 | 9.91 | 12.35 | 17.24 | 74.96 | 93.39 |
| Control | 7.60 | 6.12 | 7.23 | 16.35 | 75.86 | 127.56 |
| G4 | ADMSCs | 26.23 | 27.71 | 56.72 | 78.67 | 133.58 | 693.43 |
| Control | 27.54 | 14.66 | 19.18 | 46.43 | 65.03 | 259.84 |
Proliferation and survival of ADMSCs in
the xenografts
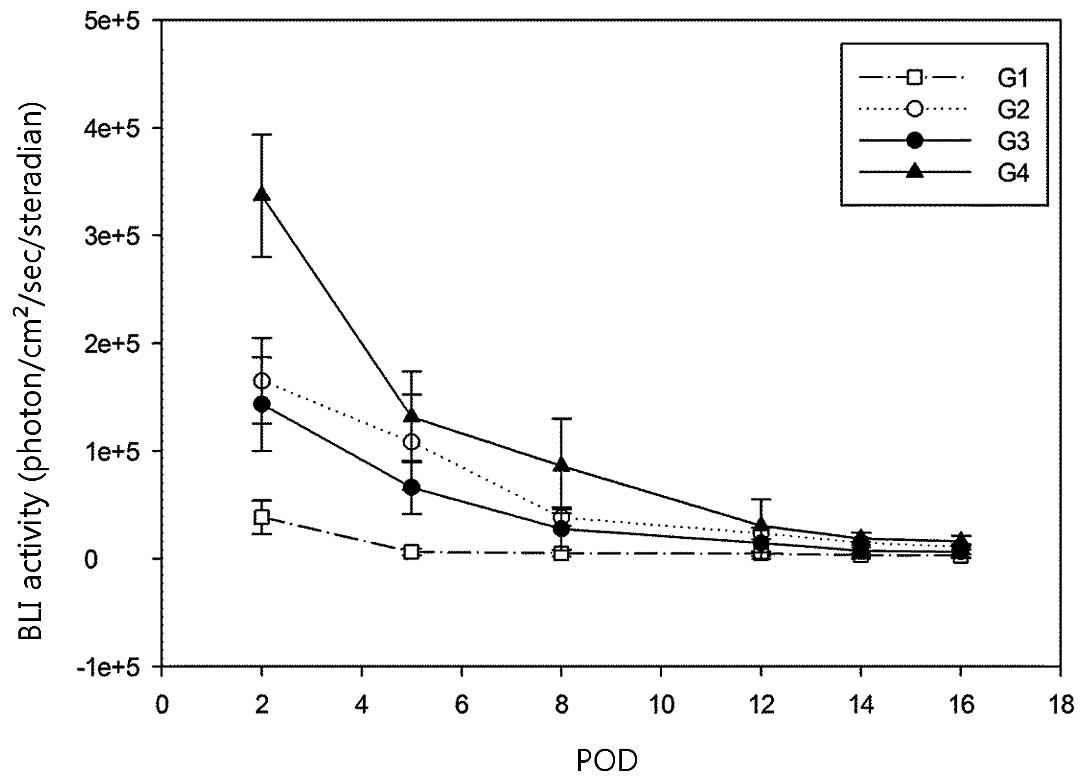
Initial BLI revealed strong luciferase activity from
the ADMSCs in all xenografts, but these activities rapidly
decreased at an early stage, and only minimal residual luciferase
activities were detected during the later stages. There was no
definite increment in BLI to suggest significant proliferation of
ADMSCs during the follow-up period and no BLI activity was detected
in contralateral tumors to suggest distant migration of the stem
cells (Fig. 4)
Histological analysis
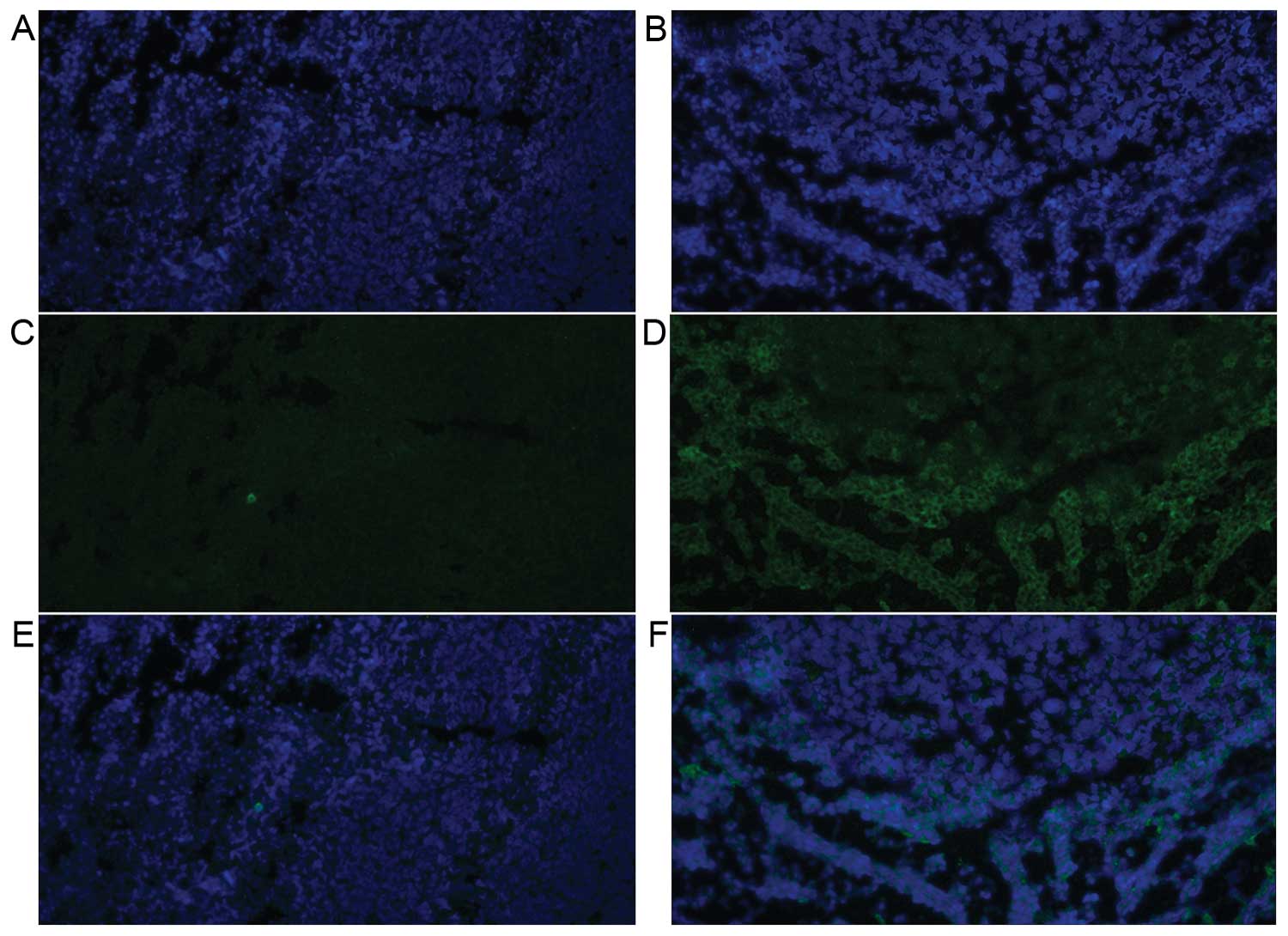
The DAPI staining of both the control side and
ADMSC-treated side revealed well stained nuclei (Fig. 5A and B) but immunohistochemical
staining for luciferase in the control side showed no positive
finding to suggest any migration of AMDSCs from the primary
administration site (Fig. 5C).
Fluorescent microscopic images of the ADMSC-treated side displayed
a few bright cells stained by the luciferase antibody at the
periphery of the tumor mass (Fig.
5D). However, these cells were not detected at the center of
the mass, and there was no visible distribution of stained cells
along the vascular structures or interstitial tissues, which might
have suggested the contribution of ADMSCs to these components.
Discussion
Stem cells are a special type of cells characterized
by self-renewal and pluripotency. They can be classified into
embryonic stem cells (ESCs) and adult stem cells by their origin.
ESCs are ideal, considering their differentiation ability and
capacity for self-renewal, but practical and ethical issues prevent
their widespread and active clinical application (3). Therefore, adult stem cells are
emerging as an alternative to ESCs due to their accessibility and
wide applicability in clinical and research fields (19). The known sources of adult stem cells
are bone marrow (10,14), fat tissue (20), peritoneum and synovium (21).
Among the adult stem cells, MSCs originating from
bone marrow were initially cultured and investigated (22). The subsequent identification of
adipose tissues as a source of MSCs has gained much attention, as
it presents an alternative source from which these cells could be
harvested more easily and in greater numbers (20). However, the characteristics of
ADMSCs and BMMSCs have not been fully defined, and data describing
the effects of these cells on tumor growth have been contradictory
(23). Several studies have
described the contribution of BMMSCs to tumor neovasculature
(14) or tumor cell growth in
vivo (11,24). Most studies on the inhibitory effect
of MSCs on tumorigenesis were also performed using BMMSCs (7–9,25,26).
Only one study addressed this issue using ADMSCs, and its analyses
were performed in vitro (18). This study was the starting point for
the present study. Although there have been several reports
concerning the trafficking of stem cells (27) and therapeutic delivery vehicles
targeting tumor stroma (5), the
proportion of stem cells migrating to target organs is very small
when using intravenous administration (28). Therefore, it has been suggested that
the clinical application of MSCs should be handled with extreme
caution, especially in malignant disease (23).
In the present study, we aimed to evaluate the
effect of the local treatment of ADMSCs in a xenografted animal
model using BLI and micro-US. In the first trial, two proportions
of ADMSCs were used at G1 (5%) and G3 (15%), and despite the same
number of UMRs being inoculated into the control side (left), the
final volume of tumors without ADMSCs was significantly different
between G1 (38.06 mm3) and G3 (127.56 mm3).
The effect of ADMSCs on tumorigenesis in G1 must be underestimated
due to the abnormally small tumor volume on the control side. The
comparison of the tumor volumes on the ADMSC-treated and control
sides of the mice revealed tumor suppression in 4/5 mice in G3, but
only 2/5 mice in G1. This was despite the average volume of the
ADMSC-treated side in G1 (72.9 mm3) and G2 (93.39
mm3) showing no statistically significant difference.
This result might be attributed to the poor condition of the frozen
UMR stock used in this experiment. In the second trial, markedly
different results were noted between mice in G2 and G4, which were
injected with UMRs containing 10 and 25% ADMSCs, respectively. G2
showed results similar to those of G3, with 4/5 mice exhibiting a
smaller tumor volume on the ADMSC-treated side. However, no mice in
G4 showed a smaller volume on the treated side than the control
side, and the ADMSC-treated side showed marked acceleration of
tumor growth as summarized in Table
II. Similar results were also observed in the cases where tumor
suppression failed in G2 and G3, although G1 data could not be used
to assess this finding due to the unreliable volume data from the
control side. The differences in the tumor volume ratios between G2
vs. G4 and G3 vs. G4 for the estimation of the tumor-suppressive
effect were confirmed by Mann-Whitney U test (P=0.0159).
Luciferase activity measured by BLI revealed a
continuously decreasing pattern, suggesting a lack of proliferation
of ADMSCs in the tumor masses, although the decrement rate was
different among the groups. This was also confirmed by
immunohistochemical staining for luciferase in the tumor specimens
after sacrificing the animals. Immunohistochemical analysis of
ADMSCs using an anti-luciferase antibody revealed only a small
number of fluorescence signals in the periphery of the tumor mass.
No significant activity was detected along the vascular structure
or interstitium of the tumor. According to the results of BLI and
immunohistochemistry, we suggest that most of the locally
administered ADMSCs perished within the tumor mass without evidence
of proliferation or contribution to the source of the stromal
structures of the tumors.
According to our results, locally administered
AMDSCs appear to act as a tumor suppressor to a certain degree at
low proportions, whereas higher proportions of ADMSCs act as a
tumor stimulator. Considering BLI and the immunohistochemistry
findings, these effects of ADMSCs appear to be more likely via
early humoral effects such as the production of various cytokines
and excretions as previously described (9,29,30),
rather than by direct effects from the continuous proliferation of
the stem cells themselves. An additional noteworthy finding is that
for two tumors in G2 and G3 where there was a failing
ADMSC-mediated suppressive effect, there was very rapid
proliferation and this finding may suggest that the
tumor-suppressive effect might be determined at an early stage.
Our study has several limitations. First, the small
size of the studied population is a major limitation, which
resulted from limitations in the numbers of animals that can be
imaged at one time point in the BLI facility, and the numbers of
ADMSCs obtained from a single batch. Therefore, a statistically
significant result could not be obtained when comparing the mean
volumes of tumors with and without ADMSCs. Nevertheless, we suggest
that this experiment may be useful as a pilot study to act as a
platform for future experiments. The second limitation is that UMRs
showed heterogeneous growth rates in the tumor xenografts despite
the same number of cells being inoculated, resulting in
difficulties in the interpretation of G1 data. The final limitation
is that in vivo BLI activity could not precisely identify
reporter expression of ADMSCs, such as light propagation in
different optical barriers such as hemorrhages or discoloration of
tumor, or any individual variation in the metabolism of
intraperitoneally administered luciferin.
However, despite all of these limitations, the
present study provides information concerning the behaviors and
actions of local treatment with ADMSCs in early tumorigenesis and
the growing period, and further study using larger numbers of
animals is necessary to elucidate the exact role of ADMSCs in tumor
growth.
References
|
1
|
Thomson JA, Itskovitz-Eldor J, Shapiro SS,
et al: Embryonic stem cell lines derived from human blastocysts.
Science. 282:1145–1147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wagner AM, Krenger W, Holzgreve W, Burkle
P and Surbek DV: Use of human embryonic stem cells and umbilical
cord blood stem cells for research and therapy: a prospective
survey among health care professionals and patients in Switzerland.
Transfusion. 53:2681–2689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faulkner SD, Vawda R and Fehlings MG:
Adult-derived pluripotent stem cells. World Neurosurg. 82:500–508.
2014. View Article : Google Scholar
|
|
4
|
Cao F, Lin S, Xie X, et al: In vivo
visualization of embryonic stem cell survival, proliferation, and
migration after cardiac delivery. Circulation. 113:1005–1014. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Serakinci N, Christensen R, Fahrioglu U,
et al: Mesenchymal stem cells as therapeutic delivery vehicles
targeting tumor stroma. Cancer Biother Radiopharm. 26:767–773.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakamizo A, Marini F, Amano T, et al:
Human bone marrow-derived mesenchymal stem cells in the treatment
of gliomas. Cancer Res. 65:3307–3318. 2005.PubMed/NCBI
|
|
7
|
Khakoo AY, Pati S, Anderson SA, et al:
Human mesenchymal stem cells exert potent antitumorigenic effects
in a model of Kaposi’s sarcoma. J Exp Med. 203:1235–1247. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiao L, Xu Z, Zhao T, et al: Suppression
of tumorigenesis by human mesenchymal stem cells in a hepatoma
model. Cell Res. 18:500–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Katsuno T, Ochi M, Tominaga K, et al:
Mesenchymal stem cells administered in the early phase of
tumorigenesis inhibit colorectal tumor development in rats. J Clin
Biochem Nutr. 53:170–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Studeny M, Marini FC, Champlin RE,
Zompetta C, Fidler IJ and Andreeff M: Bone marrow-derived
mesenchymal stem cells as vehicles for interferon-beta delivery
into tumors. Cancer Res. 62:3603–3608. 2002.PubMed/NCBI
|
|
11
|
Zhu W, Xu W, Jiang R, et al: Mesenchymal
stem cells derived from bone marrow favor tumor cell growth in
vivo. Exp Mol Pathol. 80:267–274. 2006. View Article : Google Scholar
|
|
12
|
Djouad F, Plence P, Bony C, et al:
Immunosuppressive effect of mesenchymal stem cells favors tumor
growth in allogeneic animals. Blood. 102:3837–3844. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Studeny M, Marini FC, Dembinski JL, et al:
Mesenchymal stem cells: potential precursors for tumor stroma and
targeted-delivery vehicles for anticancer agents. J Natl Cancer
Inst. 96:1593–1603. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davidoff AM, Ng CY, Brown P, et al: Bone
marrow-derived cells contribute to tumor neovasculature and, when
modified to express an angiogenesis inhibitor, can restrict tumor
growth in mice. Clin Cancer Res. 7:2870–2879. 2001.PubMed/NCBI
|
|
15
|
Patel SA, Heinrich AC, Reddy BY, Srinivas
B, Heidaran N and Rameshwar P: Breast cancer biology: the
multifaceted roles of mesenchymal stem cells. J Oncol.
2008:4258952008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dwyer RM, Khan S, Barry FP, O’Brien T and
Kerin MJ: Advances in mesenchymal stem cell-mediated gene therapy
for cancer. Stem Cell Res Ther. 1:252010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schäffler A and Büchler C: Concise review:
adipose tissue-derived stromal cells - basic and clinical
implications for novel cell-based therapies. Stem Cells.
25:818–827. 2007. View Article : Google Scholar
|
|
18
|
Zhao W, Ren G, Zhang L, et al: Efficacy of
mesenchymal stem cells derived from human adipose tissue in
inhibition of hepatocellular carcinoma cells in vitro. Cancer
Biother Radiopharm. 27:606–613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barry FP and Murphy JM: Mesenchymal stem
cells: clinical applications and biological characterization. Int J
Biochem Cell Biol. 36:568–584. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zuk PA, Zhu M, Ashjian P, et al: Human
adipose tissue is a source of multipotent stem cells. Mol Biol
Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Preston SL, Alison MR, Forbes SJ, Direkze
NC, Poulsom R and Wright NA: The new stem cell biology: something
for everyone. Mol Pathol. 56:86–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lyden D, Hattori K, Dias S, et al:
Impaired recruitment of bone-marrow-derived endothelial and
hematopoietic precursor cells blocks tumor angiogenesis and growth.
Nat Med. 7:1194–1201. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramasamy R, Lam EW, Soeiro I, Tisato V,
Bonnet D and Dazzi F: Mesenchymal stem cells inhibit proliferation
and apoptosis of tumor cells: impact on in vivo tumor growth.
Leukemia. 21:304–310. 2007. View Article : Google Scholar
|
|
24
|
Sato T, Sakai T, Noguchi Y, Takita M,
Hirakawa S and Ito A: Tumor-stromal cell contact promotes invasion
of human uterine cervical carcinoma cells by augmenting the
expression and activation of stromal matrix metalloproteinases.
Gynecol Oncol. 92:47–56. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohlsson LB, Varas L, Kjellman C, Edvardsen
K and Lindvall M: Mesenchymal progenitor cell-mediated inhibition
of tumor growth in vivo and in vitro in gelatin matrix. Exp Mol
Pathol. 75:248–255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakamura K, Ito Y, Kawano Y, et al:
Antitumor effect of genetically engineered mesenchymal stem cells
in a rat glioma model. Gene Ther. 11:1155–1164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu GD, Nolta JA, Jin YS, et al: Migration
of mesenchymal stem cells to heart allografts during chronic
rejection. Transplantation. 75:679–685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barbash IM, Chouraqui P, Baron J, et al:
Systemic delivery of bone marrow-derived mesenchymal stem cells to
the infarcted myocardium: feasibility, cell migration, and body
distribution. Circulation. 108:863–868. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Serakinci N, Fahrioglu U and Christensen
R: Mesenchymal stem cells, cancer challenges and new directions.
Eur J Cancer. 50:1522–1530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghannam S, Bouffi C, Djouad F, Jorgensen C
and Noel D: Immunosuppression by mesenchymal stem cells: mechanisms
and clinical applications. Stem Cell Res Ther. 1:22010. View Article : Google Scholar : PubMed/NCBI
|