Introduction
Despite rapid advances in diagnostic and surgical
procedures in the past decade, pancreatic cancer remains the most
lethal human malignancy with an extremely low 5-year survival rate
(1–3). In the USA, in 2014, it was estimated
that there were 46,420 newly diagnosed patients and 39,590 died of
this disease (4). A low radical
resection rate and insensitive to chemoradiotherapy are the main
reasons for the short survival time (1,5–9).
Further insights into the mechanisms causing primary or secondary
chemoresistance are urgently needed and may reveal new prospects
for therapy.
Pancreatic stellate cells (PSCs), first isolated and
cultured by Bachem et al and Apte et al in 1998, are
the main source of pancreatic fibrosis in patients with chronic
pancreatitis and pancreatic cencer (10,11).
Many studies have demonstrated that PSCs promote the progression of
pancreatic cancer including cell proliferation, migration, invasion
and even distant metastasis (12–16).
However, the role of PSCs in the chemoresistance of pancreatic
cancer has not been fully elucidated.
As an ancient cell signaling system, Notch plays a
key role in organ development, cell fate determination and stem
cell maintenance (17,18). In adults, alteration of these
functions has been associated with many types of malignancies
including pancreatic cancer (19).
A recent study demonstrated that Notch components, Notch-1, -3 and
-4, HES-1 and HEY-1 presented significantly higher nuclear
expression in locally advanced and metastatic tumors compared to
resectable cancers. In survival analyses, nuclear Notch-3 and HEY-1
expression levels were significantly associated with reduced
overall and disease-free survival following curative intent surgery
therapy (20). Targeting the Notch
signaling pathway for pancreatic cancer showed promising results in
preclinical studies (21–24). The present study revealed that PSCs
promoted expression of the Notch component, Hes 1 and
chemoresistance to gemcitabine in pancreatic cancer. The Notch
signaling pathway inhibitor (L1790) and Hes 1 siRNA reversed the
effect of PSCs on chemoresistance. In clinical study, we found that
HES 1 expression was associated with shorter overall and
disease-free survival in pancreatic cancer patients.
Materials and methods
PSC isolation and cell culture
PSCs were isolated from the normal rat pancreas
according to the method established by Apte et al (11) and were maintained in Dulbecco’s
modified Eagle’s medium (DMEM) containing 10% fetal bovine serum
(FBS). Two human pancreatic cancer cell lines (PANC-1 and BxPC-3)
were cultured in DMEM supplemented with 10% FBS, L-glutamine, and
1% penicillin and streptomycin in a 5% CO2 atmosphere at
37°C unless otherwise indicated.
Co-culture of pancreatic cancer and
stellate cells
Pancreatic cancer cells (PANC-1, 1.5×105
cells/well and BxPC-3, 1.0×105 cells/well) were seeded
in 6-well culture plates (Corning Costar, NY, USA). PSCs
(3×105 cells/culture insert) were seeded into the
culture inserts of 1.0 μm pore size (Corning Costar). On the
following day, the culture insets seeded with PSCs were placed into
the 6-well plates containing pancreatic cancer cells, and
incubation was continued up to 3 days in DMEM supplemented with 1%
FBS, penicillin and streptomycin. Cancer cells after co-culture
were collected for chemoresistance analysis.
Preparation of PSC conditioned
medium
PSC conditioned medium was prepared according to the
method as described by Hwang et al (14). Briefly, when PSCs were grown to 70
to 80% confluence in 20-cm2 dishes in DMEM/10% FCS, the
medium was replaced with serum-free DMEM, and the cells were
cultured for 48 h. Then the medium was collected, centrifuged and
the supernatant was concentrated with Centricon YM-3 filters
(Millipore Corp., Billerica, MA, USA).
Gemcitabine treatment
To explore the effect of PSCs on chemoresistance,
the cancer cells (co-cultured or not co-cultured with PSCs) were
seeded in 6-well (PANC-1, 1.5×105 cells/well and BxPC-3,
1.0×105 cells/well) or 96-well plates (4×103
and 3×103 cells/well). Cancer cells cultured with PSCs
were incubated with PSC conditional medium. The Cell Counting Kit-8
(CCK-8) was used to calculate the inhibitory rate after incubation
with gemcitabine (100 ng/ml; Sigma-Aldrich, St. Louis, MO, USA) for
48 h. To assess the IC50 value, different concentrations
of gemcitabine (100, 10, 1, 0.1 and 0.01 mg/ml; 1, 0.1, and 0.01
μg/ml; and 1 and 0.1 ng/ml) were added to the co-culture system.
GraphPad Prism 6 was used to calculate the IC50
value.
Measurement of apoptosis
Annexin V-FITC/PI (BD Pharmingen, San Diego, CA,
USA) was used for detecting apoptotic cells according to the
manufacturer’s instructions. Briefly, cells were washed,
trypsinized, centrifuged and then resuspended at 1×106
cells/ml, and then incubated in binding buffer containing Annexin
V-FITC (5 ml) and PI (10 ml) for 15 min in the dark. A BD flow
cytometer was used for analysis.
Hes 1 siRNA transfection
Pancreatic cancer cells were transfected with Hes 1
siRNA (sense, 5′-AAAGAUAGCUC CCGGCAUU-3′) using Lipofectamine 2000
according to the manufacturer’s instructions.
RNA isolation, cDNA synthesis and
real-time reverse transcription-PCR
The total RNA from pancreatic cancer cells and
siRNA-transfected cancer cells was isolated with TRIzol (Invitrogen
Life Technologies, Carlsbad, CA, USA) and purified with the RNeasy
Mini kit and RNase-free DNase set (Qiagen, Hilden, Germany)
according to the manufacturer’s protocols. Total RNA was reverse
transcribed using the High Capacity cDNA reverse transcription kit
(Applied Biosystems, Foster City, CA, USA) and then mRNA expression
was quantified using the TaqMan Gene Expression Assay (Applied
Biosystems). The primers used in the PCR reaction for Hes 1 and
Jagged-1 were: Hes 1 forward, 5′-GGGCAAGAATAAAT GAAAG-3′ and
reverse, 5′-GCGCGGTACTTCCCCAA CAC-3′ and Jagged-1 forward,
5′-GGGCCAGACTGCAGGATAAAC-3′ and reverse, 5′-CGCCGTGCCCTTTGTGGAG-3′,
respectively.
Western blot analysis
To detect changes in the protein levels of Hes 1
(sc-13844) and Jagged 1 (sc-6011) (both from Santa Cruz
Biotechnology, Santa Cruz, CA, USA), standard western
immunoblotting techniques were used. Cells were lysed in lysis
buffer by incubating for 20 min at 4°C. Total proteins were
fractionated using SDS-PAGE and transferred onto nitrocellulose
membrane for western blotting in routine manner. The blots were
then detected using ECL (Illumina, Inc., San Diego, CA, USA).
Tissue specimens and
immunohistochemistry
Human specimens from 72 patients with pancreatic
cancer who underwent R0 resection between January 2004 and 2013
were obtained from the Tissue Bank of the Department of General
Surgery, Xuanwu Hospital, Beijing, China. The study was approved by
the Ethics Committee of the hospital. Immunohistochemistry on
formalin-fixed, paraffin-embedded samples was conducted as
previously described. The slides were graded into 3 categories as
described earlier (25), from grade
1 to 3, as follows: grade 1, 0–25% staining; grade 2, 26–50%
staining; and grade 3, >50% staining.
Statistical analyses
Experiments presented in the figures are
representative of at least 3 repetitions. Continuous data are
presented as mean ± SE and were analyzed by the two-tailed
Student’s t-test. Categorical variables were analyzed using
Chi-square tests. Survival was assessed according to the
Kaplan-Meier method; the survival differences were analyzed using
the log-rank test. Univariate and multivariate survival analyses
were performed using Cox proportional hazard model. Results are
reported as relative risk (RR) and 95% confidence intervals (95%
CI). SPSS software (version 18.0; SPSS. Inc., Chicago, IL, USA) was
used for analysis with a significance level of P<0.05.
Results
PSC isolation
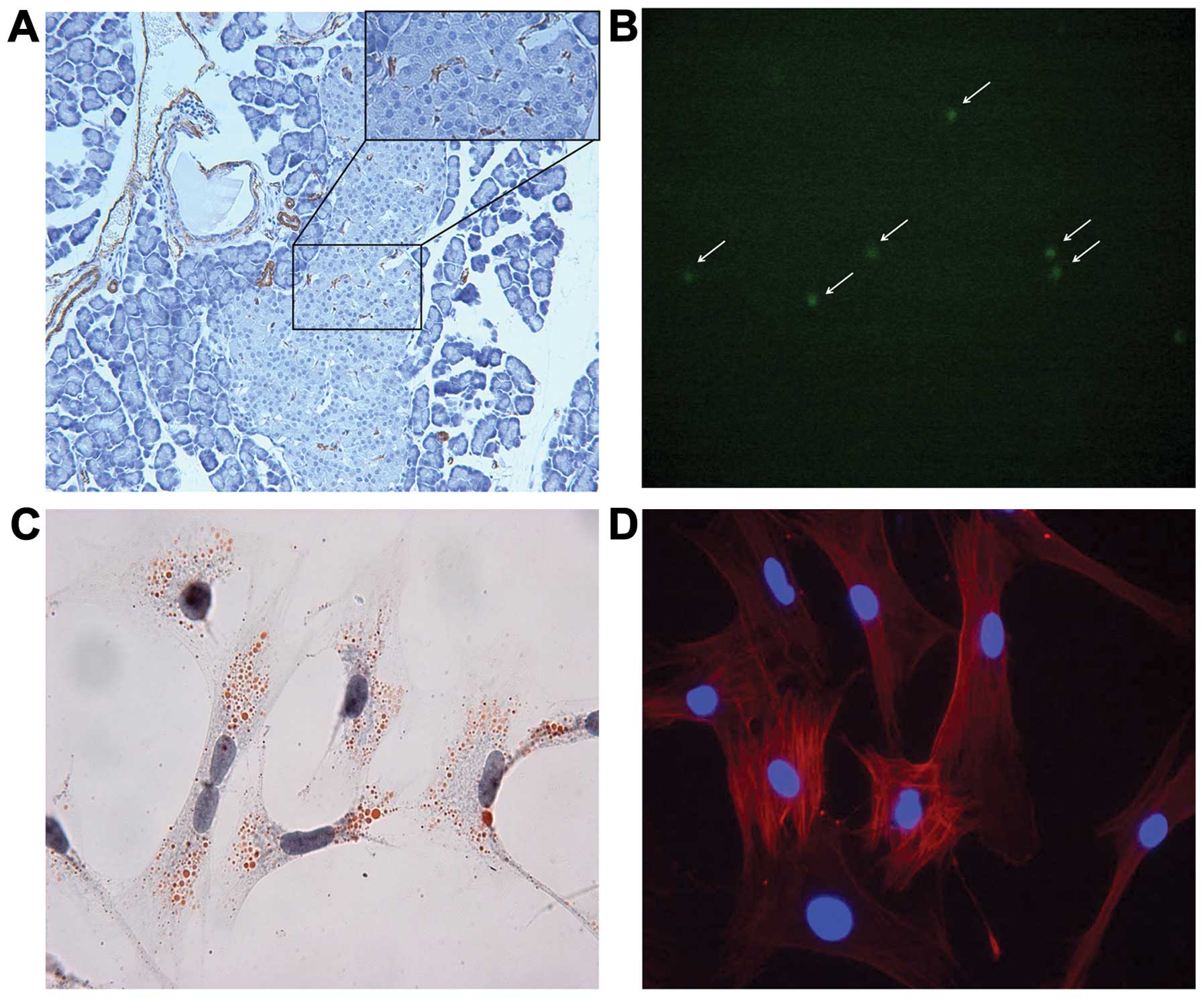
As described previously, PSCs are mainly located in
the interstitium between acini, and negative staining for α-smooth
muscle actin (α-SMA) is noted in quiescent PSCs. However, we found
that active PSCs were also occasionally visible in the islet in the
normal rat pancreas (Fig. 1A).
After isolation, Oil-red O staining and vitamin A autofluorescence
showed the droplets in the cytoplasm in quiescent PSCs (Fig. 1B and C). Cytoplasmic α-SMA staining
was detected in active PSCs which had been cultured for 7 days
(Fig. 1D).
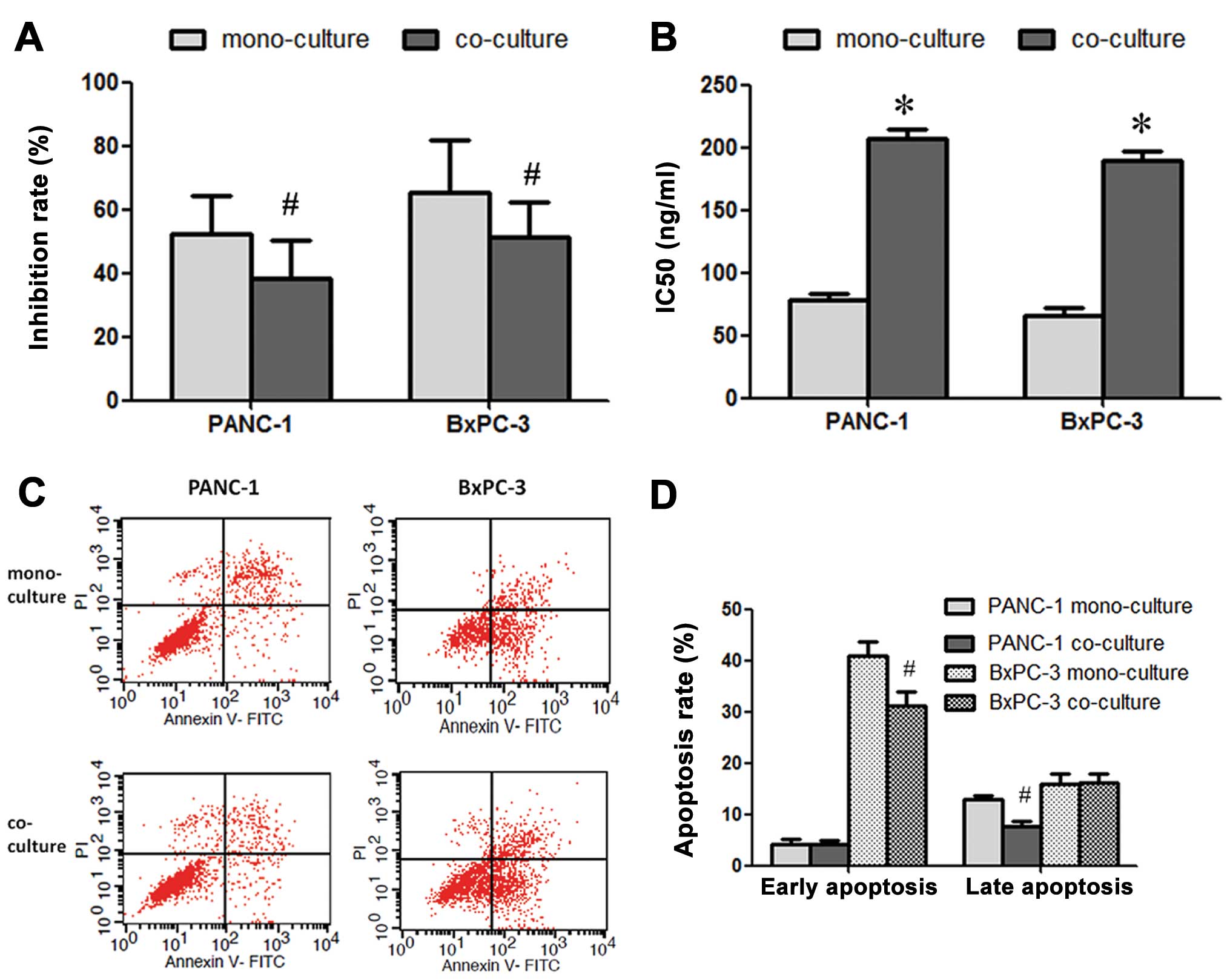
PSC promotes chemoresistance to
gemcitabine of pancreatic cancer cells
Following treatment with gemcitabine (100 ng/ml),
the growth inhibition rate was 52.3±12.1 and 65.1±16.8% in the
PANC-1 and BxPC-3 cells, respectively. After being cultured with
PSC conditioned medium, the inhibition rate significantly decreased
to 38.5±11.6 and 51.2±10.9%, respectively (Fig. 2A). The IC50 values were
also increased significantly in both pancreatic cancer cell lines
(Fig. 2B). Flow cytometric analysis
revealed that PSCs promoted the anti-apoptosis ability of the
cancer cells. The late apoptosis rate of PANC-1 and the early
apoptosis rate of BxPC-3 cells were decreased significantly after
co-culture with PSCs (Fig. 2C and
D).
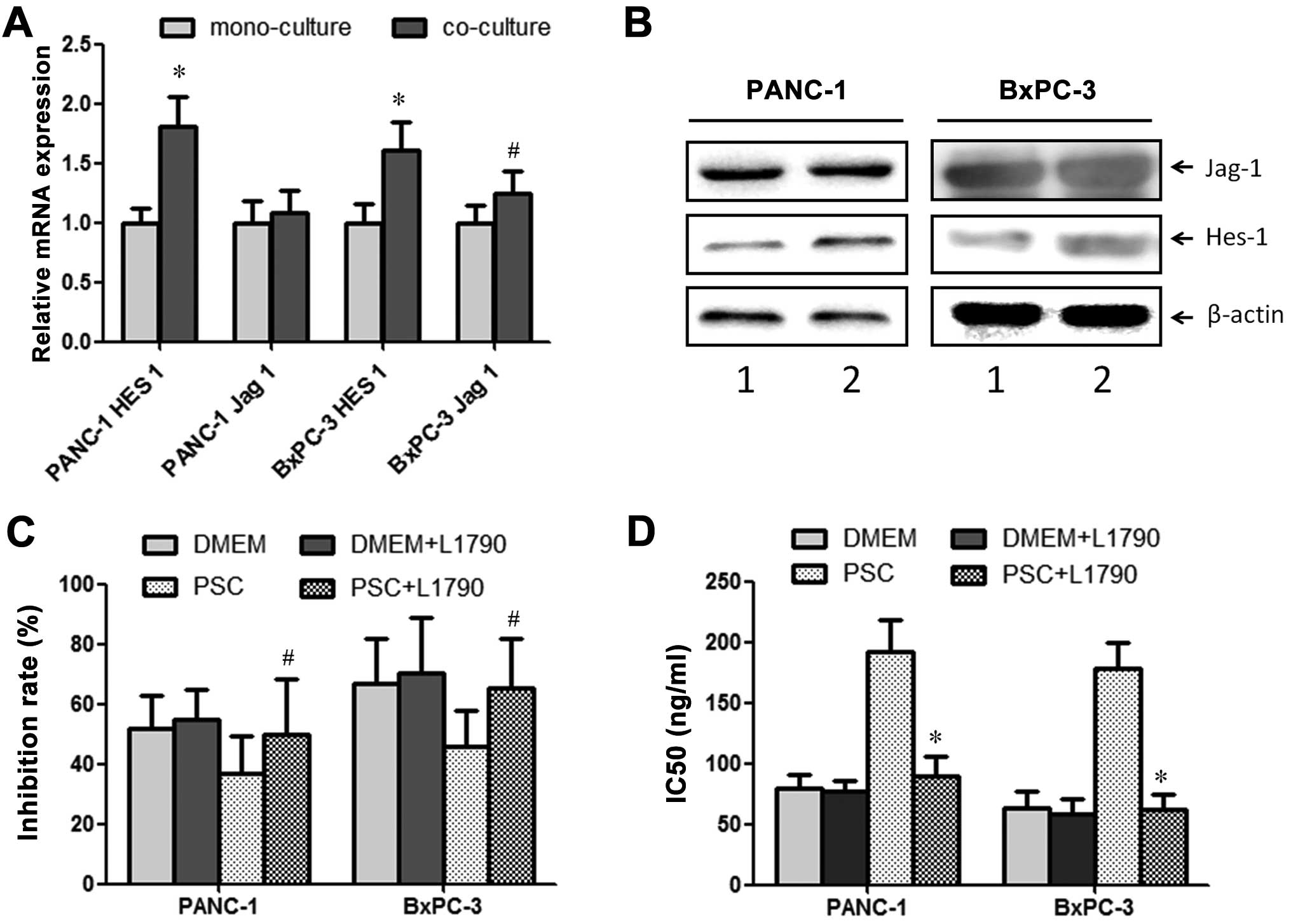
Notch signaling pathway is involved in
the chemoresistance induced by PSCs
After being co-cultured with PSCs for 48 h, the
cancer cells were collected for further analysis. RT-PCR analysis
revealed that the expression levels of Jagged 1 and Hes 1, members
of the Notch signaling pathway were significantly promoted after
co-culture with PSCs in both the PANC-1 and BxPC-3 cell lines
(Fig. 3A). Western blot analysis
showed similar results (Fig. 3B).
In order to determine the role of the Notch signaling pathway in
chemoresistance induced by PSCs, L1790 (5 μM, Notch signaling
pathway inhibitor) was added to the co-culture system. After
introduction of the inhibitor, increased chemoresistance to
gemcitabine induced by PSCs was reversed (Fig. 3C). Increased IC50 values
for PANC-1 and BxPC-3 cell lines also returned to the levels in the
mono-culture (Fig. 3D).
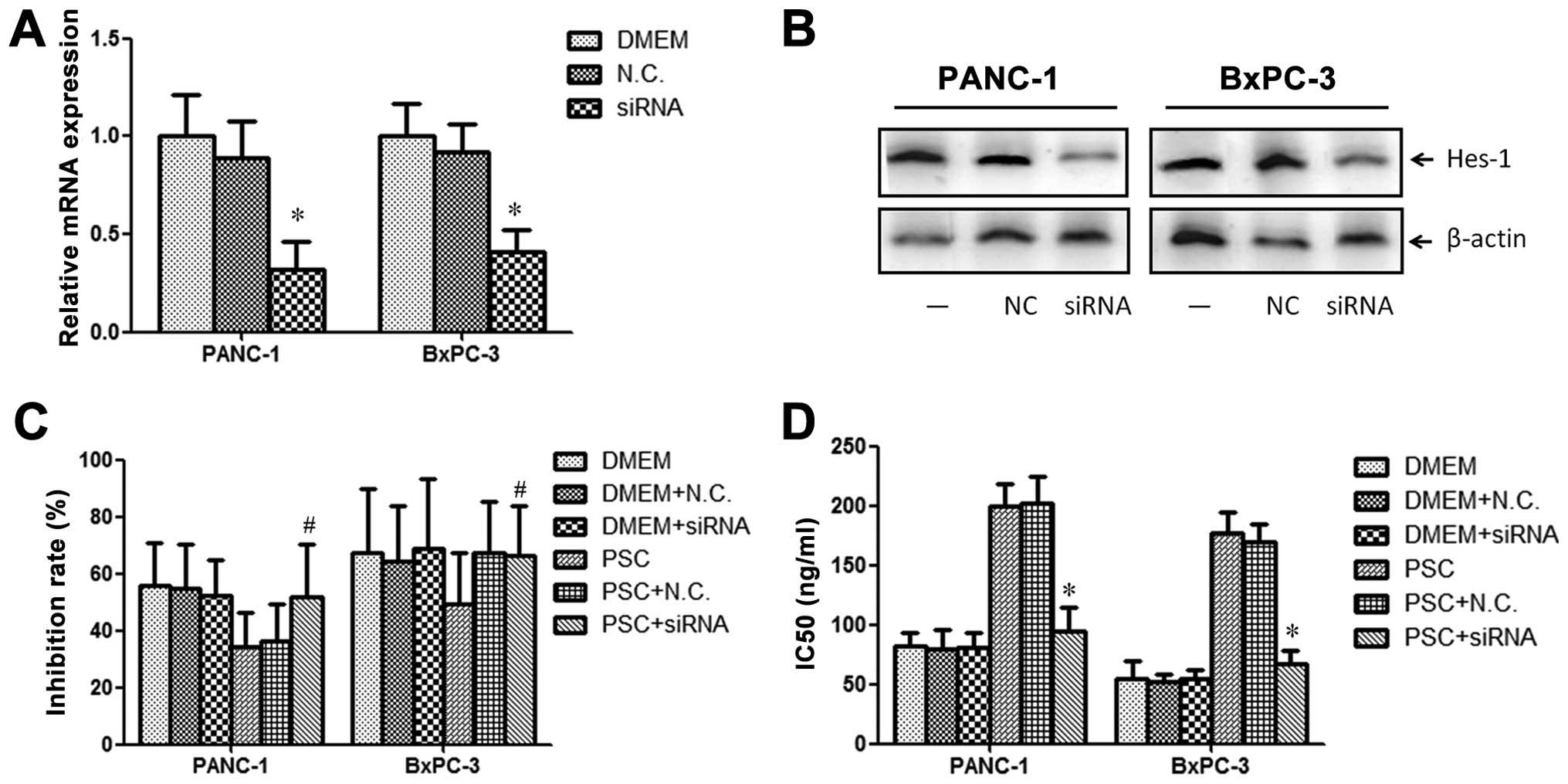
HES 1 is essential for chemoresistance
induced by PSCs
To further explore the effect of Hes 1 in the
chemoresistance induced by PSCs, we knocked down the expression by
siRNA transfection (Fig. 4A and B).
After successfully transfection of the Hes 1 siRNA, we found that
the effect of PSCs on chemoresistance of PANC-1 and BxPC-3 cells
was blocked (Fig. 4C). However, the
negative control siRNA did not have any influence on
chemoresistance. The effect of PSC on IC50 values for
both PANC-1 and BxPC-3 cells was also reversed (Fig. 4D).
HES 1 expression is associated with poor
prognosis in patients with pancreatic cancer
Seventy-two patients with pancreatic cancer who
underwent resection were included in the present study. Patient
demographics are shown in Table I.
The majority of patients were male (62.5%, 45/72) and had cancer
located in the head of the pancreas (65.2%, 47/72). Representative
staining of Hes 1 is shown in Fig.
5A–C. Nineteen, 19 and 34 patients had low, moderate and high
expression of Hes 1 and were scored as grade 1, 2 and 3. There was
no significant difference among grade 1, 2 and 3 groups (data not
shown). The overall survival and progression-free survival time for
grade 1 patients were 31.8±5.2 and 26.6±5.0 months, respectively
(Fig. 5D and E). Kaplan-Meier
analysis showed that high expression of Hes 1 was associated with
shorter overall and progression-free survival (Fig. 5D and E). Hes 1 expression was an
independent risk factor for poor prognosis in patients with
pancreatic cancer. Cox regression analysis revealed that Hes 1
expression (grade 2 and 3) was an independent risk factor for
cancer survival (RR, 2.012, 95%; CI, 1.549–10.214; P=0.001)
(Table II).
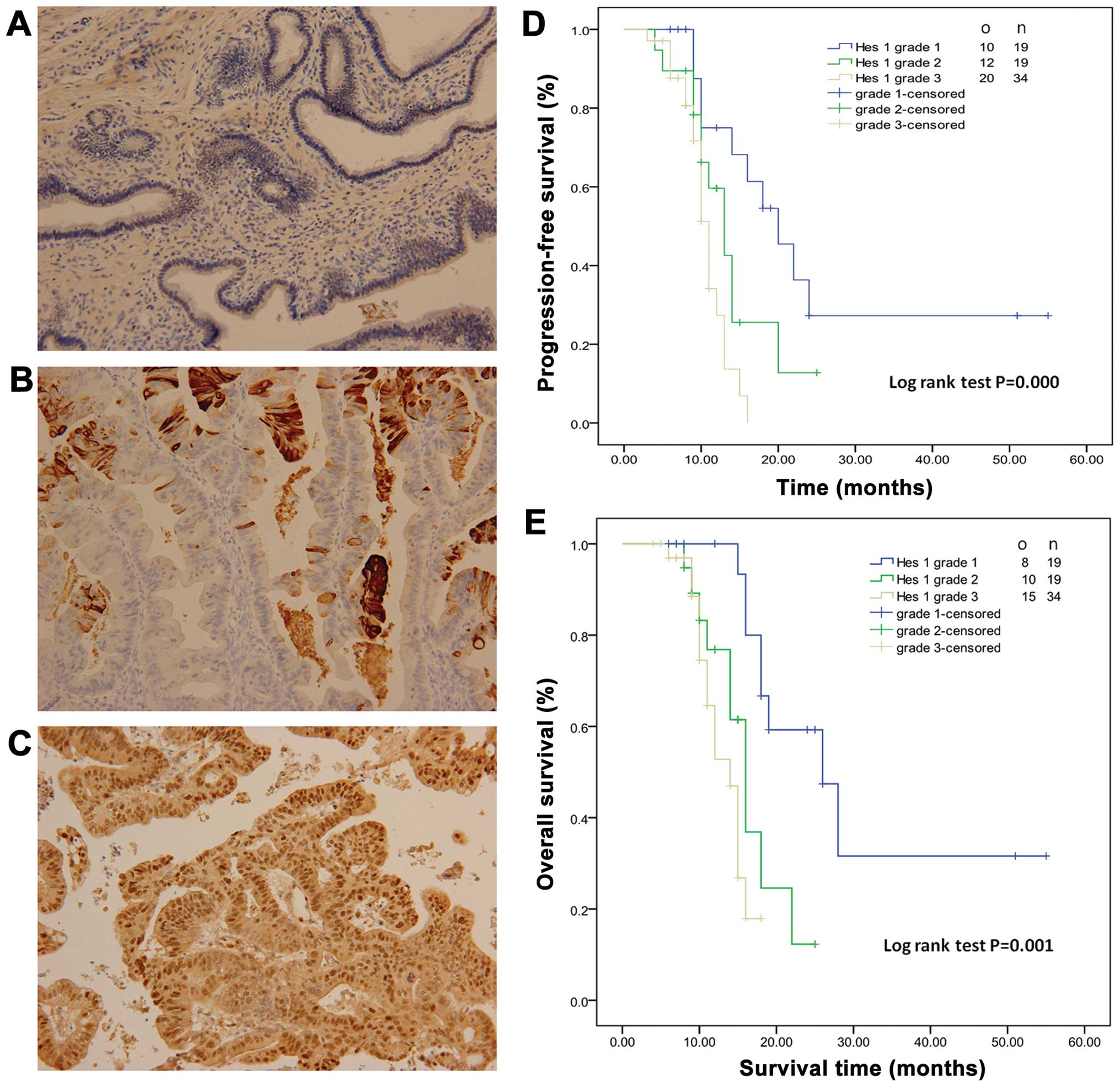 | Figure 5HES 1 expression is associated with
poor prognosis in patients with pancreatic cancer. (A–C)
Representative staining of Hes 1 in pancreatic cancer patient
tumors (magnification, ×200). (A) Grade 1, 0–25% staining; (B)
grade 2, 26–50% staining; (C) grade 3, >50% staining. (D)
Progression-free survival (PFS) analysis showed that the mean PFS
time in grade 1, 2 and 3 patients was 26.6±5.0, 13.7±1.6 and
10.7±0.6 months, respectively. (E) Overall survival (OS) analysis
revealed that the mean OS time in grade 1, 2 and 3 patients was
31.8±5.2, 16.3±1.4 and 13.3±0.7 months, respectively. o, observed
events; n, number of patients. |
 | Table IDemographics of the pancreatic cancer
patients who underwent resection (n=72). |
Table I
Demographics of the pancreatic cancer
patients who underwent resection (n=72).
|
Characteristics | Data |
|---|
| Age (years) | 66.5±11.2 |
| Gender
(male/female) | 45/27 |
| Tumor location
(head/body/tail) | 47/8/17 |
| Operation
(PD/DP) | 47/25 |
| Tumor size
(cm) | 3.3±1.5 |
| Differentiation
(well/moderate/poor) | 7/33/32 |
| Lymph node
metastasis (yes/no) | 44/28 |
| Perineural
infiltration (yes/no) | 29/43 |
| Resection margin
(−/+) | 59/13 |
| AJCC/UICC stage
(1A/1B/2A/2B) | 6/11/7/48 |
 | Table IIResults of the univariate and
multivariate Cox regression analyses for cancer survival. |
Table II
Results of the univariate and
multivariate Cox regression analyses for cancer survival.
| Univariate Cox
regression | Multivariate Cox
regression |
|---|
|
|
|
|---|
| Variable | RR (95% CI) | P-value | RR (95% CI) | P-value |
|---|
| Gender (referent,
male) | 1.001
(0.321–3.108) | 0.115 | 0.981
(0.138–5.015) | 0.305 |
| Age at diagnosis
(referent, >65 years) | 2.218
(1.234–12.408) | <0.001 | 2.799
(1.194–15.486) | <0.001 |
| Primary site of
tumor (referent, head) | 2.254
(0.993–17.134) | 0.478 | 3.096
(0.256–15.196) | 0.641 |
| T stage (referent,
T1) | 0.910
(0.219–4.952) | 0.126 | 0.887
(0.326–3.125) | 0.213 |
| N stage (referent,
N0) | 0.950
(0.275–18.031) | 0.271 | 1.010
(0.448–16.150) | 0.437 |
| Resection margin
(referent, positive) | 1.998
(0.879–11.258) | 0.021 | 2.001
(0.714–12.524) | 0.019 |
| Gemcitabine therapy
(referent, no therapy) | 0.749
(0.247–5.867) | 0.118 | 0.735
(0.312–4.129) | 0.069 |
| Hes 1 grade 2 + 3
(referent, grade 1) | 2.154
(1.987–11.212) | <0.001 | 2.012
(1.549–10.214) | 0.001 |
Discussion
The role of gemcitabine in the treatment of
pancreatic cancer has been established by a series of excellent
trials (6,7,26–30).
However, the objective response rate remains unsatisfactory
(8,9,31).
Primary chemoresistance to single-agent gemcitabine occurred in
~34.5% of metastatic pancreatic cancer patients (8). The addition of cytotoxic and targeted
agents to gemcitabine almost invariably provided no significant
survival improvement, despite an improvement in response rates in
some studies (7,32,33).
Stromal cells might play an important role in primary and secondary
chemoresistance in cancer patients. As a partner in crime with
pancreatic cancer cells, PSCs significantly promote cancer
progression in in vivo and in vitro studies (14–16,35).
Mounting evidence suggests PSCs are both direct and indirect
drivers of pancreatic cancer chemoresistance and spread, and thus
elucidation of the underlying mechanisms may potentiate current
chemotherapy. Our study demonstrated that PSCs promoted the ability
of chemoresistance to gemcitabine in both PANC-1 and BxPC-3
cells.
While the cause of chemoresistance is
multifactorial, three major processes have been largely clarified:
i) reduced drug uptake; ii) increased energy-dependent drug efflux;
and iii) alterations in cellular capabilities affecting drug
cytotoxicity, such as reduced apoptosis and dysregulated drug
metabolism (34). Our study also
showed that PSCs reduce late apoptosis in PANC-1 and in BxPC-3
cells which may contribute to chemoresistance. In addition, PSCs
stimulated the epithelial-mesenchymal transition (EMT) of cancer
cells, which resulted in a more chemoresistant phenotype (35). Cancer stem cells are also involved
in the chemoresistance induced by PSCs (36). In an in vitro study and in
pancreatic cancer patients, another major determinant of pancreatic
cancer chemoresistance was the extensive fibrosis produced by PSCs,
which resulted in significant intratumoral hypoxia and a
self-perpetuating hypoxia-fibrosis cycle. This impaired drug
delivery to cancer cells and stimulated their EMT and genetic
instability, yielding a more chemoresistant phenotype (34).
More and more evidence has revealed the fact that
the Notch signaling pathway may be a potential target for reversing
the chemoresistance of pancreatic cancer. Gungor et al
showed that Midkine-Notch-2 interaction activated Notch signaling,
induced EMT, upregulated Hes 1 and increased chemoresistance
(37). Wang et al
demonstrated that in gemcitabine-resistant pancreatic cancer cells,
the Notch signaling pathway was overactivated with Notch-2 and
Jagged-1 overexpression (38). Kang
et al showed that Notch ligand Delta-like 4 (DLL4) induced
impaired chemo-drug delivery and enhanced chemoresistance in
pancreatic cancer in vivo. Overactivation of the DLL4/Notch
pathway enhanced the phenotype of EMT and cancer stem cells, and
induced multi-chemoresistance in vitro (39). Our study also demonstrated that PSCs
promoted Hes 1 expression and overactivated the Notch signaling
pathway. L1790 (Notch signaling pathway inhibitor) and Hes 1 siRNA
reversed the chemoresistance induced by PSCs. These results provide
molecular evidence showing that Hes 1 is essential for the
chemoresistance induced by PSCs.
In view of the Notch signaling pathway in the
development of pancreatic cancer, it is not surprising that the
Notch expression status is associated with the prognosis of
pancreatic cancer patients. We found that Hes 1 high expression is
a biomarker for poor prognosis in pancreatic adenocarcinoma.
In conclusion, our results suggest that PSCs induce
Hes 1 expression and promote chemoresistance in pancreatic cancer.
Hes 1 is an effective prognostic factor and is significantly
associated with prognosis of pancreatic cancer patients. Therapy
targeting the Notch signaling pathway may reverse chemoresistance
and improve survival in patients with advanced pancreatic
cancer.
References
|
1
|
Wray CJ, Ahmad SA, Matthews JB and Lowy
AM: Surgery for pancreatic cancer: recent controversies and current
practice. Gastroenterology. 128:1626–1641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cameron JL, Riall TS, Coleman J and
Belcher KA: One thousand consecutive pancreaticoduodenectomies. Ann
Surg. 244:10–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raman SP, Horton KM, Cameron JL and
Fishman EK: CT after pancreaticoduodenectomy: spectrum of normal
findings and complications. AJR Am J Roentgenol. 201:2–13. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loehrer PJ Sr, Feng Y, Cardenes H, et al:
Gemcitabine alone versus gemcitabine plus radiotherapy in patients
with locally advanced pancreatic cancer: an Eastern Cooperative
Oncology Group trial. J Clin Oncol. 29:4105–4112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colucci G, Labianca R, Di Costanzo F, et
al: Randomized phase III trial of gemcitabine plus cisplatin
compared with single-agent gemcitabine as first-line treatment of
patients with advanced pancreatic cancer: the GIP-1 study. J Clin
Oncol. 28:1645–1651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cunningham D, Chau I, Stocken DD, et al:
Phase III randomized comparison of gemcitabine versus gemcitabine
plus capecitabine in patients with advanced pancreatic cancer. J
Clin Oncol. 27:5513–5518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Conroy T, Desseigne F, Ychou M, et al:
FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N
Engl J Med. 364:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Von Hoff DD, Ervin T, Arena FP, et al:
Increased survival in pancreatic cancer with nab-paclitaxel plus
gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bachem MG, Schneider E, Gross H, et al:
Identification, culture, and characterization of pancreatic
stellate cells in rats and humans. Gastroenterology. 115:421–432.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Apte MV, Haber PS, Applegate TL, et al:
Periacinar stellate shaped cells in rat pancreas: identification,
isolation, and culture. Gut. 43:128–133. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujita H, Ohuchida K, Mizumoto K, et al:
Tumor-stromal interactions with direct cell contacts enhance
proliferation of human pancreatic carcinoma cells. Cancer Sci.
100:2309–2317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vonlaufen A, Joshi S, Qu C, et al:
Pancreatic stellate cells: partners in crime with pancreatic cancer
cells. Cancer Res. 68:2085–2093. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hwang RF, Moore T, Arumugam T, et al:
Cancer-associated stromal fibroblasts promote pancreatic tumor
progression. Cancer Res. 68:918–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mantoni TS, Lunardi S, Al-Assar O,
Masamune A and Brunner TB: Pancreatic stellate cells radioprotect
pancreatic cancer cells through β1-integrin signaling. Cancer Res.
71:3453–3458. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Farrow B, Berger DH and Rowley D:
Tumor-derived pancreatic stellate cells promote pancreatic cancer
cell invasion through release of thrombospondin-2. J Surg Res.
156:155–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Greenwald I and Kovall R: Notch signaling:
genetics and structure. WormBook. 1–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nicolas M, Wolfer A, Raj K, et al: Notch1
functions as a tumor suppressor in mouse skin. Nat Genet.
33:416–421. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Radtke F and Raj K: The role of Notch in
tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer.
3:756–767. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mann CD, Bastianpillai C, Neal CP, et al:
Notch3 and HEY-1 as prognostic biomarkers in pancreatic
adenocarcinoma. PLoS One. 7:e511192012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yabuuchi S, Pai SG, Campbell NR, et al:
Notch signaling pathway targeted therapy suppresses tumor
progression and metastatic spread in pancreatic cancer. Cancer
Lett. 335:41–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mizuma M, Rasheed ZA, Yabuuchi S, et al:
The gamma secretase inhibitor MRK-003 attenuates pancreatic cancer
growth in preclinical models. Mol Cancer Ther. 11:1999–2009. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cook N, Frese KK, Bapiro TE, et al: Gamma
secretase inhibition promotes hypoxic necrosis in mouse pancreatic
ductal adenocarcinoma. J Exp Med. 209:437–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schott AF, Landis MD, Dontu G, et al:
Preclinical and clinical studies of gamma secretase inhibitors with
docetaxel on human breast tumors. Clin Cancer Res. 19:1512–1524.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamada S, Fuchs BC, Fujii T, et al:
Epithelial-to-mesenchymal transition predicts prognosis of
pancreatic cancer. Surgery. 154:946–954. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van Cutsem E, van de Velde H, Karasek P,
et al: Phase III trial of gemcitabine plus tipifarnib compared with
gemcitabine plus placebo in advanced pancreatic cancer. J Clin
Oncol. 22:1430–1438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abou-Alfa GK, Letourneau R, Harker G, et
al: Randomized phase III study of exatecan and gemcitabine compared
with gemcitabine alone in untreated advanced pancreatic cancer. J
Clin Oncol. 24:4441–4447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heinemann V, Quietzsch D, Gieseler F, et
al: Randomized phase III trial of gemcitabine plus cisplatin
compared with gemcitabine alone in advanced pancreatic cancer. J
Clin Oncol. 24:3946–3952. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van Cutsem E, Vervenne WL, Bennouna J, et
al: Phase III trial of bevacizumab in combination with gemcitabine
and erlotinib in patients with metastatic pancreatic cancer. J Clin
Oncol. 27:2231–2237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ueno H, Ioka T, Ikeda M, et al: Randomized
phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine
alone in patients with locally advanced and metastatic pancreatic
cancer in Japan and Taiwan: GEST study. J Clin Oncol. 31:1640–1648.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vaccaro V, Sperduti I and Milella M:
FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N
Engl J Med. 365:768–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Louvet C, Labianca R, Hammel P, et al:
Gemcitabine in combination with oxaliplatin compared with
gemcitabine alone in locally advanced or metastatic pancreatic
cancer: results of a GERCOR and GISCAD phase III trial. J Clin
Oncol. 23:3509–3516. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Colucci G, Giuliani F, Gebbia V, et al:
Gemcitabine alone or with cisplatin for the treatment of patients
with locally advanced and/or metastatic pancreatic carcinoma: a
prospective, randomized phase III study of the Gruppo Oncologia
dell’Italia Meridionale. Cancer. 94:902–910. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McCarroll JA, Naim S, Sharbeen G, et al:
Role of pancreatic stellate cells in chemoresistance in pancreatic
cancer. Front Physiol. 5:1412014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kikuta K, Masamune A, Watanabe T, et al:
Pancreatic stellate cells promote epithelial-mesenchymal transition
in pancreatic cancer cells. Biochem Biophys Res Commun.
403:380–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Izumiya M, Kabashima A, Higuchi H, et al:
Chemoresistance is associated with cancer stem cell-like properties
and epithelial-to-mesenchymal transition in pancreatic cancer
cells. Anticancer Res. 32:3847–3853. 2012.PubMed/NCBI
|
|
37
|
Gungor C, Zander H, Effenberger KE, et al:
Notch signaling activated by replication stress-induced expression
of midkine drives epithelial-mesenchymal transition and
chemoresistance in pancreatic cancer. Cancer Res. 71:5009–5019.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Z, Li Y, Kong D, et al: Acquisition
of epithelial-mesenchymal transition phenotype of
gemcitabine-resistant pancreatic cancer cells is linked with
activation of the notch signaling pathway. Cancer Res.
69:2400–2407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kang M, Jiang B, Xu B, et al: Delta like
ligand 4 induces impaired chemo-drug delivery and enhanced
chemoresistance in pancreatic cancer. Cancer Lett. 330:11–21. 2013.
View Article : Google Scholar
|