Introduction
Renal cell carcinoma (RCC) is the most common type
of kidney cancer in adults. Surgical removal of the cancer, either
by radical or partial nephrectomy, is a standard therapeutic option
for organ-confined RCC. However, ~30% of patients eventually
manifest metastatic disease after surgery (1). Recent studies have clarified the key
molecular events of oncogenesis for renal cell carcinoma such as
aberrant hypoxia-inducible factor (HIF)-α activation by Von
Hippel-Lindau (VHL) gene mutation/deletion and the resultant
overexpression of vascular endothelial growth factor (VEGF) in
clear cell carcinoma, which provided the rationale for the
development of VEGF receptor (VEGFR) inhibitors such as sunitinib
and sorafenib. Although contemporary emergence of such molecular
targeting agents has contributed to the prolonged survival of
metastatic RCC (mRCC) patients, attainment of complete response is
extremely rare (2) and improvement
in overall survival is still limited. Therefore, detailed
mechanisms of how RCC cells spread and eventually metastasize must
be clarified in order to develop better therapeutic options.
Cancer cell invasion and metastasis share many
similarities with leukocyte trafficking, which is crucially
regulated by soluble factors and their receptors (3). Anaphylatoxin C5a is an N-terminal 74
amino acid fragment derived from the α-chain of the complement
fifth component (C5), which serves as a leukocyte chemoattractant
and inflammatory mediator (4). C5a
promotes leukocyte migration by interacting with the
membrane-associated C5a receptor (C5aR; CD88). C5aR is one of the G
protein-coupled receptors (GPCRs), and its association with the C5a
ligand provokes activation of intracellular signaling pathways such
as the Raf/MEK/ERK and PI3K/Akt pathways (5,6), which
play pivotal roles in leukocyte migration (7,8).
Recently, we reported that C5aR is aberrantly expressed in a wide
variety of human cancers presumably due to the consequence of
malignant transformation while the C5a-C5aR axis promoted cancer
cell invasion by eliciting matrix metalloprotease (MMP) secretion
and cytoskeletal reorganization (9). In that study, we briefly mentioned
that C5aR was also expressed in renal cell carcinoma specimens.
However, the number of specimens in that study was limited, and the
clinical significance and biological role of the C5aR expression in
renal cell carcinoma remains unclear.
Here, we analyzed the relationship between the C5aR
expression and the clinical parameters in renal cell carcinoma
specimens. We observed that C5aR was expressed in a vast majority
of the mRCC cases, whereas only half of the organ-confined RCCs
expressed C5aR. Furthermore, we provided in vitro evidence
that the C5a-C5aR axis provoked renal cancer cell invasion. Our
results suggest that the C5a-C5aR axis-elicited renal cancer cell
invasion may be one of the critical steps for establishing renal
cancer cell metastasis.
Materials and methods
Cell line
Renca cells (ATCC: CRL-2947) were obtained in 2013
from the American Type Culture Collection. The cells were cultured
in RPMI-1640 supplemented with 10% fetal calf serum (FCS),
penicillin (40 U/ml) and streptomycin (40 μg/ml) and were
maintained at 37°C in 5% CO2.
Reagents
Recombinant C5a was purchased from Hycult Biotech
(Plymouth, PA, USA). G418 was purchased from InvivoGen (San Diego,
CA, USA). U0126 was purchased from Promega (Madison, WI, USA).
PI-103 was purchased from Merck (Darmstadt, Germany). Anti-C5aR
antibody was purchased from Santa Cruz Biotechnology (Dallas, TX,
USA). Antibodies for phospho-ERK and total ERK, phospho-Akt and
total Akt and HRP-conjugated secondary antibodies were purchased
from Cell Signaling Technology (Danvers, MA, USA).
Tissue samples, immunohistochemistry and
retrospective analysis
Renal cell carcinoma tissue samples were obtained by
surgical resection or core needle biopsy from 127 patients in
Kumamoto University Hospital, and the usage of those samples for
the present study was approved by The Internal Review Board of
Kumamoto University Hospital. Immunohistochemistry for C5aR in the
RCC samples was performed according to a previously described
protocol (9). The relationship
between the C5aR expression and baseline demographic
data/clinicopathological parameters was analyzed by Fisher’s exact
test.
Immunoblotting
Immunoblotting was performed as previously described
(10). Briefly, cell lysates were
analyzed by SDS-PAGE under reducing conditions and transferred to
nitrocellulose membranes (Protran; GE Healthcare Life Sciences,
Pittsburgh, PA, USA). After blocking with blocking buffer (1% TBS-T
buffer containing 5% bovine serum albumin), the membranes were
incubated with the primary antibodies indicated according to the
manufacturer’s instructions, followed by incubation with
appropriate HRP-conjugated secondary antibodies. The bands were
visualized by ECL Plus Western Blotting Detection System (GE
Healthcare Life Sciences) according to the manufacturer’s
instructions.
Establishment of C5aR stably expressing
Renca cells and in vivo study
pCMV-C5aR that encodes full-length murine C5aR was
purchased from OriGene Technologies (Rockville, MD, USA). Renca
cells were transfected with pCMV-C5aR using the ProFection
Mammalian Transfection System (Promega). After 48 h, the medium was
replaced with a selection medium supplemented with G418 (400 μg/ml)
then cultured for 2 weeks. G418-resistant cells were isolated by
limiting dilution and propagated. The cells were subjected to flow
cytometric analysis by FACSCan (BD Biosciences, San Jose, CA, USA)
as previously described (9) to
select cells expressing C5aR (designated as Renca/C5aR cells).
Renca cells transfected with the empty plasmid pCMV and resistant
to 400 μg/ml G418 were isolated and then designated as Renca/empty
cells. For the in vivo study, the Renca-derived cells were
injected into the renal subcapsular space in mice according to
Shvarts et al (11). This
experiment was approved by the Kumamoto University Animal
Experiment Committee.
Anoikis assay
Anoikis assay was performed based on the protocol
reported by Berezovskaya et al (12) with minor modifications.
Renca-derived cells were dissociated by Accutase (Millipore,
Billerica, MA, USA), and then washed with serum-free medium and
suspended with medium containing 0.5% FCS. The viable cells were
counted using the trypan blue dye exclusion method to confirm that
the initial viability of the cells after dissociation by Accutase
was >95%. A suspension of 1×106 viable cells was
treated with or without 10 nM recombinant C5a (rC5a) then plated in
2.0 μl of serum-free medium in 6-well ultra-low-attachment
polystyrene plates (Corning, Tewksbury, MA, USA) and incubated at
37°C in 5% CO2 overnight. The numbers of the total and
viable cells after incubation were counted as described above, and
then the percentage of the viable cells was calculated.
Immunofluorescence analysis
Cells (5×104) were seeded on glass
coverslips and incubated for 24 h. After serum starvation
overnight, the cells were stimulated with 10 nM rC5a for the stated
time periods. The cells were then fixed in 4% paraformaldehyde,
permeabilized in 0.2% Triton X-100 for 5 min, and were incubated
with 5 U/ml Alexa 488-phalloidin (Invitrogen Life Technologies,
Carlsbad, CA, USA) for 40 min, followed by washing with PBS. Nuclei
were counterstained with 1.0 mM TO-PRO®-3 (Invitrogen
Life Technologies) for 15 min. Images were obtained and processed
by FluoView 300 laser scanning confocal microscope (Olympus,
Melville, NY, USA).
Invasion assay in vitro
BioCoat Matrigel invasion chambers were utilized
(24-well plates, 8-μm pores; BD Biosciences) as previously
described (9). Renca-derived cells
(5×104) were suspended in serum-free RPMI-1640, which
were then seeded into the upper chamber. RPMI-1640 supplemented
with either 10 nM rC5a or carrier solution (PBS) was placed in the
lower chamber. PI-103, U0126 or carrier control (DMSO) was added to
the medium in both the upper and lower well at the indicated
concentrations when appropriate. The numbers of cells that migrated
through the membrane were counted in 4 microscopic fields (x20
magnification) per membrane. The average was calculated from
triplicate samples, and statistical analyses were performed by
two-tailed t-tests.
Results
Frequent expression of the C5a receptor
in metastatic renal cell carcinoma
First, we analyzed C5aR expression in 127 primary
RCC specimens using surgically removed or needle biopsy samples.
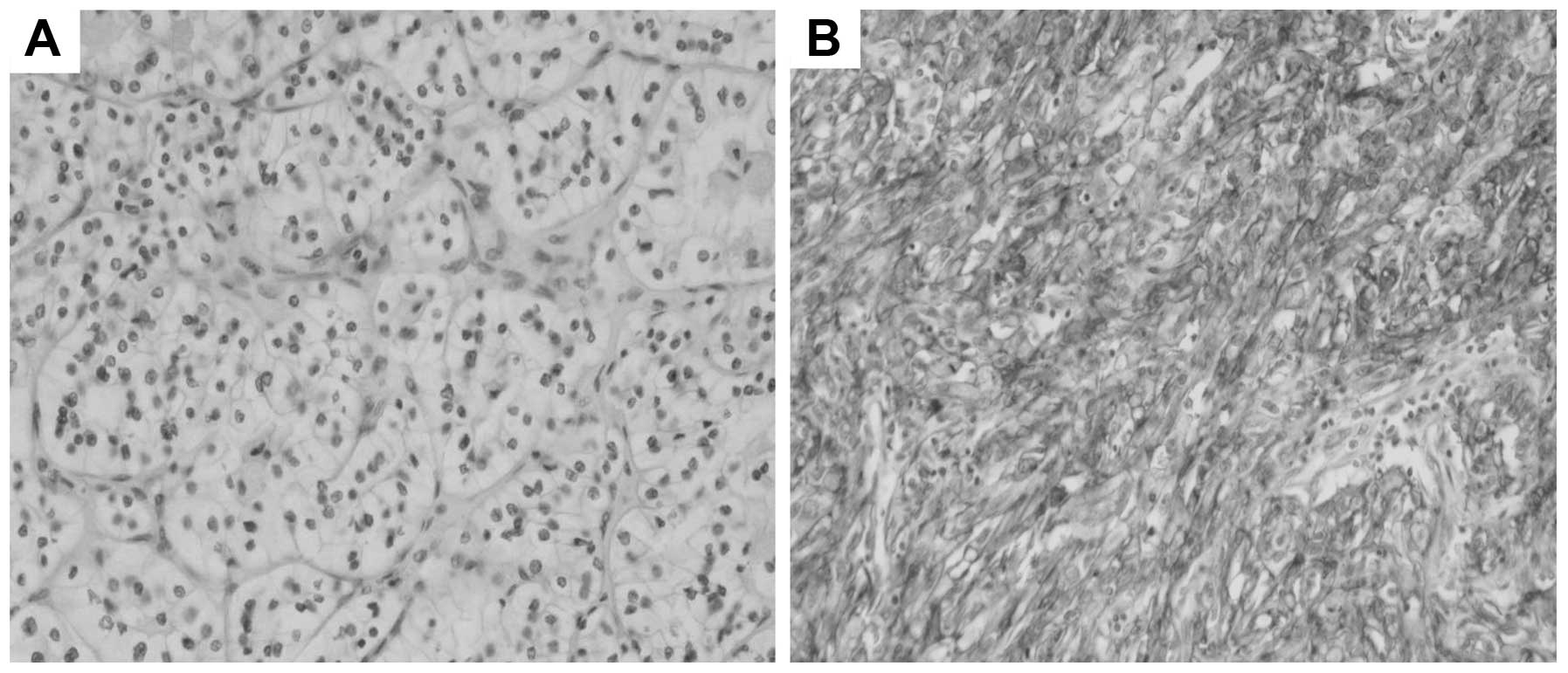
Overall, the C5aR expression was observed in 78 out of 127 samples
(61.4%). This sample cohort consisted of 97 RCCs without metastasis
and 30 mRCC (Fig. 1). As shown in
Table I, there was no significant
difference between the C5aR-positive and -negative group in regards
to age, gender and histological subtype. Regarding Fuhrman grade,
although the grade 3 population was quite low (n=6) in this sample
cohort, C5aR-positive tumors tended to exhibit a higher grade than
the C5aR-negative tumors (G1: 21.8 vs. 46.9%, G2: 69.2 vs. 49.0%).
Regarding T staging, the C5aR-positive group contained
significantly less T1 tumors (P=0.0056) and more T3 tumors
(P=0.0283) than the C5aR-negative group. As for N staging, more
C5aR-negative tumors were without lymph node metastasis (N0) than
the C5aR-positive tumors (98.0 vs. 87.2%) with marginal
significance (P=0.0497). Distant metastasis was more frequently
observed in the C5aR-positive group than in the C5aR-negative group
(37.2 vs. 2%). Of note, 96.7% (29/30) of the patients with
metastatic disease showed C5aR expression at their primary sites.
With regards to microscopic invasion, more C5aR-positive tumors
manifested microvascular invasion than C5aR-negative tumors (29.0
vs. 6.3%). These results suggest that C5aR expression contributes
to local invasion and distant metastasis of renal cell
carcinoma.
 | Table IPatients and tumor
characteristics. |
Table I
Patients and tumor
characteristics.
| C5aR(−)
(n=49) | C5aR(+)
(n=78) | P-value |
|---|
| Age (years) | 61.8 | 61.490 | 0.8962 |
| Gender, n (%) | | | |
| Male | 36 (73.5) | 50 (64.1) | 0.3313 |
| Female | 13 (26.5) | 28 (35.9) | |
| Histological
subtypes, n (%) | | | |
| Clear cell | 48 (100) | 72 (92.3) | 0.0812 |
| Chromophobe | 0 (0) | 3 (3.8) | 0.2835 |
| Papillary | 0 (0) | 3 (3.8) | 0.2835 |
| Fuhrman grade, n
(%) | | | |
| G1 | 23 (46.9) | 17 (21.8) | 0.0056 |
| G2 | 24 (49.0) | 50 (69.2) | 0.0259 |
| G3 | 1 (2.0) | 5 (6.4) | 0.4044 |
| Unknown | 1 (2.0) | 2 (2.6) | NA |
| Stagea, n (%) | | | |
| T1 | 46 (93.9) | 55 (70.5) | 0.0056 |
| T2 | 2 (4.1) | 10 (12.8) | 0.1270 |
| T3 | 1 (2.0) | 11 (14.1) | 0.0283 |
| T4 | 0 ( 0) | 2 (2.6) | NA |
| N0 | 48 (98.0) | 68 (87.2) | 0.0497 |
| N1 | 0 (0) | 5 (6.4) | 0.1555 |
| N2 | 1 (2) | 5 (6.4) | 0.4044 |
| M0 | 48 (98.0) | 49 (62.8) |
<0.0001 |
| M1 | 1 (2) | 29 (37.2) | |
| Microscopic
invasionb, n (%) | | | |
| Vascular | 3 (6.3) | 20 (29.0) | 0.0038 |
| Lymphatic | 0 (0) | 3 (4.7) | 0.2587 |
C5a-C5aR axis is not involved in Renca
cell proliferation and anoixis
To study the biological role of the C5a-C5aR axis in
renal cell carcinoma, we employed Renca cells since these cells are
the established model for studying renal cell carcinoma metastasis
(11,13). Interestingly, both western blotting
and flow cytometric analysis showed that C5aR was not expressed in
Renca cells (data not shown). Such an observation in cancer cell
lines, which does not seemingly reflect the characteristics in
clinical specimen, was described and discussed in our previous
study, suggesting that C5aR expression was lost during the process
of cell line establishment from primary culture of cancer cells in
order to prioritize the expression of other essential proteins for
clonal development in the context of 2-dimensional culture
(9). To establish renal cell
carcinoma cells expressing C5aR, C5aR cDNA was stably introduced
into Renca cells, and clones expressing C5aR were isolated and
propagated. Cells expressing C5aR on their cell surface, confirmed
by flow cytometric analysis (Fig.
2A), were selected and designated as Renca/C5aR cells. Cells
stably transfected with the empty plasmid were designated as
Renca/empty cells. The original purpose of establishing Renca/C5aR
cells was to study if C5aR can enhance the metastatic behavior of
renal carcinoma cells in a syngeneic orthotopic murine model using
BALB/c mice since Renca cells are known to establish multiple lung
metastasis in this model (11).
However, the Renca/C5aR cells, which were stimulated by recombinant
C5a and then injected into the renal subcapsular space, did not
exhibit any increase in either the number or the size of lung
metastatic nests compared to the Renca/empty cells (data not
shown). This may be due to the difficulty of attaining sustained
C5aR activation by recombinant C5a until establishing lung
metastasis, considering the stability of C5a and the period
required for tumor cell implantation into the lungs after
subcapsular injection. Because of such technical difficulty of the
in vivo study to analyze the biological role of C5aR
expression in renal cell carcinoma metastasis, we analyzed the
effect of C5aR on crucial steps of cancer metastasis in
vitro instead. Anoikis is a subtype of apoptosis induced upon
cell detachment from the extracellular matrix, which is an
indispensable step for metastasis (14). To analyze whether renal carcinoma
cells are able to acquire resistance to anoikis by C5a-C5aR axis
activation, Renca-derived cells were treated with or without C5a
and then cultured in suspension on ultra low-attachment plates and
a number of viable cells was assessed. Fig. 2B shows that the percentage of
Renca/C5aR cell survival in suspension culture was slightly higher
than that of the Renca/empty cells, which was not significantly
enhanced by C5a treatment. This result suggests that, although C5aR
expression itself may have a marginal effect on cell survival in an
adhesion-independent condition, it is unlikely that anoikis plays
an important role in the metastasis of C5aR-expressing renal
carcinoma cells.
C5a elicits cytoskeletal rearrangement
and changes in cellular morphology in C5aR-expressing Renca
cells
It is known that the chemoattractant C5a causes
actin rearrangement and stimulates the migration of leukocytes
(15,16). We previously showed that cancer
cells can exploit this mechanism to acquire the ability of
migration and invasion by activation of aberrantly expressed C5aR
using bile duct carcinoma cells (9). To test the hypothesis that C5aR
expressed in renal cell carcinoma facilitates actin reorganization
by C5a stimulation as well, the effect of C5aR activation on actin
rearrangement in Renca cells was analyzed by F-actin
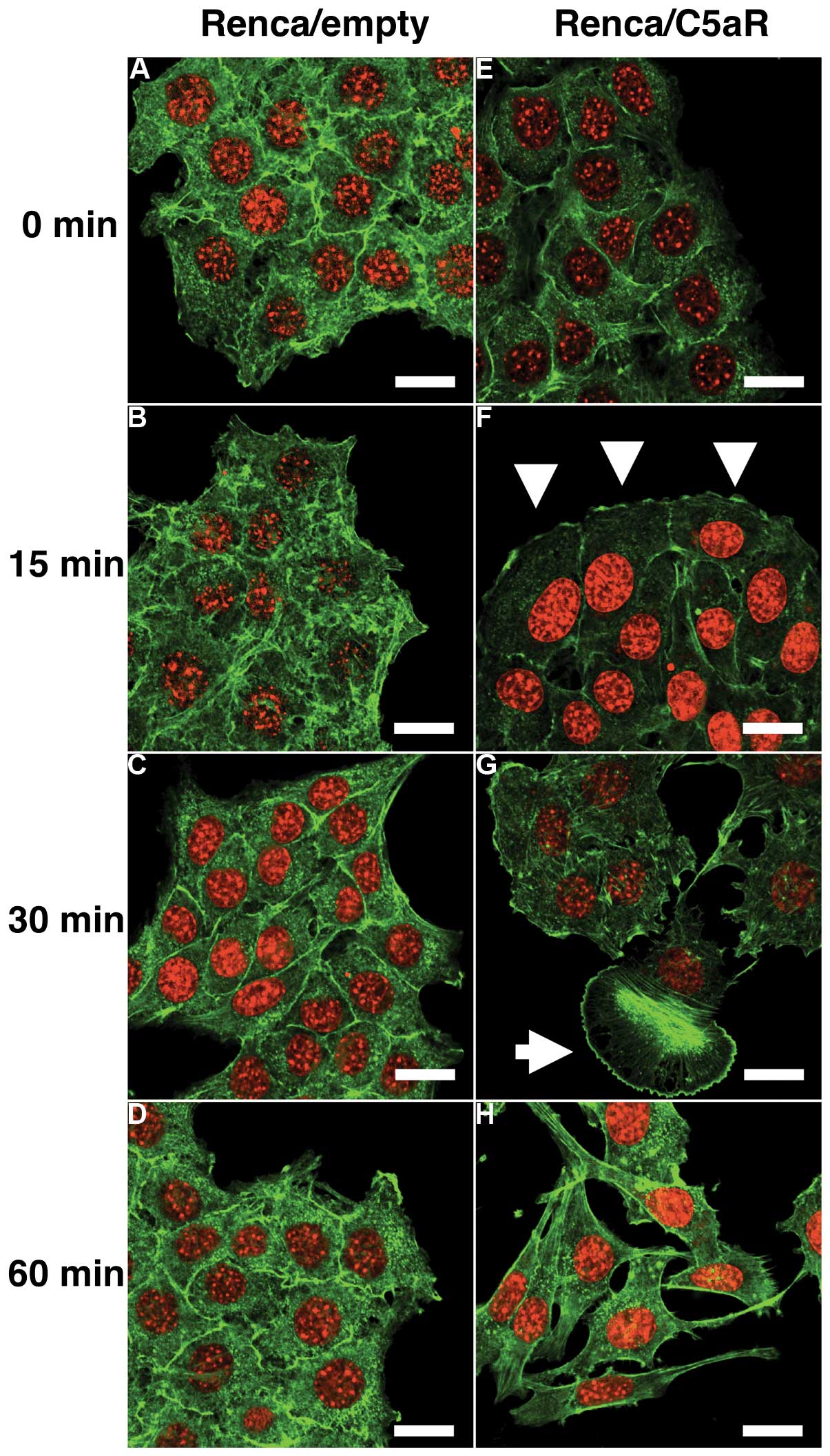
immunofluorescent staining. Without C5a stimulation, both
Renca/empty and Renca/C5aR cells showed staining of cortical
F-actin bundles at the borders of the cells (Fig. 3A and E). As early as 15 min after
C5a stimulation, Renca/C5aR cells revealed membrane ruffling
formation at the periphery of the cell clusters with reduced
F-actin bundles at the cell-cell border (Fig. 3F). Thirty minutes after stimulation,
the cell-cell contact became more loosened and some cells started
to manifest lamellipodia formation (Fig. 3G). This was followed by marked
change in cell shape such as stretched morphology and dissociation
of cell clusters accompanied by stress fiber formation (Fig. 3H). In contrast, Renca/empty cells
did not show any significant changes in both cytoskeleton and
cellular morphology during observation despite C5a stimulation
(Fig. 3A–D). These results suggest
that C5a elicits cytoskeletal rearrangement and cellular
morphological change in renal carcinoma cells via C5aR, leading to
their dissociation and scattering.
C5a-C5aR axis enhances Renca cell
invasion via the ERK and PI3K pathways
C5a is known to induce ERK (5,17) and
PI3K activation (6,18) in inflammatory/immune cells in a
C5aR-dependent manner, leading to manifestation of a variety of
biological phenomena including leukocyte migration (19). Since ERK and PI3K are crucial
mediators of extracellular stimuli-induced actin reorganization
(20,21) which was observed in Fig. 3, we investigated if C5a provokes ERK
and PI3K activation in C5aR-expressing renal carcinoma cells. As
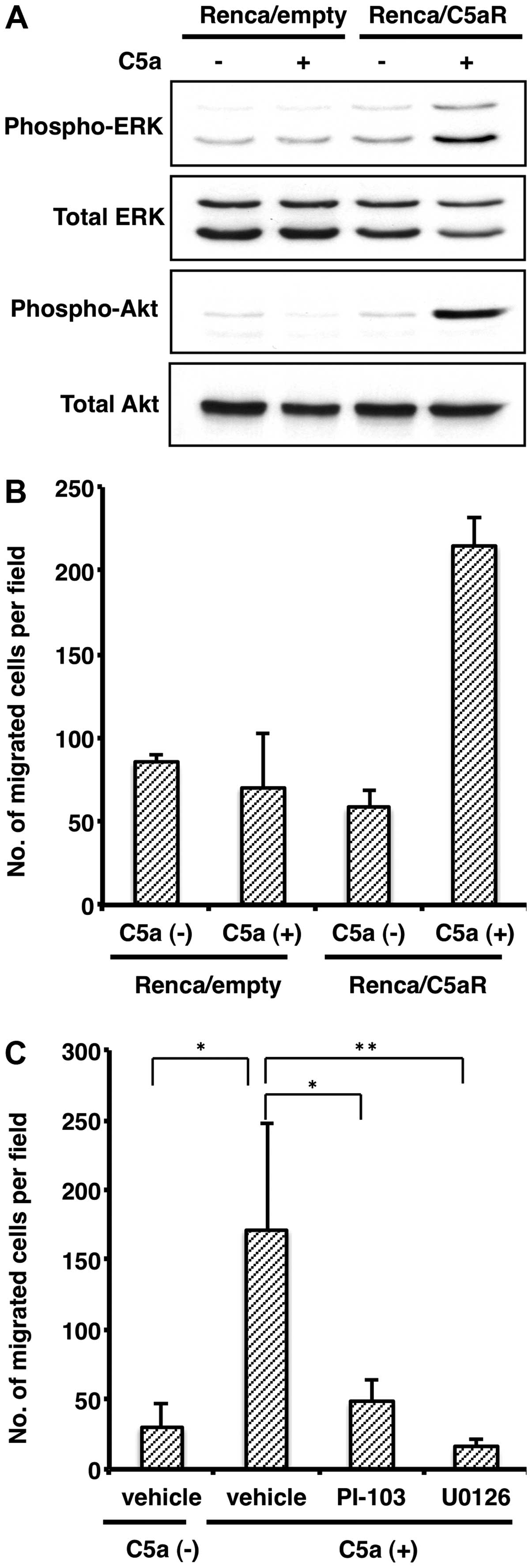
shown in Fig. 4A, Renca/C5aR cells
showed a robust increase in ERK and Akt, substrate of PI3K,
phosphorylation by C5a stimuli, whereas such phosphorylation did
not occur in the Renca-empty cells. This result indicates that the
C5a-C5aR axis can activate ERK and PI3K kinase pathways in renal
carcinoma cells. We previously reported that the C5a-C5aR axis
enhances cancer cell invasion both in vitro and in
vivo (9). To test if this is
also the case in renal carcinoma cells, we performed an invasion
assay using a Matrigel-coated Boyden chamber. Fig. 4B shows that C5a stimuli elicited
invasion of the Renca/C5aR cells, but not the Renca-empty cells, in
the Matrigel-coated boyden chamber, indicating that the C5a-C5aR
axis can enhance invasion of renal cell carcinoma as well. In
addition, treatment with either an MEK inhibitor U0126 or a PI3K
inhibitor PI-103 significantly diminished Renca cell invasion
promoted by the C5a-C5aR axis (Fig.
4C). This result indicates that the ERK and PI3K pathways are
indispensable for C5a-triggered C5aR-expressing renal carcinoma
cell invasion. All things considered, these results suggest that
the C5a-C5aR axis elicits renal carcinoma cell metastasis by
triggering actin reorganization and invasion induced by ERK and
PI3K pathway activation.
Discussion
C5aR was originally identified in cells with a
myeloid origin, and it has been shown by numerous studies that it
mediates a wide variety of biological phenomena in myeloid cells
induced by C5a such as leukocyte migration upon inflammation.
However, Cao et al (5)
reported an intriguing finding that the C5aR is also expressed in
epithelial cells, which suggested the involvement of C5aR in other
biological processes in non-myeloid cells. Recently, we showed that
C5aR is aberrantly expressed in various types of human cancers
(9), which was the first study
regarding the biological significance of C5aR expression in cancer
cells. In that study, we also showed that ~60% of RCC specimens
expressed C5aR. However, the sample number of RCC specimens in that
study was limited (n=11) and not sufficient to analyze the
relationship between C5aR expression status and clinical
parameters. In this study, we investigated 127 RCC samples using
immunohistochemistry and found a similar frequency of C5aR
expression (61.4%; 78/127) as in the previous study. It is of note
that around half of adjacent normal kidney tubular epithelial cells
already express C5aR (9), which was
confirmed in this expanded analysis (data not shown). At the
moment, the biological significance of C5aR expression in normal
renal tubular epithelial cells is unknown. The fact that around 40%
of RCC samples (49/127) did not express C5aR, implies that C5aR
could be a sublineage marker of renal tubular epithelial cells and
the expression status of C5aR in renal cell carcinoma may be
stochastic and reflect its sublineage rather than a consequence of
malignant transformation. Angelotti et al (22) reported that there are 2
subpopulations of renal progenitors with the potential to
regenerate tubular epithelial cells. It would be intriguing if
C5a-positive renal tubular epithelial cells represent either of
those populations.
In the study cohort we examined by
immunohistochemistry, C5aR-positive RCCs manifested both a locally
invasive and metastatic phenotype with a higher incidence of
microvascular invasion. In addition, we demonstrated that C5a-C5aR
axis activation elicited invasion using an in vitro invasion
assay with a renal carcinoma cell line. Hence C5aR appears to
facilitate local invasion to adjacent tissues and microvascular
infiltration thereby promoting distant metastasis of renal
carcinoma cells. Generally, metastasis requires a number of steps
including dissociation of cancer cells from primary sites, invasion
through basement membrane and into blood/lymphatic vessels,
survival when floating in blood/lymphatic stream, and implantation
and proliferation in distant target organs (23). From both clinical and experimental
results shown in the present study, it is plausible that C5aR
expression contributes at least to the invasion steps in this
metastasis model.
We performed renal subcapsular injection of
Renca-derived cells in BALB/c mice to examine if C5aR expression
promotes spontaneous renal carcinoma cell metastasis in a syngeneic
orthotopic murine model. Although we were able to observe lung
metastasis using this model as reported in the literature (11), we could not find any difference in
either the size or the number of metastatic foci in the lung
between Renca/empty and Renca/C5aR cells regardless of recombinant
C5a treatment. Our previous study showed an increased invasion of
subcutaneously injected C5aR-expressing HuCCT1 cells in nude mice
compared to HuCCT1 cells harboring empty plasmid (9). In the latter assay, the 2-day period
was sufficient to observe a significant increase in local invasion.
However, in the case of the syngeneic orthotopic murine model used
in the present study, it required up to 3 weeks to observe
spontaneous lung metastasis (11),
which may be sufficiently long to offset the effect of the
recombinant C5a stimulation to Renca/C5aR cells before injection.
In order to appropriately assess the effect of C5a-C5aR axis
activation on spontaneous metastasis in this murine model, improved
experimental methods may be required for sustained activation of
C5aR.
Acquisition of anoikis resistance by cancer cells is
also an important step for establishing cancer metastasis because
cancer cells have to survive in the floating condition during their
travel through the circulatory and lymphatic systems until
implantation to target organs (14). These facts led us to analyze further
whether C5a-C5aR axis activation contributes to this cellular
phenomenon. However, C5a stimulation did not have a significant
impact on anoikis resistance in the renal carcinoma cells
regardless of C5aR expression. Therefore we concluded that the
C5a-C5aR axis does not play a significant role in acquiring
anoikis-resistance in mRCC.
We showed that C5a induced dynamic reorganization of
actin cytoskeleton in the C5aR-expressing renal carcinoma cells,
namely, lamellipodia and stress fiber formation, resulting in
dissociation of cell clusters and scattering. It is well known that
these processes are mediated by activation of Rho family small G
proteins such as Rac1 and RhoA (24). Li et al (25) previously reported that C5a induced
activation of Rho family small G proteins in neutrophils, leading
to actin reorganization of the cell, suggesting that the C5a-C5aR
axis is one of the upstream switches of Rho family protein
activation. It would be of interest to analyze if Rho family
proteins are activated when actin rearrangement is induced in renal
carcinoma cells expressing C5aR by C5a stimulation.
In addition to inducing actin rearrangement, C5a
elicited invasion of C5aR-expressing renal carcinoma cells in a
Matrigel-coated Boyden chamber. Therefore, it is plausible that
C5a-C5aR may be involved in the metastasis of renal cell carcinoma
by prompting dissociation of cancer cells from primary sites and
invasion through the basement membrane. Furthermore, C5a
stimulation activated the ERK and PI3K pathways and inhibition of
these kinase pathways by specific inhibitors negated the
C5aR-expressing renal carcinoma cell invasion. These pathways are
known to be activated by C5a stimulation in cells with myeloid
origin to regulate numerous biological phenomena (19). In the present study, we showed that
such activation does occur in renal carcinoma cells. This is the
first study to show that the C5a-C5aR axis does trigger activation
of these kinase pathways and invasion in cancer cells. Campbell
et al (26) reported that
phosphorylated ERK is an independent prognostic biomarker that
significantly predicts the onset of metastasis in clinically
confined RCC, and Horiguchi et al (27) showed that phosphorylated Akt is
significantly associated with RCC metastasis. These studies are
consistent with our finding that C5aR, which can trigger ERK and
PI3K activation by C5a stimulation, is expressed in a vast majority
of clinical mRCC specimens.
The present study provides the proof-of-concept that
the C5a-C5aR axis can be a novel target for preventing renal cell
carcinoma progression as well as further support of the current
therapeutic concept to target the ERK and PI3K/mTOR pathways in
mRCC (28). Clinical application of
this concept may contribute to develop novel therapeutic strategies
for advanced RCC in the future.
Acknowledgements
We thank S. Nakata, M. Matsumoto and T. Kubo for
technical assistance. We are also grateful to K. Yoshinobu, The
Gene Technology Center in Kumamoto University for her important
contributions to the immunofluorescence experiments.
References
|
1
|
Zisman A, Pantuck AJ, Wieder J, et al:
Risk group assessment and clinical outcome algorithm to predict the
natural history of patients with surgically resected renal cell
carcinoma. J Clin Oncol. 20:4559–4566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wada Y, Takahashi W, Kawano Y and Eto M:
Current status of pharmacotherapy against metastatic renal cell
carcinoma in Japan. Int J Urol. 19:284–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Müller A, Homey B, Soto H, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo RF and Ward PA: Role of C5a in
inflammatory responses. Annu Rev Immunol. 23:821–852. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao Q, McIsaac SM and Stadnyk AW: Human
colonic epithelial cells detect and respond to C5a via apically
expressed C5aR through the ERK pathway. Am J Physiol Cell Physiol.
302:C1731–C1740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwan WH, van der Touw W, Paz-Artal E, Li
MO and Heeger PS: Signaling through C5a receptor and C3a receptor
diminishes function of murine natural regulatory T cells. J Exp
Med. 210:257–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiou WF, Tsai HR, Yang LM and Tsai WJ:
C5a differentially stimulates the ERK1/2 and p38 MAPK
phosphorylation through independent signaling pathways to induced
chemotactic migration in RAW264.7 macrophages. Int Immunopharmacol.
4:1329–1341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsai HR, Yang LM, Tsai WJ and Chiou WF:
Andrographolide acts through inhibition of ERK1/2 and Akt
phosphorylation to suppress chemotactic migration. Eur J Pharmacol.
498:45–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nitta H, Wada Y, Kawano Y, et al:
Enhancement of human cancer cell motility and invasiveness by
anaphylatoxin C5a via aberrantly expressed C5a receptor (CD88).
Clin Cancer Res. 19:2004–2013. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawano Y, Diez S, Uysal-Onganer P,
Darrington RS, Waxman J and Kypta RM: Secreted frizzled-related
protein-1 is a negative regulator of androgen receptor activity in
prostate cancer. Br J Cancer. 100:1165–1174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shvarts O, Janzen N, Lam JS, et al:
RENCA/carbonic anhydrase-IX: a murine model of a carbonic
anhydrase- IX-expressing renal cell carcinoma. Urology.
68:1132–1138. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berezovskaya O, Schimmer AD, Glinskii AB,
et al: Increased expression of apoptosis inhibitor protein XIAP
contributes to anoikis resistance of circulating human prostate
cancer metastasis precursor cells. Cancer Res. 65:2378–2386. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eto M, Kamiryo Y, Takeuchi A, et al:
Posttransplant administration of cyclophosphamide and donor
lymphocyte infusion induces potent antitumor immunity to solid
tumor. Clin Cancer Res. 14:2833–2840. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Frisch SM and Screaton RA: Anoikis
mechanisms. Curr Opin Cell Biol. 13:555–562. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Banks P, Barker MD and Burton DR:
Recruitment of actin to the cytoskeletons of human monocyte-like
cells activated by complement fragment C5a. Is protein kinase C
involved? Biochem J. 252:765–769. 1988.PubMed/NCBI
|
|
16
|
Monk PN and Banks P: Evidence for the
involvement of multiple signalling pathways in C5a-induced actin
polymerization and nucleation in human monocyte-like cells. J Mol
Endocrinol. 6:241–247. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li K, Fazekasova H, Wang N, et al:
Functional modulation of human monocytes derived DCs by
anaphylatoxins C3a and C5a. Immunobiology. 217:65–73. 2012.
View Article : Google Scholar
|
|
18
|
Bosmann M, Patel VR, Russkamp NF, et al:
MyD88-dependent production of IL-17F is modulated by the
anaphylatoxin C5a via the Akt signaling pathway. FASEB J.
25:4222–4232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Don MJ, Liao JF, Lin LY and Chiou WF:
Cryptotanshinone inhibits chemotactic migration in macrophages
through negative regulation of the PI3K signaling pathway. Br J
Pharmacol. 151:638–646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mendoza MC, Er EE, Zhang W, et al:
ERK-MAPK drives lamellipodia protrusion by activating the WAVE2
regulatory complex. Mol Cell. 41:661–671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Posern G, Saffrich R, Ansorge W and Feller
SM: Rapid lamellipodia formation in nerve growth factor-stimulated
PC12 cells is dependent on Rac and PI3K activity. J Cell Physiol.
183:416–424. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Angelotti ML, Ronconi E, Ballerini L, et
al: Characterization of renal progenitors committed toward tubular
lineage and their regenerative potential in renal tubular injury.
Stem Cells. 30:1714–1725. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hall A: Rho GTPases and the actin
cytoskeleton. Science. 279:509–514. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Z, Hannigan M, Mo Z, et al: Directional
sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent
activation of Cdc42. Cell. 114:215–227. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Campbell L, Nuttall R, Griffiths D and
Gumbleton M: Activated extracellular signal-regulated kinase is an
independent prognostic factor in clinically confined renal cell
carcinoma. Cancer. 115:3457–3467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Horiguchi A, Oya M, Uchida A, Marumo K and
Murai M: Elevated Akt activation and its impact on
clinicopathological features of renal cell carcinoma. J Urol.
169:710–713. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Figlin RA, Kaufmann I and Brechbiel J:
Targeting PI3K and mTORC2 in metastatic renal cell carcinoma: new
strategies for overcoming resistance to VEGFR and mTORC1
inhibitors. Int J Cancer. 133:788–796. 2013. View Article : Google Scholar : PubMed/NCBI
|