Introduction
Cervical cancer is the fourth most prevalent cause
of cancer-related mortality in women worldwide and 12,360 estimated
new cases of cervical cancer were diagnosed in 2014, with 4,020
estimated deaths in the USA (1).
Significant advances concerning the molecular mechanisms of
cervical carcinogenesis have been made during the last several
decades (2). However, the detailed
mechanisms of cervical cancer initiation and progression have yet
to be fully elucidated. Persistent infection with high-risk human
papilloma virus (HR-HPV) has been proven to be the main cause of
almost all types of cervical cancer. However, a substantial body of
evidence shows that HR-HPV infection alone is not sufficient to
induce malignant transformation, indicating that other genetic
alterations may be involved in cervical carcinogenesis (3). Identification of key factors in
cervical cancer is important for the screening, diagnosis and
treatment of cervical cancer.
The phosphoinositide 3-kinase (PI3K)/Akt signaling
pathway appears to be a key regulator in cervical carcinogenesis,
as it is activated in >90% of cervical cancer types (4). Akt signaling is the downstream target
of HPV oncoproteins which have been identified as major mediators
of cervical cancer initiation and development (5). Gene expression profiling also
demonstrated that the PI3K/Akt signaling pathway may be of
potential therapeutic target in cervical cancer (6). The phosphatase and tensin homolog
deleted on chromosome 10 (PTEN) protein is principally involved in
the homeostatic maintenance of the PI3K/Akt signaling pathway
(7). Findings of a previous study
showed that loss of PTEN resulted in persistent activation of PI3K
effectors which has an important impact on various aspects of
cancer development such as cell proliferation, cell cycle, cell
migration and metastasis (8). It
has been demonstrated that abnormal promoter methylation of the
PTEN gene was usually identified in cervical cancer and
associated with tumor differentiation, lymph-node metastasis and
FIGO staging. PTEN was identified to be important in the occurrence
and development of cervical cancer (9). Therefore, identification of the
molecular regulating PTEN expression may be an attractive strategy
for elucidating the underlying mechanism of cervical
carcinogenesis.
MicroRNAs (miRNAs) are small non-coding RNAs of 22
nucleotides in length, transcribed from non-protein coding genes or
introns, which generally act as negative regulators of gene
expression at post-transcriptional levels through mRNA degradation
and translation repression (10).
Accumulating evidence has shown that the aberrant expression of
miRNAs may function as tumor suppressors or oncogenes in cancers
according to the role of their target genes (11), which indicates miRNAs have the
potential to be diagnostic and prognostic biomarkers for cancer
(12). miR-494 has consistently
been reported to be aberrantly expressed in various types of
cancer. The functional role of miR-494 is extremely complex as it
may function as an oncogenic or tumor suppressive miRNA depending
on the cellular microenvironments (13–17).
More importantly, it has been reported that miR-494 participated in
the carcinogenesis and development of colorectal cancers by
directly targeting PTEN (18).
Aberrant miRNA expression profiles have also been identified in
cervical cancer cell lines and cervical cancer tissues (19,20).
However, to the best of our knowledge, there are few detailed
studies focusing on the role of miR-494 in cervical cancer. Given
the complexity of its functionality, it would be of interest to
explore the functional roles and relationship of miR-494 and PTEN
in cervical cancer carcinogenesis and development.
In the present study, we analyzed the expression of
miR-494 in cervical cancer cell lines and clinical specimens, and
examined the association of miR-494 with PTEN expression and
clinicopathological data of cervical cancer patients. In
vitro experiments showed that inhibition of miR-494 suppressed
cell proliferation and growth by directly targeting the
3′-untranslated region (3′-UTR) of PTEN mRNA. These findings
identified a novel molecular mechanism involved in the regulation
of PTEN expression and cervical cancer progression. Thus, targeting
miR-494 may be a promising therapeutic strategy for the treatment
of cervical cancer.
Materials and methods
Patients and tissue specimens
The tissue-based specimen collection and study were
approved by the Research Ethics Committee of Xi’an Jiaotong
University. All the patients provided written consent and indicated
willingness to donate their blood and tissue samples. A total of 89
patients were enrolled in the present study. Clinical and
pathological classification and staging were performed according to
the International Federation of Gynecology and Obstetrics criteria
(21). The clinicopathological
information of the patients is shown in Table I. The follow-up information for all
participants was updated every 3 months by telephone. Information
regarding the death of patients was ascertained from their family.
In all 89 snap-frozen cervical cancer samples, the HC2 assay was
used to detect the presence of high-risk HPV DNA, including DNA
from HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and
68 (22). High-risk HPV (HR-HPV)
was detected in 79 cases, which gave an overall infection rate of
88.8%.
 | Table IAssociation between miR-494
expression and clinico-pathological characteristics. |
Table I
Association between miR-494
expression and clinico-pathological characteristics.
|
Characteristics | No. | miR-494 expression
| P-value |
|---|
| Low | High |
|---|
| Age (years) | | | | 0.666 |
| ≤35 | 35 | 16 | 19 | |
| >35 | 54 | 28 | 26 | |
| FIGO stage | | | | 0.026a |
| IB | 58 | 34 | 24 | |
| >IB | 31 | 10 | 21 | |
| HR-HPV | | | | 0.091 |
| Yes | 79 | 38 | 41 | |
| No | 10 | 8 | 2 | |
|
Differentiation | | | | 0.833 |
| Well | 41 | 21 | 20 | |
| Moderate/poor | 48 | 23 | 25 | |
| Tumor size | | | | 0.477 |
| ≤4 cm | 70 | 33 | 37 | |
| >4 cm | 19 | 11 | 8 | |
| LN metastasis | | | | 0.027a |
| Yes | 78 | 35 | 43 | |
| No | 11 | 9 | 2 | |
| Stromal
invasion | | | | 0.045a |
| <2/3 | 68 | 38 | 30 | |
| ≥2/3 | 21 | 6 | 15 | |
Cell culture
Primary normal cervical epithelial cells (NCEC)
obtained from healthy female cervical tissue were cultured in
keratinocyte serum-free medium (Invitrogen, Carlsbad, CA, USA)
supplemented with epithelial growth factor, bovine pituitary
extract and antibiotics (1% streptomycin and 1% penicillin). The
HeLa, C33A, Caski and SiHa cervical cancer cell lines were grown in
Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen). The cells
were supplemented with 10% fetal bovine serum (FBS) (HyClone,
Logan, UT, USA) and 1% penicillin/streptomycin (Invitrogen).
Reverse transcriptase-quantitative PCR
(RT-qPCR) assay
The expression of miR-494 in cervical cancer and
corresponding adjacent tissues was detected by the RT-qPCR assay.
Briefly, total RNA was extracted from tissues using TRIzol reagent
(Invitrogen) according to the manufacturer’s instructions. miRNA
expression levels were quantified using a TaqMan miRNA real-time
RT-PCR kit (Applied Biosystems, Foster City, CA, USA) according to
the manufacturer’s instructions. Data were analyzed using 7500
software v. 2.0.1 (Applied Biosystems), with the automatic Ct
setting for adapting the baseline and threshold for Ct
determination. The universal small nuclear RNA U6 (RNU6B) was used
as an endogenous control for miRNAs. Each sample was examined in
triplicate and the amount of PCR products produced was
non-neoplasticized to RNU6B.
Oligonucleotide transfection
miR-494 inhibitors were chemically synthesized by
Shanghai GenePharma (GenePharma, Shanghai, China). When the cells
reached 80% confluence, miR-494 inhibitor was transfected into
cervical cancer cells with Lipofectamine 2000 (Invitrogen)
according to the manufacturer’s instructions. The cells were also
transfected with scramble oligonucleotide as a negative control
(NC). The expression level of mir-494 in the transfected
osteosarcoma cells were identified by RT-qPCR.
Luciferase reporter assay
Cervical cancer cells were seeded in 96-well plates
at 60% confluence. After 24 h, the cells were transfected with 120
ng of miR-494 expression vector or NC. The cells were transfected
with 30 ng of wild-type (WT) or mutant (MT) 3′-UTR of PTEN mRNA.
The cells were collected 48 h after transfection, and luciferase
activity was measured using a dual luciferase reporter assay system
according to the manufacturer’s instructions (Promega, Madison, WI,
USA).
Cell viability assay
Cells were plated in 96-well plates
(0.5×104 cells/well) and transfected with NC, and
miR-494 inhibitors. After 48 h, 10 μl of MTT reagent (5
mg/ml) was added to each well and the cells were incubated at 37°C
for another 4 h. The medium was removed, the cells were solubilized
in 150 μl of dimethyl sulfoxide, and colorimetric analysis
was performed (wavelength, 490 nm). One plate was analyzed
immediately after the cells adhered (~4 h after plating), and the
remaining plates were assayed every day for the following 4
consecutive days.
Colony formation assay
Briefly, 10 cm dishes were seeded with 500 viable
cells in complete medium and allowed to grow for 24 h. The cells
were then incubated in the presence of miR-494 inhibitors or NC for
up to 48 h. The medium was removed, and the cells were washed in
phosphate-buffered saline (PBS) and incubated for an additional 10
days in complete medium. Each treatment was carried out in
triplicate. The colonies obtained were washed with PBS and fixed in
4% formalin for 10 min at room temperature and then washed with PBS
followed by staining with 0.2% crystal violet.
Soft agar colony formation assay
Cells seeded in a 6-well plate were covered with a
layer of 0.6% agar in DMEM medium supplemented with 10% FBS. After
transfection for 48 h, the cells were trypsinized, gently mixed
with 0.3% agar medium mixture containing selective antibiotics and
reseeded in triplicate in a 6-well plate. After 4 weeks, the
resistant colonies were stained with 0.2% crystal violet and
counted under the microscope.
Flow cytometric analysis of cell
cycle
The cervical cancer cells were transfected with NC
and miR-101 inhibitors. Forty-eight hours after post-transfection,
the cells were trypsinized and analyzed for cell cycle
distribution. For cell cycle distribution, the cells of each group
were stained with propidium iodide (PI) and analyzed by flow
cytometry using FACSCalibur (BD Biosciences, San Diego, CA, USA).
For each group, 10,000 events were obtained. The percentage of
cells in G1, S and G2 phases of the cell cycle was calculated.
Statistical analysis
Data are presented as mean ±SD. Statistical analysis
was performed using IBM SPSS statistical software (version 21.0)
(International Business Machines Corporation, Armonk, NY, USA). The
differences in characteristics between the two groups were examined
by the χ2 or Fisher’s exact tests. P-values were
determined from two-sided tests, and statistical significance was
based on a P-value of 0.05.
Results
miR-494 is upregulated in cervical cancer
cell lines and tissues
To examine the levels of miR-494 expression in
cervical cancer, we conducted RT-qPCR to measure miR-494 expression
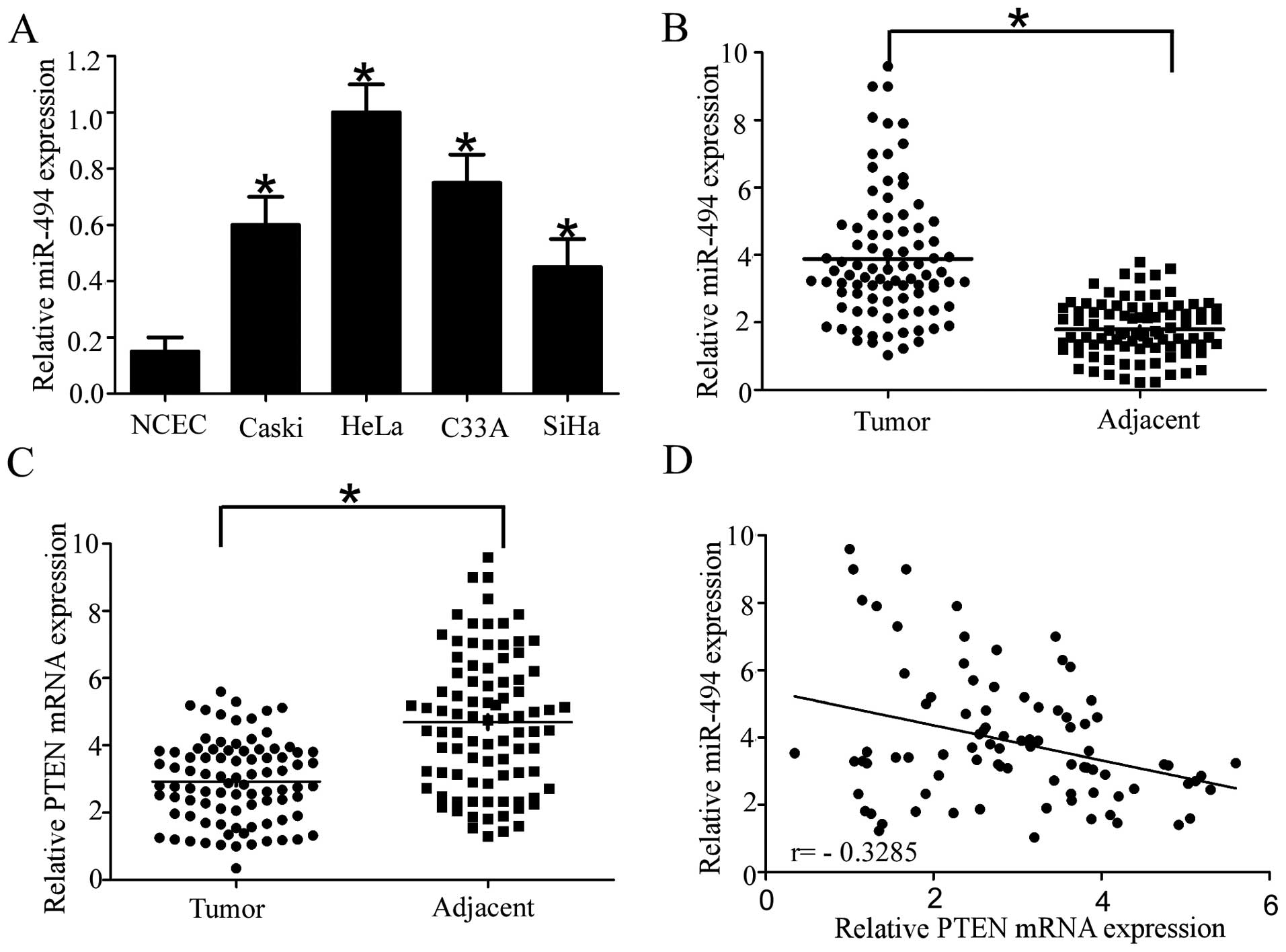
in four cervical cancer cell lines and NCEC. The result showed that
miR-494 was markedly increased in the Caski, HeLa, C33A and SiHa
cervical cell lines, particularly in HeLa and C33A, compared with
NCEC (Fig. 1A). Consistent with the
results found in cervical cell lines, miR-494 expression was
significantly higher in 89 cervical cancer tissue specimens
compared with their adjacent normal tissues (Fig. 1B). By contrast, the expression of
PTEN was significantly down-regulated in cervical cancer tissues
compared with their normal tissue counterparts (Fig. 1C), which was consistent with
previous literature (23). More
importantly, statistically significant inverse correlations were
revealed by Spearman’s correlation analysis between mRNA levels of
miR-494 and PTEN in cervical cancer specimens (r=−0.3285;
P=0.0017). Taken together, the results suggested that miR-494
played an oncogenic role and PTEN a tumor-suppressor role in
cervical cancer. Furthermore, miR-494 inversely correlated with
PTEN in cervical cancer, which indicated that PTEN was a potential
target of miR-494 in cervical cancer.
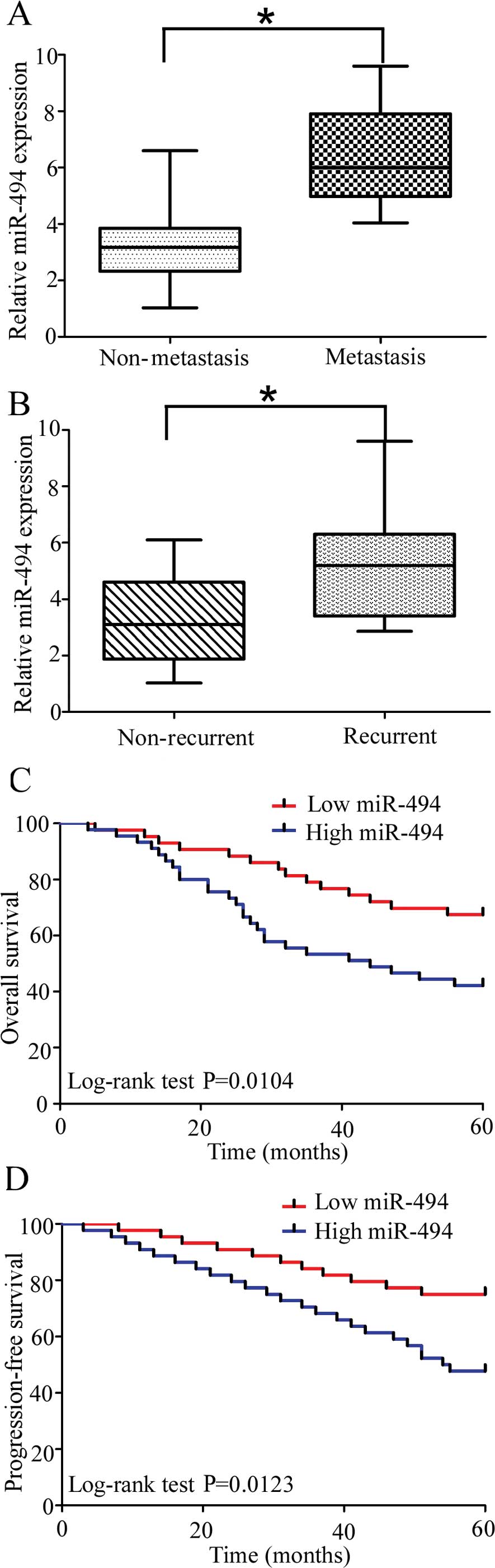
Upregulation of miR-494 is associated
with metastasis and recurrence in cervical cancer patients
To explore the relationship between miR-494 and
cervical cancer, we investigated the correlation of miR-494
expression with metastasis and recurrence of cervical cancer.
Compared with non-metastatic cervical cancer specimens, the miR-494
levels were significantly upregulated in metastatic cervical
tissues (Fig. 2A). Moreover,
miR-494 levels were significantly higher in the specimens obtained
from the patients who suffered cervical cancer recurrence (Fig. 1B). Collectively, these data
indicated that significantly upregulation of miR-494 expression was
correlated with relapse and metastasis in cervical cancer
patents.
miR-494 expression is correlated with
clinicopathological characteristics and prognosis in cervical
cancer patients
In order to determine the clinical significance of
miR-494 in cervical cancer, the 89 patients were divided into two
groups based on miR-494 expression levels (low vs. high) with the
median expression levels as a cut-off point. The Kaplan-Meier
analysis revealed that high miR-494 expression was significantly
correlated with reduced overall and progression-free survival in 89
cervical cancer patients (Fig. 2C
and D; log-rank test, P=0.0104 and P=0.0123, respectively). The
patients with a high miR-494 expression tended to have a shorter
overall and progression-free survival time when compared to
patients with a low miR-494 expression. In addition, upregulation
of miR-494 was significantly correlated with FIGO stage, lymph-node
metastasis and deep stromal invasion while no significant
correlation was observed in other clinicopathological variables
(Table I). The, univariate analysis
demonstrated that the overall and progression-free survival of
cervical cancer patients was associated with FIGO stage, lymph-node
status, and HR-HPV and miR-494 expression (Tables II and III). To determine whether the prognostic
value of miR-494 was independent of other clinicopathological
parameters for poor overall and progression-free survival in
cervical cancer patients, a multivariate analysis was performed
using a Cox proportional hazard model. The multivariate analysis
including miR-494 expression, age, FIGO stage, HR-HPV,
differentiation status, tumor size and lymph-node metastasis
demonstrated that a high miR-494 expression was an independent
prognostic biomarker for poor overall and progression-free survival
in cervical cancer patients (Tables
II and III; HR=3.279,
CI=1.177–5.192, P=0.013 and HR=4.614, CI=2.895–10.321, P<0.001
respectively). Statistically significant results were also obtained
for FIGO stage and lymph-node metastasis, where the other
parameters were not independent prognostic biomarkers for overall
and progression-free survival in cervical cancer patients. Taken
together, these results suggest the upregulation of miR-494 was
significantly correlated with a worse prognosis and was involved in
the progression of cervical cancer.
 | Table IIUnivariate and multivariate analyses
of clinical parameters in relation to overall survival. |
Table II
Univariate and multivariate analyses
of clinical parameters in relation to overall survival.
| Variables | Univariate
| Multivariate
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| miR-494
expression | 4.143
(1.751–6.397) | 0.009a | 3.279
(1.177–5.192) | 0.013a |
| Age (years) | 2.145
(0.745–2.451) | 0.658 | 1.784
(0.874–2.175) | 0.791 |
| FIGO stage | 4.156
(2.209–5.167) | 0.017a | 3.516
(2.124–5.349) | 0.009a |
| HR-HPV | 3.129
(1.296–4.719) | 0.045a | 2.891
(1.152–4.325) | 0.021a |
|
Differentiation | 1.819
(0.742–2.795) | 0.209 | 2.113
(0.696–2.782) | 0.491 |
| Tumor size | 1.361
(0.534–1.987) | 0.419 | 1.542
(0.759–2.175) | 0.219 |
| LN metastasis | 3.714
(1.892–5.562) | 0.017a | 4.115
(1.579–6.123) | 0.008a |
| Stromal
invasion | 1.193
(0.415–1.987) | 0.118 | 1.453
(0.879–2.161) | 0.374 |
 | Table IIIUnivariate and multivariate analyses
of clinical parameters in relation to progression-free
survival. |
Table III
Univariate and multivariate analyses
of clinical parameters in relation to progression-free
survival.
| Variables | Univariate
| Multivariate
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| miR-494
expression | 4.891
(2.425–6.257) | 0.007* | 4.614
(2.895–10.321) | <0.001a |
| Age (years) | 1.427
(0.628–2.162) | 0.351 | 1. 891
(0.898–2.477) | 0.519 |
| FIGO stage | 3.451
(1.679–4.129) | 0.029a | 2.915
(1.789–4.187) | 0.011a |
| HR-HPV | 3.198
(1.589–5.245) | 0.014a | 2.941
(1.497–4.827) | 0.008a |
|
Differentiation | 1.813
(0.741–2.514) | 0.295 | 2.161
(0.819–3.255) | 0.417 |
| Tumor size | 1.429
(0.711–2.287) | 0.342 | 1.827
(0.717–3.165) | 0.417 |
| LN metastasis | 5.104
(1.998–10.179) | 0.029a | 4.219
(2.326–7.619) | 0.031a |
| Stromal
invasion | 1.355
(0.611–2.341) | 0.173 | 1.625
(0.681–2.749) | 0.251a |
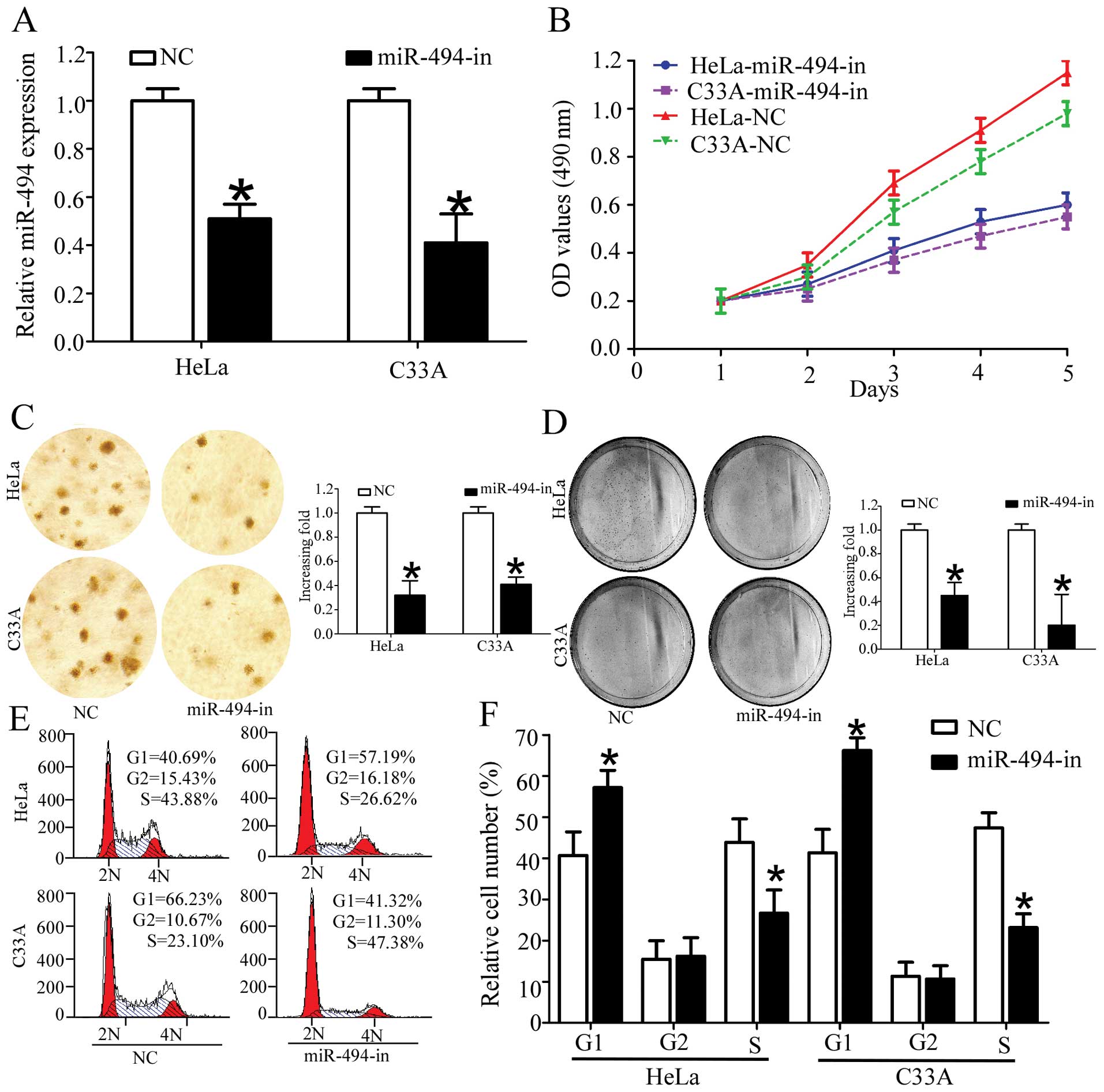
miR-494 promotes the proliferation of
cervical cancer cells
As the relative expression of miR-494 was relatively
higher in HeLa and C33A than SiHa and Caski, we chose HeLa and C33A
to investigate the physiological function of miR-494 in cervical
cancer cells. To analyze the effect of miR-494 on the proliferation
of cervical cancer cells, we transfected miR-494 inhibitors into
HeLa and C33A cell lines. As shown in Fig. 3A, transfection of miR-494 inhibitors
decreased the miR-494 expression in HeLa and C33A (Fig. 3A). After confirming the efficiency
of miR-494 inhibitors, we determined the effects of miR-494 on cell
viability using an MTT assay. Cervical cancer cells transfected
with miR-494 inhibitors showed a significant decrease in cell
viability as compared with the normal control (Fig. 3B). We determined the effect of
miR-494 on cell proliferation using colony formation and soft agar
colony formation assays. As shown in Fig. 4C and D, inhibition of miR-494
significantly decreased the growth rate of the two cervical cell
lines as compared with the normal control (Fig. 4C and D). Taken together, these
results indicated that the downregulation of miR-494 suppressed the
proliferation of cervical cancer cells.
Effect of miR-494 on cell cycle in
vitro
As miR-494 significantly affects cell proliferation
in HeLa and C33A cells, we hypothesized that miR-494 functions by
affecting the cell cycle of cervical cancer cells. Thus, we
investigated the effect of miR-494 on the cell cycle by flow
cytometry. The results revealed that overexpression of miR-494
inhibitors markedly increased the number of cells in G1 peak and
decreased those in the S peak (Fig.
3E and F). Taken together, these results indicated the
inhibition of miR-494 suppressed the proliferation of cervical
cancer cells by inducing cell cycle arrest.
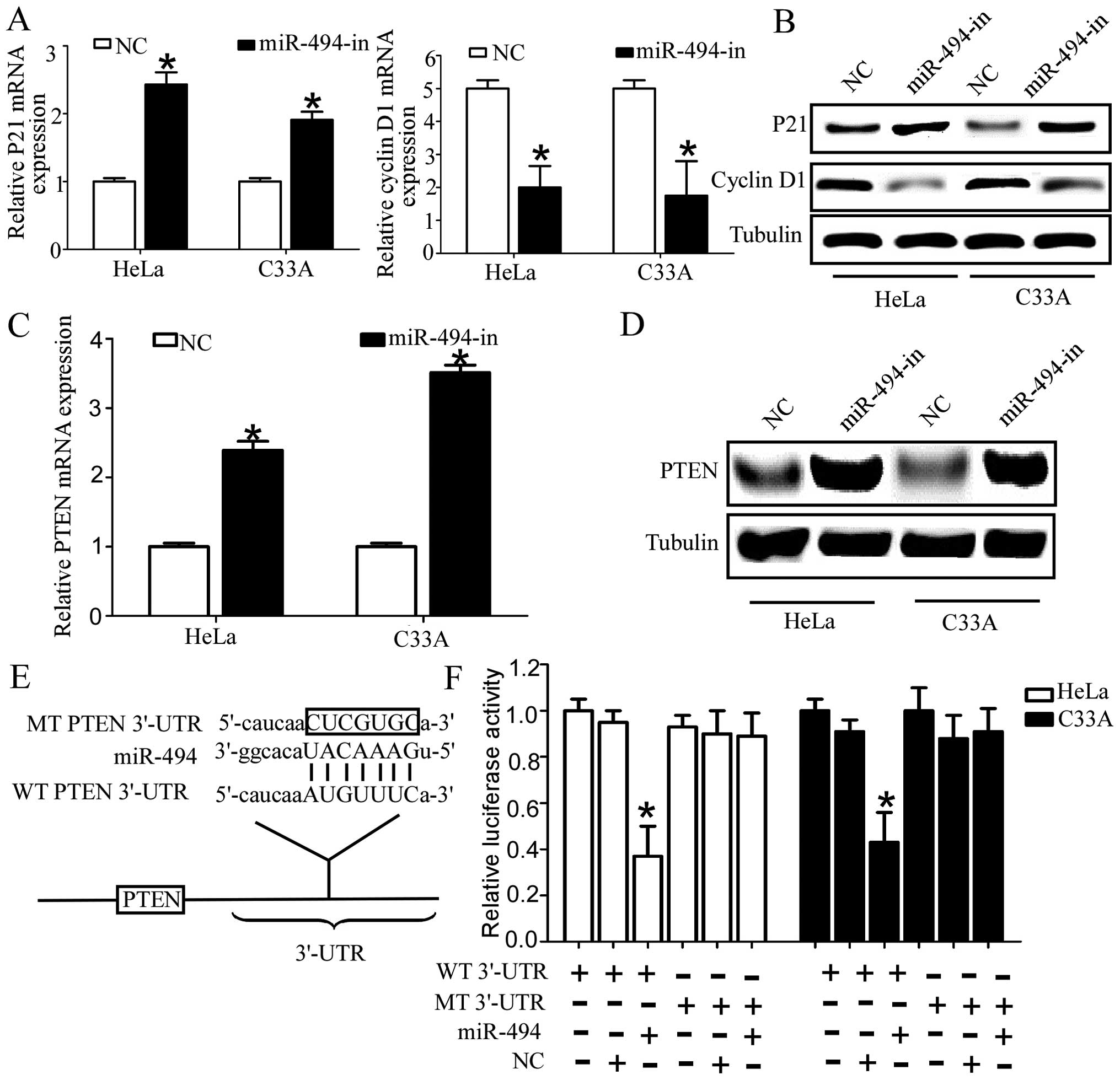
Inhibition of miR-494 increases cell
cycle inhibitors p21Cip1 and decreases cell cycle
regulator cyclin D1
As overexpression of miR-494 inhibitors appears tobe
closely linked to the proliferation of cervical cancer cells, we
further investigated whether the CDK inhibitor p21Cip1
or the CDK regulator cyclin D1 could be regulated by miR-494.
RT-qPCR and western blot analysis revealed that p21Cip1
was upregulated, whereas cyclin D1 was downregulated in cervical
cells transfected with miR-494 inhibitors compared with cells
transfected with the normal control (Fig. 4A and B). Taken together, these
results supported our hypothesis that miR-494 has a critical role
in the growth of cervical cancer cells.
PTEN is the direct target of miR-494 in
cervical cancer cells
It has been proven that PTEN was the direct target
of miR-494 in multiple solid tumors (24), and loss of protein expression of
PTEN was involved in the pathogenesis, proliferation and metastasis
of cervical cancer (25,26). Considering the tissue-specific and
developmental stage-specific manner of miRNA, we investigated the
relationship between PTEN and miR-494 in cervical cancer. In order
to confirm PTEN is the target gene for miR-494 in cervical cancer
cells, RT-qPCR and western blotting was used to detect the
expression of PTEN in HeLa and C33A. As expected, the expression of
PTEN at the mRNA and protein level was significantly upregulated in
cervical cancer cells transfected with miR-494 inhibitors (Fig. 4C and D). Our previous results
demonstrated the mRNA of PTEN was inversely correlated with miR-494
expression (Fig. 1D). Taken
together, these results suggested that PTEN was the potential
target gene of miR-494 in cervical cancer cell lines and
tissues.
Then we performed the luciferase reporter assay to
further verify whether miR-494 directly targeted the 3′-UTR of PTEN
in cervical cancer cells. The target sequence of wild-type PTEN
3′-UTR (WT 3′-UTR) or the mutant PTEN 3′-UTR (MT 3′-UTR) was cloned
into a luciferase reporter vector (Fig.
4E). As shown in Fig. 4F,
transfection of miR-494 consistently suppressed the luciferase
activity of PTEN WT3′UTR luciferase reporter plasmids in HeLa and
C33A cells, whereas point mutations in the miR-494-binding seed
region of the PTEN abrogated the repressive effect of miR-494.
Taken together, the data suggested that PTEN was a genuine target
of miR-494.
Discussion
In the present study, miR-494 expression was
significantly upregulated in human cervical cancer cell lines and
tissues. miR-494 upregulation was also significantly associated
with PTEN downregulation, adverse clinicopathological
characteristics, poor overall and progression-free survival, and
poor prognosis. In addition, inhibition of miR-494 expression
induced cell cycle arrest in G1 stage and inhibited cell
proliferation and cell growth in cervical cancer cell lines.
Additional in vitro studies showed that PTEN was the direct
target of miR-494 in cervical cancer cells. Results of the present
study show that miR-494 may have an essential role in the
carcinogenesis and progression of cervical cancer.
Several miRNAs have been identified as candidate
components of oncogene and tumor suppressor networks in cervical
cancer, and these miRNAs and their targets play critical roles in
the carcinogenesis and progression of cervical cancer. For example,
miR-135a/SIAH1/β-catenin signaling functions as an oncogene in the
transformation and progression of cervical cancer (27). Similarly, miR-31, miR-155 and
miR-1246 are found to promote cervical cancer cell proliferation
and function as oncomiRs in cervical cancer (28–30).
However, miR-507, miR-99a and miR-99b act as tumor suppressors in
cervical cancer and inhibit cervical cancer cell proliferation and
cell growth (31,32). Classical tumor suppressor miR-101
induced cell cycle arrest by targeting Fos (33). However the role, mechanism and
clinical significance of miR-494 in cervical cancer have not been
further reported, since whether miR-494 is an oncogenic or tumor
suppressor miRNA remains to be determined.
Accumulating evidence suggested that the functions
of miR-494 in cancer development are complicated. Upregulation of
miR-494 has been proven to be associated with promotion in cell
proliferation and cell growth in H460 lung and breast cancer cells,
colorectal cancer, hepatocellular carcinoma and transformed
bronchial epithelial cells (18,24,34–36).
However, miR-494 functions as a tumor suppressor and induces cell
cycle arrest in lung, gastric and prostate cancer, and
cholangiocarcinoma (17,37,38).
Different tumor microenvironments, cellular contexts, tissue
specificity and molecules which miR-494 targeted account for this
discrepancy. As the effect of miR-494 in cervical cancer was far
from defined, the present study aimed to investigate the potential
biological function of miR-494 in cervical cancers. Our results
demonstrate that suppression of miR-494 significantly inhibited the
cell proliferation and cell growth of HeLa and C33A cells by
induction of cell cycle arrest. More importantly, miR-494 was
significantly correlated with adverse clinicopathological features,
poor survival and prognosis of cervical cancer patients. All these
results suggest that miR-494 may function as an oncogenic miRNA in
the initiation and progression of cervical cancer. Of note, the
present study further supported the hypothesis that various
functions of miRNA, including miR-494, in different types of cancer
were dependent on the cancer type and cellular context (28).
To address the molecular mechanism involved in
miR-494-mediated changes of biological properties, PTEN was
selected for further study. The PTEN gene, known as mutated
in multiple advanced cancer 1 (MMAC1), is a classic
tumor-suppressor gene located at chromosome 10q23.31 (39). The PTEN protein is principally
involved in the homeostatic maintenance of PI3K/Akt signaling
(40). PTEN/PI3K/Akt is highly
involved in carcinogenesis and associated with EMT (41), and cell cycle arrest (42,43).
The PI3K/Akt signaling pathway is involved in tumor cell
proliferation during the development of cervical cancer, and
downstream effectors of PI3K/Akt signaling are promising targets
for cervical cancer therapy (4).
Gene expression profiling also suggests that the PI3K/Akt pathway
is a therapeutic target in cervical cancer (6). More importantly, it has been confirmed
that PTEN, which counteracts PI3K/Akt activity, is involved in
various aspects of cancer development, such as inhibition of cell
proliferation, apoptosis, migration and invasion (8,44,45).
In particular, PTEN expression intensity is lower in cervical
cancer than benign cervical samples (46) and a decreased expression of PTEN was
found in invasive cervical cancers (47). From the previous study, we concluded
that the PTEN/PI3K/Akt signaling pathway is important in the
carcinogenesis and development of cervical cancer, thus identifying
the molecules regulating PTEN/PI3K/Akt may be an attractive
strategy for the underlying mechanism of cervical carcinogenesis.
In the present study, the results supported the hypothesis as,
miR-494 was involved in the modulation of PTEN expression in
cervical cancer. First of all, miR-494 was inversely correlated
with PTEN expression in cervical cancer tissues. Secondly, the mRNA
and protein levels of PTEN were significantly upregulated after
knockdown of miR-494 expression in cervical cancer cell lines. In
addition, luciferase analyses indicated PTEN was the direct target
of miR-494 in cervical cancer cells. Taken together, we have
demonstrated miR-494 could directly regulate PTEN expression by
targeting its mRNA 3′-UTR. Thus, previous findings and our results
suggest that, miR-494 functions as an oncogenic miRNA and
PTEN/PI3K/Akt regulator in cervical cancer. More specific studies
are required to further elucidate the relationship between miR-494
and PI3K/Akt and more specific mechanisms that miR-494 regulated
the expression of PTEN.
In summary, to the best of our knowledge, the
present study identified for the first time the correlation between
miR-494-mediated cervical cancer cell proliferation and
downregulation of PTEN. Our findings reveal a crucial role for
miR-494 in regulating cell cycle checkpoints and cervical cancer
cell proliferation. Understanding the precise role played by
miR-494 in inducing tumor cell proliferation may increase our
understanding of the biology of cervical cancer and inhibition of
miR-494 may be a novel therapeutic strategy in the treatment of
cervical cancer.
Acknowledgments
The authors would like to thank the local doctors
and the patients who participated in the present study.
Abbreviations:
|
miR-494
|
microRNA-494
|
|
HR-HPV
|
high-risk human papilloma virus
|
|
PTEN
|
phosphatase and tensin homolog deleted
on chromosome 10
|
|
NCEC
|
normal cervical epithelial cells
|
|
WT
|
wild-type
|
|
MT
|
mutant
|
|
3′-UTR
|
3′-untranslated region
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
MMAC1
|
mutated in multiple advanced cancer
1
|
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Castellsagué X, Díaz M, de Sanjosé S, et
al: Worldwide human papillomavirus etiology of cervical
adenocarcinoma and its cofactors: implications for screening and
prevention. J Natl Cancer Inst. 98:303–315. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hildesheim A and Wang SS: Host and viral
genetics and risk of cervical cancer: a review. Virus Res.
89:229–240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du CX and Wang Y: Expression of P-Akt,
NFkappaB and their correlation with human papillomavirus infection
in cervical carcinoma. Eur J Gynaecol Oncol. 33:274–277.
2012.PubMed/NCBI
|
|
5
|
Castellsagué X: Natural history and
epidemiology of HPV infection and cervical cancer. Gynecol Oncol.
110(Suppl 2): S4–S7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwarz JK, Payton JE, Rashmi R, et al:
Pathway-specific analysis of gene expression data identifies the
PI3K/Akt pathway as a novel therapeutic target in cervical cancer.
Clin Cancer Res. 18:1464–1471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molinari F and Frattini M: Functions and
regulation of the PTEN gene in colorectal cancer. Front Oncol.
3:3262014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang S and Yu D: PI(3)king apart PTEN’s
role in cancer. Clin Cancer Res. 16:4325–4330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi Q, Ling Y, Zhu M, et al: Promoter
region methylation and loss of protein expression of PTEN and
significance in cervical cancer. Biomed Rep. 2:653–658.
2014.PubMed/NCBI
|
|
10
|
Inui M, Martello G and Piccolo S: MicroRNA
control of signal transduction. Nat Rev Mol Cell Biol. 11:252–263.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ventura A and Jacks T: MicroRNAs and
cancer: short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartels CL and Tsongalis GJ: MicroRNAs:
novel biomarkers for human cancer. Clin Chem. 55:623–631. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao JJ, Yang J, Lin J, et al:
Identification of miRNAs associated with tumorigenesis of
retinoblastoma by miRNA microarray analysis. Childs Nerv Syst.
25:13–20. 2009. View Article : Google Scholar
|
|
14
|
Kwak SY, Yang JS, Kim BY, Bae IH and Han
YH: Ionizing radiation-inducible miR-494 promotes glioma cell
invasion through EGFR stabilization by targeting p190B rhoGAP.
Biochim Biophys Acta. 1843:508–516. 2014. View Article : Google Scholar
|
|
15
|
Yamanaka S, Campbell NR, An F, et al:
Coordinated effects of microRNA-494 induce G2/M arrest
in human cholangiocarcinoma. Cell Cycle. 11:2729–2738. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Luo F, Li Q, et al: Identification
of new aberrantly expressed miRNAs in intestinal-type gastric
cancer and its clinical significance. Oncol Rep. 26:1431–1439.
2011.PubMed/NCBI
|
|
17
|
Olaru AV, Ghiaur G, Yamanaka S, et al:
MicroRNA down-regulated in human cholangiocarcinoma control cell
cycle through multiple targets involved in the G1/S checkpoint.
Hepatology. 54:2089–2098. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun HB, Chen X, Ji H, et al: miR494 is an
independent prognostic factor and promotes cell migration and
invasion in colorectal cancer by directly targeting PTEN. Int J
Oncol. 45:2486–2494. 2014.PubMed/NCBI
|
|
19
|
Lui WO, Pourmand N, Patterson BK and Fire
A: Patterns of known and novel small RNAs in human cervical cancer.
Cancer Res. 67:6031–6043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JW, Choi CH, Choi JJ, et al: Altered
microRNA expression in cervical carcinomas. Clin Cancer Res.
14:2535–2542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
FIGO Committee on Gynecologic Oncology:
FIGO staging for carcinoma of the vulva, cervix, and corpus uteri.
Int J Gynaecol Obstet. 125:97–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou T, Ou J, Zhao X, Huang X, Huang Y and
Zhang Y: MicroRNA-196a promotes cervical cancer proliferation
through the regulation of FOXO1 and p27Kip1. Br J
Cancer. 110:1260–1268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El-Mansi MT and Williams AR: Evaluation of
PTEN expression in cervical adenocarcinoma by tissue microarray.
Int J Gynecol Cancer. 16:1254–1260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Lai L, Chen Q, et al: MicroRNA-494
is required for the accumulation and functions of tumor-expanded
myeloid-derived suppressor cells via targeting of PTEN. J Immunol.
188:5500–5510. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang GW, Lin JH, Qian JP and Zhou J:
Identification of risk and prognostic factors for patients with
clonorchiasis-associated intrahepatic cholangiocarcinoma. Ann Surg
Oncol. 21:3628–3637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu D, Qian J, Yin X, Xiao Q, Wang C and
Zeng Y: Expression of PTEN and survivin in cervical cancer:
promising biological markers for early diagnosis and prognostic
evaluation. Br J Biomed Sci. 69:143–146. 2012.
|
|
27
|
Leung CO, Deng W, Ye TM, et al: miR-135a
leads to cervical cancer cell transformation through regulation of
β-catenin via a SIAH1-dependent ubiquitin proteosomal pathway.
Carcinogenesis. 35:1931–1940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang N, Zhou Y, Zheng L and Li H: MiR-31
is an independent prognostic factor and functions as an oncomir in
cervical cancer via targeting ARID1A. Gynecol Oncol. 134:129–137.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lao G, Liu P, Wu Q, et al: Mir-155
promotes cervical cancer cell proliferation through suppression of
its target gene LKB1. Tumour Biol. 35:11933–11938. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Yao D, Zhao S, et al: MiR-1246
promotes SiHa cervical cancer cell proliferation, invasion, and
migration through suppression of its target gene thrombospondin 2.
Arch Gynecol Obstet. 290:725–732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wen SY, Lin Y, Yu YQ, et al: miR-506 acts
as a tumor suppressor by directly targeting the hedgehog pathway
transcription factor Gli3 in human cervical cancer. Oncogene. Mar
10–2014.Epub ahead of print. View Article : Google Scholar
|
|
32
|
Wang L, Chang L, Li Z, et al: miR-99a and
-99b inhibit cervical cancer cell proliferation and invasion by
targeting mTOR signaling pathway. Med Oncol. 31:9342014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang X, Liu Y, Zeng L, et al: miR-101
inhibits the G1-to-S phase transition of cervical cancer cells by
targeting Fos. Int J Gynecol Cancer. 24:1165–1172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu L, Jiang Y, Zhang H, Greenlee AR and
Han Z: Overexpressed miR-494 down-regulates PTEN gene expression in
cells transformed by
anti-benzo(a)pyrene-trans-7,8-dihydro-diol-9,10-epoxide. Life Sci.
86:192–198. 2010. View Article : Google Scholar
|
|
35
|
Lim L, Balakrishnan A, Huskey N, et al:
MicroRNA-494 within an oncogenic microRNA megacluster regulates
G1/S transition in liver tumorigenesis through
suppression of mutated in colorectal cancer. Hepatology.
59:202–215. 2014. View Article : Google Scholar :
|
|
36
|
Romano G, Acunzo M, Garofalo M, et al:
MiR-494 is regulated by ERK1/2 and modulates TRAIL-induced
apoptosis in non-small-cell lung cancer through BIM
down-regulation. Proc Natl Acad Sci USA. 109:16570–16575. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ohdaira H, Sekiguchi M, Miyata K and
Yoshida K: MicroRNA-494 suppresses cell proliferation and induces
senescence in A549 lung cancer cells. Cell Prolif. 45:32–38. 2012.
View Article : Google Scholar
|
|
38
|
Shen PF, Chen XQ, Liao YC, et al:
MicroRNA-494-3p targets CXCR4 to suppress the proliferation,
invasion, and migration of prostate cancer. Prostate. 74:756–767.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Correia NC, Gírio A, Antunes I, Martins LR
and Barata JT: The multiple layers of non-genetic regulation of
PTEN tumour suppressor activity. Eur J Cancer. 50:216–225. 2014.
View Article : Google Scholar
|
|
40
|
Hafsi S, Pezzino FM, Candido S, et al:
Gene alterations in the PI3K/PTEN/AKT pathway as a mechanism of
drug-resistance (Review). Int J Oncol. 40:639–644. 2012.
|
|
41
|
Mulholland DJ, Kobayashi N, Ruscetti M, et
al: Pten loss and RAS/MAPK activation cooperate to promote EMT and
metastasis initiated from prostate cancer stem/progenitor cells.
Cancer Res. 72:1878–1889. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Choi BH, Pagano M and Dai W: Plk1 protein
phosphorylates phosphatase and tensin homolog (PTEN) and regulates
its mitotic activity during the cell cycle. J Biol Chem.
289:14066–14074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vogt PK, Gymnopoulos M and Hart JR: PI
3-kinase and cancer: changing accents. Curr Opin Genet Dev.
19:12–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stewart AL, Mhashilkar AM, Yang XH, et al:
PI3 kinase blockade by Ad-PTEN inhibits invasion and induces
apoptosis in RGP and metastatic melanoma cells. Mol Med. 8:451–461.
2002.PubMed/NCBI
|
|
45
|
Tamura M, Gu J, Matsumoto K, Aota S,
Parsons R and Yamada KM: Inhibition of cell migration, spreading,
and focal adhesions by tumor suppressor PTEN. Science.
280:1614–1617. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Loures LF, Cândido EB, Vidigal PV, Seabra
MA, Marco LA and Silva-Filho AL: PTEN expression in patients with
carcinoma of the cervix and its association with p53, Ki-67 and
CD31. Rev Bras Ginecol Obstet. 36:205–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vázquez-Ulloa E, Lizano M, Aviléss-Salas
A, Alfaro-Moreno E and Contreras-Paredes A: Abnormal distribution
of hDlg and PTEN in premalignant lesions and invasive cervical
cancer. Gynecol Oncol. 122:663–668. 2011. View Article : Google Scholar : PubMed/NCBI
|