Cancer remains a major public health issue due to
the limitations of current therapy. Despite improvements in
surgical techniques, radiation therapy and chemotherapy, there has
been no major improvement in the effective blockage of tumor
progression. Tumor development is a complex stepwise process
involving the accumulation of abnormalities in miscellaneous
molecules that drive tumor growth and progression by coordinating
critical interactions between tumor cells and the host
microenvironment, including a variety of normal stromal cell types,
the extracellular matrix (ECM), proteases and cytokines (1). Thus, an improvement in the prognosis
of cancer will require the successful development of more effective
molecular-targeted therapies. Annexin A2 (ANXA2) is one of the most
important molecules that are aberrantly expressed in a wide range
of cancers and participate in tumor cell adhesion, proliferation,
invasion, metastasis and tumor neovascularization, thereby playing
a crucial role in cancer growth and progression (2–4). In
this review, we summarize the contribution of ANXA2 to cancer
development and the underlying mechanisms, in an attempt to
highlight the effects of ANXA2 on tumor cell adhesion,
proliferation, invasion, metastasis and tumor neovascularization,
and to provide a therapeutic target for molecular-based
strategies.
ANXA2 (also called p36, calpactin I heavy chain and
lipocortin II) is a 36-kDa protein belonging to the
calcium-dependent phospholipid binding proteins (5–7). It is
structurally highly conserved and is expressed in nearly all
eukaryotes (7–10), where ANXA2 is distributed mainly in
the plasma membrane (11) and
cytoplasm with a small proportion in the nucleus (12). Similar to other annexins, the basic
structure of ANXA2 consists of a homologous C-terminal core
composed of four repeats, and a highly variable N-terminal tail
(13,14). The C-terminus of ANXA2 harbors the
binding sites of calcium, phospholipids and F-actin which are
necessary for the membrane-associated activities of ANXA2 (15–21).
This domain also interacts directly with various molecules such as
heparin and RNA, thus endowing ANXA2 with more regulating actions
(22–25). The N-terminal domain of ANXA2
contains a nuclear export signal (NES) (12) as well as multiple phosphorylation
sites such as Tyr23, Ser11 and Ser25, which can be phosphorylated
by Src kinase and protein kinase C, respectively (26–28).
The phosphorylation of ANXA2 affects its intracellular localization
and regulating actions in specific cell types (29–38).
Moreover, ANXA2 has been identified as a cellular redox regulatory
protein, and this action is related to a reactive cysteine residue
(Cys-8) in the N-terminus. These domains dictate its functional and
regulatory specificity distinct from other annexin members
(39).
ANXA2 exists as a monomer or as a heterotetramer
(AIIt). AIIt consists of 2 molecules of ANXA2 and a dimer of
S100A10 (P11), and possesses a spectrum of biological behavior
(40–42). The most well-documented function of
ANXA2 is the interaction with tissue plasminogen activator (tPA) as
well as its substrate, plasminogen, and promotes the conversion of
plasminogen into plasmin (10,42–44) by
which it regulates the fibrinolytic process (45), facilitates tissue remodeling,
degrades ECM and participates in angiogenesis (46–48)
when it locates on the extracellular surface. The expression of
ANXA2 in the cytoplasm and plasma membrane is involved in the
regulation of actin cytoskeleton dynamics (6,19,21,49),
endocytosis and exocytosis (31,50–52),
cell-cell adhesion (5,53,54),
cell polarity (55) and endosome
formation (56,57). ANXA2 has also been shown to play an
important role in DNA synthesis and mRNA transport and translation
after identification of a small population existing in the nucleus
and a NES in the N-terminus of this protein that regulates its
nuclear export (12,58). ANXA2 acting as a part of the primer
recognition protein complex and as a DNA-binding protein regulates
DNA polymerase α activity, DNA synthesis, cell proliferation and
cell cycle progression (59–66).
Other studies have revealed that ANXA2 directly binds to
ribonucleotide homopolymers, cytoskeleton-bound polysomes and is
involved in association of mRNA with the cytoskeleton and
perinuclear localization (25). The
nuclear accumulation of the ANXA2 monomer plays a role in
protecting the cells from DNA damage during oxidative stress
(67). More recently, increasing
data have shown that ANXA2 is implicated in a wide range of
biological action such as facilitating the cell cycle partly
through a p53-dependent mechanism (68), regulating signal transducer and
activator of transcription 6 (STAT6) activity (69) and participating in multiple redox
cycles (39). Post-translation
modification of ANXA2 such as acetylation and phosphorylation
regulates its localization, NES, as well as binding to S100A10 and
plasminogen, which determines its biological activation (29,31–35,37,38).
Phosphorylation of ANXA2, at Tyr23 for example, was found to induce
actin reorganization and cell scattering in MDCK cells (30); meanwhile, Tyr23 phosphorylation is
required for cell-surface localization of ANXA2 involved in
pancreatic ductal adenocarcinoma invasion (36). ANXA2 is aberrantly expressed in a
wide spectrum of tumors, and this abnormal expression of ANXA2
plays a crucial role in tumor growth and progression.
ANXA2 is one of the most common proteins that are
overexpressed in a series of cancers and are implicated in the
multistep processes of tumor development. The first association
between ANXA2 and tumorigenesis was described in hepatocellular
carcinoma (HCC) in 1990, in which an abundance of ANXA2 was
detected (70). Recently, a number
of studies have found the increased expression of ANXA2 at both the
protein and mRNA levels in many types of malignancies such as
colorectal (71,72), breast (73–76)
and lung cancer (77), HCC
(78), gastric carcinoma (79) and pancreatic cancer (80), particularly in the more aggressive
or poorer prognosis phenotype of these cancers (81–84).
As a secretory protein, the serum level of ANXA2 was also found to
be elevated in patients with cancers, including HCC (78,85,86)
and invasive breast cancer (87).
There is increasing evidence to suggest that
overexpression of ANXA2 is closely associated with the
differentiation status, histological type, lymph node metastasis
and distant metastasis in non-small cell lung cancer (NSCLC)
(68,88,89),
colorectal (71,90,91)
and gastric cancer (79,84). Statistical analysis has also shown
that ANXA2 overexpression is correlated with a reduced survival
time and a higher risk of recurrence in colorectal (71), pancreatic (80,92),
gastric (79), clear-cell renal
cell carcinoma (83) as well as
NSCLC (88,89). These studies indicate the
involvement of ANXA2 in tumor progression. In contrast, ANXA2 was
also found to have an inverse correlation with esophageal
carcinomas (93,94) as well as head and neck squamous cell
carcinoma (95). In other words,
the expression of ANXA2 was found to be significantly lower in
tumor tissues compared to its paired adjacent normal tissues in
these cancers, and the downregulation of ANXA2 was significantly
correlated with advanced clinical stage, more frequent recurrence
and regional lymph node and distant metastasis. The different
experimental techniques and the difference between primary tumors
and metastatic lesions may be responsible for these contradictory
findings.
Taken together, these data indicate that aberrant
expression of ANXA2 is an important prognostic factor in a number
of tumor types, and exerts profound effects on tumor
progression.
High ANXA2 expression in cancer cells and tumor
stroma has been implicated in tumor cell adhesion. Initial evidence
for the involvement of ANXA2 in tumor adhesion was discovered in
RAW117 large cell lymphoma cells (96). This study demonstrated that the
binding of RAW117 tumor cells to endothelial cells (ECs) was
mediated by ANXA2 expressed on the surface of the RAW117 tumor
cells, and this binding was inhibited by antibodies of ANXA2,
indicating the association of ANXA2 with tumor cell adhesion. In
the secretome of co-cultured cells, ANXA2 siRNA significantly
inhibited ovarian cancer cell adhesion to peritoneal cells,
supporting the role of ANXA2 in cell adhesion (97). Similarly, the ability of prostate
cancer PC-3 cells to bind to human bone marrow ECs and osteoblasts
was significantly blocked by an antibody to ANXA2 or the N-terminal
competing peptide of this protein. The adhesive capacity of PC-3
cells to osteoblasts derived from Anxa2+/+ mice was
significantly increased compared to those from Anxa2−/−
mice, further supporting the involvement of ANXA2 in tumor adhesion
(98). However, the underlying
mechanism responsible for the actions of ANXA2 in tumor adhesion
remains unclear. Recently studies have demonstrated that the
adhesion between breast cancer cells and ECs is mediated by
interactions between ANXA2 and S100A10. ANXA2 expressed on the
surface of breast cancer cells interacts with S100A10 located on
microvascular ECs, facilitating the process by which cancer cells
form cell-cell contact with microvascular ECs (99).
ANXA2 is a key contributor to the stimulation of
tumor cell proliferation and promotion of cancer growth under
multiple regulatory modes. A relatively early study demonstrated a
higher expression of ANXA2 in pancreatic carcinoma cell lines
compared with cells of the normal pancreas, and an inverse
relationship was noted between the levels of ANXA2 and the doubling
time of the culture cells (100).
As one of the most highly expressed genes in primary multiple
myeloma (MM) cells (101), ANXA2
was found to increase cell proliferation and inhibit cell apoptosis
(2). Downregulation of ANXA2
expression by siRNA in lung cancer A549 cells or breast cancer
MDA-MB-231 and JIMT-1 cells significantly decreased the cell
proliferative capacity (74,102).
Similarly, in human HCC cells, silencing of ANXA2 suppressed cell
proliferation and led to abnormal apoptosis, and the percentage of
cells in the S phase was markedly decreased (103). When ANXA2 was suppressed by RNA
interference (RNAi) in breast cancer cells, the treated cells were
found to accumulate in the G0/G1 phase accompanied by a decrease in
the S/G2+M phase population and a reduction in cell proliferation
(104). ANXA2 facilitates
proliferation and inhibits apoptosis via different pathways in a
wide range of cancer cell types. ANXA2 was suggested as a part of
the primer recognition protein complex and DNA binding protein
involved in DNA replication (61,62,64,65).
ANXA2 was also identified as an RNA-binding protein interacting
with specific mRNAs such as its cognate mRNA and c-myc mRNA that
are involved in the transport and/or anchorage of specific mRNAs
(25,105–107). In human HeLa, 293 and 293T cells,
for example, downregulation of ANXA2 protein levels reduced DNA
synthesis and inhibited cell division and proliferation (66). The interaction of ANXA2 and c-myc
mRNA was found to lead to the increased expression levels of c-myc
protein which is involved in cell proliferation, differentiation
and apoptosis (108). It is well
known that p53, as a tumor suppressor, plays a critical role in
cell cycle regulation and apoptosis in different cancer cells.
ANXA2 has also been proposed to be involved in p53-mediated
apoptosis based on a study that overexpression of p53 induced
apoptosis of lung cancer cells concomitantly with downregulation of
ANXA2. In addition, ANXA2 knockdown increased the levels of p53 and
its downstream gene expression, and caused p53 translocation from
the cytoplasm to the nucleus (109). In vivo and in vitro
studies showed that ANXA2 facilitates cell cycle progression and
cell proliferation in part mediated by inhibition of p53 expression
(68). Furthermore, ANXA2 was
reported as a receptor mediating proliferative and anti-apoptotic
effects of progastrin/gastrin on target cells such as colon cancer
and pancreatic cancer (110,111).
A considerable body of research has documented that
angiogenesis is one hallmark of cancer (112), and is required for tumor growth,
migration and metastasis (113,114). This process is initiated by the
activation of proangiogenic factors such as vascular EC growth
factor (VEGF), basic fibroblast growth factor (bFGF), plasminogen,
followed by degradation of the ECM and proliferation and migration
of ECs, as well as the synthesis of new matrix components (115–118). Increased ANXA2 expression in
tumors has been recognized as a key contributor to cancer
angiogenesis in vivo and in vitro (47,119).
Studies in human breast tumor xenograft models have demonstrated
that neoangiogenesis in the tumor microenvironment can be markedly
inhibited by the ANXA2 antibody, indicating the involvement of
ANXA2 in new vessel formation in cancer (120). Clinical specimens also showed that
the accumulation of tPA and ANXA2 on the surface of invasive human
breast cancer was correlated with tumor neovascularization
(48). The plasminogen/plasmin
system activated matrix metalloproteinases (MMPs) into active
protease which is required for the degradation of the ECM during
the sprouting of new blood vessels (121). ANXA2 plays an important role in
the plasminogen activation system and acts as a tPA receptor on the
cell surface of endothelial and cancer cells, which mediates the
conversion of plasminogen into plasmin (44,48).
In addition, ANXA2 also participates in VEGF-mediated
neovascularization. ANXA2 was found to be increased in a murine
model of ischemic retinopathy through a VEGF/VEGF-R2/PKCβ pathway
(122). Silencing of the ANXA2
gene by siRNA inhibited the expression of proangiogenic molecules,
including VEGF, leading to the inhibition of neovascularization
(2). Simultaneously, addition of
purified domains I and IV of ANXA2 partly inhibited VEGF-dependent
formation of capillary-like networks in a dose-dependent manner
(123). Furthermore, ANXA2 was
demonstrated to interact directly with the vascular endothelial
cadherin (VE-cad)-based complex which is required to maintain
VE-cad-dependent cell-cell junctions responsible for the
maintenance of vascular endothelium integrity (124). The domains I and IV of ANXA2
compete with endogenous ANXA2 for interaction with VE-cad, leading
to the disruption of the capillary-like network by affecting
endothelial cell-cell contacts (123). Studies also showed that under the
stimulation of sphingosine 1 and angiogenic growth factors, ANXA2
regulated Akt activation in sprouting angiogenesis, and depletion
of ANXA2 attenuated Akt activation during EC invasion which was
associated with increased phosphorylation of VE-cad and endothelial
barrier leakage (125).
Importantly, increased vasculogenesis which occurs
via mature ECs from proliferation and differentiation of bone
marrow-derived endothelial progenitor cells (EPCs) has been
recognized to contribute to tumor development (126–128). In addition to the direct cellular
contribution to new vessel formation, EPCs secrete a spectrum of
proangiogenic cytokines that promote not only angiogenesis but
vasculogenesis by different modes, thus playing a crucial role in
neovascularization during neonatal growth and tumor progression
(126–128). However, EPCs mobilized from the
bone marrow into the peripheral circulation, migrating and adhering
to the sites of new vessel formation is a complex process dependent
on cell active mobility. Cytoskeleton remodeling plays crucial
roles in cell mobility. Various cell activities, including
migration, morphological change and polarity formation are
regulated by actin filament dynamics, including actin filament
disassembly, severing and reorganization (129,130). Studies have shown that the
dysfunction of actin leads to the impairment in EPC functions,
including tube formation (131,132). ANXA2 plays a crucial role in
regulating actin cytoskeletal rearrangements by binding the regions
of free-barbed ends (19). Thus,
ANXA2 may be involved in the neovascularization of EPCs by
interacting with actin, yet this theory requires more supporting
evidence.
These findings indicate that ANXA2 plays a crucial
role in tumor progression by enhancing neovascularization. Thus,
ANXA2 may be a potential target for the therapeutic management of
cancer via blockage of ANXA2-mediated neovascularization.
Tumor invasion and metastasis is responsible for the
majority of deaths among cancer patients. This complex process
includes adhesion of tumor cells to ECM proteins, proteolysis of
ECM proteins and remodeling of ECM. Through these mechanisms, tumor
cells create intercellular spaces for migration, an event that
requires membrane synthesis and cytoskeletal rearrangements. The
contribution of ANXA2 to tumor invasion and metastasis by
interacting with its potential invasion-associated protease
proteins as well as the actin cytoskeleton has been reported in
many advanced human tumors. In breast cancer, for example, ANXA2
was found to be overexpressed in the highly invasive cell line
MDA-MB-231 compared with a poorly invasive cell line MCF-7
(75). In MCF-7/ADR cells, the
administration of adriamycin increased the expression of ANXA2
consistent with the enhancement in cell proliferation and invasion,
suggesting the involvement of ANXA2 in cancer cell invasion
(133). In two head and neck
squamous cell carcinoma cell lines, respectively, ANXA2 was found
to be upregulated in metastatic lymph node compare with the primary
tumor of the same patient (134).
Analogously, the elevated expression of ANXA2 was also detected in
lymph node metastatic tissues of lung cancers (88). ANXA2 was also differentially
expressed in a pair of canine glioma subclones that exhibited
different invasive phenotypes in rat brains yet had similar genetic
backgrounds (135). In addition,
the reduction in ANXA2 expression by siRNA or neutralizing
antibodies significantly inhibited the motility and invasion of a
number of cancer types such as ovarian cancer, human glioma and HCC
(97,103,136), further supporting the contribution
of ANXA2 in tumor invasion. Accumulating data suggest that the
plasminogen activation system plays a crucial role in various
processes of tumor development, including activation of MMPs,
degradation of ECM and switch of growth factors, which together
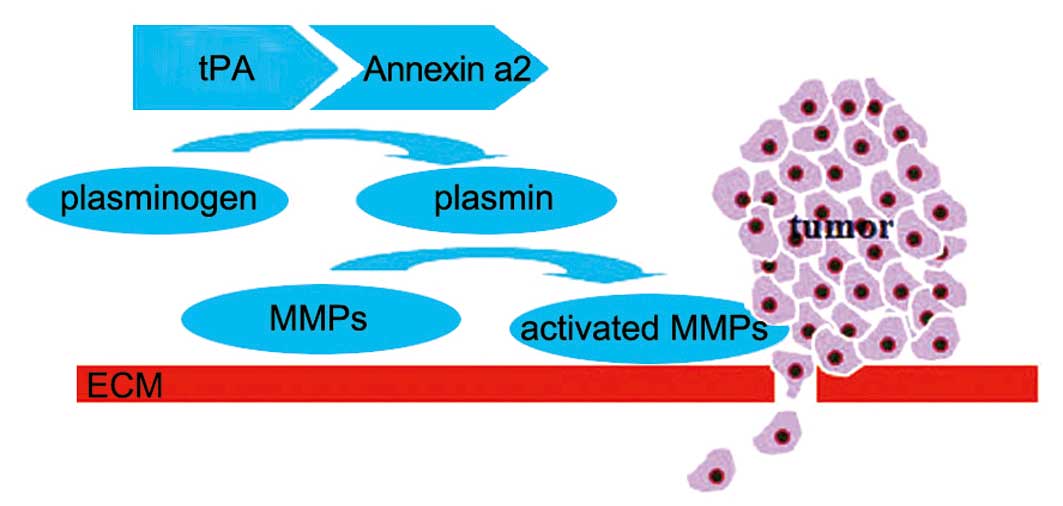
facilitate cellular migration and invasion (137–142). The convertion of inactive enzyme
plasminogen to active serine protease plasmin is a key event in
this process, which is mediated by plasminogen activators, t-PA and
urokinase plasminogen activator (uPA). ANXA2 catalyzes the
conversion via the interaction with tPA, thus efficiently enforcing
the effects of plasmin on tumor angiogenesis and tissue remodeling,
MMPs and latent growth factor activation, and ECM degration,
leading to tumor progression and metastasis (44,45,143)
(Fig. 1).
Moreover, ANXA2 has also been shown to regulate
migration and invasion of tumor cells by interaction with other
proteins. In invasive human breast cancer cell lines which
overexpress ANXA2, the invasive capacity of the cancer cells was
decreased by the siRNA of ANXA2 via inhibition of c-myc expression
(104). In contrast, upregulation
of ANXA2 in the noninvasive breast cancer cell line MCF-7 was
correlated with enhanced migration and invasion ability of cells
both in vitro and in vivo by increasing expression of
c-myc and cyclin D1 via activation of the Erk1/2 signaling pathways
(144). In HCC, the interaction
between ANXA2 and CD147 was found to regulate the trafficking of
CD147-harboring membrane microvesicles thereby promoting the
production of MMP-2 by tumor stoma fibroblasts, and suppressing the
migration and invasion of tumor cells (145,146). ANXA2 was also confirmed to promote
pancreatic cancer cell motility by interaction with S100A6
(147). Studies also showed that
the location of ANXA2 on the cell surface promoted
TGFβ-Rho-mediated epithelial-mesenchymal transition (EMT) in
pancreatic ductal adenocarcinoma (36) which is an important process for the
invasion and metastasis of this cancer (148).
These results indicate that ANXA2 may be a vital
component in the regulation of tumor invasion and metastasis, and
understanding of the mechanisms of ANXA2-mediated tumor development
is crucial.
Development of a tumor involves a complex process,
and multiple pathological events are considered to mediate and
drive tumor cell growth and development. ANXA2 is an important
molecule involved in regulating tumor cell adhesion, proliferation,
invasion, metastasis and tumor neovasculogenesis, thus playing a
crucial role in tumor development. The cellular and molecular
mechanisms of the effects of ANXA2 on tumor development require
further elucidation, and may provide a potential efficient
therapeutic target for molecular-based strategies for tumor
treatment.
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bao H, Jiang M, Zhu M, Sheng F, Ruan J and
Ruan C: Overexpression of Annexin II affects the proliferation,
apoptosis, invasion and production of proangiogenic factors in
multiple myeloma. Int J Hematol. 90:177–185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma MC and Sharma M: The role of
annexin II in angiogenesis and tumor progression: A potential
therapeutic target. Curr Pharm Des. 13:3568–3575. 2007. View Article : Google Scholar
|
|
4
|
Lokman NA, Ween MP, Oehler MK and
Ricciardelli C: The role of annexin A2 in tumorigenesis and cancer
progression. Cancer Microenviron. 4:199–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gerke V and Moss SE: Annexins: From
structure to function. Physiol Rev. 82:331–371. 2002.PubMed/NCBI
|
|
6
|
Rescher U and Gerke V: Annexins - unique
membrane binding proteins with diverse functions. J Cell Sci.
117:2631–2639. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hajjar KA, Guevara CA, Lev E, Dowling K
and Chacko J: Interaction of the fibrinolytic receptor, annexin II,
with the endothelial cell surface. Essential role of endonexin
repeat 2. J Biol Chem. 271:21652–21659. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mai J, Finley RL Jr, Waisman DM and Sloane
BF: Human procathepsin B interacts with the annexin II tetramer on
the surface of tumor cells. J Biol Chem. 275:12806–12812. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Madureira PA, Surette AP, Phipps KD,
Taboski MA, Miller VA and Waisman DM: The role of the annexin A2
heterotetramer in vascular fibrinolysis. Blood. 118:4789–4797.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hajjar KA, Jacovina AT and Chacko J: An
endothelial cell receptor for plasminogen/tissue plasminogen
activator. I. Identity with annexin II. J Biol Chem.
269:21191–21197. 1994.PubMed/NCBI
|
|
11
|
Courtneidge S, Ralston R, Alitalo K and
Bishop JM: Subcellular location of an abundant substrate (p36) for
tyrosine-specific protein kinases. Mol Cell Biol. 3:340–350.
1983.PubMed/NCBI
|
|
12
|
Eberhard DA, Karns LR, VandenBerg SR and
Creutz CE: Control of the nuclear-cytoplasmic partitioning of
annexin II by a nuclear export signal and by p11 binding. J Cell
Sci. 114:3155–3166. 2001.PubMed/NCBI
|
|
13
|
Burger A, Berendes R, Liemann S, et al:
The crystal structure and ion channel activity of human annexin II,
a peripheral membrane protein. J Mol Biol. 257:839–847. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Camors E, Monceau V and Charlemagne D:
Annexins and Ca2+ handling in the heart. Cardiovasc Res.
65:793–802. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jost M, Thiel C, Weber K and Gerke V:
Mapping of three unique Ca2+-binding sites in human
annexin II. Eur J Biochem. 207:923–930. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jost M, Weber K and Gerke V: Annexin II
contains two types of Ca2+-binding sites. Biochem J.
298:553–559. 1994.
|
|
17
|
Filipenko NR, Kang HM and Waisman DM:
Characterization of the Ca2+-binding sites of annexin II
tetramer. J Biol Chem. 275:38877–38884. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Filipenko NR and Waisman DM: The C
terminus of annexin II mediates binding to F-actin. J Biol Chem.
276:5310–5315. 2001. View Article : Google Scholar
|
|
19
|
Hayes MJ, Shao D, Bailly M and Moss SE:
Regulation of actin dynamics by annexin 2. EMBO J. 25:1816–1826.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jones PG, Moore GJ and Waisman DM: A
nonapeptide to the putative F-actin binding site of annexin-II
tetramer inhibits its calcium-dependent activation of actin
filament bundling. J Biol Chem. 267:13993–13997. 1992.PubMed/NCBI
|
|
21
|
Rescher U, Ruhe D, Ludwig C, Zobiack N and
Gerke V: Annexin 2 is a phosphatidylinositol (4,5)-bisphosphate
binding protein recruited to actin assembly sites at cellular
membranes. J Cell Sci. 117:3473–3480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su M, Shi JJ, Yang YP, Li J, Zhang YL,
Chen J, Hu LF and Liu CF: HDAC6 regulates aggresome-autophagy
degradation pathway of α-synuclein in response to
MPP+-induced stress. J Neurochem. 117:112–120. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aukrust I, Hollås H, Strand E, Evensen L,
Travé G, Flatmark T and Vedeler A: The mRNA-binding site of annexin
A2 resides in helices C-D of its domain IV. J Mol Biol.
368:1367–1378. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kassam G, Manro A, Braat CE, Louie P,
Fitzpatrick SL and Waisman DM: Characterization of the heparin
binding properties of annexin II tetramer. J Biol Chem.
272:15093–15100. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vedeler A and Hollås H: Annexin II is
associated with mRNAs which may constitute a distinct
subpopulation. Biochem J. 348:565–572. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gould KL, Woodgett JR, Isacke CM and
Hunter T: The protein-tyrosine kinase substrate p36 is also a
substrate for protein kinase C in vitro and in vivo. Mol Cell Biol.
6:2738–2744. 1986.PubMed/NCBI
|
|
27
|
Jost M and Gerke V: Mapping of a
regulatory important site for protein kinase C phosphorylation in
the N-terminal domain of annexin II. Biochim Biophys Acta.
1313:283–289. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Glenney JR Jr and Tack BF: Amino-terminal
sequence of p36 and associated p10: Identification of the site of
tyrosine phosphorylation and homology with S-100. Proc Natl Acad
Sci USA. 82:7884–7888. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo W, Yan G, Li L, et al: Epstein-Barr
virus latent membrane protein 1 mediates serine 25 phosphorylation
and nuclear entry of annexin A2 via PI-PLC-PKCalpha/PKCbeta
pathway. Mol Carcinog. 47:934–946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Graauw M, Tijdens I, Smeets MB,
Hensbergen PJ, Deelder AM and van de Water B: Annexin A2
phosphorylation mediates cell scattering and branching
morphogenesis via cofilin activation. Mol Cell Biol. 28:1029–1040.
2008. View Article : Google Scholar :
|
|
31
|
Morel E and Gruenberg J: Annexin A2
binding to endosomes and functions in endosomal transport are
regulated by tyrosine 23 phosphorylation. J Biol Chem.
284:1604–1611. 2009. View Article : Google Scholar
|
|
32
|
Hubaishy I, Jones PG, Bjorge J, Bellagamba
C, Fitzpatrick S, Fujita DJ and Waisman DM: Modulation of annexin
II tetramer by tyrosine phosphorylation. Biochemistry.
34:14527–14534. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Regnouf F, Sagot I, Delouche B, Devilliers
G, Cartaud J, Henry JP and Pradel LA: ‘In vitro’ phosphorylation of
annexin 2 heterotetramer by protein kinase C. Comparative
properties of the unphosphorylated and phosphorylated annexin 2 on
the aggregation and fusion of chromaffin granule membranes. J Biol
Chem. 270:27143–27150. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Johnstone SA, Hubaishy I and Waisman DM:
Phosphorylation of annexin II tetramer by protein kinase C inhibits
aggregation of lipid vesicles by the protein. J Biol Chem.
267:25976–25981. 1992.PubMed/NCBI
|
|
35
|
Deora AB, Kreitzer G, Jacovina AT and
Hajjar KA: An annexin 2 phosphorylation switch mediates
p11-dependent translocation of annexin 2 to the cell surface. J
Biol Chem. 279:43411–43418. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng L, Foley K, Huang L, et al: Tyrosine
23 phosphorylation-dependent cell-surface localization of annexin
A2 is required for invasion and metastases of pancreatic cancer.
PLoS One. 6:e193902011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yan G, Luo W, Lu Z, Luo X, Li L, Liu S,
Liu Y, Tang M, Dong Z and Cao Y: Epstein-Barr virus latent membrane
protein 1 mediates phosphorylation and nuclear translocation of
annexin A2 by activating PKC pathway. Cell Signal. 19:341–348.
2007. View Article : Google Scholar
|
|
38
|
Liu J, Rothermund CA, Ayala-Sanmartin J
and Vishwanatha JK: Nuclear annexin II negatively regulates growth
of LNCaP cells and substitution of ser 11 and 25 to glu prevents
nucleocytoplasmic shuttling of annexin II. BMC Biochem. 4:102003.
View Article : Google Scholar
|
|
39
|
Madureira PA, Hill R, Miller VA,
Giacomantonio C, Lee PW and Waisman DM: Annexin A2 is a novel
cellular redox regulatory protein involved in tumorigenesis.
Oncotarget. 2:1075–1093. 2011.PubMed/NCBI
|
|
40
|
Johnsson N, Marriott G and Weber K: p36,
the major cytoplasmic substrate of src tyrosine protein kinase,
binds to its p11 regulatory subunit via a short amino-terminal
amphiphatic helix. EMBO J. 7:2435–2442. 1988.PubMed/NCBI
|
|
41
|
Réty S, Sopkova J, Renouard M, Osterloh D,
Gerke V, Tabaries S, Russo-Marie F and Lewit-Bentley A: The crystal
structure of a complex of p11 with the annexin II N-terminal
peptide. Nat Struct Biol. 6:89–95. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Waisman DM: Annexin II tetramer: Structure
and function. Mol Cell Biochem. 149–150:301–322. 1995. View Article : Google Scholar
|
|
43
|
Kassam G, Choi KS, Ghuman J, Kang HM,
Fitzpatrick SL, Zackson T, Zackson S, Toba M, Shinomiya A and
Waisman DM: The role of annexin II tetramer in the activation of
plasminogen. J Biol Chem. 273:4790–4799. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cesarman GM, Guevara CA and Hajjar KA: An
endothelial cell receptor for plasminogen/tissue plasminogen
activator (t-PA). II. Annexin II-mediated enhancement of
t-PA-dependent plasminogen activation. J Biol Chem.
269:21198–21203. 1994.PubMed/NCBI
|
|
45
|
Hajjar KA and Krishnan S: Annexin II: A
mediator of the plasmin/plasminogen activator system. Trends
Cardiovasc Med. 9:128–138. 1999. View Article : Google Scholar
|
|
46
|
Jacovina AT, Deora AB, Ling Q, Broekman
MJ, Almeida D, Greenberg CB, Marcus AJ, Smith JD and Hajjar KA:
Homocysteine inhibits neoangiogenesis in mice through blockade of
annexin A2-dependent fibrinolysis. J Clin Invest. 119:3384–3394.
2009.PubMed/NCBI
|
|
47
|
Valapala M, Thamake SI and Vishwanatha JK:
A competitive hexapeptide inhibitor of annexin A2 prevents
hypoxia-induced angiogenic events. J Cell Sci. 124:1453–1464. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sharma M, Ownbey RT and Sharma MC: Breast
cancer cell surface annexin II induces cell migration and
neoangiogenesis via tPA dependent plasmin generation. Exp Mol
Pathol. 88:278–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gerke V, Creutz CE and Moss SE: Annexins:
Linking Ca2+ signalling to membrane dynamics. Nat Rev
Mol Cell Biol. 6:449–461. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ali SM, Geisow MJ and Burgoyne RD: A role
for calpactin in calcium-dependent exocytosis in adrenal chromaffin
cells. Nature. 340:313–315. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sarafian T, Pradel LA, Henry JP, Aunis D
and Bader MF: The participation of annexin II (calpactin I) in
calcium-evoked exocytosis requires protein kinase C. J Cell Biol.
114:1135–1147. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Morel E, Parton RG and Gruenberg J:
Annexin A2-dependent polymerization of actin mediates endosome
biogenesis. Dev Cell. 16:445–457. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yamada A, Irie K, Hirota T, Ooshio T,
Fukuhara A and Takai Y: Involvement of the annexin II-S100A10
complex in the formation of E-cadherin-based adherens junctions in
Madin-Darby canine kidney cells. J Biol Chem. 280:6016–6027. 2005.
View Article : Google Scholar
|
|
54
|
Mai J, Waisman DM and Sloane BF: Cell
surface complex of cathepsin B/annexin II tetramer in malignant
progression. Biochim Biophys Acta. 1477:215–230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Grieve AG, Moss SE and Hayes MJ: Annexin
A2 at the interface of actin and membrane dynamics: A focus on its
roles in endocytosis and cell polarization. Int J Cell Biol.
2012:8524302012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jost M, Zeuschner D, Seemann J, Weber K
and Gerke V: Identification and characterization of a novel type of
annexin-membrane interaction: Ca2+ is not required for
the association of annexin II with early endosomes. J Cell Sci.
110:221–228. 1997.
|
|
57
|
König J and Gerke V: Modes of
annexin-membrane interactions analyzed by employing chimeric
annexin proteins. Biochim Biophys Acta. 1498:174–180. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu J and Vishwanatha JK: Regulation of
nucleo-cytoplasmic shuttling of human annexin A2: A proposed
mechanism. Mol Cell Biochem. 303:211–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jindal HK and Vishwanatha JK: Purification
and characterization of primer recognition proteins from HeLa
cells. Biochemistry. 29:4767–4773. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kumble KD and Vishwanatha JK:
Immunoelectron microscopic analysis of the intracellular
distribution of primer recognition proteins, annexin 2 and
phosphoglycerate kinase, in normal and transformed cells. J Cell
Sci. 99:751–758. 1991.PubMed/NCBI
|
|
61
|
Vishwanatha JK, Jindal HK and Davis RG:
The role of primer recognition proteins in DNA replication:
Association with nuclear matrix in HeLa cells. J Cell Sci.
101:25–34. 1992.PubMed/NCBI
|
|
62
|
Boyko V, Mudrak O, Svetlova M, Negishi Y,
Ariga H and Tomilin N: A major cellular substrate for protein
kinases, annexin II, is a DNA-binding protein. FEBS Lett.
345:139–142. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Krutilina RI, Babich VS, Kropotov AV,
Mudrak OS, Chesnokov IN, Turoverova LV, Konstantinova IM and
Tomilin NV: The DNA-binding activity of the membrane protein
annexin II and its interaction with antibodies to the chromatin
nuclear ribonucleoprotein (alpha-RNP). Tsitologiia. 38:1106–1114.
1996.In Russian.
|
|
64
|
Vishwanatha JK and Kumble S: Involvement
of annexin II in DNA replication: Evidence from cell-free extracts
of Xenopus eggs. J Cell Sci. 105:533–540. 1993.PubMed/NCBI
|
|
65
|
Kumble KD, Iversen PL and Vishwanatha JK:
The role of primer recognition proteins in DNA replication:
Inhibition of cellular proliferation by antisense
oligodeoxyribonucleotides. J Cell Sci. 101:35–41. 1992.PubMed/NCBI
|
|
66
|
Chiang Y, Rizzino A, Sibenaller ZA, Wold
MS and Vishwanatha JK: Specific down-regulation of annexin II
expression in human cells interferes with cell proliferation. Mol
Cell Biochem. 199:139–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Madureira PA, Hill R, Lee PW and Waisman
DM: Genotoxic agents promote the nuclear accumulation of annexin
A2: Role of annexin A2 in mitigating DNA damage. PLoS One.
7:e505912012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang CY, Chen CL, Tseng YL, Fang YT, Lin
YS, Su WC, Chen CC, Chang KC, Wang YC and Lin CF: Annexin A2
silencing induces G2 arrest of non-small cell lung cancer cells
through p53-dependent and -independent mechanisms. J Biol Chem.
287:32512–32524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Das S, Shetty P, Valapala M, Dasgupta S,
Gryczynski Z and Vishwanatha JK: Signal transducer and activator of
transcription 6 (STAT6) is a novel interactor of annexin A2 in
prostate cancer cells. Biochemistry. 49:2216–2226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Frohlich M, Motté P, Galvin K, Takahashi
H, Wands J and Ozturk M: Enhanced expression of the protein kinase
substrate p36 in human hepatocellular carcinoma. Mol Cell Biol.
10:3216–3223. 1990.PubMed/NCBI
|
|
71
|
Yang T, Peng H, Wang J, Yang J, Nice EC,
Xie K and Huang C: Prognostic and diagnostic significance of
annexin A2 in colorectal cancer. Colorectal Dis. 15:e373–e381.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Duncan R, Carpenter B, Main LC, Telfer C
and Murray GI: Characterisation and protein expression profiling of
annexins in colorectal cancer. Br J Cancer. 98:426–433. 2008.
View Article : Google Scholar
|
|
73
|
Deng S, Wang J, Hou L, Li J, Chen G, Jing
B, Zhang X and Yang Z: Annexin A1, A2, A4 and A5 play important
roles in breast cancer, pancreatic cancer and laryngeal carcinoma,
alone and/or synergistically. Oncol Lett. 5:107–112. 2013.
|
|
74
|
Shetty PK, Thamake SI, Biswas S, Johansson
SL and Vishwanatha JK: Reciprocal regulation of annexin A2 and EGFR
with Her-2 in Her-2 negative and herceptin-resistant breast cancer.
PLoS One. 7:e442992012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sharma MR, Koltowski L, Ownbey RT,
Tuszynski GP and Sharma MC: Angiogenesis-associated protein annexin
II in breast cancer: Selective expression in invasive breast cancer
and contribution to tumor invasion and progression. Exp Mol Pathol.
81:146–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chuthapisith S, Bean BE, Cowley G, et al:
Annexins in human breast cancer: Possible predictors of
pathological response to neoadjuvant chemotherapy. Eur J Cancer.
45:1274–1281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yao H, Zhang Z, Xiao Z, et al:
Identification of metastasis associated proteins in human lung
squamous carcinoma using two-dimensional difference gel
electrophoresis and laser capture microdissection. Lung Cancer.
65:41–48. 2009. View Article : Google Scholar
|
|
78
|
Zhang HJ, Yao DF, Yao M, Huang H, Wu W,
Yan MJ, Yan XD and Chen J: Expression characteristics and
diagnostic value of annexin A2 in hepatocellular carcinoma. World J
Gastroenterol. 18:5897–5904. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang Q, Ye Z, Yang Q, He X, Wang H and
Zhao Z: Upregulated expression of annexin II is a prognostic marker
for patients with gastric cancer. World J Surg Oncol. 10:1032012.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Takano S, Togawa A, Yoshitomi H, et al:
Annexin II overexpression predicts rapid recurrence after surgery
in pancreatic cancer patients undergoing gemcitabine-adjuvant
chemotherapy. Ann Surg Oncol. 15:3157–3168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yang SF, Hsu HL, Chao TK, Hsiao CJ, Lin YF
and Cheng CW: Annexin A2 in renal cell carcinoma: Expression,
function, and prognostic significance. Urol Oncol.
33:22.e11–22.e21. 2014. View Article : Google Scholar
|
|
82
|
Ma RL, Shen LY and Chen KN: Coexpression
of ANXA2, SOD2 and HOXA13 predicts poor prognosis of esophageal
squamous cell carcinoma. Oncol Rep. 31:2157–2164. 2014.PubMed/NCBI
|
|
83
|
Ohno Y, Izumi M, Kawamura T, Nishimura T,
Mukai K and Tachibana M: Annexin II represents metastatic potential
in clear-cell renal cell carcinoma. Br J Cancer. 101:287–294. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Emoto K, Sawada H, Yamada Y, et al:
Annexin II overexpression is correlated with poor prognosis in
human gastric carcinoma. Anticancer Res. 21:1339–1345.
2001.PubMed/NCBI
|
|
85
|
Liu Z, Ling Q, Wang J, Xie H, Xu X and
Zheng S: Annexin A2 is not a good biomarker for hepatocellular
carcinoma in cirrhosis. Oncol Lett. 6:125–129. 2013.PubMed/NCBI
|
|
86
|
Ji NY, Park MY, Kang YH, et al: Evaluation
of annexin II as a potential serum marker for hepatocellular
carcinoma using a developed sandwich ELISA method. Int J Mol Med.
24:765–771. 2009.PubMed/NCBI
|
|
87
|
Jeon YR, Kim SY, Lee EJ, Kim YN, Noh DY,
Park SY and Moon A: Identification of annexin II as a novel
secretory biomarker for breast cancer. Proteomics. 13:3145–3156.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Luo CH, Liu QQ, Zhang PF, Li MY, Chen ZC
and Liu YF: Prognostic significance of annexin II expression in
non-small cell lung cancer. Clin Transl Oncol. 15:938–946. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Jia JW, Li KL, Wu JX and Guo SL: Clinical
significance of annexin II expression in human non-small cell lung
cancer. Tumour Biol. 34:1767–1771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pei H, Zhu H, Zeng S, et al: Proteome
analysis and tissue microarray for profiling protein markers
associated with lymph node metastasis in colorectal cancer. J
Proteome Res. 6:2495–2501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Emoto K, Yamada Y, Sawada H, Fujimoto H,
Ueno M, Takayama T, Kamada K, Naito A, Hirao S and Nakajima Y:
Annexin II overexpression correlates with stromal tenascin-C
overexpression: A prognostic marker in colorectal carcinoma.
Cancer. 92:1419–1426. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kagawa S, Takano S, Yoshitomi H, et al:
Akt/mTOR signaling pathway is crucial for gemcitabine resistance
induced by annexin II in pancreatic cancer cells. J Surg Res.
178:758–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu Z, Feng JG, Tuersun A, et al:
Proteomic identification of differentially-expressed proteins in
esophageal cancer in three ethnic groups in Xinjiang. Mol Biol Rep.
38:3261–3269. 2011. View Article : Google Scholar
|
|
94
|
Zhi H, Zhang J, Hu G, Lu J, Wang X, Zhou
C, Wu M and Liu Z: The deregulation of arachidonic acid
metabolism-related genes in human esophageal squamous cell
carcinoma. Int J Cancer. 106:327–333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Pena-Alonso E, Rodrigo JP, Parra IC,
Pedrero JM, Meana MV, Nieto CS, Fresno MF, Morgan RO and Fernandez
MP: Annexin A2 localizes to the basal epithelial layer and is
down-regulated in dysplasia and head and neck squamous cell
carcinoma. Cancer Lett. 263:89–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tressler RJ, Updyke TV, Yeatman T and
Nicolson GL: Extracellular annexin II is associated with divalent
cation-dependent tumor cell-endothelial cell adhesion of metastatic
RAW117 large-cell lymphoma cells. J Cell Biochem. 53:265–276. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lokman NA, Elder AS, Ween MP, Pyragius CE,
Hoffmann P, Oehler MK and Ricciardelli C: Annexin A2 is regulated
by ovarian cancer-peritoneal cell interactions and promotes
metastasis. Oncotarget. 4:1199–1211. 2013.PubMed/NCBI
|
|
98
|
Shiozawa Y, Havens AM, Jung Y, et al:
Annexin II/annexin II receptor axis regulates adhesion, migration,
homing, and growth of prostate cancer. J Cell Biochem. 105:370–380.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Myrvang HK, Guo X, Li C and Dekker LV:
Protein interactions between surface annexin A2 and S100A10 mediate
adhesion of breast cancer cells to microvascular endothelial cells.
FEBS Lett. 587:3210–3215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kumble KD, Hirota M, Pour PM and
Vishwanatha JK: Enhanced levels of annexins in pancreatic carcinoma
cells of Syrian hamsters and their intrapancreatic allografts.
Cancer Res. 52:163–167. 1992.PubMed/NCBI
|
|
101
|
Claudio JO, Masih-Khan E, Tang H, et al: A
molecular compendium of genes expressed in multiple myeloma. Blood.
100:2175–2186. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wang YX, Lv H, Li ZX, Li C and Wu XY:
Effect of shRNA mediated down-regulation of annexin A2 on
biological behavior of human lung adencarcinoma cells A549. Pathol
Oncol Res. 18:183–190. 2012. View Article : Google Scholar
|
|
103
|
Zhang HJ, Yao DF, Yao M, Huang H, Wang L,
Yan MJ, Yan XD, Gu X, Wu W and Lu SL: Annexin A2 silencing inhibits
invasion, migration, and tumorigenic potential of hepatoma cells.
World J Gastroenterol. 19:3792–3801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang J, Guo B, Zhang Y, Cao J and Chen T:
Silencing of the annexin II gene down-regulates the levels of
S100A10, c-Myc, and plasmin and inhibits breast cancer cell
proliferation and invasion. Saudi Med J. 31:374–381.
2010.PubMed/NCBI
|
|
105
|
Filipenko NR, MacLeod TJ, Yoon CS and
Waisman DM: Annexin A2 is a novel RNA-binding protein. J Biol Chem.
279:8723–8731. 2004. View Article : Google Scholar
|
|
106
|
Mickleburgh I, Burtle B, Hollås H,
Campbell G, Chrzanowska-Lightowlers Z, Vedeler A and Hesketh J:
Annexin A2 binds to the localization signal in the 3′ untranslated
region of c-myc mRNA. FEBS J. 272:413–421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Hollås H, Aukrust I, Grimmer S, Strand E,
Flatmark T and Vedeler A: Annexin A2 recognises a specific region
in the 3′-UTR of its cognate messenger RNA. Biochim Biophys Acta.
1763:1325–1334. 2006. View Article : Google Scholar
|
|
108
|
Pelengaris S and Khan M: The c-MYC
oncoprotein as a treatment target in cancer and other disorders of
cell growth. Expert Opin Ther Targets. 7:623–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Huang Y, Jin Y, Yan CH, Yu Y, Bai J, Chen
F, Zhao YZ and Fu SB: Involvement of annexin A2 in p53 induced
apoptosis in lung cancer. Mol Cell Biochem. 309:117–123. 2008.
View Article : Google Scholar
|
|
110
|
Rengifo-Cam W, Umar S, Sarkar S and Singh
P: Antiapoptotic effects of progastrin on pancreatic cancer cells
are mediated by sustained activation of nuclear factor-κB. Cancer
Res. 67:7266–7274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Sarkar S, Swiercz R, Kantara C, Hajjar KA
and Singh P: Annexin A2 mediates up-regulation of NF-κB, β-catenin,
and stem cell in response to progastrin in mice and HEK-293 cells.
Gastroenterology. 140:583.e4–595.e4. 2011. View Article : Google Scholar
|
|
112
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Carmeliet P: Angiogenesis in health and
disease. Nat Med. 9:653–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Lima e Silva R, Shen J, Gong YY, Seidel
CP, Hackett SF, Kesavan K, Jacoby DB and Campochiaro PA: Agents
that bind annexin A2 suppress ocular neovascularization. J Cell
Physiol. 225:855–864. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Sharma M, Blackman MR and Sharma MC:
Antibody-directed neutralization of annexin II (ANX II) inhibits
neoangiogenesis and human breast tumor growth in a xenograft model.
Exp Mol Pathol. 92:175–184. 2012. View Article : Google Scholar
|
|
121
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhao S, Huang L, Wu J, Zhang Y, Pan D and
Liu X: Vascular endothelial growth factor upregulates expression of
annexin A2 in vitro and in a mouse model of ischemic retinopathy.
Mol Vis. 15:1231–1242. 2009.PubMed/NCBI
|
|
123
|
Raddum AM, Evensen L, Hollås H, Grindheim
AK, Lorens JB and Vedeler A: Domains I and IV of annexin A2 affect
the formation and integrity of in vitro capillary-like networks.
PLoS One. 8:e602812013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Dejana E, Orsenigo F and Lampugnani MG:
The role of adherens junctions and VE-cadherin in the control of
vascular permeability. J Cell Sci. 121:2115–2122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Su SC, Maxwell SA and Bayless KJ: Annexin
2 regulates endothelial morphogenesis by controlling AKT activation
and junctional integrity. J Biol Chem. 285:40624–40634. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sun XT, Yuan XW, Zhu HT, Deng ZM, Yu DC,
Zhou X and Ding YT: Endothelial precursor cells promote
angiogenesis in hepatocellular carcinoma. World J Gastroenterol.
18:4925–4933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yu DC, Chen J, Sun XT, Zhuang LY, Jiang CP
and Ding YT: Mechanism of endothelial progenitor cell recruitment
into neo-vessels in adjacent non-tumor tissues in hepatocellular
carcinoma. BMC Cancer. 10:4352010. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Finney MR, Greco NJ, Haynesworth SE, et
al: Direct comparison of umbilical cord blood versus bone
marrow-derived endothelial precursor cells in mediating
neovascularization in response to vascular ischemia. Biol Blood
Marrow Transplant. 12:585–593. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Pollard TD and Borisy GG: Cellular
motility driven by assembly and disassembly of actin filaments.
Cell. 112:453–465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Revenu C, Athman R, Robine S and Louvard
D: The co-workers of actin filaments: From cell structures to
signals. Nat Rev Mol Cell Biol. 5:635–646. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wei J, Liu Y, Chang M, Sun CL, Li DW, Liu
ZQ and Hu LS: Proteomic analysis of oxidative modification in
endothelial colony-forming cells treated by hydrogen peroxide. Int
J Mol Med. 29:1099–1105. 2012.PubMed/NCBI
|
|
132
|
Zhang X, Cui X, Cheng L, Guan X, Li H, Li
X and Cheng M: Actin stabilization by jasplakinolide affects the
function of bone marrow-derived late endothelial progenitor cells.
PLoS One. 7:e508992012. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Zhang F, Zhang L, Zhang B, Wei X, Yang Y,
Qi RZ, Ying G, Zhang N and Niu R: Anxa2 plays a critical role in
enhanced invasiveness of the multidrug resistant human breast
cancer cells. J Proteome Res. 8:5041–5047. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wu W, Tang X, Hu W, Lotan R, Hong WK and
Mao L: Identification and validation of metastasis-associated
proteins in head and neck cancer cell lines by two-dimensional
electrophoresis and mass spectrometry. Clin Exp Metastasis.
19:319–326. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Maruo T, Ichikawa T, Kanzaki H, et al:
Proteomics-based analysis of invasion-related proteins in malignant
gliomas. Neuropathology. 33:264–275. 2013. View Article : Google Scholar
|
|
136
|
Tatenhorst L, Rescher U, Gerke V and
Paulus W: Knockdown of annexin 2 decreases migration of human
glioma cells in vitro. Neuropathol Appl Neurobiol. 32:271–277.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Mignatti P and Rifkin DB: Biology and
biochemistry of proteinases in tumor invasion. Physiol Rev.
73:161–195. 1993.PubMed/NCBI
|
|
138
|
McColl BK, Baldwin ME, Roufail S, Freeman
C, Moritz RL, Simpson RJ, Alitalo K, Stacker SA and Achen MG:
Plasmin activates the lymphangiogenic growth factors VEGF-C and
VEGF-D. J Exp Med. 198:863–868. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Bajou K, Masson V, Gerard RD, et al: The
plasminogen activator inhibitor PAI-1 controls in vivo tumor
vascularization by interaction with proteases, not vitronectin.
Implications for antiangiogenic strategies. J Cell Biol.
152:777–784. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Díaz VM, Planaguma J, Thomson TM, Reventós
J and Paciucci R: Tissue plasminogen activator is required for the
growth, invasion, and angiogenesis of pancreatic tumor cells.
Gastroenterology. 122:806–819. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Pepper MS: Extracellular proteolysis and
angiogenesis. Thromb Haemost. 86:346–355. 2001.PubMed/NCBI
|
|
142
|
Tarui T, Majumdar M, Miles LA, Ruf W and
Takada Y: Plasmin-induced migration of endothelial cells. A
potential target for the anti-angiogenic action of angiostatin. J
Biol Chem. 277:33564–33570. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Ranson M and Andronicos NM: Plasminogen
binding and cancer: Promises and pitfalls. Front Biosci.
8:s294–s304. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
144
|
Wu B, Zhang F, Yu M, Zhao P, Ji W, Zhang
H, Han J and Niu R: Up-regulation of Anxa2 gene promotes
proliferation and invasion of breast cancer MCF-7 cells. Cell
Prolif. 45:189–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhang W, Zhao P, Xu XL, Cai L, Song ZS,
Cao DY, Tao KS, Zhou WP, Chen ZN and Dou KF: Annexin A2 promotes
the migration and invasion of human hepatocellular carcinoma cells
in vitro by regulating the shedding of CD147-harboring
microvesicles from tumor cells. PLoS One. 8:e672682013. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zhao P, Zhang W, Tang J, Ma XK, Dai JY, Li
Y, Jiang JL, Zhang SH and Chen ZN: Annexin II promotes invasion and
migration of human hepatocellular carcinoma cells in vitro via its
interaction with HAb18G/CD147. Cancer Sci. 101:387–395. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Nedjadi T, Kitteringham N, Campbell F,
Jenkins RE, Park BK, Navarro P, Ashcroft F, Tepikin A, Neoptolemos
JP and Costello E: S100A6 binds to annexin 2 in pancreatic cancer
cells and promotes pancreatic cancer cell motility. Br J Cancer.
101:1145–1154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|