Introduction
Head and neck squamous cell carcinoma (HNSCC) is one
of the most common types of cancer worldwide (1). Despite advancements in the treatment
of HNSCC over the past few decades, the clinical outcomes for
advanced HNSCC patients remain dismal. Therefore, further
elucidation of the molecular basis of HNSCC is critical for
identifying molecular abnormalities that contribute to HNSCC
progression. These abnormalities may serve as potential biomarkers
for predicting clinical outcomes and/or molecular targets to
improve the treatment of HNSCC patients.
Histone demethylases control the expression of
downstream genes, which are known to promote cancer development
(2,3). PLU-1/JARID1B (jumonji AT-rich
interactive domain 1B) is a member of the JARID1/KDM5 protein
family. Similar to other members of the JARID1 family,
PLU-1/JARID1B functions as a demethylase that is responsible for
demethylation of lysine 4 of histone 3 trimethylation (H3K4me3)
(4,5). PLU-1/JARID1B is highly expressed in
testis tissues and in differentiated mammary glands, while
expressed at low levels in other adult tissues (6–8).
PLU-1/JARID1B overexpression has been observed in breast cancer,
which contributes to tumor cell proliferation (5,9–11).
PLU-1/JARID1B expression is also increased in bladder and lung
cancers (12). The downregulation
of PLU-1/JARID1B expression has been reported to repress cancer
cell proliferation via regulating the E2F/RB1 cell cycle pathway
(12). Roesch et al
(13) reported that PLU-1/JARID1B
could help characterize a subpopulation of slow-cycling cells in
melanoma, revealing that PLU-1/JARID1B-positive cells are essential
for continuous growth. Other groups have reported that KDM5 family
members, including PLU-1/JARID1B, are highly expressed in embryonic
stem cells (14,15). These results suggest that
PLU-1/JARID1B may play a role in maintaining stem cell properties
in melanoma. As a testis/cancer-related gene with a potential role
in stem cells, PLU-1/JARID1B may also contribute to the
tumorigenesis and development of HNSCCs.
The aim of the present study was to investigate the
expression patterns and explore the functional role of
PLU-1/JARID1B in HNSCCs.
Materials and methods
Patients and tissue
Ninety-nine HNSCC tissue samples were obtained from
the Ninth People’s Hospital of the Shanghai Jiao Tong University
School of Medicine. Tissues were obtained from patients diagnosed
with primary HNSCC from 1999 to 2009. Tumor tissues were stained
with hematoxylin and eosin (H&E) and classified histologically.
Patients with HNSCC were staged according to the International
Union Against Cancer TNM classification criteria (5th edition).
Three normal oral mucosa tissues were obtained from the third molar
extraction gingival tissue or palatal epithelial tissues during
palatoplasty. The research protocol was reviewed and approved by
the Institutional Review Board. All of the patients signed written
informed consent in accordance with institutional guidelines.
Immunohistochemistry and evaluation
Tissue sections were deparaffinized in xylene,
rehydrated in graded ethanol, treated with Tris-ethylene diamine
tetraacetic acid buffer (for PLU-1/JARID1B) or citrate buffer (for
Ki-67) for antigen retrieval and quenched in hydrogen peroxide. The
tissue sections were then incubated overnight at 4°C with an
anti-PLU-1/JARID1B antibody (1:1,000 dilution; Abgent, San Diego,
CA, USA) and 1 h at room temperature with a Ki-67 antibody (1:200
dilution; Shanghai Sunbio Co., Shanghai, China). The labeling index
was defined as the intensity of staining (0, 1, 2 or 3) multiplied
by the percentage of positive tumor cells (25, 50, 75 or 100%). A
pathologist examined 7 to 10 tumor areas in each section to
calculate the final scores (ranging from 0 to 300). For
PLU-1/JARID1B, scores marked as 0 were considered negative
(negative staining), scores below 100 were marked as weakly
positive (mild staining), and scores between 100 and 300 were
marked as strongly positive (intense staining). For Ki-67, scores
>100 were defined as Ki-67 high, and scores ≤100 were considered
Ki-67 low.
Cell culture
HNSCC cell lines (HN4, HN6, HN12, HN13 and HN30)
were cultured in Dulbecco’s modified Eagle’s medium (DMEM;
Gibco-BRL, Grand Island, NY, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Gibco-BRL), penicillin
(100 U/ml), and streptomycin (100 μg/ml) at 37°C in a
humidified 5% CO2 atmosphere. CAL27, SCC4, SCC9 and
SCC25 cells (purchased from the American Type Culture Collection,
Manassas, VA, USA) were cultured in DMEM/F12 medium (Gibco-BRL)
supplemented with 10% heat-inactivated FBS, penicillin (100 U/ml)
and streptomycin (100 μg/ml). Immortalized oral epithelial
cells infected with human papillomavirus type 16-E6/E7 (HPV16E6/E7)
were cultured in a defined keratinocyte serum-free medium
(Gibco-BRL) (16). HIOEC cells,
which are immortalized oral epithelial cells infected with human
papillomavirus type 16-E6E7 (HPV16 E6/E7), were cultured in a
defined keratinocyte serum-free medium (17). Normal human oral mucosa tissues were
harvested from the area adjacent to the area of tooth extraction at
the Oral and Maxillofacial Surgery Outpatient Clinic. The tissue
was incubated with dispase overnight to separate the surface
epithelium from the underlying fibrous connective tissue. Then, the
epithelium was trypsinized to prepare a single-cell suspension.
Primary normal human oral mucosa cells were cultured in the
keratinocyte serum-free medium as previously described (18).
PCR and western blot analyses
Real-time PCR reactions were performed as previously
described (19). The following
primers were used to detect PLU-1/JARID1B and β-actin mRNA:
5′-TCAGTGCAGAGAGCCAGAGA-3′ and 5′-ATCG AGGACACAGCACCTCT-3′; and
5′-CCTGGCACCCAGC ACAAT-3′ and 5′-GGGCCGGACTCGTCATACT-3′,
respectively. The ΔΔCt method was applied to calculate the
PLU-1/JARID1B expression levels in the HNSCC cell lines, as
previously described (20). For
western blotting, cells were lysed in radioimmunoprecipitation
assay buffer (Sigma-Aldrich, St. Louis, MO, USA). The lysates were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis. Primary antibodies against PLU-1/JARID1B
(Proteintech, Chicago, IL, USA) were used to evaluate protein
levels, and GAPDH or β-actin antibodies (Santa Cruz Biotechnology
Inc., Santa Cruz, CA, USA) were used to normalize protein loading
as previously described (20).
Generation of stable PLU-1/JARID1B
knockdown cell lines
Short-hairpin RNA (shRNA) synthetic
oligodeoxynucleotides were cloned into the pLKO.1-puro vector
(Sigma-Aldrich) according to the manufacturer’s instructions. The
sequences of the annealed oligonucleotides (against PLU-1/JARID1B)
were as follows: sh14760-F, 5′-CCGGTCCTCTCCAAGATGTGG
ATATActcgagTATATCCACATCTTGGAGAGGTTTTTG-3′ and sh14760-R,
5′-AATTCAAAAACCTCTCCAAGATGT GGATATActcgagTATATCCACATCTTGGAGAGGA-3′;
and 14761-F, 5′-CCGGTCCTGAGGAAGAGGAGTATCTTctcgag
AAGATACTCCTCTTCCTCAGGTTTTTG-3′ and 14761-R,
5′-AATTCAAAAACCTGAGGAAGAGGAGTATCTTctcgag AAGATACTCCTCTTCCTCAGGA-3′.
Successful cloning and the orientation of the insert were validated
by sequencing and PCR. PLU-1/JARID1B-shRNA-containing viral
particles were produced in packaging cells (HEK 293T) after
co-transfection with compatible packaging plasmids. Then, viral
particle-infected HN4 and HN13 cells were selected with puromycin
(1.0 g/ml; Gibco) for 2 weeks to obtain stable
PLU-1/JARID1B-knockdown cells.
Cell proliferation and colony formation
assays
To analyze the proliferation potential of HN4 and
HN13 cells stably expressing pLKO.1-puro-vector and
pLKO.1-puro-PLU-1/JARID1B-shRNA, cells were plated in 96-well
plates at 1×103 cells/well and maintained at 37°C in a
humidified incubator. At the indicated time-points (day 1, 3, 5, 7,
9 and 11), 10 μl of CCK-8 solution (Dojindo Laboratories,
Kumamoto, Japan) was added to the wells and incubated for 1 h, and
the absorbance was measured at 450 nm to calculate the number of
viable cells in each well. Measurements were performed in
quadruplicate, and the mean (standard deviation) optical density
was reported.
Briefly, HN4 and HN13 (1×103 cells/plate)
cells stably expressing PLU-1/JARID1B-shRNAs and pLKO.1-puro-vector
were seeded in a 10-mm plate and cultured in complete medium with 1
g/ml puromycin for 2 weeks. Cell colonies were visualized by
staining with 0.25% crystal violet. After washing, the number of
colonies containing >50 cells were counted.
Cell cycle and apoptosis assays
For cell cycle analysis, cells in the logarithmic
growth phase were collected, and single-cell suspensions were
permeabilized with 70% ethanol. Then, cells were labeled with
propidium iodide (PI) and treated with RNase at 4°C for 30 min.
Apoptosis was assessed using Annexin V-PI staining without ethanol
fixation, and the stained cells were quantified by flow cytometry
using the Annexin V-FITC apoptosis detection kit (BD Biosciences,
San Jose, CA, USA) according to the manufacturer’s protocol
(19).
In vivo tumor growth assay
Animal experiments were reviewed and approved by the
Animal Ethics Committee of the Ninth Hospital, Shanghai Jiaotong
University School of Medicine. Stable PLU-1/JARID1B-knockdown
(shRNA no: sh14761) HN4 cells and stable control (PLKO.1-vector)
HN4 cells were trypsinized and suspended in serum-free medium.
Then, 1×107 cells from each group were bilaterally
implanted subcutaneously into four nude mice (the right side
received the control cells and the left side received the
PLU-1-knockdown cells). Twenty days after inoculation, the mice
were sacrificed, and the tumor volume was analyzed using the
following formula: V = (A × B2)/2.
Statistical analysis
The data statistical analyses were performed using
SPSS (standard version 17.0; SPSS Inc., Chicago, IL, USA).
Real-time PCR analysis results were evaluated using the
Mann-Whitney test for two independent groups. The results of the
cell proliferation, colony formation assay, in vitro cell
cycle, and apoptosis assays were evaluated using Student’s t-tests.
All tests were two-sided, and a P-value <0.05 was considered to
indicate a statistically significant result.
Results
PLU-1/JARID1B is overexpressed in the
HNSCC cell lines
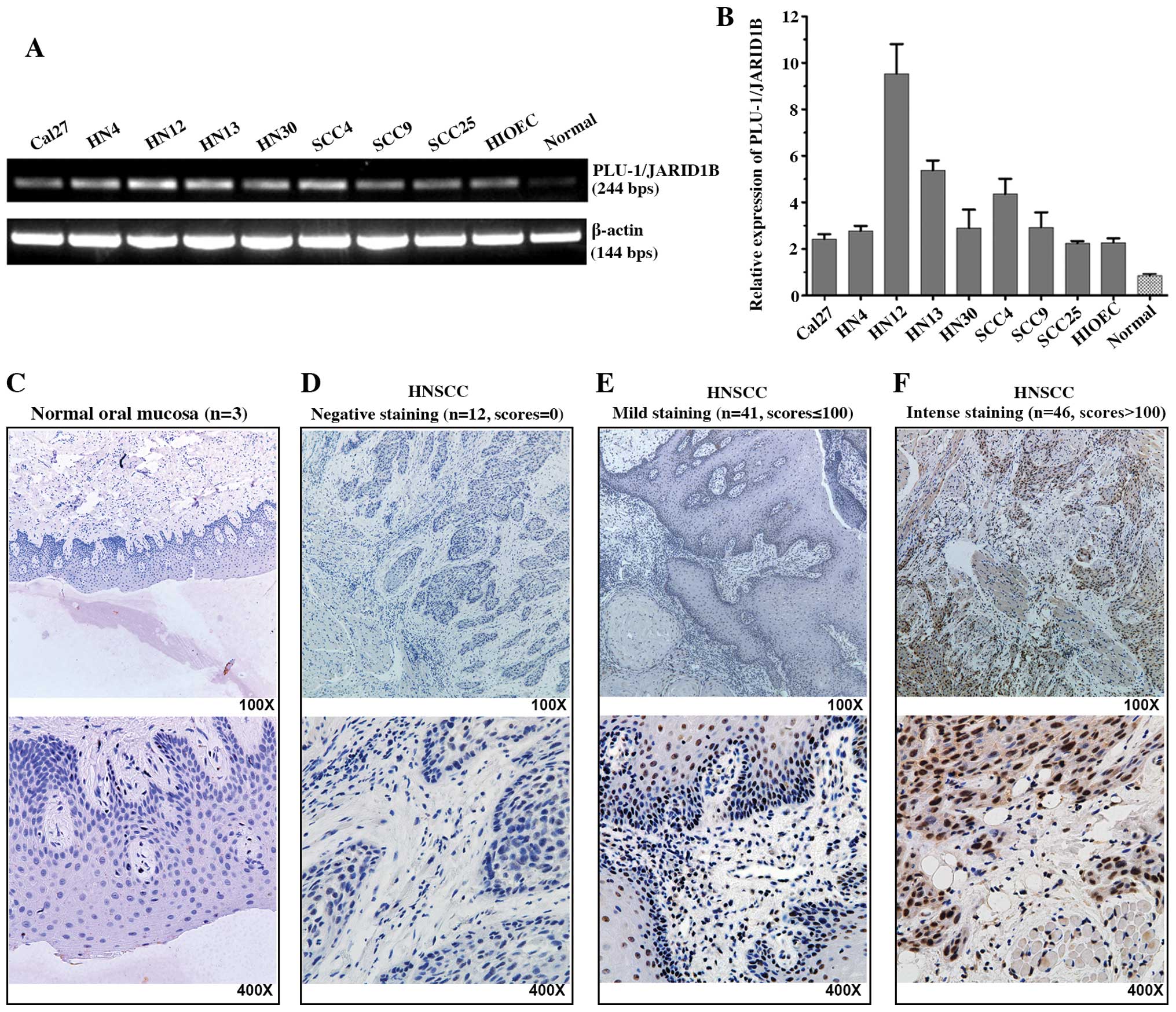
First, we examined the mRNA expression of
PLU-1/JARID1B in 8 HNSCC cell lines, 1 immortalized oral epithelial
cell line (HIOEC) and normal mucosa epithelium cells. The
PLU-1/JARID1B transcription levels were higher in the HNSCCs and
immortalized cell lines compared to the level in the primary
cultured oral normal mucosa epithelium cells (Fig. 1A and B). We analyzed primary tumor
tissues from 99 patients with HNSCC. PLU-1/JARID1B protein was
expressed in 87 (88%) of the tumors; 41 with low levels (scores
≤100) and 46 with high levels (scores >100). In contrast,
PLU-1/JARID1B protein expression was not detected in all 3 normal
oral mucosa epithelium tissues (Fig.
1C–F).
PLU-1/JARID1B expression is associated
with the Ki-67 labeling index
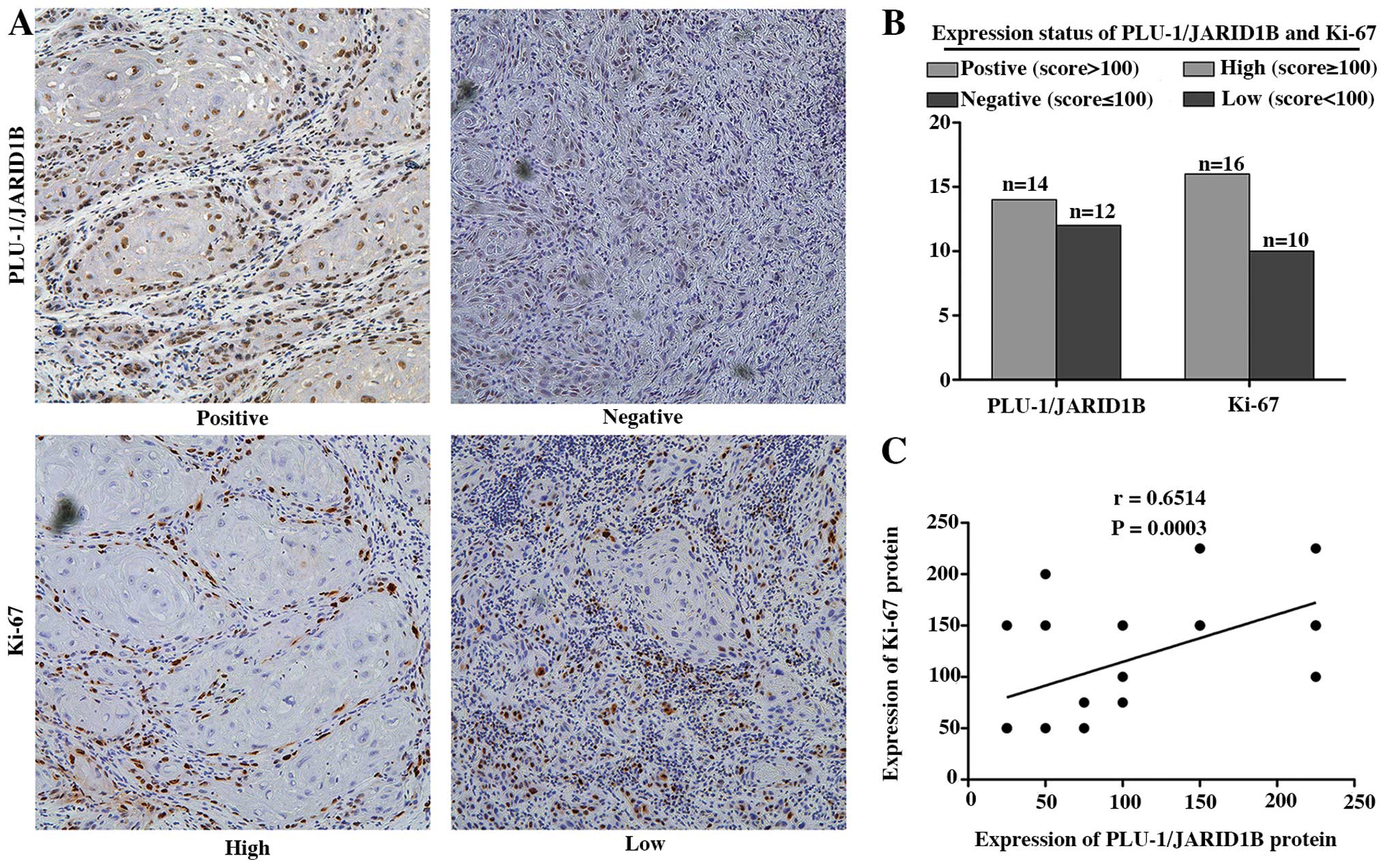
Notably, cells with high PLU-1/JARID1B expression
were concentrated on the proliferating edge of the HNSCC tissues,
leukoplakia and normal testis tissues (data not shown). To
determine a potential association between PLU-1/JARID1B expression
and HNSCC cell proliferation, we analyzed the expression pattern of
PLU-1/JARID1B and the proliferation marker Ki-67 in a set of 26
HNSCC tissues. We observed a statistically significant positive
association between PLU-1/JARID1B expression and Ki-67 indices
[Pearson correlation coefficient (r)=0.6514, P=0.0003] (Fig. 2).
PLU-1/JARID1B knockdown suppresses cell
proliferation in vitro
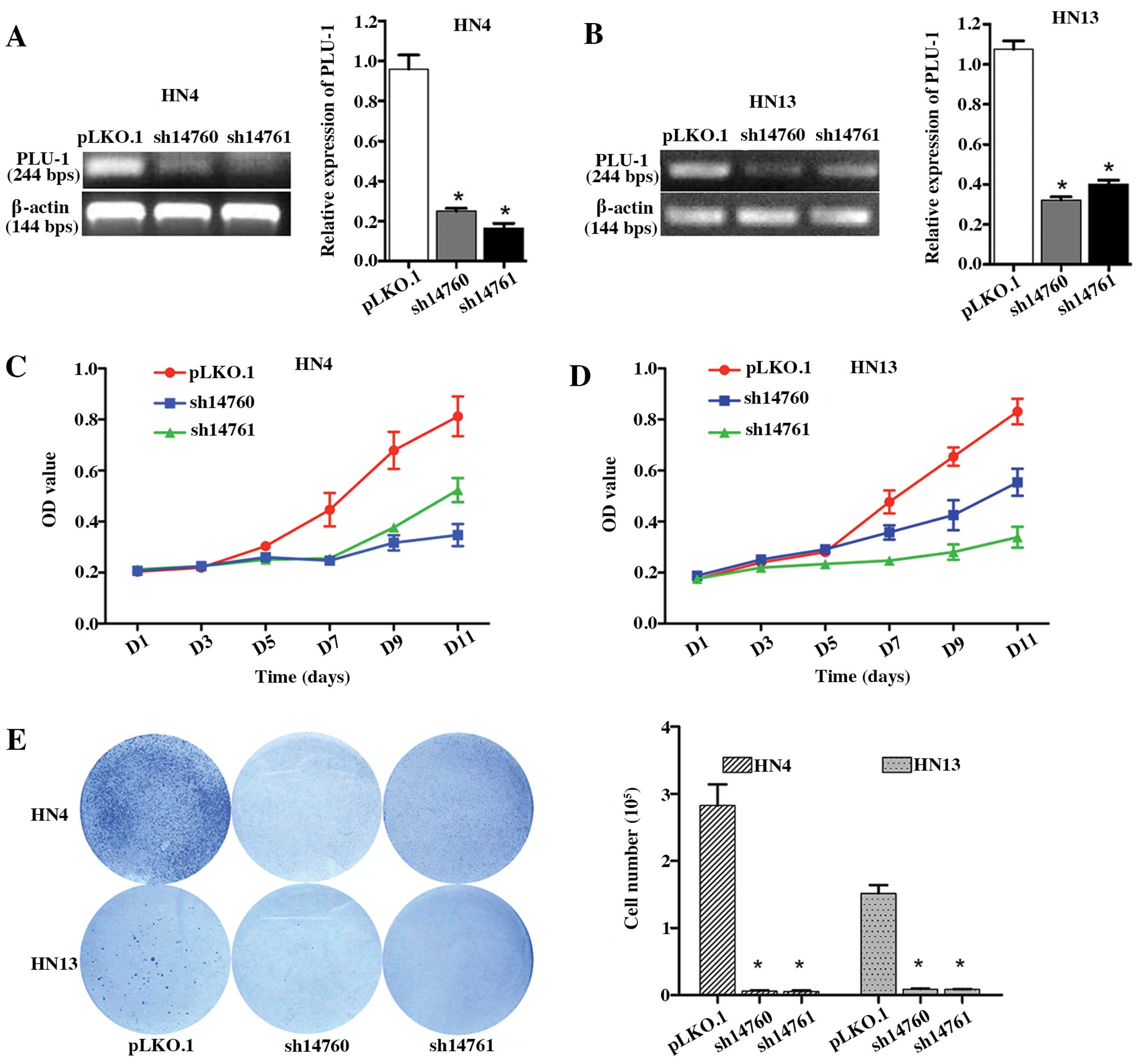
To determine the potential involvement of
PLU-1/JARID1B in HNSCC cell proliferation, we generated stable
PLU-1/JARID1B-knockdown HN4 and HN13 cells using shRNAs. Among the
five shRNAs tested, two markedly downregulated PLU-1/JARID1B
expression levels in both cell lines (Fig. 3A and B). HN4 and HN13 cells with
reduced PLU-1/JARID1B expression exhibited significantly lower
growth compared to the control group (Fig. 3C and D). Consistent with this
result, PLU-1/JARID1B downregulation significantly inhibited the
colony formation in the HN4 (P<0.01) and HN13 (P<0.01) cells
(Fig. 3E).
PLU-1/JARID1B downregulation induces G1
arrest in HNSCC cells
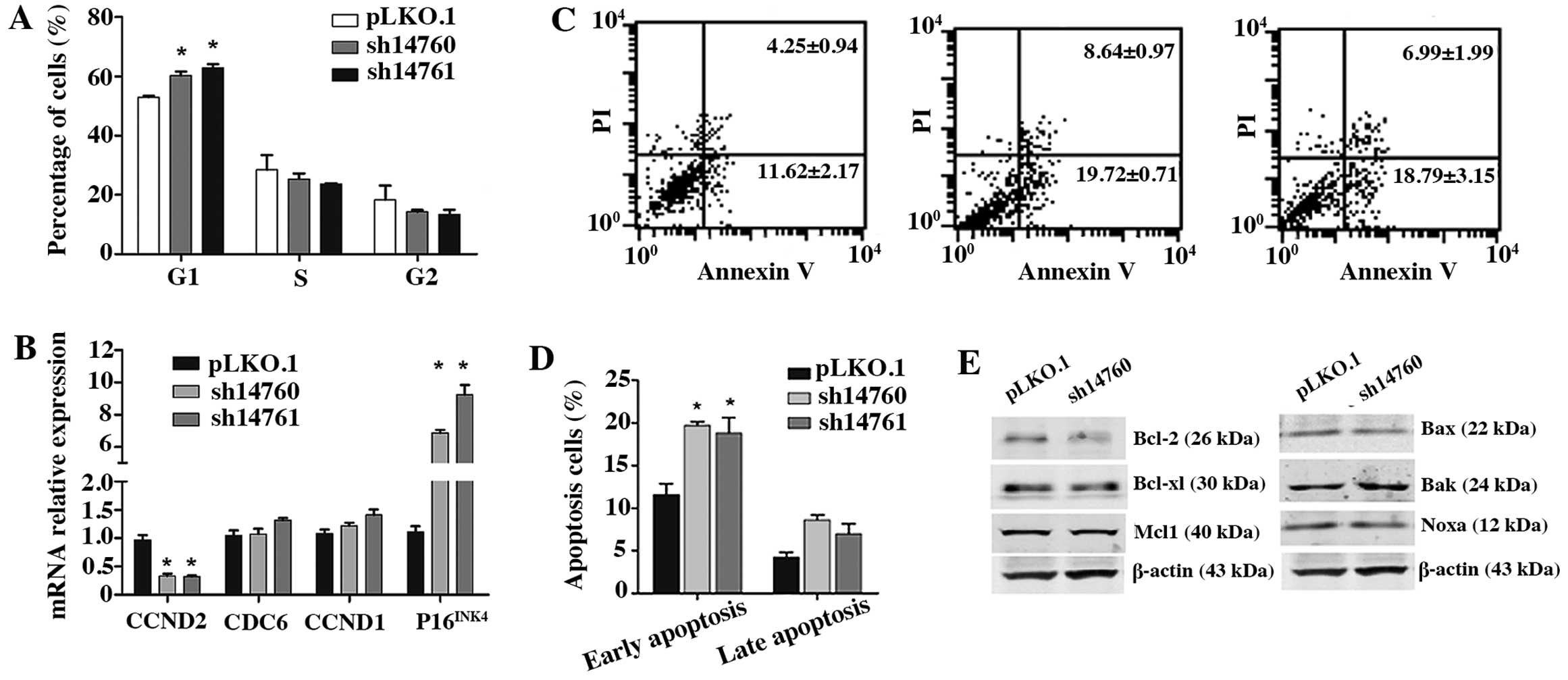
To determine the mechanisms of how PLU-1/JARID1B
downregulation inhibits HNSCC cell growth, we performed cell cycle
analysis in the HN4 cells. FACS analysis revealed that
PLU-1/JARID1B knockdown caused G1 arrest (P<0.05), with no
changes in the S and G2 phases (P>0.05) (Fig. 4A). Moreover, the mRNA level of the
G1 phase-related gene CCND2 was downregulated in the
PLU-1/JARID1B-knockdown cells, whereas P16INK4 was
upregulated (Fig. 4B). These
results suggest that PLU-1/JARID1B may facilitate G1 progression by
regulating CCND2 and P16INK4 expression.
PLU-1/JARID1B is involved in the
regulation of apoptosis
Annexin V and PI double labeling assays demonstrated
that the number of early apoptotic cells was significantly higher
in the PLU-1/JARID1B-knockdown cells compared to the controls
(P<0.05) (Fig. 4C and D).
Consistent with this result, western blot analyses indicated that
Bak protein was upregulated, whereas the anti-apoptotic protein,
Bcl-2, was downregulated in the PLU-1/JARID1B-knockdown cells
(Fig. 4E). These data suggest that
PLU-1/JARID1B maintains tumor cell survival by regulating Bcl
family members.
PLU-1/JARID1B knockdown suppresses cell
growth in vivo
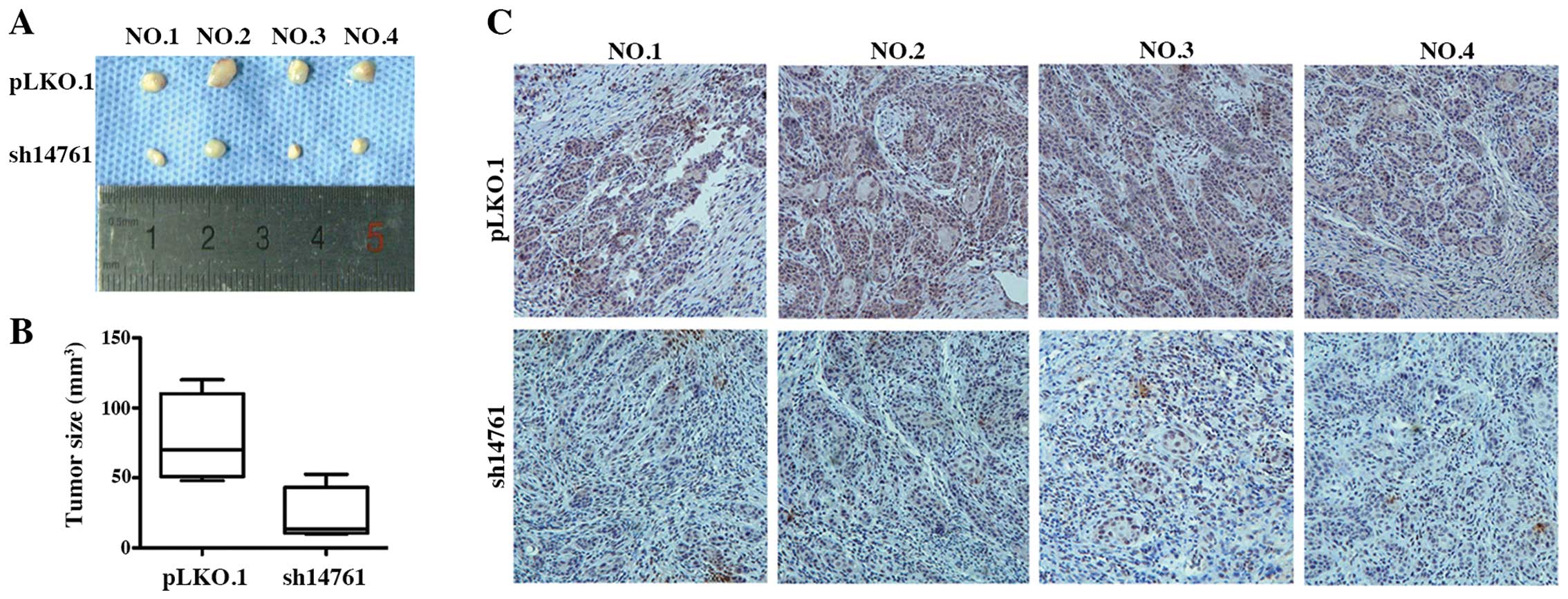
Next, we used a nude mouse xenograft model to
observe tumor formation in vivo. Six days after xenograft
implantation, tumor nodes were observed in the control mice
(HN4-PLKO.1-vector). Over 20 days, tumors in the
PLU-1/JARID1B-knockdown group (HN4-sh14761) grew significantly
slower in the nude mice compared to the control group. Thus,
PLU-1/JARID1B silencing inhibited in vivo tumor growth
(Fig. 5A and B).
Immunohistochemical staining revealed that PLU-1/JARID1B protein
was widely and strongly expressed in the tumors of the control
group, whereas weak PLU-1/JARID1B expression was observed in the
tumors of the PLU-1/JARID1B-knockdown group (Fig. 5C). These results suggest that
PLU-1/JARID1B knockdown inhibited HNSCC growth in a subcutaneous
xenograft nude mouse model.
Discussion
PLU-1/JARID1B is expressed in breast cancers but not
in normal adult tissues at both the mRNA and protein levels
(21). In the present study, we
showed that PLU-1/JARID1B was highly expressed in all of the HNSCC
cell lines tested as well as an immortalized oral epithelial cell
line but not in primary cultured oral normal mucosa cells.
Lu et al (7)
reported that PLU-1/JARID1B is only expressed in the adult testis
and cancer tissues. We confirmed this finding in different cancer
tissues and normal testis samples (data not shown), suggesting that
PLU-1/JARID1B is a cancer/testis antigen. The aberrant expression
of cancer/testis antigens is correlated with DNA promoter
methylation status in different types of cancers (22,23).
In the present study, we observed that several transcription
factors might bind to the PLU-1/JARID1B promoter and regulate its
transcriptional activity, but further studies are needed to confirm
this observation. Since the PLU-1/JARID1B promoter is GC rich, we
hypothesized that promoter hypermethylation may block the binding
of these transcription factors to DNA promoters in HNSCC, leading
to the abnormal expression of PLU-1/JARID1B (24–26).
Similar to other cancer/testis antigens, such as MAGE, BAGE and
GAGE, systemic PLU-1/JARID1B knockout led to early embryonic
lethality in mice (9). Given the
similarities between embryonic stem cells and cancer stem cells, it
is possible that PLU-1/JARID1B may play a role in HNSCC progression
and could serve as a diagnostic biomarker. Further studies are
warranted to determine the stem cell-related properties of
PLU-1/JARID1B.
The Ki-67 labeling index has been linked to cancer
cell proliferation and poor clinical outcomes in HNSCC patients
(27–29). We observed a positive association
between PLU-1/JARID1B expression and Ki-67 labeling in HNSCC
tissues. Unfortunately, the small sample size and incomplete
follow-up data from the patient cohort prevented us from drawing a
definitive conclusion for the potential associations between
PLU-1/JARID1B expression and clinical parameters.
Roesch et al (13) reported that the expression pattern
of PLU-1/JARID1B in HNSCC was quite different from the expression
pattern in melanomas. They explained that this observation was
because PLU-1/JARID1B is highly expressed in differentiated
carcinoma cells, and epithelial carcinomas generally harbor more
differentiated cells than melanomas. They concluded that
PLU-1/JARID1B could be used as a biomarker for characterizing a
subpopulation of slow-cycling melanoma cells. They also found that
PLU-1/JARID1B knockdown initially accelerated tumor growth in
melanoma cells, similar to the proliferative role of PLU-1/JARID1B
protein observed in the present study. Thus, the potential cancer
stem cell property of PLU-1/JARID1B warrants investigation, as it
may represent another significant role for PLU-1/JARID1B in HNSCC
progression. Researchers reported that PLU-1/JARID1B knockdown
upregulated HOXA5 (5), which could
induce p53 expression and promote apoptosis in breast cancer cells
(30). In our
PLU-1/JARID1B-knockdown HNSCC cells, Bcl-2 family members were
regulated in response to decreased PLU-1/JARID1B expression.
In conclusion, PLU-1/JARID1B is overexpressed in
HNSCCs and is associated with tumor proliferation. PLU-1/JARID1B
knockdown induces tumor apoptosis by suppressing Bcl-2 family
members. The mechanism underlying how these proteins are regulated
by histone demethylases need to be studied in the future.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (30973343, 81072171 and
91229103) and by projects of the Shanghai Science and Technology
Committee (11DZ2291800 and 10DZ1951300) and by Shanghai Jiaotong
University School of Medicine Doctoral Innovation Foundation
(BXJ201227).
References
|
1
|
Sun Q, Zhang J, Cao W, Wang X, Xu Q, Yan
M, Wu X and Chen W: Dysregulated miR-363 affects head and neck
cancer invasion and metastasis by targeting podoplanin. Int J
Biochem Cell Biol. 45:513–520. 2013. View Article : Google Scholar
|
|
2
|
Amente S, Lania L and Majello B: The
histone LSD1 demeth-ylase in stemness and cancer transcription
programs. Biochim Biophys Acta. 1829:981–986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jithesh PV, Risk JM, Schache AG, Dhanda J,
Lane B, Liloglou T and Shaw RJ: The epigenetic landscape of oral
squamous cell carcinoma. Br J Cancer. 108:370–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang PH, Chen CH, Chou CC, Sargeant AM,
Kulp SK, Teng CM, Byrd JC and Chen CS: Histone deacetylase
inhibitors stimulate histone H3 lysine 4 methylation in part via
transcriptional repression of histone H3 lysine 4 demethylases. Mol
Pharmacol. 79:197–206. 2011. View Article : Google Scholar :
|
|
5
|
Yamane K, Tateishi K, Klose RJ, Fang J,
Fabrizio LA, Erdjument-Bromage H, Taylor-Papadimitriou J, Tempst P
and Zhang Y: PLU-1 is an H3K4 demethylase involved in
transcriptional repression and breast cancer cell proliferation.
Mol Cell. 25:801–812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barrett A, Santangelo S, Tan K, Catchpole
S, Roberts K, Spencer-Dene B, Hall D, Scibetta A, Burchell J,
Verdin E, et al: Breast cancer associated transcriptional repressor
PLU-1/JARID1B interacts directly with histone deacetylases. Int J
Cancer. 121:265–275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu PJ, Sundquist K, Baeckstrom D, Poulsom
R, Hanby A, Meier-Ewert S, Jones T, Mitchell M, Pitha-Rowe P,
Freemont P and Taylor-Papadimitriou J: A novel gene (PLU-1)
containing highly conserved putative DNA/chromatin binding motifs
is specifically up-regulated in breast cancer. J Biol Chem.
274:15633–15645. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Madsen B, Spencer-Dene B, Poulsom R, Hall
D, Lu PJ, Scott K, Shaw AT, Burchell JM, Freemont P and
Taylor-Papadimitriou J: Characterisation and developmental
expression of mouse Plu-1, a homologue of a human nuclear protein
(PLU-1) which is specifically up-regulated in breast cancer. Mech
Dev. 119(Suppl 1): S239–S246. 2002. View Article : Google Scholar
|
|
9
|
Catchpole S, Spencer-Dene B, Hall D,
Santangelo S, Rosewell I, Guenatri M, Beatson R, Scibetta AG,
Burchell JM and Taylor-Papadimitriou J: PLU-1/JARID1B/KDM5B is
required for embryonic survival and contributes to cell
proliferation in the mammary gland and in ER+ breast
cancer cells. Int J Oncol. 38:1267–1277. 2011.PubMed/NCBI
|
|
10
|
Coleman JA, Correa I, Cooper L, Bohnenkamp
HR, Poulsom R, Burchell JM and Taylor-Papadimitriou J: T cells
reactive with HLA-A*0201 peptides from the histone demethylase
JARID1B are found in the circulation of breast cancer patients. Int
J Cancer. 128:2114–2124. 2011. View Article : Google Scholar
|
|
11
|
Ohta K, Haraguchi N, Kano Y, Kagawa Y,
Konno M, Nishikawa S, Hamabe A, Hasegawa S, Ogawa H, Fukusumi T, et
al: Depletion of JARID1B induces cellular senescence in human
colorectal cancer. Int J Oncol. 42:1212–1218. 2013.PubMed/NCBI
|
|
12
|
Hayami S, Yoshimatsu M, Veerakumarasivam
A, Unoki M, Iwai Y, Tsunoda T, Field HI, Kelly JD, Neal DE, Yamaue
H, et al: Overexpression of the JmjC histone demethylase KDM5B in
human carcinogenesis: involvement in the proliferation of cancer
cells through the E2F/RB pathway. Mol Cancer. 9:592010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roesch A, Fukunaga-Kalabis M, Schmidt EC,
Zabierowski SE, Brafford PA, Vultur A, Basu D, Gimotty P, Vogt T
and Herlyn M: A temporarily distinct subpopulation of slow-cycling
melanoma cells is required for continuous tumor growth. Cell.
141:583–594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmitz SU, Albert M, Malatesta M, Morey
L, Johansen JV, Bak M, Tommerup N, Abarrategui I and Helin K:
Jarid1b targets genes regulating development and is involved in
neural differentiation. EMBO J. 30:4586–4600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stalker L and Wynder C: Evaluation of
histone-modifying enzymes in stem cell populations. Methods Mol
Biol. 809:411–426. 2012. View Article : Google Scholar
|
|
16
|
Zhong LP, Pan HY, Zhou XJ, Ye DX, Zhang L,
Yang X, Chen WT and Zhang ZY: Characteristics of a cancerous cell
line, HIOEC-B(a)P-96, induced by benzo(a)pyrene from human
immortalized oral epithelial cell line. Arch Oral Biol. 53:443–452.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao W, Feng Z, Cui Z, Zhang C, Sun Z, Mao
L and Chen W: Up-regulation of enhancer of zeste homolog 2 is
associated positively with cyclin D1 overexpression and poor
clinical outcome in head and neck squamous cell carcinoma. Cancer.
118:2858–2871. 2012. View Article : Google Scholar
|
|
18
|
Oda D and Watson E: Human oral epithelial
cell culture. I Improved conditions for reproducible culture in
serum-free medium. In Vitro Cell Dev Biol. 26:589–595. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao W, Zhang ZY, Xu Q, Sun Q, Yan M, Zhang
J, Zhang P, Han ZG and Chen WT: Epigenetic silencing of MAL, a
putative tumor suppressor gene, can contribute to human epithelium
cell carcinoma. Mol Cancer. 9:2962010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui Z, Cao W, Li J, Song X, Mao L and Chen
W: TRIM24 overexpression is common in locally advanced head and
neck squamous cell carcinoma and correlates with aggressive
malignant phenotypes. PLoS One. 8:e638872013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barrett A, Madsen B, Copier J, Lu PJ,
Cooper L, Scibetta AG, Burchell J and Taylor-Papadimitriou J: PLU-1
nuclear protein, which is upregulated in breast cancer, shows
restricted expression in normal human adult tissues: a new
cancer/testis antigen? Int J Cancer. 101:581–588. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Glazer CA, Smith IM, Ochs MF, Begum S,
Westra W, Chang SS, Sun W, Bhan S, Khan Z, Ahrendt S and Califano
JA: Integrative discovery of epigenetically derepressed cancer
testis antigens in NSCLC. PLoS One. 4:e81892009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yegnasubramanian S, Haffner MC, Zhang Y,
Gurel B, Cornish TC, Wu Z, Irizarry RA, Morgan J, Hicks J, DeWeese
TL, et al: DNA hypomethylation arises later in prostate cancer
progression than CpG island hypermethylation and contributes to
metastatic tumor heterogeneity. Cancer Res. 68:8954–8967. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SG, Kim AS, Jeong JH, Choi JY and
Kweon H: 4-Hexyl-resorcinol stimulates the differentiation of SCC-9
cells through the suppression of E2F2, E2F3 and Sp3 expression and
the promotion of Sp1 expression. Oncol Rep. 28:677–681.
2012.PubMed/NCBI
|
|
25
|
Kulkarni P, Shiraishi T, Rajagopalan K,
Kim R, Mooney SM and Getzenberg RH: Cancer/testis antigens and
urological malignancies. Nat Rev Urol. 9:386–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao WF, Wang HB, Xie B, Hu LJ, Xu LH,
Kuang BH, Li MZ and Zhang X: Sp1 and Sp3 are involved in the full
transcriptional activity of centromere protein H in human
nasopharyngeal carcinoma cells. FEBS J. 279:2714–2726. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clahsen PC, van de Velde CJ, Duval C,
Pallud C, Mandard AM, Delobelle-Deroide A, van den Broek L and van
de Vijver MJ: The utility of mitotic index, oestrogen receptor and
Ki-67 measurements in the creation of novel prognostic indices for
node-negative breast cancer. Eur J Surg Oncol. 25:356–363. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Colozza M, Azambuja E, Cardoso F, Sotiriou
C, Larsimont D and Piccart MJ: Proliferative markers as prognostic
and predictive tools in early breast cancer: where are we now? Ann
Oncol. 16:1723–1739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Trihia H, Murray S, Price K, Gelber RD,
Golouh R, Goldhirsch A, Coates AS, Collins J, Castiglione-Gertsch M
and Gusterson BA: International Breast Cancer Study Group: Ki-67
expression in breast carcinoma: its association with grading
systems, clinical parameters, and other prognostic factors - a
surrogate marker? Cancer. 97:1321–1331. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raman V, Martensen SA, Reisman D, Evron E,
Odenwald WF, Jaffee E, Marks J and Sukumar S: Compromised HOXA5
function can limit p53 expression in human breast tumours. Nature.
405:974–978. 2000. View
Article : Google Scholar : PubMed/NCBI
|