Introduction
Mammary cancer accounts for ~30% of all cancers
observed in female dogs, displaying pathological and clinical
heterogeneity (1) and exhibiting
several similarities with breast cancer in humans (2,3).
Approximately 50% of the tumors are malignant (4–6). To
date, surgery is the treatment of choice for this disease; however,
this therapeutic option is not feasible in the case of unresectable
or extensive metastatic tumors (4,5).
Currently, there is no effective therapy since some tumor cells may
acquire resistance to commonly available drugs (7). A possible explanation for this
situation is the presence of cancer stem-like cells (CSCs) within
the tumor mass; CSCs exhibit self-renewal, resistance to several
antitumor treatments such as chemotherapy and radiotherapy and
possess tumor-initiating capacity (7,8).
Several signaling pathways have been identified as relevant for
maintaining the capacity of self-renewal and pluripotency of CSCs,
including Wnt/β-catenin, hedgehog and Notch. The canonical
Wnt/β-catenin pathway regulates several cell activities such as
proliferation, migration and self-renewal, important features of
CSCs (9). The chemoresistance
appears to be related with the expression of ATP-binding
transporters such as MDR, assuring efflux capability to CSCs
(7,10). Mammary CSCs are characterized by a
low expression of heat stable antigen CD24 and a high expression of
hyaluronan receptor CD44 (7,9). The
expression of other surface markers, such as CD133 and aldehyde
dehydrogenase (ALDH), has been detected in these cells (9,11).
Mammary CSCs exhibit the capacity to form spheres, structures that
grow from disaggregated solid tumors cultured under harsh
conditions in which only the more undifferentiated cells can
survive and proliferate (7,8).
The statins are a group of commercially available
therapeutic drugs used for the reduction in the circulating levels
of cholesterol (12). These drugs
exhibit additional effects, including an apparent prevention of the
abnormal growth of tissues such as the prostate and mammary gland
(12–15). In humans, several studies have
suggested a link between statin use and a decrease in overall
cancer incidence (15); however,
other groups describe no association between the use of these drugs
and a reduction in cancer risk (16). These controversial findings require
further research to specify the role of statins in cancer. In
veterinary medicine, no data on this topic have been published
making the generation of information vital. Statins reduce serum
cholesterol levels by competitively inhibiting
3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoAR)
preventing the synthesis of cholesterol (13–15).
The inhibition of HMG-CoAR causes a deficit in mevalonate,
decreasing the formation of isoprenoid intermediates such as
farnesyl pyrophosphate (Fpp) and geranylgeranyl pyrophosphate
(GGPP); these participate in protein isoprenylation, a process that
provides lipid attachment sites for some proteins, allowing them to
participate in the regulation of cell survival and migration
(17,18). In addition, it has been described
that the effects of statins may occur via other mechanisms, such as
reduction in the expression of CD44 via a transcriptional
mechanism, non-related with HMG-CoAR (19). Thus, the precise mechanisms by which
statins prevent mammary carcinogenesis are not completely
understood; however, the participation of cell cycle-regulating
proteins appears to be essential for this effect. Lipophilic
statins such as simvastatin and lovastatin show antiproliferative,
antimetastasic and pro-apoptotic effects in mammary tumor cells
(20–22). These effects seem to be more
powerful in estradiol receptor (ER)-negative breast cancer cells
with permanently activated Ras or ErbB2 (mutated epidermal growth
factor receptor) (23–25). Due to the high expression of CD44 in
CSCs, we investigated the effect of statins on CSCs derived from
canine mammary tumor cells. It should be mentioned that Gopalan
et al recently demonstrated that simvastatin and
γ-tocotrienol alone or in mixture decreased CSCs in drug-resistant
human breast tumor cells (26);
these observations appear to be related to the antiproliferative
effect induced by simvastatin on karyotypically abnormal embryonic
stem cells (27).
Based on the above-described data, it was imperative
for us to explore new cytotoxic strategies to increase the efficacy
of antitumor therapies on mammary CSCs, particularly in veterinary
oncology. Therefore, in the present study, we characterized spheres
derived from a canine mammary carcinoma cell line, analyzing the
effects of simvastatin on sphere-forming capacity, cell viability,
apoptosis and cell invasion. In addition, we explored various
proteins (β-catenin and p53) associated with clonogenic ability and
cell survival in response to simvastatin.
Materials and methods
Materials
Cell culture material was obtained from Nalge Nunc
(Rochester, NY, USA). Ultra-low attachment plates were purchased
from Corning (Corning, NY, USA). TGFβ, simvastatin, GGPP and
paclitaxel were purchased from Sigma-Aldrich Inc. (St. Louis, MO,
USA). Doxorubicin was purchased from Tocris Bioscience (Ellisville,
MO, USA). CellTiter 96 Aqueous One Solution Cell Proliferation
Assay and Apo-One Homogeneous Caspase-3/7 assay were purchased from
Promega Corporation (Madison, WI, USA). ApopTag Peroxidase In
Situ Apoptosis Detection kit was obtained from Millipore Co.
(Billerica, MA, USA). BD BioCoat was obtained from BD Biosciences
(Bedford, MA, USA). Most of the other biochemicals used were
purchased from Sigma-Aldrich Inc. and Gibco by Life Technologies
(Carlsbad, CA, USA).
Antibodies
APC rat anti-mouse CD44 clone IM7 (559250), PE rat
anti-mouse CD24 clone M1/69 (553262) and mouse anti-β-catenin
(610154) monoclonal antibodies were obtained from BD Pharmingen
(San Jose, CA, USA). Anti-mouse CD133 monoclonal antibody clone
13A4 (14–1331) was obtained from eBioscience (San Diego, CA, USA).
Polyclonal goat anti-p53 (sc-1311) and polyclonal rabbit anti-pp53
(sc-7997) antibodies were from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). FITC-conjugated goat anti-rat IgG
(pA1–28775) was purchased from Thermo Scientific (Rockford, IL,
USA). Mouse monoclonal anti-β-actin antibody (ab8226) was from
Abcam (Cambridge, UK). Peroxidase-conjugated rabbit anti-goat IgG
(A5420), peroxidase-conjugated goat anti-rabbit IgG (A6667) and
peroxidase-conjugated goat anti-mouse IgG (A9917) were purchased
from Sigma-Aldrich Inc.
Cell line
CF41.Mg epithelial cells from canine mammary cancer
tissue (CRL-6232; ATCC, Manassas, VA, USA), were cultured in
Dulbecco’s modified Eagle’s medium (DMEM) high glucose containing
10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 μg/ml
penicillin, 100 μg/ml streptomycin and 0.25 μg/ml
amphotericin B. In each of the experiments, the cells were cultured
at 37°C in a humidified incubator, in a 5% CO2
atmosphere.
Sphere formation assay
These experiments were performed as describes by
Cocola et al (8). in brief,
cultured CF41.Mg cells were detached and re-suspended in ultra-low
attachment plates with serum-free DMEM/F12 culture medium
containing 10 ng/ml bFGF, 10 ng/ml EGF, 5 μg/ml insulin, 4
μg/ml heparin, B27 and 20 μg/ml penicillin, 20
μg/ml streptomycin and 0.05 μg/ml amphotericin B
(sphere medium). To assess self-renewal capacity, cells derived
from spheres were disaggregated and reseeded in 6-well ultra-low
attachment plates. The spheres that formed were observed and
photographed at low magnification every other day up to 7 days of
culture. The effect of TGFβ (10 ng/ml) on the sphere formation
efficiency was studied by counting at 7 days of culture at low
magnification using an Olympus phase contrast microscope (CKX41
model; Tokyo, Japan).
Flow cytometric analysis
Cells derived from the spheres were dissociated with
0.25% trypsin-EDTA, washed and resuspended with phosphate-buffered
saline (PBS) plus 2% FBS, and then incubated with specific labelled
antibodies against CD44, CD24 and CD133 at 4°C for 45 min, as
described by Michishita et al (7). For CD133 detection, secondary
FITC-conjugated goat anti-rat antibody was used. The cell
populations were analyzed within 30 min by flow cytometry using a
BD FACSCalibur cytometer (BD Biosciences, San Jose, CA, USA).
Cell viability studies
CF41.Mg cells (~5,000/cm2) were seeded in
ultra-low attachment plates and incubated for 24 h; then, the cells
were incubated with several concentrations of doxorubicin and
paclitaxel. After that, incubations with different concentrations
of simvastatin (0–20 μM), in the absence or presence of 30
μM GGPP and doxorubicin were performed. In parallel, similar
experiments on parental cells (grown in DMEM-high glucose plus 10%
FBS) were run. At the completion of the incubation period, cell
viability was estimated using a
3-(4,5-dimethylthiazol-2-yl)-5-(3-carbo-xymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay (CellTiter 96 Aqueous One Solution); MTS is reduced to
formazan by viable cells. The quantity of formazan was measured by
absorbance on a BioTek Synergy MX microplate reader at 490 nm
(Winooski, VT, USA). In addition, the effects of simvastatin on
sphere formation efficiency were conducted. Each experiment was
performed at least 3 times in triplicate.
Caspase activity
Apoptosis was analyzed by measuring the activity of
caspase-3/7 (Apo-One Homogeneous Caspase-3/7 assay), following the
manufacturer’s instructions. Briefly, 5,000 cells/cm2
were seeded in the sphere medium without or with different
concentrations of simvastatin, for 3–6 days. Caspase-3/7 activity
was measured with a BioTek Synergy Mx microplate reader at an
excitation wavelength of 485 nm and emission wavelength of 520 nm
(Winooski, VT, USA). Caspase-3/7 activity was expressed relative to
the number of total living cells.
TUNEL assay
The in situ staining of DNA strand breaks, a
typical characteristic of end apoptotic cells was detected by the
TUNEL (ApopTag Peroxidase In Situ Apoptosis Detection kit)
assay according to the manufacturer’s instructions. Cells derived
from the spheres were exposed to 10 μM simvastatin for 3 and
6 days, fixed in alcoholic glyoxal for 6 h and sedimented in warmed
1% agarose. After solidification, the cell blocks were dehydrated,
embedded in paraffin, cut (4 μm) and rehydrated. The
sections were digested with proteinase K and endogenous peroxidase
quenched. After several washes, the slides were incubated with TdT
enzyme for 60 min in a humidified chamber at 37°C. The samples were
then washed and incubated with anti-digoxigenin conjugate for 30
min at room temperature. After extensive washes, the samples were
developed with 3,3′-diaminobenzidine, counterstained with Mayer’s
hematoxylin, dehydrated and mounted with Eukitt (Foster City, CA,
USA). Finally, the samples were inspected with an Olympus light
microscope (FSX100 model) fitted with a color CCD camera. In each
experiment, the images were captured under fixed settings of
illumination, exposure times and camera gain.
Immunocytochemistry assays
Spheres were exposed to 10 μM simvastatin for
1, 6 and 16 h, fixed in alcoholic glyoxal for 6 h and sedimented in
warmed 1% agarose. After that, the cell blocks were dehydrated,
embedded in paraffin, cut and rehydrated. The sections were
subjected to heat antigen retrieval in a microwave with 0.01 M
citrate buffer (pH 6.0) for 20 min. After cooling at room
temperature for 20 min, blocking of endogenous peroxidase was
performed with 10% H202. After several
washes, the slides were blocked with 5% bovine serum albumin for 30
min in a humidified chamber at room temperature. The samples were
then washed and incubated with the anti-β-catenin antibody diluted
1:100 at 4°C overnight. Then, the sections were incubated with
Vector Universal reagent anti-mouse/rabbit IgG (Vector
Laboratories, Inc., Burlingame, CA, USA) for 30 min at room
temperature, following the manufacturer’s instructions. After 3
washes, the samples were developed with vector
3,3′-diaminobenzidine, counterstained with Mayer’s hematoxylin,
dehydrated and mounted with Eukitt. Finally, the samples were
inspected with an Olympus light microscope (FSX100 model) fitted
with a color CCD camera. In each experiment, the images were
obtained under fixed settings of illumination, exposure times and
camera gain.
Invasion assays
These were carried out using BD BioCoat™ Matrigel™
invasion chambers (Transwell® 8-μm pore size,
24-wells; BD Biosciences, Bedford, MA, USA). Cells in the sphere
medium containing 5 μM simvastatin and/or GGPP were
incubated for 48 h against a gradient of 5% FBS. Non-invading cells
were wiped from the upper side of the filter, and the nuclei of the
invading cells were stained with DAPI. After fixation with cold
methanol, the nuclei were inspected by epifluorescence (Olympus
FSX100). For each condition, 3 Transwell units were used in the
experiments; 5 microscopic fields were counted/insert.
Western blot analyses
For total cell protein extraction, statin-exposed
and control cells were washed and harvested by scraping with RIPA
lysis buffer containing 20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM
Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate,
2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4, 1 μg/ml leupetin and protease
inhibitors. Cellular lysates were centrifugated and the protein
content was determined by the Micro BCA™ assay (thermo Scientific,
Rockford, IL, USA). For electrophoresis, 40 μg protein
samples was boiled for 5 min and loaded on 10–15% polyacrylamide
gels. Electrophoresis was run using Bio-Rad’s Mini-PROTEAN
chambers. Bands were electro-transferred onto pVDF membranes.
Immunodetection using appropriate primary antibodies and peroxidase
labelled secondary antibodies, and development with enhanced
chemiluminescence were carried out. Relative levels of total
protein in each sample were determined by stripping the
phospho-specific antibodies from the membrane and re-probing with
antibodies against the non-phosphorylated proteins. The bands were
analyzed with NIH’s ImageJ software.
Statistical analyses
Student’s t-test and ANOVA followed by post
hoc comparisons of means were used to evaluate differences
between samples and the respective controls. p<0.05 was
considered to indicate a statistically significant result. Data
were analyzed with InfoStat for Windows Software, AR.
Results
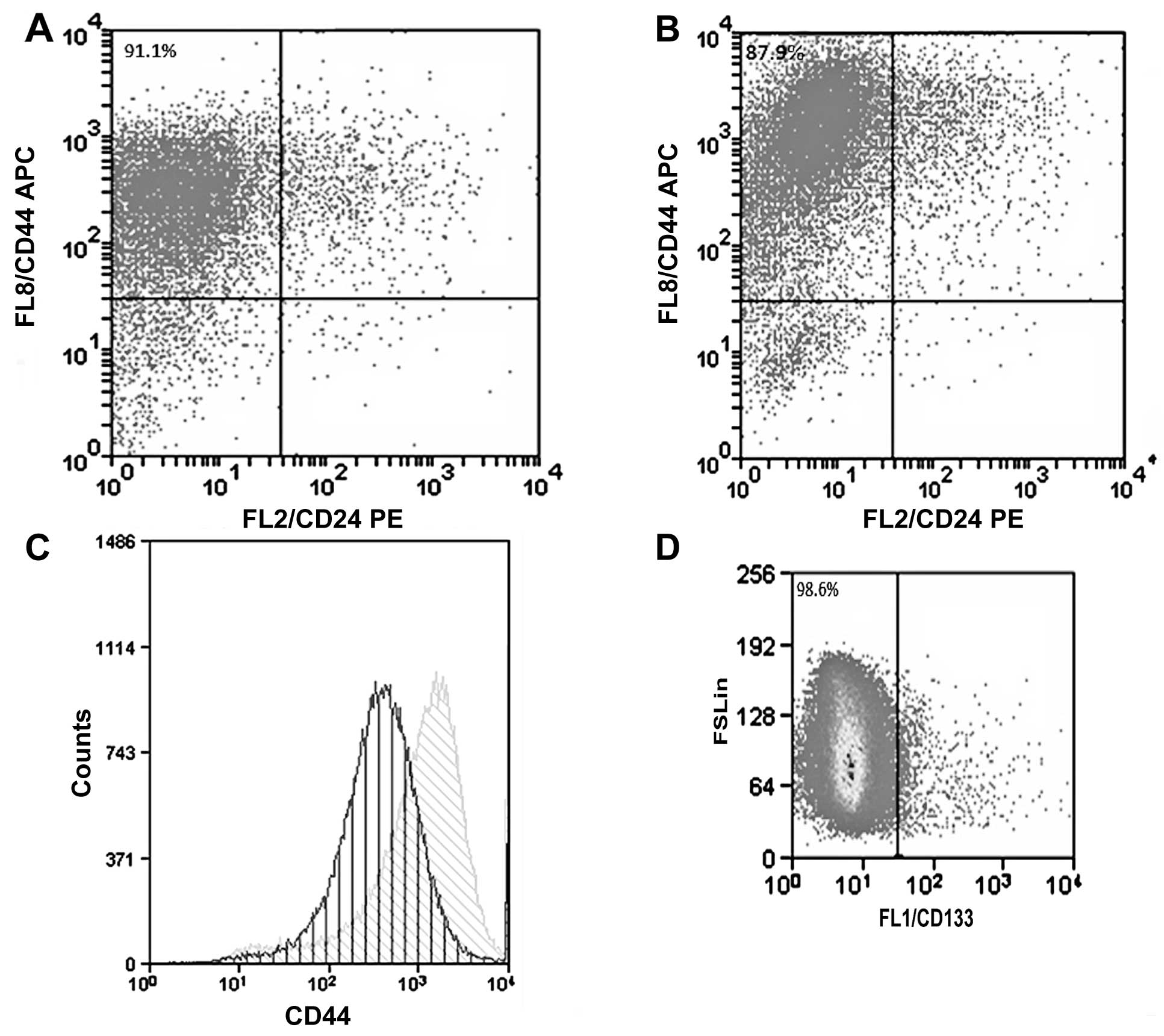
The culture of the CF41.Mg (parental) cells under
anchorage-independent conditions and the absence of FBS allowed the
isolation of spheres. These cell formations showed some
characteristics of stemness, such as the preference for expression
of the CD44+/CD24−/low phenotype; the cells,
however, did not displayed CD133, a surface molecule frequently
present in CSCs. The majority of parental CF41.Mg cells expressed
high levels of CD44+/CD24−/low phenotype
(91.1%), nevertheless spheres expressed a higher amount of CD44
(Fig. 1).
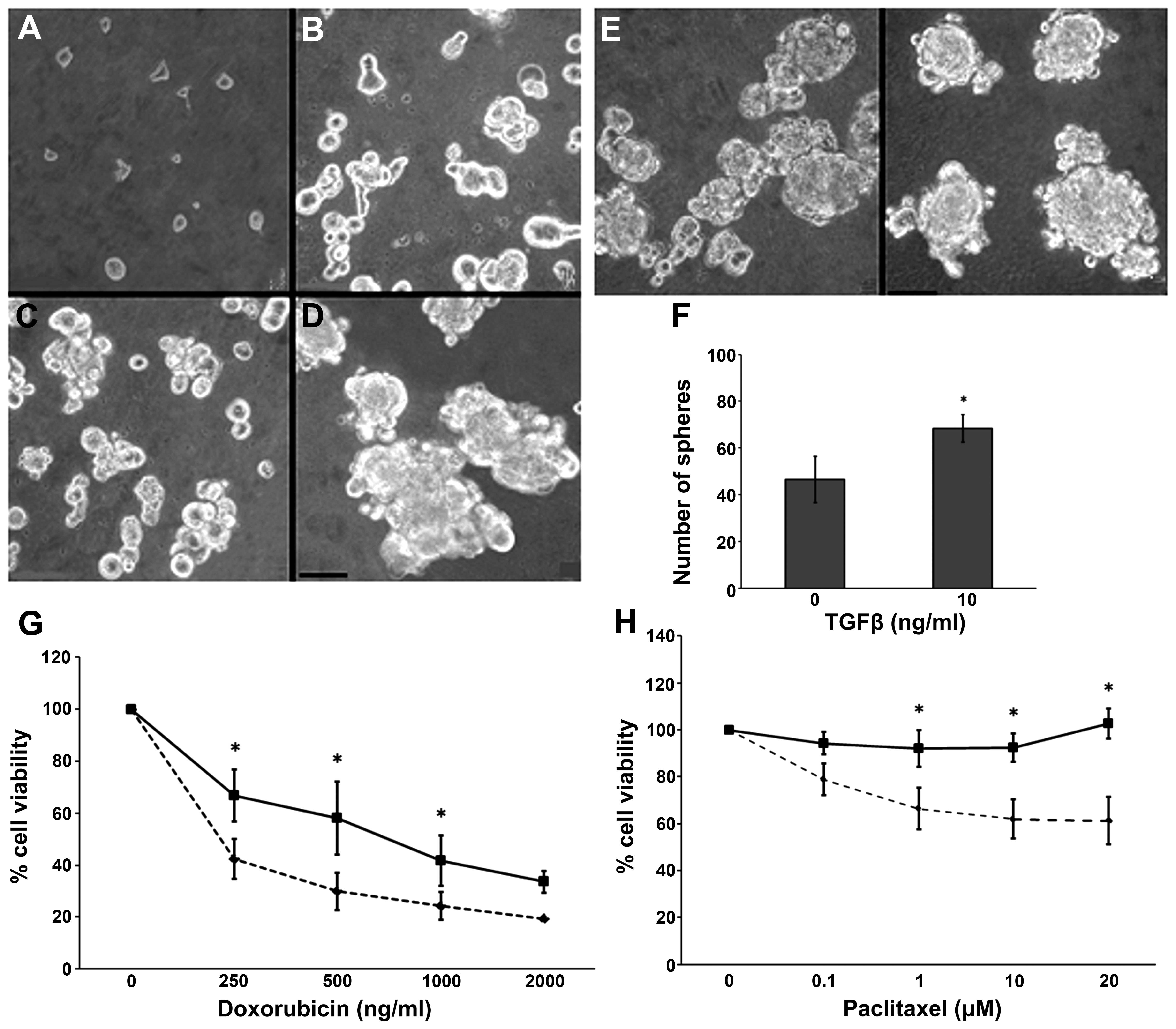
Cells derived from the spheres were disaggregated
and seeded; they showed self-renewal capacity, a classical property
of CSCs (Fig. 2A–D).
Epithelial-mesenchymal transition (EMT) is a process that can be
elicited by TGFβ and increases the proportion of CSCs in certain
tumors. We assessed this effect on the spheres by adding 10 ng/ml
TGFβ to the culture medium. We obtained an increased number of
spheres without any effect on their size (Fig. 2E and F). As compared to the parental
CF41.Mg cells, the sphere-derived cells presented a relative drug
resistance to doxorubicin and paclitaxel. The results from the
resistance analyses are summarized in Fig. 2G and H.
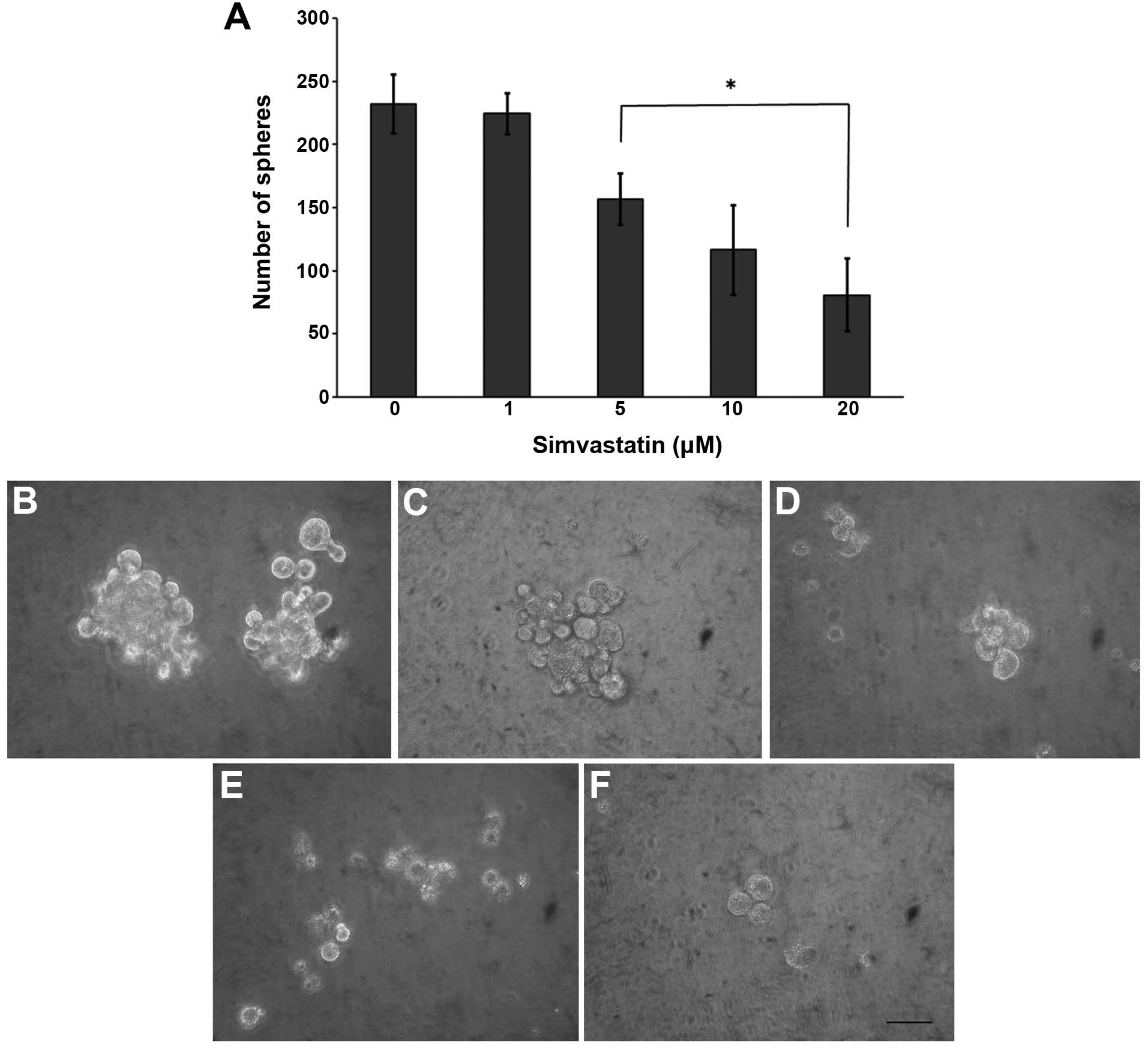
Since simvastatin impairs tumor cells with high CD44
expression, we analyzed its potential antiproliferative effect on
CSCs. The statin significantly decreased the sphere forming
efficiency in a concentration-dependent manner (Fig. 3), reducing both the number and size.
In response to simvastatin, we observed a reduction in β-catenin
expression. It has been speculated that this protein complex is
involved in the self-renewal ability of CSCs (Fig. 4).
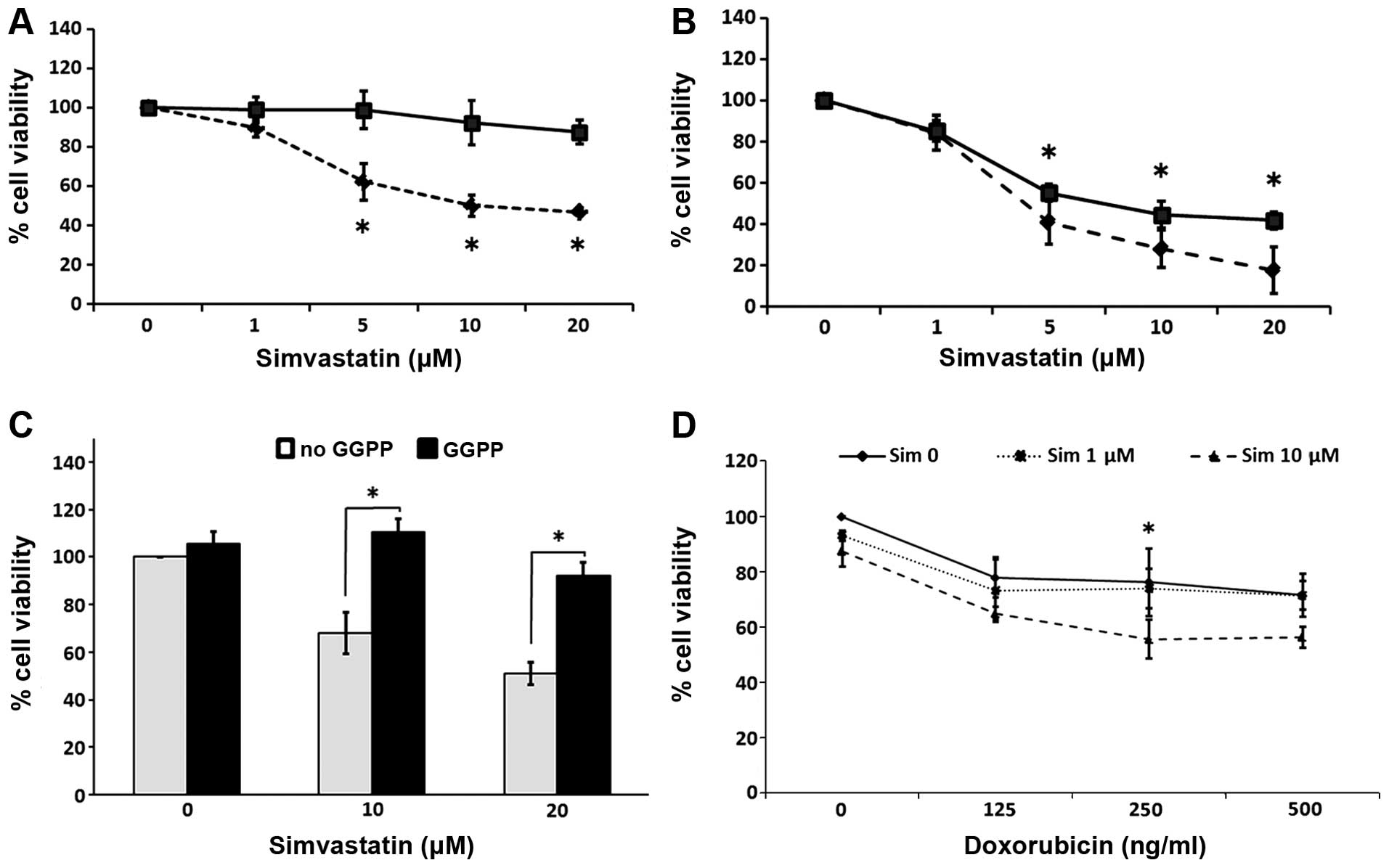
Simvastatin decreased the cell viability in a
concentration-and exposure time-dependent manner, reaching a
maximum effect at 20 μM and after 6 days of contact with the
drug. The spheres exhibited a relative resistance to simvastatin in
comparison with the parental cells, which showed a high sensitivity
to the statin. Representative graphs of these experiments are
presented in Fig. 5A and B. The
simultaneous addition of 30 μM GGPP efficiently blocked the
effects of simvastatin on the spheres (Fig. 5C). To determine whether simvastatin
exerts a synergistic effect with doxorubicin, we assessed the cell
viability in spheres grown in the presence of both drugs. By adding
10 μM of simvastatin, the antiproliferative effect induced
by doxorubicin was enhanced. This cytotoxicity was compared to the
effects of statin alone at 6 days of culture (Fig. 5D).
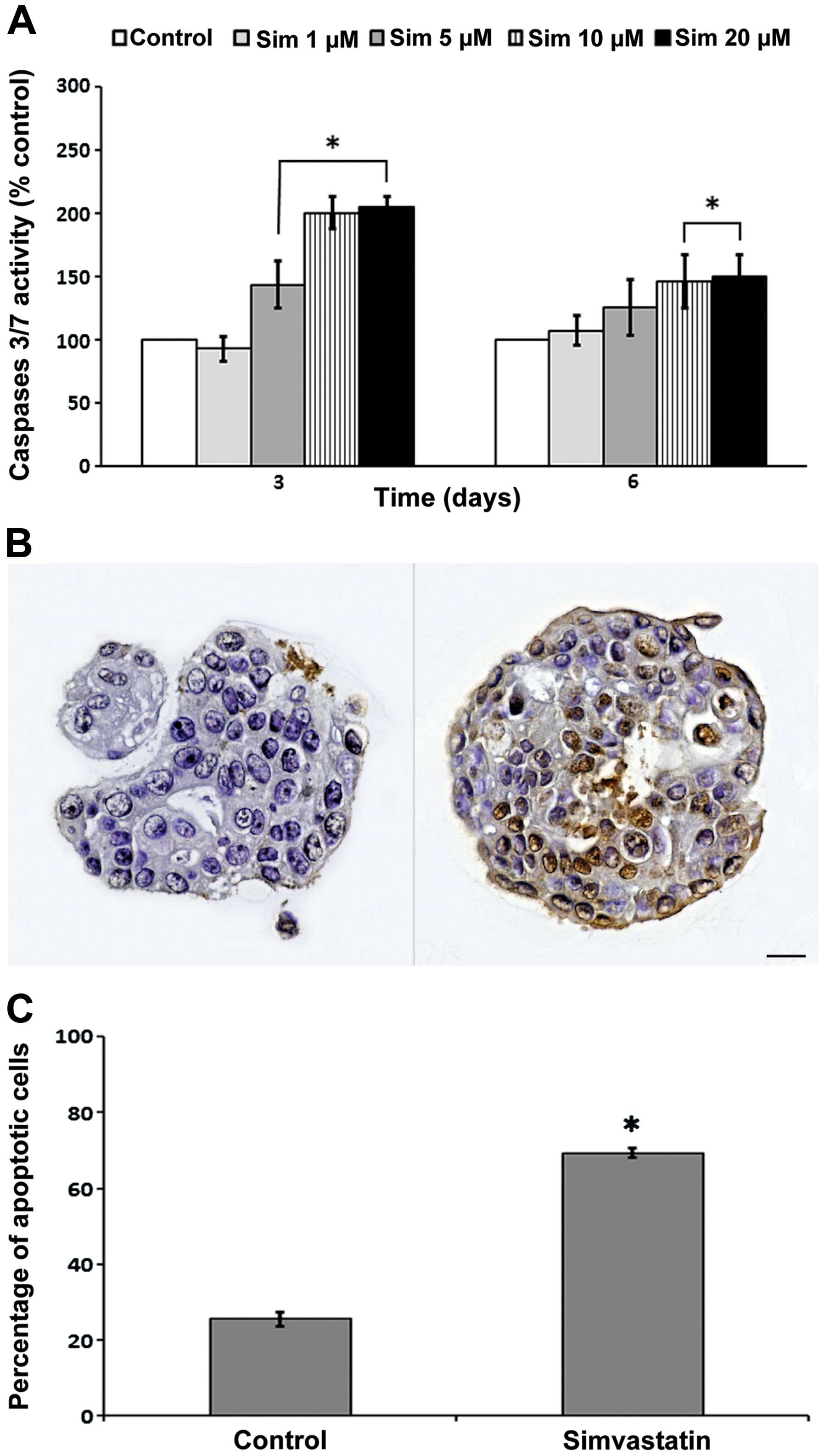
Exposure to simvastatin for 72 and 144 h
significantly induced the activity of caspase-3/7 in relation to
the control (Fig. 6A). This
increased activity was directly proportional to the concentration.
In spite of no evident changes in the proportion of living cells in
response to simvastatin during 3 days of exposure, an increase in
caspase-3/7 activity was observed, suggesting the activation of
apoptotic processes. Therefore, we evaluated apoptosis through the
TUNEL method, based on the detection of DNA strand breaks
characteristic of DNA fragmentation. For this purpose, spheres
grown in presence of 10 μM simvastatin were included in cell
blocks, and then cut and stained. The spheres exposed to
simvastatin for 6 days exhibited a 45.8% increase in apoptotic cell
death compared to the control condition in replicated experiments;
the differences was statistically significant (Fig. 6B and C).
As shown in Fig. 7,
a progressive increase in both p53 expression and its
phosphorylation was detected in the spheres exposed to simvastatin
for 16 h. This was consistent with the antiproliferative and
apoptotic effects of the statin.
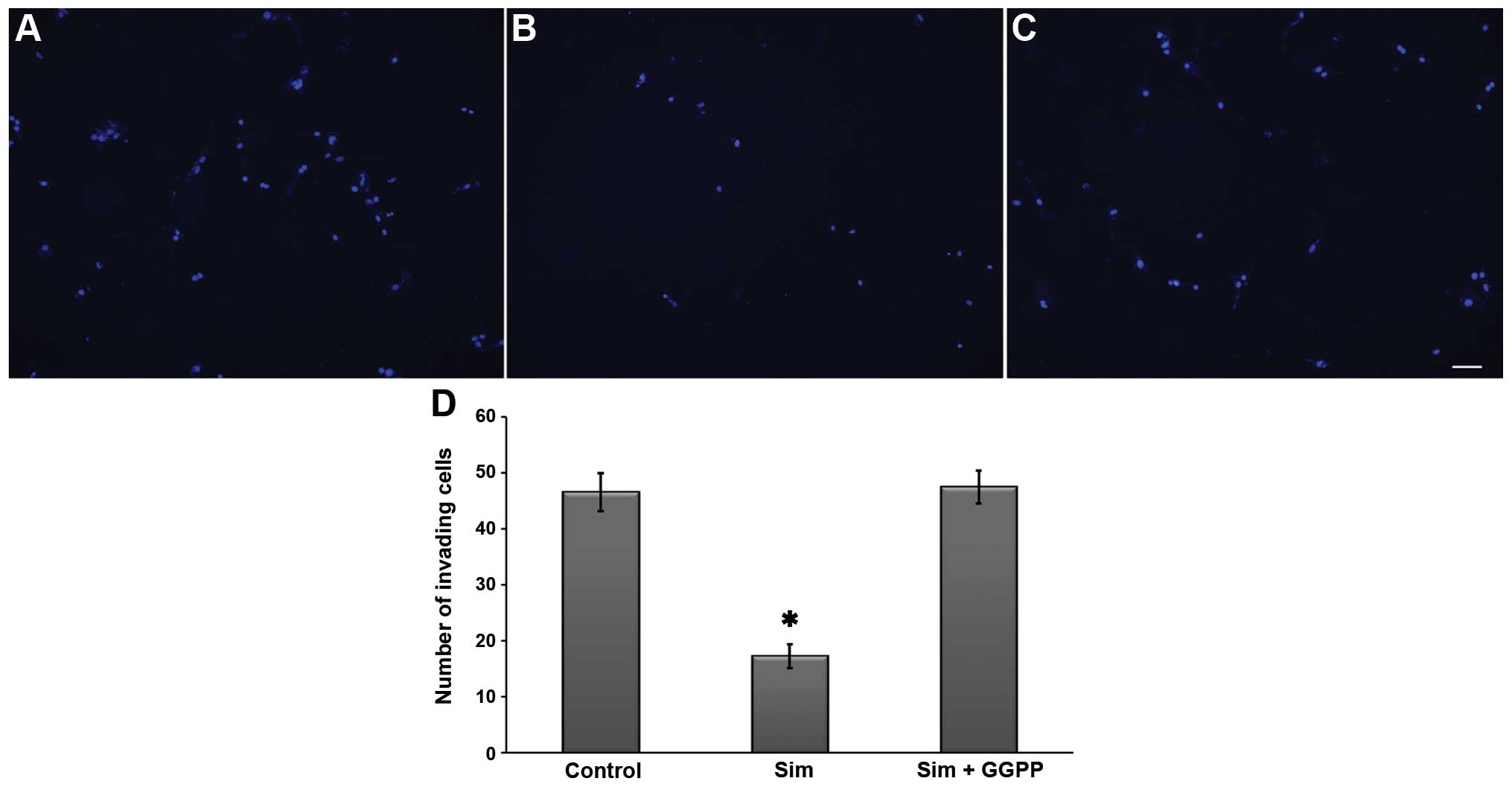
Since spheres have invasive potential, we performed
Transwell invasion assays with 5 μM simvastatin in the
presence or absence of GGPP. As shown in Fig. 8, the results indicated the
inhibition of invasiveness in the presence of the statin. The
effect was hindered by 30 μM GGPP, suggesting that the
anti-invasive effect of simvastatin is dependent on the inhibition
of protein prenylation as observed in the cell viability
analysis.
Discussion
Similar to the mammary gland, solid tumors are
heterogeneous, presenting different cell populations with varying
rates of proliferation and differentiation, In addition, tumors
exhibit variable metastatic capacity and response to treatments
(28,29). According to the cancer stem cell
model, also known as the ʻtumor initiating cell modelʼ, a small
subpopulation of tumorigenic cells located within the tumor exhibit
capacities associated with stemness, that is, exhibit the ability
for continuous self-renewal, differentiation, initiation and
perpetuation of a tumor (30–32).
To date, several groups have reported in vitro and in
vivo data supporting the concept that CSCs are associated with
malignancy (33,34). Yang et al found a high
proportion of cancer stem-like cells in breast cancer tissues of
poor prognosis (basal-like type and triple-negative form),
suggesting that CSCs have prognostic value in this type of tumor
(35). In canine mammary cancer,
preliminary studies have shown that the presence of the
CD44+/CD24− phenotype is directly related to
high histological grade carcinomas (36).
The tumor microenvironment is significant for CSC
development since it regulates the stemness level (31), explaining in part the plasticity
shown by these cells. In our experiments, spheres derived from
CF41.Mg cells exhibited different features consistent with stemness
(anchorage-independent growth, high expression of CD44,
auto-renewal and chemoresistance). However, the cells did not
express CD133, a biomarker usually present in CSCs. In veterinary
literature, there are discordant data concerning the expression of
CD133 in canine mammary CSCs derived from different cell lines
(7,11), supporting the view of a high
phenotypic variability for CSCs. Among others, the tumor
microenvironment is the product of various cell types present,
which promote a distinct pro-inflammatory and pro-tumorigenic
niche. There is evidence that macrophages and adipocytes
participate in this process, releasing molecules involved in CSC
development, such as TNFα and TGFβ that modulate the Wnt/β-catenin
pathway and EMT, respectively (37). In this context, we analyzed the
effect of TGFα on the clonogenic capacity of spheres. The number of
spheres was significantly increased by the treatment confirming
that this growth factor is important to induce CSCs, as previously
proposed (10).
In both canine and human mammary cancers, some CSCs
become resistant to conventional antitumor treatments, including
chemotherapy with doxorubicin, weakening the treatment of many
mammary tumors (7,26,38).
Concordant with the above, we observed that spheres showed
chemoresistance to doxorubicin and paclitaxel in relation to the
parental cells. In the presence of the highest concentrations of
paclitaxel, the spheres tended to disaggregate, yet this did not
mean greater cytotoxicity, suggesting this drug may impair
sphere-forming capacity without changing their viability (data not
shown).
The present study is the first report showing the
antitumor properties of simvastatin on canine mammary carcinoma
cell-derived CSCs. The statin impaired both the sphere-forming
ability and cell viability, generating a maximum effect at 20
μM. The spheres exhibited resistance to simvastatin at 3
days of exposure, with almost no cytotoxicity as compared to the
parental cells.
The canonical Wnt/β-catenin signaling pathway
regulates critical processes related to cancer cells such as
proliferation, migration and differentiation, among others
(39). The self-renewal of cancer
stem cells is also modulated by this pathway (40). Thus, the development of drugs that
inhibit this signaling route is desirable. β-catenin is a protein
that acts as a transcriptional co-regulator, and in conjunction
with E-cadherin, is also involved in cell-to-cell adhesion
(41). In the present study,
β-catenin was expressed in the plasma membrane of cell spheres and
the expression was reduced by simvastatin. These observations
suggest that simvastatin partially impairs sphere-forming ability
and cell viability through this pathway. Wang et al recently
reported that β-catenin signaling is associated with drug
resistance to upregulate ATP binding cassette subfamily G2 (ABCG2)
proteins in high malignant breast cancer (42,43).
On the other hand, activation of Akt and Wnt/β-catenin signaling
confers chemoresistance to CSCs inducing a more efficient DNA
repair mechanism (44). As shown in
Fig. 5D, the addition of 10
μM of simvastatin exerted a synergistic effect with
doxorubicin on spheres at 3 days of exposure, a time point at which
they slightly respond to statin. The above described drug
resistance mechanisms mediated by β-catenin may be inhibited by the
statin through an unclear mechanism, which warrants further
investigation explore in future studies.
According to of our results, simvastatin activate
apoptosis, a process evaluated through caspase-3/7 activity and DNA
fragmentation assays Despite the fact that simvastatin did not
generate changes in cell viability at 3 days of incubation, the
statin induced the activity of caspase-3/7 as a pro-apoptotic
event. After 6 days of exposure to simvastatin, caspase activity
remained and DNA fragmentation was increased, indicating a late
apoptotic event. In this context, these changes have been
associated with an increase in the expression of cell
cycle-negative regulatory proteins such as p53 (19) and p21 (45). We observed a higher expression and
phosphorylation of p53 in response to simvastatin, confirming the
results described in the literature (45,46).
Apoptosis generated by simvastatin is probably related to the
induction of intracellular radical oxygen species (45); the oxidative stress leads to an
increase in p53 by inducing cell arrest and apoptosis (19). Thus, treatment with statin leads to
an increase in pro-apoptotic Bax protein and activation of
caspase-9 and -3 (46). In addition
to the antiproliferative effects produced by simvastatin, this drug
impaired invasiveness of spheres derived from CF41.Mg cells,
promoting an antimetastatic potential effect. Mandal et al
demonstrated that simvastatin decreased the invasive and metastatic
potential of MDA-MB-231 CD44-positive cells (19), which is consistent with our
observations.
Since the main mechanism of action of simvastatin is
the disruption of this pathway through inhibition of HMGCoAR
(14), we evaluated whether some of
the effects of the drug were mediated by post-translational
modifications associated with the inhibition of the mevalonate
pathway. For this purpose, we conducted experiments adding the
isoprenoid GGPP to the culture medium (14). The inhibitory effect induced by the
statin on the cell viability and invasiveness was completely
reverted, suggesting that these properties are dependent on protein
isoprenylation. Several studies have shown that the effects of
simvastatin and other statins are reversed in the presence of GGPP
or mevalonate, but not by cholesterol or farnesyl pyrophosphate
(46). This means that GGPP
synthesis is relevant for the modulation of the activity of
proteins involved in cell migration and survival, through an
association with the plasma membrane. Our results suggest that
simvastatin plays a role in the proliferative behavior of cancer
stem-like cells. Therefore, statins represent valuable potential
agents against CSCs by enhancing the effects of conventional
chemotherapy in canines with mammary tumors.
Acknowledgments
The present study was supported by the Fondecyt
Chile, grant 11110148. We wish to thank Dr Walter D. Sierralta and
Dr Jose I. Arias for their technical assistance.
References
|
1
|
Torres CG, Pino AM and Sierralta WD: A
cyclized peptide derived from α-fetoprotein inhibits the
proliferation of ER-positive canine mammary cancer cells. Oncol
Rep. 21:1397–1404. 2009.PubMed/NCBI
|
|
2
|
Paoloni M and Khanna C: Translation of new
cancer treatment from pets dogs to humans. Nat Rev Cancer.
8:147–156. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khanna C, Lindblad-Toh K, Vail D, London
C, Bergman P, Barber L, Breen M, Kitchell B, Mcneil E, Modiano JF,
Niemi S, Comstock KE, Ostrander E, Westmoreland S and Withrow S:
The dog as a cancer model. Nat Biotechnol. 24:1065–1066. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sorenmo KU, Rasotto R, Zappulli V and
Goldschmidt MH: Development, anatomy, histology, lymphatic
drainage, clinical features, and cell differentiation markers of
canine mammary gland neoplasms. Vet Pathol. 48:85–97. 2011.
View Article : Google Scholar
|
|
5
|
Klopfleisch R, von Euler H, Sarli G, Pinho
SS, Gärtner F and Gruber AD: Molecular carcinogenesis of canine
mammary tumors: news from an old disease. Vet Pathol. 48:98–116.
2011. View Article : Google Scholar
|
|
6
|
Rivera P and von Euler H: Molecular
biological aspects on canine and human mammary tumors. Vet Pathol.
48:132–146. 2011. View Article : Google Scholar
|
|
7
|
Michishita M, Akiyoshi R, Yoshimura H,
Katsumoto T, Ichikawa H, Ohkusu-Tsukada K, Nakagawa T, Sasaki N and
Takahashi K: Characterization of spheres derived from canine
mammary gland adenocarcinoma cell lines. Res Vet Sci. 91:254–260.
2011. View Article : Google Scholar
|
|
8
|
Cocola C, Anastasi P, Astigiano S,
Piscitelli E, Pelucchi P, Vilardo L, Bertoli G, Beccaglia M,
Veronesi MC, Sanzone S, Barbieri O, Reinbold RA, Luvoni GC and
Zucchi I: Isolation of canine mammary cells with stem cell
properties and tumour-initiating potential. Reprod Domest Anim.
44(Suppl 2): S214–S217. 2009. View Article : Google Scholar
|
|
9
|
Pang LY and Argyle D: Cancer stem cells
and telomerase as potential biomarkers in veterinary oncology. Vet
J. 185:15–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pang LY, Cervantes-Arias A, Else RW and
Argyle DJ: Canine mammary cancer stem cells are radio- and
chemo-resistant and exhibit an epithelial-mesenchymal transition
phenotype. Cancers. 3:1744–1762. 2011. View Article : Google Scholar
|
|
11
|
Blacking TM, Waterfall M, Samuel K and
Argyle DJ: Flow cytometric techniques for detection of candidate
cancer stem cell subpopulations in canine tumour models. Vet Comp
Oncol. 10:252–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boudreau DM, Yu O and Johnson J: Statin
use and cancer risk: a comprehensive review. Expert Opin Drug Saf.
9:603–621. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Behbod F and Rosen JM: Will cancer stem
cells provide new therapeutic targets? Carcinogenesis. 26:703–711.
2005. View Article : Google Scholar
|
|
14
|
Demierre MF, Higgins PDR, Gruber SB, Hawk
E and Lippman SM: Statins and cancer prevention. Nat Rev Cancer.
5:930–942. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Campbell MJ, Esserman LJ, Zhou Y,
Shoemaker M, Lobo M, Borman E, Baehne F, Kumar AS, Adduci K, Marx
C, Petricoin EF, Liotta LA, Winters M, Benz S and Benz CC: Breast
cancer growth prevention by statins. Cancer Res. 66:8707–8714.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taylor ML, Wells BJ and Smolak MJ: Statins
and cancer: a meta-analysis of case-control studies. Eur J Cancer
Prev. 17:259–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mück AO, Seeger H and Wallwiener D:
Inhibitory effect of statins on the proliferation of human breast
cancer cells. Int J Clin Pharmacol Ther. 42:695–700. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kusama T, Mukai M, Tatsuta M, Nakamura H
and Inoue M: Inhibition of transendothelial migration and invasion
of human breast cancer cells by preventing geranylgeranylation of
Rho. Int J Oncol. 29:217–223. 2006.PubMed/NCBI
|
|
19
|
Mandal CC and Ghosh-Choudhury N, Yoneda T,
Choudhury GG and Ghosh-Choudhury N: Simvastatin prevents skeletal
metastasis of breast cancer by an antagonistic interplay between
p53 and CD44. J Biol Chem. 286:11314–11327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Borgquist S, Djerbi S, Pontén F,
Anagtosnaki L, Goldman M, Gaber A, Manjer J, Landberg B and
Jirström K: HMG-CoA reductase expression in breast cancer is
associated with a less aggressive phenotype and influenced by
anthropometric factors. Int J Cancer. 123:1146–1153. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu X, Luo Y, Zhou Y, Zhang Q, Wang J, Wei
N, Mi M, Zhu J, Wang B, Chang H and Tang Y: BRCA1 overexpression
sensitizes cancer cells to lovastatin via regulation of cyclin
D1-CDK4-p21WAF1/Cip1 pathway: Analyses using a breast
cancer cell line and tumoral xenograft model. Int J Oncol.
33:555–563. 2008.PubMed/NCBI
|
|
22
|
Kato S, Smalley S, Sadarangani A, Chen-Lin
K, Oliva B, Brañes J, Carvajal J, Gejman R, Owen GI and Cuello M:
Lipophilic but not hydrophilic statins selectively induce cell
death in gynecological cancers expressing high levels of HMGCoA
reductase. J Cell Mol Med. 14:1180–1193. 2010.
|
|
23
|
Kumar AS, Benz CC, Shim V, Minami CA,
Moore DA and Esserman LJ: Estrogen receptor-negative breast cancer
is less likely to arise among lipophilic statin users. Cancer
Epidemiol Biomarkers Prev. 17:1028–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herrero-Martin G and López-Rivas A:
Statins activate a mitochondria-operated pathway of apoptosis in
breast tumor cells by a mechanism regulated by ErbB2 and dependent
on the prenylation of proteins. FEBS Lett. 582:2589–2594. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang S, Kim ES and Moon A: Simvastatin and
lovastatin inhibit breast cell invasion induced by H-Ras. Oncol
Rep. 21:1317–1322. 2009.PubMed/NCBI
|
|
26
|
Gopalan A, Yu W, Sanders BG and Kline K:
Eliminating drug resistant breast cancer stem-like cells with
combination of simvastatin and γ-tocotrienol. Cancer Lett.
328:285–296. 2013. View Article : Google Scholar
|
|
27
|
Gauthaman K, Manasi N and Bongso A:
Statins inhibit the growth of variant human embryonic stem cells
and cancer cells in vitro but not normal human embryonic stem
cells. Br J Pharmacol. 157:962–973. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geng SQ, Alexandrou AT and Li JJ: Breast
cancer stem cells: multiple capacities in tumor metastasis. Cancer
Lett. 349:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lorico A and Rappa G: Phenotypic
heterogeneity of breast cancer stem cells. J Oncol.
2011:1350392011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Ejeh F, Smart CE, Morrison BJ,
Chenevix-trench G, Lopez JA, Lakhani SR, Brown MP and Khanna KK:
Breast cancer stem cells: treatment resistance and therapeutic
opportunities. Carcinogenesis. 32:650–658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cruz MH, Sidén A, Calaf GM, Delwar ZM and
Yakisich JS: The stemness phenotype model. ISRN Oncol.
2012:3926472012.PubMed/NCBI
|
|
32
|
Tang DG: Understanding cancer stem cell
heterogeneity and plasticity. Cell Res. 22:457–472. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou BB, Zhang H, Damelin M, Geles KG,
Grindley JC and Dirks PB: Tumour-initiating cells: challenges and
opportunities for anticancer drug discovery. Nat Rev Drug Discov.
8:806–823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Geng SQ, Alexandrou AT and Li JJ: Breast
cancer stem cells: multiple capacities in tumor metastasis. Cancer
Lett. 349:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang G, Xue F and Chen X: prognostic value
of different amounts of cancer stem cells in different molecular
subtypes of breast cancer. Gland Surg. 1:20–24. 2012.PubMed/NCBI
|
|
36
|
Magalhães GM, Terra EM, de Oliveira
Vasconcelos R, de Barros Bandarra M, Moreira PR, Rosolem MC and
Alessi AC: Immunodetection of cells with a
CD44+/CD24− phenotype in canine mammary
neoplasms. BMC Vet Res. 9:2052013. View Article : Google Scholar
|
|
37
|
Pérez-Hernández AI, Catalán V,
Gómez-Ambrosi J, Rodríguez A and Frühbeck G: Mechanisms linking
excess adiposity and carcinogenesis promotion. Front Endocrinol.
5:652014.
|
|
38
|
Tanabe A, Deguchi T, Sato T, Nemoto Y,
Maruo T, Madarame H, Shida T, Naya Y, Ogihara K and Sahara H:
Radioresistance of cancer stem-like cell derived from canine
tumours. Vet Comp Oncol. Jul 28–2014.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li J and Zhou BP: Activation of β-catenin
and Akt pathways by Twist are critical for the maintenance of EMT
associated cancer stem cell-like characters. BMC Cancer. 11:492011.
View Article : Google Scholar
|
|
40
|
Roarty K and Rosen JM: Wnt and mammary
stem cells: hormones cannot fly wingless. Curr opin pharmacol.
10:643–649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Torres CG, Ramírez ME, Cruz P, Epuñan MJ,
Valladares L and Sierralta WD: 27-Hydroxycholesterol induces the
transition of MCF7 cells into a mesenchymal phenotype. Oncol Rep.
26:389–397. 2011.PubMed/NCBI
|
|
42
|
Wang N, Wang Z, Peng C, You J, Shen J, Han
S and Chen J: Dietary compound isoliquiritigenin targets GRP78 to
chemosensitize breast cancer stem cells via β-catenin/ABCG2
signaling. Carcinogenesis. 35:2544–2554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Z, Wang N, Li W, Liu P, Chen Q, Situ
H, Zhong S, Guo L, Lin Y, Shen J and Chen J: Caveolin-1 mediates
chemoresistance in breast cancer stem cells via β-catenin/ABCG2
signaling pathway. Carcinogenesis. 35:2346–2356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zafarana G and Bristow RG: Tumor
senescence and radioresistant tumor-initiating cells (TICs): let
sleeping dogs lie! Breast Cancer Res. 12:1112010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guterres FA, Martinez GR, Rocha ME and
Winnischofer SM: Simvastatin rises reactive oxygen species levels
and induces senescence in human melanoma cells by activation of
p53/p21 pathway. Exp Cell Res. 319:2977–2988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang Y, Xu SL, Wu YZ, Zao MS, Xu WJ, Yang
HY and Li YX: Simvastatin induces caspase-dependent apoptosis and
activates P53 in OCM-1 cells. Exp Eye Res. 113:128–134. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zanfardino M, Spampanato C, De Cicco R,
Buommino E, De Filippis A, Baiano S, Barra A and Morelli F:
Simvastatin reduces melanoma progression in a murine model. Int J
Oncol. 43:1763–1770. 2013.PubMed/NCBI
|