Introduction
OCT4, also known as POU5F1, is an
essential transcription factor of embryonic development, and
maintains the pluripotency and self-renewal of embryonic stem cells
(ESCs) (1–3). It is a main factor in iPS cell
generation (4–6). OCT4 is expressed in several types of
cancer and is involved in maintaining cancer stem cell (CSC)
properties (7–10), and is associated with the degree of
malignancy and the drug-resistance of cancer (11–14).
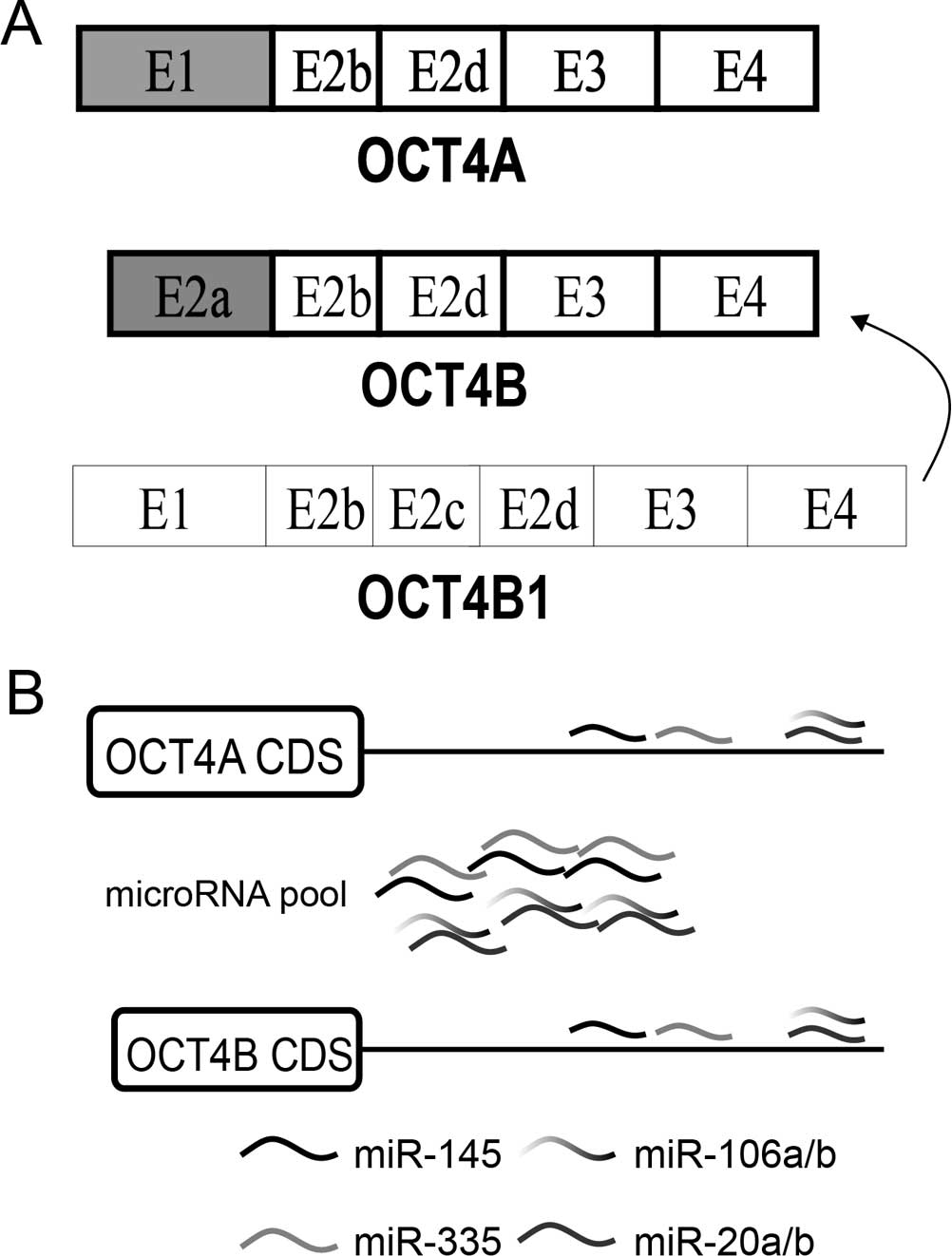
There are 3 alternative spliced mRNA isoforms
generated by the OCT4 gene: OCT4A, OCT4B and OCT4B1
(15,16). OCT4A mRNA translates into the OCT4A
protein which is the canonical OCT4 protein exerting the known
function of OCT4 as a transcription factor. OCT4B mRNA generates
three protein isoforms through alternative translation initiation:
OCT4B-265, OCT4B-190 and OCT4B-164 (17). OCT4B1 mRNA is predicted to generate
a potential truncated peptide (18). OCT4A is highly expressed in the
nuclei of blastocysts and compacted embryos, whereas OCT4B is found
in the cytoplasm of all cells from the four-cell stage onward
(19,20). Many studies have focused on the
function of OCT4A in contrast to OCT4B. Recently, OCT4B-265 has
been reported to be upregulated by genotoxic stress treatment in ES
cell lines, and overexpression of OCT4B-265 was found to promote
cell apoptosis in a p53-dependent manner (21). In oncology research, most types of
cancer express a relatively low level of the OCT4 protein,
particularly the OCT4B isoforms (22).
Compared to the low protein level, OCT4A and OCT4B
mRNA are co-expressed in several types of tumor cell lines
(22,23), yet the OCT4B protein fails to be
upregulated after proteasome inhibitor treatment (21). These findings may suggest that in
tumor cells OCT4B executes its functions at the RNA level but not
as a fully functional protein.
Regulation of gene expression by microRNAs which
guide the RNA-induced silencing complex (RISC) to microRNA response
elements (MREs) on target transcripts is ubiquitous among mammals,
usually resulting in degradation of the transcript or translation
inhibition (24). Aberrant
expression-fluctuation of a large number of miRNAs has been found
in cancer (25,26). Recently, numerous experiments
provide support to the hypothesis that RNA molecules that share
MREs can regulate each other by competing for microRNA binding, for
which the process is termed competing endogenous RNA (ceRNA)
regulation (27–32). An OCT4 pseudogene,
OCT4-pg4, has been observed to regulate OCT4A expression as
an miR-145 sponge in hepatocellular carcinoma (33). Unfortunately, miR-145 is
downregulated in most types of cancers, which confines the strength
of this regulation (34–36). ceRNA regulation mediated by several
pseudogenes has been demonstrated. Yet, whether or not this
regulation exists among transcript isoforms and the related
mechanism have not been elucidated. Consistent with the
co-expression of OCT4A and OCT4B, we hypothesized that OCT4B
regulates OCT4A as ceRNA.
In the present study, we demonstrated that in the
cancer cell lines: i) expression of OCT4B was relatively low at the
protein level, yet not at the RNA level; ii) OCT4B regulated OCT4A
expression in an miRNA-dependent manner (ceRNA regulation) at the
post-transcription level; iii) in addition to miR-145, miR-20a,
miR-20b, miR-106a, miR-106b and miR-335 were capable of regulating
OCT4. This is the first time that ceRNA regulation has been
observed among spliced isoforms, and OCT4B acts as a modulator of
OCT4A expression. These findings may pave a way for identification
of new targets for cancer treatment.
Materials and methods
Materials
The primary antibody of OCT4 (ab19857, rabbit pAb,
1:500) was obtained from Abcam (Cambridge, MA, USA), and antibodies
against β-actin (sc-47778, HP-conjugated, 1:500) were purchased
from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and
HP-conjugated anti-rabbit mouse secondary antibodies were obtained
from the Beyotime Institute of Biotechnology (Beyotime, Haimen,
China). Three independent anti-Oct4B stealth RNAi siRNAs, Si-1
5-AAGGGATGCAGAGCATCGTGAAAGG-3, Si-2 5-TTTCCATTCGGGATTCAAGAACCTA-3
and Si-3 5-TAAACACACCAGTTATCAATCTCCC-3, were mixed into an siRNA
pool (20 and 6.7 μM each). Lipofectamine® 2000,
RNAiMAX, Opti-MEM® reduced serum media,
Stealth® RNAi siRNA and SYBR® Select Master
Mix, and NCode® miRNA First-Strand cDNA Synthesis kit
were obtained from Invitrogen (Life-Technologies, Carlsbad, CA,
USA). X-tremeGENE® 9 was purchased from Roche (Roche
Applied Science, Basel, Switzerland). RPMI-1640, MEM-EBSS and
McCoy’s 5A medium and fetal bovine serum (FBS) were obtained from
HyClone (Thermo Fisher Scientific, Waltham, MA, USA). MG132 was
purchased from Beyotime Institute of Biotechnology.
PrimeScript® RT reagent kit with gDNA Eraser was
obtained from Takara Bio (Dalian, China). microRNA mimics were
obtained from RiboBio (Guangzhou, China).
Plasmid construction
The 3′UTR of OCT4 was amplified from genomic DNA of
PA-1 cells and cloned into the EGFP ORF C-terminal of pEGFP-N1
(Clontech, Mountain View, CA, USA) using NotI and
XbaI sites, and then subcloned into the psiCHECK-2 vector
(Promega, Madison, WI, USA) by the XhoI and NotI
sites. The primer sequences were: OCT4 3′UTR-F,
5-TGCCTGCCCTTCTAGGAA-3 and OCT4 3′UTR-R,
5-AAGTGTGTCTATCTACTGTGTCC-3. microRNA-specific mutation psi-CHECK-2
OCT4 3′UTR plasmid (Δ145, Δ17, Δ335, Δ384 and Δ339), were generated
using the QuikChange® Site-Directed Mutagenesis kit
(Stratagene, Agilent Technologies, Wilmington, DE, USA).
Cell culture and transfection
The human ovarian teratoma cell line PA-1, and the
human gastric cancer cell lines MGC803, SGC7901 and NCI-N87, were
purchased from the Cell Resource Center, IBMS, CAMS/PUMC. The
prostate cancer cell line DU145 was obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA). The colon cancer cell
line HCT116 wild-type and Dicer−/−
(HCT116−/−) cell line were kindly donated by Professor
B. Vogelstein of Johns Hopkins University. PA-1 cells were
maintained in MEM-EBSS medium, HCT116 and HCT116−/− were
maintained in McCoy’s 5A medium and the other cell lines were
maintained in RPMI-1640 medium. All culture media were supplemented
with 10% FBS and 100 U/ml penicillin/streptomycin (both from
HyClone, Thermo Fisher Scientific), at 37°C in a humidified
atmosphere (5% CO2/95% air).
OCT4 3′UTR was cloned into the pEGFP-N1 vector and
marked as pEGFP-Oct4 3′UTR. DNA transfection was performed using
X-tremeGENE® 9 when cells reached 80% confluency. For
siRNA/miRNA mimic transfection (100 nM), Lipofectamine®
RNAiMAX was utilized, when cells reached a 50% confluency.
Lipofectamine® 2000 was used for reporter gene plasmid
and miRNA mimic co-transfection. Transfections were performed
according to the manufacturer’s recommendations in 6-well
plates.
Cell proliferation analysis
Eight hours post-transfection, the cells were
trypsinized, resuspended and seeded in four separate 24-well plates
at a final density of 10,000/well. Starting from the following day
(day 0), one plate/day was washed once with PBS, fixed in 4%
paraformaldehyde solution for 10 min at room temperature, and then
stained with 0.05% crystal violet for 30 min. After lysis with
methanol, the absorbance was read at 592 nm on a microplate
spectrophotometer (SpectraMax® M3; Molecular Devices,
Sunnyvale, CA, USA).
RNA extraction and real-time PCR
For real-time PCR analyses, total RNA was extracted
from the cells using TRIzol® (Ambion, Life-Technologies)
reagent as per the manufacturer’s instructions. Several types of
RNA were donated by Dr Wang (HMU). Clearance of DNA contamination
in RNA and cDNA synthesis was performed using the
PrimeScript® RT reagent kit with gDNA Eraser according
to the manufacturer’s instructions. MicroRNA reverse transcription
was performed using NCode® miRNA First-Strand cDNA
synthesis kit according to the manufacturer’s instructions.
Real-time PCR was subsequently performed using the ABI-7500 system
employing SYBR® Select Master Mix. Primer sequences
were: OCT4A F, 5-CCCCTGGTGCCGTGAA-3 and OCT4A R,
5-GCAAATTGCTCGAGTTCTTTCTG-3; OCT4B F, 5-CAG GGAATGGGTGAATGAC-3 and
OCT4B R, 5-AGGCAGAAGACTTGTAAGAAC-3; GAPDH F, 5-GAGTCAACG
GATTTGGTCGT-3 and GAPDH R, 5-GACAAGCTTCCCGTTCTCAG-3.
Luciferase assays
Validation of miRNA regulation by
OCT4
HCT116 cells were seeded 24 h before transfection at
a density of 50,000 cells/well in 24-well plates. psiCHECK-2 OCT4
3′UTR WT vector (500 ng) or miR-145/miR-335/miR-384/miR-339/miR-17a
site mutation vector was co-transfected with corresponding miRNA
mimics with Lipofectamine® 2000 according to the
manufacturer’s instructions.
Detection of OCT4A/B reciprocal
regulation
HCT116 and PA-1 cells were seeded 24 h before
transfection at a density of 50,000 or 60,000 cells/well in 24-well
plates. psiCHECK-2 OCT4 3′UTR WT vector (500 ng) was co-transfected
with the corresponding 800 ng pEGFP-Oct4 3′UTR/pEGFP empty vector
with Lipofectamine 2000 according to the manufacturer’s
instructions.
In all cases, firefly luciferase gene in psiCHECK-2
was used as a normalization control for transfection efficiency. At
48/72 h after transfection, firefly and Renilla luciferase
activities were measured consecutively with the Dual-Luciferase
reporter assay system using a luminometer (both from Promega).
Western blotting assay
Whole-cell lysate preparation and western blot
analysis were carried out as previously described (37).
Bisulfite sequencing analysis
Bisulfite sequencing PCR (BS-PCR) was performed with
genomic DNA from the PA-1, HCT116, HCT116−/−, MGC803 and
DU145 cells with EpiTect Fast LyseAll Bisulfite kit (Qiagen,
Hilden, Germany). PCR reactions were performed using EpiTaq HS
(Takara Bio) with Nest PCR primers: OCT4 distal enhancer (DE) outer
F, 5-AG GAGTTATTAGGAAAATGGGTAGTAG-3 and OCT4 DE outer R,
5-TACCTTCTAAAAAAATAAATATCCC-3; OCT4 DE inner F,
5-ATTTGTTTTTTGGGTAGTTAAAGGT-3 and OCT4 DE inner R,
5-CCAACTATCTTCATCTTAATAACA TCC-3.
The second PCR products were subcloned using pMD-19
T vector (Takara Bio) according to the manufacturer’s protocol, and
individual clones were subsequently sequenced (Sangon Biotech,
Shanghai, China). Clones were only accepted if there was at least
90% cytosine conversion, and all possible clonalities were excluded
based on criteria from the BiQ Analyzer software (Max Planck
Society, Munich, Germany). At least 10 replicates were performed
for each of the selected regions in each cell line.
Co-expression of OCT4A and OCT4B
analysis
For qRT-PCR assessment, data were analyzed using the
ΔΔCt method; for co-expression in invasive breast cancer, prostate
and colon cancer samples, processed and normalized expression data
were downloaded from the TCGA database, and then analyzed with RSEM
(RNA-Seq by Expectation-Maximization).
Statistical analysis
Each experiment was repeated at least in triplicate.
Statistical analyses (Student’s t-test) were performed using
Microsoft Excel. p<0.05 (p<0.05, p<0.01, p<0.001 as
indicated in the figures) was considered to indicate a
statistically significant result, and results are expressed as mean
± SD.
Results
Ubiquitous expression of OCT4A and OCT4B
mRNA but not protein in the tumor cell lines
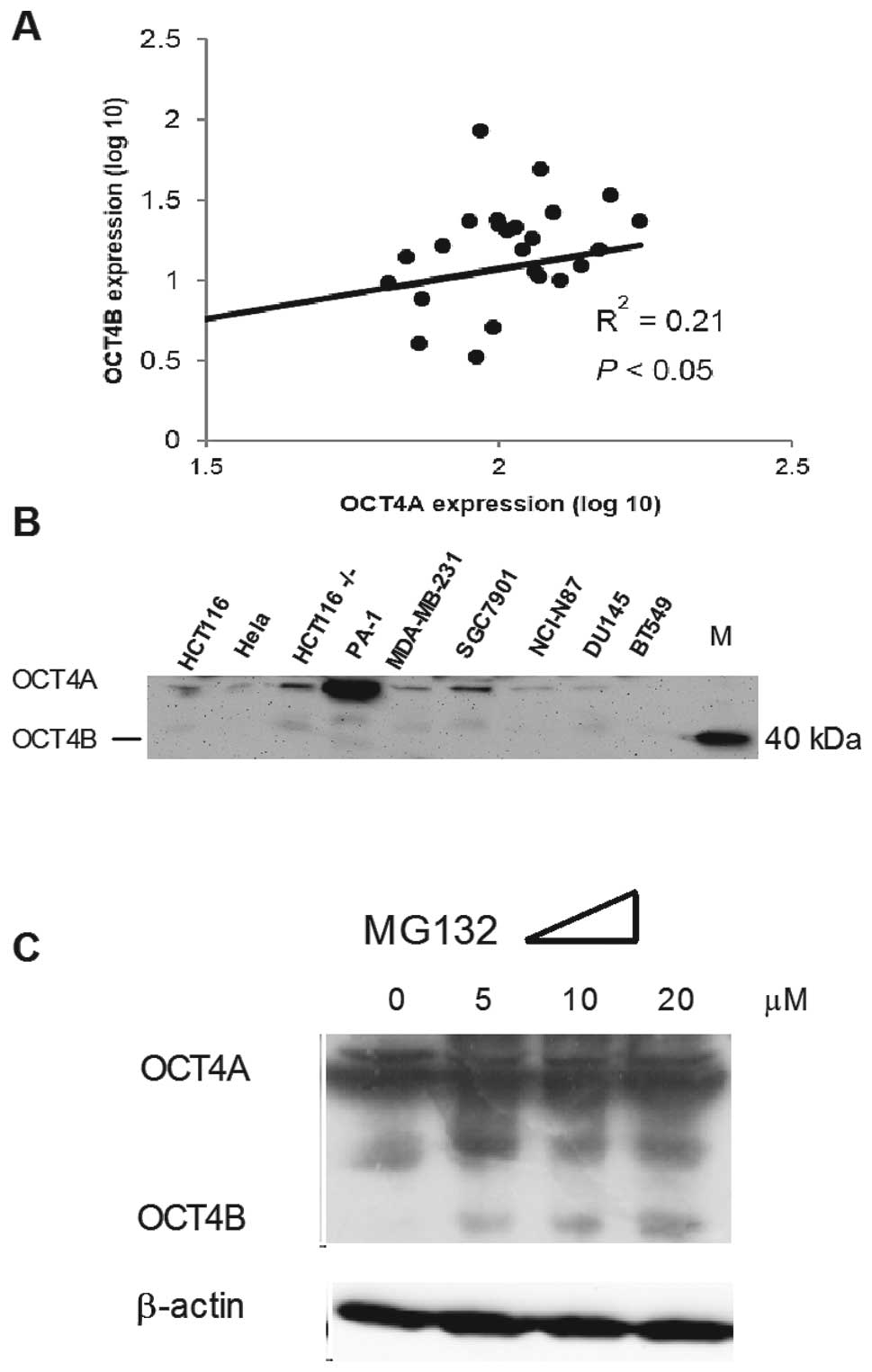
To measure expression of OCT4 in the tumor cells, we
used qRT-PCR and western blotting to detect the RNA and protein
expression levels of OCT4 (Fig. 1A
and B). Consistent with a previous study, OCT4 protein was absent
in most of the somatic cancer cell lines (22). At the RNA level, we found that OCT4A
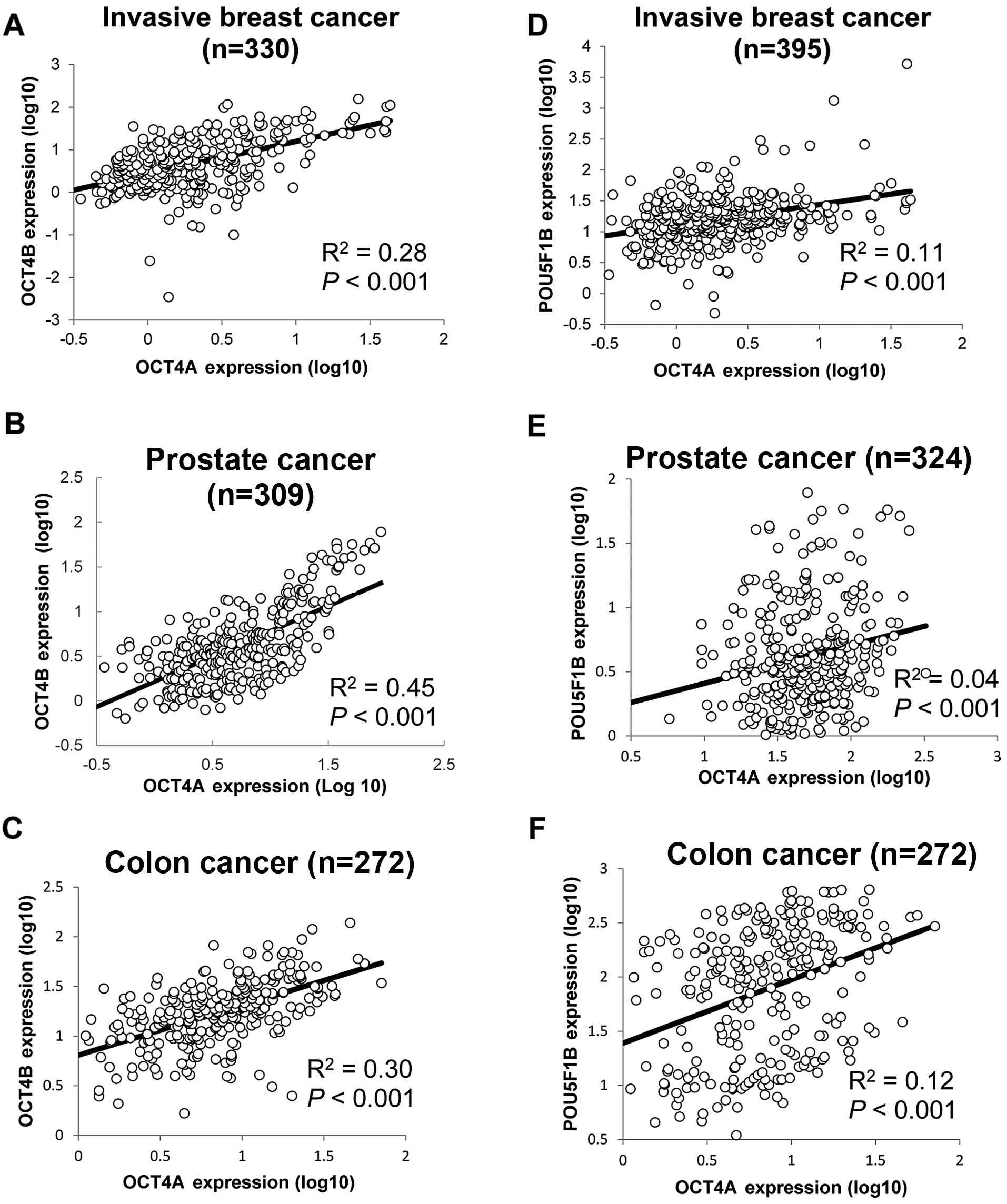
and OCT4B expression were correlated to each other (Fig. 1A). In order to confirm whether
co-expression of OCT4A/B exists in the tumor samples, we analyzed
expression data from the TCGA database. After removing samples that
did not express OCT4A or OCT4B (no raw sequencing read), expression
of OCT4A/B was found to be correlated in invasive breast, prostate
and colon cancer samples (Fig.
2A–C). To eliminate any bias generated by our analysis method,
an OCT4 pseudogene transcript, POU5F1B, acting as a valid
microRNA-145 sponge that regulates OCT4A expression was also
analyzed (33). The results
indicated that POU5F1B also showed a positive correlation to OCT4A
expression in the aforementioned cancers (Fig. 2D–F).
We further exposed PA-1 (a teratoma cell line) to
MG132, a proteasome inhibitor, which has been proven to upregulate
OCT4B in these cells (21). As
expected, OCT4B was upregulated following a 10-h treatment with
MG132 in a concentration-dependent manner (Fig. 1C). Yet, MG132 was unable to
upregulate OCT4B protein in the colon cancer cell lines HCT116 and
HCT15 and in the prostate cancer cell lines DU145 and PC-3 (data
not shown).
OCT4B modulates OCT4A expression as
ceRNA
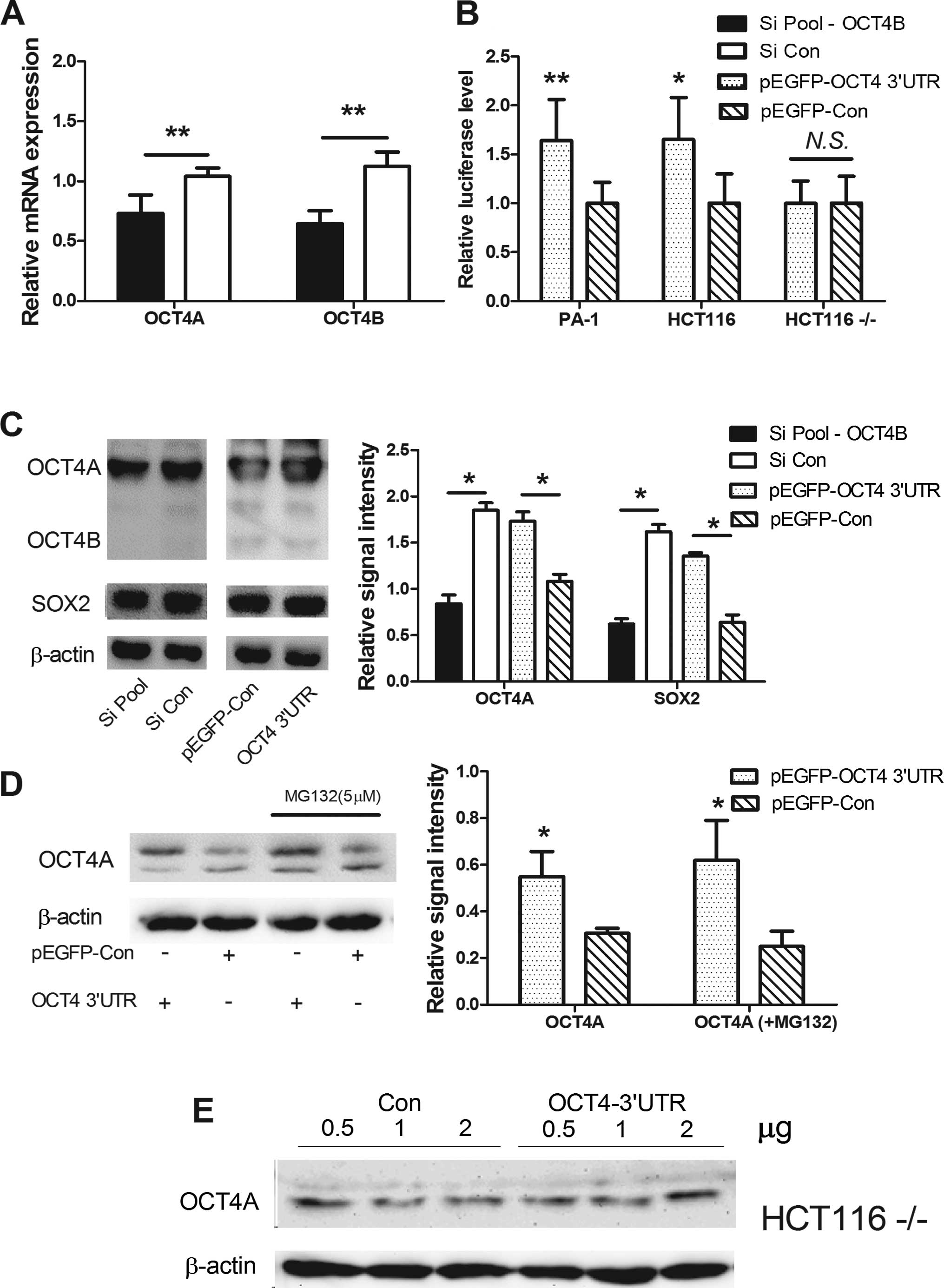
To confirm our hypothesis, we utilized anti-OCT4B
siRNA knockdown of OCT4B or overexpression of OCT4B 3′UTR (as for
OCT4 3′UTR, due to the fact that OCT4B 3′UTR and OCT4A 3′UTR are
identical). In the PA-1 cells, anti-OCT4B siRNA inhibited OCT4B and
OCT4A at the RNA and protein levels. Overexpression of OCT4 3′UTR
increased OCT4A and OCT4B protein expression (Fig. 3A and C). Since miR-145 has been
confirmed to regulate SOX2, we assessed whether SOX2 expression was
affected. SOX2 protein also fluctuated in the process with the same
tendency as OCT4 (Fig. 3C).
To ascertain whether this observed effect is
dependent upon OCT4 3′UTR, we constructed a chimeric luciferase
plasmid tagged with the OCT4 3′UTR (Luc-OCT4 3′UTR). In the PA-1
and HCT116 cells, overexpression of OCT4 3′UTR enhanced Luc-OCT4
3′UTR activity, but in Dicer-deficit HCT116 cells
(HCT116−/− cells do not express the majority of mature
miRNAs), Luc-OCT4 3′UTR activity was not affected, indicating that
mature microRNAs are essential for the regulation (Fig. 3B). Furthermore, overexpression of
OCT4 3′UTR increased OCT4A protein expression in the HCT116 cells,
but not in the Dicer-deficit HCT116−/− cells (Fig. 3D and E), and MG132 was unable to
elevate OCT4B protein expression (Fig.
3D).
microRNA pool regulates OCT4
expression
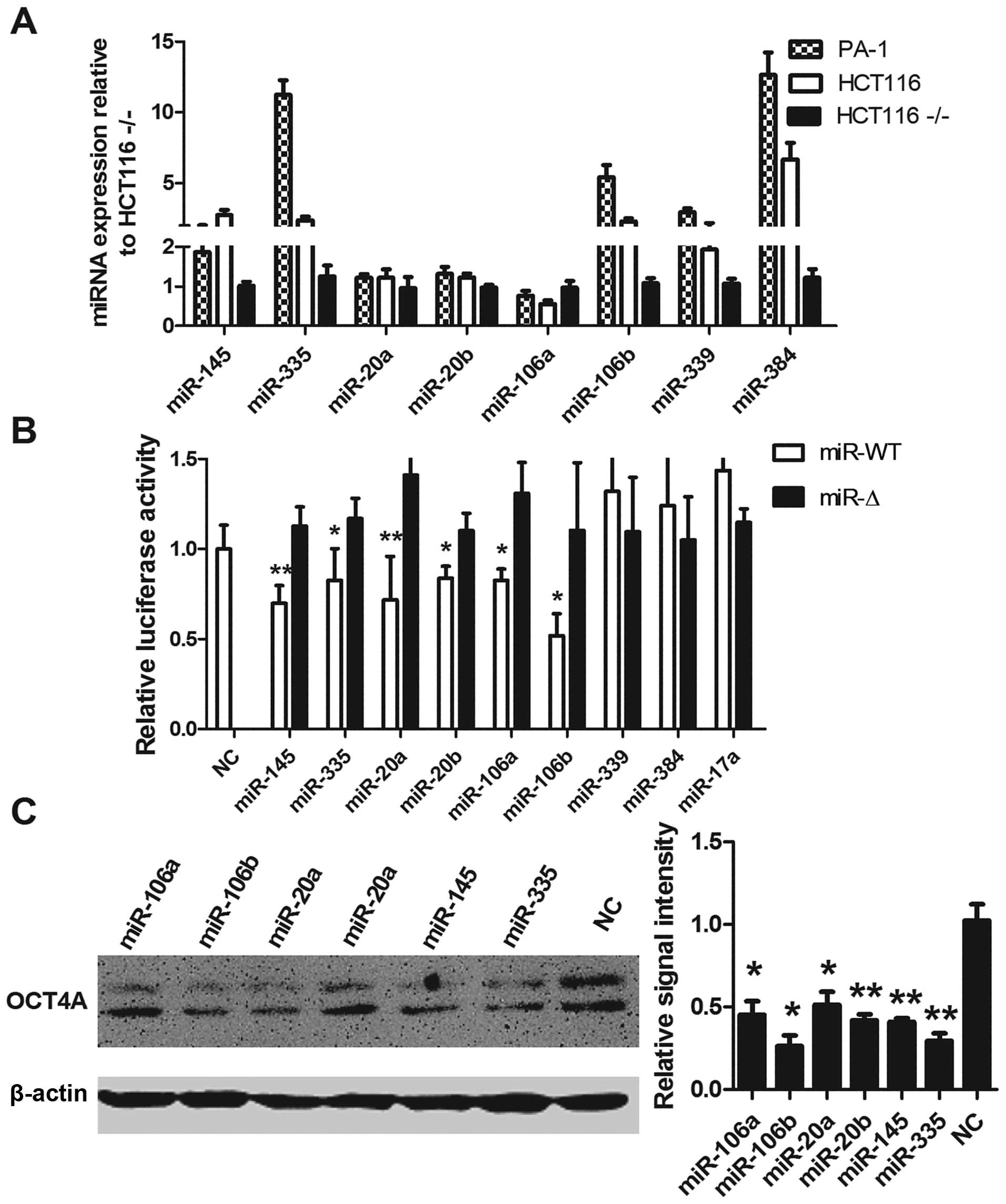
To verify the microRNAs that mediate OCT4A/B ceRNA
modulation, we used the miRanda algorithm to predict OCT4-targeting
miRNAs and detected their expression levels; apart from miR-106a,
predicted miRNAs, miR-145, miR-20a, miR-20b, miR-384, miR-106b,
miR-335 and miR-339 were expressed at a higher level in the PA-1
and HCT116 cells, in contrast to the levels in the
HCT116−/− cells (Fig.
4A). miR-145 has been reported to regulate OCT4 expression
(38). In the HCT116 cells,
expression of miR-145, miR-335, miR-20a, miR-20b, miR-106a and
miR-106b significantly reduced Luc-OCT4 3′UTR activity according to
mutation construct, respectively. Overexpression of miR-339 and
miR-384 did not obviously affect Luc-OCT4 3′UTR activity (Fig. 4B). Since miR-106a/b and miR-20a/b
belong to the miR-17 family, we further tested the effect of
miR-17a expression on Luc-OCT4 3′UTR activity, and the results
demonstrated a negative effect (Fig.
4B).
Consistent with the luciferase results,
overexpression of miR-145, miR-335, miR-20a, miR-20b, miR-106a and
miR-106b caused a significant downregulation of OCT4 protein in the
HCT116 cells (Fig. 4C).
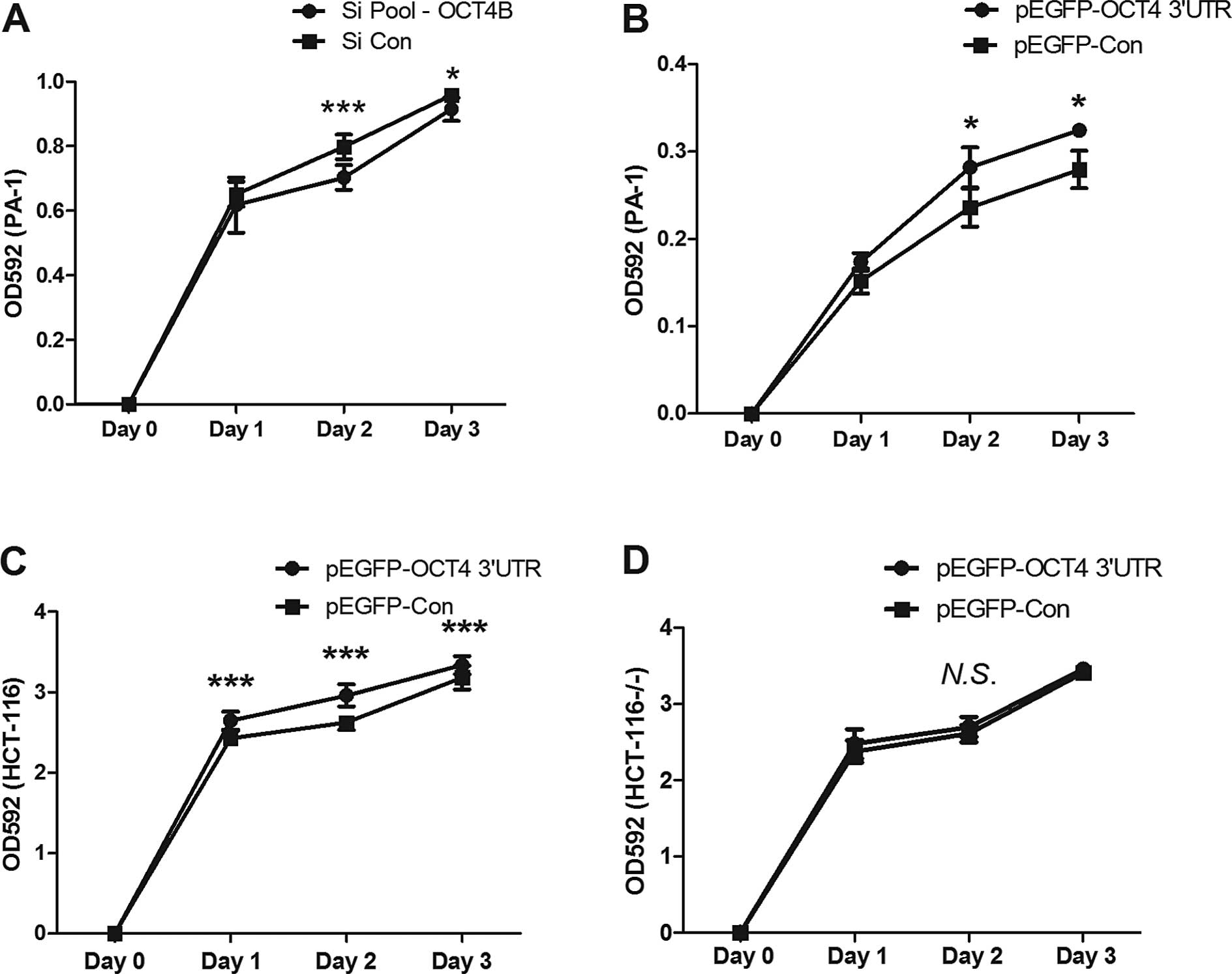
Cell proliferation is regulated by
OCT4A/B ceRNA interaction
We next investigated the biological functions of
ceRNA regulation among OCT4A/B. Previous studies indicated that
suppression of OCT4A inhibited cell proliferation, and suppression
of OCT4B sensitized A549 cells to cisplatin (39,40).
To evaluate the effects of anti-OCT4B siRNA or overexpression of
OCT4 3′UTR on cell proliferation, a cell proliferation assay was
performed. The results revealed that in the PA-1 cells knockdown of
OCT4B significantly inhibited cell growth (Fig. 5A). In contrast, overexpression of
OCT4 3′UTR in the PA-1 and HCT116 cells promoted cell proliferation
(Fig. 5B and C), but not in the
HCT116−/− cell line (Fig.
5D).
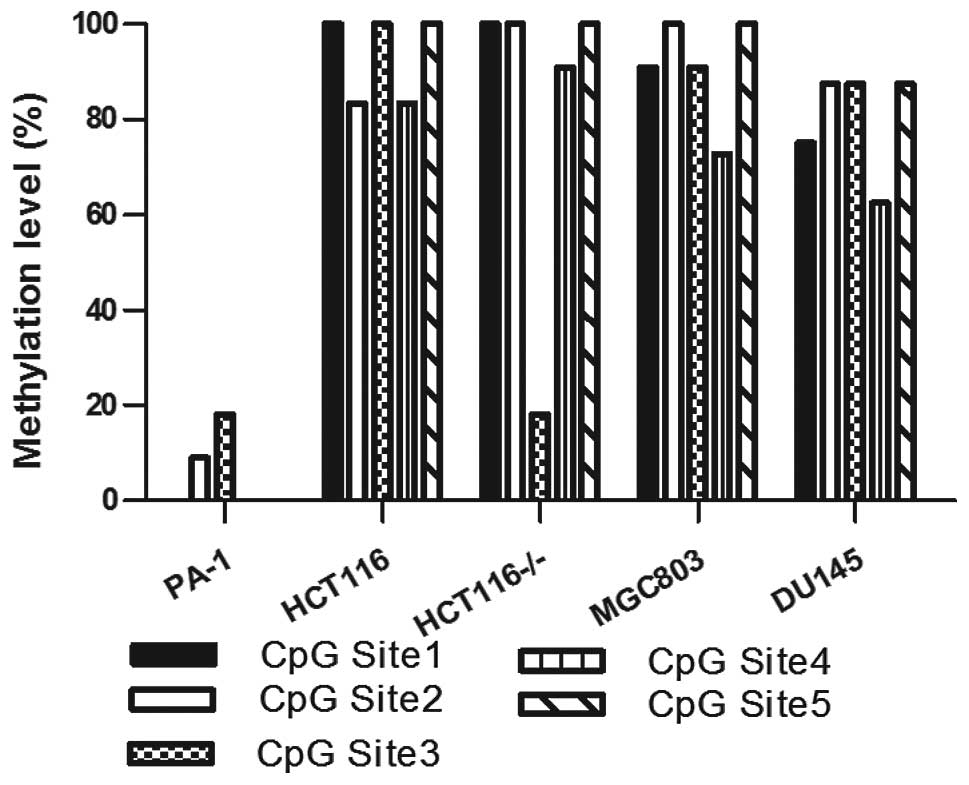
OCT4 promoter is highly methylated in
somatic cancer cell lines
To elucidate the underlying mechanisms involved in
the low level of expression of OCT4A/B in tumor cells, the
epigenetic factors were investigated.
Methylation of a distal enhancer region of the OCT4
promoter was reported to be associated with OCT4 expression
(22). We utilized bisulfite
sequencing to detect methylation of this region in the teratoma
cell line PA-1 and in 4 different somatic cancer cell lines. The
results revealed that in the HCT116, HCT116−/−, MGC803
and DU145 cell lines, all 5 CpG sites showed a relative high level
of methylation, in contrast to the PA-1 cells (Fig. 6). Yet, notably, DNA methylation
inhibitor, decitabine, failed to enhance the expression of OCT4A/B
in the HCT116 and DU145 cells (data not shown).
Discussion
OCT4 is an essential transcription factor of
embryonic development, involving maintenance of the pluripotency
and self-renewal of embryonic stem cells (ESCs) (1–3), and
is a main factor in iPS cell generation (4–6). OCT4
is expressed in several types of cancer facilitating the
maintenance of cancer stem cell (CSCs) properties (7–10), and
is associated with the degree of malignancy and the drug-resistance
character of cancer (11–14).
Three alternative spliced mRNA isoforms are
generated by the OCT4 gene: OCT4A, OCT4B and OCT4B1 (Fig. 7A) (15,16).
OCT4A mRNA translates into the OCT4A protein which is the canonical
OCT4 protein exerting the known function of OCT4 as a transcription
factor. OCT4B mRNA generates three protein isoforms through
alternative translation initiation: OCT4B-265, OCT4B-190 and
OCT4B-164 (17). OCT4B1 mRNA has
been predicted to generate a potential truncated peptide and could
convert to OCT4B (18).
Based on previous research and our results,
expression of OCT4, particularly OCT4B, was relative lower in the
tumor cell lines when compared to embryonic cells. At the protein
level, OCT4B was undetectable by western blotting (Fig. 1B). (21,22).
Evidence in ES cells shows that OCT4B proteins are degraded quickly
by the proteasome pathway, and MG132 blocks the degradation of
OCT4B (21,41). In several tumor cell lines, MG132
was unable to upregulate expression of OCT4B, whereas OCT4A and
OCT4B RNA expression were correlated with each other in the tumor
cell lines and tumor samples (Figs.
1A and 2A-C). Thus, we
hypothesized that OCT4B may function as a non-coding RNA, yet not a
coding RNA in tumors.
Recently, the boundary between coding RNAs and
non-coding RNAs has become obscure (42). A coding transcript may exert a
non-coding function by competive binding with miRNAs and act as a
trans-modulator of other gene expression for the same miRNA-target
(27,29,30).
This ceRNA regulation has been found between gene/pseudogene and
among diverse gene transcripts. Here, in the present study, for the
first time, we found a ceRNA relationship between 2 alternative
spliced transcripts of one gene; OCT4B regulated OCT4A expression
by competive binding with microRNAs (Figs. 3 and 7). During the process, we also found a
group of miRNAs that target OCT4; agreeing with most research,
miRNA target-predicting algorithms have a low precision, compared
to the experimental detection methods (Fig. 4B) (43). Our results also support that
manipulation of OCT4B expression may alter cell proliferation,
consistent with a previous study on OCT4B (39). We believe that the reported
anti-apoptotic property of OCT4B is based on its impact on OCT4A,
acting as ceRNA. Our subsequent research will focus on this aspect.
In the course of overexpression of OCT4 3′UTR, SOX2 was also
upregulated. Yet, due to the absence of SOX2 expression in the
HCT116−/− cells, we could not ascertain whether or not
the underlying mechanisms are dependent upon the competive binding
of miR-145 by OCT4 3′UTR.
Confusingly, anti-OCT4B siRNA was unable to
downregulate OCT4A in the HCT116 cells (data not shown). One
explanation may be that knockdown of the low expression of OCT4B
was not enough to perturb the abundance of OCT4-targeting miRNAs.
Abundance of miRNAs and endogenous target sites may play a key role
in ceRNA regulation (44).
DNA methylation has been reported to be associated
with gene expression; hypermethylated CpG site at CpG islands in
the gene promoter is associated with gene transcription (45–47).
Methylation of a distal enhancer region of the OCT4 promoter was
reported to be related to OCT4 expression (Fig. 6) (22). According to our results, DNA
methylation inhibitor, decitabine, was unable to increase the
expression of OCTA or OCT4B in the HCT116 and DU145 cells. This
demonstrated that more complicated epigenetic modifications are
involved, apart from DNA methylation (48–52).
In conclusion, we demonstrated that: i) expression
of OCT4B is low at the protein level, yet not at the RNA level; ii)
OCT4B modulates OCT4A expression via an miRNA-dependent manner
(ceRNA regulation) at the post-transcription level; iii) in
addition to miR-145, miR-20a, miR-20b, miR-106a, miR-106b and
miR-335 are capable of targeting OCT4. This is the first time that
ceRNA regulation was observed among spliced isoforms, and OCT4B
acts as a modulator of OCT4A expression.
Acknowledgments
We thank the members of the laboratory of Dr Yu for
critical assessment of the manuscript, and H.B. Bao, H.Y. Liu, N.
Wang, X.C. Wang, L.L. Yang, Y. Chen and W.J. Li. (Harbin Medical
University) for discussions and the several types of cell RNA. We
thank B. Vogelstein for the DICER−/− cells.
Co-expression results were entirely or partly based upon data
generated by the TCGA Research Network: http://cancergenome.nih.gov/. D.L. was supported by a
Challenge Cup Undergraduate Innovation Award of Heilongjiang
Province and Spring Thunder Project of Harbin Medical University.
The present study was supported by a grant from Harbin Science and
Technology Bureau (grant no. RC2014XK004031) to X.L.Z., a grant
from the National Youth Natural Science Foundation of China (grant
no. 81101942) to P.L., and a grant from Undergraduate Enterprise
Program of Heilongjiang Province to D.L., in part.
References
|
1
|
Nichols J, Zevnik B, Anastassiadis K, Niwa
H, Klewe-Nebenius D, Chambers I, Schöler H and Smith A: Formation
of pluripotent stem cells in the mammalian embryo depends on the
POU transcription factor Oct4. Cell. 95:379–391. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niwa H, Miyazaki J and Smith AG:
Quantitative expression of Oct-3/4 defines differentiation,
dedifferentiation or self-renewal of ES cells. Nat Genet.
24:372–376. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boyer LA, Lee TI, Cole MF, et al: Core
transcriptional regulatory circuitry in human embryonic stem cells.
Cell. 122:947–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hay DC, Sutherland L, Clark J and Burdon
T: Oct-4 knockdown induces similar patterns of endoderm and
trophoblast differentiation markers in human and mouse embryonic
stem cells. Stem Cells. 22:225–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park IH, Zhao R, West JA, Yabuuchi A, Huo
H, Ince TA, Lerou PH, Lensch MW and Daley GQ: Reprogramming of
human somatic cells to pluripotency with defined factors. Nature.
451:141–146. 2008. View Article : Google Scholar
|
|
7
|
Kumar SM, Liu S, Lu H, Zhang H, Zhang PJ,
Gimotty PA, Guerra M, Guo W and Xu X: Acquired cancer stem cell
phenotypes through Oct4-mediated dedifferentiation. Oncogene.
31:4898–4911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reers S, Pfannerstill AC, Maushagen R,
Pries R and Wollenberg B: Stem cell profiling in head and neck
cancer reveals an Oct-4 expressing subpopulation with properties of
chemoresistance. Oral Oncol. 50:155–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ,
Tsai TH, Chou SH, Chien CS, Ku HH and Lo JF: Positive correlations
of Oct-4 and Nanog in oral cancer stem-like cells and high-grade
oral squamous cell carcinoma. Clin Cancer Res. 14:4085–4095. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen YC, Hsu HS, Chen YW, et al: Oct-4
expression maintained cancer stem-like properties in lung
cancer-derived CD133-positive cells. PLoS One. 3:e26372008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Atlasi Y, Mowla SJ, Ziaee SA and Bahrami
AR: OCT-4, an embryonic stem cell marker, is highly expressed in
bladder cancer. Int J Cancer. 120:1598–1602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Linn DE, Yang X, Sun F, Xie Y, Chen H,
Jiang R, Chen H, Chumsri S, Burger AM and Qiu Y: A role for OCT4 in
tumor initiation of drug-resistant prostate cancer cells. Genes
Cancer. 1:908–916. 2010. View Article : Google Scholar
|
|
13
|
de Resende MF, Chinen LT, Vieira S,
Jampietro J, da Fonseca FP, Vassallo J, Campos LC, Guimarães GC,
Soares FA and Rocha RM: Prognostication of OCT4 isoform expression
in prostate cancer. Tumour Biol. 34:2665–2673. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen K, Fu Z, Wu X, Feng J, Chen W and Qian
J: Oct-4 is required for an antiapoptotic behavior of
chemoresistant colorectal cancer cells enriched for cancer stem
cells: Effects associated with STAT3/Survivin. Cancer Lett.
333:56–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takeda J, Seino S and Bell GI: Human Oct3
gene family: cDNA sequences, alternative splicing, gene
organization, chromosomal location, and expression at low levels in
adult tissues. Nucleic Acids Res. 20:4613–4620. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Asadi MH, Mowla SJ, Fathi F, Aleyasin A,
Asadzadeh J and Atlasi Y: OCT4B1, a novel spliced variant of OCT4,
is highly expressed in gastric cancer and acts as an antiapoptotic
factor. Int J Cancer. 128:2645–2652. 2011. View Article : Google Scholar
|
|
17
|
Wang X, Zhao Y, Xiao Z, et al: Alternative
translation of OCT4 by an internal ribosome entry site and its
novel function in stress response. Stem Cells. 27:1265–1275. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Atlasi Y, Mowla SJ, Ziaee SA, Gokhale PJ
and Andrews PW: OCT4 spliced variants are differentially expressed
in human pluripotent and nonpluripotent cells. Stem Cells.
26:3068–3074. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cauffman G, Liebaers I, Van Steirteghem A
and Van de Velde H: POU5F1 isoforms show different expression
patterns in human embryonic stem cells and preimplantation embryos.
Stem Cells. 24:2685–2691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cauffman G, Van de Velde H, Liebaers I and
Van Steirteghem A: Oct-4 mRNA and protein expression during human
preimplantation development. Mol Hum Reprod. 11:173–181. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao Y, Wei J, Han J, Wang X, Su G, Zhao Y,
Chen B, Xiao Z, Cao J and Dai J: The novel function of OCT4B
isoform-265 in genotoxic stress. Stem Cells. 30:665–672. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cantz T, Key G, Bleidissel M, Gentile L,
Han DW, Brenne A and Schöler H: Absence of OCT4 expression in
somatic tumor cell lines. Stem Cells. 26:692–697. 2008. View Article : Google Scholar
|
|
23
|
Zhao S, Yuan Q, Hao H, et al: Expression
of OCT4 pseudogenes in human tumours: Lessons from glioma and
breast carcinoma. J Pathol. 223:672–682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ebert MS and Sharp PA: Roles for microRNAs
in conferring robustness to biological processes. Cell.
149:515–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tay Y, Kats L, Salmena L, et al:
Coding-independent regulation of the tumor suppressor PTEN by
competing endogenous mRNAs. Cell. 147:344–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karreth FA, Tay Y, Perna D, et al: In vivo
identification of tumor- suppressive PTEN ceRNAs in an oncogenic
BRAF-induced mouse model of melanoma. Cell. 147:382–395. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miranda KC, Huynh T, Tay Y, Ang YS, Tam
WL, Thomson AM, Lim B and Rigoutsos I: A pattern-based method for
the identification of microRNA binding sites and their
corresponding heteroduplexes. Cell. 126:1203–1217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fang L, Du WW, Yang X, et al: Versican
3′-untranslated region (3′-UTR) functions as a ceRNA in inducing
the development of hepatocellular carcinoma by regulating miRNA
activity. FASEB J. 27:907–919. 2013. View Article : Google Scholar
|
|
33
|
Wang L, Guo ZY, Zhang R, et al: Pseudogene
OCT4-pg4 functions as a natural micro RNA sponge to regulate OCT4
expression by competing for miR-145 in hepatocellular carcinoma.
Carcinogenesis. 34:1773–1781. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshino H, Enokida H, Itesako T, Kojima S,
Kinoshita T, Tatarano S, Chiyomaru T, Nakagawa M and Seki N:
Tumor-suppressive microRNA-143/145 cluster targets hexokinase-2 in
renal cell carcinoma. Cancer Sci. 104:1567–1574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iio A, Takagi T, Miki K, Naoe T, Nakayama
A and Akao Y: DDX6 post-transcriptionally down-regulates
miR-143/145 expression through host gene NCR143/145 in cancer
cells. Biochim Biophys Acta. 1829:1102–1110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kojima S, Enokida H, Yoshino H, et al: The
tumor-suppressive microRNA-143/145 cluster inhibits cell migration
and invasion by targeting GOLM1 in prostate cancer. J Hum Genet.
59:78–87. 2014. View Article : Google Scholar
|
|
37
|
Lin F, Lin P, Zhao D, et al: Sox2 targets
cyclinE, p27 and survivin to regulate androgen-independent human
prostate cancer cell proliferation and apoptosis. Cell Prolif.
45:207–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu N, Papagiannakopoulos T, Pan G, Thomson
JA and Kosik KS: MicroRNA-145 regulates OCT4, SOX2, and KLF4 and
represses pluripotency in human embryonic stem cells. Cell.
137:647–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cortes-Dericks L, Yazd EF, Mowla SJ,
Schmid RA and Karoubi G: Suppression of OCT4B enhances sensitivity
of lung adenocarcinoma A549 cells to cisplatin via increased
apoptosis. Anticancer Res. 33:5365–5373. 2013.PubMed/NCBI
|
|
40
|
Lin H, Sun LH, Han W, et al: Knockdown of
OCT4 suppresses the growth and invasion of pancreatic cancer cells
through inhibition of the AKT pathway. Mol Med Rep. 10:1335–1342.
2014.PubMed/NCBI
|
|
41
|
Gao Y, Wang X, Han J, Xiao Z, Chen B, Su G
and Dai J: The novel OCT4 spliced variant OCT4B1 can generate three
protein isoforms by alternative splicing into OCT4B. J Genet
Genomics. 37:461–465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Smith JE, Alvarez-Dominguez JR, Kline N,
Huynh NJ, Geisler S, Hu W, Coller J and Baker KE: Translation of
small open reading frames within unannotated RNA transcripts in
Saccharomyces cerevisiae. Cell Rep. 7:1858–1866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li J, Zhang Y, Wang Y, Zhang C, Wang Q,
Shi X, Li C, Zhang R and Li X: Functional combination strategy for
prioritization of human miRNA target. Gene. 533:132–141. 2014.
View Article : Google Scholar
|
|
44
|
Denzler R, Agarwal V, Stefano J, Bartel DP
and Stoffel M: Assessing the ceRNA hypothesis with quantitative
measurements of miRNA and target abundance. Mol Cell. 54:766–776.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ramsahoye BH, Davies CS and Mills KI: DNA
methylation: Biology and significance. Blood Rev. 10:249–261. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Leonhardt H, Rahn HP and Cardoso MC:
Functional links between nuclear structure, gene expression, DNA
replication, and methylation. Crit Rev Eukaryot Gene Expr.
9:345–351. 1999. View Article : Google Scholar
|
|
47
|
Ruvinsky A: Basics of gametic imprinting.
J Anim Sci. 77(Suppl 2): S228–S237. 1999.
|
|
48
|
Amente S, Lania L and Majello B: The
histone LSD1 demeth-ylase in stemness and cancer transcription
programs. Biochim Biophys Acta. 1829:981–986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chase A and Cross NC: Aberrations of EZH2
in cancer. Clin Cancer Res. 17:2613–2618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kellner S and Kikyo N: Transcriptional
regulation of the Oct4 gene, a master gene for pluripotency. Histol
Histopathol. 25:405–412. 2010.PubMed/NCBI
|
|
51
|
Lee YH and Wu Q: Chromatin regulation
landscape of embryonic stem cell identity. Biosci Rep. 31:77–86.
2011. View Article : Google Scholar
|
|
52
|
Das S and Levasseur D: Transcriptional
regulatory mechanisms that govern embryonic stem cell fate. Methods
Mol Biol. 1029:191–203. 2013. View Article : Google Scholar : PubMed/NCBI
|