Introduction
Breast cancer (BC) is the most frequently diagnosed
cancer and the leading cause of cancer-related deaths in females
worldwide, accounting for 23% (1.38 million) of the total new
cancer cases and 14% (458,400) of the total cancer deaths in 2008
(1). Even though treatment options
have been greatly improved, resistance to classical
chemotherapeutics is still a tremendous challenge for BC therapy
(2). To date, there is no validated
drug-response/resistant biomarker available in the clinical
settings, and the underlying mechanisms of acquisition of
resistance to chemotherapeutic agents are still poorly understood.
Hence, an advancement of the treatment by avoiding drug resistance
and a better prediction of chemotherapy efficacy would improve the
clinical outcome for BC patients.
Several different mechanisms of chemoresistance in
cancer have been elucidated. Changes in the intracellular metabolic
machinery, induction of growth signaling and impairment of
apoptosis all lead to the acquisition of drug resistance (3,4). As a
semisynthetic analogue of paclitaxel, docetaxel is widely used in
the treatment of advanced BC and other solid tumors. It leads to
cell cycle arrest and apoptosis by binding to and inhibiting the
depolymerisation of the β-tubulin subunit of microtubules (5,6). As
with other chemotherapeutic agents, numerous patients are either
intrinsically resistant or acquire resistance to docetaxel during
the course of treatment.
The resistance to docetaxel results from both
genetic and epigenetic dysregulation of key genes, involving drug
transporters, changes in drug metabolism and pathway alterations of
cell cycle and apoptosis (7–9).
Identified as important post-transcriptional regulators, microRNAs
(miRNAs) have expanded the definition of epigenetic regulation.
miRNAs are endogenous, non-coding RNAs of ~22 nucleotides that
target various genes either by degrading the mRNA or by repressing
the translation (10). An altered
expression of miRNAs in primary human types of cancers has been
used for tumor diagnosis, classification, staging and prognosis
(11). Moreover, they control the
cell growth, proliferation, metabolism and apoptosis. In addition,
the current research highlights their role in drug resistance in
various types of cancers (12,13).
As an oncogene or a tumor-suppressor gene, miR-141
has been reported to be either upregulated (ovarian and colorectal
cancers) (14,15) or downregulated (prostate, renal cell
and hepatocellular carcinoma) (16–18) in
various types of cancers. Recently, a study showed that
downregulated miR-141 may confer cisplatin resistance in esophageal
squamous cell carcinoma (19).
Moreover, miR-141 modulates cisplatin sensivitity in ovarian cancer
cells (20). Even though miR-141
has been intensively studied, no study has investigated the role of
miR-141 expression in docetaxel-resistance in BC or the underlying
mechanisms.
The present study is the first to report that
increased miR-141 expression was associated with acquired docetaxel
resistance in vitro and changes in miR-141 expression
modulated response to docetaxel in BC cells, at least in part by
targeting the eukaryotic translation initiation factor 4E (EIF4E).
Furthermore, we showed that suppression of miR-141 or EIF4E
significantly promoted or reduced docetaxel induced apoptosis,
respectively. Our study revealed an increased expression of miR-141
in an acquired model of docetaxel resistance in breast cancer.
Materials and methods
Cell lines and culture
Human BC MCF-7 and MDA-MB-231 cell lines were
obtained from ATCC. The parental cell line (termed
docetaxel-sensitive cells) was step wide cultured and made
resistant to docetaxel (termed docetaxel-resistant cells) following
sequential exposure to docetaxel (Sanofi-Aventis, Surrey, UK) at
increasing concentrations as previously described (21). Cells were cultured and maintained in
RPMI-1640 medium including L-glutamine, supplemented with 10% (v/v)
fetal calf serum and 1% (v/v) penicillin/streptomycin (100,000 U/l
penicillin, 10 mg/l streptomycin) at 37°C in a humidified
atmosphere containing 5% carbon dioxide.
RNA isolation
Total RNA and miRNA fractions were isolated from the
cell lines by TRIzol agent (Invitrogen, Carlsbad, CA, USA). The
quality and quantity of the RNA was assessed with a NanoDrop
ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE,
USA) at 260 and 280 nm (A260/280).
Real-time quantitative reverse
transcription-PCR
For miRNA expression detection, reverse
transcription (RT) reaction was performed with PrimeScript RT
reagent kit and real-time quantitative RT-PCR (qRT-PCR) was
performed using SYBR Premix Ex Taq™ II (both from Takara
Biotechnology Co., Ltd., Dalian, China) on the basis of the
protocol provided by the manufacturer. The expression of EIF4E mRNA
was detected by quantitative PCR using paired primers. The designed
PCR primers were as follows: EIF4E forward primer,
5′-ATGGCGACTGTCGAACCG-3′ and reverse primer,
5′-ATTAGATTCCGTTTTCTCCTCTTCTG-3′; GAPDH forward primer,
5′-AAGGGAAGGTTGCTGGATAGG-3′ and reverse primer,
5′-CACATCCACCTCCTCCACATC-3′. The expression of the target miRNA was
normalized relative to that of the internal control, U6 and the
expression of the target gene was normalized relative to the
expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH),
which was used as an internal control. Data were analyzed according
to the comparative Ct method also referred to as the
2−ΔΔCt method.
miRNA mimics, miRNA inhibitors and
siRNA
miR-141 mimics and inhibitors were purchased from
Shanghai GenePharma Co. (Shanghai, China) along with the negative
controls (miR-NC mimics or anti-miR-NC). siRNAs against EIF4E were
designed and synthesized by RiboBio Co. (Guangzhou, China). EIF4E
siRNA oligonucleotide duplexes (or control siRNA) using
Lipofectamine 2000 (Invitrogen) according to the manufacturer’s
protocol, respectively.
miR-141 transfection
miRNA-141 was overexpressed in MCF-7 or MDA-MB-231
docetaxel-sensitive cells using a miRNA-141 mimics. In MCF-7/DTX or
MDA-MB-231/DTX docetaxel-resistant cells, miRNA-141 was knocked
down using a miRNA-141 inhibitor. Cells (1×106) cultured
in a well of a 6-well cell culture plate were transiently
transfected with 50 pmol of miR-141 inhibitor and miR-141 mimics,
and its negative control oligonucleotides. Transfected cells were
resuspended and cultured in regular culture medium for 48–72 h
before analysis.
Western blotting
Total cellular proteins were extracted by cell
lysis, separated on an 8–10% SDS-PAGE, transferred onto a PVDF
membrane and incubated with primary antibodies against human EIF4E
(Santa Cruz Biotechnology) at 1:500 overnight at 4°C and then with
anti-rabbit IgG (horseradish peroxidase-conjugated secondary
antibody) for 1 h at room temperature. After being washed, the
membranes were developed with an ECL Plus Western Blotting
Detection System (Amersham, UK). The loading control for western
blotting was β-actin.
In vitro drug sensitivity assay
The sensitivity of the BC cells to docetaxel was
evaluated using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay as previously described (22). After 72-h culture, cell viability
was assessed using the MTT assay. The absorbance of each well at
490 nm (A490) was read on a spectrophotometer. The concentration at
which each drug produced 50% inhibition of growth (IC50)
was estimated by the relative survival curve. Four independent
experiments were performed in quadruplicate.
Luciferase assay
The pGL3-EIF4E wild-type and pGL3-EIF4E mutant
constructs were constructed and either 400 ng pGL3-EIF4E wild-type
or pGL3-EIF4E mutant and 8 ng pGL4.70 [hRluc] plasmid was
co-transfected with 50 nM miRNA-141 precursor. Transfected cells
were incubated for 24 h before performing the dual luciferase assay
according to the manufacturer’s instructions (Promega). Firefly
luciferase activity of the pGL3-control plasmid was normalized to
the Renilla luciferase activity of pGL4.70 [hRluc]. Each
assay was performed in triplicate.
Cell cycle and apoptosis analyses
At 24 h after miR-141 inhibitor transfection, cells
were incubated with docetaxel for 48 h. For cell cycle analysis,
cells were trypsinized, pelleted and then resuspended in propidium
iodide (PI) solution (Sigma-Aldrich, St. Louis, MO, USA), including
100 ng/ml of PI, 0.1 mg/ml of RNase A and 0.05% Triton X-100, for a
30-min incubation at 37°C and analyzed by flow cytometry using a
FACS calibur instrument with CellQuest Software (BD Biosciences,
Franklin Lakes, NJ, USA).
The Annexin V-FITC Apoptosis Detection kit (BD
Biosciences, La Jolla, CA, USA) was used for the apoptosis assays.
Cells (1×106) were stained according to the manuf
acturer’s protocol and sorted using a FACS sorter and the data were
analyzed using ModFit (both from BD Biosciences).
Bioinformatic and statistical
analysis
Online miRNA databases (TargetScan, miRBase Targets
and PicTarget) were used for prediction of miR-141 target genes.
Each experiment was repeated at least 3 times. Numerical data are
presented as the mean ± SD. The difference between means was
analyzed with ANOVA and then Student’s t-test. All statistical
analyses were performed using SPSS 19.0 software (SPSS, Inc.,
Chicago, IL, USA). Differences were considered to indicate a
statistically significant result when *P<0.05.
Results
miR-141 expression in docetaxel-sensitive
and-resistant human BC cell lines
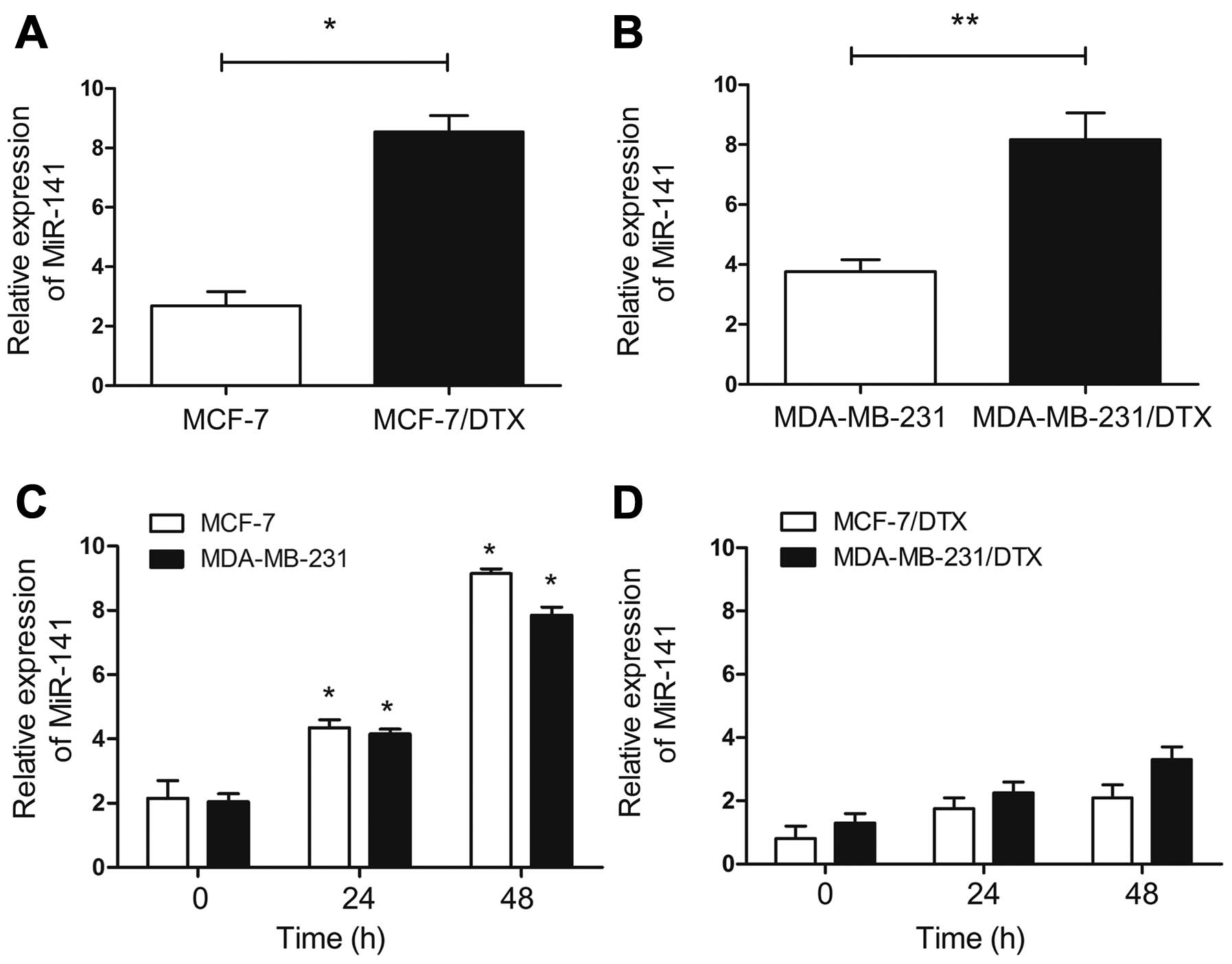
Two docetaxel-resistant BC cell lines (MCF-7/DTX and
MDA-MB-231/DTX) were developed from the docetaxel-sensitive BC cell
lines (MCF-7 and MDA-MB-231), respectively. To investigate whether
miR-141 regulates the acquired docetaxel-resistance in BC cells,
qRT-PCR was performed to detect the expression of miR-141 in
docetaxel-resistant and parental BC cells. Notably, the results
showed that miR-141 was expressed at significantly higher levels in
the MCF-7/DTX and MDA-MB-231/DTX cells than that in their parental
cells, respectively (P<0.05, Fig.
1A; P<0.01, Fig. 1B).
Then, we further verified whether docetaxel
treatment could affect the expression of miR-141 in BC cells. As
shown in Fig. 1C and D, the miR-141
levels were increased in the parental MCF-7 or MDA-MB-231 cells
after treatment with docetaxel (5.0 μg/l), but not in the
MCF-7/DTX or MDA-MB-231/DTX cells.
Downregulation of miR-141 reverses the
resistance of docetaxel-resistant BC cells to docetaxel
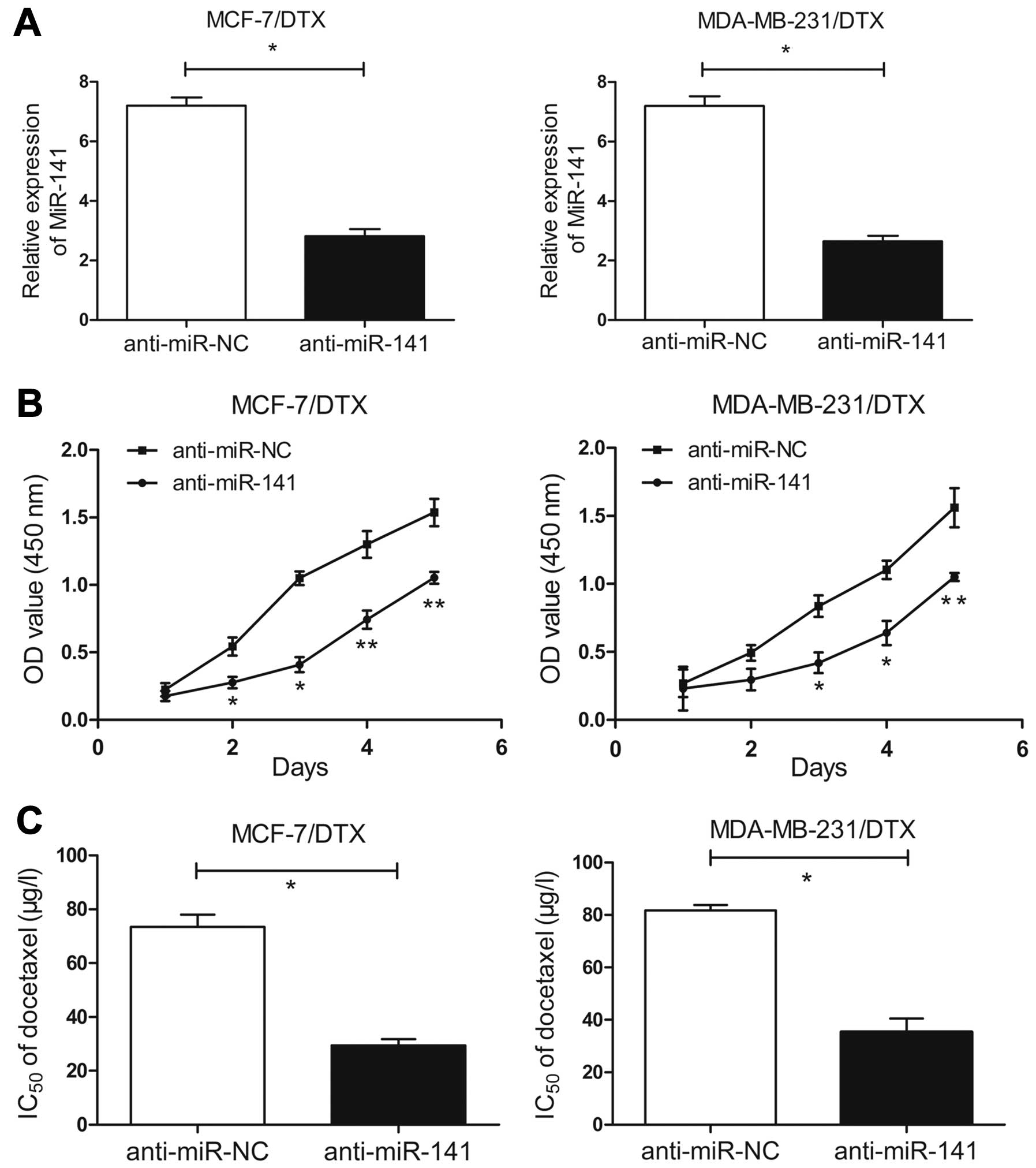
We further characterized the role of miR-141 in
regulating chemotherapy resistance by modulating its levels in BC
cells. Transient transfection of miR-141 inhibitor resulted in a
significant decrease in docetaxel-resistant BC cells. As shown in
Fig. 2A, the expression of miR-141
in miR-141 inhibitor-transfected MCF-7/DTX and MDA-MB-231/DTX cells
was significantly decreased compared with that in the
anti-miR-NC-transected cells (P<0.05). Further, the results of
the MTT assay showed that miR-141 inhibitor decreased the growth of
MCF-7/DTX and MDA-MB-231/DTX cells compared with anti-miR-NC cells
(Fig. 2B).
Next, the changes of docetaxel sensitivity of
chemoresistant BC cells induced by miR-141 inhibitor were analyzed.
Compared with the anti-miR-NC-transfected cells, the
IC50 value of docetaxel in the miR-141
inhibitor-transfected MCF-7/DTX and MDA-MB-231/DTX cells was
significantly decreased by ~44.0 and 46.4%, respectively
(P<0.05, Fig. 2C). From these
experimental data, it was concluded that the downregulation of
miR-141 could significantly reverse the resistance of
docetaxel-resistant BC cells to docetaxel by inhibiting their
growth and viability.
Upregulation of miR-141 reduces the in
vitro sensitivity of parental BC cells to docetaxel
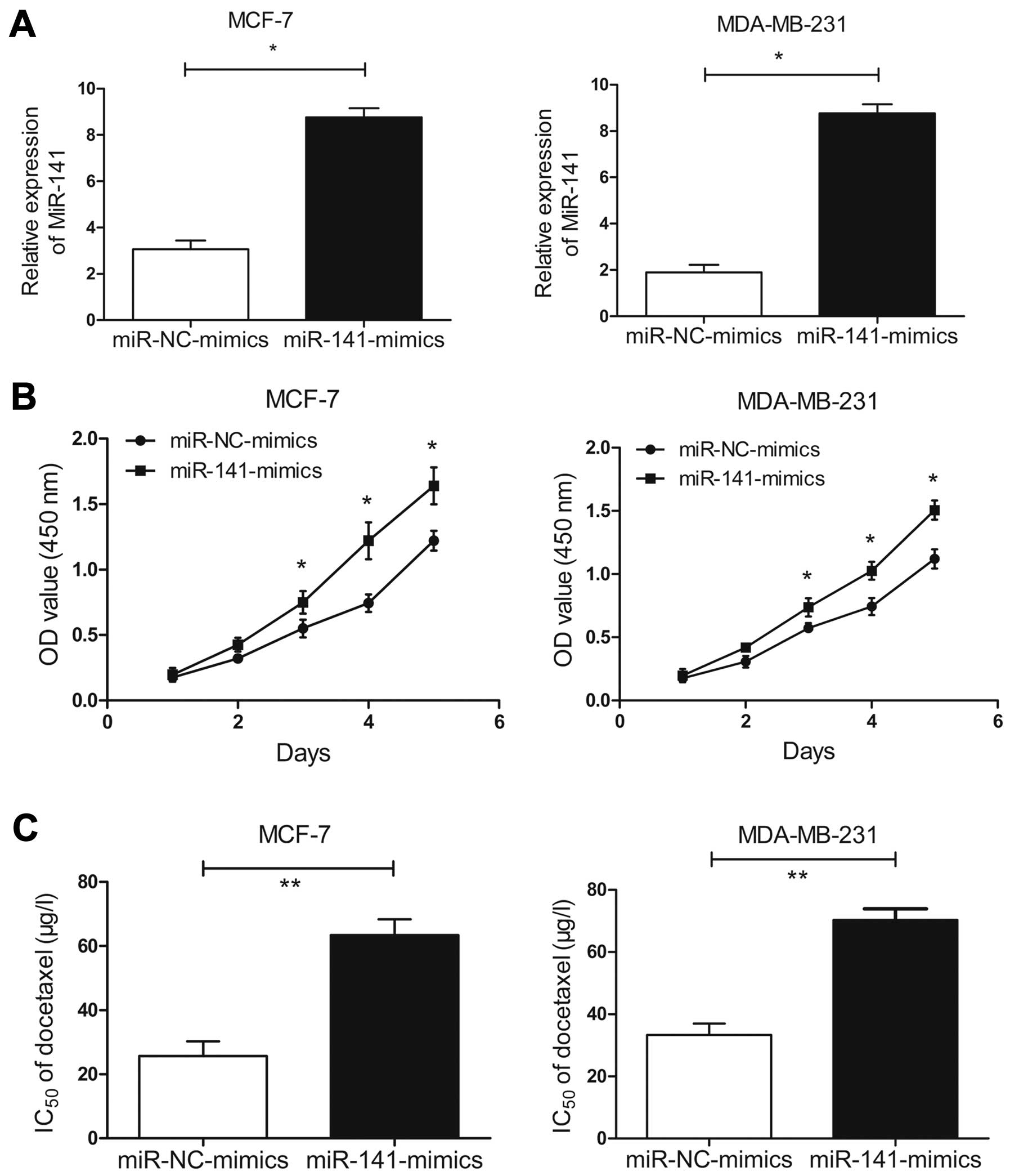
On the other hand, we investigated whether
upregulation of miR-141 affected the sensitivity of BC cells to
docetaxel. Then, miR-141 mimics were transfected into the parental
MCF-7 or MDA-MB-231 cells. After 48 h transfection, qRT-PCR assay
showed that the expression of miR-141 in the miR-141
mimic-transfected MCF-7 or MDA-MB-231 cells could be significantly
increased (P<0.05, Fig. 3A). MTT
assay indicated that miR-141 mimics could induce the increased
growth ability of the parental MCF-7 or MDA-MB-231 cells compared
with miR-NC mimics (Fig. 3B). More
importantly, the IC50 value of docetaxel in the miR-141
mimic-transfected MCF-7 or MDA-MB-231 cells were increased by ~33.4
or 42.3%, respectively, compared with the miR-NC mimic-transfected
cells (P<0.05, Fig. 3C). These
data suggested that upregulation of miR-141 promotes the resistance
of docetaxel in BC cells.
EIF4E is a target of miR-141 and
responsible for the miR-141-induced resistance in BC cells
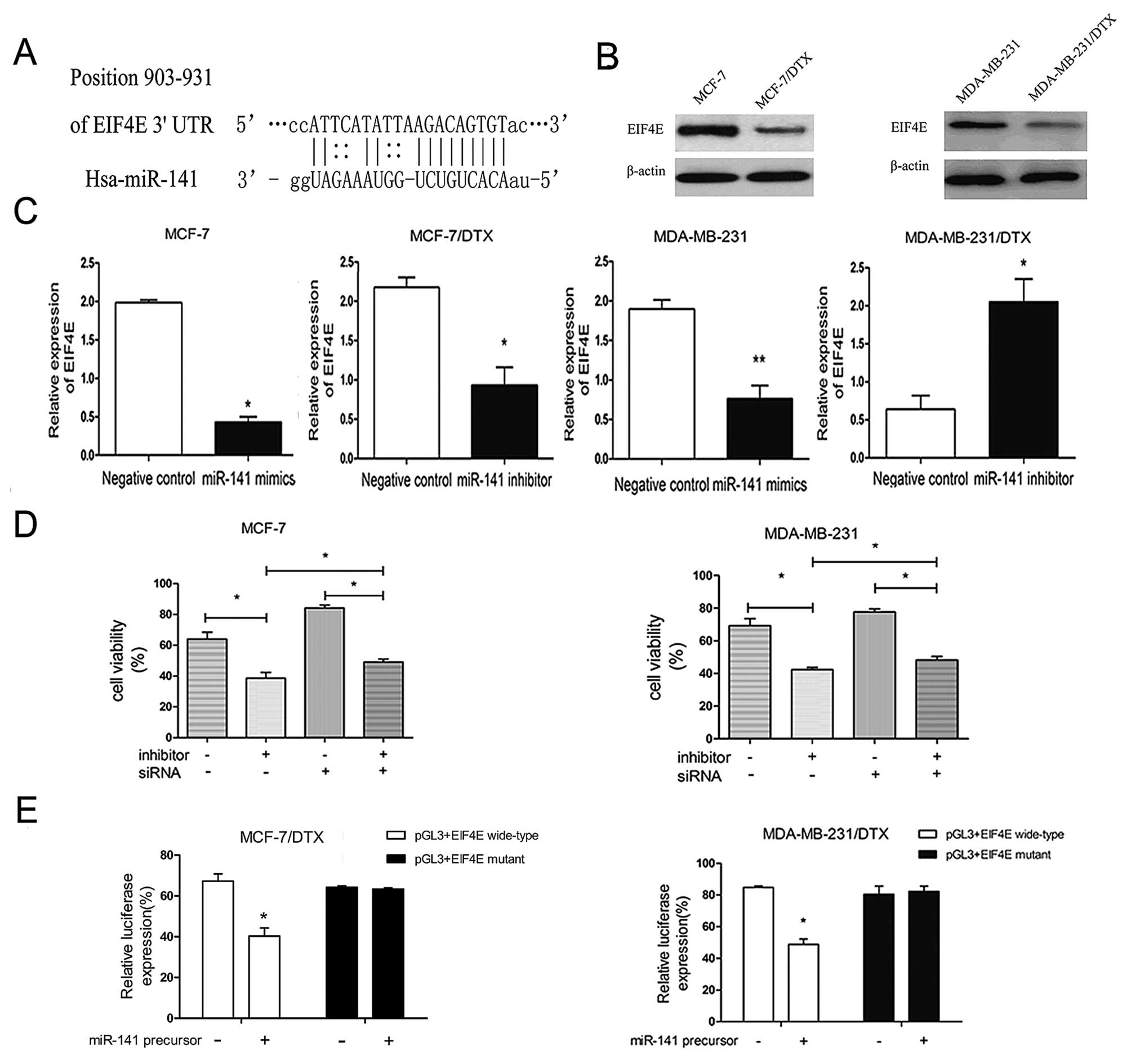
To examine the molecular mechanisms by which miR-141
regulates chemoresistance, we investigated its target genes and
focused on a few that relate to cancer progression. By employing
open access software (TargetScan, miRBase Targets and PicTarget),
transcription factor EIF4E was chosen as a preferred candidate
target gene of miR-141 due to the complementary site of miR-141 in
its 3′-untranslated region (3′-UTR) (Fig. 4A). To investigate whether the EIF4E
gene could exert functional effects in BC cells, western blot
analysis was performed and downregulation of EIF4E protein
expression was detected in the MCF-7/DTX and compared with MCF-7
cells. A similar trend was also found in the MDA-MB-231/DTX cells
(Fig. 4B).
We investigated the correlation between miR-141 and
EIF4E. As shown in Fig. 4C,
compared with the NC-transfected cells, the level of EIF4E mRNA
expression was significantly increased in the miR-141
inhibitor-transfected MCF-7/DTX and MDA-MB-231/DTX cells, and
markedly decreased in the miR-141 mimic-transfected MCF-7 and
MDA-MB-231 cells (P<0.05). While downregulation of the
expression of EIF4E by siRNAs, MCF-7/MDA-MB-231 cells became more
resistant to the therapy of docetaxel (Fig. 4D). In addition, the enhanced
growth-inhibitory effect by the miR-141 inhibitor transfection was
weakened after the addition of EIF4E siRNA (Fig. 4D). Those results suggest that the
EIF4E expression is regulated by miR-141 in BC and is responsible
for the miR-141-induced resistance.
To further confirm the possibility that miR-141
targets EIF4E, a dual luciferase reporter system was used to
measure the direct interaction between miR-141 and EIF4E. Upon
transfection of the miR-141 precursor, luciferase activity
significantly decreased by 27.0 and 36.1% (P<0.05) in the
MCF-7/DTX/MDA-MB-231/DTX cells that contained the 3′-UTR with the
miR-141 seed region of EIF4E, respectively (Fig. 4E). In contrast, addition of the
miR-141 precursor did not alter luciferase activity in
docetaxel-resistant cells that contained a mutated seed region in
the 3′-UTR of the EIF4E sequence (Fig.
4E). By reducing luciferase activity, this shows that miR-141
directly interacts with the 3′-UTR region of the EIF4E gene.
Transfection of miR-141 inhibitor or
siRNA-EIF4E promotes or reduces docetaxel-induced apoptosis,
respectively
To explore the mechanism(s) by which miR-141
inhibitor induces sensitivity of BC cells to docetaxel, we analyzed
the cell cycle and apoptosis alterations in MCF-7 cells transfected
with the miR-141 inhibitor. Suppression of miR-141 had no
significant alterations in the cell cycle (data not shown). We next
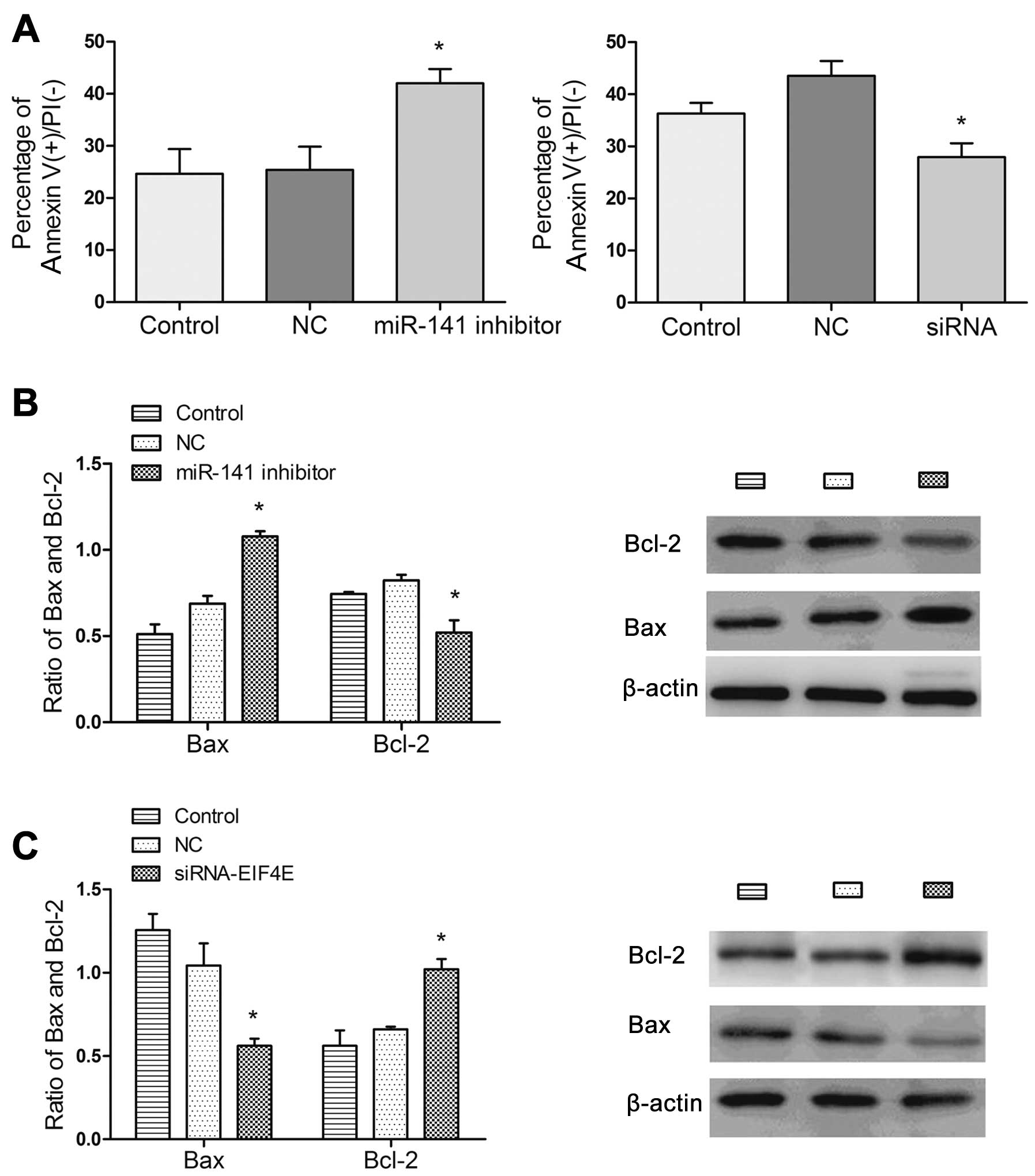
measured cell apoptosis using an Annexin V/PI assay. The results
showed a significant increase in the percentages of apoptosis of
miR-141 inhibitor-transfected MCF-7 cells treated with docetaxel
than the control cells (Fig. 5A,
left panel) and significant decrease in the percentages of
apoptosis of siRNA-EIF4E-transfected cells with docetaxel than the
control cells (Fig. 5A, right
panel). Therefore, the results suggested that downregulation of
miR-141 could lead to an apoptosis-inducing effect similarly to
that in upregulation of EIF4E.
Next, we measured the expression of
apoptosis-related proteins in BC cells. As a result, treatment with
docetaxel elevated the expression of Bax and lowered the expression
of Bcl-2 in the miR-141 inhibitor-transfected cells compared to the
mock-transfected cells (Fig. 5B).
After treatment with docetaxel, decreased expression of Bax and
increased Bcl-2 expression was noted when the cells were
transfected with siRNA-EIF4E (Fig.
5C).
Discussion
The key finding of the present study is that miR-141
is correlated with breast cancer (BC) chemoresistance to docetaxel
and serves as a phenotypic regulator of chemoresistance. While our
studies focused on the role of miR-141 as a regulator of
chemotherapy response, data showed that downregulation of miR-141
reversed the resistance of docetaxel-resistant BC cells.
Furthermore, EIF4E was a target of miR-141 and partly responsible
for the miR-141-induced resistance in BC cells. Transfection of
miR-141 inhibitor and siRNA-EIF4E significantly promoted
docetaxel-induced apoptosis and changed the expression of Bcl-2 and
BAX. These data suggest that miR-141 is a potential target for
chemosensitizing BCs.
miR-141 is a widely studied human miRNA that was
associated with cancer progression and metastasis in a variety of
cancers (23–27) and recently, evidence emerged that
miR-141 is also associated with drug resistance in cancer. Zhang
et al reported that miR-141 can predict tumor progression in
prostate cancer (28). Also, high
miR-141 plasma level is associated with poor prognosis in
colorectal cancer and was proposed as a novel biomarker to find
distant metastases (15). All the
above research reported that miR-141 not only to acts as a tumor
suppressor, but also as an oncogene. Equally important, Imanaka
et al highlighted an important regulatory role for miR-141
in the development of cisplatin resistance in esophageal squamous
cell carcinoma (19). Furthermore,
the miR-141-mediated regulation of KEAP1 has a crucial role in the
cellular response to cisplatin in ovarian cancer cells (20). Moreover, the downregulated miR-141
was involved in Helicobacter pylori-modulated cisplatin
sensitivity in gastric cancer (29). However, there are no previous
reports concerning the association of miR-141 expression with the
chemoresistance of BC tumor cells. In the present study, we firstly
showed that miR-141 was significantly upregulated in
docetaxel-resistant BC cells (MCF-7/DTX and MDA-MB-231/DTX)
compared with parental BC cells. By modulating the miR-141 level in
BC cells, we revealed that miR-141 mediated the docetaxel
sensitivity of BC cells in vitro, which was consistent with
the majority of the above studies.
Although several studies identified the role of
miR-141 in chemoresistance, the underlying mechanism of the
miR-141-induced chemoresistance was still unclear. Most miRNAs
function through the inhibition of effective mRNA translation of
target genes, which may be involved in the progression of cancer
and chemoresistance. Previous research showed that the NF-κB
pathway, which can be regulated by KEAP1, is activated upon miR-141
overexpression, and the inhibition of this pathway partially
reverses miR-141-mediated cisplatin resistance in ovarian cancer
(29). van Jaarsveld et al,
stressed that miR-141 is an oncogene that can enhance anoikis
resistance in ovarian cancer cells through downregulation of KLF12.
Indeed, the known targets of miR-141, KEAP1 and KLF12 played an
important role in regulating drug-resistance and cancer development
(20).
Eukaryotic initiation factor 4E (EIF4E), is a
eukaryotic translation initiation factor involved in directing
ribosomes to the cap structure of mRNAs and plays an important role
in the development and progression of cancer (30,31).
It contributes to malignancy by selectively enabling the
translation of a limited pool of mRNAs that encode key proteins
involved in cell proliferation, angiogenesis and survival, as well
as in transformation and metastasis (32,33).
Increased eIF4E expression has been shown in several cancer cells
(including those of the breast, head and neck, bladder, colon, lung
and prostate) (34–36) and its overexpression also appears to
be related to disease progression (37). Moreover, studies showed that
cellular overexpression of eIF4E results in increased proliferation
and suppression of apoptosis, whereas reduction in eIF4E
expression, reduces tumorigenesis (38). In our previous study, we
demonstrated that siRNA-mediated EIF4E knockdown yielded very
similar effects as that of ectopic miR-141 expression in BC cells.
EIF4E is responsible for the miR-141 modulating chemosensitivity of
BC cells to docetaxel and it was a direct target of miR-141.
Moreover, EIF4E is partly responsible for the miR-141-induced
apoptosis. This suggests that miR-141-mediated docetaxel resistance
occurs through regulation of EIF4E and EIF4E is involved in the
regulation of the apoptosis process. Therefore, the biological role
of decreased EIF4E in docetaxel resistance remains unclear and can
only be speculated upon. The EIF4E family contains a variety of
genes which may also be a target of miR-141. Therefore, alterations
of miR-141 may trigger the apoptotic gene changes, whereby EIF4E
expression is predominated by regulating the expression of other
anti-apoptotic genes, which in turn may lead to resistance to
docetaxel. It was concluded that downregulation of EIF4E may be a
molecular mechanism by which miR-141 exerted its functions of
chemosensitivity regulator on human BC cells.
Furthermore, we showed that transfection of miR-141
inhibitor could significantly promote docetaxel-induced apoptosis
and change the expression of Bax and Bcl-2. However, when the BC
cells were transfected with siRNA-EIF4E, the data showed opposite
results. It suggested that EIF4E is partly responsible for the
miR-141-induced apoptosis which is related to the mitochondrial
apoptosis pathway. In the previous studies, antisense Bcl-2
treatment enhanced sensitivity to tamoxifen in HER2-positive cells
in tamoxifen-resistant BC cells (39). In addition, Bcl-2 overexpression,
causes paclitaxel resistance in MCF-7 cells (40). These studies showed, that even
though Bcl-2 is known as an anti-apoptotic protein, increased Bcl-2
expression can be beneficial in BC treatment, whereas decreased
Bcl-2 expression is associated with drug resistance or poorer
outcome. In the present study, the mechanisms by which the
decreased Bcl-2 expression negatively influenced the activity of
docetaxel are currently not known. The apoptotic pathway is a
complex machinery that involves a variety of apoptotic genes,
therefore it may be possible that other factors influence the cell
to enter or evade docetaxel-induced apoptosis.
In conclusion, the results of the present study
demonstrated that miR-141 affected the chemosensitivity of BC cells
to docetaxel by directly targeting EIF4E, due to its anti-apoptotic
properties. Our data suggest that miR-141 may serve as a potential
target for BC therapy. The study highlights the potentially
important role of miR-141 in the development of drug resistance,
and suggests that miR-141 may serve as biomarkers for response to
chemotherapy. Further investigation of a larger patient population
will be necessary to confirm the association of miR-141 and its
target EIF4E with the responses of BC patients to docetaxel-based
chemotherapy.
Acknowledgments
The present study was supported by the Shandong
Natural Science Foundation (Y2006C23).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chuthapisith S, Eremin JM, El-Sheemy M and
Eremin O: Neoadjuvant chemotherapy in women with large and locally
advanced breast cancer: chemoresistance and prediction of response
to drug therapy. Surgeon. 4:211–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y, Du F, Chen W, Yao M, Lv K and Fu P:
Knockdown of dual specificity phosphatase 4 enhances the
chemosensitivity of MCF-7 and MCF-7/ADR breast cancer cells to
doxorubicin. Exp Cell Res. 319:3140–3149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li M, Yin J, Mao N and Pan L: Upregulation
of phosphorylated cofilin 1 correlates with taxol resistance in
human ovarian cancer in vitro and in vivo. Oncol Rep. 29:58–66.
2013.
|
|
5
|
Yvon AM, Wadsworth P and Jordan MA: Taxol
suppresses dynamics of individual microtubules in living human
tumor cells. Mol Biol Cell. 10:947–959. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang TH, Wang HS and Soong YK:
Paclitaxel-induced cell death: where the cell cycle and apoptosis
come together. Cancer. 88:2619–2628. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hopper-Borge E, Chen ZS, Shchaveleva I,
Belinsky MG and Kruh GD: Analysis of the drug resistance profile of
multidrug resistance protein 7 (ABCC10): resistance to docetaxel.
Cancer Res. 64:4927–4930. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rouzier R, Rajan R, Wagner P, et al:
Microtubule-associated protein tau: a marker of paclitaxel
sensitivity in breast cancer. Proc Natl Acad Sci USA.
102:8315–8320. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kastl L, Brown I and Schofield AC:
miRNA-34a is associated with docetaxel resistance in human breast
cancer cells. Breast Cancer Res Treat. 131:445–454. 2012.
View Article : Google Scholar
|
|
10
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Voorhoeve PM: MicroRNAs: oncogenes, tumor
suppressors or master regulators of cancer heterogeneity? Biochim
Biophys Acta. 1805:72–86. 2010.
|
|
12
|
Hao J, Zhao S, Zhang Y, et al: Emerging
role of microRNAs in cancer and cancer stem cells. J Cell Biochem.
115:605–610. 2014. View Article : Google Scholar
|
|
13
|
Blower PE, Chung JH, Verducci JS, et al:
MicroRNAs modulate the chemosensitivity of tumor cells. Mol Cancer
Ther. 7:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iorio MV, Visone R, Di Leva G, et al:
MicroRNA signatures in human ovarian cancer. Cancer Res.
67:8699–8707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin J, Bai Z, Song J, et al: Differential
expression of serum miR-126, miR-141 and miR-21 as novel biomarkers
for early detection of liver metastasis in colorectal cancer. Chin
J Cancer Res. 26:95–103. 2014.PubMed/NCBI
|
|
16
|
Porkka KP, Pfeiffer MJ, Waltering KK,
Vessella RL, Tammela TL and Visakorpi T: MicroRNA expression
profiling in prostate cancer. Cancer Res. 67:6130–6135. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Wang X, Ruan A, et al: miR-141 is
a key regulator of renal cell carcinoma proliferation and
metastasis by controlling EphA2 expression. Clin Cancer Res.
20:2617–2630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Ding Y, Huang J, et al: MiR-141
suppresses the migration and invasion of HCC cells by targeting
Tiam1. PLoS One. 9:e883932014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Imanaka Y, Tsuchiya S, Sato F, Shimada Y,
Shimizu K and Tsujimoto G: MicroRNA-141 confers resistance to
cisplatin-induced apoptosis by targeting YAP1 in human esophageal
squamous cell carcinoma. J Hum Genet. 56:270–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Jaarsveld MT, Helleman J, Boersma AW,
et al: miR-141 regulates KEAP1 and modulates cisplatin sensitivity
in ovarian cancer cells. Oncogene. 32:4284–4293. 2013. View Article : Google Scholar
|
|
21
|
Brown I, Shalli K, McDonald SL, et al:
Reduced expression of p27 is a novel mechanism of docetaxel
resistance in breast cancer cells. Breast Cancer Res. 6:R601–R607.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Juengel E, Nowaz S, Makarevi J, et al:
HDAC-inhibition counteracts everolimus resistance in renal cell
carcinoma in vitro by diminishing cdk2 and cyclin A. Mol Cancer.
13:1522014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao G, Wang B, Liu Y, et al: miRNA-141,
downregulated in pancreatic cancer, inhibits cell proliferation and
invasion by directly targeting MAP4K4. Mol Cancer Ther.
12:2569–2580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mateescu B, Batista L, Cardon M, et al:
miR-141 and miR-200a act on ovarian tumorigenesis by controlling
oxidative stress response. Nat Med. 17:1627–1635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morales-Prieto DM, Schleussner E and
Markert UR: Reduction in miR-141 is induced by leukemia inhibitory
factor and inhibits proliferation in choriocarcinoma cell line
JEG-3. Am J Reprod Immunol. 66(Suppl 1): S57–S62. 2011. View Article : Google Scholar
|
|
26
|
Zhang L, Deng T, Li X, et al: microRNA-141
is involved in a nasopharyngeal carcinoma-related genes network.
Carcinogenesis. 31:559–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Du Y, Xu Y, Ding L, et al: Down-regulation
of miR-141 in gastric cancer and its involvement in cell growth. J
Gastroenterol. 44:556–561. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang HL, Qin XJ, Cao DL, et al: An
elevated serum miR-141 level in patients with bone-metastatic
prostate cancer is correlated with more bone lesions. Asian J
Androl. 15:231–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou X, Su J, Zhu L and Zhang G:
Helicobacter pylori modulates cisplatin sensitivity in gastric
cancer by down-regulating miR-141 expression. Helicobacter.
19:174–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sonenberg N: eIF4E, the mRNA cap-binding
protein: from basic discovery to translational research. Biochem
Cell Biol. 86:178–183. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thoreen CC: Many roads from mTOR to eIF4F.
Biochem Soc Trans. 41:913–916. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fischer PM: Cap in hand: targeting eIF4E.
Cell Cycle. 8:2535–2541. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mathonnet G, Fabian MR, Svitkin YV, et al:
MicroRNA inhibition of translation initiation in vitro by targeting
the cap-binding complex eIF4F. Science. 317:1764–1767. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Graff JR and Zimmer SG: Translational
control and metastatic progression: enhanced activity of the mRNA
cap-binding protein eIF-4E selectively enhances translation of
metastasis-related mRNAs. Clin Exp Metastasis. 20:265–273. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nasr Z, Robert F, Porco JA Jr, Muller WJ
and Pelletier J: eIF4F suppression in breast cancer affects
maintenance and progression. Oncogene. 32:861–871. 2013. View Article : Google Scholar
|
|
36
|
Furic L, Rong L, Larsson O, et al: eIF4E
phosphorylation promotes tumorigenesis and is associated with
prostate cancer progression. Proc Natl Acad Sci USA.
107:14134–14139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
De Benedetti A and Graff JR: eIF-4E
expression and its role in malignancies and metastases. Oncogene.
23:3189–3199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Avdulov S, Li S, Michalek V, et al:
Activation of translation complex eIF4F is essential for the
genesis and maintenance of the malignant phenotype in human mammary
epithelial cells. Cancer Cell. 5:553–563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim R, Tanabe K, Emi M, Uchida Y and Toge
T: Modulation of tamoxifen sensitivity by antisense Bcl-2 and
trastuzumab in breast carcinoma cells. Cancer. 103:2199–2207. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang Y, Ray S, Reed JC, et al: Estrogen
increases intracellular p26Bcl-2 to p21Bax ratios and inhibits
taxol-induced apoptosis of human breast cancer MCF-7 cells. Breast
Cancer Res Treat. 42:73–81. 1997. View Article : Google Scholar : PubMed/NCBI
|