Introduction
Breast cancer is the most common malignancy and the
leading cause of cancer-related mortality among women worldwide
(1). In patients with breast
cancer, survival rates have improved steadily over the past two
decades. Death resulting from breast cancer is due primarily to
cancer cell invasion of the surrounding tissues and metastasis to
distal organs followed by secondary tumor formation (2). However, triple-negative breast cancer
(TNBC) exhibits aggressive characteristics associated with shorter
disease-free survival (3). TNBC
tumors are characterized by the absence of estrogen receptor (ER)
and progesterone receptor (PR) expression as well as human
epidermal growth factor receptor 2 (HER-2) amplification.
Therefore, patients with TNBCs do not benefit from commonly used
antiestrogen and herceptin-based therapies (4,5).
Although chemotherapy is currently the mainstay of systemic
treatment for breast cancer, patients with TNBC disease have a
worse outcome after chemotherapy than patients with other subtypes
of breast cancer (6,7). Previous studies revealed that
neoadjuvant treatment involving the administration of chemotherapy
before surgery was effective in a minority of women with TNBCs who
showed a complete pathologic response and an excellent outcome;
however, a relatively poor outcome was observed for the majority of
the population with residual disease after treatment.
Ubiquitin is a small regulatory protein that is
found in almost all tissues of eukaryotic organisms.
Ubiquitination, the covalent attachment of ubiquitin to a target
protein, is a post-translational modification that regulates the
stability, function and/or localization of the modified proteins
(8,9). USP39, also known as 65-kDa SR-related
protein of the U4/U6·U5 tri-snRNP, has been implicated in the
assembly of the mature spliceosome complex (10). USP39 is also required to maintain
the spindle checkpoint and to support successful cytokinesis.
Consistent with its previously described role in mRNA processing,
depletion of USP39 leads to a specific reduction of the mRNA levels
of Aurora B (11), and mutation of
zebrafish USP39 leads to rb1 splicing defect and pituitary lineage
expansion (12). In addition, USP39
and USP4 can form a stable complex in the cell (13). The USP4 contains a Ubl
(ubiquitin-like) domain, and this Ubl domain can bind to the
catalytic domain and compete with the ubiquitin substrate,
partially inhibiting USP4 activity against different substrates.
Notably, USP39 relieves this inhibition (14).
In the field of mammalian cell biology, a major
approach to reveal one or several gene functions by
transient/stable overexpression or knockdown of the target gene,
involves manipulation of the expression of genes of interest in
selected cell lines, Unfortunately, for various cell biological
investigations, this approach is unsuitable when the gene
expression manipulations result in cell growth/proliferation
defects or unwanted cell differentiation. Therefore, researchers
have adapted the tetracycline repressor protein (TetR) from the
E. coli tetracycline resistance operon to generate extremely
efficient systems for the expression of cDNAs in mammalian cells
(15,16). In short, TetR has been modified to
either block transcription initiation by binding to the
Tet-operator (TO) in the promoter region following addition of
tetracycline (termed Tet-off system) or bind to the TO in the
absence of tetracycline (termed Tet-on system). Given the
inconvenience that the Tet-off system requires the continuous
presence of tetracycline, the Tet-on system has been more
extensively optimized, resulting in the development of very
efficient vector systems for cDNA expression. The aim of the
present study was to elucidate the role of USP39 in TNBC cells
using the Tet inducible system. Using real-time PCR analysis, we
found that the USP39 gene was more active in TNBC cells than in
non-TNBC cells. Therefore, we investigated USP39 in TNBC cells.
Materials and methods
Tissue samples and clinical data
Informed consent was obtained for the use of 25
human triple-negative breast cancer (TNBC) tissue samples from
adult patients diagnosed at the Affiliated Hospital of Qingdao
University, Qingdao, China. Twenty-five normal breast tissue
samples were also obtained. The present study was approved by the
hospital institutional review board and written informed consent
was obtained from all patients included in the present study.
Cell culture and reagents
The human embryonic kidney cell line 293FT, human
breast cancer cell lines MDA-MB-231 and HCC1937, and the normal
breast cell line HS578BST, were obtained from the American Type
Culture Collection (Rockville, MD, USA) and cultured in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine
serum (FBS) and 100 IU/ml penicillin/streptomycin in a 37°C
humidified incubator with 5% CO2 (17).
Lentiviral production and
transduction
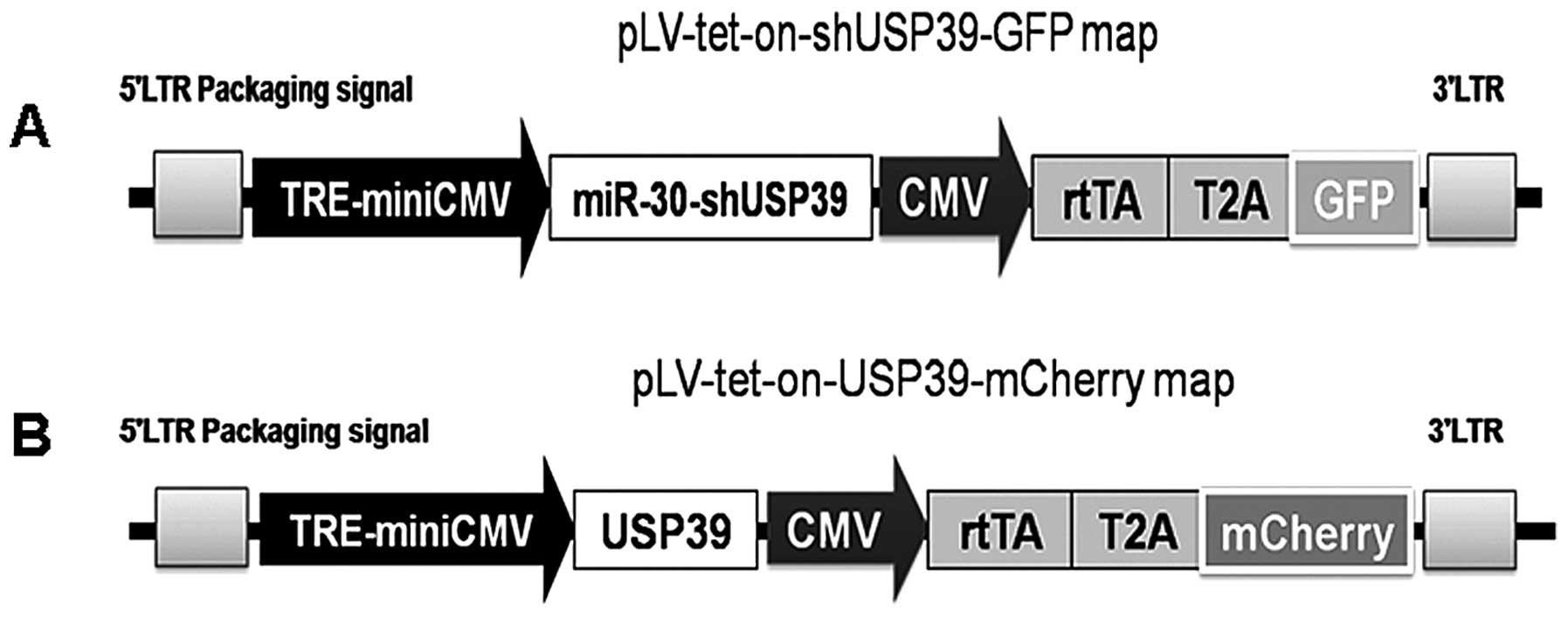
We reconstituted the Tet-on (tetracycline-regulated
transgene expression) two-component system in a single lentiviral
vector by inserting a CMV-rtTA-T2A-reporter gene element after the
TRE- miniCMV expression cassettes based on the pLVTHM system. To
develop the miR-30 hairpin-based gene knockdown vector, the miR-30
backbone sequences were further modified to incorporate the target
gene. To test miR-30 hairpin-based knockdown of USP39 in a
lentiviral vector, we designed miR-30-shUSP39 to the USP39 gene.
The forward (miR-30-shUSP39-F) and reverse (miR-30-shUSP39-R)
oligonucleotides used were
5′-TCGAGAAGGTATATTGCTGTTGACAGTGAGCGAAAGGTTAAGGTGAGCTCATCGTAGTGAAGCCACAGATGTACGATGAGCTCACCTTAACCTTGTGCCTACTGCCTCGG-3′
and
5′-AATTCCGAGGCAGTAGGCACAAGGTTAAGGTGAGCTCATCGTACATCTGTGGCTTCACTACGATGAGCTCACCTTAACCTTTCGCTCACTGTCAACAGCAATATACCTTC-3′,
respectively. These sequences were synthesized, annealed into new
oligonucleotides, then ligated into the pLV-tet-on-GFP vector and
designated pLV-tet-on-shUSP39-GFP. The USP39 full-length fragment
was inserted into the pLV-tet-on-mCherry vector to generate the
pLV-tet-on-USP39-mCherry shuttle vector. Lentiviruses were
generated by cotransfecting 15 μg of lentiviral vector and
7.5 μg of each packaging vector (coding for Gag, Pol, Tat,
Rev and VSVG) into 293FT cells using the calcium phosphate method.
Supernatants were collected 48 h after transfection, filtered
through a 0.4-μm membrane and used to infect the cells. The
cells were infected and the percentage of GFP/mCherry-positive
cells was determined to ensure that the majority of cells contained
the lentiviruses (18).
RNA extraction and quantitative reverse
transcription-PCR
Total RNA was isolated using RNAiso (Takara)
(19). Quantitative real-time PCR
was performed using the LightCycler 480 instrument (Roche) with the
SYBR-Green PCR kit (Takara). The primers used were: USP39,
5′-TTTTCCTCAACCTCCACA-3′ and 5′-ATTCAGTCCCA CAATACCC-3′; and GAPDH,
5′-TCATGGGTGTGAACC ATGAGAA-3′ and 5′-GGCATGGACTGTGGTCATGAG-3′. The
parameters for the PCR were 1 cycle at 95°C for 1 min followed by
40 cycles at 95°C for 5 sec and then 60°C for 20 sec. Fold-changes
in mRNA levels were calculated according to the 2−ΔΔCt
method using GAPDH mRNA for normalization.
Protein extraction and western blot
analysis
The mock-infected and infected cells in 6-well
plates were washed once with phosphate-buffered saline and lysed
directly in 1X sodium dodecyl sulfate (SDS) sample buffer. Proteins
were denatured by boiling. Equivalent amounts of protein were
subjected to 10% SDS-polyacrylamide gel electrophoresis and
transferred to polyvinylidene fluoride membranes (Millipore). The
membranes were blocked in Tris-buffered saline-Tween containing 5%
milk and probed overnight in Tris-buffered saline-Tween at 4°C with
anti-caspase-3 (1:2,000), anti-Bcl-2 (1:1,000), anti-Bax (1:1,000)
or anti-GAPDH (1:5,000). Horseradish peroxidase-conjugated goat
anti-rabbit and anti-mouse immunoglobulin secondary antibodies were
purchased from Sigma and used at a 1:5,000 dilution. Secondary
antibody was detected using ECL Plus detection reagents (Amersham
Biosciences) and captured with Fusion FX6 (Vilber Lourmat). The
results were quantified using ImageJ software.
MTT assay
MTT assays were performed to analyze the cell
proliferation as previously described (19). In brief, 5,000 cells/well were
cultured in 96-well plates. Cells were incubated with 100
μl/well of
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-tetrazolium bromide (MTT,
0.5 mg/ml; Sigma) for 4 h. After removing the medium, 100 μl
of DMSO was added to each well, and the plates were shaken for 10
min. The absorbance of each well was measured at 490 nm using a
Safire II spectrophotometer reader (Tecan, Mannedorf,
Switzerland).
Colony formation assay
For the colony formation assay, cells were seeded
evenly in 6-well plates (2×102 cells/well) and cultured
for 14 days. Next, the cells were fixed with methanol for 10 min,
stained with 0.1% crystal violet for 20 min, and washed 3 times
with phosphate-buffered saline. Each treatment group was assayed in
triplicate.
Statistical analysis
The Student’s t-test and one-way analysis of
variance were used to determine significance. Error bars were used
to represent the standard error of the mean. The Pearson
correlation coefficient was calculated to test the association
between normal and tumor samples. A P-value <0.05 was considered
to indicate a statistically significant result.
Results
USP39 is upregulated in TNBC tissue and
TNBC cancer cells
In the present study, we examined the effect of
USP39 on TNBC. To evaluate USP39 expression in TNBC and normal
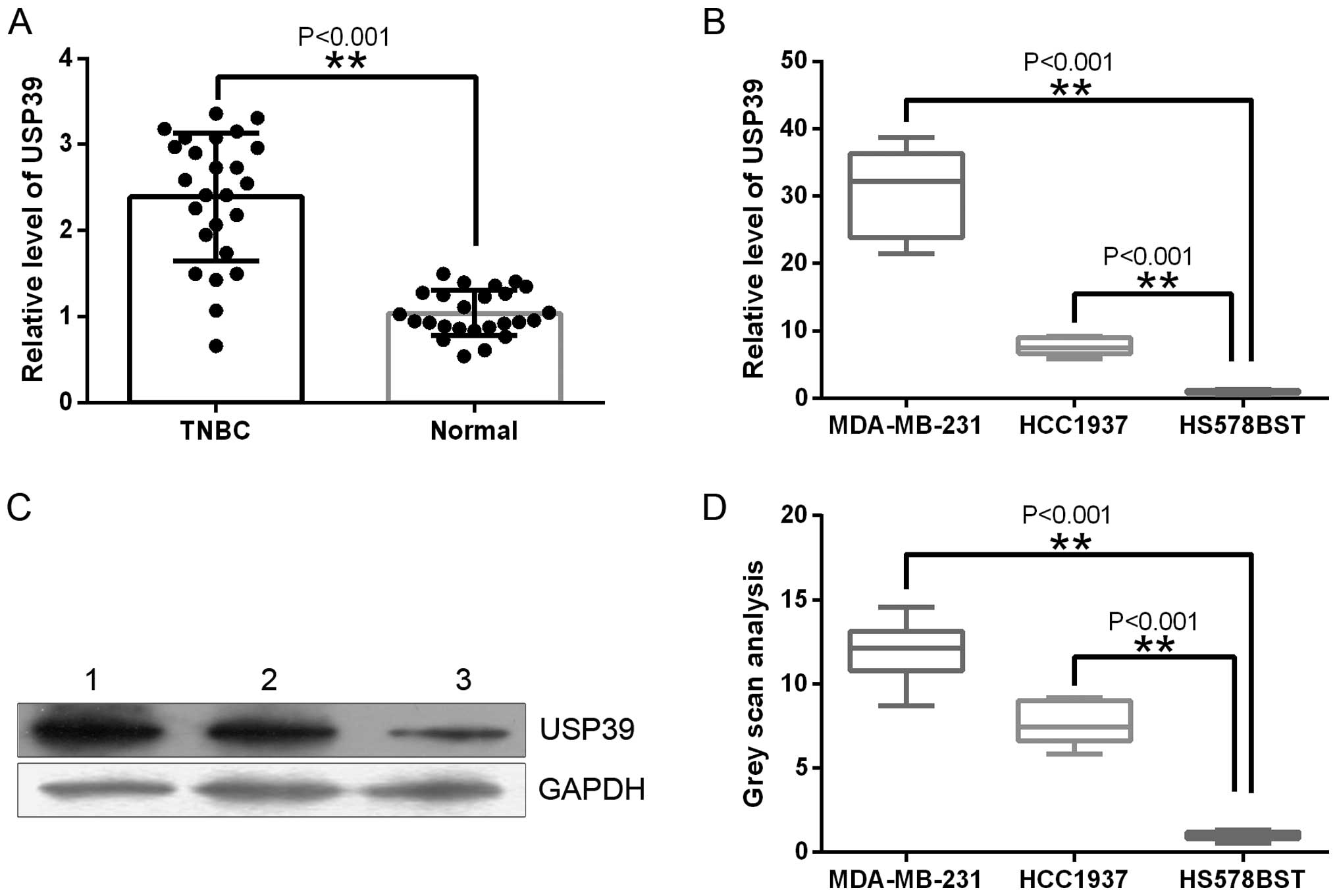
breast tissues, RT-qPCR was used. Fig.
1A shows that USP39 was highly expressed in TNBC tissues
compared with normal breast tissues (P<0.001). We also examined
the expression levels of USP39 in TNBC cell lines (MDA-MB-231 and
HCC1937) and the normal breast cell line HS578BST by RT-qPCR and
western blot analysis. These cell lines displayed the same
expression patterns as USP39 in the breast tissue samples (Fig. 1B–D).
Establishment of an inducible
USP39-overexpression and USP39-downregulation lentiviral
system
The principal aim of the present study was to
determine the cellular and molecular mechanisms underlying the
biological functions of USP39 in TNBC and normal breast cells.
Since USP39 was normally expressed at different levels in cultured
TNBC and normal breast cells (Fig.
1B–D), we engineered a tetracycline (doxycycline,
DOX)-inducible lentiviral system to overexpress or downregulate
USP39 (Fig. 2). To evaluate the
relationship between USP39 expression and the TNBC-initiating
capacity, TNBC cells (MDA-MB-231) were infected with
LV-tet-on-USP39-mCherry or LV-tet-on-shUSP39-GFP (Fig. 3) in which the USP39 shRNA (Fig. 3A) or the USP39 cDNA (Fig. 3B) was cloned downstream of
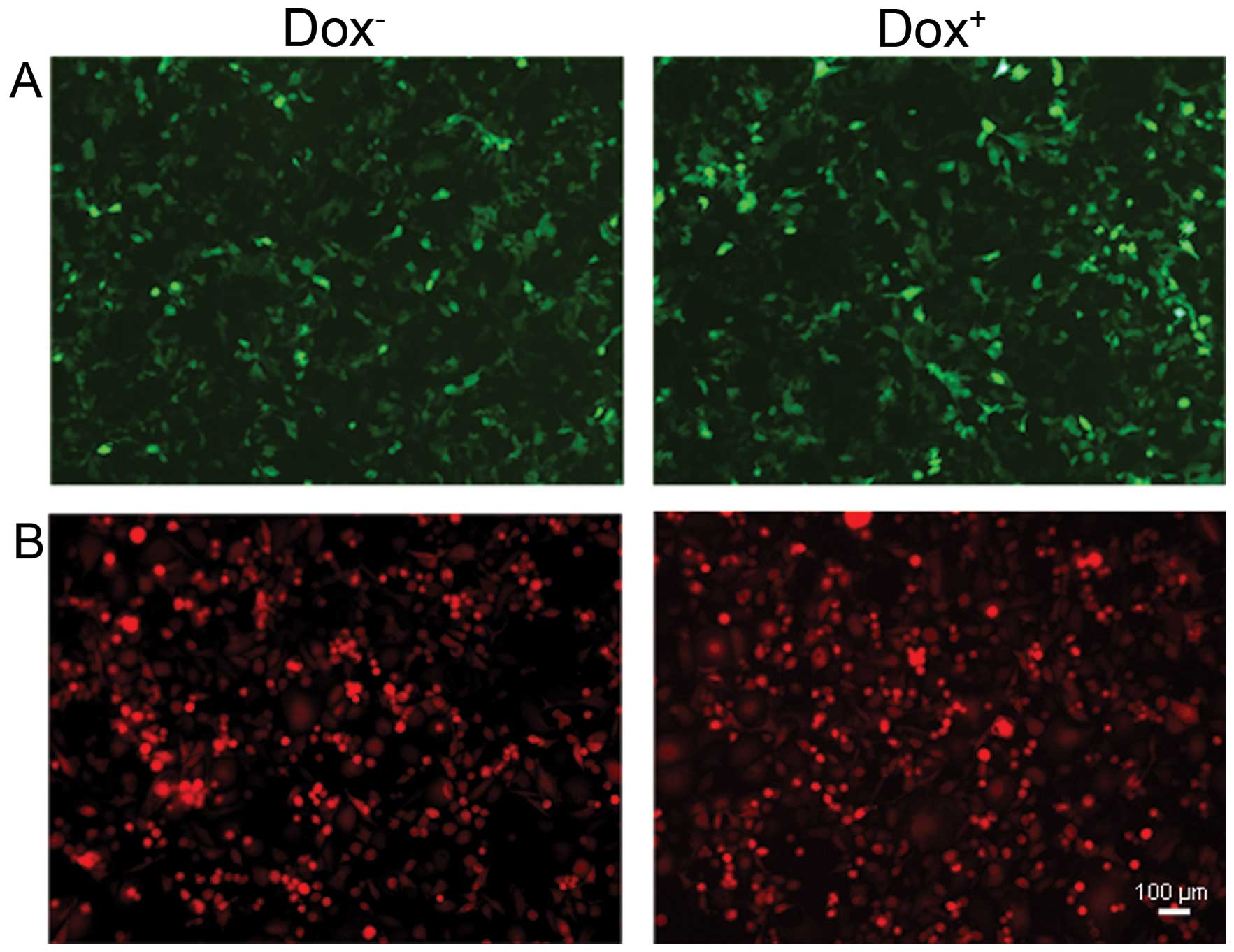
TRE-miniCMV. MDA-MB-231 cells infected with LV-tet-on-USP39-mCherry
or LV-tet-on-shUSP39-GFP were grown either in the absence (left
panel) or the presence (right panel) of DOX.
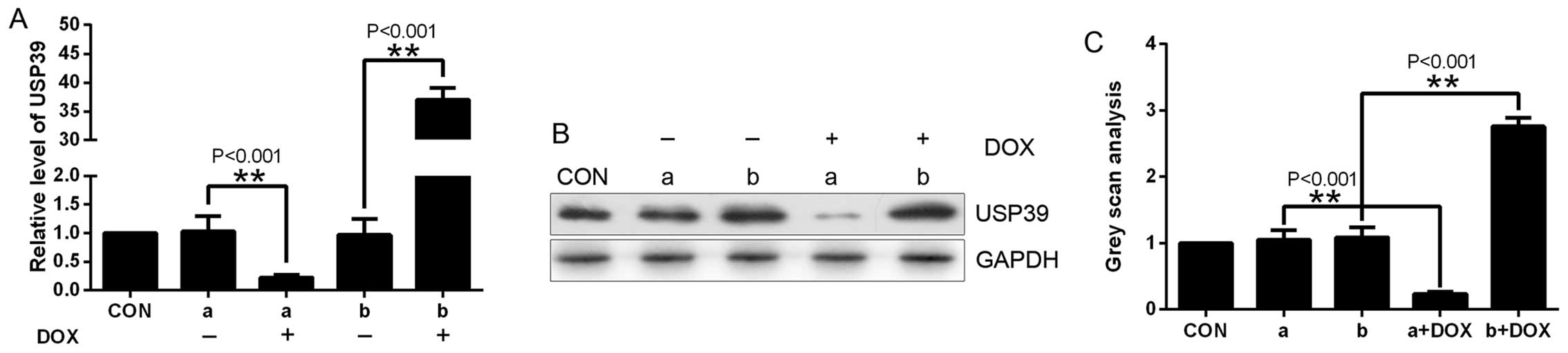
In the cells transfected with these lentiviruses,
USP39 expression was evaluated by real-time PCR and western blot
analysis. USP39 expression was significantly elevated in the
mCherry-positive cells treated with DOX compared with the control
cells (Fig. 4), and USP39 was much
lower in the GFP-positive cells treated with DOX compared with the
control cells, as assessed using real-time PCR (Fig. 4A) and western blot analysis
(Fig. 4B and C).
USP39 overexpression does not promote
cancer cell proliferation, while downregulation of USP39 suppresses
the growth of TNBC cells
Having successfully established an inducible system
to overexpress or downregulate USP39, we next sought to determine
the biological responses of TBNC cells to elevated USP39 protein.
As the downregulation of USP39 was previously reported to suppress
the growth of breast cancer cells in our laboratory (18), we first performed short-term assays
in which our inducible-USP39 cell lines were stimulated with DOX
prior to counting the total number of cells.
For functional live cell staining, it is important
to verify that, after MTT formazan staining, cells can still
maintain their biological functions and/or form characteristic cell
aggregates. Therefore, we investigated the feasibility of staining
cells with MTT formazan multiple times to detect cell growth. In
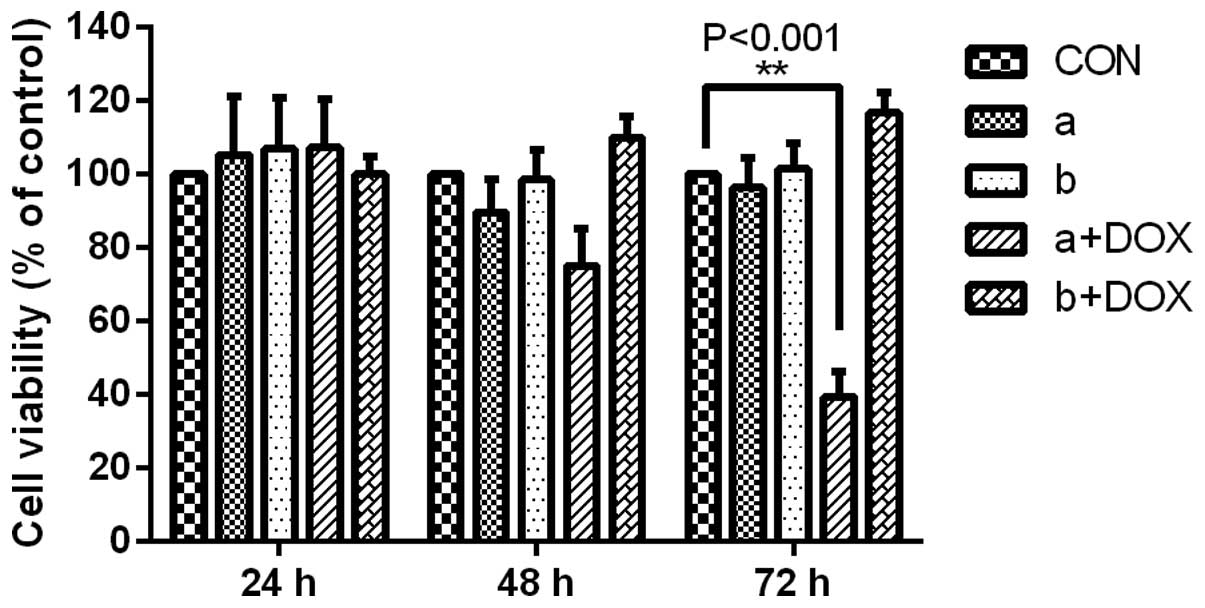
parallel experimental groups, MDA-MB-231 cells without DOX
induction were incubated with MTT from 24 to 72 h. At specific
time-points, the cells were washed with PBS, stained with MTT and
subjected to OD490 detection. Under standard culture conditions, a
significant difference was apparent in the MDA-MB-231 cells
containing LV-tet-on-shUSP39-GFP in the presence of DOX (Fig. 5). However, no significant
differences were detected between the CON and
LV-tet-on-USP39-mCherry groups (Fig.
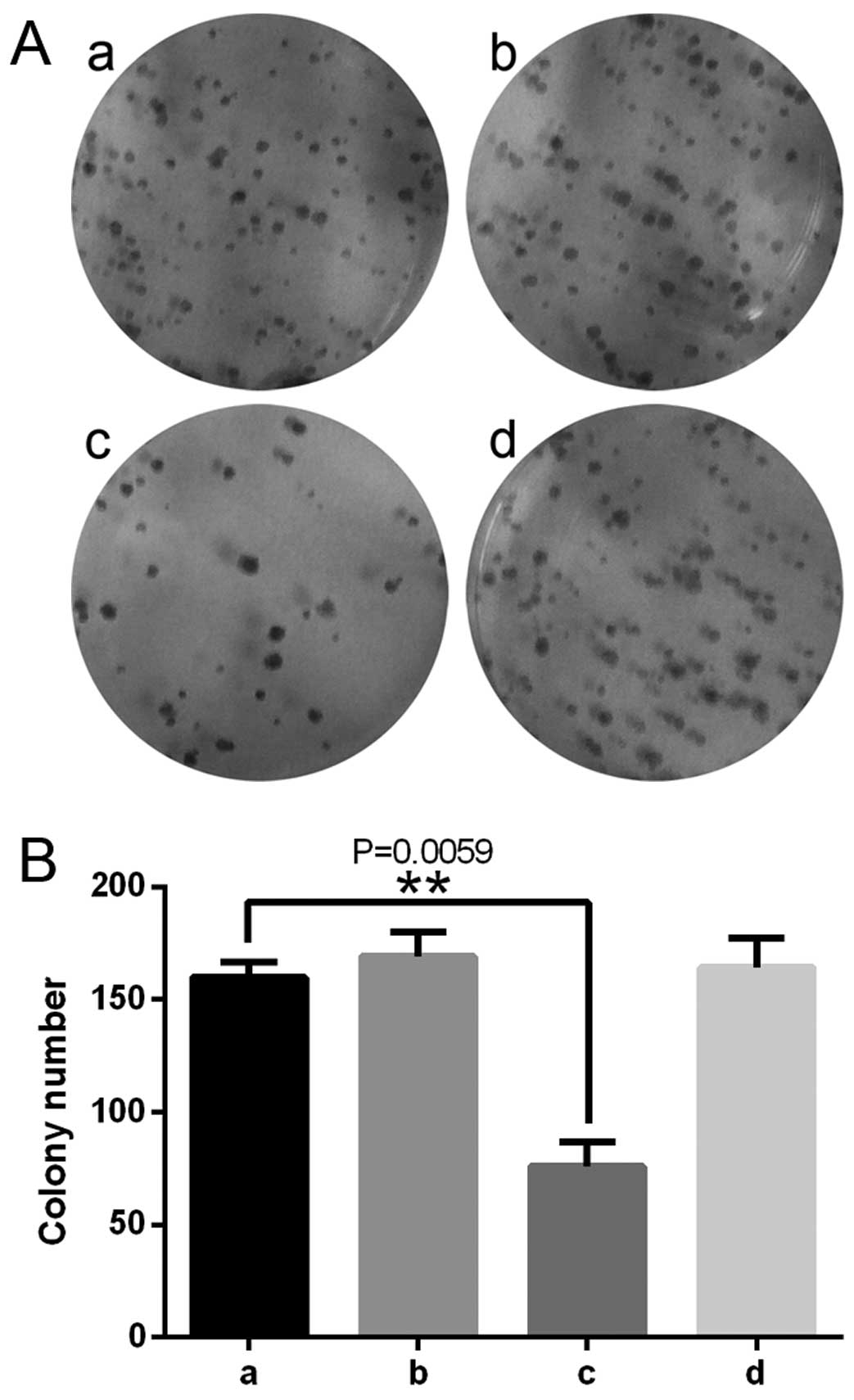
5). Colony formation assays revealed differences between CON
and LV-tet-on-shUSP39-GFP, which suggested that decreased USP39
expression inhibited MDA-MB-231 cell growth in the TNBC cancer
cells (Fig. 6).
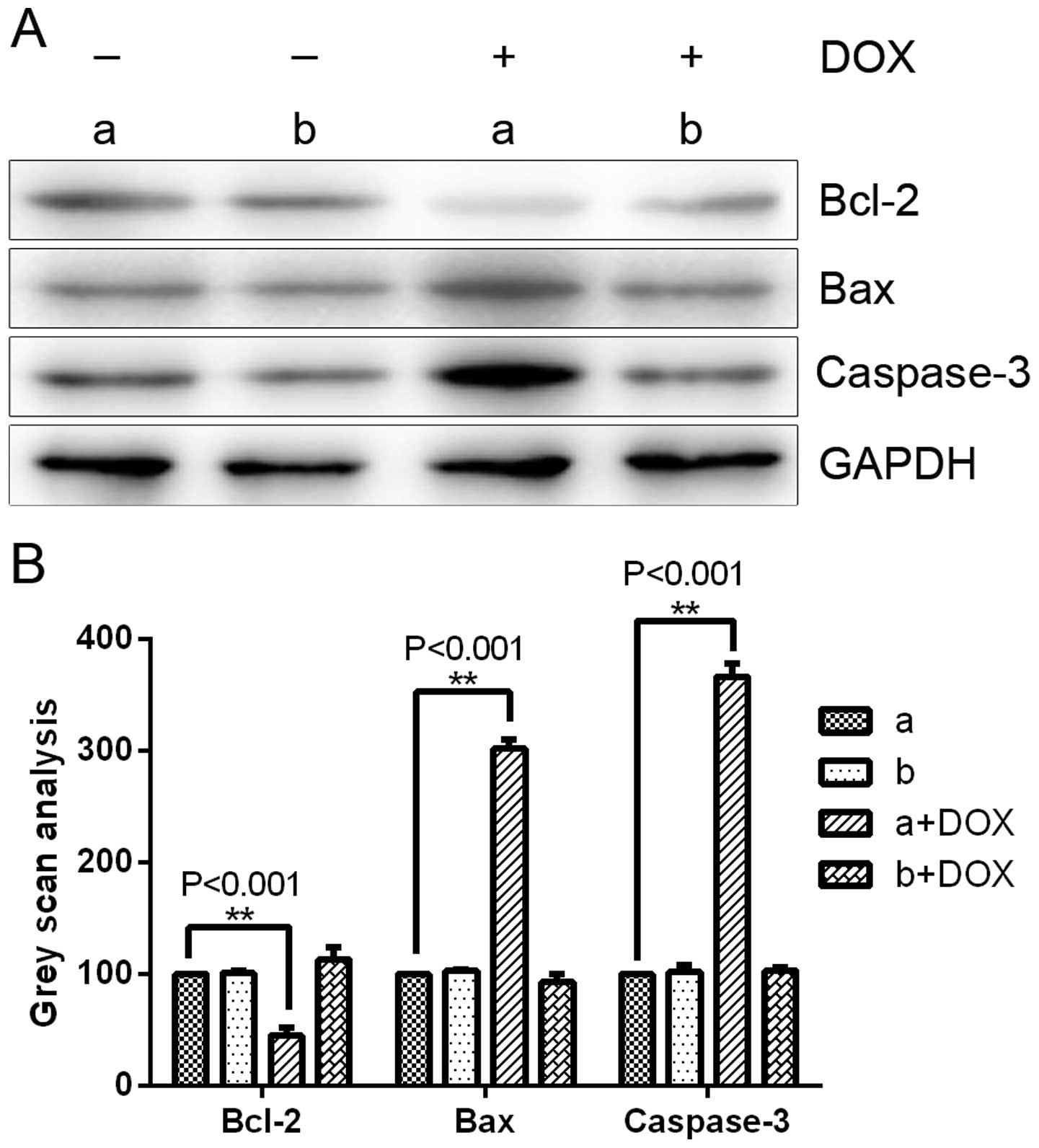
Consistent with the observed phenotype, western blot
analysis of DOX-treated MDA-MB-231 cells revealed that with the
clear downregulation of USP39 protein, there was a detectable
change in the levels of several regulators of cell apoptosis,
including Bax and caspase-3, both of which have been previously
reportedly to be transcriptionally upregulated in response to
apoptosis in several types of human cancer cells (20,21).
Similar to these cell apoptosis regulators, the apoptosis-related
gene Bcl-2 also showed significant downregulation following DOX
induction in the LV-tet-on-shUSP39-GFP infection groups (Fig. 7). In contrast, MDA-MB-231 cells
infected with LV-tet-on-USP39-mCherry with DOX induction, compared
to the control cells without DOX induction showed no significant
changes in the MTT, colony formation or western blot assays
(Figs. 5–7).
Discussion
The treatment of patients with triple-negative
breast cancer (TNBC), which lacks estrogen receptor (ER) and
progesterone receptor (PR) expression as well as human epidermal
growth factor receptor 2 (HER2) amplification, is challenging due
to the heterogeneity of the disease and the absence of well-defined
molecular targets (22,23). TNBC tumors are generally larger in
size, are of higher grade with other breast cancers, have lymph
node involvement at diagnosis and are biologically more aggressive
(24).
Recently, miRNA-based lentiviral vectors have been
used for the expression of shRNAs (25–27).
The shRNA embedded in the microRNA scaffold provides more robust
expression of the siRNA and gene silencing compared with
conventional shRNA constructs (28). Pol II-driven polycistronic
transcripts containing multiple shRNA sequences can be efficiently
expressed from the miRNA backbone (29). The present observations are
consistent with the recent discovery that endogenous shRNA
expression is enhanced by miRNAs (30,31).
However, it is important to note that conventional stem-loop
shRNAs, such as those used previously in lentiviruses (18), are not processed into functional
siRNAs under certain conditions. The inducible expression of
transgenes provides an improved level of safety since it avoids
much of the unintended consequences of viral vector-mediated
delivery and gene silencing. We believe that Tet-on systems greatly
facilitate the analysis of gene function, particularly in cell
systems that are difficult to manipulate and/or when the
manipulated gene is essential for cell survival. Furthermore, the
ability to control gene expression with highly specific Tet-on
systems offers the opportunity to study gene functions at different
stages (for example during cell cycle progression or well-defined
differentiation processes). The lentiviral-mediated inducible
expression of USP39-shRNA, under the control of the hU6 promoter,
decreased the growth of MDA-MB-231 cancer cells. Our data provide
evidence that the lentiviral-mediated Tet-on inducible system may
have viable therapeutic application. Our data strongly indicate
that if the expression of certain genes can be controlled by drugs
in various types of cancers, the lentiviral-mediated Tet-on
inducible system can be utilized for therapeutic purposes to cure
cancers via the induction of cancer cell-specific death. The method
presented herein will enable researchers to generate the desired
Tet-on cell lines within a reasonable time frame. In addition, this
approach may be applicable to other therapeutic areas through the
use of different therapeutic genes in vitro and even in
vivo.
USP39, which harbors a Dub domain, belongs to the
ubiq-uitin-specific protease family. Previous investigations have
described the role of USP39 in mRNA processing (32). It is essential for the assembly of
mature spliceosomes and mitotic spindle checkpoint integrity
(10,11). However, no USP39-specific substrate
has been identified, and other functions of USP39 remain poorly
understood. We found that suppression of USP39 in TNBC cell lines
was key to their capacity for growth. Transient shRNA silencing by
the USP39 lentivirus in MCF-7 cells partially recapitulated the
aggressive basal phenotype (18).
These effects on migration were independent of cell
replication.
In summary, the present study demonstrated that the
elements needed for DOX-regulated gene expression were delivered
using lentiviral vectors. This system may be useful for long-term
gene therapy applications and for the functional identification of
genes.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundations of China (81372632/H1617), the
Natural Science Foundation of Shandong Province of China (Y2008C48)
and the Department of Education of Shandong Province of China
(J11LF05).
References
|
1
|
Shin HR, Carlos MC and Varghese C: Cancer
control in the Asia Pacific region: current status and concerns.
Jpn J Clin Oncol. 42:867–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Foulkes WD, Smith IE and Reis-Filho JS:
Triple-negative breast cancer. N Engl J Med. 363:1938–1948. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferguson LL, Curran B, Martinez M, et al:
Triple-negative breast cancer: what is known about it? Clin J Oncol
Nurs. 18:E6–E11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirshfield KM and Ganesan S:
Triple-negative breast cancer: molecular subtypes and targeted
therapy. Curr Opin Obstet Gynecol. 26:34–40. 2014. View Article : Google Scholar
|
|
6
|
Gangi A, Chung A, Mirocha J, et al:
Breast-conserving therapy for triple-negative breast cancer. JAMA
Surg. 149:252–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chin YR, Yoshida T, Marusyk A, et al:
Targeting Akt3 signaling in triple-negative breast cancer. Cancer
Res. 74:964–973. 2014. View Article : Google Scholar :
|
|
8
|
Dikic I, Wakatsuki S and Walters KJ:
Ubiquitin-binding domains - from structures to functions. Nat Rev
Mol Cell Biol. 10:659–671. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pickart CM and Eddins MJ: Ubiquitin:
structures, functions, mechanisms. Biochim Biophys Acta.
1695:55–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Makarova OV, Makarov EM and Luhrmann R:
The 65 and 110 kDa SR-related proteins of the U4/U6.U5 tri-snRNP
are essential for the assembly of mature spliceosomes. EMBO J.
20:2553–2563. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Leuken RJ, Luna-Vargas MP, Sixma TK,
et al: Usp39 is essential for mitotic spindle checkpoint integrity
and controls mRNA-levels of aurora B. Cell Cycle. 7:2710–2719.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rios Y, Melmed S, Lin S, et al: Zebrafish
usp39 mutation leads to rb1 mRNA splicing defect and pituitary
lineage expansion. PLoS Genet. 7:e10012712011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song EJ, Werner SL, Neubauer J, et al: The
Prp19 complex and the Usp4Sart3 deubiquitinating enzyme control
reversible ubiquitination at the spliceosome. Genes Dev.
24:1434–1447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luna-Vargas MP, Faesen AC, van Dijk WJ, et
al: Ubiquitin-specific protease 4 is inhibited by its
ubiquitin-like domain. EMBO Rep. 12:365–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gossen M and Bujard H: Tight control of
gene expression in mammalian cells by tetracycline-responsive
promoters. Proc Natl Acad Sci USA. 89:5547–5551. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gossen M, Freundlieb S, Bender G, et al:
Transcriptional activation by tetracyclines in mammalian cells.
Science. 268:1766–1769. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kao J, Salari K, Bocanegra M, et al:
Molecular profiling of breast cancer cell lines defines relevant
tumor models and provides a resource for cancer gene discovery.
PLoS One. 4:e61462009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Ji X, Liu X, et al:
Lentivirus-mediated inhibition of USP39 suppresses the growth of
breast cancer cells in vitro. Oncol Rep. 30:2871–2877.
2013.PubMed/NCBI
|
|
19
|
Wang H, Zhao G, Liu X, et al: Silencing of
RhoA and RhoC expression by RNA interference suppresses human
colorectal carcinoma growth in vivo. J Exp Clin Cancer Res.
29:1232010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Butt AJ, Dickson KA, McDougall F, et al:
Insulin-like growth factor-binding protein-5 inhibits the growth of
human breast cancer cells in vitro and in vivo. J Biol Chem.
278:29676–29685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McIlroy D, Sakahira H, Talanian RV, et al:
Involvement of caspase 3-activated DNase in internucleosomal DNA
cleavage induced by diverse apoptotic stimuli. Oncogene.
18:4401–4408. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pegram MD, Lipton A, Hayes DF, et al:
Phase II study of receptor-enhanced chemosensitivity using
recombinant humanized anti-p185HER2/neu monoclonal antibody plus
cisplatin in patients with HER2/neu-overexpressing metastatic
breast cancer refractory to chemotherapy treatment. J Clin Oncol.
16:2659–2671. 1998.PubMed/NCBI
|
|
23
|
Carey LA, Dees EC, Sawyer L, et al: The
triple negative paradox: primary tumor chemosensitivity of breast
cancer subtypes. Clin Cancer Res. 13:2329–2334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haffty BG, Yang Q, Reiss M, et al:
Locoregional relapse and distant metastasis in conservatively
managed triple negative early-stage breast cancer. J Clin Oncol.
24:5652–5657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bauer M, Kinkl N, Meixner A, et al:
Prevention of interferon-stimulated gene expression using
microRNA-designed hairpins. Gene Ther. 16:142–147. 2009. View Article : Google Scholar
|
|
26
|
Sun BS, Dong QZ, Ye QH, et al:
Lentiviral-mediated miRNA against osteopontin suppresses tumor
growth and metastasis of human hepatocellular carcinoma.
Hepatology. 48:1834–1842. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stegmeier F, Hu G, Rickles RJ, et al: A
lentiviral microRNA-based system for single-copy polymerase
II-regulated RNA interference in mammalian cells. Proc Natl Acad
Sci USA. 102:13212–13217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boden D, Pusch O, Silbermann R, Lee F,
Tucker L and Ramratnam B: Enhanced gene silencing of HIV-1 specific
siRNA using microRNA designed hairpins. Nucleic Acids Res.
32:1154–1158. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Snyder LL, Esser JM, Pachuk CJ, et al:
Vector design for liver-specific expression of multiple interfering
RNAs that target hepatitis B virus transcripts. Antiviral Res.
80:36–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu XY, Tang QS, Chen HC, et al:
Lentiviral miR30-based RNA interference against heparanase
suppresses melanoma metastasis with lower liver and lung toxicity.
Int J Biol Sci. 9:564–577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee Y, Kim M, Han J, et al: MicroRNA genes
are transcribed by RNA polymerase II. EMBO J. 23:4051–4060. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lygerou Z, Christophides G and Seraphin B:
A novel genetic screen for snRNP assembly factors in yeast
identifies a conserved protein, Sad1p, also required for pre-mRNA
splicing. Mol Cell Biol. 19:2008–2020. 1999.PubMed/NCBI
|