Introduction
Nasopharyngeal carcinoma (NPC) has an unusual
geographical and ethnic distribution, and is one of the most common
cancers in Southeast Asia and Southern China, especially among
individuals of Cantonese origin (1). The highest incidence of this disease
is found in this area (peaking at 50/100,000 people/year) (2). Although detection of NPC by imaging
and advanced radiotherapy techniques have led to an improvement in
the management and treatment of NPC, radioresistance remains a
serious barrier to successful treatment in many cases (3,4).
Radiotherapy in cancer therapy directly or indirectly damages DNA
and induces apoptosis. Defects in the apoptotic machinery can lead
to radioresistance (5). The exact
molecular mechanism involved in NPC radioresistance remains poorly
understood. Therefore, it is essential to investigate the potential
mechanism of NPC radioresistance.
The Slug protein belongs to the Snail superfamily of
zinc finger transcription factors (6). It is closely related to
transcriptional repressors implicated in embryonic development,
where they have been shown to be vital for the formation of the
mesoderm and neural crest through epithelial-mesenchymal transition
(7). Studies have shown that in
malignant tumors, Slug not only participates in the regulation of
carcinogenesis, invasiveness and metastasis in various cancers
(8–13), but also has an anti-apoptotic effect
(14–18). However, few studies have been
undertaken to assess whether Slug can be involved in the
radioresistance of cancers. Recently, Findlay et al reported
that calcitriol (1α,25-dihydroxyvitamin D3) enhanced radiation
sensitivity in colorectal cancer, but overexpression of Slug
inhibited this effect (19). It was
reported that in cholangiocarcinoma and melanoma cells, Slug
inhibition can enhance radiosensitivity by upregulation of the
activity of p53 upregulated modulator of apoptosis (PUMA), which
has been shown to be involved in the control of apoptosis (20,21).
In ovarian cancer cells, Slug was found to promote radioresistance
by antagonizing p53-mediated apoptosis (22). However, the function of Slug
associated with radioresistance in NPC has never been previously
investigated.
In the present study, we successfully established
radioresistant CNE-2 cells (CNE-2-RES) by exposing CNE-2 cells to
gradually increasing doses of irradiation (IR). It was demonstrated
that upregulation of Slug expression contributed to the
radioresistance of CNE-2-RES cells which was associated with
downregulated PUMA expression. By inhibition of Slug, the
radiosensitivity of NPC was enhanced both in vitro and in
vivo. These results have implications for the treatment of
NPC.
Materials and methods
Cell culture and the establishment of
radioresistant CNE-2-RES cells
The poorly differentiated NPC cell line CNE-2 and
the high differentiated NPC cell line CNE-1 (Shanghai Bogoo
Biological Technology Co., Ltd., Shanghai, China) were cultured in
Roswell Park Memorial Institute (RPMI)-1640 medium (Invitrogen Life
Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS), with 100 IU/ml penicillin and streptomycin at 37°C in
a humidified atmosphere with 5% CO2. The selection
procedure of CNE-2-RES cells was performed as previously described
(23). The parental CNE-2 cells,
which were used as the control, were treated according to the same
procedure except for the IR step. CNE-2-RES cells were cultured in
the same culture medium as the CNE-2 cells. Exponentially growing
cells were used for all experiments.
Cell viability and colony forming
assay
A Cell Counting Kit-8 (Beyotime Biotechnology Co.,
Ltd., Shanghai, China) was used according to the manufacturer’s
instructions to determine the growth of both cell lines. Cells were
plated onto 96-well plates at a density of 2×103
cells/well in triplicate. After 12 h of culture, the cells were
exposed to different doses of IR (2, 4, 6, 8 and 10 Gy). Absorbance
values were expressed as percentages relative to the controls. For
the colony forming assay (24),
cells were seeded into 6-well culture plates at 1×103
cells/well for 12 h and were exposed to IR with doses ranging from
2 to 10 Gy. The cells were then cultured for an additional 14 days.
Next, the cells were washed twice with phosphate-buffered saline,
fixed with methanol/acetic acid (3:1; v/v) (both from SunShine
Biotechnology Co., Ltd., Nanjing, China) and stained with 0.5%
crystal violet (C3886; Sigma Chemical Co., St. Louis, MO, USA). The
number of colonies was counted under a microscope. The number of
surviving colonies (a colony was defined as >50 cells) was
counted under a microscope (Nikon TE2000; Nikon Corporation,
Japan).
Silencing of Slug by shRNA
Three pairs of shRNAs targeting different regions of
the human Slug transcript (GenBank: U97060) and 1 control shRNA
were designed and synthesized (Invitrogen Life Technologies). They
were cloned into the pLentiLox 3.7 lentiviral vector between
HpaI and XhoI. The packaged lentiviruses which showed
the highest knockdown efficiency of Slug mRNA in the two cell lines
was used for the experiments. Cells were seeded into 6-well plates
at a density of 1.5×103 cells (70% confluency) and were
infected with control lentiviral shRNA and lentiviral shRNA Slug
which were referred to as negative and LV-sh-Slug. The cells were
assayed by RT-PCR and western blotting on the second day after
infection.
Silencing of p53 by siRNA
In order to silence the p53 gene, p53 siRNA
(sc-45917; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was
used to transfect the CNE-2-RES cells. After infection with
LV-sh-Slug, CNE-2-RES cells were then transfected with p53 siRNA
according to the protocol. The transfection efficiency was detected
by western blotting as described below.
Reverse transcription-PCR
Total RNA was isolated using Trizol according to a
standard protocol from cells of each group. Total RNA was treated
with DNase I (Invitrogen Life Technologies) to remove the
contaminating genomic DNA. PCR analysis was conducted using the
One-Step reverse transcription-PCR kit (Invitrogen Life
Technologies). Actin was used as an internal control. The following
primers were used: Slug sense, 5′-CATGCCTGTCATACCACAAC-3′ and
anti-sense, 5′-GGTGTCAGATGGAGGAGGG-3′; PUMA sense,
5′-GACGACCTCAACGCACAGTA-3′ and antisense,
5′-AGGAGTCCCATGATGAGATTGT-3′; p53 sense, 5′-AATCTC
ACCCCATCCCACAC-3′ and antisense, 5′-GACCCTGAG CATAAAACAAGTCT-3′;
actin sense, 5′-TGATGGGTGTGAACCACGAG-3′ and antisense,
3′-TTGAGTCGCAGGAGACAACC-5′. The PCR conditions were as follows:
95°C for 3 min, followed by 40 cycles of 95°C for 30 sec, 55°C for
20 sec and 72°C for 20 sec. The melting curve program was 95°C for
15 sec, 60°C for 15 sec, and 20 min for warming. The final
extension was at 95°C for 15 sec. PCR was performed under a
quantitative PCR instrument (Bio-Rad, Shanghai, China).
Western blotting
Western blot analyses were performed as described
previously (25). Briefly, total of
50 μg proteins were extracted from the cells of each group.
After electrophoresis, transmembrane and blocking, the blotted
membranes were incubated with primary monoclonal anti-Slug
(sc-166476, 1:500); polyclonal anti-PUMA (sc-20534, 1:500) and
monoclonal anti-p53 (sc-126, 1:1,000) antibodies, which were
purchased from Santa Cruz Biotechnology, Inc., at 4°C overnight.
Subsequent to being washed, the membranes were incubated with
HRP-labeled anti-goat or anti-mouse (Boster Biological Engineering
Co., Ltd., Wuhan, China) for 1 h at room temperature. Bands were
visualized by employing the BeyoECL Plus Detection system (Beyotime
Institute of Biotechnology, Jiangsu, China). The intensity of
protein fragments was quantified with Quantity One software (4.5.0
Basic; Bio-Rad, Hercules, CA, USA) and represented as the
densitometric ratio of the targeted protein to β-actin. Cell
protein lysates were assayed in triplicate.
Xenograft tumor experiments
Male, 4- to 6-week-old BALB/c nude mice were
purchased from Shanghai Animal Center (Shanghai, China). The mice
were then observed daily for their diet consumption, stools and
mental state. The body weight was measured every three days. On the
day of tumor cell inoculation, tumor cells at 70–80% confluency
were trypsinized and resuspended in FBS-free culture medium.
Xenograft tumors were established by subcutaneous injection of
2×106 NPC cells (CNE-2, CNE-2-RES, CNE-2-RES LV-sh-Slug)
into the groin area of the 4- to 6-week-old male nude mice (n=6, a
total of 2 subgroup). Two weeks later, the mice in one subgroup
were exposed to an IR dose of 4 Gy, while the other did not. The
lengths and widths of the tumors were measured with Vernier
calipers and calculated using the following formula: Tumor volume =
length × width2 × 0.5. The mice were sacrificed 2 weeks
later in accordance with institutional regulations for animal
experiments. The use of animals in the present study complied with
the Guide for the Care and Use of Laboratory Animals. The study was
approved by the Institutional Animal Care and Use Committee, Wuxi,
Jiangsu, China. The tissue sections were viewed at ×100
magnification, and images were captured with a digital camera.
Statistical analysis
The SPSS 11.5 for Windows statistical analysis
software package (SPSS, Inc., Chicago, IL, USA) was employed for
the analysis of data. The Student’s t-test and Mann-Whitney U test
were used for the statistical analysis of data. A P<0.05 was
considered to indicate a statistically significant difference.
Results
Slug expression in the NPC cells
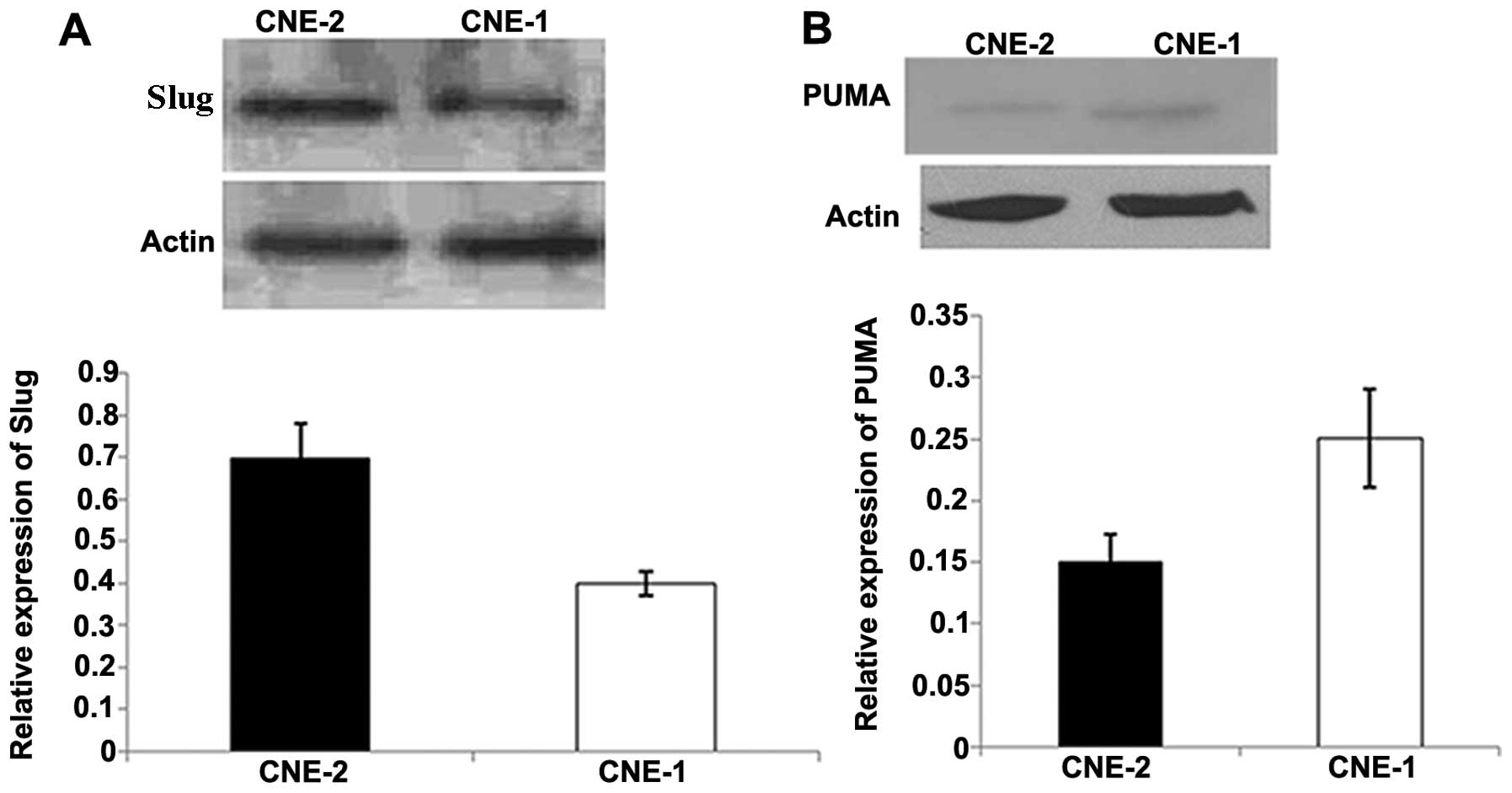
We detected the mRNA expression of Slug in the CNE-1
and CNE-2 cells. As shown in Fig.
1, CNE-2 cells showed relatively higher expression of Slug mRNA
than that in the CNE-1 cells. Thus, we chose CNE-2 cells for the
following experiments.
Radioresistant CNE-2-RES cell line is
successfully established and its radioresistant capacity is
validated
The cell radioresistant capacity was validated by
CCK-8 and colony forming assays. The survival rates of CNE-2-RES
cells following gradually increasing doses of IR (0, 2, 4, 6, 8 and
10 Gy) were higher than those of the CNE-2 cells. The two cell
lines were then exposed to a 4-Gy dose of IR, and at various
time-points (1, 2, 3, 4 and 5 days) we observed that the survival
rates of the CNE-2-RES cells were significant higher than those of
the CNE-2 cells (Fig. 2A and B).
The effect of IR on NPC cell growth was examined under a 4-Gy dose
of IR by CCK-8, After a 4-Gy dose of IR, at various time-points (1,
2, 3, 4 and 5 days), the growth rate of the CNE-2-RES cells was
significantly inhibited compared with the parental cells (Fig. 2C). A colony forming assay indicated
that a greater number of CNE-2-RES cell colonies survived when
compared with the CNE-2 cells (Fig.
2D).
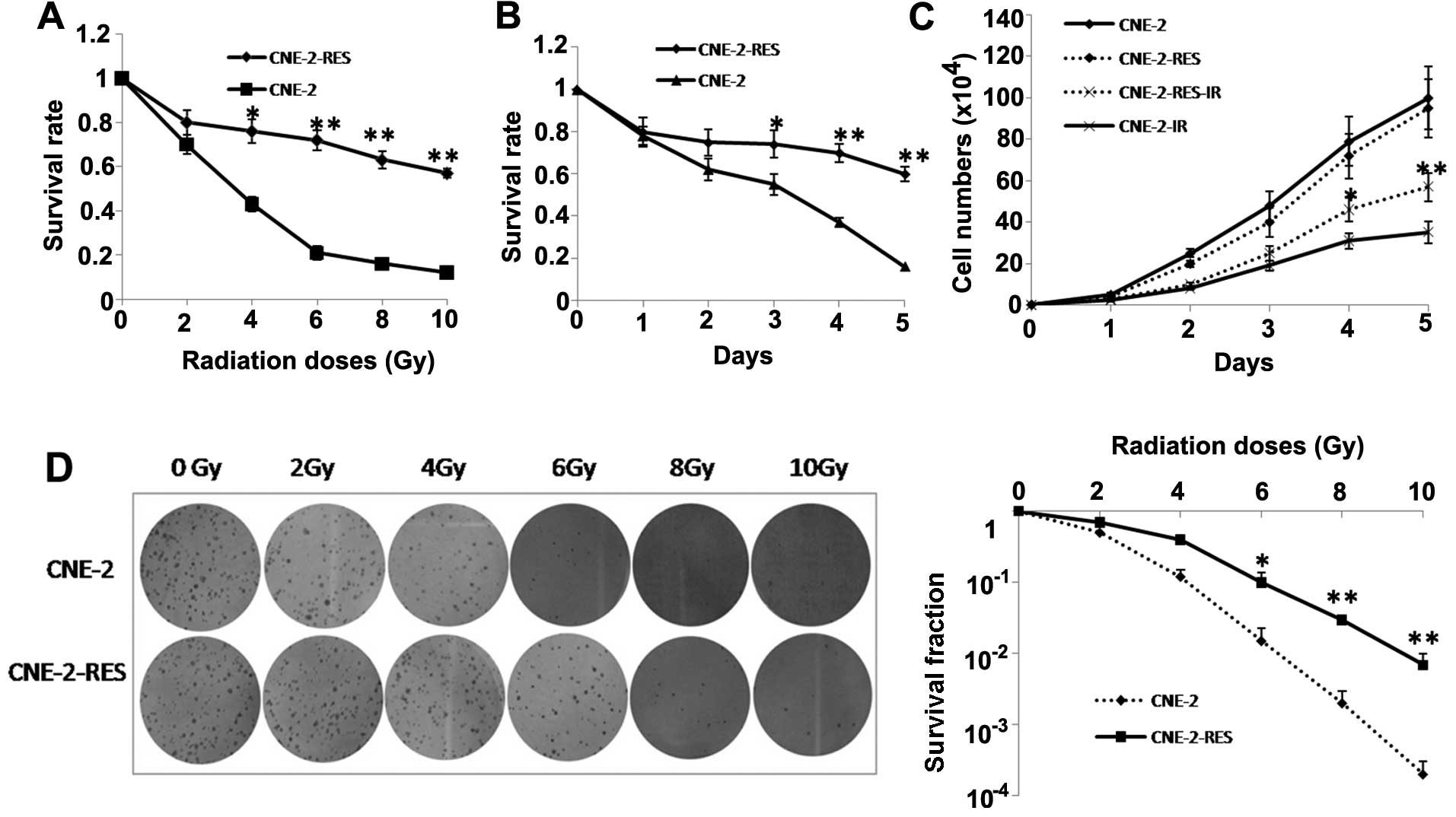 | Figure 2Radioresistant CNE-2 cells (CNE-2-RES)
are established and validated. The survival rates for CNE-2-RES and
CNE-2 cells at (A) different irradiation (IR) doses (0, 2, 4, 6, 8
and 10 Gy) and at (B) different time-points (1, 2, 3, 4 and 5 days)
were determined using a CCK-8 assay. (C) The cell growth curves of
CNE-2-RES and CNE-2 cells exposed or not exposed to 4-Gy IR at
different time-points (1, 2, 3, 4 and 5 days). (D) A representative
image of colony formation in CNE-2-RES and CNE-2 cells exposed or
not exposed to different doses of IR (0, 2, 4, 6, 8 and 10 Gy)
after 14 days (left). Survival fractions of CNE-2-RES and CNE-2
cells were obtained from the results of the colony forming assays
(right). The results are the average of three independent
experiments ± standard deviation (SD) (*P<0.05,
**P<0.01). |
Slug expression is increased in the
radioresistant CNE-2-RES cells
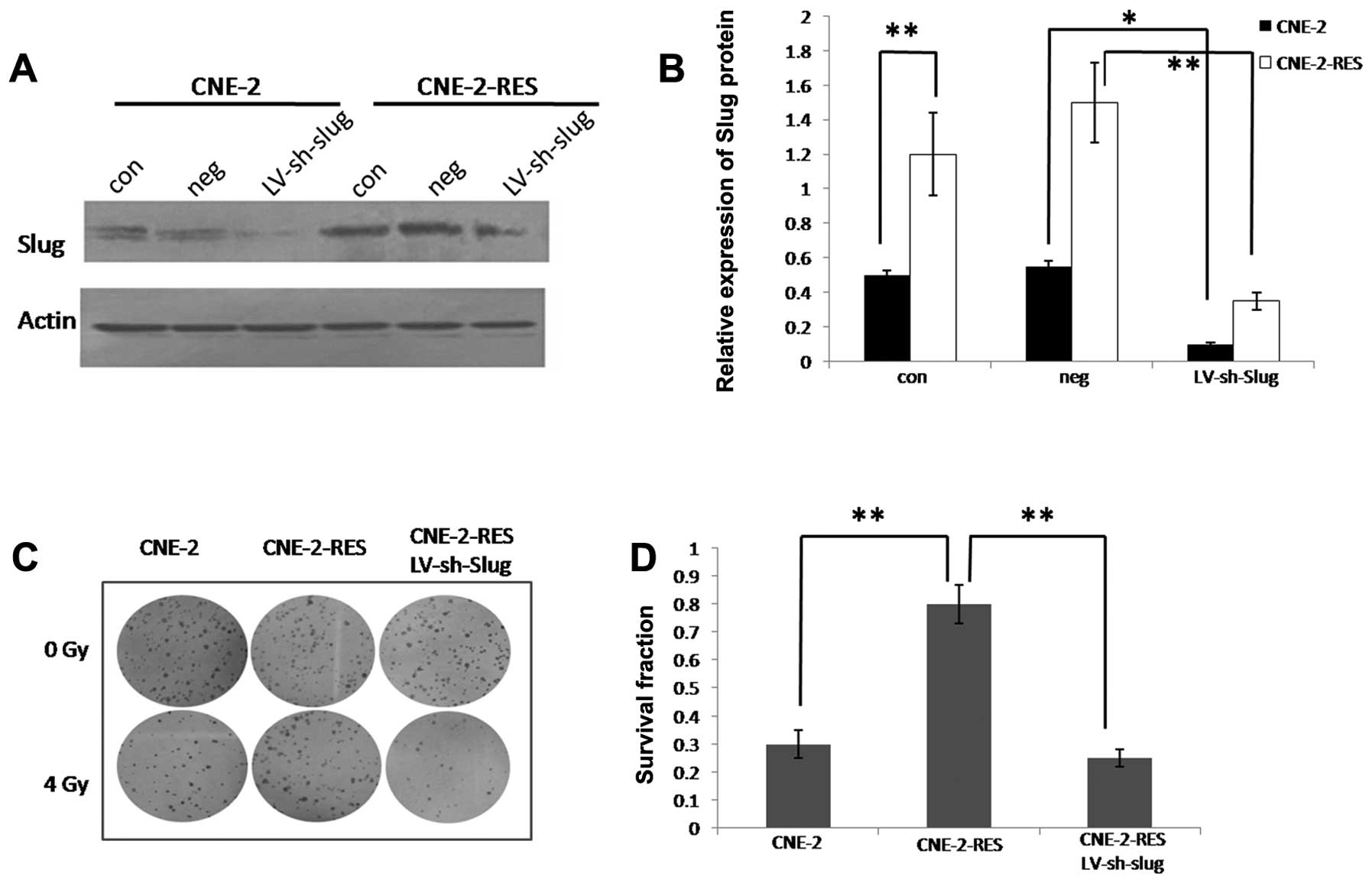
We detected the expression of Slug protein by
western blotting. When the CNE-2 cells acquired the ability of
resistance, Slug protein expression was significantly increased
(P<0.05, Fig. 3A and B).
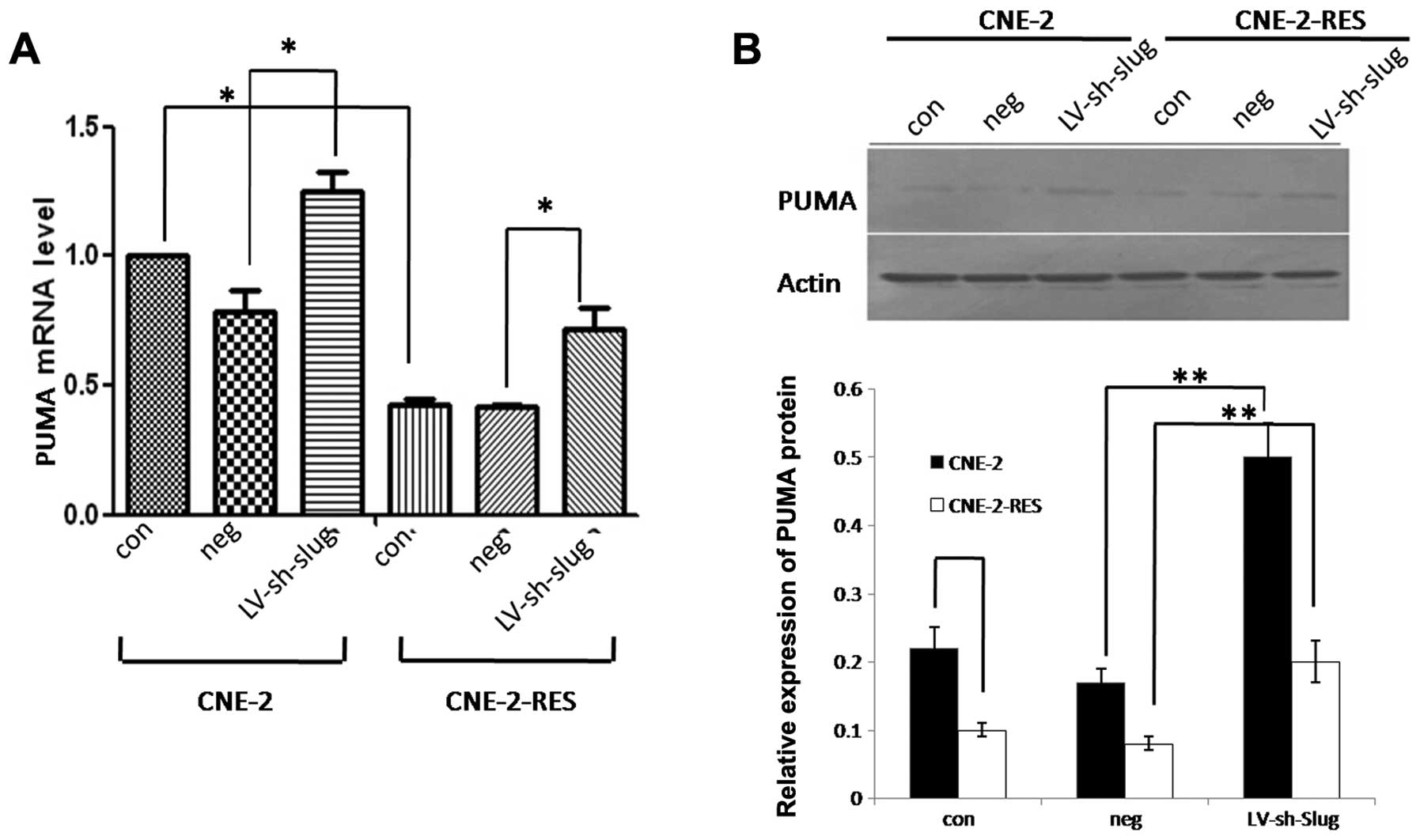
PUMA expression is decreased in the
radioresistant CNE-2-RES cells
Results showed that when the CNE-2 cells acquired
the ability of resistance, both the PUMA mRNA and protein levels
were significantly decreased (P<0.05, Fig. 4A and B).
Knockdown of Slug in the CNE-2-RES cells
increases their sensitivity to IR
Slug protein was significantly inhibited in the
CNE-2-RES cells following knockdown of Slug (Fig. 3A and B); the infection efficiency
was 89.38±2.1%. As shown by the colony forming assay, following a
4-Gy dose of IR, inhibition of Slug in the CNE-2-RES cells led to a
decreased number of surviving clones compared to the negative
control (25.9±5.2 vs. 79.4±10.1%; P<0.01, Fig. 3C and D).
Slug regulates PUMA expression in the
CNE-2 and CNE-2-RES cells
After Slug was knocked down in both cell lines
(CNE-2 and CNE-2-RES), we detected the expression of PUMA by real
time RT-PCR and western blotting. The results showed that both the
expression levels of PUMA mRNA and protein were significantly
increased in both cell lines (P<0.05, Fig. 4A and B). This result revealed that
Slug might promote the radioresistant ability of CNE-2-RES cells by
downregulating PUMA.
Slug mediates CNE-2-RES cell
radioresistance via the p53-independent pathway
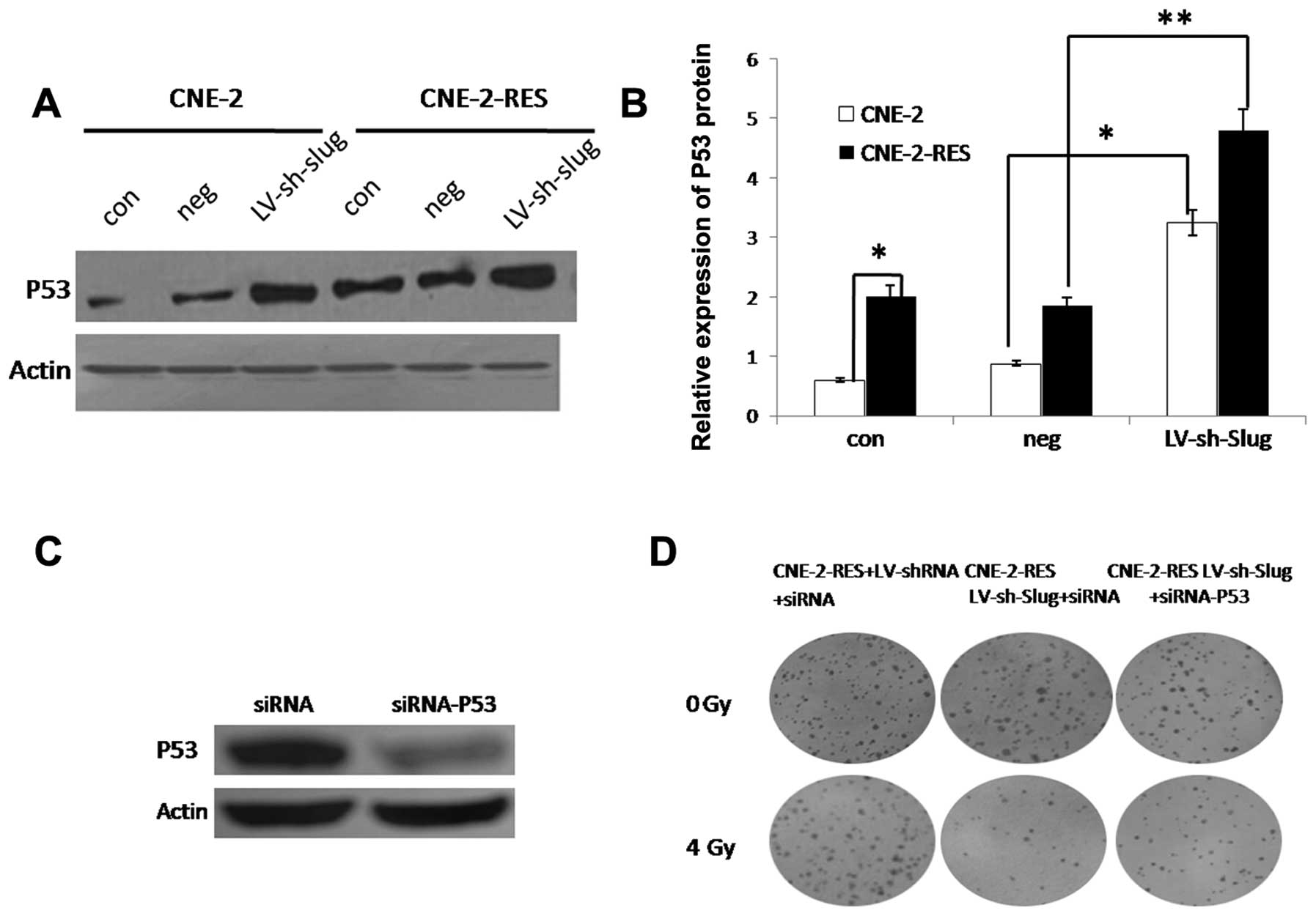
We further detected expression of the
tumor-suppressor gene p53. Surprisingly, the radioresistant
CNE-2-RES cells showed significantly increased p53 expression when
compared with that in the CNE-2 cells. This result demonstrated
that the p53-independent pathway may play an important role in
Slug-induced radioresistance in CNE-2-RES cells.
Slug regulates p53 expression in the
CNE-2 and CNE-2-RES cells
We detected p53 after downregulation of Slug.
Knockdown of Slug resulted in increased p53 expression (Fig. 5A and B). To further demonstrate that
Slug mediates CNE-2 radioresistance via downregulation of PUMA in a
p53-dependent manner, we used p53 siRNA to silence p53 expression
in the LV-sh-Slug infected CNE-2-RES cells. The results showed that
after transfection, p53 protein in the CNE-2-RES cells was
significantly inhibited; the transfected efficiency was 85.26±3.9%
(Fig. 5C), It was shown by colony
forming assay that following a 4-Gy dose of IR, inhibition of p53
in the LV-sh-Slug-infected CNE-2-RES cells partly restored the
ability of radioresistance under 4 Gy of IR. The number of
surviving clones in the CNE-2-RES+LV-shRNA+siRNA,
CNE-2-RES+LV-sh-Slug+siRNA-p53 and CNE-2-RES +LV-sh-Slug+siRNA
cells were 76.32±9.5, 29.1±4.8 and 53.4±6.1%, respectively,
(P<0.01, Fig. 5D). These results
suggest that Slug mediates CNE-2 radioresistance partly via
downregulation of PUMA in a p53-dependent manner.
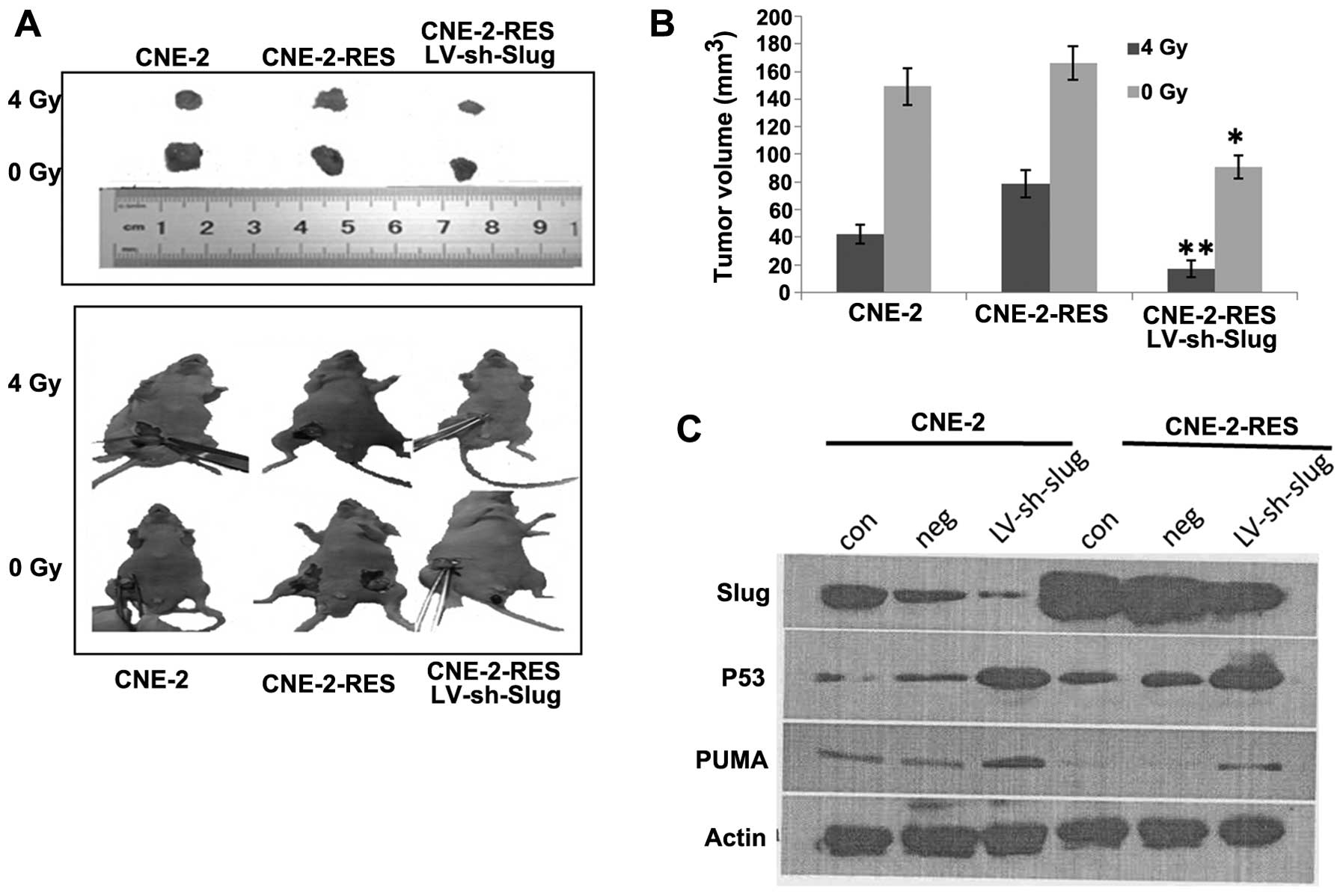
Knockdown of Slug decreases the
radioresistance of NPC xenografts in vivo
After 4 weeks, when receiving no radiation, the
xenograft tumors that were established by subcutaneous injection of
CNE-2-RES, LV-sh-Slug cells showed tumor regression of 45.3±3.6%
compared with the CNE-2-RES group; there was no difference between
the CNE-2 and CNE-2-RES groups (P>0.05). After receiving a 4-Gy
dose of radiation at 2 weeks, the tumor volumes of xenografts that
were established by subcutaneous injection of CNE-2, CNE-2-RES and
CNE-2-RES LV-shRNA-Slug cells decreased by 42.3±7, 78.6±9.3 and
17.4±5.6%, respectively. These findings indicated that when CNE-2
cells acquired radioresistant ability, the corresponding xenograft
tumors also acquired radioresistance. Yet, following knockdown of
Slug, the radioresistance of the CNE-2-RES xenografts was
significantly reduced (Fig. 6A and
B).
We further detected Slug, PUMA and p53 protein in
the xenograft tumor tissues. CNE-2-RES xenograft tumor tissues
showed relatively higher Slug and p53 expression and lower PUMA
expression than the CNE-2 xenograft tumor tissues. Following
knockdown of the Slug gene, the corresponding xenograft tumor
tissues showed higher p53 and PUMA expression (Fig. 6C). These results were consistent
with the previous in vitro experiment.
Discussion
Resistance of NPC to radiotherapy is a major problem
in cancer treatment (26). Slug, a
snail family transcription factor, is a suppressor of PUMA, which
has been shown to be involved in the radioresistance and
chemoresistance of several types of cancers (19–22).
In the present study, we successfully established radioresistant
CNE-2-RES cells to study the role of Slug in NPC. When CNE-2 cells
acquired the ability of radioresistance, Slug expression was
significantly upregulated. Colony forming assay further showed that
knockdown of Slug significantly increased the radiosensitivity of
CNE-2-RES cells following a 4-Gy dose of IR. These results suggest
that Slug overexpression in CNE-2-RES cells may result in the
radioresistance of cells.
It is known that Slug can repress PUMA gene
transcription in many types of cells (19–22,27).
PUMA-induced apoptosis mainly occurs through activation of the
tumor-suppressor protein p53 (28).
PUMA-induced apoptosis may also be promoted independently of p53
activation by other stimuli, such as oncogenic stress, growth
factors and/or cytokine withdrawal and kinase inhibition, ER
stress, altered redox status, ischemia, immune modulation, and
infection (28). But the function
of PUMA in p53-independent apoptosis remains to be fully elucidated
(28). You et al (29) and Adlakha and Saini (30) domonstrated that nuclear-activated
FOXO3A binds the PUMA promoter regardless of the p53 genotype,
thereby demonstrating that FOXO3A can act directly on the PUMA
promoter in a p53-independent manner.
In the present study, radioresistant CNE-2-RES cells
showed downregulated PUMA expression and upregulated p53
expression. The change in PUMA which was inconsistent with p53
suggests that Slug might mediate radiation resistance in CNE-2-RES
cells via inhibition of PUMA but not antagonizing p53-dependent
apoptosis. There is probably some p53-independent signaling
pathways involved in the Slug/PUMA axis associated with apoptosis
in CNE-2 cells, or Slug might act through another signaling pathway
other than the PUMA/P53 axis. Our results were not consistent with
an ovarian cancer report, which showed that Slug mediated radiation
resistance mainly by p53-dependent apoptosis (22). Following knockdown of Slug, we
detected PUMA and p53 expressions in both the CNE-2 and CNE-2-RES
cell lines. Slug inversely regulated PUMA and p53 expression in
both cell lines. Knockdown of p53 in the LV-sh-Slug-infected
CNE-2-RES cells partly restored the ability of radioresistance
under 4 Gy of IR. These results suggest that Slug mediates CNE-2
radioresistance partly via downregulation of PUMA in a
p53-dependent manner. The result demonstrates that the
Slug/PUMA/p53 axis does exit and act in the Slug induced radiation
resistance of CNE-2-RES cells, but Slug-induced radiation
resistance is the result of joint action of many signaling
pathways. Slug-induced radioresistance is mediated both by
antagonizing p53-mediated apoptosis and not in CNE-2-RES cells.
Yet, identification of the exact signaling pathway involved in the
p53-independent apoptosis needs further investigation.
Notably, Slug expression was relatively higher in
the CNE-2 cells than that in the CNE-1 cells, and PUMA expression
is relatively lower in CNE-2 cells than that in CNE-1 cells. To the
best of our knowledge, CNE-2 is relatively more radiosensitive than
CNE-1 (31); this phenomenon was
inconsistent with our above results. We believe that this may be
because CNE1 and CNE2 are two different types of NPC cells. The
degree of differentiation and other features are not the same, thus
the two cell lines are not comparable. Moreover, the
radiosensitivity of NPC cells is determined by the effects of
multiple factors, and is not limited to Slug.
Slug-induced radioresistance was further verified in
animal experiments. When CNE-2 cells acquired radioresistant
ability, the corresponding xenograft tumors also acquired
radioresistance. But following knockdown of Slug, the
radio-resistance of the CNE-2-RES xenografts was significantly
reduced. The trends of protein expressed in the animal tissues were
similar to the results we detected in vitro.
Taken together, our results demonstrated that Slug
is a valuable radioresistance-associated biomarker and a promising
therapeutic target in the management of NPC. Slug inhibition may be
useful for chemoprevention and/or therapy of NPC. Yet, the
p53-independent signaling pathway needs further study in subsequent
research.
Acknowledgments
This study was supported by grants from the General
Program of Health Bureau of Wuxi City (no. ML201314) and the Key
Program of Nanjing Medical University (no. 2014NJMUZD034).
References
|
1
|
Lo KW, Chung GT and To KF: Deciphering the
molecular genetic basis of NPC through molecular, cytogenetic, and
epigenetic approaches. Semin Cancer Biol. 22:79–86. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wee JT, Ha TC, Loong SL and Qian CN: Is
nasopharyngeal cancer really a ‘Cantonese cancer’? Chin J Cancer.
29:517–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yeh SA, Tang Y, Lui CC, Huang YJ and Huang
EY: Treatment outcomes and late complications of 849 patients with
nasopharyngeal carcinoma treated with radiotherapy alone. Int J
Radiat Oncol Biol Phys. 62:672–679. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coultas L and Strasser A: The molecular
control of DNA damage-induced cell death. Apoptosis. 5:491–507.
2000. View Article : Google Scholar
|
|
6
|
Nieto MA: The Snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ros MA, Sefton M and Nieto MA: Slug, a
zinc-finger gene previously implicated in the early patterning of
the mesoderm and the neural crest, is also involved in chick limb
development. Development. 124:1821–1829. 1997.PubMed/NCBI
|
|
8
|
Lee HJ, Jeng YM, Chen YL, Chung L and Yuan
RH: Gas6/Axl pathway promotes tumor invasion through the
transcriptional activation of Slug in hepatocellular carcinoma.
Carcinogenesis. 35:769–775. 2014. View Article : Google Scholar
|
|
9
|
Shih JY and Yang PC: The EMT regulator
slug and lung carcinogenesis. Carcinogenesis. 32:1299–1304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shirley SH, Greene VR, Duncan LM, Torres
Cabala CA, Grimm EA and Kusewitt DF: Slug expression during
melanoma progression. Am J Pathol. 180:2479–2489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu WS, Heinrichs S, Xu D, Garrison SP,
Zambetti GP, Adams JM and Look AT: Slug antagonizes p53-mediated
apoptosis of hematopoietic progenitors by repressing puma. Cell.
123:641–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang CH, Yang WH, Chang SY, Tai SK, Tzeng
CH, Kao JY, Wu KJ and Yang MH: Regulation of membrane-type 4 matrix
metalloproteinase by SLUG contributes to hypoxia-mediated
metastasis. Neoplasia. 11:1371–1382. 2009.PubMed/NCBI
|
|
13
|
Zhang KJ, Wang DS, Zhang SY, Jiao XL, Li
CW, Wang XS, Yu QC and Cui HN: The E-cadherin repressor slug and
progression of human hilar cholangiocarcinoma. J Exp Clin Cancer
Res. 1:882010. View Article : Google Scholar
|
|
14
|
Inukai T, Inoue A, Kurosawa H, Goi K,
Shinjyo T, Ozawa K, Mao M, Inaba T and Look AT: SLUG, a
ces-1-related zinc finger transcription factor gene with
antiapoptotic activity, is a downstream target of the E2A-HLF
oncoprotein. Mol Cell. 4:343–352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cha HS, Bae EK, Ahn JK, Lee J, Ahn KS and
Koh EM: Slug suppression induces apoptosis via Puma transactivation
in rheumatoid arthritis fibroblast-like synoviocytes treated with
hydrogen peroxide. Exp Mol Med. 42:428–436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dhasarathy A, Phadke D, Mav D, Shah RR and
Wade PA: The transcription factors Snail and Slug activate the
transforming growth factor-beta signaling pathway in breast cancer.
PLoS One. 6:e265142011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kurrey NK, Amit K and Bapat SA: Snail and
Slug are major determinants of ovarian cancer invasiveness at the
transcription level. Gynecol Oncol. 97:155–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martin TA, Goyal A, Watkins G and Jiang
WG: Expression of the transcription factors snail, slug, and twist
and their clinical significance in human breast cancer. Ann Surg
Oncol. 12:488–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Findlay VJ, Moretz RE, Wang C, Vaena SG,
Bandurraga SG, Ashenafi M, Marshall DT, Watson DK and Camp ER: Slug
expression inhibits calcitriol-mediated sensitivity to radiation in
colorectal cancer. Mol Carcinog. (Suppl 53): E130–E139. 2014.
View Article : Google Scholar
|
|
20
|
Zhang K, Zhang B, Lu Y, Sun C, Zhao W,
Jiao X, Hu J, Mu P, Lu H and Zhou C: Slug inhibition upregulates
radiation-induced PUMA activity leading to apoptosis in
cholangiocarcinomas. Med Oncol. (Suppl 28): S301–S309. 2011.
View Article : Google Scholar
|
|
21
|
Arienti C, Tesei A, Carloni S, Ulivi P,
Romeo A, Ghigi G, Menghi E, Sarnelli A, Parisi E, Silvestrini R, et
al: SLUG silencing increases radiosensitivity of melanoma cells in
vitro. Cell Oncol (Dordr). 36:131–139. 2013. View Article : Google Scholar
|
|
22
|
Kurrey NK, Jalgaonkar SP, Joglekar AV,
Ghanate AD, Chaskar PD, Doiphode RY and Bapat SA: Snail and slug
mediate radioresistance and chemoresistance by antagonizing
p53-mediated apoptosis and acquiring a stem-like phenotype in
ovarian cancer cells. Stem Cells. 27:2059–2068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li G, Liu Y, Su Z, Ren S, Zhu G, Tian Y
and Qiu Y: MicroRNA-324–3p regulates nasopharyngeal carcinoma
radio-resistance by directly targeting WNT2B. Eur J Cancer.
49:2596–2607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar
|
|
25
|
Xu T, Yu CY, Sun JJ, Liu Y, Wang XW, Pi
LM, Tian YQ and Zhang X: Bone morphogenetic protein-4-induced
epithelial-mesenchymal transition and invasiveness through
Smad1-mediated signal pathway in squamous cell carcinoma of the
head and neck. Arch Med Res. 42:128–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee N, Xia P, Quivey JM, Sultanem K, Poon
I, Akazawa C, Akazawa P, Weinberg V and Fu KK: Intensity-modulated
radiotherapy in the treatment of nasopharyngeal carcinoma: an
update of the UCSF experience. Int J Radiat Oncol Biol Phys.
53:12–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Perez-Mancera PA, Gonzalez-Herrero I,
Perez-Caro M, Gutiérrez-Cianca N, Flores T, Gutiérrez-Adán A,
Pintado B, Sánchez-Martín M and Sánchez-García I: SLUG in cancer
development. Oncogene. 24:3073–3082. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu J and Zhang L: PUMA, a potent killer
with or without p53. Oncogene. (Suppl 27): S71–S83. 2008.
View Article : Google Scholar
|
|
29
|
You H, Pellegrini M, Tsuchihara K,
Yamamoto K, Hacker G, Erlacher M, Villunger A and Mak TW:
FOXO3a-dependent regulation of Puma in response to cytokine/growth
factor withdrawal. J Exp Med. 203:1657–1663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adlakha YK and Saini N: miR-128 exerts
pro-apoptotic effect in a p53 transcription-dependent and
-independent manner via PUMA-Bak axis. Cell Death Dis. 4:e5422013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qu Y, Zhang H, Zhao S, Hong J and Tang C:
The effect on radioresistance of manganese superoxide dismutase in
nasopharyngeal carcinoma. Oncol Rep. 23:1005–1011. 2010.PubMed/NCBI
|