Introduction
Regional or distal lymph-node metastasis is the
major cause of mortality for patients with malignant tumors.
Although tumor metastasis has been extensively studied, the exact
mechanisms remain to be determined. Epithelial-mesenchymal
transition (EMT) is a process whereby epithelial cells transform
morphologically and phenotypically into fibroblasts or mesenchymal
cells, and gain migratory ability (1). EMT is considered to be the first step
of tumor metastasis (2,3). During EMT, epithelial cells lose their
polarity and cell-cell adhesion, develop cyto-skeletal changes and
obtain increased migratory potential and mobility (4). This process is concurrent with the
alteration of various molecular markers, including downregulation
of the epithelial markers E-cadherin and β-catenin, and
upregulation of the mesenchymal phenotypic markers vimentin and
N-cadherin (5). EMT has been shown
to be closely associated with the invasion and metastasis of
various malignancies, including gastric, colorectal, breast, liver
and ovarian cancer (6–10). Numerous factors and processes are
involved in the EMT of cancer cells, such as transforming growth
factor (TGF)-β, hepatocyte growth factor (HGF), c-Met
amplification, epidermal growth factor receptor (EGFR) mutation,
and transcription factors ZEB1/2, Snail, Twist and Tiam1 (3). Among these, TGF-β is considered one of
the most important inducers of EMT. TGF-β exerts an antitumor
activity through the suppression of tumor growth at the early stage
of tumor development, while at the late stage, it promotes tumor
metastasis and EMT of tumor cells by reducing the adhesion molecule
expression, accelerating tumor neovascularization and activating
proteases that promote tumor metastasis (11).
MicroRNAs (miRNAs) are small non-coding RNA
molecules (containing ~22 nucleotides) that are widely found in
eukaryotes and viruses and regulate various cell functions. The
mature miRNA binds to the non-coding region at the 3′-terminus of
the target mRNA to regulate gene expression post-transcriptionally.
miRNAs are also closely associated with tumor development and
progression (12). The miR-200
family, an important class of EMT mediators, is found to mediate
the proliferation, migration, invasion and metastasis of epithelial
cell-derived malignant tumor cells in response to transforming
growth factor β1 (TGF-β1) by regulating the expression of ZEB1/2
(13). In addition, to maintain the
integrity of intestinal epithelium, TGF-β1 induces EMT by
suppressing Smad2 in a miR-200b-dependent manner (14).
Tumor metastasis is known to be organ specific. as
tumor cells undergo EMT and become migratory, they may develop
directional migration via chemokines. Chemokines are preferentially
expressed in organs or tissues, and along with cell surface
proteins, including integrins, they regulate the homing of multiple
blood cell subsets to specific anatomic sites. During tumor
development, the stromal cell-derived factor 1 (SDF-1)/CXC
chemokine receptor type 4 (CXCR4) axis has been shown to be
involved in the metastasis of various types of human cancer
(15,16).
The role of platelets in tumor progression has
attracted increasing attention. Platelets, a major component of
blood, contain α and dense granules and lysosomes, and play an
important role in hemostasis, thrombosis and inflammation (17). In addition, platelets promote tumor
metastasis. An increase in the platelet count is positively
correlated with tumor metastasis, while a reduction in platelet
count or inhibition of platelet function markedly inhibits tumor
metastasis (18–21). The platelet α granules contain many
substances, such as vascular endothelial growth factor (vEGF),
SDF-1, platelet-derived growth factor (PDGF) and TGF-β, which are
involved in tumor progression when excreted (22,23).
Activation of the platelet α granule is mediated by the
proteinase-activated receptor (PAR) signaling pathway (23,24).
PAR is a subfamily of the seven-pass transmembrane
G-protein-coupled receptors, including PAR1, PAR2, PAR3 and PAR4
(25). PAR1 is expressed on
platelets and is activated by thrombin or tissue factor in the
tumor microenvironment (26,27).
Notably, PAR1 is also expressed in multiple tumor cells, and
inhibition of PAR1 may suppress tumor growth and metastasis
(28).
In clinical practice, thrombocythemia is
predominantly found in patients with advanced tumors. It was
generally thought that thrombocythemia develops only at the late
stages of cancer, and platelets are only involved in the growth and
metastasis of advanced tumors (29). However, it has been demonstrated
that a large number of tumor cells migrate into the bloodstream
even at an early stage, and these circulating tumor cells (CTCs)
constitute nidi for tumor metastasis and recurrence (30). EMT and chemotaxis are essential for
CTCs to enter the vasculature. In addition, platelet activation has
been found to occur in the early stages of tumor development
(30). Previous findings have shown
that only direct contact between platelets and breast cancer cells
induces EMT of breast cancer cells via the TGF-β signaling pathway,
suggesting that platelets induce EMT of tumor cells thus
facilitating their entry into the bloodstream (31). Since tumor cells develop EMT prior
to entering the bloodstream, we speculate that platelets may not
need to directly contact tumor cells to induce their EMT, and may
instead have a chemotactic effect on tumor cells. The aim of the
present study was to investigate whether PAR1-activated platelets
induce a colon cancer cell line to undergo EMT without direct
contact with cancer cells, and to understand the role of platelets
in the early-phase metastasis of colon cancer cells.
Materials and methods
Cell lines
The SW620 human colon cancer cell line was purchased
from the Cell Bank of the Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). The cells were cultured in
McCoy’s 5A medium containing 10% fetal bovine serum (FBS), 1%
penicillin and 1% streptomycin at 37°C in 5% CO2.
Antibodies and reagents
The PAR1 agonist TFLLR-NH2 was obtained
from Sigma-Aldrich (St. Louis, MO, USA). The human CD62P-FITC
antibody was purchased from AK-4 (eBioscience, inc., San Diego, CA,
USA). E-cadherin and vimentin rabbit anti-human antibodies were
purchased from Abcam (Cambridge, MA, USA). PathScan® EMT
Duplex IF kit was purchased from Cell Signaling Technology, Inc.
(Shanghai, China). The ELISA kit for TGF-β1 was obtained from
Neobioscience Technology Co., Ltd. (Beijing, China). Transwell
chambers (Corning Inc., Lowell, MA, USA) were used for the EMT
assays. The cells were stained for flow cytometry using PE
anti-human CD184/CXCR4 (12G5; BioLegend, San Diego, CA, USA).
Preparation of the platelets
Whole blood samples were collected from 10 healthy
volunteers (8 men and 2 women, none of whom had any medical history
or had received anticoagulants one week prior to blood sample
collection). The blood samples were centrifuged at 200 x g for 10
min to yield three cell layers: platelet rich plasma (PRP) on the
upper layer, white cells at the middle layer, and red cells at the
lower layer. Approximately 2/3 of the PRP layer were transferred to
a centrifugation tube containing 50 ng/ml prostaglandin E1 (PGE1),
centrifuged at 22°C for 10 min and the supernatant was removed. The
sediment platelets were washed twice with 5 ml of
phosphate-buffered saline (PBS) without calcium or magnesium. The
platelets were re-suspended in solutions containing 5 mM glucose
and 0.25% bovine serum albumin (BSA), and adjusted to a
concentration of 150,000/μl for the subsequent
experiments.
Test for the optimal dose of platelet
PAR1 agonist
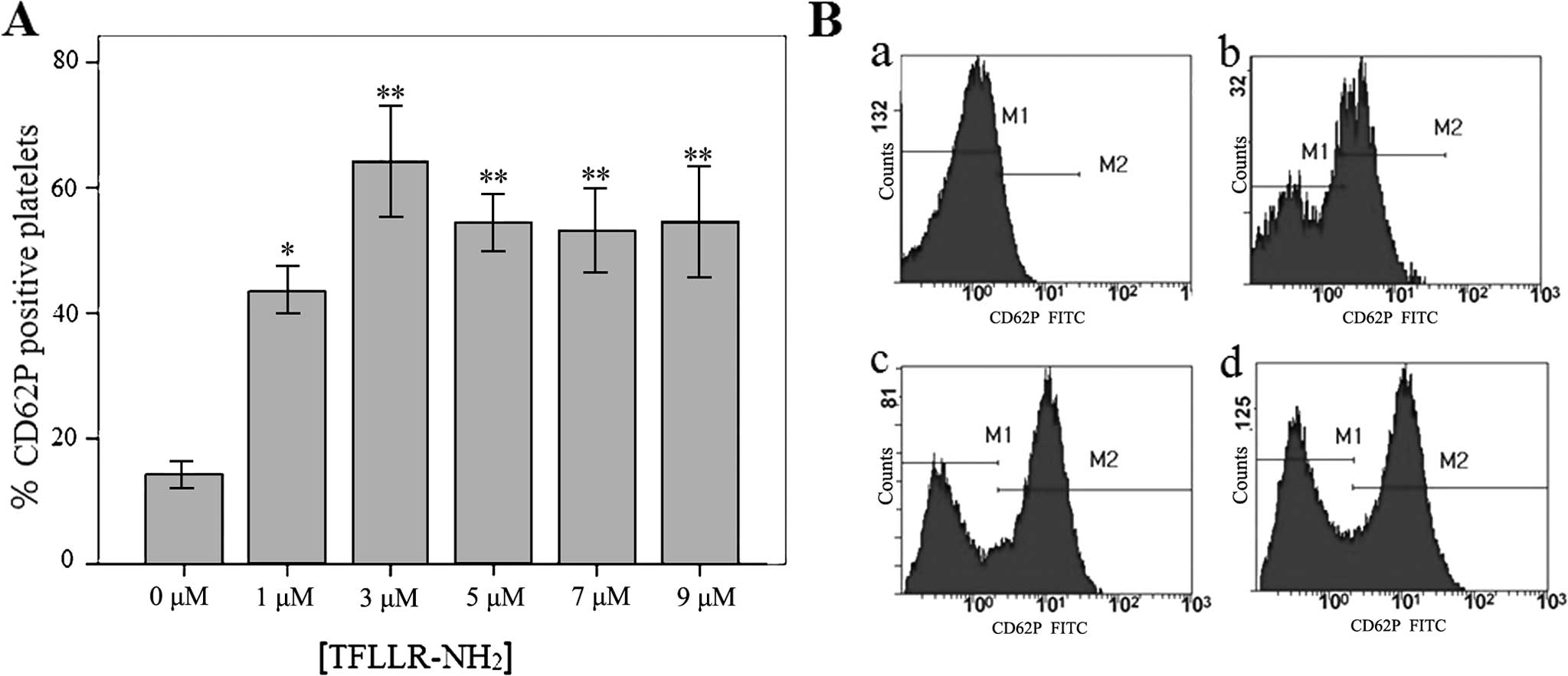
To investigate platelet activation following
treatment with various concentrations of TFLLR-NH2,
CD62P (P-selectin) was used as a marker of platelet activation, and
the rate of CD62P-positive platelets was detected using flow
cytometry. TFLLR-NH2 was added to the platelet culture
at final concentrations of 1, 3, 5, 7 and 9 μM in McCoy’s 5A
medium containing 10% FBS, and incubated at 37°C for 15 min, while
non-treated platelets served as controls. The CD62P expression in
the platelets was determined using flow cytometry (FC500 flow
cytometer; Beckman Coulter, Inc., Chaska, MN, USA).
Detection of EMT
Based on the results of the above tests, 3 μM
TFLLR-NH2 was selected for subsequent experiments to
determine whether activation of platelet PAR-1 induces EMT of the
SW620 colon cancer cell line. Three groups were assigned: i) group
1, 100 μl of activated platelet supernatant + SW620 cells,
ii) group 2, 100 μl of supernatant of untreated platelets +
SW620 cells and iii) group 3, 100 μl of medium + SW620
cells. Three independent experiments were conducted.
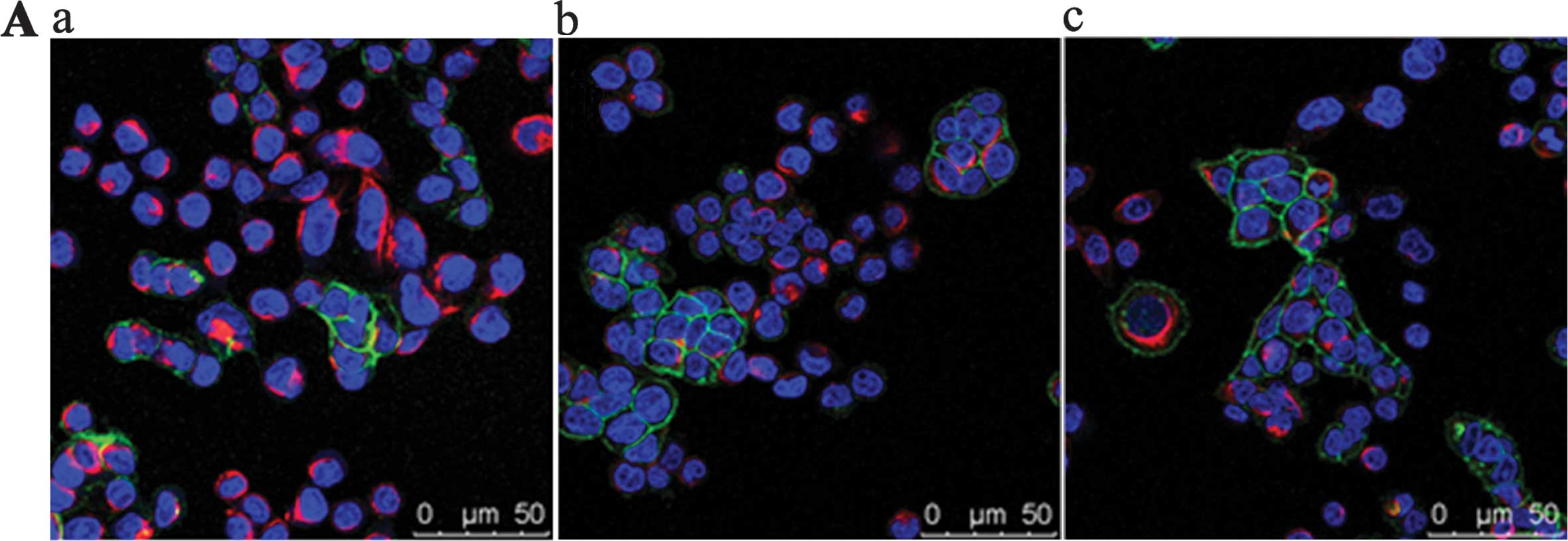
After 24 h of culture at 37°C, the SW620 cells were
stained for E-cadherin and vimentin using the PathScan®
EMT Duplex IF kit, and their expression was observed under a
four-channel CLS-4HS laser confocal microscope (Thorlabs Inc.,
Newton, NJ, USA). Three independent experiments were conducted.
After incubation in 6-well plates for 24 h, the
E-cadherin and vimentin protein expression in the SW620 cells was
detected using western blotting. Briefly, the cultured cells were
washed once with PBS buffer and lysed in lysis buffer (1% SDS, 50
mM Tris, pH 7.4, 0.15 M NaCl, 1 mM NaF, 10 mM phenylmethylsulfonyl
fluoride, 1 mM sodium orthovanadate, 1 mM EDTA) for 5 min and
passed through a 27-gauge needle. Lysates were centrifuged at
12,000 x g for 1 min, the supernatants were collected, and protein
concentrations were determined using a Bio-Rad DC protein assay
(Bio-Rad, Hercules, CA, USA). Equal amounts of protein were
separated by 10% SDS-PAGE and transferred to nitrocellulose
membranes. The membranes were blocked with 3% BSA in TBST (10 mM
Tris, pH 7.5, 150 mM NaCl, 0.1% Tween-20) for 1 h. Primary
antibodies (1:200) and secondary antibodies were used according to
the manufacturer’s instructions. The blots were analyzed and
quantified with the MCID imaging software (Imaging Research Inc.,
St. Catharines, ON, Canada). β-actin served as an internal
reference.
Transwell migration assay
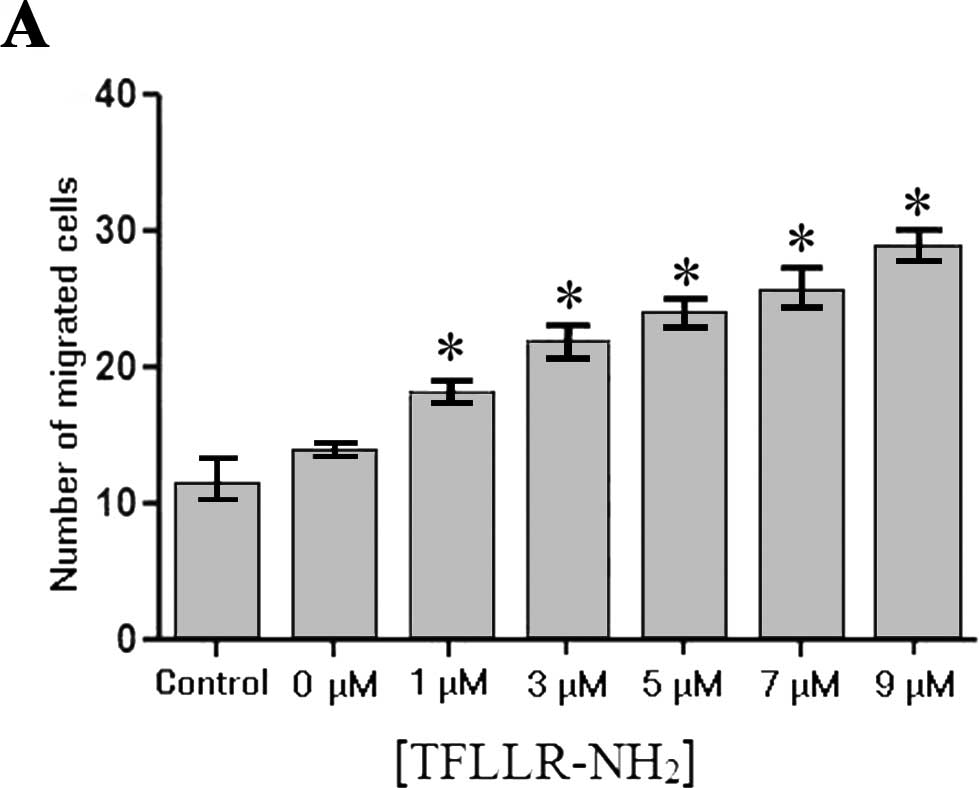
We assessed whether activation of platelet PAR-1
induces chemotaxis of the SW620 colon cancer cell line using a
Transwell migration assay. Transwell chambers with an 8-μm
pore diameter and a 6.5 mm membrane diameter were used in a
chemotaxis assay. Three groups were assigned: i) activated platelet
supernatant group (cells were incubated in 600 μl of the
supernatant of the platelets activated by TFLLR-NH2 at a
final concentration of 1, 3, 5, 7 or 9 μM in McCoy’s 5A
medium containing 10% FBS), ii) non-activated platelet supernatant
group (same as above but without TFLLR-NH2 treatment),
and iii) blank control group (cells were treated with McCoy’s 5A
medium containing 10% FBS).
The upper Transwell chamber was filled with SW620
cells at a concentration of 2.5×105/100 μl, and
the chemotaxis chamber was incubated at 37°C in 5% CO2
for 18 h. The nuclei of the cells migrating through the Transwell
membrane were stained with 5 μg/ml of DAPI for 10 min, and
its fluorescence signal was observed under an inverted fluorescence
microscope. The number of cells at five randomly selected fields
was counted, and the mean count was calculated. Each experiment was
repeated three times.
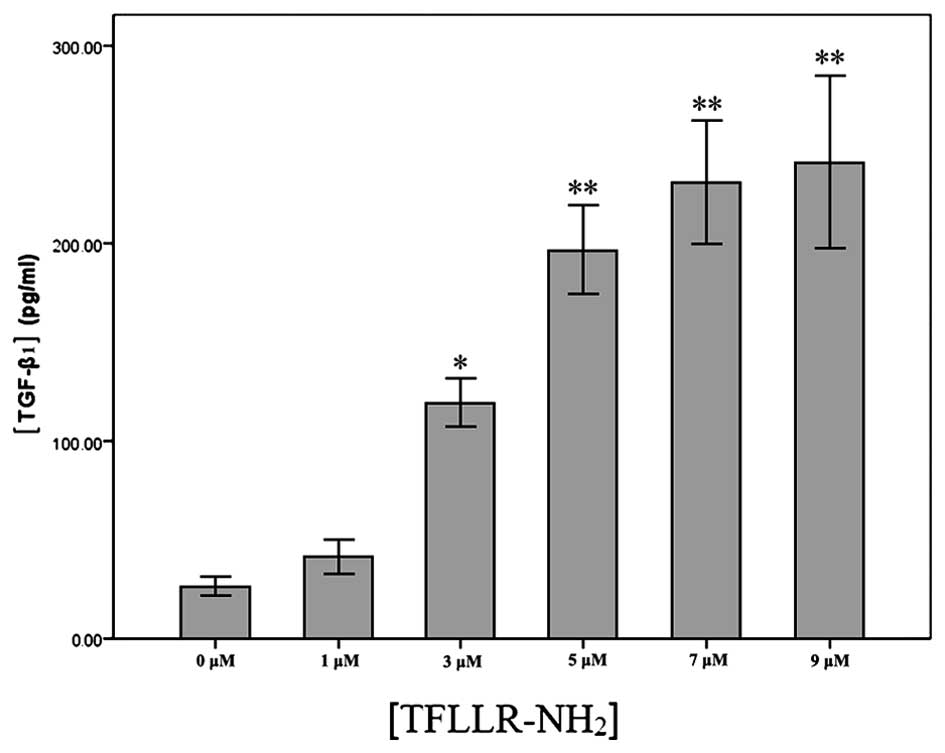
Quantification of TGF-β1 levels in the
platelet supernatant by ELISA
The supernatants of the platelets treated with
TFLLR-NH2 at a final concentration of 1, 3, 5, 7 or 9
μM in McCoy’s 5A medium containing 10% FBS and PBS were
collected, and the TGF-β1 released from platelets α granules was
detected using an ELISA kit according to the manufacturer’s
instructions.
Quantitative PCR of miR-200b
expression
Quantitative PCR (qPCR) was performed to detect the
effect of the activated platelet supernatant on miR-200b expression
in the SW620 cells. Platelets from healthy donors were incubated in
3 μM TFLLR-NH2 in McCoy’s 5A medium containing
10% FBS for 15 min, and the platelet supernatants were collected.
The SW620 cells were treated with 0, 60, 120, 240 or 480 μl
of the supernatant for 24 or 48 h. Total RNA was isolated from the
cells using TRIzol® reagent, and its integrity was
checked on agarose gel. After reverse transcription, qPCR was
performed using a miR-200b primer (part no.: 4427975; assay ID:
002251) based on the specific cDNA template in 20 μl of the
reaction system containing 10 μl of TaqMan®
Universal PCR Master Mix, 1 μl of diluted PreAmp product, 1
μl of microRNA assay (all from ABI, Santa Monica, CA, USA)
and 8 μl of nuclease-free water under the following
conditions: at 95°C for 10 min, followed by 40 cycles at 95°C for
15 sec, and at 60°C for 60 sec. U6 snRNA (part no.: 4427975; assay
ID: 001973; ABI) served as an internal reference. The relative
miR-200b expression was estimated using the 2−ΔΔCt
method.
Flow cytometric detection of CXCR4
expression on the SW620 cell surface
The TFLLR-NH2 and non-activated platelet
groups were assigned. SW620 cells were seeded in 6-well plates at a
concentration of 1.0×106 cells/well. The platelet
supernatant (100 μl) activated at a final concentration of 3
μM TFLLR-NH2 in McCoy’s 5A medium containing 10%
FBS was added to the TFLLR-NH2 group, while the same
volume of medium was transferred to the blank control. After a 24 h
culture, the cells were collected, digested with pancreatic enzymes
and the cell density was adjusted to 1.5×107 cells/ml.
The CXCR4 level on the SW620 cell surface was determined using flow
cytometry. Experiments were repeated three times.
Statistical analysis
Data are presented as mean ± standard deviation
(SD). Statistical analyses were performed using the statistical
software SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA). The
statistical significance of the differences between the means was
determined by the Student’s t-test when analyzing two independent
samples, while analysis of variance (ANOVA) was employed for
multiple independent samples. Rank sum test was used to make
comparisons among multiple groups when the variances of the groups
were heterogeneous. P<0.05 was considered to indicate a
statistically significant result.
Results
Activation of platelets by the PAR
agonist TFLLR-NH2
To detect platelet activation following treatment
with various concentrations of TFLLR-NH2, the rate of
CD62P (P-selectin)-positive platelets was determined using flow
cytometry. The rates of CD62P-positive platelets were (43.52±1.5)%,
(64.22±3.60)%, (54.46±1.82)%, (53.15±2.7)% and (54.63±3.66)% in the
platelets treated with TFLLR-NH2 at a final
concentration of 1, 3, 5, 7 and 9 μM, respectively, all of
which were significantly greater (P<0.05) than the rates in the
blank control (no TFLLR-NH2) group (14.35±0.86)%
(Fig. 1A and B). Thus, 3 μM
was selected as the dose of TFLLR-NH2 for platelet
activation.
EMT of SW620 cells
To detect whether platelet activation induced EMT of
the SW620 cells, immunofluorescence and western blotting were
employed to determine the expression of EMT markers E-cadherin and
vimentin. Laser confocal microscopy showed that the supernatant of
TFLLR-NH2-activated platelets triggered the
downregulation of E-cadherin expression and the upregulation of
vimentin expression (Fig. 2A) in
the SW620 cells compared to the cells treated with medium or with
the supernatant of untreated platelets. Western blot analysis
showed a similar finding (Fig. 2B).
The grey value of E-cadherin was 0.225 for cells treated with
activated platelet supernatant, which was lower than that for the
cells treated with medium or with the supernatant of untreated
platelets (0.758 and 0.769, respectively). However, the grey value
of vimentin was 0.616 for cells treated with activated platelet
supernatant, which was greater than that for the cells treated with
medium or with the supernatant of untreated platelets (0.211 and
0.234, respectively). These findings suggested that platelet
activation may induce the EMT of the SW620 cells.
The supernatant of
TFLLR-NH2-activated platelets induces SW620 cell
migration
A chemotaxis assay was performed to investigate the
chemotactic effect of platelet activation on tumor cells. Our
findings showed no significant difference in the number of SW620
cells that migrated through the Transwell membrane/field between
the group with the supernatant of untreated platelets and the blank
control group (11.33±2.08 vs. 13.67±0.58, P>0.05). When using
conditioned media from the platelets treated with
TFLLR-NH2 at a final concentration of 1, 3, 5, 7 and 9
μM, the number of SW620 cells migrating through the membrane
to the platelet supernatant was 18±1, 21.67±1.53, 23.67±1.53,
25.33±2.08 and 28.67±1.53, respectively (Fig. 3A), all of which were significantly
greater (P<0.05) than the numbers in the blank control (no
TFLLR-NH2) group and in the group with the supernatant
of the untreated platelets (P<0.05). A dose-dependent increase
in the number of the cells migrating through the Transwell membrane
was observed (Fig. 3B). These
findings indicated that the supernatant of the
TFLLR-NH2-activated platelets had a chemotactic effect
on the SW620 cells.
TGF-β1 levels in the
TFLLR-NH2-activated platelet supernatant
The TGF-β1 levels were 41.42±3.60, 119.27±5.16,
197.11±9.21, 231.37±7.26 and 241.33±17.60 pg/ml in the supernatants
of the platelets activated by TFLLR-NH2 at a final
concentration of 1, 3, 5, 7 and 9 μM, respectively,
reflecting a dose-dependent increase in the TGF-β1 secretion, and
the TGF-β1 level was significantly higher in the activated platelet
supernatant than in that of the control group (26.31±1.96)
(Fig. 4).
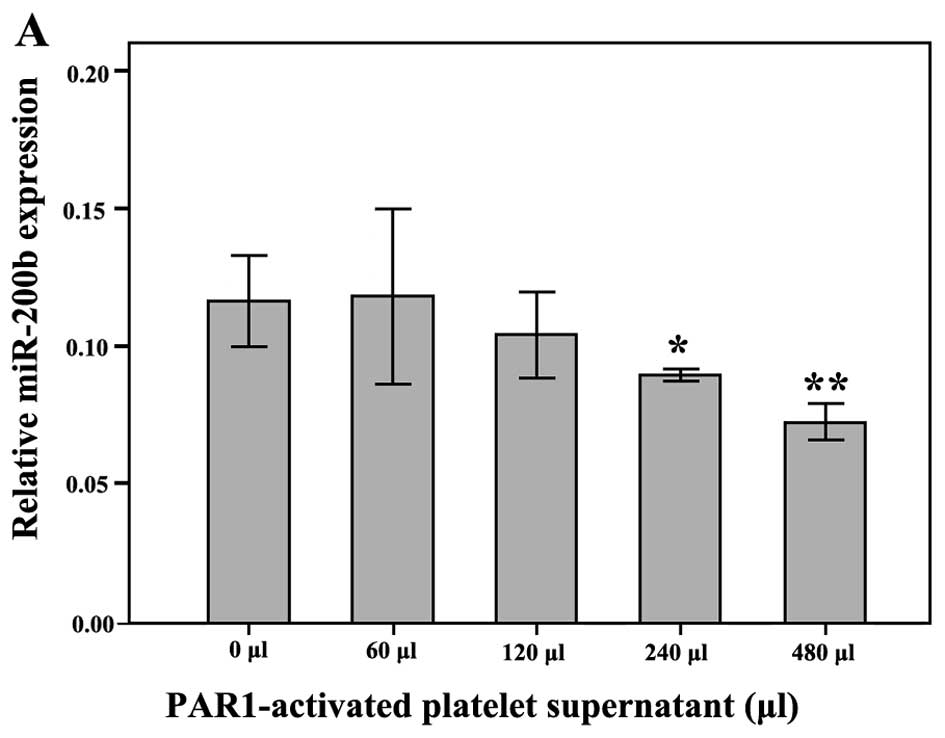
miR-200b expression in SW620 cells
Although miR-200b has been shown to be involved in
the regulation of EMT (14), the
effect of the platelet activation on miR-200b expression remains
unclear. qPCR was performed to investigate the effect of 3
μM TFLLR-NH2-treated platelet supernatant on the
miR-200b expression in the SW620 cells. Our findings showed that
following treatment with activated platelet supernatant for 48 h,
no significant difference was found in the relative miR-200b
expression between the non-treated platelet supernatant control and
the TFLLR-NH2 treatment group (P>0.05) (Fig. 5B). The relative miR-200b expression
was 0.118±0.013, 0.105±0.006, 0.090±0.001 and 0.073±0.003 in SW620
cells treated with 60, 120, 240 and 480 μl of the platelet
supernatant for 24 h, respectively, compared to 0.117±0.007 in the
blank control group. However, the relative miR-200b expression was
lower in the SW620 cells treated with 240 and 480 μl of the
platelet supernatant than in the control group (Fig. 5A, P<0.05), suggesting that the
miR-200b expression appeared to decline with increasing volumes of
the platelet supernatant, while in the SW620 cells treated with 60
and 120 μl of the platelet supernatant there were no
significant differences with the non-treated platelet supernatant
control group (P>0.05).
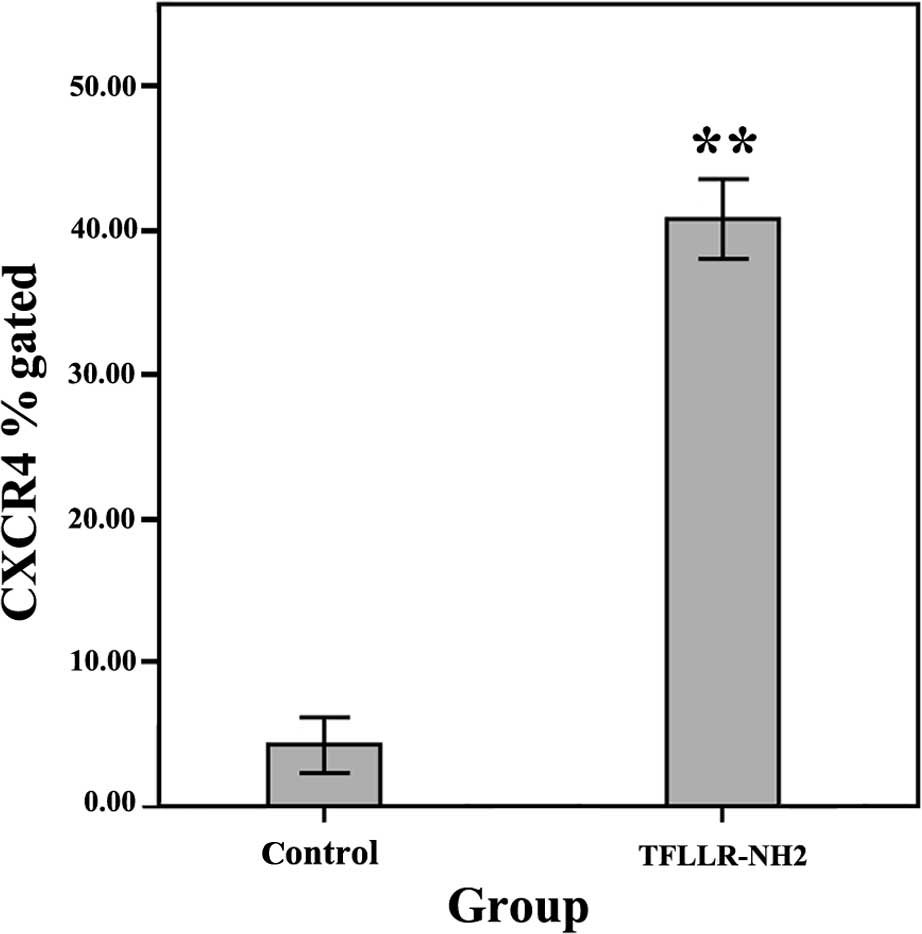
Effect of TFLLR-NH2-activated
platelet supernatant on CXCR4 expression in the SW620 cells
Cell surface CXCR4, when bound to its ligand SDF-1,
induces the directional migration of tumor cells (32). The aforementioned results showed
that TFLLR-NH2-activated platelets, not only induced the
chemotaxis of the SW620 cells, but also stimulated TGF-β1 secretion
by platelets. Flow cytometry was used to investigate the effect of
platelet activation on CXCR4 expression on SW620 cells. Our
findings showed that (40.89±6.74)% of the SW620 cells were positive
for CXCR4 24 h after treatment with the supernatant of the
platelets activated by 3 μM TFLLR-NH2, which was
significantly greater than that (3.47±1.40)% in the non-activated
platelet group (P<0.01; Fig.
6).
Discussion
In the present study, we found that PAR1 agonist
TFLLR-NH2-activated platelets released TGF-β1 leading to
the upregulation of CXCR4 and the inhibition of miR-200b expression
in SW620 cells, ultimately inducing EMT-phenotype and migration in
vitro. Our findings suggest that the activation of platelet PAR1
may be important in the initial stages of tumor metastasis, and
early antiplatelet therapy may be of great significance to suppress
colorectal cancer metastasis and improve the survival rate.
Platelet-tumor cell interaction is extremely complex
in tumor microenvironments. It has been reported that tumor cells
upregulate the expression of tissue factor (TF) leading to an
increase in thrombin, which activates platelet PAR1 or PAR4
(33,34). In addition, tumor cells can directly
secrete thrombin into microenvironments to activate platelets
(35). inflammation and
angiogenesis are usually present inside tumors, and the
distribution of new blood vessels is disorganized. These
pathological changes may cause the slowdown of blood circulation in
and surrounding the tumors, thereby increasing the probability of
tumor-activating platelet deposition (36), which may promote tumor cell growth
and metastasis.
Labelle et al (37) found that the platelet-breast cancer
cell interaction promoted tumor cell EMT and metastasis. Since the
supernatant of the thrombin-activated platelets did not induce
tumor cell EMT within 48 h, the direct contact between platelets
and tumor cells was considered essential for the development of EMT
(38). However, this does not
concur with our findings. in the present study, the supernatant of
platelets activated by a PAR1 agonist TFLLR-NH2, was
found to induce EMT of the SW620 cells within 24 h, indicating that
tumor cell EMT may occur without the direct contact between
platelets and tumor cells. In addition, Labelle et al (37) reported that the induction of breast
cancer EMT by platelets required at least one week. However, the
sensitivity to cytokines may vary in tumor cells, resulting in
varying potentials and speeds of EMT. For example, it has been
shown that EMT occurs in the tumor cells NMuMG, A549 and MDA-MB-231
24 h after the addition of TGF-β (38).
Our findings show that the activated platelets
secreted TGF-β1, and conditioned media from the activated platelet
culture led to the downregulation of miR-200b expression in the
SW620 cells. Accumulating evidence has demonstrated that multiple
factors released by platelets induce tumor cell EMT, of which TGF-β
is the most extensively investigated (37,38).
TGF-β has been found to promote the metastasis of multiple tumors
through the induction of cancer cell EMT (12,14,39).
Besides activating the Smad pathway, TGF-β induces tumor cell EMT
through other means, including the Ras-Erk/MAPK, p38/MAPK, JNK, Rho
GTPase and PI3K/Akt pathways (12).
In addition, TGF-β has been reported to mediate EMT via the
regulation of miRNA transcription (14). It has been shown that TGF-β1
signaling downregulates the expression of the miR-200 family by
activating Akt2, leading to the upregulation of ZEB1 and ZEB2
(39). Moreover, ZEB2 binds to the
miR-200 promoter to inhibit its transcription, resulting in the
formation of a negative feedback loop, which further stimulates the
EMT of tumor cells (40).
In addition, results of the present study have shown
that PAR1 agonist-activated platelets had a chemotactic effect on
the SW620 colon cancer cell line. It is well known that activated
platelets may be involved in the chemotaxis of various cells, and
thus participate in various pathological processes, such as
inflammation, thrombogenesis and arteriosclerosis (41). During inflammation, platelets
promote their adhesion to other cells in the blood by expressing
receptors such as β3 integrins, and are involved in the chemotaxis
of inflammatory cells by releasing a large number of chemokines
(42). Our findings confirm the
chemotactic effect of platelets on malignant tumor cells. If a
solid tumor exceeds 2 mm in diameter, new blood vessels form, a
process accompanied by the infiltration of inflammatory cells. When
platelets pass through tumors via the bloodstream, they may be
activated by specific cytokines released by tumor cells or by
mesenchymal cells in tumor microenvironments, and activated
platelets may in turn induce tumor cell EMT and migration into
blood vessels. Therefore, some clinically diagnosed early-stage
tumors are virtually at an ‘advanced phase’. This may explain the
reason for some cancer patients developing early-stage metastasis.
It has been shown that an antiplatelet agent such as aspirin may
reduce the metastatic rate of malignant tumors and improve
prognosis (43).
In addition, our findings show that the activated
platelet culture supernatant upregulated the expression of CXCR4 on
the surface of SW620 cells, suggesting platelets actively induce
the migration of tumor cells into blood vessels. The chemokine
receptor CXCR4 is widely present in multiple tumors, and a high
CXCR4 expression is a predictive marker of tumor metastasis and
results in a worse prognosis (44–46).
For example, a low CXCR4 expression is detected in normal breast
tissues, while a high expression is found in breast cancer tissues
(47). The CXCR4-SDF-1 interaction
may lead to actin polymerization and pseudopod formation, thereby
resulting in chemotaxis and invasion (47), while the specific blockade of CXCR4
inhibits the metastasis of breast cancer cells to lymph nodes and
bone marrow (47). In addition, a
high CXCR4 expression was found to correlate with bone metastasis
in prostate cancer, while a neutralizing antibody against CXCR4 may
block the bone metastasis of prostate cancer (48).
The interaction between tumor and microenvironment
is complex, and the interaction between tumor and the cell
components in the microenvironment is potent. The present study
only investigated the effect of static platelets on tumor cells.
Future studies are required to evaluate the impact of platelets on
tumor cells in dynamic bloodstream. In addition to TGF-β1,
platelet-derived PDGF plays an important role in inducing cell EMT
(49). Notably, platelets have
recently been found to enter tumor tissues in animal models
(50). Further studies are
warranted to evaluate the role of platelets in tumor progression
and the difference between intratumoral and intra-vascular
platelet-mediated tumor activation.
In conclusion, PAR1-activated platelets may induce
EMT of the SW620 colon cancer cell line via the TGF-β pathway, and
they provide chemotactic signals to the SW620 cells which lead to
the upregulation of CXCR4 on the cancer cell surface. The present
study provides evidence showing a molecular mechanism potentially
underlying colorectal cancer metastasis and contributes to
supporting the potential value of early antiplatelet therapy for
colorectal cancer.
Acknowledgments
This study was supported by a Hebei Province
Department of Education Major Project Grant (grant no.
ZD20131052).
References
|
1
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kang Y and Massagué J:
Epithelial-mesenchymal transitions: Twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Findlay VJ, Wang C, Watson DK and Camp ER:
Epithelial-to-mesenchymal transition and the cancer stem cell
phenotype: Insights from cancer biology with therapeutic
implications for colorectal cancer. Cancer Gene Ther. 21:181–187.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wendt MK, Allington TM and Schiemann WP:
Mechanisms of the epithelial-mesenchymal transition by TGF-beta.
Future Oncol. 5:1145–1168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thiery JP: Epithelial-mesenchymal
transitions in cancer onset and progression. Bull Acad Natl Med.
193:1969–1979. 2009.In French.
|
|
6
|
Murai T, Yamada S, Fuchs BC, Fujii T,
Nakayama G, Sugimoto H, Koike M, Fujiwara M, Tanabe KK and Kodera
Y: Epithelial-to-mesenchymal transition predicts prognosis in
clinical gastric cancer. J Surg Oncol. 109:684–689. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang W, Wu Y, Yan Q, Ma F, Shi X, Zhao Y,
Peng Y, Wang J and Jiang B: Deferoxamine enhances cell migration
and invasion through promotion of HIF-1α expression and
epithelial-mesenchymal transition in colorectal cancer. Oncol Rep.
31:111–116. 2014.
|
|
8
|
Leibovich-Rivkin T, Liubomirski Y,
Bernstein B, Meshel T and Ben-Baruch A: Inflammatory factors of the
tumor microenvironment induce plasticity in nontransformed breast
epithelial cells: EMT, invasion, and collapse of normally organized
breast textures. Neoplasia. 15:1330–1346. 2013. View Article : Google Scholar
|
|
9
|
Yau WL, Lam CS, Ng L, Chow AK, Chan ST,
Chan JY, Wo JY, NG KT, Man K, Poon RT, et al: Over-expression of
miR-106b promotes cell migration and metastasis in hepatocellular
carcinoma by activating epithelial-mesenchymal transition process.
PLoS One. 8:e578822013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lili LN, Matyunina LV, Walker LD, Wells
SL, Benigno BB and McDonald JF: Molecular profiling supports the
role of epithelial-to-mesenchymal transition (EMT) in ovarian
cancer metastasis. J Ovarian Res. 6:492013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pardali K and Moustakas A: Actions of
TGF-beta as tumor suppressor and pro-metastatic factor in human
cancer. Biochim Biophys Acta. 1775:21–62. 2007.
|
|
12
|
Nelson KM and Weiss GJ: MicroRNAs and
cancer: Past, present, and potential future. Mol Cancer Ther.
7:3655–3660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Xiao Y, Ge W, Zhou K, Wen J, Yan
W, Wang Y, Wang B, Qu C, Wu J, et al: miR-200b inhibits TGF-β
1-induced epithelial-mesenchymal transition and promotes growth of
intestinal epithelial cells. Cell Death Dis. 4:e5412013. View Article : Google Scholar
|
|
15
|
Jankowski K, Kucia M, Wysoczynski M, Reca
R, Zhao D, Trzyna E, Trent J, Peiper S, Zembala M, Ratajczak J, et
al: Both hepatocyte growth factor (HGF) and stromal-derived
factor-1 regulate the metastatic behavior of human rhabdomyosarcoma
cells, but only HGF enhances their resistance to radiochemotherapy.
Cancer Res. 63:7926–7935. 2003.PubMed/NCBI
|
|
16
|
Yang P, Liang SX, Huang WH, Zhang HW, Li
XL, Xie LH, Du CW and Zhang GJ: Aberrant expression of CXCR4
significantly contributes to metastasis and predicts poor clinical
outcome in breast cancer. Curr Mol Med. 14:174–184. 2014.
View Article : Google Scholar
|
|
17
|
Bazzoni G, Dejana E and Del Maschio A:
Platelet-neutrophil interactions. Possible relevance in the
pathogenesis of thrombosis and inflammation. Haematologica.
76:491–499. 1991.PubMed/NCBI
|
|
18
|
Hwang SG, Kim KM, Cheong JH, Kim HI, An
JY, Hyung WJ and Noh SH: Impact of pretreatment thrombocytosis on
blood-borne metastasis and prognosis of gastric cancer. Eur J Surg
Oncol. 38:562–567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao J, Zhang HY and Xia YF: Increased
platelet count is an indicator of metastasis in patients with
nasopharyngeal carcinoma. Tumour Biol. 34:39–45. 2013. View Article : Google Scholar
|
|
20
|
Sasaki K, Kawai K, Tsuno NH, Sunami E and
Kitayama J: Impact of preoperative thrombocytosis on the survival
of patients with primary colorectal cancer. World J Surg.
36:192–200. 2012. View Article : Google Scholar
|
|
21
|
Kaneko MK, Kunita A, Abe S, Tsujimoto Y,
Fukayama M, Goto K, Sawa Y, Nishioka Y and Kato Y: Chimeric
anti-podoplanin antibody suppresses tumor metastasis through
neutralization and antibody-dependent cellular cytotoxicity. Cancer
Sci. 103:1913–1919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blair P and Flaumenhaft R: Platelet
alpha-granules: Basic biology and clinical correlates. Blood Rev.
23:177–189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chatterjee M, Huang Z, Zhang W, Jiang L,
Hultenby K, Zhu L, Hu H, Nilsson GP and Li N: Distinct platelet
packaging, release, and surface expression of proangiogenic and
antiangiogenic factors on different platelet stimuli. Blood.
117:3907–3911. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tamura S, Suzuki H, Hirowatari Y, Hatase
M, Nagasawa A, Matsuno K, Kobayashi S and Moriyama T: Release
reaction of brain-derived neurotrophic factor (BDNF) through PAR1
activation and its two distinct pools in human platelets. Thromb
Res. 128:e55–e61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cottrell GS, Coelho AM and Bunnett NW:
Protease-activated receptors: The role of cell-surface proteolysis
in signalling. Essays Biochem. 38:169–183. 2002.PubMed/NCBI
|
|
26
|
Schaffner F and Ruf W: Tissue factor and
PAR2 signaling in the tumor microenvironment. Arterioscler Thromb
Vasc Biol. 29:1999–2004. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang L, Xu C, Yu S, Liu P, Luo D, Zhou Q,
Gao C and Hu H: A critical role of thrombin/PAR-1 in ADP-induced
platelet secretion and the second wave of aggregation. J Thromb
Haemost. 11:930–940. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fujimoto D, Hirono Y, Goi T, Katayama K,
Matsukawa S and Yamaguchi A: The activation of proteinase-activated
receptor-1 (PAR1) mediates gastric cancer cell proliferation and
invasion. BMC Cancer. 10:4432010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O’Keefe SC, Marshall FF, Issa MM, Harmon
MP and Petros JA: Thrombocytosis is associated with a significant
increase in the cancer specific death rate after radical
nephrectomy. J urol. 168:1378–1380. 2002. View Article : Google Scholar
|
|
30
|
Lim SH, Becker TM, Chua W, Caixeiro NJ, Ng
WL, Kienzle N, Tognela A, Lumba S, Rasko JE, de Souza P, et al:
Circulating tumour cells and circulating free nucleic acid as
prognostic and predictive biomarkers in colorectal cancer. Cancer
Lett. 346:24–33. 2014. View Article : Google Scholar
|
|
31
|
Almog N and Klement GL: Platelet proteome
and tumor dormancy: Can platelets content serve as predictive
biomarkers for exit of tumors from dormancy? Cancers. 2:842–858.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang K, Zhao X, Kuang C, Qian D, Wang H,
Jiang H, Deng M and Huang L: Overexpression of SDF-1α enhanced
migration and engraftment of cardiac stem cells and reduced
infarcted size via CXCR4/PI3K pathway. PLoS One. 7:e439222012.
View Article : Google Scholar
|
|
33
|
Zhou H, Hu H, Shi W, Ling S, Wang T and
Wang H: The expression and the functional roles of tissue factor
and protease-activated receptor-2 on SW620 cells. Oncol Rep.
20:1069–1076. 2008.PubMed/NCBI
|
|
34
|
Bambace NM and Holmes CE: The platelet
contribution to cancer progression. J Thromb Haemost. 9:237–249.
2011. View Article : Google Scholar
|
|
35
|
Hu L, Lee M, Campbell W, Perez-Soler R and
Karpatkin S: Role of endogenous thrombin in tumor implantation,
seeding, and spontaneous metastasis. Blood. 104:2746–2751. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nishimura S, Manabe I, Nagasaki M, Seo K,
Yamashita H, Hosoya Y, Ohsugi M, Tobe K, Kadowaki T, Nagai R, et
al: In vivo imaging in mice reveals local cell dynamics and
inflammation in obese adipose tissue. J Clin Invest. 118:710–721.
2008.PubMed/NCBI
|
|
37
|
Labelle M, Begum S and Hynes RO: Direct
signaling between platelets and cancer cells induces an
epithelial-mesenchymal-like transition and promotes metastasis.
Cancer Cell. 20:576–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bae E, Kim SJ, Hong S, Liu F and Ooshima
A: Smad3 linker phosphorylation attenuates Smad3 transcriptional
activity and TGF-β1/Smad3-induced epithelial-mesenchymal transition
in renal epithelial cells. Biochem Biophys Res Commun. 427:593–599.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xiong M, Jiang L, Zhou Y, Qiu W, Fang L,
Tan R, Wen P and Yang J: The miR-200 family regulates
TGF-β1-induced renal tubular epithelial to mesenchymal transition
through Smad pathway by targeting ZEB1 and ZEB2 expression. Am J
Physiol Renal Physiol. 302:F369–F379. 2012. View Article : Google Scholar
|
|
40
|
Brabletz S and Brabletz T: The ZEB/miR-200
feedback loop - a motor of cellular plasticity in development and
cancer? EMBO Rep. 11:670–677. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gawaz M, Langer H and May AE: Platelets in
inflammation and atherogenesis. J Clin Invest. 115:3378–3384. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Blair P and Flaumenhaft R: Platelet
α-granules: Basic biology and clinical correlates. Blood Rev.
23:177–189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rothwell PM, Wilson M, Elwin CE, Norrving
B, Algra A, Warlow CP and Meade TW: Long-term effect of aspirin on
colorectal cancer incidence and mortality: 20-year follow-up of
five randomised trials. Lancet. 376:1741–1750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin MS, Huang JX, Zhu J and Shen HZ:
Elevation of platelet count in patients with colorectal cancer
predicts tendency to metastases and poor prognosis.
Hepatogastroenterology. 59:1687–1690. 2012.PubMed/NCBI
|
|
45
|
Zhang SS, Han ZP, Jing YY, Tao SF, Li TJ,
Wang H, Wang Y, Li R, Yang Y, Zhao X, et al:
CD133+CXCR4+ colon cancer cells exhibit
metastatic potential and predict poor prognosis of patients. BMC
Med. 10:852012. View Article : Google Scholar
|
|
46
|
Balabanian K, Lagane B, Infantino S, Chow
KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M and
Bachelerie F: The chemokine SDF-1/CXCL12 binds to and signals
through the orphan receptor RDC1 in T lymphocytes. J Biol Chem.
280:35760–35766. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun YX, Schneider A, Jung Y, Wang J, Dai
J, Wang J, Cook K, Osman NI, Koh-Paige AJ, Shim H, et al: Skeletal
localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis
blocks prostate cancer metastasis and growth in osseous sites in
vivo. J Bone Miner Res. 20:318–329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu Q, Hou X, Xia J, Qian X, Miele L,
Sarkar FH and Wang Z: Emerging roles of PDGF-D in EMT progression
during tumorigenesis. Cancer Treat Rev. 39:640–646. 2013.
View Article : Google Scholar :
|
|
50
|
Devarajan E, Song YH, Krishnappa S and Alt
E: Epithelial-mesenchymal transition in breast cancer lines is
mediated through PDGF-D released by tissue-resident stem cells. Int
J Cancer. 131:1023–1031. 2012. View Article : Google Scholar
|