Introduction
Retinoblastoma is the most common and malignant
intra-ocular tumor in children (1).
Chemotherapy combined with local therapy has become the first-line
treatment for early retinoblastoma, and over 95% of patients with
retinoblastoma survive their malignancy in developed countries
(2). However, once extraocular
dissemination occurred, the rate of ocular salvage and patient
survival were low. The leading cause of treatment failure and
cancer-associated mortalities in retinoblastoma are chemoresistance
and metastasis to distant organs. This is particularly prominent in
developing countries due to the ignorance of the parents, and delay
in referral which is associated with delayed diagnosis and
treatment (3,4).
Hypoxia is a common feature of solid tumor including
retinoblastoma and responses of tumor cells to hypoxia are
important for tumor progression as well as tumor therapy. Previous
studies demonstrated that hypoxic responses of tumor cells were
involved in various biological processes including cellular
proliferation, apoptosis, chemotherapy resistance, angiogenesis and
migration (5–7). Thus, the presence of a hypoxic region
in retinoblastoma may be a common pathway mediating chemoresistance
and metastasis, rendering hypoxia a new therapeutic target. Hypoxic
responses are an outcome of the interplay between the tumor cells
and microenvironment. However, the exact mechanism was a
complicated process involving the coordination of different factors
that have not been adequately understood yet.
MicroRNAs (miRNAs) are a family of small non-coding
RNA oligonucleotides that regulate gene expression by binding to
sites in the 3′-untranslated region (3′-UTR) of the corresponding
mRNA. Dysregulation of miRNAs is a key process involved in each
stage of many cancer types from initiation to metastasis. Studies
have shown there is a functional link between hypoxia and miRNA
dysregulation (also termed hypoxia-regulated miRNAs, HRMs) which
plays an important role in cell survival in low oxygen environment
in cancer (8–10).
We identified miR-181b as an HRM in retinoblastoma
cells that stimulated proliferation of retinoblastoma cells in our
previous study (11). However, the
function of miR-181b in retinoblastoma has yet to be elucidated. In
the present study, we aim to investigate the regulatory mechanism
of miR-181b under hypoxic conditions and evaluate the possible
roles of miR-181b in hypoxic responses of retinoblastoma cells.
Materials and methods
Cell and culture
HXO-RB44 cells (human retinoblastoma cells) were
provided by Professor Heping Xu (Zhongnan University, China) and
human umbilical vein endothelial cells (HUVECs) were purchased from
the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). HXO-RB44 cells were cultured in RPMI-1640 medium
containing 10% fetal bovine serum (FBS) (both from Gibco-BRL, Grand
Island, NY, USA) in an incubator (37°C, 5% CO2). HUVECs
were grown in M-199 medium (Gibco-BRL) with 20% FBS, 100 U/ml
penicillin (Biological Industries, Bet Haemek, Israel) and 1 U/ml
fibroblast growth factor 2 (FGF2; Upstate Biotechnology, Lake
Placid, NY, USA) in a humidified incubator (37°C, 5%
CO2). To induce hypoxic conditions, the cells were
maintained in an InVivo200 hypoxia workstation (Biotrace
International, Ruskinn Life Sciences, UK) with a steady flow of gas
mixture (1% O2, 5% CO2 and 94%
N2).
Small-interfering RNA (siRNA)-mediated
hypoxia-inducible factor-1α (HIF-1α) silencing
siRNAs targeting HIF-1α and a scrambled sequence
(GenePharma, Shanghai, China) were transfected into HOX-RB44 cells
according to the manufacturer’s instructions. Briefly, the cells
were plated into 24-well plates (5×105 cells/well) with
FBS-free medium overnight. The cells were tranfected with siHIF-1α
and scrambled sequence using Lipofectamine 2000 reagent
(Invitrogen, Carlsbad, CA USA). After 6 h, the cells were re-fed
with fresh medium containing 10% FBS and experiments were performed
48 h later. Sequences for the siRNA of HIF-1α used in this study
were: siHIF-1α-816 forward, GCUCAAUUUAUGAAUAUUATT and reverse,
UAAUAUUCAUAAAUUGAGCGG; siHIF-1α-1171 forward, GAAGGAACCUGAUGCUUUATT
and reverse, UAAAGCAUCAGGUUCCUUCTT; siHIF-1α-1504 forward,
CGAUGGAAGCACUAGACAATT and reverse, UUGUCUAGUGCUUCCAUCGGA.
Detection of the expression of
HIF-1α
Total RNA was isolated from HXO-RB44 cells using
TRIzol reagent (Invitrogen) according to the manufacturer’s
instructions. RNA integrity was assessed using NanoDrop (Thermo
Fisher Scientific Inc., Wilmington, DE, USA). RNA (2 μg) was
reverse transcribed into single-strand DNA using the First Strand
Synthesis kit (Invitrogen). The resulting cDNA was used to perform
quantitative PCR with Rotor-Gene 3000 Real-Time PCR System (Corbett
Robotics, Australia) using SYBR-Green reporter dye (Invitrogen) and
β-actin was used for normalization. The PCR amplification protocol
was 95°C for 1 min followed by 40 cycles of 95°C for 15 sec, 62°C
for 15 sec, 72°C for 30 sec, then followed by 95°C for 15 sec, 60°C
for 1 min, 95°C for 30 sec and a 30-sec final extension at 37°C.
Specific primers (Sangon Biotech Inc., Shanghai, China) used for
HIF-1α were: forward, CGTTCCTTCGATCAGTTGTC and reverse,
TCAGTGGTGGCAGTGGTAGT. The primer sequences for β-actin were:
forward, CTCCATCCTGGCCTCGCTGT and reverse, GCTGTCACCTTCACCGTTCC.
The relative expression was analyzed using the 2−ΔΔCt
method and all the experiments were performed in triplicate.
Transfection of miRNAs
HXO-RB44 cells in the exponential growth phase were
seeded in 24-well plates (4×104 cells/well). miR-181b
precursor and inhibitor and the negative control (Ambion, Foster
City, CA, USA) were transfected into the cells at a final
oligonucleotide concentration of 30 pmol/well using Lipofectamine
2000 (Invitrogen) according to the manufacturer’s instructions. The
cells were incubated in a hypoxia workstation for 48 h prior to
experiments.
Detection of miR-181b by TaqMan
RT-PCR
Total RNA was extracted from cells using TRIzol
(Invitrogen). The RT and PCR reactions were performed using the
Hairpin-it™ miRNAs Real-Time PCR Quantitation kit (GenePharma,
Shanghai, China). For reverse transcription (RT), the reaction was
carried out as follows: 30 min at 16°C, 30 min at 42°C, 5 min at
85°C, and then kept at 4°C. The primer used for RT was
GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACACCCAC. The PCR
amplification was conducted as follows: 95°C for 1 min followed by
40 cycles of 95°C for 15 sec, 62°C for 15 sec, 72°C for 30 sec, and
a 30-sec final extension at 37°C. The relative amounts of miRNAs
were normalized against the U6 RNA using the 2−ΔΔCt
method, all experiments were performed in triplicate for each
sample. The primers used for PCR amplification were: miR-181b
forward, GGGAACATTCATTGCTG and reverse, CAGTGCGTGTCGTGGAGT; U6
forward, GCTTCGGCAGCACATATACTAAAAT and reverse,
CGCTTCACGAATTTGCGTGTCAT.
Tube formation assay
Matrigel-coated (BD Pharmingen, San Jose, CA, USA)
24-well plates were incubated at 37°C for 2 h prior to the
experiment. HUVECs were seeded onto the plates (4×104
cells/well) and cultured at 37°C for 6 h with i) M-199 medium in
normal conditions, ii) M-199 medium in hypoxic conditions and iii)
culture medium of pre-transfected retinoblastoma cells in hypoxic
conditions. The number of the capillary-like structures was scanned
and quantified under a light microscope (Zeiss, Aoboer Cohen,
Germany) in five lower-power fields (magnification, x100).
Cell cytotoxicity assay
Vincristine (VCR), etoposide VP-16 (VP-16) and
carboplatin were purchased from the Pharmaceutical Corporation of
Qilu, China. HXO-RB44 cells were seeded into 96-well plates
(6×105 cells/well) and treated with VCR (0.1, 1, 5, and
10 μg/ml), VP-16 (2, 20, 100, and 200 μg/ml) and
carboplatin (2, 20, 100, and 200 μg/ml) at different
concentrations, respectively. Wells with medium only were set as
blank and wells with cells but no drugs as control. After being
cultured under normal and hypoxic conditions for 72 h, cell
viability was determined by a CCK-8 Kit (Dojindo Laboratories,
Kumamoto, Japan) according to the manufacturer’s instructions. The
50% inhibitory concentration (IC50) for each drug was
calculated according to a dose-response curve by non-linear
regression analysis. Pre-transfected HXO-RB44 cells (miR-181b
precursor, and inhibitor and negative control) were treated with
drugs as mentioned above and the IC50 value was
calculated. The experiments were performed three times and three
parallel samples were measured each time.
Bioinformatics analysis of target genes
of miR-181b
Target genes of miR-181b were predicted by
retrieving the miRBase (http://microrna.sanger.ac.uk/), TargetScan (http://www.targetscan.org) and Pictar (http://pictar.mdcberlin.de) databases. Target
prediction was performed by applying the three algorithms and two
experimentally validated databases: TarBase and miRecords
(http://mirsystem.cgm.ntu.edu.tw/)
provided by the miRSystem. Targets receiving more than three
positive votes were selected as miR-181b targets for subsequent
analysis to reduce the quantity of false-positive results.
Reverse transcription and PCR
amplification
Extraction and assessment of RNA were performed as
described above. RNA was reverse transcribed with reverse
transcriptase to synthesize the cDNA using a random primer. Two
micrograms of total RNA in a 25-μl reaction was carried out
at 42°C for 60 min, then the reaction mixture was incubated at 95°C
to inactivate the reverse transcriptase and denature the template.
The PCR reaction was conducted in a final reaction volume of 25
μl containing 2 μl of cDNA, 1 μl of
gene-specific primers (10 μM), 0.2 μl of dNTPs (25
mM), 1 μl Taq DNA polymerase (1 U/μl) and 2.5
μl of 10X buffer. The cycling conditions for the PCR
reaction were: 95°C for 10 min, followed by 35 cycles of 95°C for
20 sec, 60°C for 20 sec, 72°C for 30 sec, then followed by 72°C for
7 min and then kept at 4°C. The amplified products were visualized
using 2% agarose gel and ethidium bromide under UV
transillumination. Expression was considered positive if the
expected band of gene was observed. The gene-specific primers used
for PCR were: visinin-like 1 (VSNL1) forward, ATGGGGAAACAGAATAGCAA
and reverse, TCATTTCTGAATGTCACACTG; pleiomorphic adenoma gene 1
(PLAG1) forward, CAAGATTCTCAAGCAT CGTCA and reverse,
TCCAAGGCTCCCCACTG; glutamate receptor 1 (GRIA1) forward,
AAATCTACAGCAATGCTGGCGA and reverse, CTTCGATGACTTCTCTGTC; protease-
activated receptors 4 (PAR-4) forward, GGGACCTCGGAACTCAAC
and reverse, TGTATCTGCCTGGGACTG; programmed cell death-10
(PDCD10) forward, CCTAAACGAAAAGGCACGAG and reverse,
GCCCTGCGGTTCTGGTA; T-cell leukemia/lymphoma 1 (TCL1)
forward, ATCATCGAGCTCCAGGCTGGAGCTGGTTTCCATG and reverse,
ATCATCAGATCTCGTCCAAATACACGAACTTCTCCC; E2F transcription factor 5
(E2F5) forward, ATCCAGCATGGCAACTCAA and reverse,
TCATCTGCCGGGGTAGGAG; GATA binding protein 6 (GATA6) forward,
CTCCAACTTCCACCTCTTCTAAC and reverse, GCCCATCTTGACCCGAAT.
Western blotting
The cells were incubated with buffer containing
protease inhibitor cocktail (0.01 mg/ml of aprotinin, pepstatin A
and leupeptin) and PMSF (Sigma-Aldrich, St. Louis, MO, USA) at 4°C
for 5 min. The cells were lysed by sonicator (Episonic, Santa Ana,
CA, USA) with 25% power for 1 min, then centrifuged at 4°C (50,000
× g for 20 min) and the supernatant was transferred to a new
sterile 1.5 ml EP tube. Protein concentrations were measured using
the BCA Protein Assay kit (Bio-Rad, Hercules, CA, USA). The western
blot analysis was subsequently performed. Briefly, 50 μg of
total protein was separated by SDS-PAGE on 10% gel and transferred
onto PVDF membranes (Millipore, Billerica, MA, USA) at 200 mA for
1.5 h. The membranes were incubated with primary antibodies (1:50
in 1% BSA/TBST) overnight at 4°C. The primary antibodies (mouse
monoclonal) against PDCD10, GATA6, PLAG1 and E2F5 were all
purchased from Bioworld (St. Louis, MN, USA). The membranes were
incubated with secondary antibodies [1:5,000 in 1% BSA/TBST, goat
anti-mouse IRDye 800CW, LI-COR Biosciences (Lincoln, Ne, USA)] for
1 h at room temperature. The immunoreactivity of proteins was
detected using an ECL reagent (Millipore). The mean density of each
band was quantified using Image J software with tubulin
(Sigma-Aldrich; 1:1,000 in 1% BSA/TBST), used as an internal
control.
Statistical analysis
Data were presented as means ± SD if not specified
ortherwise. Statistical analysis was performed using SPSS 15.0
(SPSS Inc., Chicago, IL, USA). The difference between groups was
analyzed using the unpaired Student’s t-test (only two groups) or
one-way analysis of variance (three or more groups) with
significance accepted as P<0.05.
Results
Upregulation of miR-181b in
retinoblastoma cells under hypoxia is HIF-1α independent
Our previous findings (11) confirmed that hypoxia upregulated the
expression of miR-181b in retinoblastoma cells. Since HIF-1α is the
master regulator of transcription factors under hypoxic conditions,
we aimed to determine whether the expression of miR-181b is
regulated by HIF-1α. We detected the expression of HIF-1α in
retinoblastoma cells under normal and hypoxic conditions,
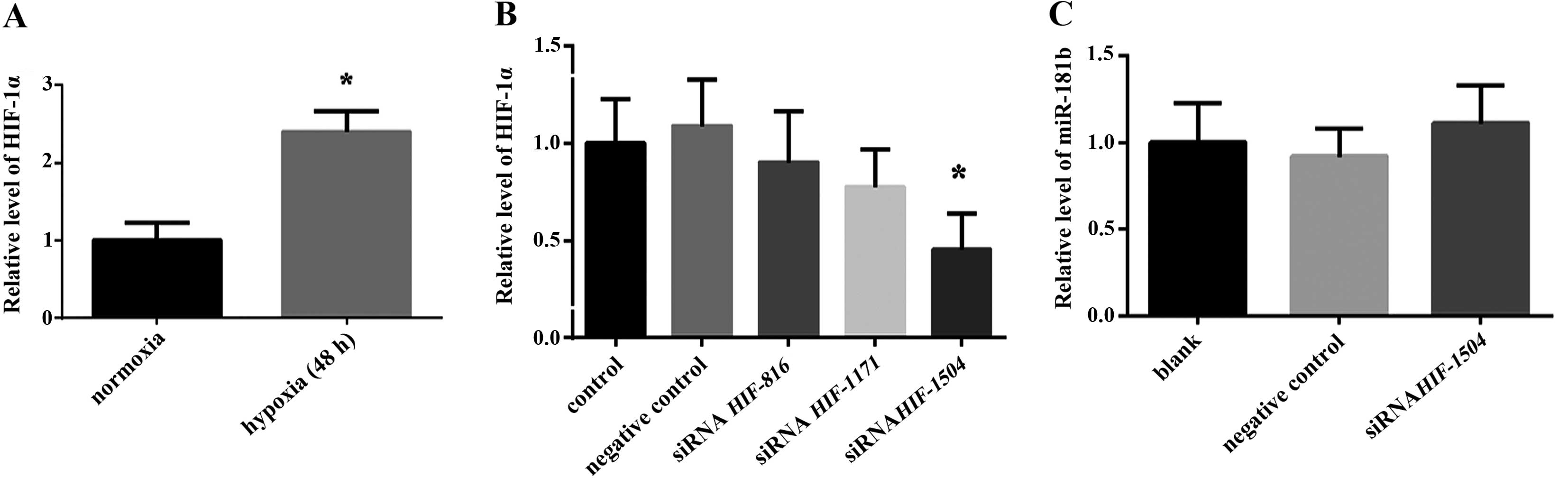
respectively, and found a 2.4-fold increased expression of HIF-1α
under hypoxia as compared to normal conditions (Fig. 1A). To better understand the
connection between HIF-1α and miR-181b, we reduced the level of
HIF-1α using siRNA technology. In order to silence the target gene
more effectively, we designed three pairs of different sequences of
siRNA (HIF-1α-816 targeted 816–836 sites, HIF-1α-1171 targeted
1171–1191 sites and HIF-1α-1504 targeted 1504-1524 sites).
Quantitative PCR results showed HIF-1α-1504 reduced the level of
HIF-1α >50% (Fig. 1B). However,
the expression of HIF-1α was largely blocked by siHIF-1α-1504, and
no significant change of the expression of miR-181b (Fig. 1C) was identified, indicating that
the increased expression of miR-181b under hypoxia was not
regulated by HIF-1α.
Hypoxia enhances the capacity of tube
formation of HUVECs
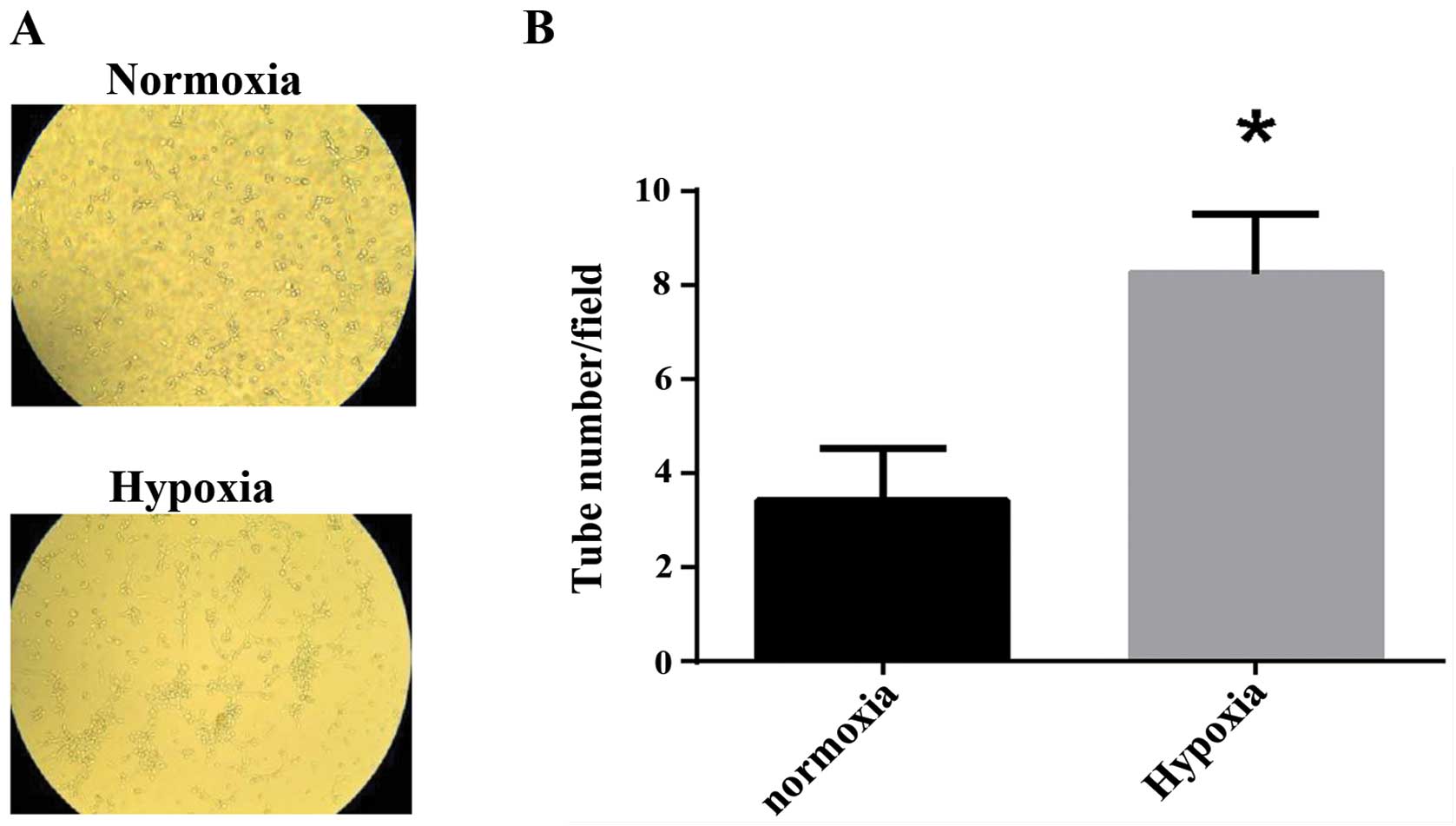
To examine the effect of hypoxia on the ability of
tube formation of HUVECs, HUVECs were exposed to normoxia or
hypoxia for 6 h, and the capillary-like structures were calculated.
The results showed HUVECs formed more capillary-like structures
under hypoxia than under normoxia (Fig.
2).
miR-181b significantly promotes capillary
tube formation of HUVECs under hypoxia
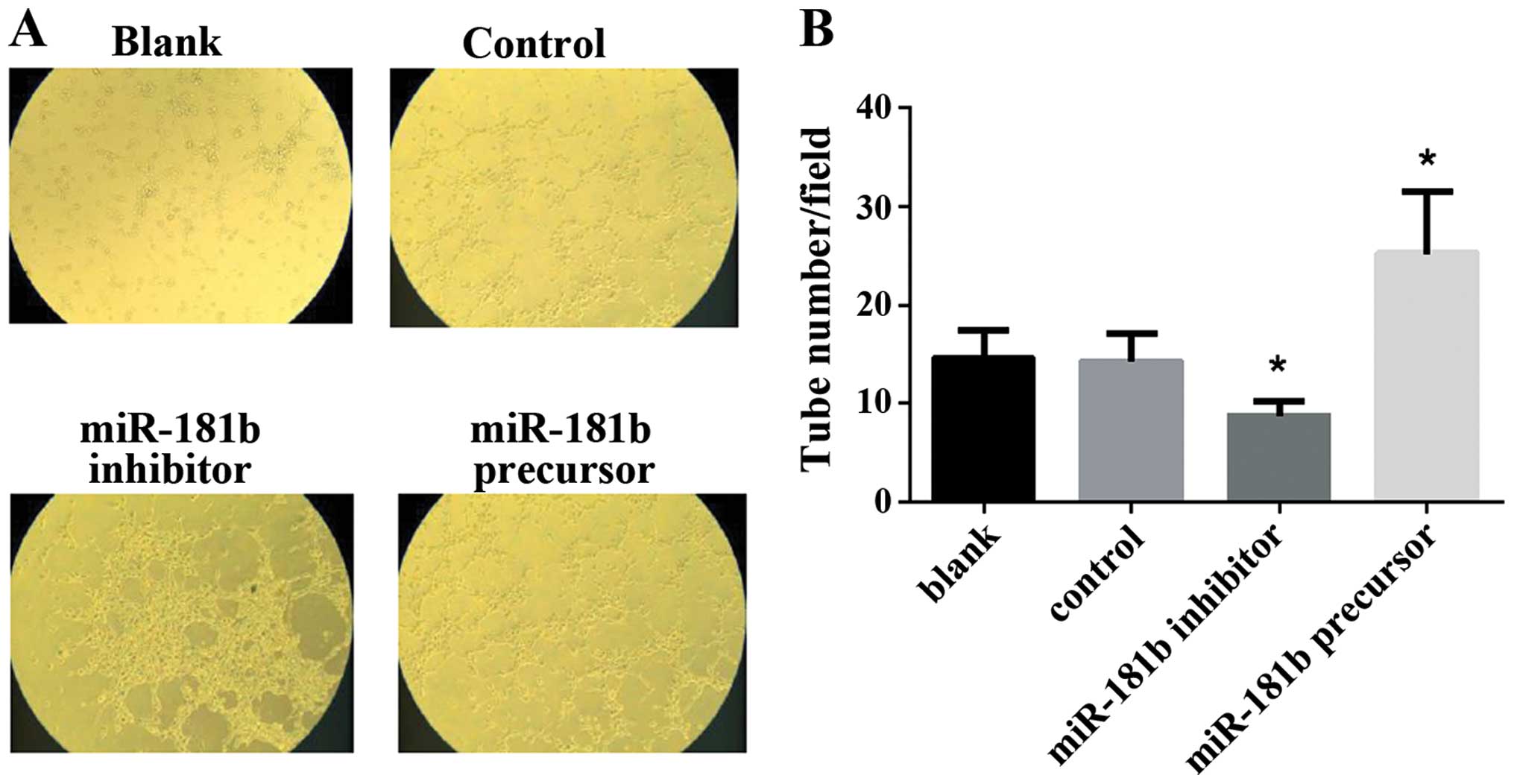
To examine the biological mechanism of miR-181b in
tumor-hypoxic responses, we further detected the effect of miR-181b
on the ability of capillary tube formation of HUVECs in
vitro. Retinoblastoma cells were transfected with miR-181b
precursor and inhibitor to upregulate or down-regulate their
miR-181b levels. Their culture medium was added to HUVECs. The
culture medium of miR-181b precursor-transfected retinoblastoma
cells significantly increased the capillary tube formation of
HUVECs. By contrast, the medium of miR-181b inhibitor led to the
suppression of tube formation of HUVECs. The results suggested that
miR-181b enhanced the angiogenesis of HUVECs in vitro
(Fig. 3).
Induced chemoresistance to VP-16 by
hypoxia is independent of miR-181b in retinoblastoma cells
To elucidate the possible role of miR-181b as a
hypoxic responses regulator, we examined the effect of miR-181b on
chemoresistance in retinoblastoma cells. VCR, VP-16 and carboplatin
are the most widely used chemotherapeutic agents in retinoblastoma.
The regimen containing these three drugs has become the first-line
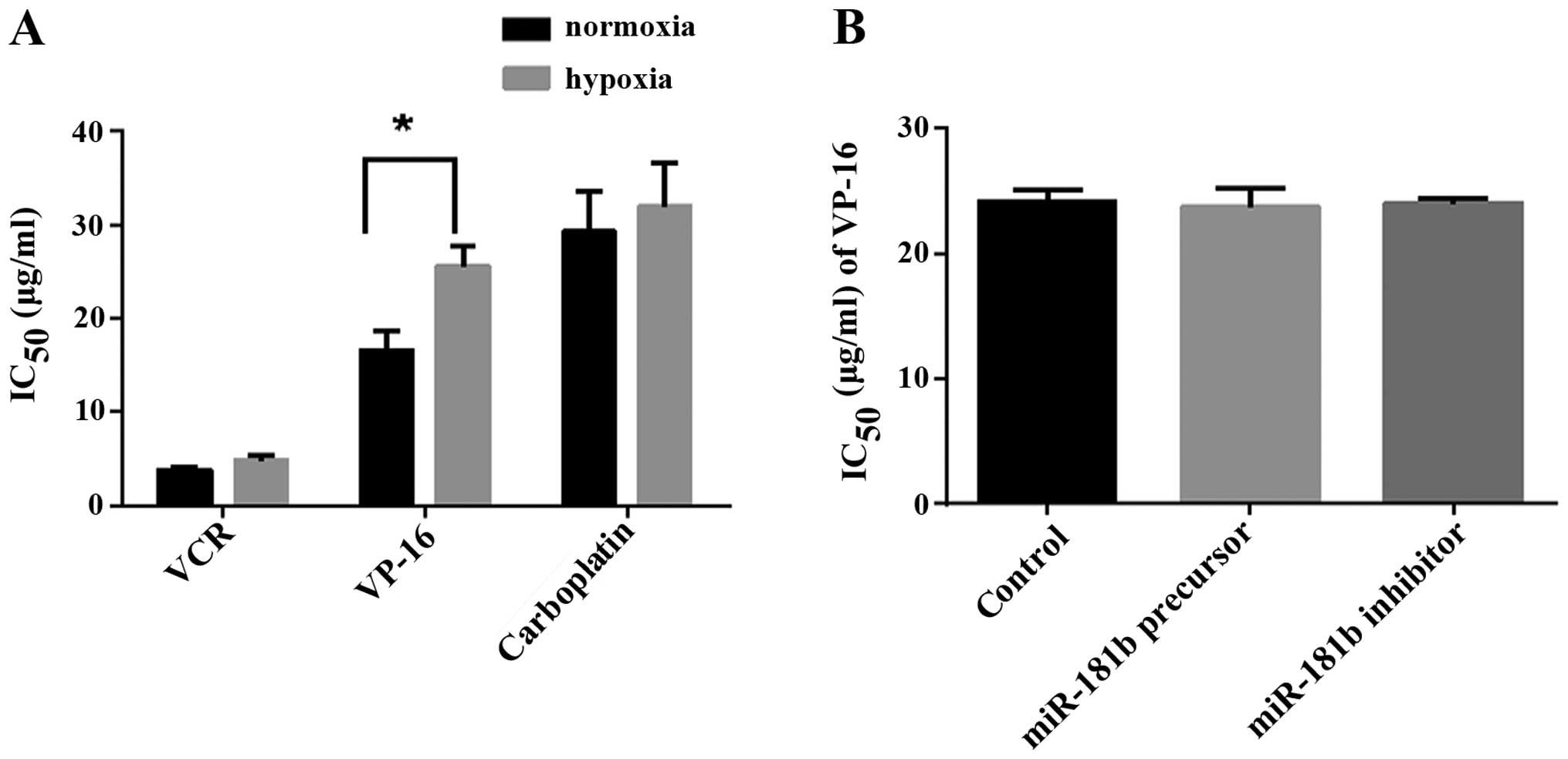
therapy for retinoblastoma in recent years. We used IC50
which describes the concentration of the drugs that lead to
inhibition of cell growth in 50% of the treated cells to represent
the chemoresistance. If a drug has a higher IC50,
stronger cell resistance to drugs should be considered. When
exposed to hypoxic conditions for 72 h, the IC50 values
of three drugs were higher than those of the normal conditions,
with only the difference of VP-16 (25.66 vs. 16.57 μg/ml)
being statistically significant (Fig.
4A). It meant that hypoxia was one of the incentives of
acquired resistance of VP-16. We further characterized the role of
miR-181b in regulating hypoxia-induced chemoresistance of VP-16 by
modulating its levels in retinoblastoma cells. But transient
transfection of miR-181b precursor and inhibitor did not show any
effect on the IC50 of VP-16 compared to control (23.76,
24.03 and 24.17 μg/ml, respectively, Fig. 4B). The date indicated
hypoxia-induced chemoresistance of VP-16 in retinoblastoma cells
was miR-181b-independent.
Prediction of target genes of
miR181b
The conservation of the miRNA binding sites in the
3′-UTR of the orthologous genes is a significant feature in
predicting miRNA targets. It is conceivable that the use of
multiple prediction tools lead to a higher confidence in predicting
miRNA target gene pairs. We adopted a consensus target prediction
approach of using multiple miRNA target prediction databases:
miRBase, TargetScan and PicTar to identify target genes of
miR-181b. Eight genes contained predicted binding site for miR-181b
at 3′-UTR (Fig. 5). Further
investigation was performed to exclude the false-positive. We
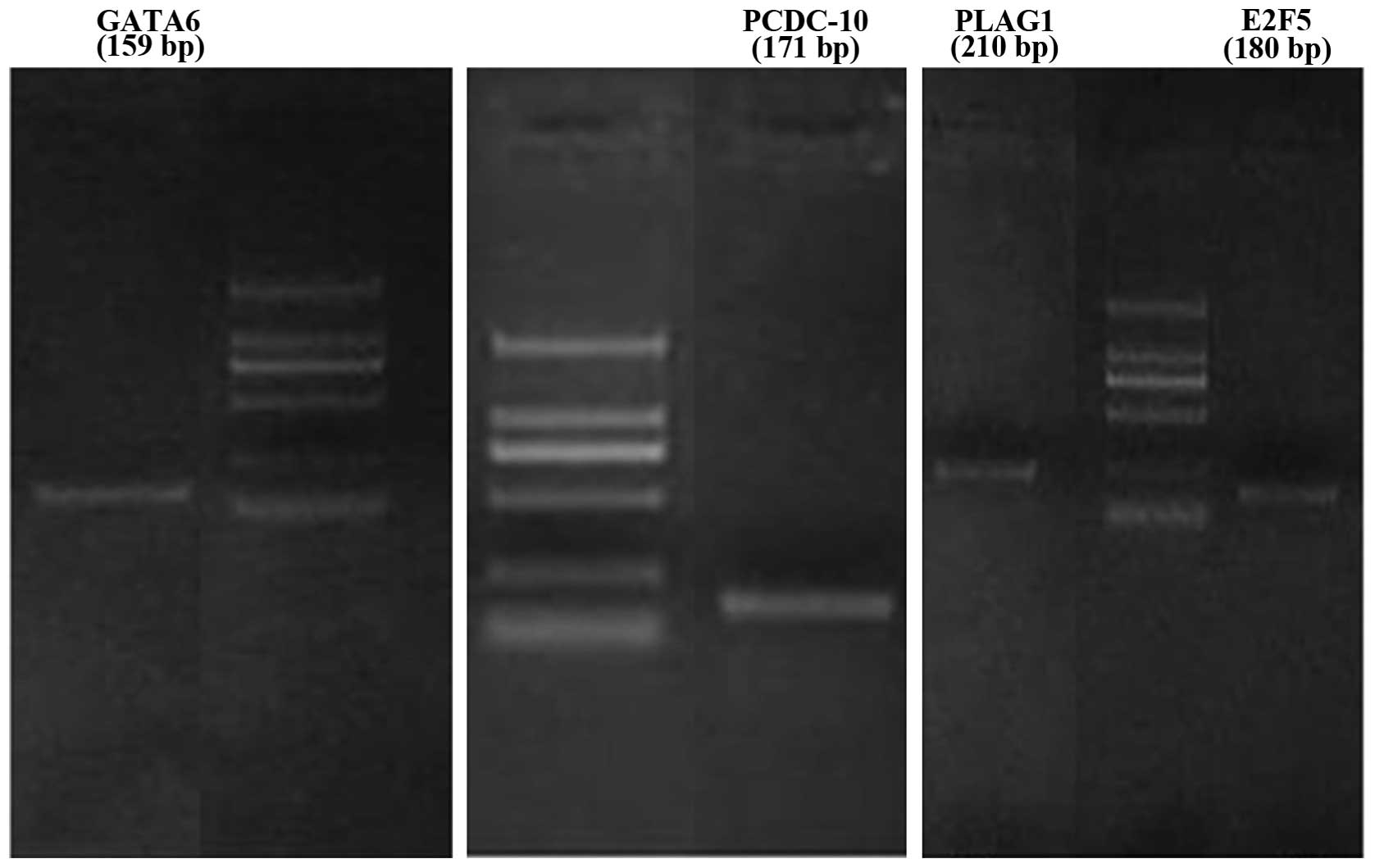
detected the mRNA level of these genes and found GATA6,
PDCD10, PLAG1 and E2F5 were steadily expressed
in retinoblastoma cells (Fig. 6).
The four genes were selected as candidates of miR-181b.
miR-181b inhibits PDCD10 and GATA6
production in retinoblastoma cells
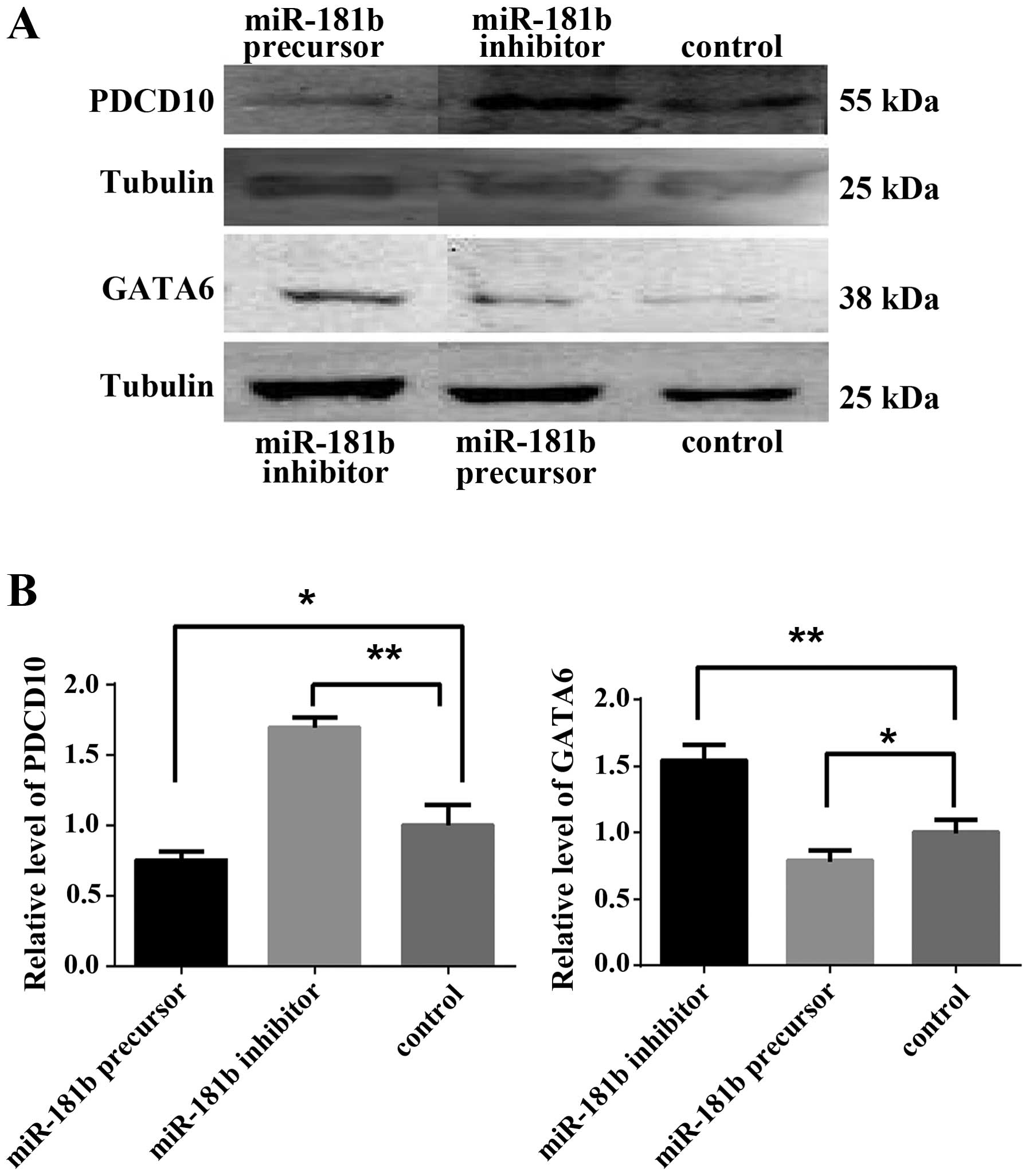
We examined the regulation of miR-181b on the
expression of the four genes mentioned above by western blotting.
The results showed that pretreatment with miR-181b precursor
decreased PDCD10 and GATA6 expression while pretreated miR-181b
inhibitor increased PDCD10 and GATA6 expression (Fig. 7). These results suggested that
miR-181b acted as a negative regulator of PDCD10 and GATA6 under
hypoxia in retinoblastoma cells.
Discussion
Hypoxia is an important pathological process of
solid tumors, and hypoxic regions are present in retinoblastoma
(12). The findings of our previous
study showed that miR-181b was upregulated by hypoxia in
retinoblastoma cells, although the molecular mechanisms of
responses to hypoxia remained unknown. HIF-1 is the most
studied gene that plays a central role in cancer hypoxic responses
(13). HIF-1 is composed of HIF1α
and HIF1β subunits. HIF-1α is considered as the most important
regulator under hypoxic conditions in various solid tumors and a
high expression of HIF-1α was confirmed in the progression of
retinoblastoma (14–16). Many miRNAs, such as miR-21, -210,
-103 and -195 were reported to be induced and upregulated by HIF-1α
in cancer cells under hypoxic conditions (17,18).
Thus, whether miR-181b is also a controlled candidate of HIF-1α
remains to be determined. Results of this study showed that the
expression of HIF-1α in retinoblastoma cells was elevated
substantially following exposure to hypoxia for 48 h. When using
HIF-1α siRNA to reduce the level of HIF-1α, the repression of
miR-181b was not observed synchronously. These results suggested
the expression of miR-181b under hypoxia was not regulated by
HIF-1α. A possible explanation for this finding is that HIF-1α may
be crucial under hypoxic conditions but it is not the single
regulatory factor. Mounting evidence has indicated HIF alone is
insufficient to implement the full program of adaptive changes of
cancer under hypoxic stress. Recently, transcription factors
responding to hypoxia, such as p53 and NF-κB, have been shown to
affect the expression of selected miRNAs (19–21).
Instead, mTOR, endoplasmic reticulum (ER) stress were also reported
to be involved in hypoxic responses via HIF-independent pathways
(22,23). It seems that miRNAs interface with
both HIF-driven and -independent pathways to form an interconnected
regulatory network under hypoxic stress. We have demonstrated that
miR-181b transcriptional upregulation in hypoxia in retinoblastoma
is HIF-1α-independent. However, additional studies are needed to
identify the network between miR-181b and hypoxia in
retinoblastoma.
miR181-b is a type of miRNA endogenously expressed
in cells of the inner and middle retina. Dysregulated expression of
miR-181b has been demonstrated in different tumors (24,25).
Retinoblastoma is a malignancy originating from the retina.
However, to the best of our knowledge, there are no reports
available concerning the possible roles of miR-181b in the
development of retinoblastoma. We have identified miR-181b as an
HRM of retinoblastoma in a previous study (11). Since chemoresistance and
angiogenesis were main responses to hypoxia in many tumors
including retinoblastoma, we examined the functions of miR-181b in
hypoxic responses of retinoblastoma. Firstly, we determined the
influence of hypoxia on the ability of tube formation of HUVECs and
found HUVECs formed more capillary tubes under hypoxic as compared
to normal conditions. Subsequently, through up- and downregulation
of miR-181b expression in retinoblastoma cells, we demonstrated
that miR-181b enhanced the ability of capillary tube formation of
HUVECs in vitro. Additionally, we identified that
retinoblastoma cells showed a stronger resistance to VP-16 in
hypoxic conditions, although the same chemoresistance was not
observed in VCR and carboplatin (these three were the most
popularly used chemotherapy drugs in retinoblastoma). Further
investigation suggested the chemoresistance induced by hypoxia was
miR-181b-independent. These results, however, were not consistent
with those of previous studies and there is controversy regarding
the roles of miR-181b in human malignancies. For instance, Sun
et al reported overexpression of miR-181b increased the
sensitivity of glioma cells to teniposide, while Takiuchi et
al found miR-181b led to the resistance of pancreatic cancer
cells to gemcitabine by activating NF-κB (26,27).
These discordant findings suggested that miR-181b may play
organ-specific roles in part due to the different cell contexts of
tumors and pharmacological characteristics. To the best of our
knowledge, the present study provides the first evidence that
hypoxia-induced miR-181b may play a critical role in angiogenesis
and metastasis in retinoblastoma.
To gain insight into the molecular mechanism
involved in the function of miR-181b, we predicted eight target
genes of miR-181b using bioinformatics analysis. This prediction
was further confirmed by evaluating the effects of up- and
downregulation of miR-181b on specific target gene expression in
retinoblastoma cells. We also determined PDCD10 and
GATA6 as downstream genes of miR-181b. PDCD10 (also known as
cerebral cavernous malformation 3, CCM3) was expressed in
endothelial cells and was essential for vascular development
(28). Previous results showed
silencing CCM3 or loss of CCM3 (decreased expression of CCM3)
stimulated sprouting and tube branching or activated endothelial
angiogenesis (29–31). These data indicated the roles of
PDCD10 as an anti-angiogenic transcription factor in vascular
development/maturation. GATA6 is a transcription factor belonging
to the GATA family which controls the development and
differentiation of a wide spectrum of cell lineages especially in
HUVECs. Considerable evidence suggests the importance of GATA6 in
the regulation of vascular endothelial cells, which enables
angiogenic function and endothelial cell survival (32). GATA gene families may be involved in
regulating homeostasis of the eye vasculature (33). Evidence also suggested GATA6 as a
target for repression by miR-181s (34). In agreement with the abovementioned
results, we found that upregulation of miR-181b in retinoblastoma
cells inhibited the expression of PDCD10 and GATA6, weakened the
anti-angiogenic functions of PDCD10 and GATA6, and promoted the
angiogenesis of retinoblastoma cells.
In conclusion, there were three important results in
the present study: hypoxia-induced overexpression of miR-181b in
retinoblastoma cells is HIF-1α independent; miR-181b significantly
increased the ability of capillary tube formation of HUVECs; and
the enhanced effects of miR-181b on angiogenesis may be due to the
decreased expression of PDCD10 and GATA6. Our results suggest that
miR-181b acts as an oncogenic miRNA in retinoblastoma cells to
promote metastasis under a hypoxic microenvironment. These results
add to the mounting evidence that miR-181b is crucial in promoting
cancer development and may assist in the development of new
therapeutic regimens against hypoxic tumors.
Acknowledgments
This study was supported by the Scientific Research
Program of the National Health and Family Planning Commission of
China (no. 2014040), the National Nature Science Foundation of
China (nos. 81372469 and 81372909), and the Science and Technology
Commission of Shanghai (nos. 12ZR1417300 and 13JC1406202).
References
|
1
|
Kivelä T: The epidemiological challenge of
the most frequent eye cancer: retinoblastoma, an issue of birth and
death. Br J Ophthalmol. 93:1129–1131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chintagumpala M, Chevez-Barrios P, Paysse
EA, Plon SE and Hurwitz R: Retinoblastoma: review of current
management. Oncologist. 12:1237–1246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chantada GL, Qaddoumi I, Canturk S, Khetan
V, Ma Z, Kimani K, Yeniad B, Sultan I, Sitorus RS, Tacyildiz N, et
al: Strategies to manage retinoblastoma in developing countries.
Pediatr Blood Cancer. 56:341–348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Canturk S, Qaddoumi I, Khetan V, Ma Z,
Furmanchuk A, Antoneli CB, Sultan I, Kebudi R, Sharma T,
Rodriguez-Galindo C, et al: Survival of retinoblastoma in
less-developed countries impact of socioeconomic and health-related
indicators. Br J Ophthalmol. 94:1432–1436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee YJ, Lee JH, Moon JH and Park SY:
Overcoming hypoxic resistance of tumor cells to TRAIL-induced
apoptosis through melatonin. Int J Mol Sci. 15:11941–11956. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gruber M and Simon MC: Hypoxia-inducible
factors, hypoxia, and tumor angiogenesis. Curr Opin Hematol.
13:169–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Greco S and Martelli F: MicroRNAs in
hypoxia response. Antioxid Redox Signal. 21:1164–1166. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McCarthy N: Hypoxia: micro changes. Nat
Rev Cancer. 14:382–383. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qin Q, Furong W and Baosheng L: Multiple
functions of hypoxia-regulated miR-210 in cancer. J Exp Clin Cancer
Res. 33:502014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu X, Jia R, Zhou Y, Song X, Wang J, Qian
G, Ge S and Fan X: Microarray-based analysis: Identification of
hypoxia-regulated microRNAs in retinoblastoma cells. Int J oncol.
38:1385–1393. 2011.PubMed/NCBI
|
|
12
|
Boutrid H, Jockovich ME, Murray TG, Piña
Y, Feuer WJ, Lampidis TJ and Cebulla CM: Targeting hypoxia, a novel
treatment for advanced retinoblastoma. Invest Ophthalmol Vis Sci.
49:2799–2805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lan KL, Lan KH, Sheu ML, Chen MY, Shih YS,
Hsu FC, Wang HM, Liu RS and Yen SH: Honokiol inhibits hypoxia-
inducible factor-1 pathway. Int J Radiat Biol. 87:579–590. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai YP and Wu KJ: Hypoxia-regulated
target genes implicated in tumor metastasis. J Biomed Sci.
19:1022012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Voss MJ, Niggemann B, Zänker KS and
Entschladen F: Tumour reactions to hypoxia. Curr Mol Med.
10:381–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sudhakar J, Venkatesan N, Lakshmanan S,
Khetan V, Krishnakumar S and Biswas J: Hypoxic tumor
microenvironment in advanced retinoblastoma. Pediatr Blood Cancer.
60:1598–1601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bao B, Ali S, Ahmad A, Azmi AS, Li Y,
Banerjee S, Kong D, Sethi S, Aboukameel A, Padhye SB, et al:
Hypoxia-induced aggressiveness of pancreatic cancer cells is due to
increased expression of VEGF, IL-6 and miR-21, which can be
attenuated by CDF treatment. PLoS One. 7:e501652012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kulshreshtha R, Ferracin M, Wojcik SE,
Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM,
Negrini M, et al: A microRNA signature of hypoxia. Mol Cell Biol.
27:1859–1867. 2007. View Article : Google Scholar :
|
|
19
|
He L, He X, Lim LP, De Stanchina E, Xuan
Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sermeus A and Michiels C: Reciprocal
influence of the p53 and the hypoxic pathways. Cell Death Dis.
2:e1642011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kluiver J, van den Berg A, De Jong D,
Blokzijl T, Harms G, Bouwman E, Jacobs S, Poppema S and Kroesen BJ:
Regulation of pri-microRNA BIC transcription and processing in
Burkitt lymphoma. Oncogene. 26:3769–3776. 2007. View Article : Google Scholar
|
|
22
|
Koritzinsky M, Magagnin MG, van den
Beucken T, Seigneuric R, Savelkouls K, Dostie J, Pyronnet S,
Kaufman RJ, Weppler SA, Voncken JW, et al: Gene expression during
acute and prolonged hypoxia is regulated by distinct mechanisms of
translational control. EMBO J. 25:1114–1125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao L and Ackerman SL: Endoplasmic
reticulum stress in health and disease. Curr Opin Cell Biol.
18:444–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xi Y, Formentini A, Chien M, Weir DB,
Russo JJ and Ju J, Kornmann M and Ju J: Prognostic values of
microRNAs in colorectal cancer. Biomark Insights. 2:113–121.
2006.
|
|
25
|
Nakajima G, Hayashi K, Xi Y, Kudo K,
Uchida K, Takasaki K, Yamamoto M and Ju J: Non-coding MicroRNAs
hsa-let-7g and hsa-miR-181b are associated with chemoresponse to
S-1 in colon cancer. Cancer Genomics Proteomics. 3:317–324.
2006.
|
|
26
|
Sun YC, Wang J, Guo CC, Sai K, Wang J,
Chen FR, Yang QY, Chen YS, Wang J, To TS, et al: miR-181b
sensitizes glioma cells to teniposide by targeting MDM2. BMC
Cancer. 14:6112014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takiuchi D, Eguchi H, Nagano H, Iwagami Y,
Tomimaru Y, Wada H, Kawamoto K, Kobayashi S, Marubashi S, Tanemura
M, et al: Involvement of microRNA-181b in the gemcitabine
resistance of pancreatic cancer cells. Pancreatology. 13:517–523.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He Y, Zhang H, Yu L, Gunel M, Boggon TJ,
Chen H and Min W: Stabilization of VEGFR2 signaling by cerebral
cavernous malformation 3 is critical for vascular development. Sci
Signal. 3:ra262010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu Y, Wu Q, Xu JF, Miller D, Sandalcioglu
IE, Zhang JM and Sure U: Differential angiogenesis function of CCM2
and CCM3 in cerebral cavernous malformations. Neurosurg Focus.
29:E12010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schleider E, Stahl S, Wüstehube J, Walter
U, Fischer A and Felbor U: Evidence for anti-angiogenic and
pro-survival functions of the cerebral cavernous malformation
protein 3. Neurogenetics. 12:83–86. 2011. View Article : Google Scholar :
|
|
31
|
You C, Sandalcioglu IE, Dammann P, Felbor
U, Sure U and Zhu Y: Loss of CCM3 impairs DLL4-Notch signalling:
implication in endothelial angiogenesis and in inherited cerebral
cavernous malformations. J Cell Mol Med. 17:407–418. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perlman H, Suzuki E, Simonson M, Smith RC
and Walsh K: GATA-6 induces p21(Cip1) expression and G1 cell cycle
arrest. J Biol Chem. 273:13713–13718. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Crawford SE, Qi C, Misra P, Stellmach V,
Rao MS, Engel JD, Zhu Y and Reddy JK: Defects of the heart, eye,
and megakaryocytes in peroxisome proliferator activator
receptor-binding protein (PBP) null embryos implicate GATA family
of transcription factors. J Biol Chem. 277:3585–3592. 2002.
View Article : Google Scholar
|
|
34
|
Ji J, Yamashita T, Budhu A, Forgues M, Jia
HL, Li C, Deng C, Wauthier E, Reid LM, Ye QH, et al: Identification
of microRNA-181 by genome-wide screening as a critical player in
EpCAM-positive hepatic cancer stem cells. Hepatology. 50:472–480.
2009. View Article : Google Scholar : PubMed/NCBI
|