Introduction
Infantile hemangiomas are a type of benign tumors
with high incidence in infancy (1).
Typically, the lesions undergo a rapid proliferative phase during
early infancy and gradually involute over the first few years
(2). Although infantile hemangiomas
are benign tumors and usually harmless, they can cause destruction
and deformation of facial features, obstruction of breathing and
vision, and even more life-threatening complications (3–5).
However, the molecular pathogenesis of infantile hemangiomas
remains largely unknown and effective treatment still needs to be
developed.
Corticosteroids, as well as other drugs such as
interferon α and vincristine, have been traditionally regarded as
the first-line therapeutic approaches for the treatment of
infantile hemangiomas (6–10). However, these drugs are accompanied
by multiple and serious side-effects that are very painful for the
infants (11–13). Recently, a serendipitous discovery
found that propranolol effectively regresses infantile hemangiomas
when used to treat obstructive hypertrophic cardiomyopathy
accompanied by an infantile hemangioma (14). Propranolol is widely used to treat
infantile hemangiomas with satisfactory outcomes and no obviously
serious side-effects (15–19). Furthermore, infantile hemangiomas
resistant to corticosteroids and interferon or severe infantile
hemangiomas may be effectively treated by propranolol (20,21).
It has been suggested that propranolol exerts its effects as a
non-selective β-adrenergic receptor (AR) blocker that inhibits cell
growth and induces cell apoptosis of the endothelial cells
(21,22). However, the precise molecular
mechanism of its action remains poorly understood.
In recent years, the role of β-AR in tumorigenesis
has attracted increasing attention, which is associated with cell
proliferation, apoptosis, invasion and metastasis of tumor cells
(23). β-AR, including β1-AR and
β2-AR, are G-protein-coupled receptors on endothelial cells that
cause vasodilation of vessels upon activation (24). Moreover, activation of β-AR results
in overexpression of the proangiogenic factors, including basic
fibroblast growth factor and endothelial growth factor (VEGF) and
inhibits cell apoptosis (21,22,25).
It has been reported that β-AR inhibitors inhibit cell growth and
angiogenesis and enhance cell apoptosis of tumors, thereby
representing novel antitumor drugs (23). Hypoxia-inducible factor (HIF)-1
consists of two subunits, HIF-1α and HIF-1β, and plays an important
role in regulating tumor progression (26,27).
Of these two subunits, it has been suggested that HIF-1α is the
major regulator for angiogenic factors such as VEGF (27). However, whether propranolol exerts
its effects by regulating HIF-1α-mediated signaling needs to be
further investigated.
In the present study, we speculated that
β-AR-mediated and HIF-1α signaling may be associated with the
therapeutic effects of propranolol in the treatment of infantile
hemangiomas. We found that the elevated HIF-1α expression levels in
infantile hemangioma patients were downregulated by propranolol.
Using the hemangioma-derived endothelial cell line, propranolol was
found to reduce the expression of HIF-1α in a dose- and
time-dependent manner. Our results further demonstrated that
propranolol inhibited HIF-1α expression through its action on
β2-AR. Moreover, we revealed that propranolol suppressed cell
growth by inhibiting HIF-1α-VEGF signaling. Additionally, the
signal transducer and activator of transcription 3 (STAT3) and
Bcl-2, which are critical oncogenic signaling molecules, were found
to be increased in infantile hemangiomas, whereas propranolol was
found to inhibit STAT3 and Bcl-2 expressions in a HIF-1α-dependent
manner. Overexpression of HIF-1α attenuated the therapeutic effects
of propranolol on hemangiomas in a mouse model, which further
confirmed that propranolol regressed infantile hemangiomas in a
HIF-1α-dependent manner. Collectively, we represent a potential
mechanism of propranolol in which propranolol represses infantile
hemangioma cell growth by inhibiting VEGF, STAT3 and Bcl-2
expression in a HIF-1α-dependent manner, which leads to cell growth
arrest and the induction of cell apoptosis.
Materials and methods
Sample collections
A total of 11 infantile hemangioma patients were
recruited in the present study from June 2012 to May 2013 at the
Second Affiliated Hospital of Xi’an Jiaotong University. The
patients were orally treated with propranolol (Tianjin Lisheng
Pharmaceutical Co., Ltd., Tianjing, China). The patients had
received no surgery or drug treatments prior to the propranolol
treatment. Before and after the propranolol treatment, venous
blood, urine and tumor tissues (d=2 mm) were collected with the
informed consent of the family members and the approval of the
Ethics Committee of the Second Affiliated Hospital of Xi’an
Jiaotong University.
Animals and cell culture
Six-week-old female BAlB/c nude mice (20–30 g) were
provided by the Experimental Animal Centre of the College of
Medicine of Xi’an Jiaotong University. The mice were housed in a
standard pathogen-free room according to the standard feeding
protocols and the animal experimental procedures were handled in
accordance with the Institutional Animal Care and Use Committee of
Xi’an Jiaotong University. The hemangioma-derived endothelial cell
line from proliferating infantile hemangioma tissues was previously
prepared and established in our laboratory (28). The cell line was cultured in a
RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with
10 ng/ml epidermal growth factor and 15% fetal bovine serum (FBS)
plus 100 U/ml penicillin and 100 μg/ml streptomycin in a
humidified atmosphere chamber containing 5% CO2 at
37°C.
Enzyme-linked immunosorbent assay
(ELISA)
The collected blood and urine samples were
centrifuged at 1,000 x g for 15 min. The supernatants were
collected and the concentration of HIF-1α was measured using an
ELISA reagent kit (R&D Systems, Minneapolis, MN, USA) as per
the supplier’s instructions and analyzed by an ELISA reader (BioTek
Instruments Inc., Winooski, VT, USA).
Cell treatments and transfections
For propranolol treatment, hemangioma endothelial
cells were treated with various concentrations of propranolol (0,
10, 50 and 100 μM) and incubated for 24, 48 and 72 h. For
gene overexpression or gene knockdown, recombinant lentiviral
vectors (Shanghai GenePharma Co., ltd., Shanghai, China) or
specific siRNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were
transfected with the cells and incubated for 48 h prior to being
collected for analysis.
Western blot analysis
Total proteins were extracted from tumor tissues or
cells and quantified using a BCA protein assay kit (Thermo Fisher
Scientific, Rockford, Il, USA). Approximately 25 μg protein
was run on a precast 12.5% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis followed by electro-blotting onto a
nitrocellulose membrane (Amersham, Little Chalfont, UK). The
membranes were blocked by blocking buffer (3% skimmed milk
solution) at 37°C for 1 h. The membranes were then blotted with
primary antibodies diluted in blocking buffer overnight at 4°C.
After being washed three times with Tris-buffered saline (TBS) and
Tween (TBST) (each for 5 min), the membranes were incubated with
horseradish peroxidase conjugated secondary antibody (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) in the blocking buffer
for 1 h. The membranes were then washed three times with TBST and
once with TBS, and the blots were developed with an enhanced
chemiluminescence (ECL) detection system (Amersham). The following
primary antibodies were used: anti-HIF-1α, anti-VEGF, anti-STAT3,
anti-p-STAT3, anti-β1-AR, anti-β2-AR, anti-Bcl-2 and anti-GAPDH
(Santa Cruz Biotechnology).
MTT assay
Cell growth was detected by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, the cells were grown in 96-well plates
(1×104 cells/well) and cultured until they reached 80%
confluence. Thereafter, the cells were treated with HIF-1α, siRNA
or lV-HIF-1α with 50 μM propranolol and incubated for 48 h.
MTT diluted in PBS (5 mg/ml) was added at 20 μl/well and
continually incubated for 4 h. Dimethylsulfoxide (150
μl/well) was added to dissolve the formazan crystals. The
optical density value was measured using an ELISA reader at 490
nm.
Mouse xenograft experiment
A xenograft of hemangioma cells in the mice was
prepared as previously described (28). Briefly, the cells
(4×1010) that were diluted in 200 μl PBS were
injected subcutaneously into the right groin. Two days after the
tumor cell implantation, recombinant lentivirus (5×1010
plaque-forming units) in 200 μl PBS was injected
subcutaneously into the left groin and this was performed every two
weeks. The nude mice were intragastrically administered with
propranolol solution (0.25 mg/ml) at 0.2 ml/10 g body weight every
two days. After 40 days, the mice were euthanized by subcutaneous
injection with sodium pentobarbital (40 mg/kg) and tumor tissues
were harvested for analysis.
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) assay
Cell apoptosis was detected by a TUNEL staining
assay using a one-step TUNEL apoptosis assay kit (Beyotime, Haimen,
China). Briefly, tumor tissues were dissected, fixed with 4%
paraformaldehyde for 24 h at 4°C, gradient-dehydrated, embedded in
paraffin and then serially cut into 5 μm-thick sections. The
sections were dewaxed, gradient-rehydrated and digested with 20
μM proteinase for 10 min. After being washed with PBS, the
apoptotic cells were examined using a TUNEl apoptosis assay kit
according to the supplier’s instructions. The sections were
observed under a fluorescence microscope (Olympus, Tokyo, Japan)
and the TUNEl staining cells were calculated in five random fields
and averaged per field.
Statistical analysis
The data were expressed as mean ± standard deviation
(SD). Statistical analyses were performed using one-way ANOVA
followed by the Bonferroni post hoc test among multiple groups. A
value of P<0.05 was regarded as statistically significant.
Results
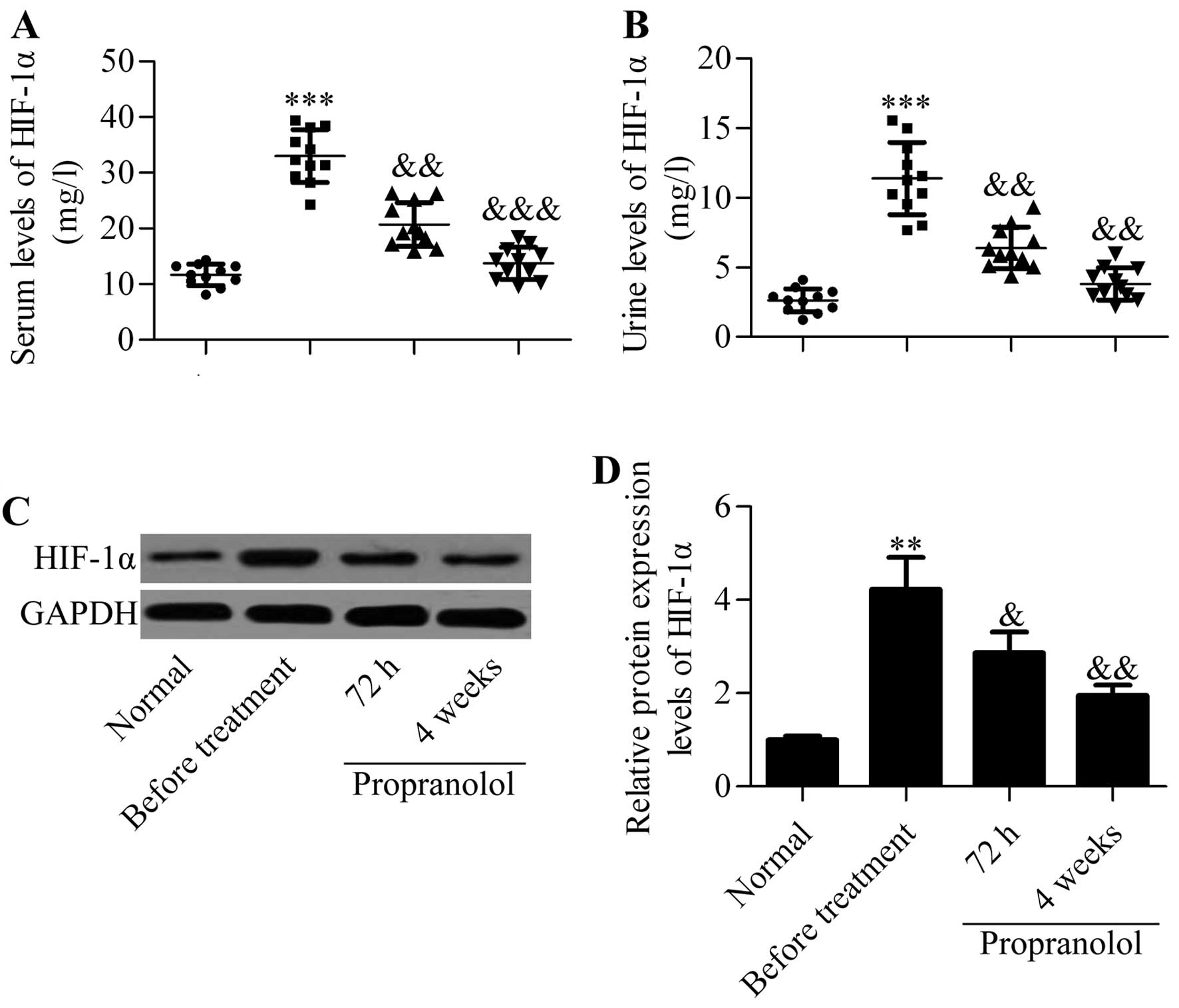
HIF-1α is downregulated by propranolol
treatment in infantile hemangioma patients
To explore the molecular basis of propranolol in the
treatment of infantile hemangioma, we evaluated the effect of
propranolol on the levels of HIF-1α in infantile hemangioma
patients. By using ELISA detection methods, we found that the
concentrations of HIF-1α in the serum and urine were significantly
increased in infantile hemangioma patients compared with those from
healthy subjects. Surprisingly, the serum and urine levels of
HIF-1α were significantly decreased after treatment with
propranolol (Fig. 1A and B). To
further validate that propranolol regulated the expression of
HIF-1α, we detected the expression of HIF-1α in the hemangioma
tumor tissues by western blot analysis. Similar results were
obtained showing that propranolol treatment significantly decreased
the protein levels of HIF-1α, which had been upregulated in
infantile hemangioma tissues without treatment (Fig. 1C and D). Collectively, the results
suggested that propranolol inhibited HIF-1α in infantile hemangioma
patients.
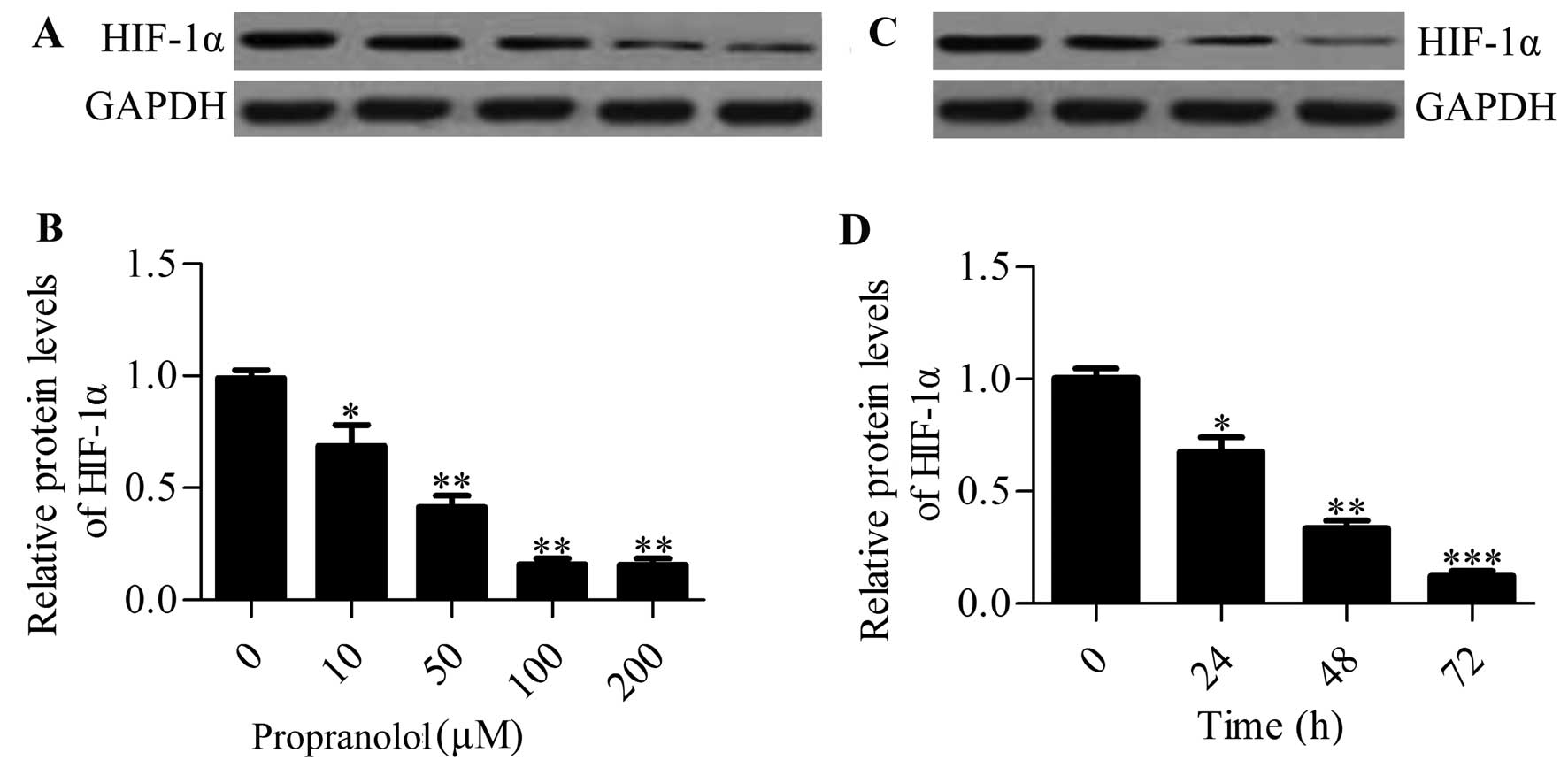
Propranolol reduces the expression of
HIF-1α in infantile hemangioma cells in a dose- and time-dependent
manner
To further verify the regulated effect of
propranolol on HIF-1α expression, we used the hemangioma-derived
endothelial cell line to investigate the effect of propranolol on
HIF-1α expression in vitro. Hemangioma endothelial cells
were treated with varying concentrations of propranolol (0, 10, 50,
100 and 200 μM) for 48 h and the HIF-1α protein expression
was determined by western blot analysis. The results showed that
propranolol administration decreased the HIF-1α expression in a
dose-dependent manner (Fig. 2A and
B). Next, we treated the cells with 100 μM propranolol
and incubated them for 24, 48 and 72 h (Fig. 2C and D). The results showed that
HIF-1α expression was increased at 24 h and continuously increased
up to 72 h. In conclusion, these results further indicated that
propranolol regulated the expression of HIF-1α in the infantile
hemangioma.
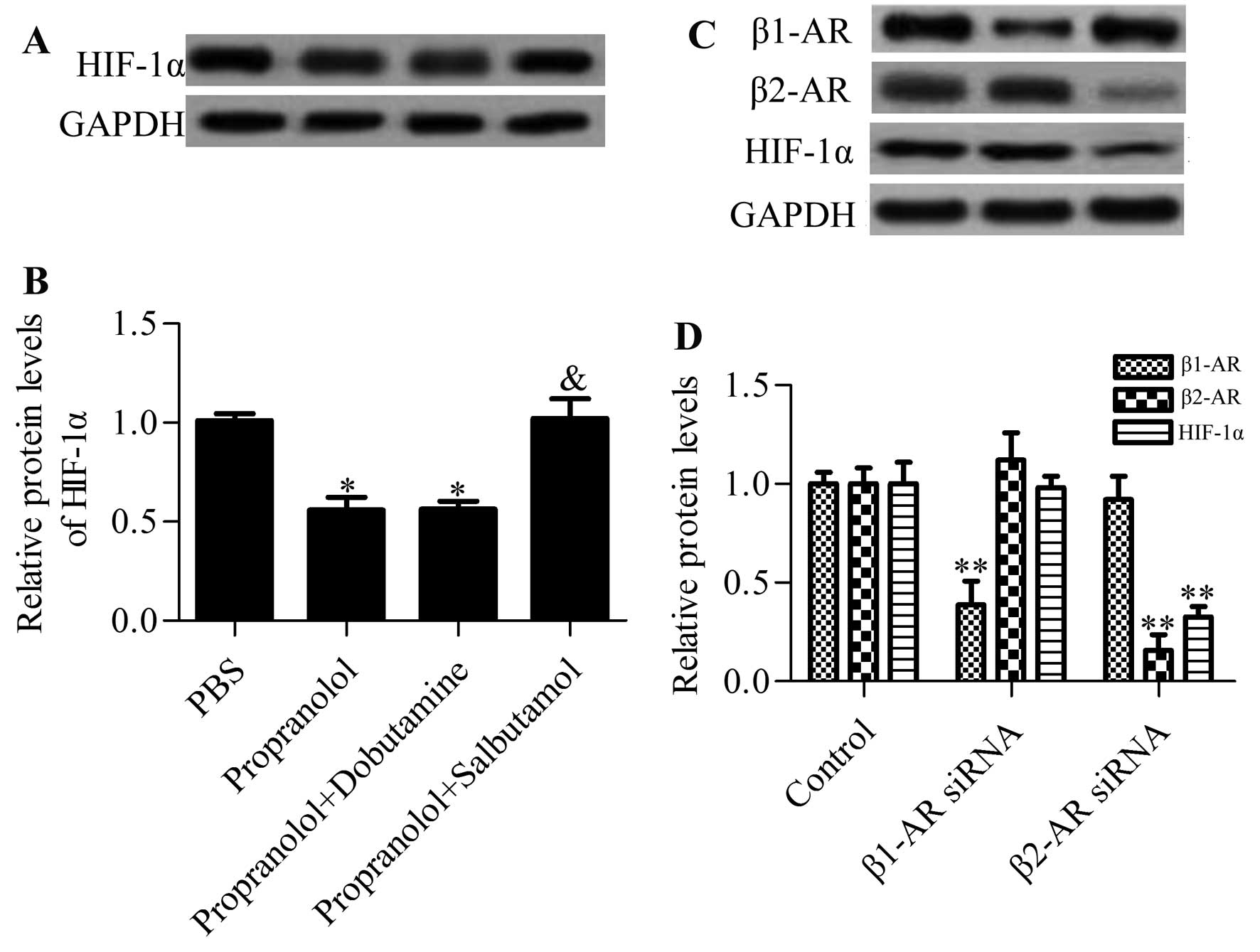
Propranolol inhibits the expression of
HIF-1α mainly through acting on the β2-adrenergic receptor
(AR)
Propranolol is a non-selective antagonist for β1-AR
and β2-AR (21). To determine which
β-AR played an important role in mediating the inhibitory effect of
propranolol on the expression of HIF-1α, we added the β1-AR agonist
(dobutamine) or the β2-AR agonist (salbutamol) to
propranolol-treated cells and examined their effects on the
expression of HIF-1α. The results showed that the β1-AR agonist had
no apparent effect on the propranolol-induced HIF-1α decrease,
whereas the β2-AR agonist significantly reversed the inhibitory
effects of propranolol on HIF-1α expression (Fig. 3A and B). Additionally, inhibition of
β2-AR by specific β2-AR siRNA had the same effect as propranolol on
HIF-1α. However, knockdown of β1-AR had no apparent effect on
HIF-1α expression (Fig. 3C and D).
Collectively, these results suggested that propranolol repressed
the expression of HIF-1α mainly through acting on β2-AR, not
β1-AR.
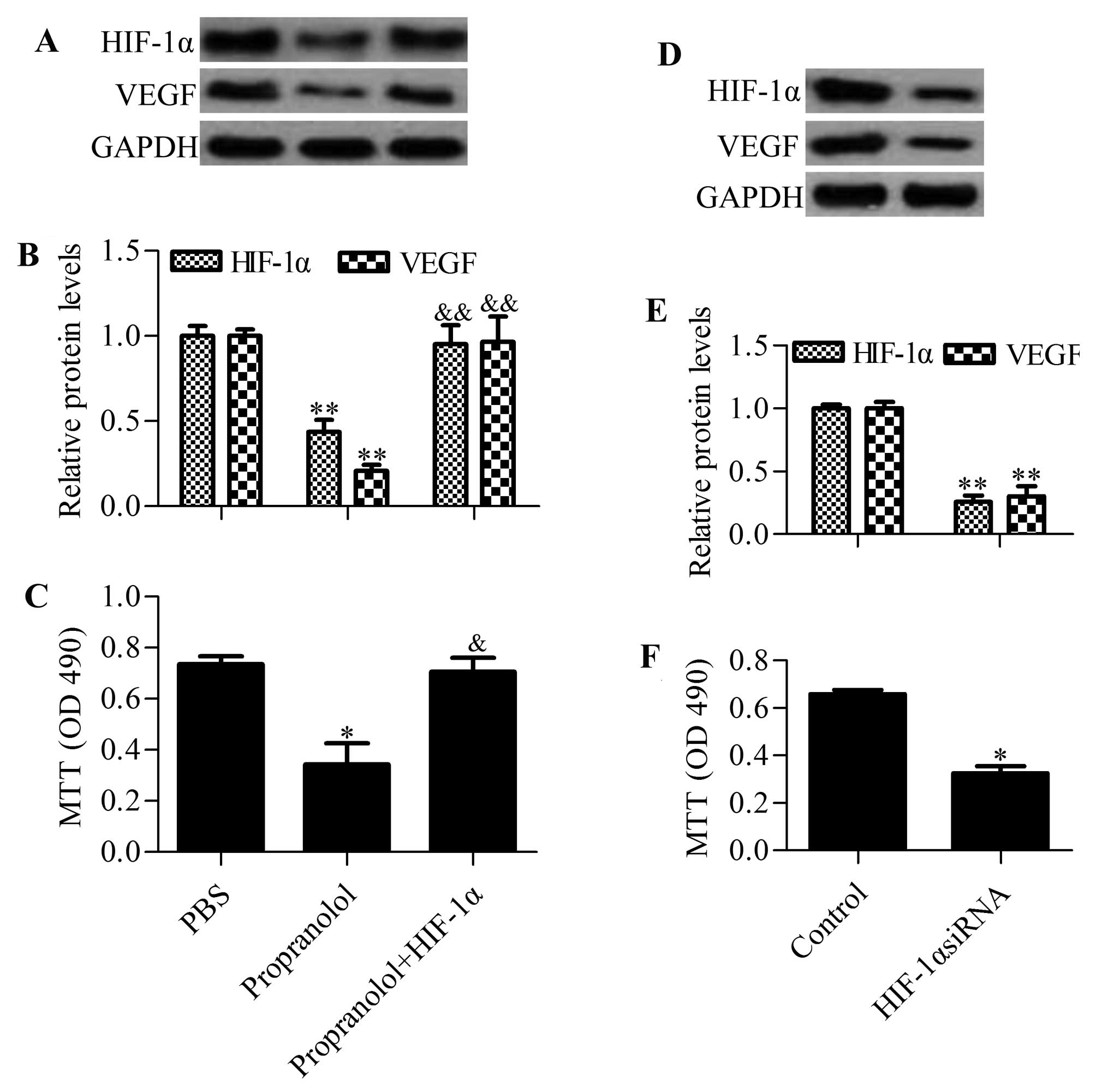
Overexpression of HIF-1α abrogates the
inhibitory effects of propranolol on hemangioma cell growth
To gain insight into HIF-1α in propranolol-induced
cell growth arrest, we examined the effect of HIF-1α overexpression
on propranolol-treated cells. The results showed that propranolol
treatment inhibited the protein expression of HIF-1α and VEGF,
whereas overexpression of HIF-1α blocked the inhibitory effects of
propranolol on VEGF expression (Fig. 4A
and B). Furthermore, propranolol-induced cell growth arrest was
also abrogated by HIF-1α overexpression (Fig. 4C). Similarly, knockdown of HIF-1α
resulted in a decreased VEGF expression (Fig. 4D and E) and cell growth inhibition
(Fig. 4F), which had the same
effect as propranolol treatment. These results implied that
propranolol suppressed hemangioma cell growth by regulating
HIF-1α.
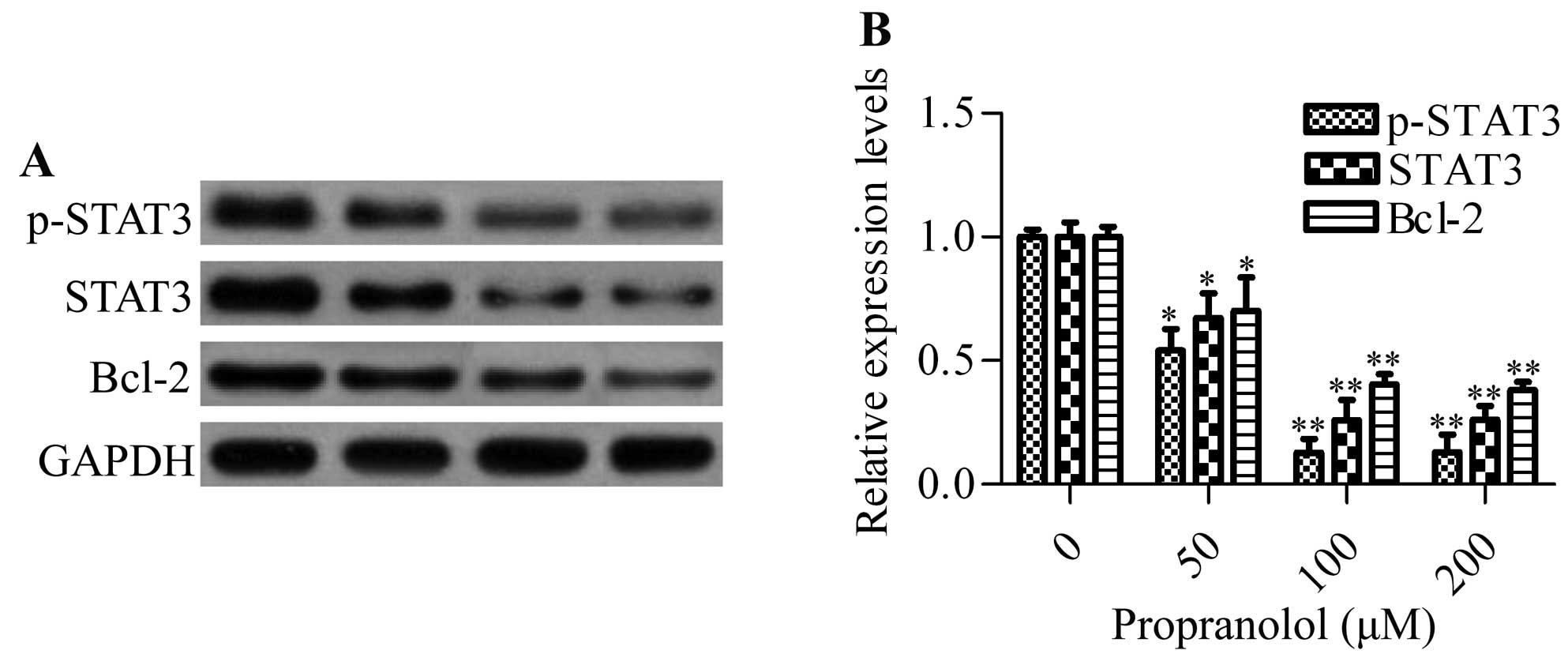
Propranolol inhibits the expression of
STAT3 and Bcl-2 in hemangioma cells
STAT3, a critical molecule for regulating oncogenic
signaling, has been previously reported to be overexpressed in
proliferating infantile hemangiomas (29). To investigate whether propranolol
had an inhibitory effect on the expression of STAT3, we detected
the protein expression of STAT3 in propranolol-treated hemangioma
cells by western blot analysis. The results showed that different
concentrations of propranolol significantly inhibited the
expression of total STAT3 and phosphorylated STAT3 (p-STAT3).
Furthermore, the downstream gene Bcl-2, an anti-apoptotic gene, was
also markedly decreased by propranolol (Fig. 5). These data indicated that
propranolol was capable of inhibiting STAT3 signaling.
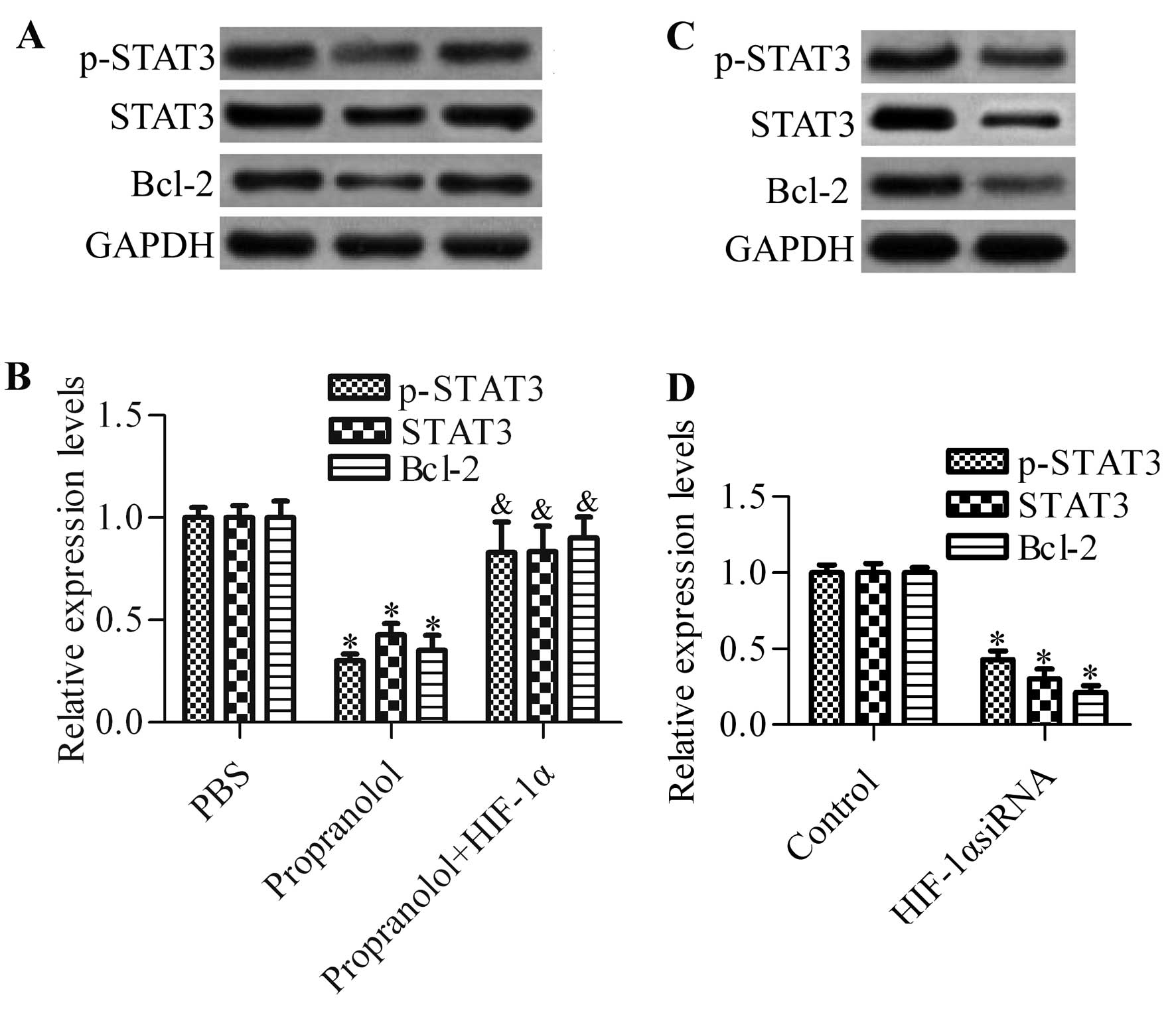
Overexpression of HIF-1α abrogates the
inhibitory effects of propranolol on STAT3 signaling
To investigate whether HIF-1α was involved in the
regulation of STAT3 signaling, we detected the effect of HIF-1α
overexpression on STAT3 signaling activation in propranolol-treated
cells by western blot analysis (Fig.
6A). The results showed that overexpression of HIF-1α
significantly abrogated the inhibitory effects of propranolol on
the protein expression of p-STAT3, STAT3 and Bcl-2 (Fig. 6B). Knockdown of HIF-1α by siRNA
markedly decreased the protein expression of p-STAT3, STAT3 and
Bcl-2, which mimics the effect of propranolol on STAT3 signaling.
Collectively, these results suggested that HIF-1α played an
important role in propranolol-mediated inhibition of STAT3
signaling in hemangioma cells.
Overexpression of HIF-1α reduces the
therapeutic effects of propranolol on hemangiomas in a mouse
model
To further validate the important role of HIF-1α
involved in propranolol treatment for hemangiomas, we infected a
mouse xenograft hemangioma model with lV-HIF-1α overexpressing
HIF-1α. Using the TUNEL method, we found that propranolol-induced
hemangioma apoptosis was apparently inhibited by HIF-1α
overexpression (Fig. 7A).
Furthermore, the protein expression of VEGF, p-STAT3, STAT3 and
Bcl-2, downregulated by propranolol in tumor tissues, was
significantly upregulated by HIF-1α overexpression (Fig. 7B and C). Collectively, these results
further confirmed that propranolol repressed hemangiomas in a
HIF-1α-dependent manner.
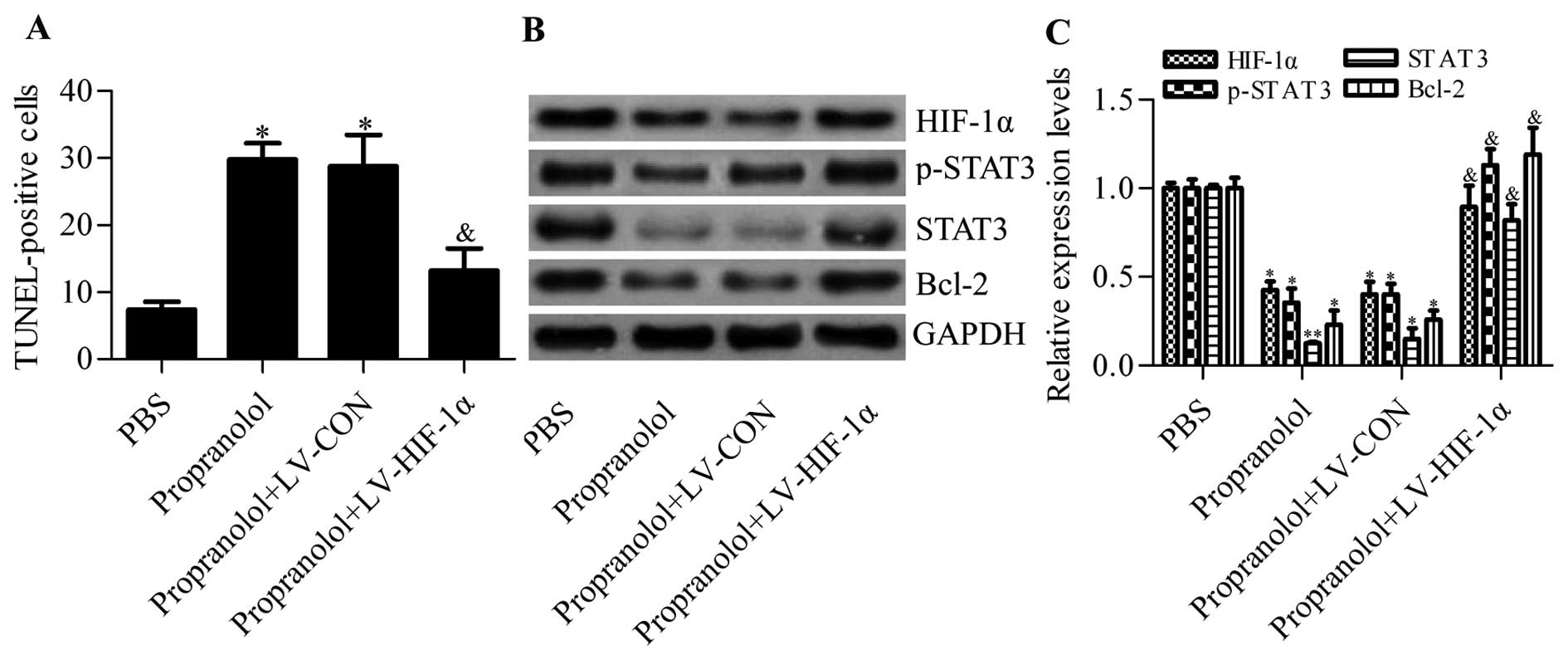 | Figure 7Detection of the effects of HIF-1α
overexpression on the therapeutic effects of propranolol on a mouse
xenograft hemangioma model. (A) Quantitative analysis of
TUNEL-positive cells in tumor tissues from different treated
groups. PBS, nude mice bearing hemangioma tumors treated with PBS;
propranolol, nude mice bearing hemangioma tumors treated with
propranolol; propranolol+LV-CON, nude mice bearing hemangioma
tumors were treated with propranolol and control lV-CON;
propranolol+HIF-1α, nude mice bearing hemangioma tumors were
treated with propranolol and lV-HIF-1α. N=5, *P<0.05
vs. PBS; &P<0.05 vs. propranolol. (B) Detection
of the effects of HIF-1α overexpression on the protein levels of
VEGF, p-STAT3, STAT3 and Bcl-2 in tumor tissues by western blot
analysis, and (C) representative histograms showing quantification
of relative protein levels. N=3, *P<0.05,
**P<0.01 vs. PBS; &P 0.05 vs.
propranolol. |
Discussion
Although the use of propranolol has been broadly
applied in the clinical treatment of infantile hemangiomas, the
underlying mechanism remains largely unknown. Therefore, it is of
great importance to gain better insight into propranolol in the
treatment of infantile hemangiomas to enable better patient care as
well as to contribute to the development of novel therapies. In the
present study, we sought to delineate the molecular mechanism of
propranolol in the treatment of infantile hemangiomas. Furthermore,
evidence was presented that propranolol represses infantile
hemangioma cell growth through its action on β2-AR and inhibits
VEGF, STAT3 and Bcl-2 expression in an HIF-1α-dependent manner,
which leads to cell growth arrest and the induction of cell
apoptosis using a hemangioma endothelial cell model.
Formerly, propranolol was generally used for the
treatment of cardiovascular diseases including hypertension,
supraventricular tachycardia, ischemic heart disease and
arrhythmia, and has been proved to be safe and well tolerated
(30). In 2008 Léauté-Labrèze et
al (14) used propranolol to
treat obstructive hypertrophic cardiomyopathy accompanied by
infantile hemangiomas and unexpectedly found that propranolol
effectively regresses infantile hemangiomas. Since then, an
increasing number of studies have reported the use of propranolol
in the treatment of infantile hemangiomas (31–33).
Currently, propranolol is regarded as the first-line drug for the
treatment of infantile hemangiomas due to its capacity to provide
instant gratification and be more effective, and its few
side-effects, as well as its low cost (34–36).
However, the underlying mechanism is still elusive. In recent
years, numerous studies have been devoted to investigating the
molecular basis of propranolol in the treatment of infantile
hemangiomas. Some possible mechanisms have been proposed, including
vasoconstriction, decreased expression of VEGF and the triggering
of apoptosis (22). Nonetheless,
there is still a lack of precise understanding.
β-AR has been reported to be expressed in various
tumor cells and the activation of β-AR results in an increase in
the cyclic AMP (cAMP) that activates the cAMP-dependent protein
kinases and the multiple signaling pathways (37–39).
Many studies have demonstrated that activation of β-AR exhibits the
tumor-promoting function in various tumor cells (40,41).
β-AR antagonists have been found to inhibit cell proliferation,
invasion and migration of the tumor cells (42–44).
Propranolol has been reported as a non-selective β-AR blocker of
β1-AR and β2-AR (21). In the
present study, we found that propranolol inhibited cell growth of
hemangioma endothelial cells mainly through its action on β2-AR,
and knockdown of β2-AR had the same effect as propranolol on cell
growth, while knockdown of β1-AR had no apparent effect on cell
growth. Our results indicated that propranolol repressed infantile
hemangiomas by inhibiting β2-AR-mediated signaling pathways.
Consistently, Truong et al (45) reported that β2-AR expression and
phosphorylation responded to propranolol treatment of infantile
hemangiomas.
HIF-1α has been proposed as an important mediator in
regulating tumor progression (26,27).
In the present study, we found that HIF-1α was upregulated in the
serum, urine and tumor tissues in infantile hemangioma patients,
and treatment of propranolol markedly inhibited the expression
levels of HIF-1α. The results also implied that HIF-1α was involved
in regulating infantile hemangiomas. Our data further identified
that propranolol suppressed HIF-1α by inhibiting β2-AR. The link
between HIF-1α and β-AR has been reported in a variety of tumors,
including breast, prostate and pancreatic cancer cells (46,47).
β-AR agonists have been shown to increase HIF-1α expression, and
β-AR agonist-induced HIF-1α and VEGF expression was abrogated by
propranolol (47). More recently,
propranolol was suggested to repress hemangioma cells by
downregulating HIF-1α, VEGF and downstream signaling pathways
(48). The present study presented
evidence that propranolol blocks β2-AR leading to decreased HIF-1α
and VEGF expression, which may explain the molecular basis of
propranolol action. Furthermore, our data demonstrated that
propranolol inhibited the expression and phosphorylation of STAT3
in infantile hemangioma cells. The overexpression of STAT3 has been
reported in infantile hemangiomas (29) and angiosarcomas (49).
In summary, our results suggested that propranolol
repressed infantile hemangioma cell growth through its action on
β2-AR and inhibits VEGF, STAT3 and Bcl-2 expression leading to cell
growth arrest and the induction of cell apoptosis using a
hemangioma endothelial cell model. Propranolol treatment also
decreased the expression of HIF-1α, which is a critical regulator
of tumor progression. Overexpression of HIF-1α significantly
abrogated the effects of propranolol, implying that propranolol
regressed infantile hemangiomas in a HIF-1α-dependent manner. These
findings suggested a plausible mechanism of propranolol in the
regression of infantile hemangiomas and provided novel insights for
the development of novel therapeutic methods for infantile
hemangiomas.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81172589).
Abbreviations:
|
AR
|
β-adrenergic receptor
|
|
HIF
|
hypoxia inducible factor
|
|
VEGF
|
vascular endothelial growth factor
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
dUTP nick-end labeling
|
Referencs
|
1
|
Kilcline C and Frieden IJ: Infantile
hemangiomas: How common are they? (A systematic review of the
medical literature). Pediatr Dermatol. 25:168–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang LC, Haggstrom AN, Drolet BA, Baselga
E, Chamlin SL, Garzon MC, Horii KA, Lucky AW, Mancini AJ, Metry DW,
et al: Growth characteristics of infantile hemangiomas:
Implications for management. Pediatrics. 122:360–367. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leaute-Labreze C, Prey S and Ezzedine K:
Infantile haemangioma: Part I. Pathophysiology, epidemiology,
clinical features, life cycle and associated structural
abnormalities. J Eur Acad Dermatol Venereol. 25:1245–1253. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Metry DW, Haggstrom AN, Drolet BA, Baselga
E, Chamlin S, Garzon M, Horii K, Lucky A, Mancini AJ, Newell B, et
al: A prospective study of PHACE syndrome in infantile hemangiomas:
Demographic features, clinical findings, and complications. Am J
Med Genet A. 140:975–986. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pandey A, Gangopadhyay AN and Upadhyay VD:
Evaluation and management of infantile hemangioma: An overview.
Ostomy Wound Manage. 54:16–18. 2008.PubMed/NCBI
|
|
6
|
Bennett ML, Fleischer AB Jr, Chamlin SL
and Frieden IJ: Oral corticosteroid use is effective for cutaneous
hemangiomas: An evidence-based evaluation. Arch Dermatol.
137:1208–1213. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ezekowitz RA, Mulliken JB and Folkman J:
Interferon alfa-2a therapy for life-threatening hemangiomas of
infancy. N Engl J Med. 326:1456–1463. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perez J, Pardo J and Gomez C: Vincristine
- an effective treatment of corticoid-resistant life-threatening
infantile hemangiomas. Acta Oncol. 41:197–199. 2002. View Article : Google Scholar
|
|
9
|
Perez Payarols J, Pardo Masferrer J and
Gomez Bellvert C: Treatment of life-threatening infantile
hemangiomas with vincristine. N Engl J Med. 333:691995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moore J, Lee M, Garzon M, Soffer S, Kim E,
Saouaf R, del Toro G, Yamashiro D and Kandel J: Effective therapy
of a vascular tumor of infancy with vincristine. J Pediatr Surg.
36:1273–1276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boon LM, MacDonald DM and Mulliken JB:
Complications of systemic corticosteroid therapy for problematic
hemangioma. Plast Reconstr Surg. 104:1616–1623. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dubois J, Hershon L, Carmant L, Belanger
S, Leclerc JM and David M: Toxicity profile of interferon alfa-2b
in children: A prospective evaluation. J Pediatr. 135:782–785.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barlow CF, Priebe CJ, Mulliken JB, Barnes
PD, MacDonald D, Folkman J and Ezekowitz RA: Spastic diplegia as a
complication of interferon Alfa-2a treatment of hemangiomas of
infancy. J Pediatr. 132:527–530. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Léauté-Labrèze C, Dumas de la Roque E,
Hubiche T, Boralevi F, Thambo JB and Taieb A: Propranolol for
severe hemangiomas of infancy. N Engl J Med. 358:2649–2651. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bigorre M, Van Kien AK and Valette H:
Beta-blocking agent for treatment of infantile hemangioma. Plast
Reconstr Surg. 123:195e–196e. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holmes WJ, Mishra A, Gorst C and Liew SH:
Propranolol as first-line treatment for rapidly proliferating
infantile haemangiomas. J Plast Reconstr Aesthet Surg. 64:445–451.
2011. View Article : Google Scholar
|
|
17
|
Buckmiller LM, Munson PD, Dyamenahalli U,
Dai Y and Richter GT: Propranolol for infantile hemangiomas: Early
experience at a tertiary vascular anomalies center. Laryngoscope.
120:676–681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Solman L, Murabit A, Gnarra M, Harper JI,
Syed SB and Glover M: Propranolol for infantile haemangiomas:
Single centre experience of 250 cases and proposed therapeutic
protocol. Arch Dis Child. 99:1132–1136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baetz J, Eigelshoven S, Marquard J,
Bruch-Gerharz D, Homey B and Meissner T: Infantile hemangioma.
Successful treatment with propranolol. Hautarzt. 61:290–292.
2010.In German. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sarialioglu F, Erbay A and Demir S:
Response of infantile hepatic hemangioma to propranolol resistant
to high-dose methylprednisolone and interferon-α therapy. Pediatr
Blood Cancer. 55:1433–1434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Storch CH and Hoeger PH: Propranolol for
infantile haemangiomas: Insights into the molecular mechanisms of
action. Br J Dermatol. 163:269–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
D’Angelo G, Lee H and Weiner RI:
cAMP-dependent protein kinase inhibits the mitogenic action of
vascular endothelial growth factor and fibroblast growth factor in
capillary endothelial cells by blocking Raf activation. J Cell
Biochem. 67:353–366. 1997. View Article : Google Scholar
|
|
23
|
Perez-Sayans M, Somoza-Martin JM,
Barros-Angueira F, Diz PG, Gandara Rey JM and Garcia-Garcia A:
Beta-adrenergic receptors in cancer: therapeutic implications.
Oncol Res. 19:45–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guimaraes S and Moura D: Vascular
adrenoceptors: An update. Pharmacol Rev. 53:319–356.
2001.PubMed/NCBI
|
|
25
|
Iaccarino G, Ciccarelli M, Sorriento D,
Galasso G, Campanile A, Santulli G, Cipolletta E, Cerullo V, Cimini
V, Altobelli GG, et al: Ischemic neoangiogenesis enhanced by
beta2-adrenergic receptor overexpression: A novel role for the
endothelial adrenergic system. Circ Res. 97:1182–1189. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Semenza GL: Regulation of mammalian O2
homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol.
15:551–578. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li P, Xiao X, Xu Q and Guo Z:
Establishment of human infancy hemangioma-derived endothelial cell
line XPTS-1 and animal model of human infancy hemangioma. Zhonghua
Kou Qiang Yi Xue Za Zhi. 46:129–133. 2011.In Chinese. PubMed/NCBI
|
|
29
|
Itinteang T, Tan ST, Brasch HD, Steel R,
Best HA, Vishvanath A, Jia J and Day DJ: Infantile haemangioma
expresses embryonic stem cell markers. J Clin Pathol. 65:394–398.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Loaldi A, Polese A, Montorsi P, De Cesare
N, Fabbiocchi F, Ravagnani P and Guazzi MD: Comparison of
nifedipine, propranolol and isosorbide dinitrate on angiographic
progression and regression of coronary arterial narrowings in
angina pectoris. Am J Cardiol. 64:433–439. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harikrishna B, Ganesh A, Al-Zuahibi S,
Al-Jabri S, Al-Waily A, Al-Riyami A, Al-Azri F, Masoud F and
Al-Mujaini A: Oral propranolol for the treatment of periorbital
infantile hemangioma: A preliminary report from Oman. Middle East
Afr J Ophthalmol. 18:298–303. 2011. View Article : Google Scholar
|
|
32
|
Manunza F, Syed S, Laguda B, Linward J,
Kennedy H, Gholam K, Glover M, Giardini A and Harper JI:
Propranolol for complicated infantile haemangiomas: A case series
of 30 infants. Br J Dermatol. 162:466–468. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Solomon T, Ninnis J, Deming D, Merritt TA
and Hopper A: Use of propranolol for treatment of hemangiomas in
PHACE syndrome. J Perinatol. 31:739–741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Price CJ, Lattouf C, Baum B, McLeod M,
Schachner LA, Duarte AM and Connelly EA: Propranolol vs
corticosteroids for infantile hemangiomas: A multicenter
retrospective analysis. Arch Dermatol. 147:1371–1376. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bertrand J, McCuaig C, Dubois J, Hatami A,
Ondrejchak S and Powell J: Propranolol versus prednisone in the
treatment of infantile hemangiomas: A retrospective comparative
study. Pediatr Dermatol. 28:649–654. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lou Y, Peng WJ, Cao Y, Cao DS, Xie J and
Li HH: The effectiveness of propranolol in treating infantile
haemangiomas: a meta-analysis including 35 studies. Br J Clin
Pharmacol. 78:44–57. 2014. View Article : Google Scholar
|
|
37
|
Montminy M: Transcriptional regulation by
cyclic AMP. Annu Rev Biochem. 66:807–822. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang X, Odom DT, Koo SH, Conkright MD,
Canettieri G, Best J, Chen H, Jenner R, Herbolsheimer E, Jacobsen
E, et al: Genome-wide analysis of cAMP-response element binding
protein occupancy, phosphorylation, and target gene activation in
human tissues. Proc Natl Acad Sci USA. 102:4459–4464. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cole SW and Sood AK: Molecular pathways:
beta-adrenergic signaling in cancer. Clin Cancer Res. 18:1201–1206.
2012. View Article : Google Scholar :
|
|
40
|
Powe DG and Entschladen F: Targeted
therapies: Using beta-blockers to inhibit breast cancer
progression. Nat Rev Clin Oncol. 8:511–512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang J, Li Z, Lu L and Cho CH:
beta-Adrenergic system, a backstage manipulator regulating tumour
progression and drug target in cancer therapy. Semin Cancer Biol.
23:533–542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shi M, Yang Z, Hu M, Liu D, Hu Y, Qian L,
Zhang W, Chen H, Guo L, Yu M, et al: Catecholamine-induced
β2-adrenergic receptor activation mediates desensitization of
gastric cancer cells to trastuzumab by upregulating MUC4
expression. J Immunol. 190:5600–5608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Entschladen F, Drell Tl IV, Lang K, Joseph
J and Zaenker KS: Tumour-cell migration, invasion, and metastasis:
navigation by neurotransmitters. Lancet Oncol. 5:254–258. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sood AK, Bhatty R, Kamat AA, Landen CN,
Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S and Cole SW:
Stress hormone-mediated invasion of ovarian cancer cells. Clin
Cancer Res. 12:369–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Truong MT, Chang KW, Berk DR,
Heerema-McKenney A and Bruckner AL: Propranolol for the treatment
of a life-threatening subglottic and mediastinal infantile
hemangioma. J Pediatr. 156:335–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hu HT, Ma QY, Zhang D, Shen SG, Han L, Ma
YD, Li RF and Xie KP: HIF-1alpha links beta-adrenoceptor agonists
and pancreatic cancer cells under normoxic condition. Acta
Pharmacol Sin. 31:102–110. 2010. View Article : Google Scholar
|
|
47
|
Park SY, Kang JH, Jeong KJ, Lee J, Han JW,
Choi WS, Kim YK, Kang J, Park CG and Lee HY: Norepinephrine induces
VEGF expression and angiogenesis by a hypoxia-inducible
factor-1alpha protein-dependent mechanism. Int J Cancer.
128:2306–2316. 2011. View Article : Google Scholar
|
|
48
|
Chim H, Armijo BS, Miller E, Gliniak C,
Serret MA and Gosain AK: Propranolol induces regression of
hemangioma cells through HIF-1alpha-mediated inhibition of VEGF-A.
Ann Surg. 256:146–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin N, Uchi H, Moroi Y, Fukiwake N,
Dainichi T, Takeuchi S, Takahara M, Tu Y, Furue M and Urabe K:
Significance of the expression of phosphorylated signal transducer
and activator of transcription-3, -Akt, and -cyclin D1 in
angiosarcoma. J Dermatol Sci. 48:64–66. 2007. View Article : Google Scholar : PubMed/NCBI
|