Introduction
Adenoid cystic carcinoma (ACC) is the most common
malignant tumor of the lacrimal glands and is characterized by
slow-growing, marked diffuse invasion into adjacent tissues and
late onset of distant metastases (i.e., lungs, bones and other
organs). Its prevalence is estimated to be 1.6% of all orbital
tumors, 4.8% of all primary orbital neoplasms, and 25–30% of all
epithelial neoplasms of the lacrimal gland (1–3).
Patients with ACC of the lacrimal glands are difficult to cure due
to the high recurrence rate, intracranial invasion and poor
clinical outcome after long-term observation (4). However, ACC cell lines from human
lacrimal glands have rarely been established. As no suitable cell
lines or animal models exist, the characteristics of this tumor are
not fully understood. Therefore, establishing an ACC cell line from
human lacrimal glands (LACC-1) and an animal model is essential to
analyze its biological behavior. In the present study, we describe
the methods of establishment and the characteristics of a new ACC
cell line isolated from human lacrimal glands and a
heterotransplantation model of human lacrimal gland ACC into nude
mice.
Materials and methods
Ethics statement
The use of tumor tissue for the present study was
approved by the Human Research Ethics Committee of the Tianjin
Medical University. The patient provided consent for her tumor
samples to be used for scientific research purposes. Written
informed consent was obtained from the patient. All of the animal
experimental procedures were approved by the Institutional Animal
Care and Use Committee (IACUC) of the Tianjin Medical University
(permit no. SYXK 2009–0001), and were performed in compliance with
the Association for Research in vision And ophthalmology Statement
for the Use of Animals in ophthalmic and vision Research. Prior to
sacrifice, general anesthesia was induced by intraperitoneal
injection of chloral hydrate to alleviate pain. Mice were
sacrificed using the cervical dislocation method.
Establishment of a new cell line
Tumor tissue was obtained from surgical material of
a 35-year-old female patient with a solid pattern of ACC on the
right lacrimal gland (Fig. 1A–C).
The tumor tissue was placed into precooled RPMI-1640 medium
(Hyclone Laboratories, Logan, UT, USA) immediately after being
excised and then transported to the laboratory. The tumor was
rinsed two to three times with RPMI-1640 medium containing 100 U/ml
penicillin and 100 μg/ml streptomycin (Hyclone Laboratories)
under sterile conditions. All necrotic tissue, fat, vessels and
conjunctival tissue were removed. The tumor was cut into 0.5–1
mm3 pieces with scissors and seeded on the bottom of
25-cm cell culture flasks, which had been pretreated with serum.
The distance between each tumor piece was ~5 mm. Each flask
contained 25–30 tumor pieces. The tumor pieces were cultured in
RPMI-1640 medium supplemented with 20% fetal bovine serum (FBS;
HyClone Laboratories), 2 mM L-glutamine (Gibco, Grand Island, NY,
USA), 100 U/ml penicillin and 100 μg/ml streptomycin as
growth medium at 37°C in a 5% CO2 incubator (Thermo
fisher Scientific, waltham, MA, USA, model: 3111). Approximately 5
days later, many cells grew from the edge of the explants. Most of
these cells contained fibroblasts and epithelioid tumor cells
(Fig. 1D–F). Approximately 32 days
later, the cells reached 80% confluency and were subcultured after
dissociation with 0.25% trypsin and 0.53 mM EDTA mixture (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China). In
order to discard the fibroblasts and purified tumor cells, we
combined continuous passage, mechanical scraping, repeated
adherence and dissociation methods. ACC cell clones appeared 69
days later. The medium was replaced every two to three days. The
ACC cells were subcultured when they reached 80% confluency.
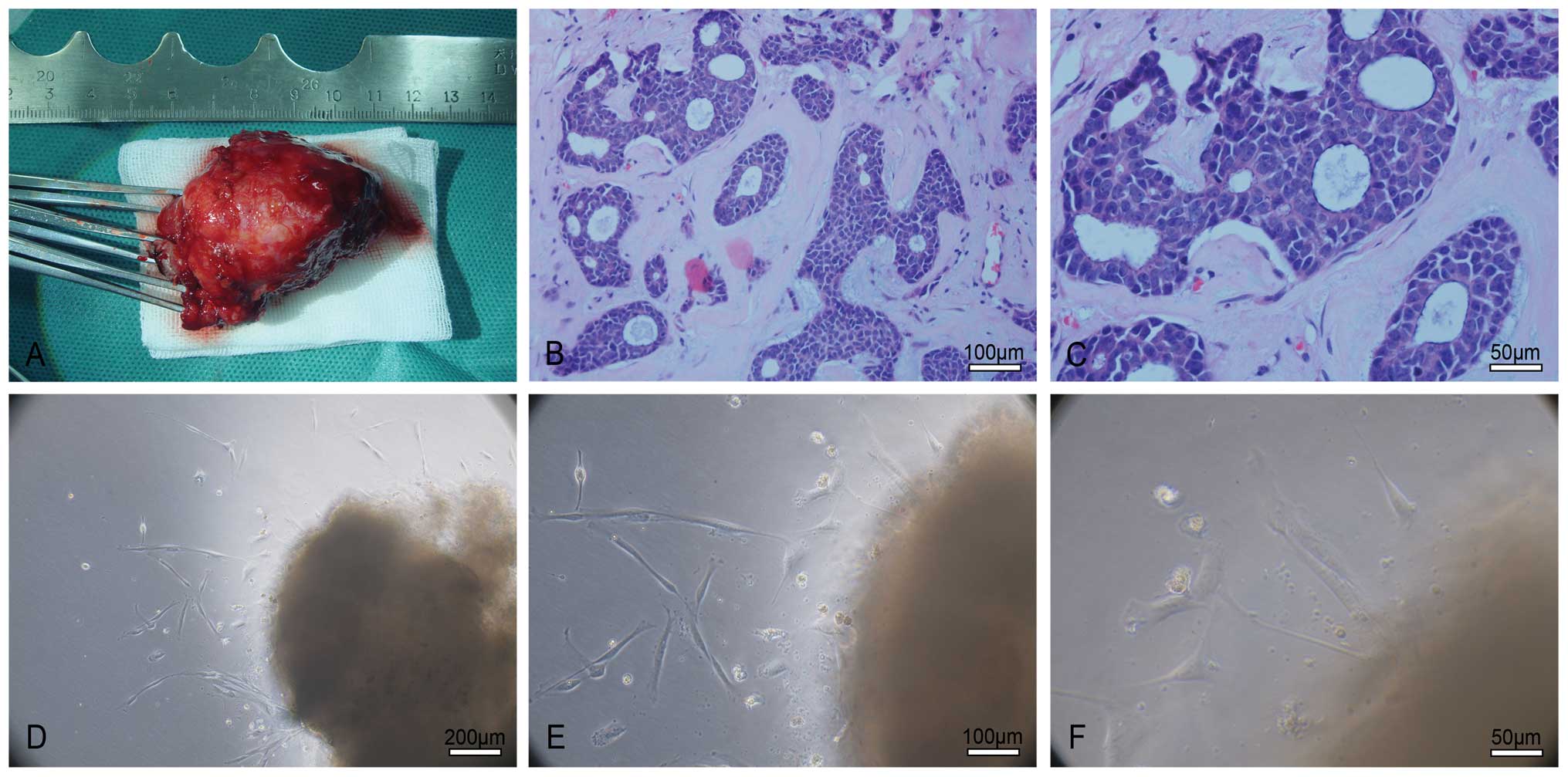 | Figure 1Establishment of the LACC-1 cell line.
(A) The surgical specimen of a patient suffering from right
lacrimal gland ACC. (B and C) H&E staining. The tumor exhibits
a solid pattern, and a cribriform structure is noted in the local
area. Tumor cells are round or oval and have rich hyperchromatic
nuclei and less cytoplasm. B: original magnification, ×200; scale
bar, 100 μm; C: Original magnification, ×400; scale bar, 50
μm. (D–F) Approximately 5 days later, many cells grew from
the edge of the explants. Most of the cells consist of fibroblasts
and epithelioid tumor cells. D: Original magnification, ×100; scale
bar, 200 μm; E: original magnification, ×200; scale bar, 100
μm; F: original magnification, ×400; scale bar, 50
μm. |
Cell morphology
Tumor pieces were observed under a phase contrast
microscope (Olympus IX70; Olympus Corp., Tokyo, Japan) every
day.
Transmission electron microscopy (TEM)
observation
When purified tumor cells were subcultured at the
exponential stage, they were seeded in 75-cm cell culture flasks
(Corning, Acton, MA, USA). The medium was replaced one day before
the cells reached 100% confluency. The cells were collected by
scraping and then the fixative was added. The cells were
centrifuged at 1,000 rpm for 10 min, and the supernatant was
discarded carefully. The fixative was added again, and the cells
were placed at 4°C for 2 h. The fixative was replaced with fresh
buffer every 10 min for three times. The cell pellets were
preserved in fresh buffer at 4°C and sent to the Neurosurgery
Institute in Beijing the next day. The institute was responsible
for embedding, sectioning and conducting the TEM observation.
Scanning electron microscopy (SEM)
observation
Purified tumor cells were seeded on slides in 6-well
plates (Corning) and incubated at 37°C in a 5% CO2
incubator when they grew at the logarithmic phase. The size of the
slide was 10 mm × 10 mm. The medium was changed one day before the
cells reached 100% confluency. The following day, the medium was
extracted and gently rinsed with phosphate-buffered solution (0.01
M PBS; Gibco) for three times. The electron microscopy fixative was
added and preserved at 4°C in the refrigerator. The next day, the
samples were sent to the Neurosurgery Institute in Beijing. The
institute was in charge of dehydration, coating the film and
conducting the SEM observation.
Immunocytochemical (IHC) detection of
protein expression
Purified tumor cells were plated on 25 mm × 75 mm
glass slides at a density of 5×104 cells/ml in a 10-cm
dish (Corning) and incubated at 37°C in a 5% CO2
incubator. The medium was discarded when the tumor cells reached
70–80% confluency. The cells were rinsed with 0.01 M PBS (pH 7.2 to
7.4) three times and fixed in 4% paraformaldehyde (analytical
grade; Yingdaxigui Chemical Reagent factory, Tianjin, China) for 10
min at room temperature. The cells were separately incubated with
primary antibodies against S-100 (rabbit polyclonal anti-S-100
protein; Invitrogen Carlsbad, CA, USA), cytokeratin (CK; rabbit
anti-cytokeratin Pan; Invitrogen), vimentin (mouse anti-vimentin,
clone V9; Invitrogen), α-SMA (actin, smooth muscle, mouse
monoclonal antibody, clone 1A4; Invitrogen) and desmin (mouse
anti-desmin; Invitrogen) overnight at 4°C in a humidified box.
Streptavidin-peroxidase immunohisto-chemistry detection kit
(SP-9000 Histostain™-Plus kit; Zymed Laboratories, San Francisco,
CA, USA) was used to detect protein expression. The brown color was
developed using DAB kit (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd, Beijing, China) and observed by light
microscopy (Olympus BX51; Olympus). The primary antibody of the
negative controls was omitted and replaced wtih PBS.
Animals
Ten 4- to 5-week-old female nude mice (BALB/c
nu/nu), which were purchased from the Beijing Academy of Military
Medical Sciences Laboratory Animal Center, were used for the animal
experiments. The mice weighed 18–20 g and were maintained under
specific pathogen-free (SPF) conditions. The animals had free
access to food and water and were maintained under a 12-h:12-h
light-dark cycle at 22–25°C. The no. 84 generation LACC-1 cells
growing at a logarithmic stage were trypsinized, washed twice and
suspended in PBS. Then, cells (1×107/ml) suspended in
0.1 ml PBS were inoculated subcutaneously into the subaxillary of
the nude mice. The longest length (L) and shortest width (W) of
each xenograft tumor forming at the transplanted site were measured
by an electronic caliper (Shanghai Shen Han Measuring Tools Co.,
Ltd., Shanghai, China; 0–150 mm) every day. Tumor volume (V) of the
xenograft tumors was calculated using the formula: V =
(L×w2)/2 and the tumor growth curve was constructed. At
day 14 after the xenograft tumors were implanted, TEM observation
was conducted. At 14, 28, 35, 42 and 49 days after implantation,
two mice were sacrificed. Before sacrifice, general anesthesia was
induced by intraperitoneal injection of chloral hydrate to
alleviate pain. The lungs, livers and alar lymph nodes were excised
for hematoxylin and eosin (H&E) staining to observe tumor
metastasis. The heterotransplanted tumors were removed for H&E
and immunohistochemistry (IHC) studies. Reverse
transcription-polymerase chain reaction (RT-PCR) was performed on
the tumors to detect human and mouse β-actin genes to confirm that
they originated from human tissue and not from mouse tissue.
H&E staining and IHC
The heterotransplanted tumors and the lungs, livers
and alar lymph nodes of the mice were fixed in 10% formalin
(analytical grade; Yingdaxigui Chemical Reagent factory) for 24 h
at room temperature, and gradient ethanol (analytical grade;
Tianjin Fengchuan Chemical Reagent Science and Technology Co.,
Ltd., Tianjin, China) and dehydrated xylene (analytical grade;
Tianjin Fengchuan Chemical Reagent Science and Technology), made
transparent, embedded in paraffin (Shanghai Hualing Recovery
Appliance factory, Shanghai, China), and cut into 4-μm
sections. The sections were stained with H&E for histological
examination. The tumor tissue sections of the LACC-1 cells grown in
nude mice were incubated with primary antibodies against S-100, CK,
vimentin, α-SMA and desmin overnight at 4°C in a humidified box.
Streptavidin-peroxidase IHC detection kit was used to detect
protein expression. The brown color was developed using DAB kit and
observed by light microscopy. The primary antibody of the negative
controls was omitted and replaced by PBS.
RT-PCR analysis
Total-RNAs from the xenograft tumors, LACC-1 cells
and nude mouse livers were extracted using TRIzol (Invitrogen). PCR
of human β-actin cDNA was conducted using a sense primer,
5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ and an antisense primer,
5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′, at 95°C for 4 min and for 35
cycles at 95°C for 30 sec, 66°C for 30 sec, 72°C for 1 min and 72°C
for 7 min. The primers for mouse β-actin were sense,
5′-GATGACGATATCGCTGCGCTG-3′, and antisense,
5′-GTACGACCAGAGGCATACAGG-3′, and PCR was conducted at 95°C for 4
min and for 35 cycles at 95°C for 30 sec, 58°C for 30 sec 72°C for
1 min and 72°C for 7 min. PCR products were electrophoresed on
agarose gels (Biowest; Gene Tech Co., Ltd., Shanghai, China),
stained with ethidium bromide and visualized under ultraviolet
illumination (BioSpectrum AC Chemi HR 410; UVP LLC, Upland, CA,
USA).
Results
Establishment of a new ACC cell line
This newly established cell line called LACC-1 was
subcultured in >100 passages during the last two years. After
eight passages, homogeneous epithelioid tumor cells appeared when
we combined continuous passage, mechanical scraping, repeated
adherence, and dissociation methods to remove the fibroblast cells.
LACC-1 cells had long elliptical, triangular, spindle-like,
cuboidal and polygonal morphologies when they were single or did
not reach full confluency. They appeared as an epithelioid
monolayer culture on a cell culture flask and presented with a
cobblestone-like appearance when they reached confluence. The
nuclei were large and round with many abnormal mitoses. The
nucleoplasm ratio was high. Multinucleated giant cells appeared.
LACC-1 cells showed a tendency to have overlapping growth without
contact inhibition when the cell density continued to increase
(Fig. 2).
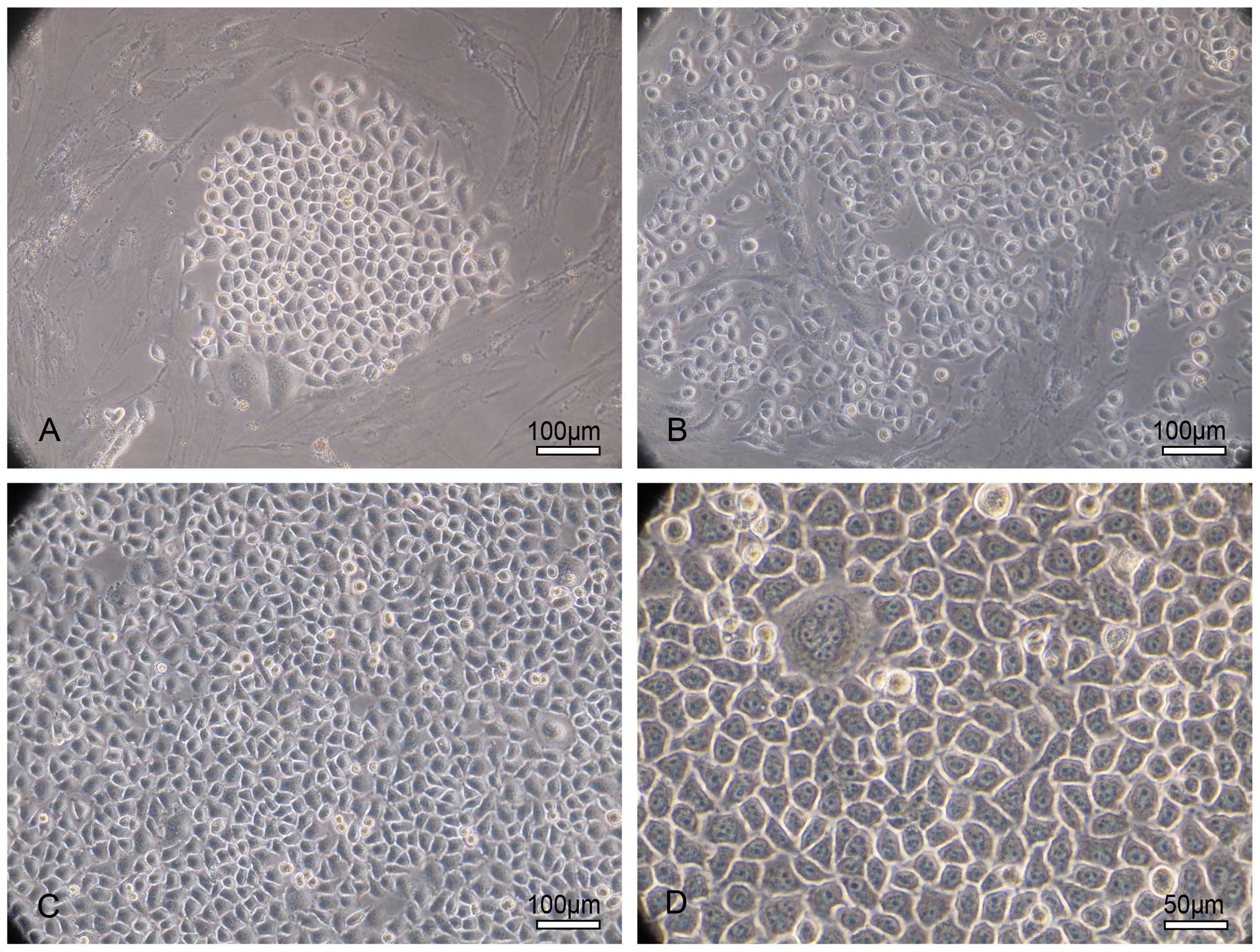 | Figure 2Morphology of the LACC-1 cells. (A)
Phase contrast photomicrograph of epithelioid tumor cell clones in
culture observed by day 72 after seeding. The cell clone presents
with a cobblestone-like appearance and is surrounded by fibroblasts
that grow in a whirl. Original magnification, ×200; scale bar, 100
μm. (B) At 2–7 passages, the tumor cells become the dominant
cells, and the fibroblasts are fewer. Original magnification, ×200;
scale bar, 100 μm. (C) After 8 passages, homogeneous
epithelioid tumor cells appear. Fibroblast cells are absent. LACC
cells exhibit triangular, cuboidal and polygonal morphologies as
they reach full confluency. Original magnification, ×200; scale
bar, 100 μm. (D) After 8 passages, the cells grow as an
epithelioid monolayer and present with a cobblestone-like
appearance when they reached confluency. The nuclei are large and
round with many abnormal mitoses. The nucleoplasm ratio is high.
Multinuclear giant cells appear. LACC cells show a tendency to have
overlapping growth without contact inhibition as the cell density
increases. Original magnification, ×400; scale bar, 50
μm. |
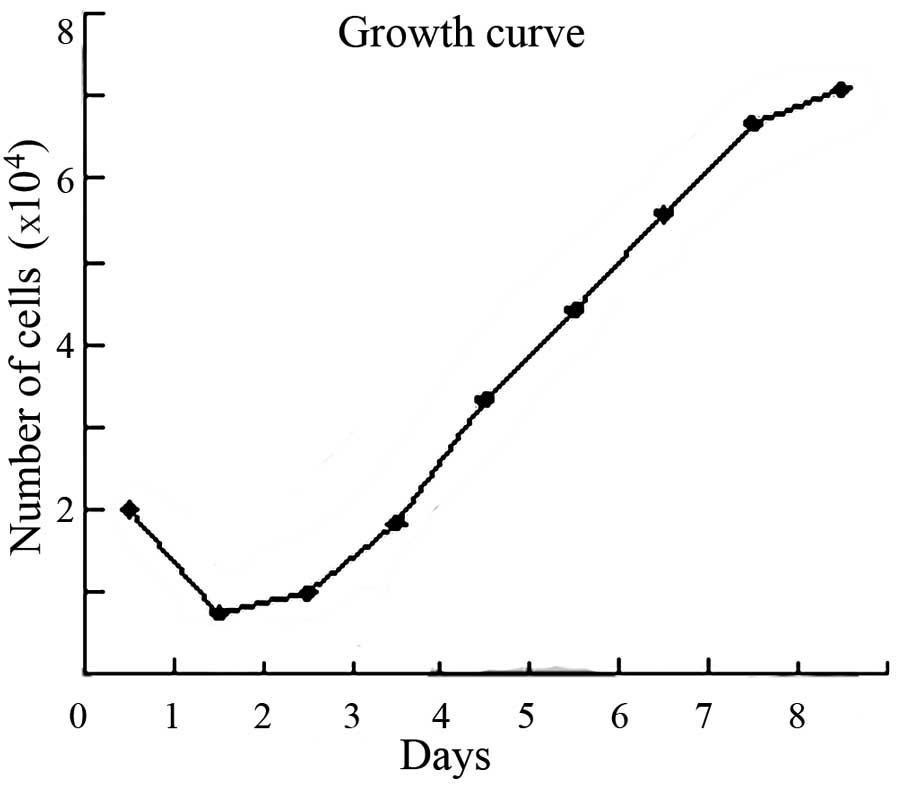
As shown in the cell growth curve, the population
doubling time was ~37.1 h (Fig.
3).
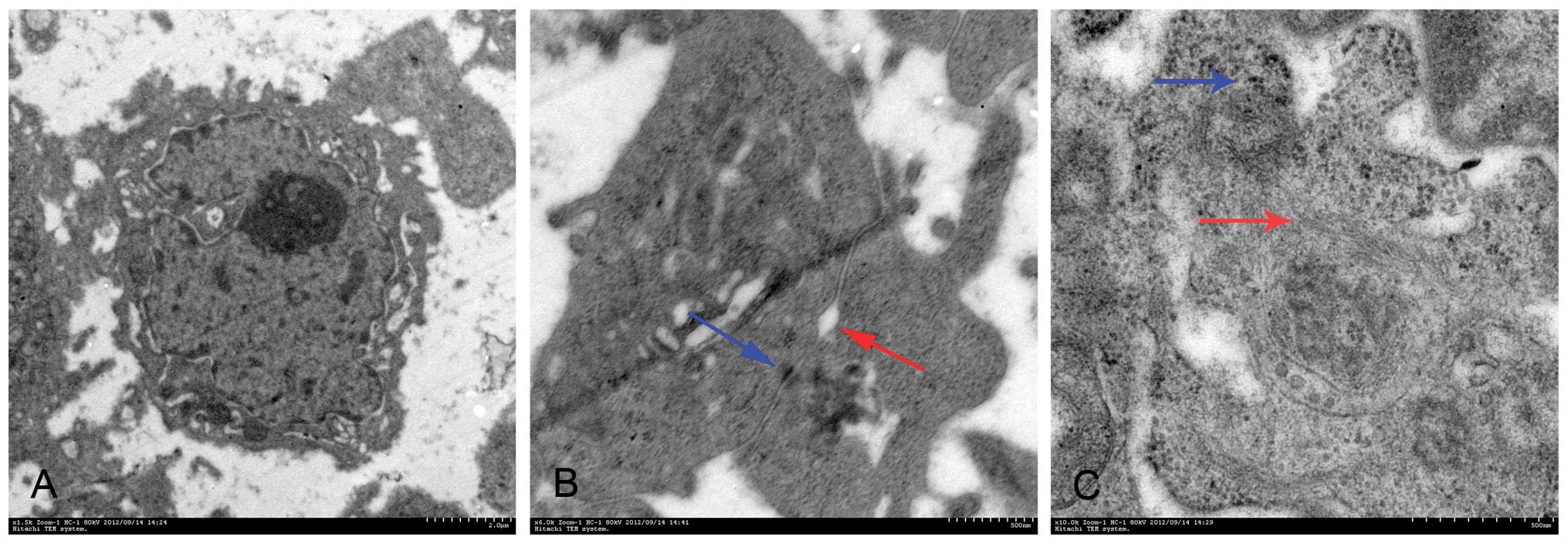
TEM observation
Poorly differentiated LACC-1 cells were different in
size and shape. Mitosis and multinucleated tumor giant cells were
common. Cytoplasm was small, and cell nuclei were large and
markedly atypical. The karyoplasmic ratio was reversed. One to
several nucleoli and many chromatins in the nuclei were found.
Euchromatin was abundant, and hetero-chromatin was highly
marginated along the nuclear membrane. Irregular dents accompanied
by occasional intranuclear pseudoinclusions were found on the
surface of most nuclear membranes. In the cytoplasm, spherical
secretory granules, which had uniform high-electron density,
appeared. Many free ribosomes were found in the cytoplasm. Large
ribosomes also appeared. The development of the rough endoplasmic
reticulum was not balanced in different cells. Golgi complexes were
occasionally visible. Other organelles were poorly developed.
Lacunas were observed between cancer cells. The free surface of the
cells was covered with many microvilli, which were uneven in
length. Cancer cells were connected by a tight junction instead of
a typical desmosome junction (Fig.
4).
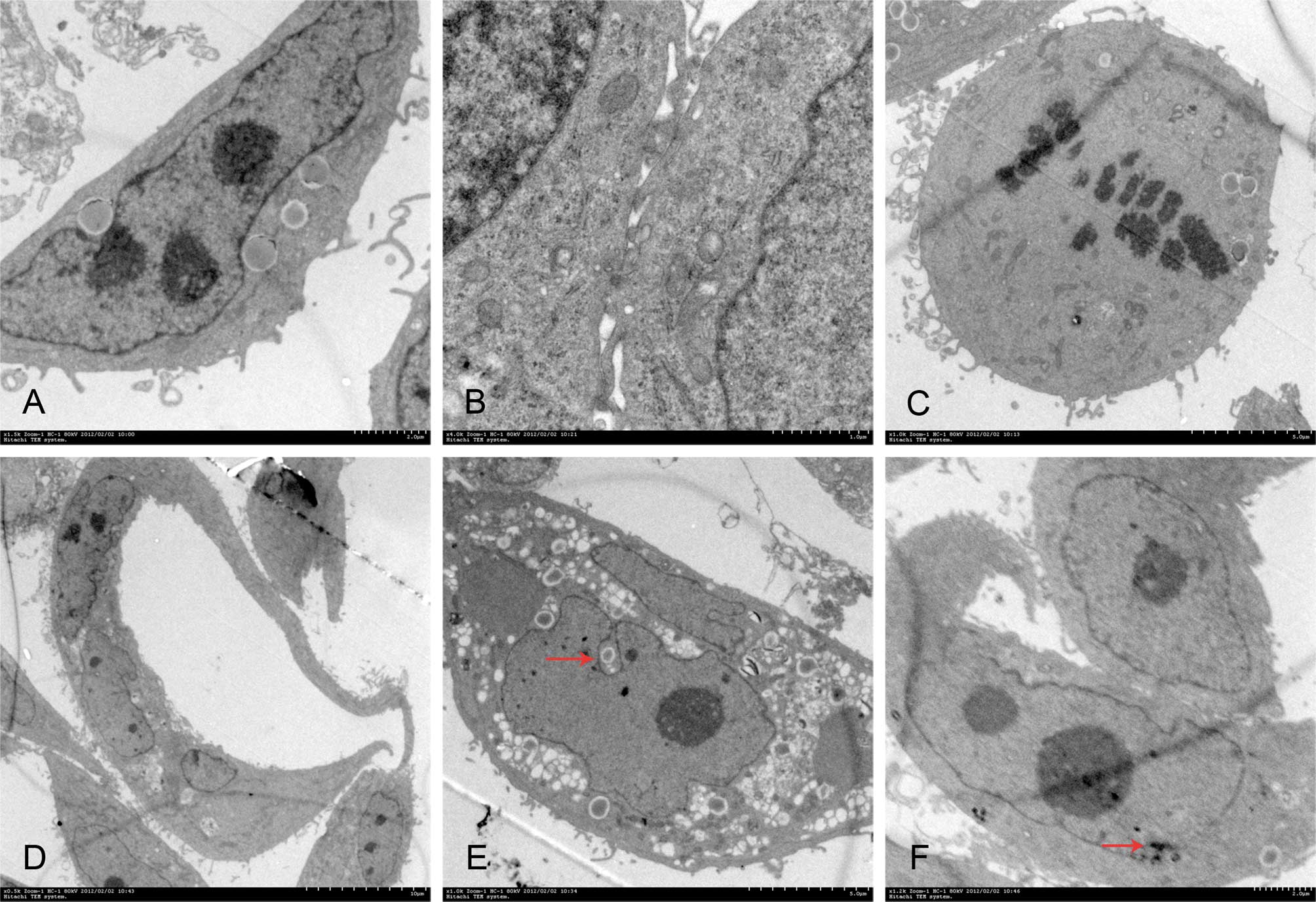 | Figure 4Transmission electron microscopic
observation. (A) The karyoplasmic ratio is reversed. Three large
nuclei appear. Original magnification, ×2.5k; scale bar, 2.0
μm. (B) Cancer cells are connected by a tight junction.
Original magnification, ×4k; scale bar, 1.0 μm. (C) The cell
undergoes division, and its free surface is covered with
microvilli. Original magnification, ×1k; scale bar, 5.0 μm.
(D) Multinucleated tumor giant cells. Original magnification,
×0.5k; scale bar, 10.0 μm. (E) Intranuclear pseudoinclusion
(red arrow). Original magnification, ×1k; scale bar, 5.0 μm.
(F) Huge ribosomes appear (red arrow). Original magnification,
×1.2k; scale bar, 2.0 μm. |
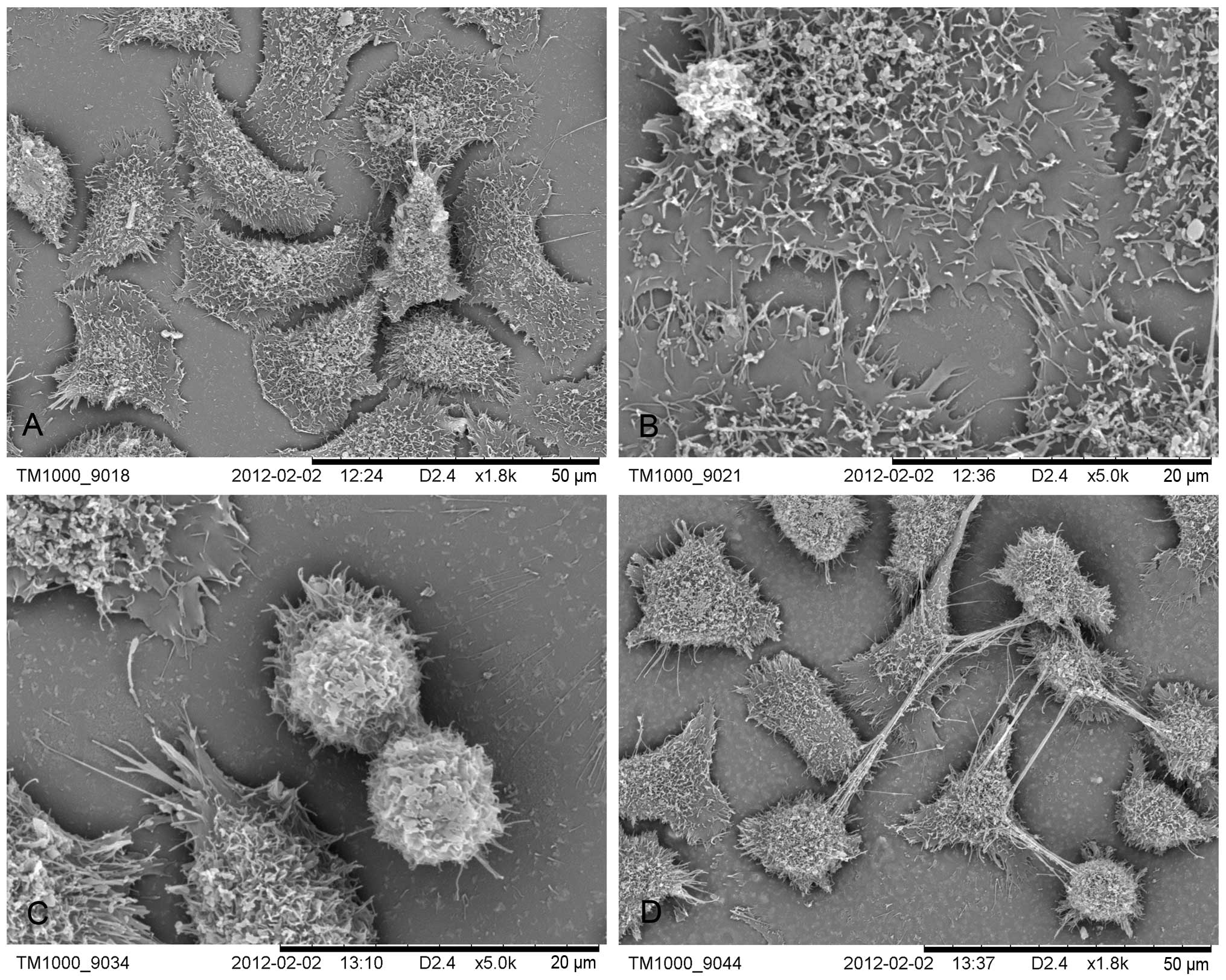
SEM observation
The LACC-1 cells were mostly polygonal but appeared
in other sizes and shapes. Abundant microvilli were apparent on the
surface of the cells. Cellular processes looked like lamellar or
vesicular structures. Adjacent cell processes were interconnected.
In the mitotic phase, the cells were apophysis. Numerous filopodia
appeared on the surface of the cancer cells except dense
microvilli. Filopodia appeared as a multistage branch that could
support the apophysis of the cancer cells. Adjacent cells were
connected by active filopodia (Fig.
5).
Animals
Two days after subcutaneous injection of
1×106 cells, some of the mice developed nodules under
the subaxillary. All 10 mice developed solid tumors at 7 days after
inoculation. The formation rate of the transplanted tumor was 100%.
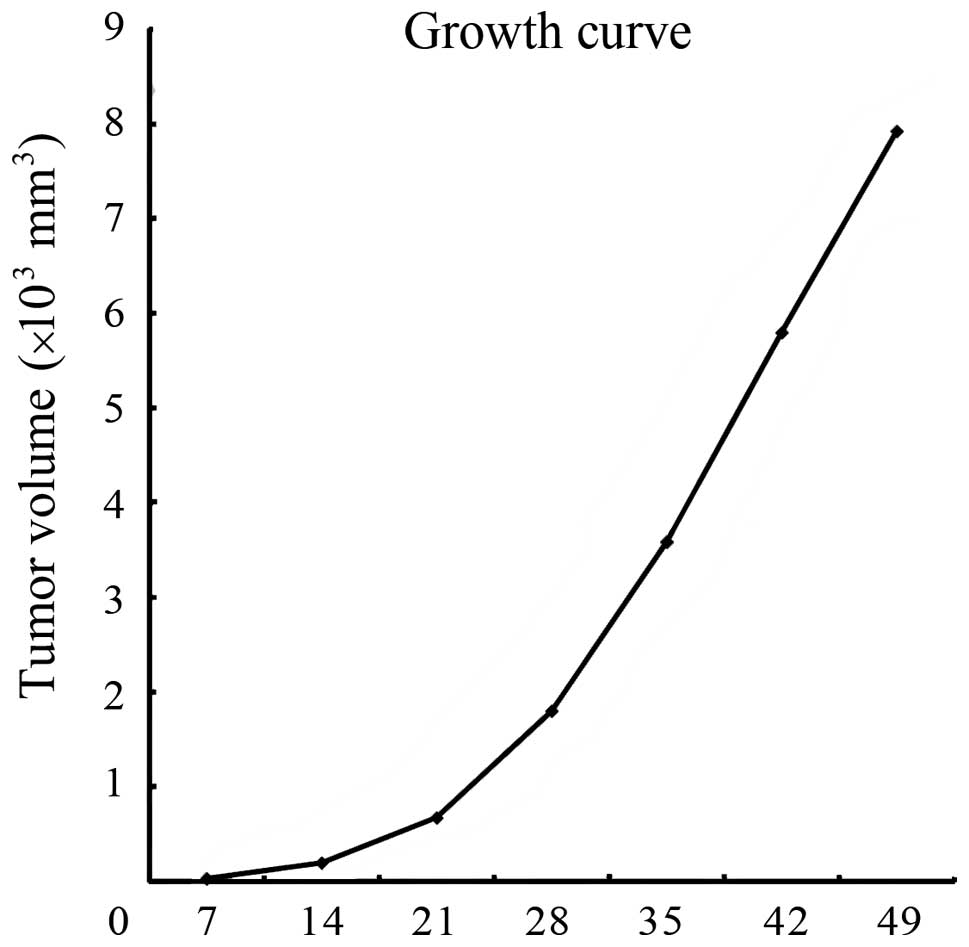
The tumor volumes were observed at 7, 14, 21, 28, 35, 42 and 49
days after injection, and the growth curve was determined (Fig. 6). The transplanted tumors grew
faster in the first three weeks after injection.
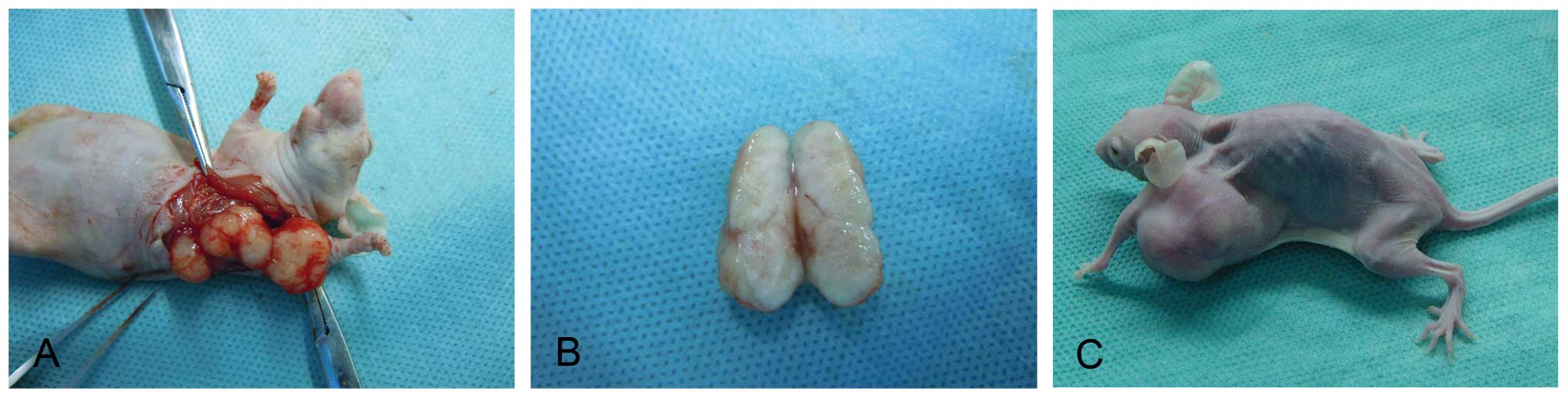
Fourteen days after injection, the xenograft tumors
became round or oval and covered with a capsule. The graft tumors
exhibited no adhesion to the adjacent tissue. The texture of the
tumor was medium. The color of the section was pale. At 49 days
after injection, the xenograft tumors were still covered with a
capsule. They did not adhere to the viscera but intruded into the
chest wall and thoracic cavity. No lung, liver and axillary lymph
node metastases occurred (Fig.
7).
The xenograft tumors were excised on day 14, 28, 35,
42 and 49 after injection. The main structure of the tumors was the
same but more necrotic tissue was present in the center of the
tumor as time passed. As observed under a light microscope, the
tumors were covered with a thin connective tissue. The tumors
exhibited mostly a solid pattern and mucoid degeneration occurred
in the local area. Tumor cells were round or oval. They had large
hyperchromatic nuclei and less cytoplasm. Mitosis and
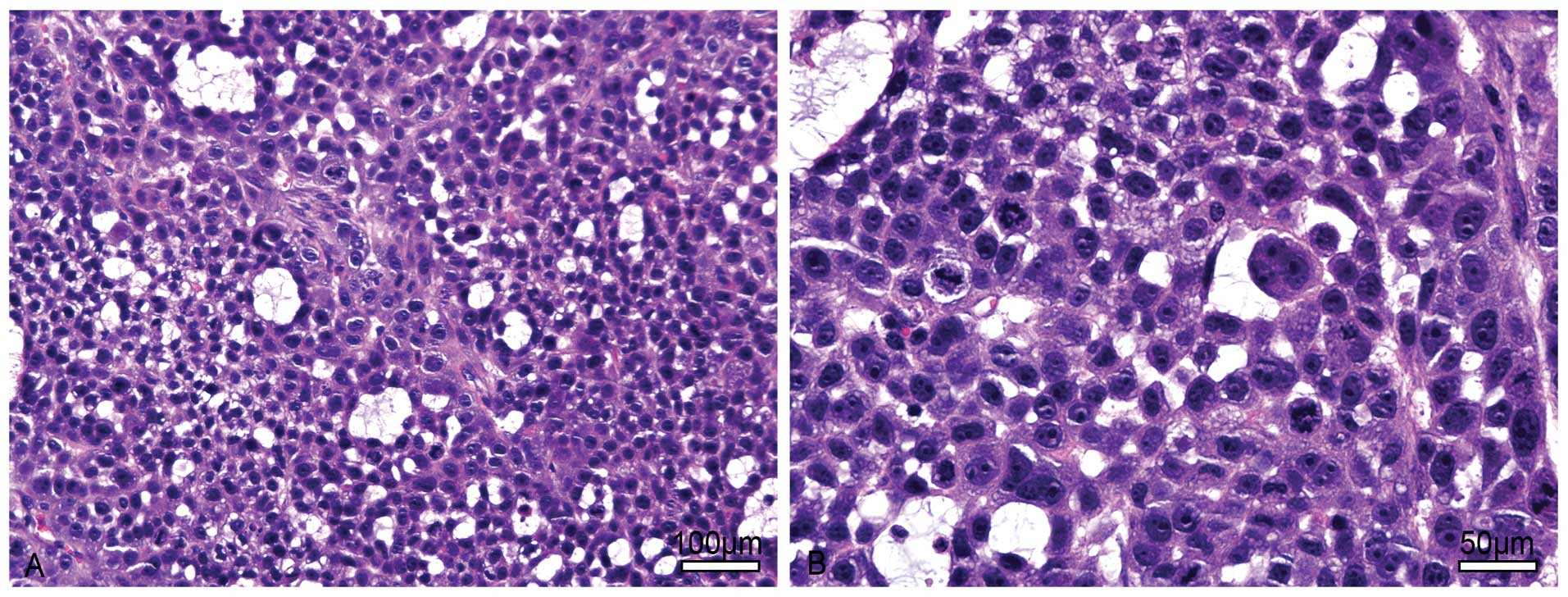
multinucleated giant tumor cells were commonly noted (Fig. 8).
The xenograft tumor cells had the same
ultrastructure as the LACC-1 cells under TEM observation. However,
the tumor cells were mostly connected by a gap junction instead of
a tight junction. Some dysplastic desmosomes appeared occasionally.
A small amount of microvilli covered the free surface of the tumor
cells. No form of mature glandular tubes was noted (Fig. 9).
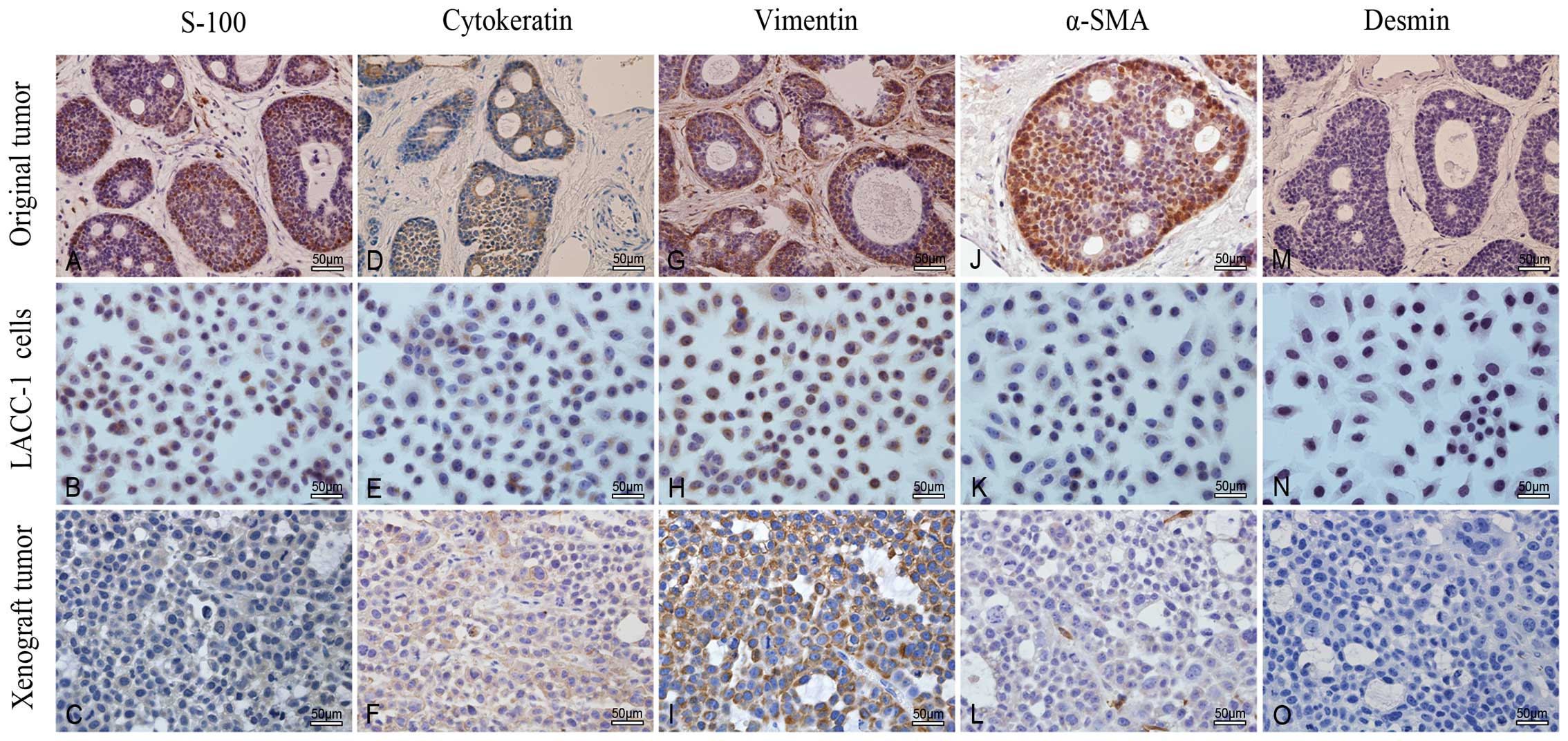
Immunocytochemical detection of protein
expression
The immunohistochemical examinations revealed the
similarity of cytoskeletal protein (e.g., CK, vimentin, α-SMA and
desmin) and S-100 expression in the original tumor, LACC-1 cells
and xenograft tumors. Immunoreactivity of these proteins gradually
decreased in the three tissues (Fig.
10 and Table I).
 | Table IImmunocytochemistry of S-100, CK,
vimentin, α-SMA and desmin in the original tumor, LACC-1 cells and
xenograft tumors. |
Table I
Immunocytochemistry of S-100, CK,
vimentin, α-SMA and desmin in the original tumor, LACC-1 cells and
xenograft tumors.
| Protein | Original tumor | LACC-1 cells | Xenograft tumors |
|---|
| S-100 | ++ | + | + |
| Cytokeratin | +++ | +++ | + |
| Vimentin | +++ | +++ | +++ |
| α-SMA | +++ | + | + |
| Desmin | − | − | − |
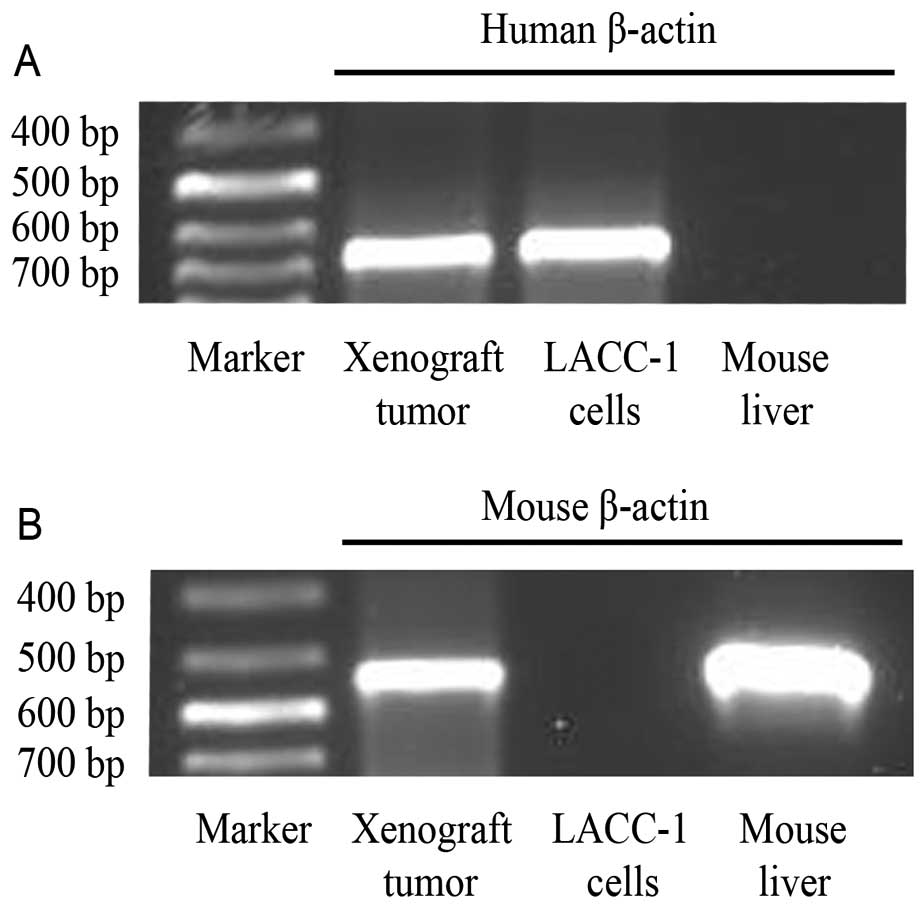
RT-PCR
RT-PCR analysis showed a strong positive reaction to
the human β-actin gene and weak reaction with the mice β-actin gene
in the xenograft tumors. It also showed a human β-actin gene band
in the LACC-1 cell line and a mouse β-actin gene band in the nude
mouse livers (Fig. 11).
Discussion
ACC of the human lacrimal glands is generally
considered to originate from the lacrimal duct epithelium. It is a
highly malignant neoplasm capable of diffuse invasion into adjacent
tissues and distant metastasis. To investigate the biological
characteristics of lacrimal gland ACC, we established the LACC-1
cell line. Many ACC cell lines have been established, such as KoA-1
(5), KoA-1L3 (6), ACC2 (7), ACC3 (7), ACCS (8), ACCY (8), ACC-M (9), ACCNS (10), ACCI (11) and ACCIM (12). However, all these ACC cell lines
were derived from the salivary glands. A recent study reports that
sinonasal, lacrimal and tracheobronchial ACCs have significantly
worse outcomes than ACC of the major salivary glands (13). Therefore, establishing a lacrimal
gland ACC cell line is essential to study its unique biological
behavior.
The most common cell primary culture methods are
tissue block culture method and enzyme digestion. The former is
simpler and more effective than the latter in solid tumor primary
culture. Many ACC cell lines have been established through tissue
block culture method, such as ACC2 (7), ACC3 (7), ACCS (8), ACCY (8) and SACC-83 (14).
To ensure the survival of isolated LACC-1 cells, a
complete medium containing 20% FBS was used for the optimal growth.
At the same time, the cell culture flasks were pretreated with
serum to promote tissue block adherence. At the early stage, tissue
block adherence was not strong. Changing the medium required us to
act slowly and gently. The fibroblasts predominated when the cells
grew from the edge of the explants. To discard the fibroblasts and
purified tumor cells, many different methods can be used. Tanaka
et al (11) established the
ACCNS cell line by culturing the cell mixture on a feeder layer of
fibroblasts to remove the fibroblasts. Fibroblasts have a
mono-layer culture in a flask and are easy to dissociate, whereas
epitheloid cells have a multilayer culture and are difficult to
dissociate at the original stage. Thus, we used 0.25% trypsin and
0.02% EDTA mixture to detach the fibroblasts from the bottom of the
flask while the epitheloid cells remained adhered to the flask.
When the digestion procedure was stopped, the epitheloid tumor
cells adhered to the flask faster and stronger than the
fibroblasts. Thus, many fibroblasts were suspended and easily
discarded. We used dissociation and repeated adherence methods to
discard most of the fibroblasts and then combined continuous
passage with the mechanical scraping method. Finally, we obtained
pure epitheloid tumor cells after 8 passages. The malignant tumor
characteristics then emerged.
LACC-1 cells appeared as a histologically solid
pattern and continuous passage culture. They showed an epithelioid
culture in the flask and presented with a cobblestone-like
appearance when they reached confluency. The nuclei were large and
round with many abnormal mitoses. The nucleoplasm ratio was high,
and multinucleated giant cells were evident. LACC-1 cells showed a
tendency to have overlapping growth without contact inhibition when
the cell density continued to increase.
Under TEM, the LACC-1 cells were poorly
differentiated. Mitosis and multinucleated giant tumor cells were
common. The cytoplasm was small, whereas the cell nuclei were large
and markedly atypical. The karyoplasmic ratio was reversed.
Many differences were noted between ACC-2 (7) and the LACC-1 cells under TEM. For
example, in the LACC-1 cytoplasm, spherical zymogen secretory
granules were found, and they had a uniform high-electron density.
Large ribosomes appeared in the LACC-1 cells. The development of
rough endoplasmic reticulum was not balanced in the different
cells. Golgi complexes were occasionally visible. Other organelles
were poorly developed. LACC-1 cells were connected by a tight
junction instead of a typical desmosome junction.
The surface of the LACC-1 cells exhibited affluent
microvilli, protuberances and filopodia under the SEM. This finding
may be related to their high invasiveness and metastatic potential.
When LACC-1 cell protuberances or filopodia adhere to adjacent
connective tissues and nerves, they may increase basement membrane
invasiveness and spread along the way or even invade blood vessels
to cause hematogenous metastasis. The exact mechanisms of the
invasive and metastatic ability of LACC-1 must be thoroughly
studied further.
As detected by IHC, the original tumor, the LACC-1
cell line and the xenograft tumors expressed both CK and vimintin.
CK is a key component of intermediate fibers of the cytoskeleton of
epithelial cells and is expressed in epithelial cells, epithelial
tumors and metastatic cells (15).
Therefore, it is useful for identifying whether or not the
metastatic tumor comes from the epithelium. Vimentin is an
intermediate filament expressed in the tissues of normal
mesenchymal origin. It is also a tumor biomarker for identifying a
mesenchymal-derived tumor. However, it is known to be expressed
aberrantly in epithelial cancers of the prostate, gastrointestinal
tract, breast, central nervous system, lung and malignant melanomas
(16). According to Chu et
al (17), the coexpression of
vimentin and CK intermediate filaments by epithelial or
nonepithelial tumors is advantageous for migratory and invasive
functions due to unique interactions between cell surface
receptors, the cytoskeleton and ECM. Desmin is an intermediate
filament protein that is widely distributed in skeletal muscle,
cardiac muscle, smooth muscle and myoepithelial cells. However, it
was not demonstrated in either the LACC-1 cell line or the original
and xenograft tumors. The S-100 proteins are expressed in neural
tissues and are involved in the regulation of cellular processes,
such as cell cycle progression and differentiation. S-100 is
localized in the cytoplasm and nuclei of astrocytes, Schwann cells,
ependymomas and astrogliomas. Actin filaments can form both stable
and labile structures and are crucial components of microvilli and
the contractile apparatus of muscle cells. Furuse et al
(18) found that the
α-smooth-muscle actin (α-SMA) is useful for the identification of
myoepithelial cells, especially in cribriform and tubular ACC. In
the present study, the original tumor, the LACC-1 cell line and
xenograft tumors all showed a positive reaction to S-100 protein
and α-SMA. The LACC-1 tumors had a perineural invasion biological
feature. Under TEM, spherical zymogen secretory granules were
observed in the LACC-1 cytoplasm. Thus, we speculated that the
LACC-1 tumor could have neuroendocrine tumor characteristics.
To date, many heterotransplanted human ACCs have
been established (5,6,11,12,19),
and the characterization of ACC proliferation, invasion and
metastasis have been frequently reported. However, most of these
xenograft model systems have been derived from the salivary glands
or oral cavity. The present study first established a human ACC
cell line of the lacrimal glands. The no. 84 generation LACC-1 cell
line was inoculated subcutaneously into the subaxillary of nude
mice. The tumorigenic potential was then evident. The formation
rate of the transplanted tumors was 100% on day 7 after
inoculation. This finding demonstrated that the LACC-1 cell line
was malignant with tumorigenic ability. The xenograft tumors
retained the same histological characteristics of a solid pattern
as the LACC-1 original tumor after inoculation for 49 days.
Lacrimal gland ACC usually exhibits a combination of three
distinctive growth patterns: cribriform, tubular and solid. The
predominant growth pattern is predictive of prognosis. For example,
the solid pattern generally indicates worse prognosis when compared
with the tubular or cribriform pattern.
RT-PCR was used to elucidate human xenograft tumors.
A strong human β-actin gene band was detected in the PCR product
and confirmed human origin. The weak mouse β-actin gene band
remained probably because adjacent thin connective tissues covered
the xenograft tumors and were combined with the tumors when
total-RNA was extracted.
When the xenograft tumors were removed 49 days
later, we observed that the tumors intruded into the chest wall and
thoracic cavity of the mice instead of metastasizing to the lung,
liver or axillary lymph nodes. In the clinic, ACCs derived from
lacrimal glands have a different biological behavior than those
derived from the salivary glands. For example, ~40–60% of salivary
gland ACCs develop distant metastases (i.e., lungs, bones and soft
tissues) despite good local control of the tumor (20). Lacrimal gland ACCs may spread into
the intracranial cavity, nasal cavity and temporal fossa or
metastasize to the lung, bone, liver and lymph nodes. For lacrimal
gland ACC, intracranial invasion is the leading cause of death, and
the 5-year metastasis rate is 25.71% (9/35) (4), which is lower than that of the
salivary glands. However, no metastatic phenomenon occurred during
the 49 days possibly because the observation time was not long
enough.
Overall, the newly established LACC-1 cell line was
subcultured for more than 100 passages during the last two years.
This cell line originated from a solid pattern of human lacrimal
gland ACC and produced tumors through subcutaneous transplantation
into nude mice. In conclusion, the LACC-1 cell line is the first
established cell line derived from human lacrimal glands and to
exhibit tumorigenicity in nude mice. The LACC-1 cell line provides
a beneficial model for studying the biological characteristics of
human lacrimal gland ACC.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 31100991).
References
|
1
|
Lee DA, Campbell RJ, Waller RR and Ilstrup
DM: A clinicopathologic study of primary adenoid cystic carcinoma
of the lacrimal gland. Ophthalmology. 92:128–134. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Font RL and Gamel JW: Epithelial tumors of
the lacrimal gland: an analysis of 265 cases. Ocular and Adnexal
Tumors. Jakobiec FA: Aesculapius; Birmingham, AL: pp. 787–805.
1978
|
|
3
|
Font RL, Smith SL and Bryan RG: Malignant
epithelial tumors of the lacrimal gland; a clinicopathologic study
of 21 cases. Arch Ophthalmol. 116:613–616. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin TT, He YJ, Zhang H, Song GX, Tang DR
and Zhao HF: Analysis of treatment and prognosis of orbital adenoid
cystic carcinoma. Zhonghua Yan Ke Za Zhi. 45:309–313. 2009.In
Chinese. PubMed/NCBI
|
|
5
|
Umeda M, Komatsubara H, Nishimatsu N, Oku
N, Shibuya Y, Yokoo S and Komori T: Establishment and
characterization of a human adenoid cystic carcinoma line of the
salivary gland which is serially transplantable and spontaneously
metastasises to the lung in nude mice. Oral Oncol. 38:30–34. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Umeda M, Komatsubara H, Ojima Y,
Minamikawa T, Shibuya Y, Yokoo S and Komori T: Establishment and
characterization of metastasizing cell lines from a
heterotransplanted human adenoid cystic carcinoma. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 98:211–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He RG, Zhang XS, Zhou XJ, et al: The
establishment of cell lines of adenoid cystic carcinoma of human
salivary glands (ACC2, ACC3) and a study of morphology. West Chin J
Stomatol. 6:1–4. 1988.
|
|
8
|
Shirasuna K, Watatani K, Furusawa H, Saka
M, Morioka S, Yoshioka H and Matsuya T: Biological characterization
of pseudocyst-forming cell lines from human adenoid cystic
carcinoma of minor salivary gland origin. Cancer Res. 50:4139–4145.
1990.PubMed/NCBI
|
|
9
|
Guan X, Qiu W and He R: The selection of
highly lung metastatic salivary adenoid cystic carcinoma clone.
Zhonghua Kou Qiang Yi Xue Za Zhi. 31:74–77. 1996.In Chinese.
PubMed/NCBI
|
|
10
|
Guan XF, Qiu WL, He RG and Zhou XJ:
Selection of adenoid cystic carcinoma cell clone highly metastatic
to the lung: an experimental study. Int J Oral Maxillofac Surg.
26:116–119. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka N, Urabe K, Hashitani S, Sakurai K
and Urade M: Establishment and characterization of a human adenoid
cystic carcinoma cell line forming colonies cultured in collagen
gel and transplantable in nude mice. Oncol Rep. 17:335–340.
2007.PubMed/NCBI
|
|
12
|
Hashitani S, Urade M, Zushi Y, Segawa E,
Okui S and Sakurai K: Establishment of nude mouse transplantable
model of a human adenoid cystic carcinoma of the oral floor showing
metastasis to the lymph node and lung. Oncol Rep. 17:67–72.
2007.
|
|
13
|
Lin YC, Chen KC, Lin CH, Kuo KT, Ko JY and
Hong RL: Clinicopathological features of salivary and non-salivary
adenoid cystic carcinomas. Int J Oral Maxillofac Surg. 41:354–360.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li SL, Liu XP and Zhang KH: Establishment
of a human cancer cell line from adenoid cystic carcinoma of the
minor salivary gland. Zhonghua Kou Qiang Yi Xue Za Zhi. 25:29–31.
621990.In Chinese.
|
|
15
|
Zhang X, Xie J, Yu C, Yan L and Yang Z:
mRNA expression of CK19, EGFR and LUNX in patients with lung cancer
micrometastasis. Exp Ther Med. 7:360–364. 2014.PubMed/NCBI
|
|
16
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chu YW, Seftor EA, Romer LH and Hendrix
MJ: Experimental coexpression of vimentin and keratin intermediate
filaments in human melanoma cells augments motility. Am J Pathol.
148:63–69. 1996.PubMed/NCBI
|
|
18
|
Furuse C, Sousa SO, Nunes FD, Magalhães MH
and Araújo VC: Myoepithelial cell markers in salivary gland
neoplasms. Int J Surg Pathol. 13:57–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moskaluk CA1, Baras AS, Mancuso SA, Fan H,
Davidson RJ, Dirks DC, Golden WL and Frierson HF Jr: Development
and characterization of xenograft model systems for adenoid cystic
carcinoma. Lab Invest. 91:1480–1490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ellis GL and Auclair PL: Tumors of the
salivary glands. Atlas of Tumor Pathology. 3rd edition. Armed
Forces Institute of Pathology; Bethesda, MD: pp. 203–216. 1996
|