Introduction
Despite treatment advances in other types of cancer,
pancreatic cancer remains the fourth deathliest cancer worldwide
with a 5-year survival rate of only 4% (1) in part since it is rarely detected at
an early stage. For patients with inoperable locally advanced
pancreatic cancer, standard treatment with chemotherapy or
chemoradiotherapy results in a median survival time of 6–9 months
(2) with combined radiochemotherapy
resulting in improved 6-, 12- and 18-month survival rates (3). Therefore, current efforts have focused
on identifying novel therapeutic targets for the treatment of
pancreatic cancer.
Angiogenesis is crucial for the growth and
progression of tumors. In isolated perfused thyroid glands,
transplanted melanoma cells grew into tumors of only 1–2 mm in
diameter and were not vascularized, suggesting that
neovascularization is required for tumor growth (4). Tumors may become necrotic or even
apoptotic in the absence of vascular support (5,6);
therefore, angiogenesis inhibition has become an important
therapeutic strategy in the prevention of tumor expansion and
metastasis (7). Evidence suggests
that specific microRNAs (miRNAs) can regulate angiogenesis through
the downregulation of angiogensis-related genes by interacting with
their 3′ untranslated region (UTR) (8). Recent studies that disrupted the
function of Dicer and Drosha have revealed the important roles of
certain miRNAs, known as angiomiRs, in regulating angiogenesis and
modulating endothelial cells (ECs) (9–11).
Dicer hypomorphic mouse lines have defects in vascular
remodeling during development and ovarian angiogenesis (9,10). In
addition, in vitro knockdown of Dicer or Drosha in human ECs
decreased angiogenesis (11).
AngiomiRs regulate angiogenesis either
cell-autonomously or non-cell-autonomously (12). miR-296 targets hepatocyte growth
factor-regulated tyrosine kinase substrate mRNA, thereby
suppressing the degradation of vascular endothelial growth factor
receptor 2 (VEGFR2) and platelet-derived growth factor receptor-β
(PDGFR-β) (13). In addition,
miR-296 inhibition decreased tumor xenograft angiogenesis in
vivo (13). Conversely, ectopic
expression of miR-18a inhibited gastric cancer cell xenograft
growth in vivo by reducing tumor angiogenesis (14). miRNAs also modulate the angiogenic
properties of human umbilical vein ECs (HUVECs) (15); suppression of miR200a expression
inhibited HUVEC viability and migration (16). Ectopic expression of miR-199a
suppressed EC migration and reduced the expression of vascular cell
adhesion molecule-1 (VCAM-1) and intracellular adhesion molecule-1
(ICAM-1) (17). miR-149 regulated
fibroblast growth factor 2 (FGF2)-induced EC proliferation and
migration (18).
Given the importance of angiogenesis in tumor
progression, the present study was undertaken to test the
hypothesis that certain miRNAs expressed in cancer-associated ECs
(CECs) may regulate angiogenesis. We identified miRNAs that were
differentially expressed in primary EC cultures derived from three
pancreatic cancer tissues as compared to those from adjacent normal
tissues. Inhibition of miR-139 or miR-200c significantly reduced
cancer endothelial cell (CEC) migration, the average tube length,
and total loop number, suggesting that they play a role in tumor
angiogenesis.
Materials and methods
Patient tissue samples
Pancreatic tumor and normal adjacent tissues were
obtained from three pancreatic cancer patients who underwent
surgery in the Department of General Surgery, Shanghai First
People’s Hospital, Shanghai Jiaotong University from October 2012
to May 2013. According to the tumor-node-metastasis (TNM) staging
system (7th edition) of the American Joint Commission on Cancer
(AJCC), all patients had stage I or II (T1-3, N0-1, M0) pancreatic
cancer. None of the patients had received chemotherapy or cytotoxic
agents within the last 12 months prior to the inclusion into this
study. None of the patients showed clinical signs for active
infectious diseases. Patients with stage III and IV pancreatic
cancer with locally advanced unresectable foci (T4) and/or systemic
metastases (M1) were excluded. Informed consent was obtained from
all study participants. The present study was approved by the
Ethics Committee of Shanghai First People’s Hospital, Shanghai
Jiaotong University, Shanghai, China.
Establishment of primary endothelial cell
cultures from human pancreatic tumor samples
Primary endothelial cell cultures derived from human
pancreatic tumor masses or adjacent normal tissue were prepared
according to the method reported by Naschberger et al
(19). Briefly, after tissues were
cut into 1-mm3 blocks, they were digested with
collagenase II (17,100 U/g) in 5 ml of endothelial basal medium
(EBM-2) supplemented with 0.5% fetal bovine serum (FBS) (EBM-low;
both from Lonza, Cologne, Germany) at 37°C with 5% CO2
for 1 h under constant agitation. Following digestion, cells were
filtered (cell strainer 100 µm; BD Biosciences, Franklin
Lakes, NJ, USA) and isolated by centrifugation at 500 × g for 5 min
at 20°C. The cell pellet was resuspended in 5 ml of EGM-2-MV
(EGM-2-MV BulletKit; Lonza), and the cell suspension was added to
flasks pre-coated with 1.5% gelatin. The growth medium was
refreshed every 2 days for 5–7 days until the cell confluence
reached 70–80%. After the cells were detached with 1–2 ml of
Accutase (Life Technologies, Carlsbad, CA, USA), they were washed
with EGM-2-MV. The cells were resuspended in 60 µl of MACS
buffer (1X PBS pH 7.2, 0.5% bovine serum albumin, 2 mM EDTA), and
then were incubated with 20 µl of CD31 beads (CD31 MicroBead
kit; Miltenyi Biotec, Bergisch Gladbach, Germany) at 4°C for 15
min. After washing with MACS buffer, the cells were resuspended in
1 ml MACS buffer, which was applied to a MACS separation column
(Miltenyi Biotec), and the endothelial cells were isolated
following the manufacturer’s instructions. The cell concentration
was adjusted to 1−2×104/cm2 and cultured on
25 T flasks pre-coated with 0.5% gelatin. Cells were cultured until
complete confluency was reached, and the medium was refreshed every
2 days.
miRNA extraction
Total RNA was isolated using the mirVana™ microRNA
isolation kit according to the manufacturer’s instructions (Ambion,
Austin, TX, USA). In brief, the sample was first lysed in a
denaturing lysis solution, and the lysate was then subjected to
phenol-chloroform extraction. After purification over a glass-fiber
filter, the total RNA integrity was confirmed with an Agilent 2100
Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA).
miRNA microarray and data analysis
The miRNA expression profiles in CECs were compared
with those of normal endothelial cells (NECs) derived from the same
patient. Affymetrix FlashTag® Biotin HSR was used for
miRNA labeling and hybridization onto the Affymetrix
GeneChip® MicroRNA 3.0 Array (both from Affymetrix,
Santa Clara, CA, USA) following the manufacturer’s instructions.
The miRNA expression profile was scanned through a
GeneChip® Scanner 3000 (cat. #00-00212, Affymetrix), and
primary data were analyzed using GeneChip-compatible™, Command
Console Software 3.1 and Expression Console Software. The CEC and
NEC miRNA expression profiles of the same patient were compared to
identify differentially expressed mRNAs. In addition, the array
results were also compared against the miRNA databases, microrna.org and TargetScan, to identify candidate
miRNAs that are relevant to angiogenesis.
Candidate miRNA validation
The differential expression of miR-200c, miR-182,
miR-139-5p and miR-200b was validated by qPCR analysis using the
stem-loop TaqMan MicroRNA Assay (Applied Biosystems, Foster City,
CA, USA), according to the manufacturer’s instructions. Briefly,
mature candidate miRNAs were reverse transcribed into cDNA from 10
ng of total RNA with mature miRNA-specific looped RT primers from
the TaqMan MicroRNA assays kit and reagents from the TaqMan
MicroRNA Reverse Transcription kit (Applied Biosystems) following
the manufacturer’s instructions. Real-time PCR was performed on the
5′-extended cDNA with Applied Biosystems TaqMan 2X Universal PCR
Master Mix and the appropriate 5X TaqMan MicroRNA Assay Mix (both
from Applied Biosystems) for each miRNA of interest. For each
sample, the threshold cycle (Ct) was calculated by the ABI 7500
Sequence Detection System software. Standard curves were used to
determine miRNA concentration in the samples, which were then
normalized to small nuclear RNA U (RNU) RNA.
miRNA knockdown in CECs
The specific inhibitors for miR-139 or miR-200c
(MiScript microRNA Inhibitor; Qiagen, Hilden, Germany) were added
into CEC cultures to evaluate the effect of these miRNAs on CEC
proliferation. In addition, since miR-1 regulates angiogenesis
(i.e., enhanced tube formation and migration of human-derived
cardiomyocyte progenitor cells) (20), a miR-1 inhibitor (Qiagen) was used
as a positive control. An inhibitor with scrambled sequence that
has no homology to any known mammalian gene (Qiagen) was used as a
negative control. Briefly, cells were seeded in a 24-well plate at
a density of 0.4−1.6×105 cells/well in 500 µl of
culture medium containing serum and antibiotics. After 4 h, cells
were incubated with 50 nM miRNA inhibitor diluted in 50 µl
of culture medium without serum and with 1.5 µl HiPerfect
transfection reagent (Qiagen). The cells were incubated with the
transfection complexes under normal growth conditions, and gene
expression was monitored after 12 h by qPCR. qRT-PCR data were
calculated as 2−ΔΔCt after normalizing to the
control.
Cell proliferation assay
Cell proliferation was assessed using the MTT assay
(Sigma, St. Louis, MO, USA) 48 h after siRNA inhibition following
the manufacturer’s instructions.
Cell migration assay
Transwell migration assay were conducted 48 h after
siRNA inhibition using a fluorometric cell migration assay kit with
polycarbonate membrane inserts (5-µm pore size; Cell
Biolabs, San Diego, CA, USA) using a modified protocol described by
Chim et al (21). Briefly,
cells were serum-starved overnight in Dulbecco’s modified Eagle’s
medium (DMEM) prior to initiation of the experiment. The lower
chambers were filled with 1 ml of medium containing 10% serum.
Cells (4×104) were resuspended in 200 µl of
Opti-MEM and added to the upper chamber. After 24 h at 37°C,
migrating cells were counted after staining with crystal
violet.
Tube formation assay
After cells were serum-starved overnight in DMEM,
they were seeded in 24-well plates that were coated with Geltrex™
reduced growth factor basement membrane matrix (Invitrogen,
Carlsbad, CA, USA) and incubated at 37°C for 30 min with Opti-MEM.
Medium was then removed and replaced with the medium containing 10%
serum and incubated at 37°C for 24 h. Four randomly selected fields
of view were analyzed, and tube formation was quantified by
measuring the length of tube-like structures and the number of
branching points. Tube length was assessed by drawing lines along
the tube-like structure and measuring the length of the line in
pixels using a modified protocol as previously described (21).
Statistical analysis
For cell proliferation and migration as well as tube
length and total loops, data are presented as mean ± standard
deviation (SD). One-sample t-tests were performed to evaluate the
cell proliferation with the mean of the control group set to 1.
Differences among the negative control, miR-1, mir-139 and miR-200c
inhibitor groups were also assessed by one-way ANOVA with a
post-hoc LSD test as for pair-wise comparisons. One-sample
t-tests were also performed to evaluate the mean tube length or
total loops set as 1. Moreover, differences between the negative
control and miR-1 inhibitor groups were compared using the
two-sample t-test. Statistical assessments were two-tailed, and
P-values <0.05 were considered to indicate statistically
significant results. SPSS 18.0 statistics software (SPSS, Inc.,
Chicago, IL, USA) was used for all statistical analyses.
Results
miRNA expression profiles in CECs and
NECs
The miRNA expression profiles of CECs were compared
with those of the NECs in three pancreatic patients. As shown in
Table I, 14 miRNAs were upregulated
by >20-fold in the CECs as compared to NECs, including
hsa-miR-139-5p, hsa-miR-182, hsa-miR-183, hsa-miR-192, hsa-miR-194,
hsa-miR-200a, hsa-miR-200b*, hsa-miR-200b, hsa-miR-200c,
hsa-miR-203, hsa-miR-25*, hsa-miR-27a*, hsa-miR-375 and
hsa-miR-92a-1*.
 | Table IDifferentially expressed miRNAs with
a >20-fold increased expression in CECs relative to NECs
(N=3). |
Table I
Differentially expressed miRNAs with
a >20-fold increased expression in CECs relative to NECs
(N=3).
| miRNA | Fold-change in
expression (CEC/NEC)a |
|---|
| hsa-miR-25* | 63.47±17.96 |
| hsa-miR-27a* | 86.33±32.15 |
| hsa-miR-92a-1* | 62.63±22.31 |
| hsa-miR-139-5p | 356.53±181.85 |
| hsa-miR-182 | 951.97±86.51 |
| hsa-miR-183 | 99.9±57.27 |
| hsa-miR-192 | 47.77±20.83 |
| hsa-miR-194 | 55.2±15.14 |
| hsa-miR-200a | 46±15.83 |
| hsa-miR-200b* | 93±39.66 |
| hsa-miR-200b | 151.07±67.69 |
| hsa-miR-200c |
4,574.97±2,209.62 |
| hsa-miR-203 | 77.87±37.57 |
| hsa-miR-375 | 58.2±33.34 |
Validation of differential expression of
candidate angiomiRs in CECs and NECs
We compared the primary miRNA data with available
miRNA databases (http://www.microrna.org; www.targetscan.org) to identify candidate
angiomiRs.
Of the miRNAs with >20-fold increased expression,
miR-182, miR-183, miR-192, miR-194, the miR-200 family, miR-203,
miR-27a*, miR-375 and miR-92a-1* have been implicated in regulating
tumor angiogenesis (16,22–32).
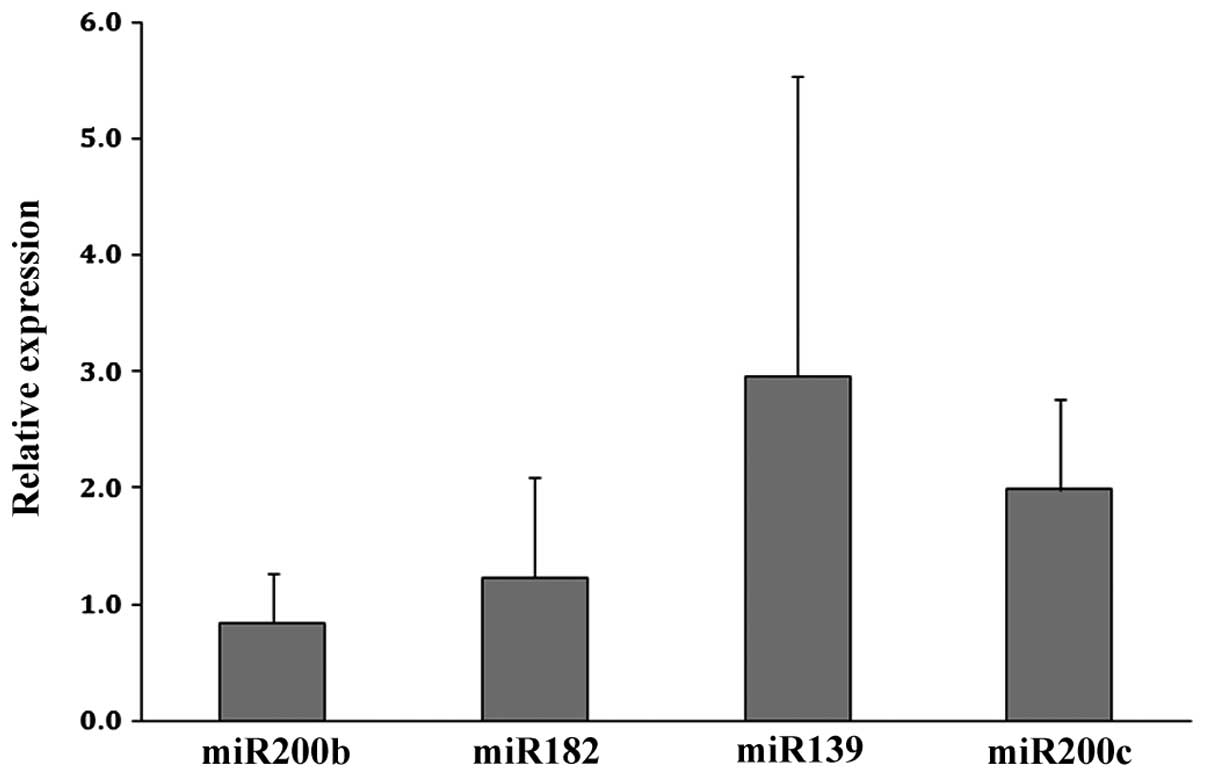
We chose to validate the upregulated expression of miR-200b,
miR-182, miR-139 and miR-200c using qPCR analysis as these
candidate angiomiRs had the greatest increase in fold-expression in
the CECs (Table I). As shown in
Fig. 1, the upregulated expression
of miR-139 and miR-200c was confirmed in CECs relative to NECs.
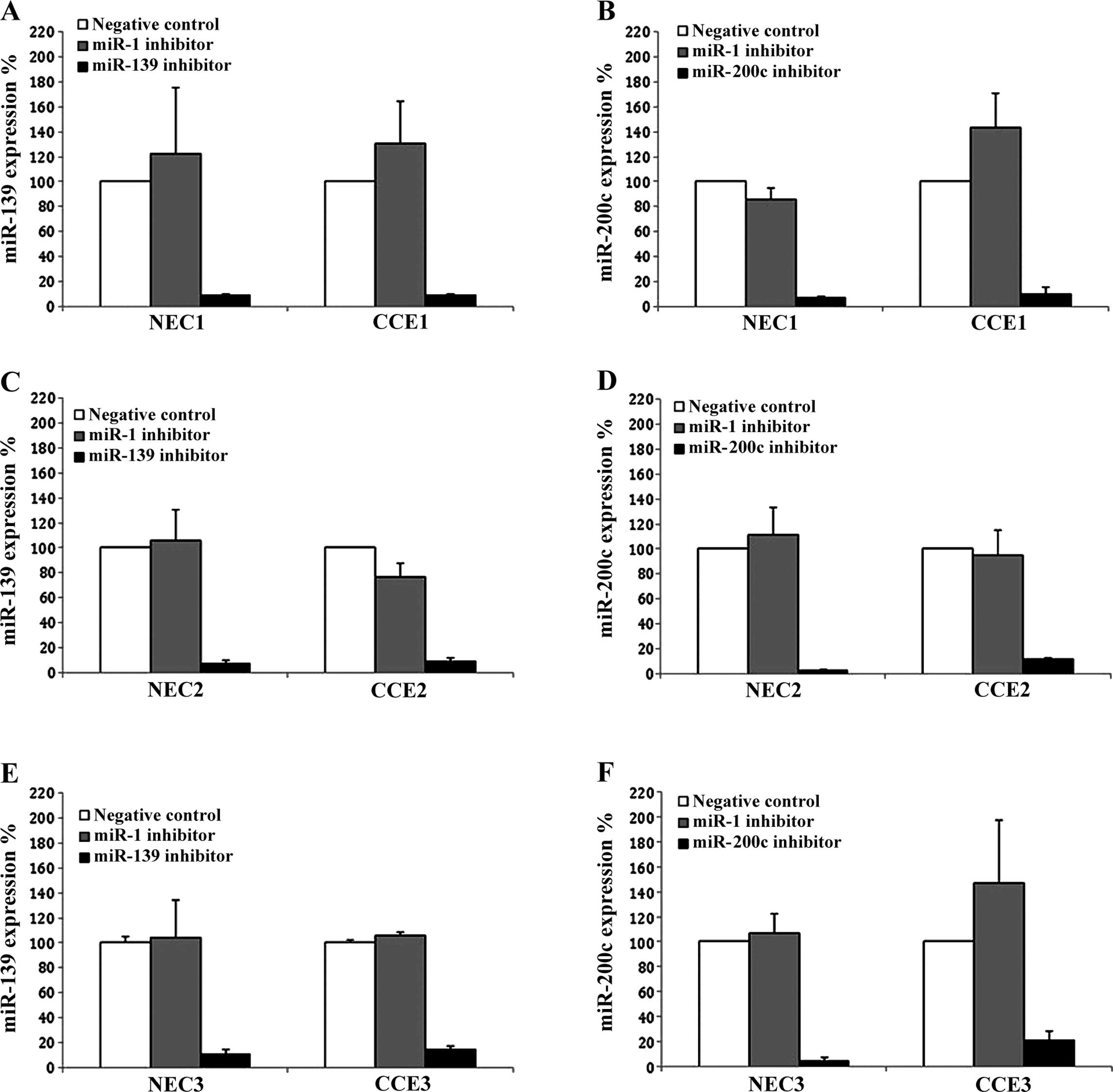
Effect of miRNA on CEC proliferation
To determine whether either miR-139 or miR-200c
influences CEC proliferation, specific inhibitors against them were
utilized. As shown in Fig. 2, the
efficiency of the miRNA inhibition was confirmed in three different
pairs of NEC and CEC cultures. Each miRNA inhibitor suppressed the
expression of their target miRNA by at least 80%. We next
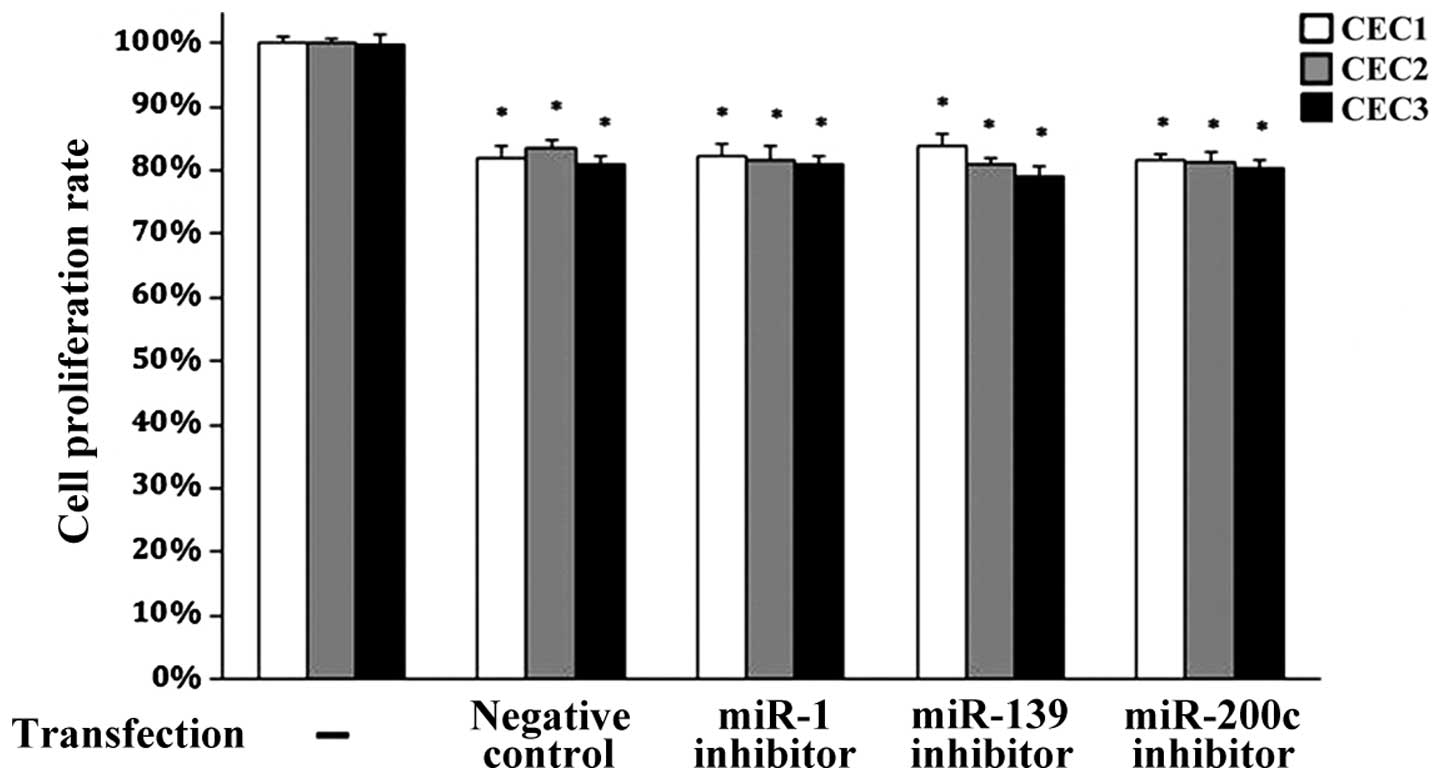
determined whether inhibition of either miR-139 or miR-200c could
influence CEC proliferation using MTT assays. As shown in Fig. 3, cell proliferation was not altered
upon inhibition of miR-139 or miR-200c as compared to the negative
control group. Similar results were obtained after inhibition of
miR-1, which is a known regulator of angiogenesis (20). However, cell proliferation was
significantly decreased in all groups as compared to the
untransfected control cells (all P<0.05). These data suggest
that neither miR-139 nor miR-200c influences pancreatic CEC
proliferation.
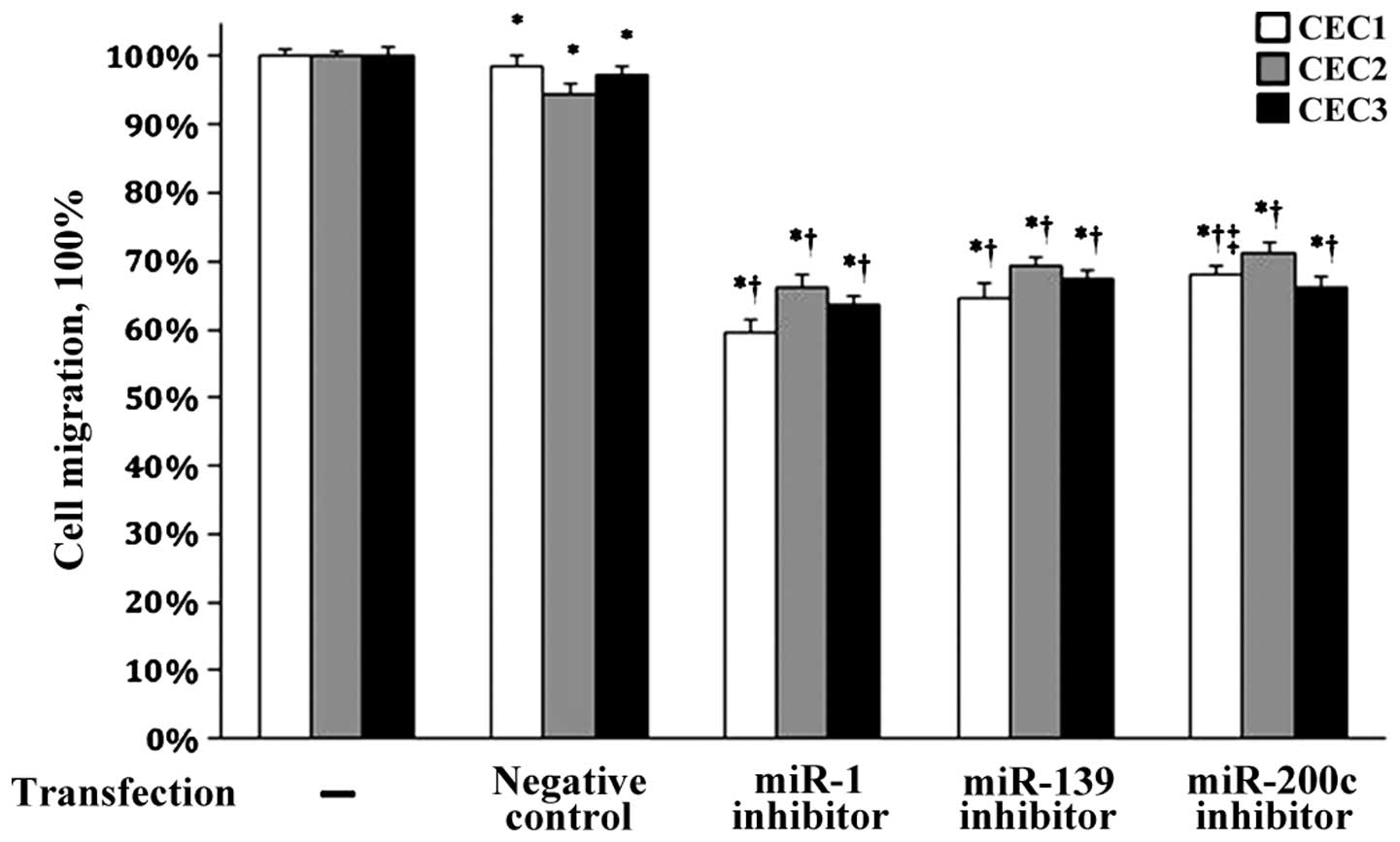
Effect of miR-139 and miR-200c on CEC
migration
The effects of miR-139 and miR200c on CEC migration
were next evaluated using their specific inhibitors. As compared to
the untransfected cells, cell migration in all the samples was
significantly decreased (all P<0.05, Fig. 4). Notably, significantly reduced CEC
migration was observed after transfection with the miR-1, miR-139
and miR-200c inhibitors compared to the negative control group (all
P<0.05). These data suggest that miR-139 and miR-200c regulate
CEC migration.
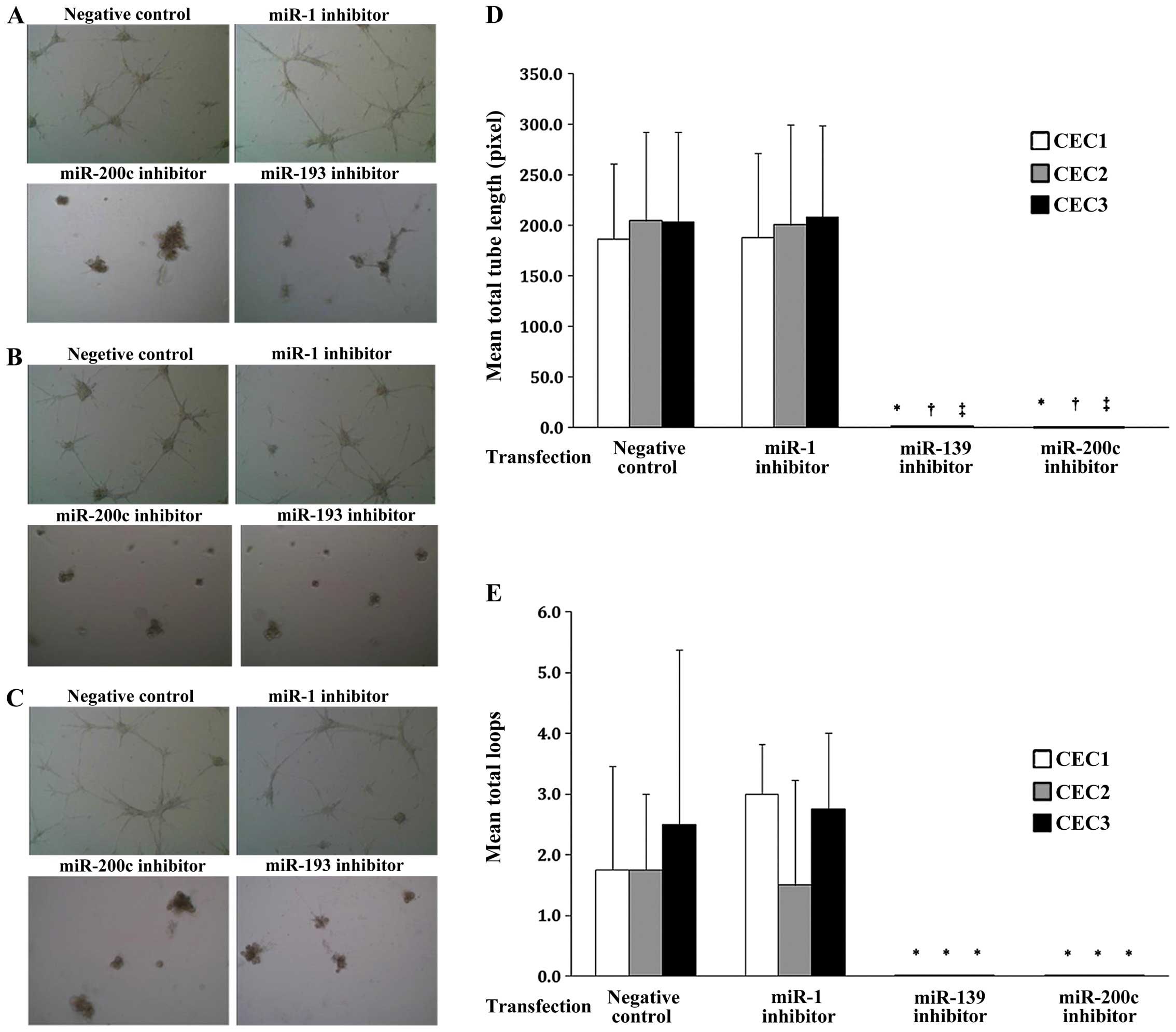
Effect of miR-139 and miR-200c on
angiogenesis in CECs
The effects of miR-139 and miR200c on CEC tube
formation were next evaluated as an in vitro measure of
their effects on angiogenesis. Representative images of the cells
from all three CEC cultures are shown in Fig. 5A–C. As shown in Fig. 5D and E, the average tube length and
total loop number were significantly decreased with miR-139 and
miR-200c inhibition in all three CEC cultures compared to those of
the negative control group (all P<0.05). No such changes were
observed with miR-1 inhibition. Thus, miR-139 and miR-200c may
regulate vasculature formation during angiogenesis.
Discussion
Inhibition of angiogenesis to suppress tumor
expansion and metastasis in pancreatic cancer has become a
promising therapeutic strategy for many types of cancer. Although
the importance of miRNAs in vasculogenesis was illustrated in
Dicer-null mice (10) and several
angiomiRs have been identified (9–11,13),
there is limited information regarding their role in pancreatic
carcinoma. Therefore, the present study aimed to identify angiomiRs
in pancreatic CECs. Fourteen miRNAs were differentially expressed
by >20-fold in the CECs of all three patients analyzed.
Subsequent inhibition studies revealed that miR-139 and miR-200c
may regulate CEC migration and tube formation but not
proliferation.
In laryngeal squamous cell carcinoma, miR-139
expression decreased with disease progression, and in vitro
and in vivo studies suggest that it inhibits proliferation,
migration and metastasis (33).
Similar tumor-suppressive functions have been reported in
glioblastomas, hepatocellular carcinomas and gliomas (34–36).
In contrast, miR-139 expression was upregulated in CECs as compared
to NECs in the present study, which may be due to cell
type-specific differences.
In human embryonic stem cells, miR-200c was
necessary for EC differentiation and in vivo vasculogenesis
through inhibition of the transcription repressor, zinc finger
E-box-binding homeobox (ZEB1) (37). Reduced expression of miR-200c in
leiomyomas, which are benign, fibrotic uterine tumors, altered
ZEB1/ZEB2, VEFGA, FBLN5 and TIMP2 expression (38). In contrast, miR-200c expression is
increased in endometrial cancer (39), and ectopic expression increased
Ishikawa cell proliferation (40).
Although we also observed increased miR-200c expression in the CECs
as compared to NECs, its inhibition did not influence cell
proliferation. However, inhibition of miR-200c reduced CEC
migration and tube formation, which is similar to that reported for
miR-200a (15). Given the role of
the putative miR-200c target genes in EMT, angiogenesis and matrix
remodeling, further studies will evaluate the effects of miR-200c
on tumor growth and metastasis.
Fourteen miRNAs were differentially expressed in the
CECs of all three patients analyzed, among which the roles for
miR-182, miR-183, miR-192, miR-194, the miR-200 family, miR-203,
miR-27a*, miR-375 and miR-92a-1* in regulating tumor angiogenesis
have been reported (16,22–32).
Subsequent qPCR analysis of miR-200b, miR-182, miR-139 and miR-200c
confirmed that miR-139 and miR-200c levels were increased in the
CECs relative to the NECs. Such differences between the microarray
results and qPCR validation may be attributed to the distance
between the PCR primers and microarray probes for a specific gene
(41) as well as spot intensity
(42) and microarray data filtering
(i.e., p-value) (43). Although
both miR139 and miR-200c were selected for further analysis on the
basis of their high expression and validation by qPCR, further
studies will analyze the roles of the other miRNAs in
angiogenesis.
In zebrafish embryos, inhibition of miR-1 inhibited
angiogenesis and reduced EC levels (44). It also regulates cardiomyocyte
progenitor cells (20). Although
knockdown of miR-1 influenced CEC migration in the present study,
no effects were observed on tube length and loop numbers. These
differential effects may be due to tissue-specific regulation by
miR-1.
The present study is limited in that the mechanism
by which miR-139 and miR-200c influence cell migration and tube
formation were not explored. Previous studies have reported that
miR-139 targets chemokine receptor 4 (CXCR4) (33) and miR200c targets ZEBs, which
regulate EMT during cancer development by repressing E-cadherin
(45,46), as well as VEGFA, FLT1, IKKβ, KLF9,
FBLN5 and TIMP2 (38,40). Therefore, these putative miRNA
targets will be explored further. In addition, the expression of
CEC miRNAs was analyzed in only three pancreatic cancer patients;
therefore, larger studies are necessary to determine the full
significance of altered miRNA expression in pancreatic CECs.
Furthermore, the differential miRNA expression observed in the
present study may only be applicable for Asian patients as
differential expression of miR-200c was noted in the leiomyomas of
African-Americans vs. Caucasians (38). Finally, the effects of miR-139 and
miR-200c on the proliferation, migration and vasculogenesis of NECs
also need to be determined to fully explore the therapeutic
potential of these miRNAs.
In summary, the present study identifies two miRNAs,
miR-139 and miR-200c, that were upregulated in CECs derived from
pancreatic tumors and that regulate CEC migration and tube
formation. The therapeutic value of targeting these miRNAs in
pancreatic cancer will be assessed in further in vivo
studies.
Acknowledgments
We are deeply grateful to all the participants as
well as to the doctors assisting with this study. This study was
supported by the National Nature Science Foundation of China
(81072006).
References
|
1
|
Maitra A and Hruban RH: Pancreatic cancer.
Annu Rev Pathol. 3:157–188. 2008. View Article : Google Scholar
|
|
2
|
Niederhuber JE, Brennan MF and Menck HR:
The National Cancer Data Base report on pancreatic cancer. Cancer.
76:1671–1677. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Y, Sun XJ, Jiang TH and Mao AW:
Combined radiochemotherapy in patients with locally advanced
pancreatic cancer: a meta-analysis. World J Gastroenterol.
19:7461–7471. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Folkman MJ, Long DM Jr and Becker FF:
Growth and metastasis of tumor in organ culture. Cancer.
16:453–467. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holmgren L, O’Reilly MS and Folkman J:
Dormancy of micrometastases: balanced proliferation and apoptosis
in the presence of angiogenesis suppression. Nat Med. 1:149–153.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parangi S, O’Reilly M, Christofori G,
Holmgren L, Grosfeld J, Folkman J and Hanahan D: Antiangiogenic
therapy of transgenic mice impairs de novo tumor growth. Proc Natl
Acad Sci USA. 93:2002–2007. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Djuranovic S, Nahvi A and Green R: A
parsimonious model for gene regulation by miRNAs. Science.
331:550–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Otsuka M, Zheng M, Hayashi M, Lee JD,
Yoshino O, Lin S and Han J: Impaired microRNA processing causes
corpus luteum insufficiency and infertility in mice. J Clin Invest.
118:1944–1954. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang WJ, Yang DD, Na S, Sandusky GE, Zhang
Q and Zhao G: Dicer is required for embryonic angiogenesis during
mouse development. J Biol Chem. 280:9330–9335. 2005. View Article : Google Scholar
|
|
11
|
Kuehbacher A, Urbich C, Zeiher AM and
Dimmeler S: Role of Dicer and Drosha for endothelial microRNA
expression and angiogenesis. Circ Res. 101:59–68. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S and Olson EN: AngiomiRs – key
regulators of angiogenesis. Curr Opin Genet Dev. 19:205–211. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Würdinger T, Tannous BA, Saydam O, Skog J,
Grau S, Soutschek J, Weissleder R, Breakefield XO and Krichevsky
AM: miR-296 regulates growth factor receptor overexpression in
angiogenic endothelial cells. Cancer Cell. 14:382–393. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng Y, Li S, Ding Y, Wang Q, Luo H, Shi
Q, Hao Z, Xiao G and Tong S: The role of miR-18a in gastric cancer
angiogenesis. Hepatogastroenterology. 60:1809–1813. 2013.
|
|
15
|
Poliseno L, Tuccoli A, Mariani L,
Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S and
Rainaldi G: MicroRNAs modulate the angiogenic properties of HUVECs.
Blood. 108:3068–3071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li YX, Liu DQ, Zheng C, Zheng SQ, Liu M,
Li X and Tang H: miR-200a modulate HUVEC viability and migration.
IUBMB Life. 63:553–559. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raimondi L, Amodio N, Di Martino MT,
Altomare E, Leotta M, Caracciolo D, Gullà A, Neri A, Taverna S,
D’Aquila P, et al: Targeting of multiple myeloma-related
angiogenesis by miR-199a-5p mimics: In vitro and in vivo anti-tumor
activity. Oncotarget. 5:3039–3054. 2014.PubMed/NCBI
|
|
18
|
Chamorro-Jorganes A, Araldi E, Rotllan N,
Cirera-Salinas D and Suárez Y: Autoregulation of glypican-1 by
intronic microRNA-149 fine tunes the angiogenic response to FGF2 in
human endothelial cells. J Cell Sci. 127:1169–1178. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Naschberger E, Schellerer VS, Rau TT,
Croner RS and Stürzl M: Isolation of endothelial cells from human
tumors. Methods Mol Biol. 731:209–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Mil A, Vrijsen KR, Goumans MJ, Metz
CH, Doevendans PA and Sluijter JP: MicroRNA-1 enhances the
angiogenic differentiation of human cardiomyocyte progenitor cells.
J Mol Med. 91:1001–1012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chim SM, Qin A, Tickner J, Pavlos N, Davey
T, Wang H, Guo Y, Zheng MH and Xu J: EGFL6 promotes endothelial
cell migration and angiogenesis through the activation of
extracellular signal-regulated kinase. J Biol Chem.
286:22035–22046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amodeo V, Bazan V, Fanale D, Insalaco L,
Caruso S, Cicero G, Bronte G, Rolfo C, Santini D and Russo A:
Effects of anti-miR-182 on TSP-1 expression in human colon cancer
cells: there is a sense in antisense? Expert Opin Ther Targets.
17:1249–1261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Donnem T, Fenton CG, Lonvik K, Berg T,
Eklo K, Andersen S, Stenvold H, Al-Shibli K, Al-Saad S, Bremnes RM,
et al: MicroRNA signatures in tumor tissue related to angiogenesis
in non-small cell lung cancer. PLoS One. 7:e296712012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Geng L, Chaudhuri A, Talmon G, Wisecarver
JL, Are C, Brattain M and Wang J: MicroRNA-192 suppresses liver
metastasis of colon cancer. Oncogene. 33:5332–5340. 2014.
View Article : Google Scholar :
|
|
25
|
Sundaram P, Hultine S, Smith LM, Dews M,
Fox JL, Biyashev D, Schelter JM, Huang Q, Cleary MA, Volpert OV, et
al: p53-responsive miR-194 inhibits thrombospondin-1 and promotes
angiogenesis in colon cancers. Cancer Res. 71:7490–7501. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pecot CV, Rupaimoole R, Yang D, Akbani R,
Ivan C, Lu C, Wu S, Han HD, Shah MY, Rodriguez-Aguayo C, et al:
Tumour angiogenesis regulation by the miR-200 family. Nat Commun.
4:24272013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu X, Er K, Mao C, Yan Q, Xu H, Zhang Y,
Zhu J, Cui F, Zhao W and Shi H: miR-203 suppresses tumor growth and
angiogenesis by targeting VEGFA in cervical cancer. Cell Physiol
Biochem. 32:64–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang W, Yu F, Yao H, Cui X, Jiao Y, Lin L,
Chen J, Yin D, Song E and Liu Q: miR-27a regulates endothelial
differentiation of breast cancer stem like cells. Oncogene.
33:2629–2638. 2014. View Article : Google Scholar
|
|
29
|
Urbich C, Kaluza D, Frömel T, Knau A,
Bennewitz K, Boon RA, Bonauer A, Doebele C, Boeckel JN,
Hergenreider E, et al: MicroRNA-27a/b controls endothelial cell
repulsion and angiogenesis by targeting semaphorin 6A. Blood.
119:1607–1616. 2012. View Article : Google Scholar
|
|
30
|
Reddi HV, Driscoll CB, Madde P, Milosevic
D, Hurley RM, McDonough SJ, Hallanger-Johnson J, McIver B and
Eberhardt NL: Redifferentiation and induction of tumor suppressors
miR-122 and miR-375 by the PAX8/PPARγ fusion protein inhibits
anaplastic thyroid cancer: a novel therapeutic strategy. Cancer
Gene Ther. 20:267–275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ando H, Okamoto A, Yokota M, Shimizu K,
Asai T, Dewa T and Oku N: Development of a miR-92a delivery system
for anti-angiogenesis-based cancer therapy. J Gene Med. 15:20–27.
2013. View
Article : Google Scholar
|
|
32
|
Mendell JT: miRiad roles for the miR-17-92
cluster in development and disease. Cell. 133:217–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luo HN, Wang ZH, Sheng Y, Zhang Q, Yan J,
Hou J, Zhu K, Cheng Y, Xu YL, Zhang XH, et al: miR-139 targets
CXCR4 and inhibits the proliferation and metastasis of laryngeal
squamous carcinoma cells. Med Oncol. 31:7892014. View Article : Google Scholar
|
|
34
|
Skalsky RL and Cullen BR: Reduced
expression of brain-enriched microRNAs in glioblastomas permits
targeted regulation of a cell death gene. PLoS One. 6:e242482011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wong CC, Wong CM, Tung EK, Au SL, Lee JM,
Poon RT, Man K and Ng IO: The microRNA miR-139 suppresses
metastasis and progression of hepatocellular carcinoma by
down-regulating Rho-kinase 2. Gastroenterology. 140:322–331. 2011.
View Article : Google Scholar
|
|
36
|
Li RY, Chen LC, Zhang HY, Du WZ, Feng Y,
Wang HB, Wen JQ, Liu X, Li XF, Sun Y, et al: MiR-139 inhibits Mcl-1
expression and potentiates TMZ-induced apoptosis in glioma. CNS
Neurosci Ther. 19:477–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo Z, Wen G, Wang G, Pu X, Ye S, Xu Q,
Wang W and Xiao Q: MicroRNA-200C and -150 play an important role in
endothelial cell differentiation and vasculogenesis by targeting
transcription repressor ZEB1. Stem Cells. 31:1749–1762. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chuang TD, Panda H, Luo X and Chegini N:
miR-200c is aberrantly expressed in leiomyomas in an
ethnic-dependent manner and targets ZEBs, VEGFA, TIMP2, and FBLN5.
Endocr Relat Cancer. 19:541–556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Snowdon J, Zhang X, Childs T, Tron VA and
Feilotter H: The microRNA-200 family is upregulated in endometrial
carcinoma. PLoS One. 6:e228282011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Panda H, Pelakh L, Chuang TD, Luo X,
Bukulmez O and Chegini N: Endometrial miR-200c is altered during
transformation into cancerous states and targets the expression of
ZEBs, VEGFA, FLT1, IKKβ, KLF9, and FBLN5. Reprod Sci. 19:786–796.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Etienne W, Meyer MH, Peppers J and Meyer
RA Jr: Comparison of mRNA gene expression by RT-PCR and DNA
microarray. Biotechniques. 36:618–626. 2004.PubMed/NCBI
|
|
42
|
Beckman KB, Lee KY, Golden T and Melov S:
Gene expression profiling in mitochondrial disease: assessment of
microarray accuracy by high-throughput Q-PCR. Mitochondrion.
4:453–470. 2004. View Article : Google Scholar
|
|
43
|
Morey JS, Ryan JC and Van Dolah FM:
Microarray validation: factors influencing correlation between
oligonucleotide microarrays and real-time PCR. Biol Proced Online.
8:175–193. 2006. View
Article : Google Scholar
|
|
44
|
Lin CY, Lee HC, Fu CY, Ding YY, Chen JS,
Lee MH, Huang WJ and Tsai HJ: miR-1 and miR-206 target different
genes to have opposing roles during angiogenesis in zebrafish
embryos. Nat Commun. 4:28292013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Davalos V and Esteller M: Opening the
treasure chest of miR-200s family members. Cell Cycle. 8:2141–2142.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brabletz S and Brabletz T: The ZEB/miR-200
feedback loop -a motor of cellular plasticity in development and
cancer? EMBO Rep. 11:670–677. 2010. View Article : Google Scholar : PubMed/NCBI
|