Introduction
Hepatoblastoma is the most frequently diagnosed
malignant liver tumor in children (1), accounting for ~50% of the liver tumors
and 1.3% of malignant tumors in children (2). A recent pediatric cancer
epidemiological analysis showed that the average annual percentage
in the incidence of hepatoblastoma has been on the increase in the
last 30 years (3). Despite the fact
that surgical therapy combined with chemotherapy has improved the
prognosis of patients with hepatoblastoma, disease-free survival
rates remain <50% for patients with advanced hepatoblastoma,
especially the patients with distant metastases (cancer spreads to
other organs and tissues of the body) (4). Cisplatin (CDDP) is a chemotherapeutic
agent that is often used for the treatment of hepatoblastoma
(5). However, many patients acquire
resistance to chemotherapeutic agents, which resulted in treatment
failures (6). Therefore,
understanding the molecular mechanisms that contribute to drug
resistance is crucial to optimize therapeutic options or develop
novel effective therapy for hepatoblastoma.
Survivin, an apoptotic inhibitor, is involved in the
suppression of apoptosis and regulation of mitosis in cancer
(7). Survivin is extensively
expressed in the majority of cancer types including lung, breast,
liver, colon, pancreatic, and head and neck cancer, but is not
expressed or is expressed at a substantially lower level in normal
tissue (8,9), which makes it a potential target for
anticancer therapy (10). Mounting
evidence suggested that survivin expression is associated with drug
resistance in cancer cells and a poor clinical outcome in patients
with cancer (11–14). In addition, survivin inhibition by
siRNA or by survivin antisense oligonucleotides (SAO) has been
demonstrated to sensitize tumors to cytotoxic drugs inculding
cisplatin in vitro and in vivo (11–16).
Sepantronium bromide (YM155), a novel small molecule
inhibitor of survivin, was obtained from high throughput screening
using a survivin promoter luciferase assay (17). YM155 demonstrates potent antitumor
activities against a various types of cancer, such as non-small
cell lung cancer, non-Hodgkin lymphoma, melanoma and prostate
cancer (17–22). Of note, phase I and II trials with
YM155 have shown its safety and tolerability in patients with
advanced refractory non-small cell lung carcinoma (23) or unresectable melanoma (24). Recent findings have shown that YM155
enhanced the antitumor activity of cytotoxic agents in several cell
lines (25).
Thus, we hypothesized that inhibition of survivin
expression by YM155 in hepatoblastoma cells may enhance the
therapeutic efficacy of CDDP by inhibiting the acquisition of
chemoresistance in cancer cells. Therefore, in this study, we
assessed the therapeutic potential of YM155 alone or in combination
with cisplatin in preclinical hepatoblastoma models in vitro
and in vivo.
Materials and methods
Tissue and cell lines
Thirty patients were treated at the Department of
Pediatric Surgery, The First Hospital of Jilin University
(Changchun, China) between August 2011 and August 2014. The present
study was approved by the Research Ethics Committee of Jilin
University (Changchun, China). Written informed consent was
obtained from all of the patients. Fresh hepatoblastoma and
corresponding normal liver tissues 5 cm away from the edge of the
hepatoblastoma tissue were taken from the patients with
hepatoblastoma who had not received any chemotherapy or
radiotherapy prior to surgery. The samples were then snap-frozen in
liquid nitrogen immediately after resection and kept at −80°C.
Human HepG2 and HuH6 hepatoblastoma cell lines,
purchased from the Cell bank of Type Culture Collection of Chinese
Academy of Sciences, Shanghai Institute of Cell biology (Shanghai,
China) were cultured in DMEM and RPMI-1640 medium, respectively,
with 10% fetal bovine serum (FBS; Gibco-Life Technologies,
Carlsbad, CA, USA), 100 IU/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified atmosphere containing 5%
CO2. Primary human fibroblasts were isolated from the
skin biopsies of healthy adult donors, and were cultured as
adherent monolayers in DMEM containing 15% FBS, 1% glutamine and 1%
penicillin/streptomycin. The cells were subcultured every 2 or 3
days.
Reagents and antibodies
YM155 was obtained from Selleck Chemicals (Houston,
TX, USA). Cisplatin was purchased from Sigma-Aldrich Co. (St.
Louis, MO, USA). The antibodies used for the western blot analysis
were: mouse anti-human survivin, and mouse anti-human GAPDH, mouse
anti-human cleaved caspase-3, mouse anti-human cleaved caspase-8
and mouse anti-human RARP monoclonal antibodies were purchased from
Cell Signaling Technology (Beverly, MA, USA).
Quantification by quantitative polymerase
chain reaction (qPCR)
Total RNA of liver tissue and cultured cells was
extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer’s instructions and quantified with
the NanoDrop 2000 (Thermo Fisher Scientific, Tokyo, Japan). Reverse
transcription of 1 µg of total RNA was performed using
oligo(dT) primers (Invitrogen) and reverse transcriptase (Toyobo,
Co., Osaka, Japan) to obtain complementary deoxyribonucleic acid
(cDNA). qPCR assays were carried out using SYBR-Green Real-Time PCR
Master Mix (Toyobo) and amplification equipment ABI PRISM 7900HT
(Applied Biosystems, Foster City, CA, USA). GADPH was used as the
endogenous control for quantifying mRNA levels. The survivin and
GAPDH primer sequences were designed as described by Zhang et
al (26). The 2−ΔΔCT
method was used to calculate the relative abundance of target gene
expression generated by Rotor-Gene Real-Time Analysis software
6.1.81 (Qiagen, Hilden, Germany).
Cell proliferation assay
To determine the inhibitory effect of YM155 alone or
in combination on cell proliferation, a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was performed. Cells (1×104/well) were plated in
96-well plates and then cultured in medium with or without various
concentrations of YM155 and/or CDDP alone or in combination.
Control cultures received 0.1% DMSO. Three days after the
treatment, the percentage of viable cells in each well was examined
by MTT assay (Sigma Chemical), using the SpectraMax Plus
spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) at a
wavelength of 570 nm by a spectrophotometer. The percentage of
viable cells for each treatment group was calculated by adjusting
the untreated control group to 100%.
Colony formation assays
Cells were seeded in 6-well plates at a density of
1×103 cells and were exposed to their respective half
maximal inhibitory concentration (IC50) values of YM155,
CDDP or both for 48 h. The cells were then washed with drugs-free
medium and allowed to grow for 14 days in drugs-free conditions.
The percentage colony formation was calculated by adjusting
untreated cells to 100%. All the experiments were performed in
triplicate.
Apoptosis assay
Apoptosis was performed using an Annexin V binding
assay (ApoAlert Annexin V-FITC apoptosis kit; Clontech
Laboratories, Inc., Mountain view, CA, USA). Briefly, the cells
were treated with their respective IC50 values of YM155,
CDDP or both for 48 h. Then cells were washed with PBS twice and
resuspended in 100 µl binding buffer (Sigma). Subsequently,
5 µl Annexin V FITC was added and incubated for 15 min in
the dark at room temperature. The cells were analyzed with a
Becton-Dickinson FACScan flow cytometer using the CellsQuest
program 2.6 (Becton-Dickinson).
In addition, the expression of cleaved caspase-3,
cleaved caspase-8 and cleaved PARP was determined by western blot
analysis 24 h after treatment with their respective IC50
values of YM155, CDDP or both.
Western blot analysis
Protein was extracted from tissues or cells using
lysis buffer (RIPA Buffer; Sigma Chemical). Protein concentrations
of lysates were determined using a BCA protein assay reagent kit
(Tiangen Biotech, Beijing, China). Equal amounts of total protein
(20 µg) were resolved by 10–12% SDS-PAGE and transferred to
a polyvinylidene fluoride membrane. After blocking at room
temperature with with 5% dry milk in TBS-T, each membrane was
incubated overnight at 4°C with the primary antibodies. After
washing with TBS-T twice, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibody for 1 h at
room temperature. Proteins of interest were visualized by enhanced
chemiluminescence using ECL Prime (GE Healthcare, Buckinghamshire,
UK). Blots were stripped and reprobed with anti-GAPDH to control
for loading variations. Quantity One software (Bio-Rad, Hercules,
CA, USA) was used for quantification of the protein bands.
Xenograft model
To assess the effect of CDDP and/or YM155 on tumor
growth in vivo, we used a nude mouse tumor xenograft model.
Six-week-old male nude mice (BALB/c nu/nu) were purchased from the
Experiments Animal Center of Changchun Biological Institute
(Changchun, China). HepG2 cells (2×106) were injected
into the flanks of the mice and allowed to reach a tumor volume of
>100 mm3 in tumor volume (length × width2)/2. The
mice were randomly divided into four groups (n=10 mice/group). The
control group received 1% polysorbate resuspended in deionized
water. The remaining three groups were treated with YM155 (10 mg/kg
body weight), CDDP (3 mg/kg body weight), or YM155 plus CDDP (3 and
1 mg/kg body weight, respectively) intraperitoneally for three days
over 4 weeks. The tumor volume was measured on day 7, 14, 21 and 28
of the treatment. On day 28, the animals were euthanized using
chloroform, tumor tissues were resected and volume and weight were
measured. The survivin expression levels of tumor tissues were
analyzed by western blot analysis. The study was approved by the
Jilin University Animal Care and Experimentation Committee
(Changchun, China).
Statistical analysis
Data were presented as mean ± SD (standard
deviation). Comparisons between two groups were analyzed by the
Student’s paired t-test. A comparison of ≥2 groups was performed
using one-way ANOVA followed by a Tukey’s post hoc test.
Statistical analyses were performed using GraphPad Prism, version 5
(GraphPad Software, Inc., San Diego, CA, USA) and SPSS software
(version 16.0; SPSS Inc., Chicago, IL, USA). P<0.05 was
considered statistically significant.
Results
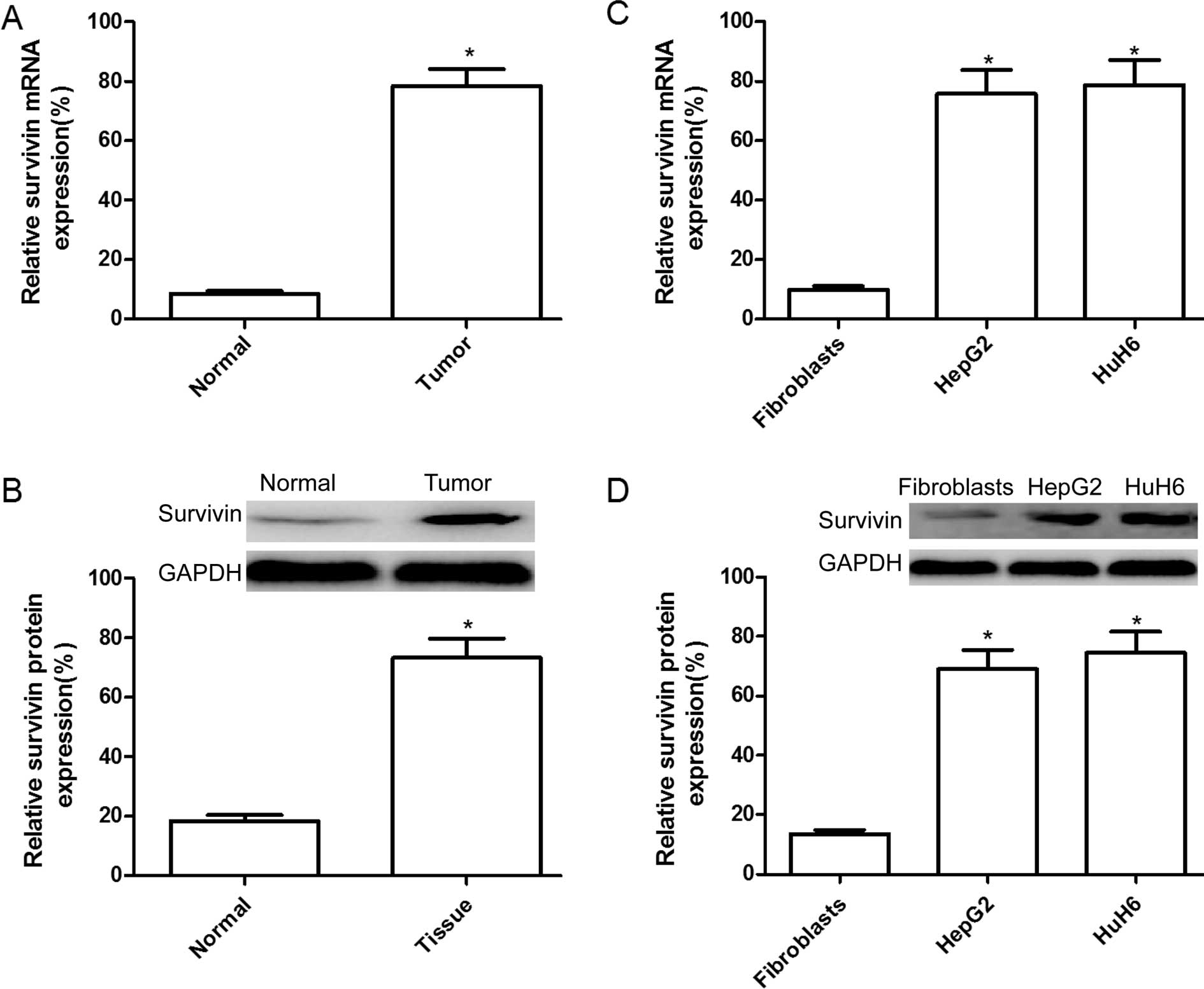
Expression of survivin is upregulated in
hepatoblastoma tissues and cells
To identify the potential roles of survivin in the
occurrence and development of hepatoblastoma, we detected survivin
expression levels in tumor tissues and adjacent normal tissues from
30 patients with hepatoblastoma by reverse-transcriptase
quantitative PCR (RT-qPCR) and western blot analysis. Results of
RT-qPCR showed that the mRNA expression level of surivivn in
hepatoblastoma tumor tissues was significantly increased compared
to matched adjacent normal tissues (Fig. 1A; P<0.05). In addition, elevated
levels of survivin protein were found in hepatoblastoma tumor
tissues compared with the paired adjacent normal tissues by western
blot analysis (Fig. 1B).
The expression levels of survivin in human HepG2 and
HuH6 hepatoblastoma cell lines, and normal fibroblasts were
detected by RT-qPCR and western blot analysis. It was found that
the expression of survivin on the mRNA (Fig. 1C) and protein (Fig. 1D) levels were clearly upregulated in
HepG2 and HuH6 cells compared to the normal fibroblasts
(P<0.05).
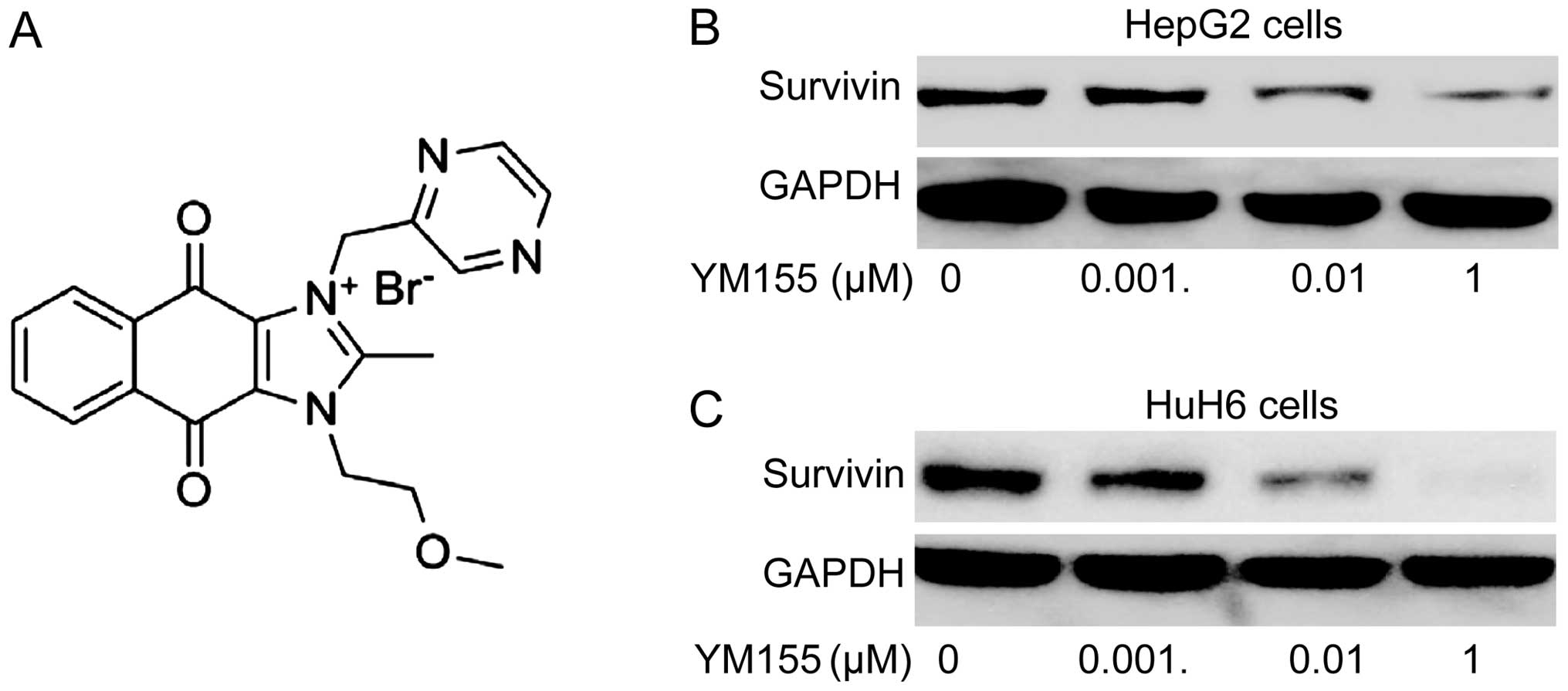
YM155 inhibits survivin expression in
hepatoblastoma cells
YM155 (Fig. 2A), a
novel small molecule inhibitor of survivin, has been shown to
inhibit survivin expression in various types of cancer (17–22).
We examined whether YM155 was effective in inhibiting survivin
expression on protein level in hepatoblastoma cell lines by western
blot analysis 24 h following treatment with different doses of
YM155 (0, 0.001, 0.01 and 1 µM). It was found that YM155
significantly downregulated survivin protein expression in HepG2
and HuH6 cells in a dose-dependent manner (Fig. 2B and C).
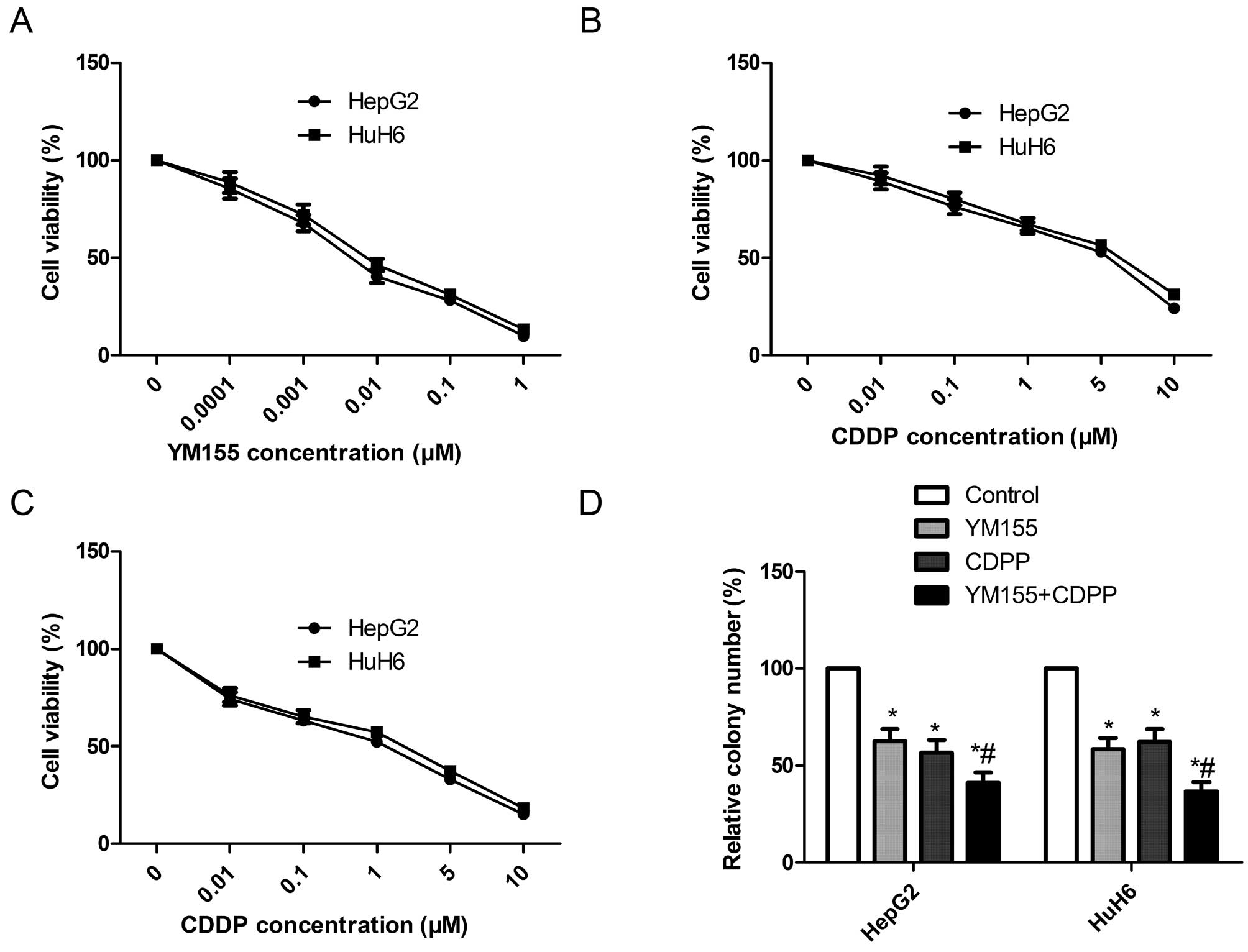
YM155 and CDDP alone or in combination
inhibit cell proliferation and colony formation in hepatoblastoma
cell lines
To assess the effect of YM155 and CDDP alone or in
combination on the cell viability of hepatoblastoma cells in
vitro, HepG2 and HuH6 cells were treated with different drug
concentrations of YM155 (0–1 µM), CDDP (0–10 µM), or
both (0.001 µM YM155 plus 0–10 µM CDDP) for 72 h,
respectively, and an MTT assay were performed. It was found that
YM155 inhibited the cell proliferation of the HepG2 and HuH6 in a
dose-dependent manner. The IC50 values of YM155 were 64
and 76 nM for HepG2 and HuH6 cell line, respectively (Fig. 3A). CDDP alone also reduced cell
proliferation in hepatoblastoma cell lines in a dose-dependent
manner with an IC50 of 5.4 µM in HepG2 cells and
an IC50 of 6.1 µM in HuH6 cells (Fig. 3b). Combination treatment (0–10
µM CDDP in the presence of 0.001 µM YM155) reduced
cell proliferation in hepatoblastoma cell lines in a dose-dependent
manner with an IC50 of 2.1 µM in HepG2 cells and
an IC50 of 2.4 µM in HuH6 cells (Fig. 3C), indicating that YM155 enhanced
the sensitivity of CDDP to hepatoblastoma cell lines. Based on the
results, we selected respective IC50 values of drugs for
subsequent treatments throughout the study.
The effects of YM155 and CDDP alone or in
combination on the cell colony formation of hepatoblastoma cell
lines were also determined. As shown Fig. 3D, the colony formation number of the
HepG2 and HuH6 hepatoblastoma cells was significantly reduced in
the YM155 and CDDP alone or combination groups. Combination
treatment with YM155 and CDDP resulted in an even greater
percentage of reduction.
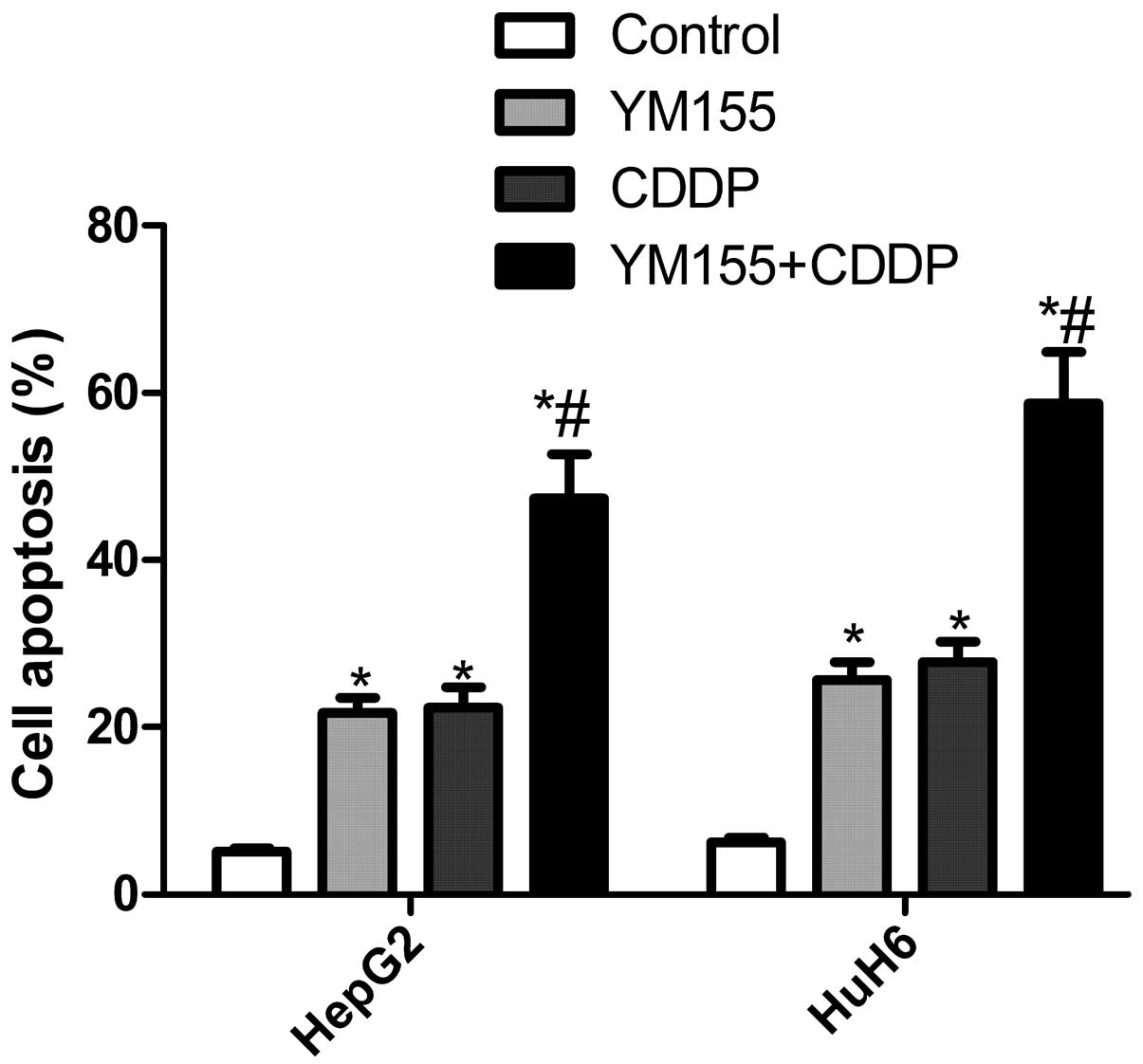
YM155 and CDDP alone or in combination
induce cell apoptosis in hepatoblastoma cell lines
We examined the in vitro effect of YM155 and
CDDP alone or in combination on cell apoptosis in hepatoblastoma
cell lines. The results showed that exposure to YM155 increased the
apoptotic cells by 21.7% in HepG2 cells and 25.6% in HuH6 cells
(Fig. 4), and exposure to CDDP
increased the apoptotic cells by 22.4% in HepG2 cells and 27.8% in
HuH6 cells. By contrast, the combination of YM155 and CDDP showed
that the apoptotic cells were increased by 47.3 and 58.8% in HepG2
and HuH6 cells, respectively, which was greater than either agent
alone (Fig. 4).
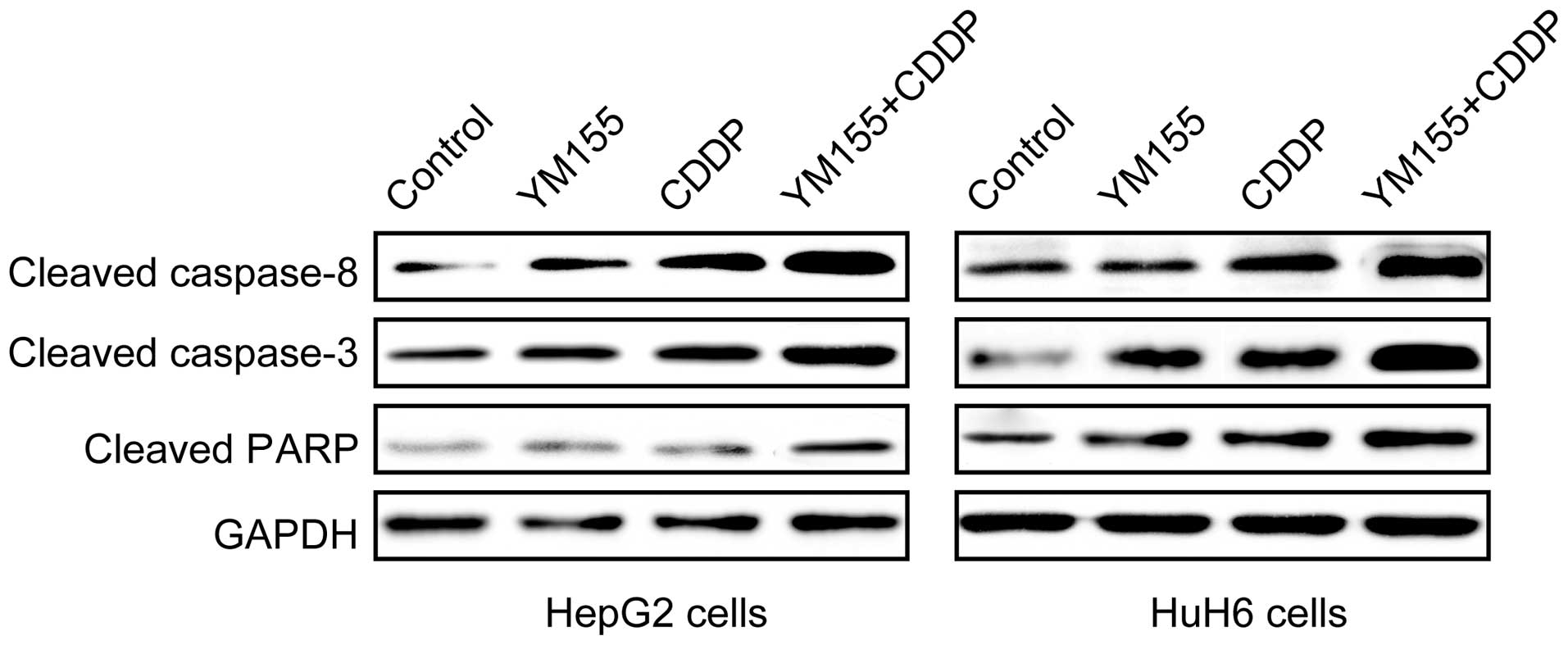
YM155 and CDDP alone or in combination
induces caspase activation
To assess the ability of YM155 and CDDP alone or in
combination to activate caspase-3 and -8 activity, we determined
cleaved caspase-3 and -8 expression by western blot analysis
following treatment with YM155 and CDDP alone or in combination. We
found that cleaved caspase-3 and -8 expression was significantly
upregualted in the YM155 and CDDP alone or combination treatment
groups as compared to the controls group (Fig. 5). Combination treatment
significantly increased the cleaved caspase-3 and -8 expression
levels as compared to YM155 or CDDP treatment alone (Fig. 5). Furthermore, we determined the
cleaved PARP expression in the hepatoblastoma cell lines following
treatment with YM155 and CDDP alone or in combination. Western blot
analysis revealed that the expression of cleaved PARP was
upregulated in cells treated with YM155 and CDDP alone or in
combination as compared to the control (Fig. 5). Combination treatment led to
maximal addition for cleaved PARP expression.
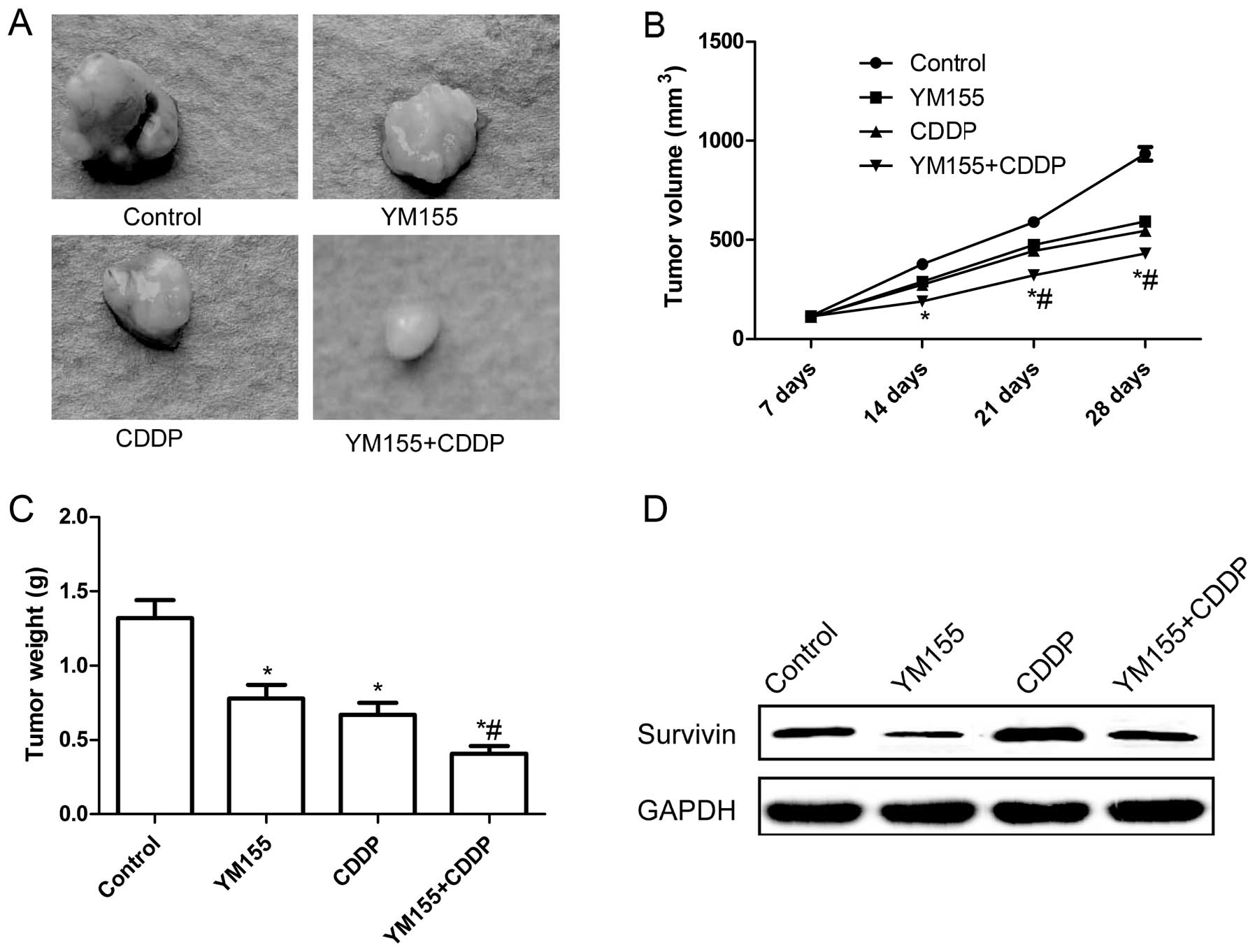
Antitumor effect of YM155 combined with
CDDP in a xenograft model
The in vitro data suggested that YM155 and
CDDP alone or in combination significantly inhibited cancer cell
growth. To validate our in vitro results further, we carried
out YM155 and CDDP combination treatment experiment in a HepG2
xenograft mouse model. Tumors grown in nude mice were respectively
treated with YM155, CDDP or both for four weeks. The tumors were
then extracted and it was found that they were significantly
smaller in the YM155 and CDDP alone or in combination groups than
that of the control group. The combination treatment group led to
marked inhibition of tumor growth as compared to the monotherapy
groups (Fig. 6A). Additionally,
tumor volume (Fig. 6b) and tumor
weight (Fig. 6C) following
treatment with YM155 and CDDP alone or in combination were
significantly reduced as compared to the control group (P<0.05;
Fig. 6B and C). Treatment with the
combination of YM155 and CDDP resulted in marked inhibitoin of
tumor growth as compared to monotherapy (P<0.05; Fig. 6B and C). We also determined survivin
expression in xenograft tumors by western blot analysis. The
results revealed that survivin expression was upregulated in the
CDDP treatment group as compared to the remaining groups, while
survivin expression was downregulated in the YM155 treatment group
as compared to the remaining groups (Fig. 6D). Of note, survivin expression had
no significant change in the combination group as compared to the
control group (Fig. 6D). These
results showed that YM155 in combination with CDDP treatment
markedly suppressed hepatoblastoma tumorigenicity in nude mice.
Discussion
In the present study, we examined the validity of
targeting survivin and the therapeutic potential of YM155 alone and
in combination with CDDP in vitro and in vivo. We
found that survivin expression was increased in hepatoblastoma
tumor tissues and hepatoblastoma cell lines. In an in vitro
combination study, YM155 synergistically enhanced the antitumor
activity of CDDP in hepatoblastoma cell lines by inhibiting
hepatoblastoma cell proliferation and colony formation, and
inducing cell apoptosis. In addition, YM155 concomitantly combined
with CDDP led to greater tumor reduction than single YM155 or CDDP
treatment in established xenograft models. These findings suggest
that YM155 in combination with CDDP is a promising candidate for
hepatoblastoma therapy.
Survivin, the smallest member of the inhibitor of
apoptosis (IAP) family, consist of 142 amino acids. It has been
reported that it is strongly expressed in children with various
types of malignancies, including neuroblastoma (27), soft tissue sarcoma and bone sarcoma
(28). It was recently shown that
the survivin expression was significantly increased in
hepatoblastoma tumor tissues and HuH6 cells after chemotherapy
(29). The present study has shown
that survivin expression was significantly upregulated in
hepatoblastoma tumor tissue and HepG2 and HuH6 hepatoblastoma cell
lines as compared to adjacent normal live tissue or normal
fibroblasts. Mounting evidence has shown that survivin promotes
cell proliferation by facilitating accurate mitotic progression and
inhibits cell apoptosis by inhibiting caspase activation (30,31).
Thus, survivin is an attractive target for treating hepatoblastoma
using a small molecule antagonist or immunotherapy to inhibit
survivin expression. YM155, a novel small-molecule survivin
suppressant, has demonstrated potent anticancer activity against a
broad spectrum of human cancer cell lines and various human-derived
tumor xenograft mouse models by altering intratumoral survivin
expression levels and a subsequent direct apoptosis induction
(19). To the best of our
knowledge, our results are the first to show that YM155 alone or in
combination with CDDP may effectively suppress tumor growth of
hepatoblastoma in vitro and in vivo by inhibiting
survivin expression.
It is well known that combinatorial chemotherapy
potentially achieves an improved response rate as compared to that
of monotherapy since combination treatment mainly depends on the
synergism between different therapeutic agents with different
mechanisms of action to increase efficacy, while maintaining a
favorable side-effect and decreasing overlapping toxicity.
Accumulating evidence suggests YM155 alone or in combination with
other types of chemotherapy suppresses tumor growth in several
cancers in vitro or in vivo. For example, Nakahara
et al (32) have reported
that tumor regression induced by YM155 in combination with
docetaxel was accompanied by decreased intratumoral survivin,
resulting in an apoptotic rate as compared to either treatment
alone. Kaneko et al (33)
have shown that YM155 enhances the antitumor activity of
bendamustine against DLBCL models through inhibition of DNA damage
responses as well as survivin-mediated cytoprotection at the G2-M
phase. Liang et al (34)
showed that the concomitant combination of YM155 with cisplatin
induced more intense apoptosis in SH-SY5Y human neuroblastomas
cells compared with monotherapy in vitro, and that this
combination shows greater efficacy than either agent alone in mouse
xenograft models. Kumar et al (25) found that YM155 significantly
enhanced the antitumor and antiangiogenic effects of CDDP in head
and neck squamous cell carcinoma, with no added systemic toxicity.
In the present study, we found that YM155 could enhance sensitivity
to the antitumor effect of CDDP in human HepG2 and HuH6
hepatoblastoma cell lines and that the combination treatment of
YM155 and CDDP promoted tumor regression of hepatoblastoma in
vitro and in vivo, suggesting a potentially novel
strategy to employing YM155 to overcome resistance in tumor cells,
thereby enhancing the effectiveness of CDDP in hepatoblastoma.
Survivin may block apoptosis by inhibiting caspase-3
and -7 activities directly (35).
Previous findings have shown that inhibiting survivin by YM155 may
increase cleaved caspase-3 expression levels, indicating activation
of caspase-3, resulting in cell apoptosis in human malignant cells
(36). In addition, it has been
shown that caspases-8 plays an important role in the apoptotic cell
death via the extrinsic apoptosis signaling cascade (37). Recent results have shown that YM155
induced apoptosis in human leukemia cells concomitant with the
activation of caspase-3 and -8 (38). Consistent with these results, the
present study has shown that YM155 alone or in combination with
CDDP significantly induced cell apoptosis by increasing cleaved
caspase-3 and -8 expression.
In conclusion, the present study has shown that
survivin expression was upregulated in hepatoblastoma tumor tissues
and cell lines, and that YM155 significantly enhanced the
therapeutic efficacy of cisplatin treatment. Additionally, YM155 in
combination with CDDP synergistically decreased hepatoblastoma cell
proliferation and formation, induced cell apoptosis in
vitro, and suppressed the tumor growth of hepatoblastoma in a
nude mouse model. These findings suggest that the combination of
YM155 and CDDP is a promising drug candidate for the treatment of
hepatoblastoma.
Acknowledgments
The present study was supported by the Innovation
Platform Construction Approach and Strategy on the Single-Disease
Clinical Pathway of Jilin Province (20120626), and the Fundamental
Public Service System Construction Approach and Strategy on
Single-Disease Clinical Pathway of Changchun City (2012117).
References
|
1
|
Stocker JT: Hepatoblastoma. Semin Diagn
Pathol. 11:136–143. 1994.PubMed/NCBI
|
|
2
|
Schoofs G, Braeye L, Vanheste R,
Verswijvel G, Debiec-Rychter M and Sciot R: Hepatic
rhabdomyosarcoma in an adult: A rare primary malignant liver tumor.
Case report and literature review. Acta Gastroenterol Belg.
74:576–581. 2011.
|
|
3
|
Czauderna P, Lopez-Terrada D, Hiyama E,
Haberle B, Malogolowkin MH and Meyers RL: Hepatoblastoma state of
the art: Pathology, genetics, risk stratification, and
chemotherapy. Curr Opin Pediatr. 26:19–28. 2014. View Article : Google Scholar
|
|
4
|
Davies JQ, de la Hall PM, Kaschula RO,
Sinclair-Smith CC, Hartley P, Rode H and Millar AJ: Hepatoblastoma
- evolution of management and outcome and significance of histology
of the resected tumor. A 31-year experience with 40 cases. J
Pediatr Surg. 39:1321–1327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang YT, Feng LH, Zhong XD, Wang LZ and
Chang J: Single-agent cisplatin treatment of children with
high-risk hepatoblastoma. J Pediatr Hematol Oncol. 36:271–275.
2014. View Article : Google Scholar
|
|
6
|
Qayed M and Katzenstein HM: Dose-intensive
cisplatin for hepatoblastoma: Have you heard? Lancet Oncol.
14:791–792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Colnaghi R, Connell CM, Barrett RM and
Wheatley SP: Separating the anti-apoptotic and mitotic roles of
survivin. J Biol Chem. 281:33450–33456. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Altieri DC: Validating survivin as a
cancer therapeutic target. Nat Rev Cancer. 3:46–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duffy MJ, O’Donovan N, Brennan DJ,
Gallagher WM and Ryan BM: Survivin: A promising tumor biomarker.
Cancer Lett. 249:49–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asechi H, Hatano E, Nitta T, Tada M,
Iwaisako K, Tamaki N, Nagata H, Narita M, Yanagida A, Ikai I, et
al: Resistance to cisplatin-induced apoptosis via PI3K-dependent
survivin expression in a rat hepatoma cell line. Int J Oncol.
37:89–96. 2010.PubMed/NCBI
|
|
12
|
Chandele A, Prasad V, Jagtap JC, Shukla R
and Shastry PR: Upregulation of survivin in G2/M cells and
inhibition of caspase 9 activity enhances resistance in
staurosporine-induced apoptosis. Neoplasia. 6:29–40. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tirro E, Consoli ML, Massimino M, Manzella
L, Frasca F, Sciacca L, Vicari L, Stassi G, Messina L, Messina A,
et al: Altered expression of c-IAP1, survivin, and Smac contributes
to chemotherapy resistance in thyroid cancer cells. Cancer Res.
66:4263–4272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zaffaroni N, Pennati M, Colella G, Perego
P, Supino R, Gatti L, Pilotti S, Zunino F and Daidone MG:
Expression of the anti-apoptotic gene survivin correlates with
taxol resistance in human ovarian cancer. Cell Mol Life Sci.
59:1406–1412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kunze D, Erdmann K, Froehner M, Wirth MP
and Fuessel S: siRNA-mediated inhibition of antiapoptotic genes
enhances chemotherapy efficacy in bladder cancer cells. Anticancer
Res. 32:4313–4318. 2012.PubMed/NCBI
|
|
16
|
Hayashi N, Asano K, Suzuki H, Yamamoto T,
Tanigawa N, Egawa S and Manome Y: Adenoviral infection of survivin
antisense sensitizes prostate cancer cells to etoposide in vivo.
Prostate. 65:10–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakahara T, Kita A, Yamanaka K, Mori M,
Amino N, Takeuchi M, Tominaga F, Hatakeyama S, Kinoyama I,
Matsuhisa A, et al: YM155, a novel small-molecule survivin
suppressant, induces regression of established human
hormone-refractory prostate tumor xenografts. Cancer Res.
67:8014–8021. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawano H, Shakushiro K, Nakata M, Kita A,
Maeda A, Watanabe S, Sako K and Oku N: Antitumor efficacy and
biodistribution of liposomal sepantronium bromide (YM155), a novel
small-molecule survivin suppressant. Eur J Pharm Biopharm.
88:283–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakahara T, Kita A, Yamanaka K, Mori M,
Amino N, Takeuchi M, Tominaga F, Kinoyama I, Matsuhisa A, Kudou M,
et al: Broad spectrum and potent antitumor activities of YM155, a
novel small-molecule survivin suppressant, in a wide variety of
human cancer cell lines and xenograft models. Cancer Sci.
102:614–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Minematsu T, Iwai M, Sugimoto K, Shirai N,
Nakahara T, Usui T and Kamimura H: Carrier-mediated uptake of
1-(2-methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)-4,9-dihydro-1H-naphtho[2,3-d]imidazolium
bromide (YM155 monobromide), a novel small-molecule survivin
suppressant, into human solid tumor and lymphoma cells. Drug Metab
Dispos. 37:619–628. 2009. View Article : Google Scholar
|
|
21
|
Kita A, Nakahara T, Yamanaka K, Nakano K,
Nakata M, Mori M, Kaneko N, Koutoku H, Izumisawa N and Sasamata M:
Antitumor effects of YM155, a novel survivin suppressant, against
human aggressive non-Hodgkin lymphoma. Leuk Res. 35:787–792. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tolcher AW, Quinn DI, Ferrari A, Ahmann F,
Giaccone G, Drake T, Keating A and de Bono JS: A phase II study of
YM155, a novel small-molecule suppressor of survivin, in
castration-resistant taxane-pretreated prostate cancer. Ann Oncol.
23:968–973. 2012. View Article : Google Scholar
|
|
23
|
Giaccone G, Zatloukal P, Roubec J, Floor
K, Musil J, Kuta M, van Klaveren RJ, Chaudhary S, Gunther A and
Shamsili S: Multicenter phase II trial of YM155, a small-molecule
suppressor of survivin, in patients with advanced, refractory,
non-small-cell lung cancer. J Clin Oncol. 27:4481–4486. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lewis KD, Samlowski W, Ward J, Catlett J,
Cranmer L, Kirkwood J, Lawson D, Whitman E and Gonzalez R: A
multicenter phase II evaluation of the small molecule survivin
suppressor YM155 in patients with unresectable stage III or IV
melanoma. Invest New Drugs. 29:161–166. 2011. View Article : Google Scholar
|
|
25
|
Kumar B, Yadav A, Lang JC, Cipolla MJ,
Schmitt AC, Arradaza N, Teknos TN and Kumar P: YM155 reverses
cisplatin resistance in head and neck cancer by decreasing
cytoplasmic survivin levels. Mol Cancer Ther. 11:1988–1998. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang K, Li Y, Liu W, Gao X and Zhang K:
Silencing survivin expression inhibits the tumor growth of
non-small-cell lung cancer cells in vitro and in vivo. Mol Med Rep.
11:639–644. 2015.
|
|
27
|
Ito R, Asami S, Motohashi S, Ootsuka S,
Yamaguchi Y, Chin M, Shichino H, Yoshida Y, Nemoto N, Mugishima H,
et al: Significance of survivin mRNA expression in prognosis of
neuroblastoma. Biol Pharm Bull. 28:565–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kappler M, Köhler T, Kampf C,
Diestelkötter P, Würl P, Schmitz M, Bartel F, Lautenschläger C,
Rieber EP, Schmidt H, et al: Increased survivin transcript levels:
an independent negative predictor of survival in soft tissue
sarcoma patients. Int J Cancer. 95:360–363. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Uehara S, Oue T, Kawatsu M, Nara K and
Fukuzawa M: Increased expression of survivin in hepatoblastoma
after chemotherapy. Eur J Pediatr Surg. 23:400–404. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ryan BM, O’Donovan N and Duffy MJ:
Survivin: A new target for anti-cancer therapy. Cancer Treat Rev.
35:553–562. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakahara T, Yamanaka K, Hatakeyama S, Kita
A, Takeuchi M, Kinoyama I, Matsuhisa A, Nakano K, Shishido T,
Koutoku H, et al: YM155, a novel survivin suppressant, enhances
taxane-induced apoptosis and tumor regression in a human Calu 6
lung cancer xenograft model. Anticancer Drugs. 22:454–462. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaneko N, Mitsuoka K, Amino N, Yamanaka K,
Kita A, Mori M, Miyoshi S and Kuromitsu S: Combination of YM155, a
survivin suppressant, with bendamustine and rituximab: A new
combination therapy to treat relapsed/refractory diffuse large
B-cell lymphoma. Clin Cancer Res. 20:1814–1822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang H, Zhang L, Xu R and Ju XL:
Silencing of survivin using YM155 induces apoptosis and
chemosensitization in neuroblastomas cells. Eur Rev Med Pharmacol
Sci. 17:2909–2915. 2013.PubMed/NCBI
|
|
35
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
caspases, and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
36
|
Yamanaka K, Nakahara T, Yamauchi T, Kita
A, Takeuchi M, Kiyonaga F, Kaneko N and Sasamata M: Antitumor
activity of YM155, a selective small-molecule survivin suppressant,
alone and in combination with docetaxel in human malignant melanoma
models. Clin Cancer Res. 17:5423–5431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Graf RP, Keller N, Barbero S and Stupack
D: Caspase-8 as a regulator of tumor cell motility. Curr Mol Med.
14:246–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feng W, Yoshida A and Ueda T: YM155
induces caspase-8 dependent apoptosis through downregulation of
survivin and Mcl-1 in human leukemia cells. Biochem Biophys Res
Commun. 435:52–57. 2013. View Article : Google Scholar : PubMed/NCBI
|