Introduction
Uveal melanoma is the most common primary
intraocular tumor in adults, and has a reported annual incidence of
6.3/million among Caucasians, 0.9 among Hispanics and 0.24 among
individuals of African descent (1).
The incidence is much lower within Asian populations although uveal
melanoma may occur earlier (2,3). It
was previously reported that 20% of uveal melanomas occurred in
patients between 19 and 30 years of age (4). The 5-year survival rate of patients
with uveal melanoma has remained at 81.6% over the past three
decades regardless of the development of local eye treatment
(5). Therefore, it is critical to
identify patients at high risk of metastatic disease, which may be
useful in selecting patients that may benefit from adjuvant
treatment (6). The organs to which
uveal melanoma most commonly metastasize are the liver (95%), lungs
(24%), bone (16%) and skin (11%). These metastases may
significantly influence the patient survival rate. Patients with
liver metastases have a median survival rate of ~4–6 months and a
1-year survival rate of ~10–15%. By contrast, patients with no
liver metastases have a median survival rate of ~19–28 months and a
1-year survival rate of ~76% (7).
GNAQ and GNA11 mutants (encoding Gαq and Gα11,
respectively) occur in ~5% of all tumors and contribute to cell
viability and migration (8).
Daniels et al (9)
demonstrated that mutations in GNAQ (47%) or GNA11 (44%) were
detected in the majority (91%) of large uveal melanomas. However,
these oncogenic mutations have rarely been identified in other
types of cancer. G protein α subunits may transduce external
signals to intracellular signaling pathways, which activate the
phospholipase C and mitogen-activated protein kinase pathways,
promoting proliferation and the cell survival rate of melanoma cell
lines (10). Constitutively active
GNAQ and GNA11 mutants may activate the extracellular
signal-regulated kinase (ERK) pathway, and knockdown of mutant GNAQ
in uveal melanoma cells may lead to mitogen-activated protein
kinase inhibition, cell growth reduction and apoptosis induction
(11). However, the effect of
mutant GNAQ on uveal melanomas has not been completely
elucidated.
Four Notch receptors (Notch1–4) are found in mammals
and play an essential role in tumorigenesis. Two ligand families,
Jagged (Jag-1 and Jag-2) and δ-like ligand, are required for the
activation of canonical Notch signaling (12). Binding of these ligands to the Notch
receptor results in its cleavage by the tumor necrosis
factor-α-converting enzyme (13).
The final cleavage event, which is induced by the γ-secretase
complex, releases the Notch intracellular domain (NICD), stimulates
nuclear translocation and modifies the gene expression (14). The best studied genes that are
modified by NICD are the Drosophila proteins hairy and
enhancer of split, homologs to human hairy and enhancer of split
(Hes), and hairy and enhancer of split related with YRPW motif
(Hey) families (15). Activation of
Notch1 has been reported to promote cell growth and tumor invasion
in human cutaneous melanoma (16).
Notch1 activation also induces a transformed cell phenotype in
cutaneous melanocytes in vitro (17). The role of Notch signaling has been
examined in uveal melanoma and was found to promote proliferation,
clonogenic growth and invasion in tumor cells (18). Nevertheless, to the best of our
knowledge, few studies have reported the interaction between Notch
signaling and mutant GNAQ in uveal melanoma.
The present study revealed that mutant GNAQ promotes
uveal melanoma cell viability and migration through the activation
of Notch signaling. Inhibition of Notch by MRK003 resulted in
decreased cell viability and migration. Furthermore, Yes-associated
protein (YAP) dephosphorylation and translocation were stimulated
by GNAQ and mediated Notch signaling activation in uveal melanoma
cells. Thus, we found an association between the GNAQ mutation and
Notch signaling, which may facilitate the identification of
therapies to treat uveal melanoma.
Materials and methods
Cell culture and tumor tissues
Five cell lines, including 92.1, OMM2.2, OMM2.5,
Mel285 and Mel290 (provided by Dr Martine Jager of Leiden
University, The Netherlands), were cultured in RPMI-1640 medium
supplemented with 10% fetal calf serum (FCS), 2.5 μg/ml
fungizone®/amphotericin B, 50 μg/ml gentamicin
and 2 mM L-glutamine (Gibco, Invitrogen, Carlsbad, CA, USA) as
previously described (19). The
cells were cultured at 37°C in a humidified air/CO2
atmosphere. Excess tumor tissues not required for diagnosis were
obtained from primary uveal melanoma tumors of 12 patients who were
enrolled at the Xi’an No. 4 Hospital following approval by the
Institutional Research Committee. The tissues were preserved at
−80°C. Human samples were obtained after informed consent was
provided by the patients, and in accordance with the Declaration of
Helsinki. The present study was approved by the Ethics Committee of
Xi’an No. 4 Hospital.
MTT assay
An MTT assay was employed for the evaluation of
melanoma cell growth and proliferation. The experiments were
carried out in 96-well plates according to the manufacturer’s
instructions (Roche GmbH, Mannheim, Germany). In the MTT test,
tetrazolium salts were transformed by active enzymes of the cells
into intracellular formazan deposits and the cells were incubated
for 4 h with the tetrazolium salts. After 4 h, the purple formazan
salts formed became soluble. Absorbance was determined at 490
nm.
Migration assay
Cell migration assays were performed with a
two-chamber Transwell® device (Corning, Edison, NJ,
USA). The cells were collected and suspended in RPMI-1640 medium
containing 10% FCS. A Transwell apparatus with an 8-μm pore
size membrane (Corning) was used to analyze the migration activity.
The cells were suspended in 120 ml RPMI-1640 medium containing 0.1%
FCS and then seeded in the upper chamber of the device, while 500
ml RPMI-1640 medium containing 10% FCS was added to the lower
chamber. The device was incubated at 37°C for 12 h. The inner side
of the upper chamber was wiped with a wet cotton swab to remove the
cells, while the outer side of the chamber was gently rinsed with
phosphate-buffered saline (PBS) and treated with 95% ethanol for 30
min. The membrane was then washed with PBS again and stained with a
0.1% crystal violet staining solution for 30 min. After being
dried, images of the membrane were taken for >5 fields and the
cells were counted. At least three experiments were performed using
the Transwell assays for each cell line.
DNA construct
The plasmids pGEM-HA-GαqQL were constructed as
described in a previous study (20,21).
The 1.1 kb HindIII-NotI fragment containing the
coding sequence for HA-Gαq was ligated into the blunted SalI
sites of pGEM-9Zf, which was confirmed by restriction mapping and
nucleotide sequencing.
Small interfering RNA transfection
Scrambled small interfering RNA (siRNA) and siRNA
targeting GNAQ (sc-35429-SH) were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). The cells were
transfected with scrambled or GNAQ siRNA according to the
manufacturer’s instructions. Briefly, the scrambled and GNAQ siRNAs
(30 pmol) were diluted in 500 μl Dulbecco’s modified Eagle’s
medium (DMEM) and mixed with 5 μl Lipofectamine®
RNAiMAX (Invitrogen). After 15 min of incubation at room
temperature, the complexes were added to the cells to a final
volume of 3 ml medium. The cells were then harvested at the
indicated time periods for subsequent analysis. The efficiency of
the GNAQ siRNA was confirmed by western blot analysis of the flag
expression.
Fluorescence staining
The Mel285 cells were tranfected with blank vectors
or HA-GαqQL. Following fixation with 4% formaldehyde in PBS for 10
min, the cells were permeabilized with 0.3% Triton X-100 in PBS for
10 min, followed by 1 h of blocking with 5% bovine serum albumin in
PBS. The cells were stained with the primary antibody, rabbit
anti-YAP, overnight at 4°C. Fluorescence staining was performed
using Alexa 488-conjugated goat anti-rabbit (green) antibody.
Confocal microscopy was performed using a multiphoton Zeiss LSM 510
laser scanning microscope (Carl Zeiss, Jena, Germany). Confocal
images were obtained with LSM 510 software.
Quantitative polymerase chain reaction
analysis
The mRNA of uveal melanoma cells or tumor tissues
was extracted with TRIzol® RNA-extraction reagent
(Gibco, Rockville, MD, USA). Approximately 5 μg of total RNA
for each sample were reverse-transcribed into first-strand cDNA for
quantitative polymerase chain reaction (RT-qPCR) analysis. RT-qPCR
was performed in a final volume of 10 μl, containing 5
μl of SsoFast™ EvaGreen® Supermix (Bio-Rad,
Hercules, CA, USA), 1 μl of cDNA (1:50 dilution) and 2
μl each of the forward and reverse primers (1 mM). The steps
in the RT-qPCR were performed as follows: 94°C for 2 min for
initial denaturation; 94°C for 20 sec, 58°C for 15 sec and 72°C for
15 sec; 2 sec were used for plate reading for 40 cycles; and a melt
curve was generated between 65 and 95°C. β-actin was used as a
quantitative and qualitative control to normalize the gene
expression. Data are analyzed using the formula: R = 2−(ΔCt
sample – ΔCt control). The primers used in this experiment
are shown in Table I.
 | Table IPrimers for quantitative real-time
PCR. |
Table I
Primers for quantitative real-time
PCR.
| Gene name | Sequence |
|---|
| GNAQ |
5′-CCCTAATGGCTGCTACCC-3′ |
|
5′-AAATCGTGGCCCAAACAC-3′ |
| Jag-1 |
5′-TACTACTGCGACTGTCTTCCC-3′ |
|
5′-CAGCGATAACCATTAACCAA-3′ |
| Hes-1 |
5′-AGCTCGCGGCATTCCAAG-3′ |
|
5′-AGCGGGTCACCTCGTTCA-3′ |
| β-actin |
5′-CTCCATCCTGGCCTCGCTGT-3′ |
|
5′-GCTGTCACCTTCACCGTTCC-3′ |
Western blot analysis
Proteins were extracted from uveal melanoma cells
and the protein concentration was quantified by the Bradford assay.
A total of 20 μg of protein was separated by 12% sodium
dodecyl sulfate polyacrylamide gel electrophoresis and transferred
to a nitrocellulose membrane (BioTeke, Beijing, China), which was
incubated with 2% non-fat dry milk in Tris-buffered saline (TBS) to
block non-specific binding at room temperature for 1 h. After being
blocked, the membrane was incubated in blocking buffer containing
primary antibodies overnight at 4°C. Antibodies including
anti-Jag-1, anti-NICD, anti-Hes-1 and anti-β-actin were purchased
from Santa Cruz Biotechnology, Inc.. Subsequently, the membrane was
washed with TBS-Tween for 10 min and incubated with horseradish
peroxidase-conjugated secondary antibody (Tiangen Corporation,
Beijing, China) diluted in blocking buffer for 1 h at room
temperature. After being washed again with TBS-Tween buffer for 10
min, proteins were detected using enhanced chemiluminescence
(Pierce, Rockford, IL, USA).
Statistical analysis
The statistical significance of differences was
assessed using SPSS 15.0 (SPSS Science, Chicago, IL, USA).
Differences between the two groups were analyzed by the Student’s
t-test. Correlations were assessed using the Spearman’s rank
correlation coefficient. Differences between multiple groups were
analyzed by the ANOVA. P<0.05 was considered to indicate a
statistically significant result.
Results
Oncogene GNAQ contributes to uveal
melanoma cell viability and migration
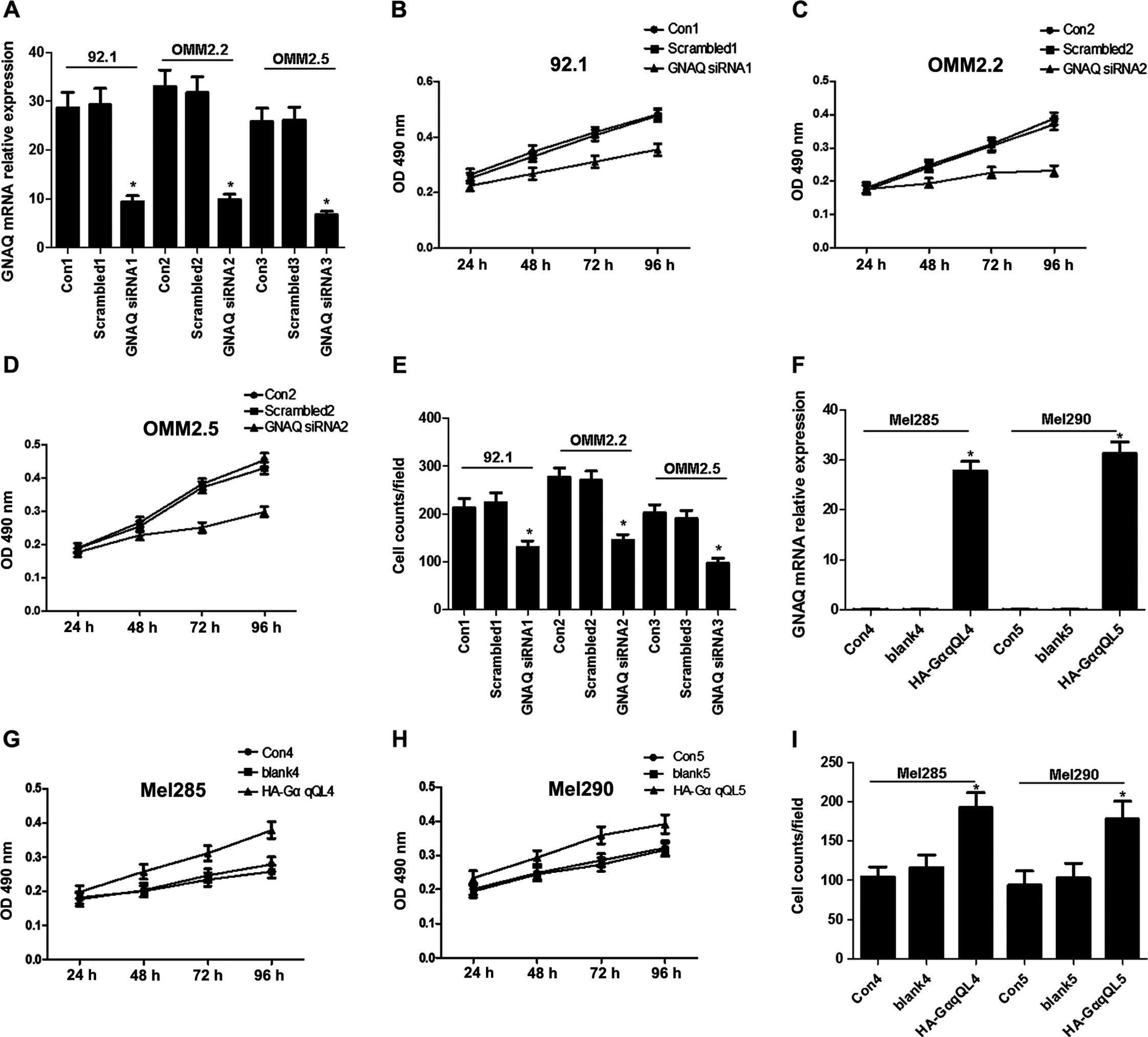
Mutant GNAQ has been identified in certain uveal
melanoma cell lines including 92.1, OMM2.2 and OMM2.5 (22). To determine the effect of GNAQ on
uveal melanoma cells, the cultured 92.1, OMM2.2 and OMM2.5 cells
were transfected with scrambled or GNAQ siRNA. Transfection of GNAQ
siRNA resulted in a 67, 70 and 74% inhibition of GNAQ mRNA
expression in 92.1, OMM2.2 and OMM2.5 cells, respectively (Fig. 1A). The viability of these
transfected cells was evaluated by MTT over 24–96 h. The results
showed that GNAQ knockdown significantly inhibited the viability of
92.1, OMM2.2 and OMM2.5 cells (Fig.
1B–D). GNAQ knockdown also reduced the migratory rates of 92.1,
OMM2.2 and OMM2.5 cells compared with the respective controls
(Fig. 1E). Moreover, cells such as
Mel285 and Mel290 were absent from the mutant GNAQ. Therefore, we
examined the effect of the GNAQ expression on these cells through
transfection with human influenza hemagglutinin A epitope
(HA)-tagged GαqQL. Consequently, HA-GαqQL transfection resulted in
a significant increase in the GNAQ mRNA expression (Fig. 1F), and increased the viability and
migration of the Mel285 and Mel290 cells (Fig. 1G–I). These results indicated that
mutant GNAQ may promote uveal melanoma cell viability and
migration.
GNAQ activates Notch signaling in uveal
melanoma cells
Activation of Notch signaling is involved in the
carcinogenesis of various tissues including ocular tissues
(23–25). As mutant GNAQ was highly expressed
in uveal melanoma cells (26), we
hypothesized that GNAQ causes Notch signal activation and
contributed to cell growth and migration. To validate our
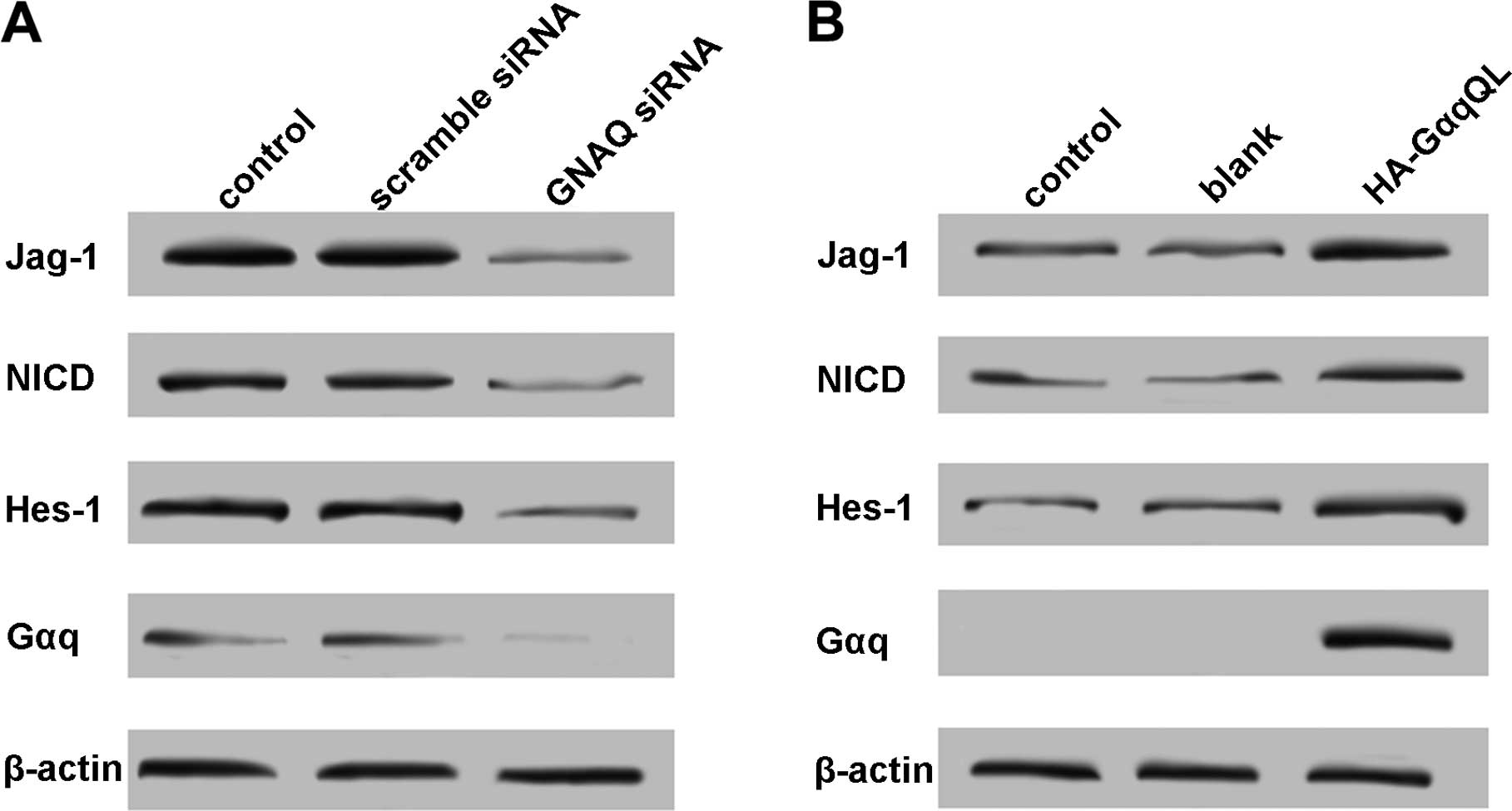
hypothesis, GNAQ expression in 92.1 cells was knocked down by GNAQ
siRNA, and the expression of Jag-1 (Notch ligand), NICD and Hes-1
(Notch target gene) was analyzed by western blotting. A decreased
expression of Jag-1, NCID and Hes-1 was observed in the GNAQ
knockdown cells (Fig. 2A).
Furthermore, HA-GαqQL transfection in the Mel285 cells notably
upregulated the expression of Jag-1, NCID and Hes-1 (Fig. 2B). These results revealed that the
Notch signaling may be triggered by mutant GNAQ in uveal melanoma
cells.
Inhibition of Notch signaling blocks
uveal melanoma cell viability and migration induced by GNAQ
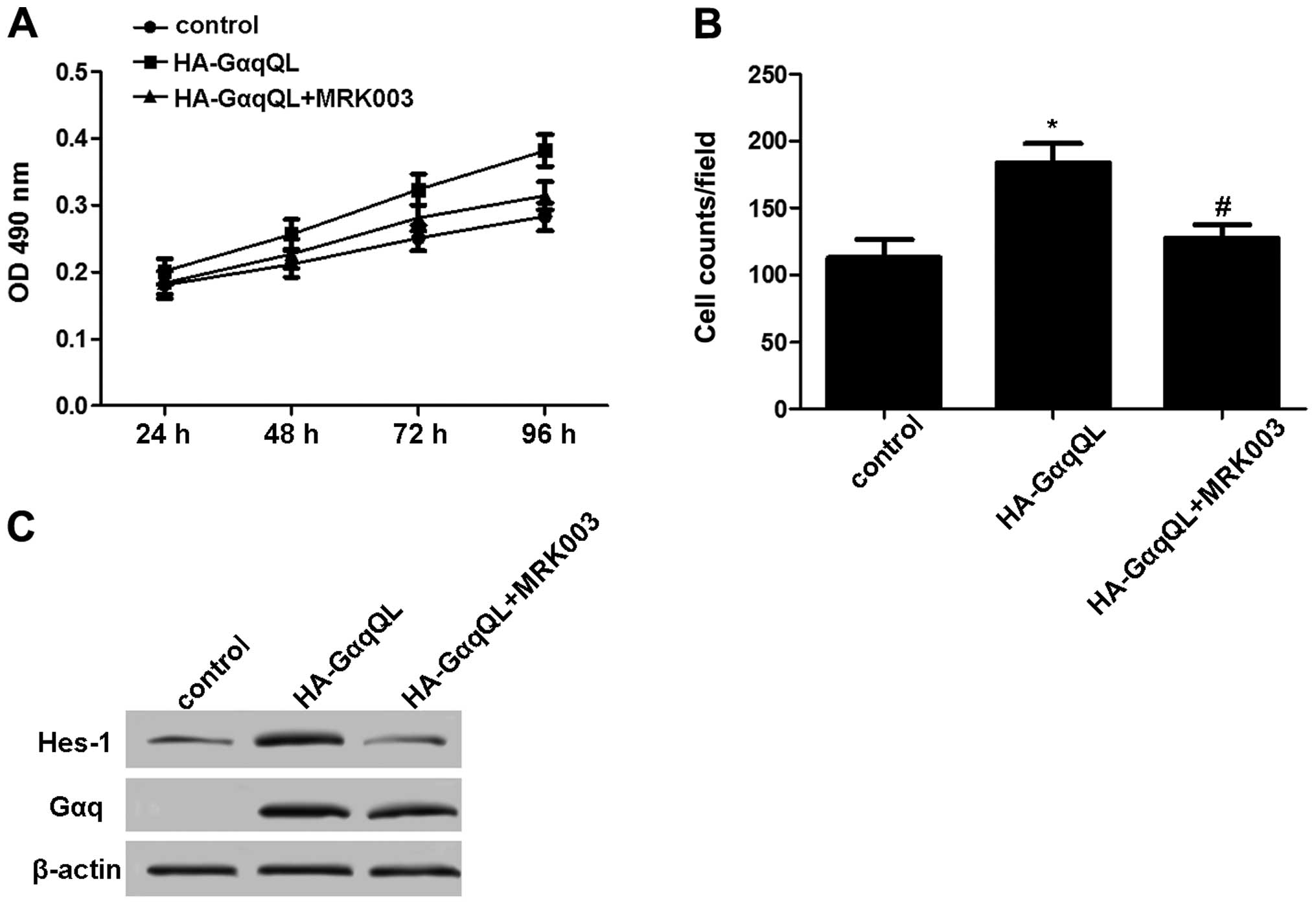
To confirm the effect of Notch activation induced by
GNAQ on uveal melanoma cells, the Mel285 cells were transfected
with HA-GαqQL and treated with or without 5 μmol/l MRK003 (a
potent γ-secretase inhibitor). Compared with the HA-GαqQL
transfection group, treatment with MRK003 significantly inhibited
cell viability and migration (Fig. 3A
and B). Furthermore, Hes-1 expression in these cells was
decreased by MRK003 (Fig. 3C).
These results indicated that inactive Notch impeded the effect of
GNAQ on uveal melanoma cells.
YAP mediates GNAQ-induced Notch
activation
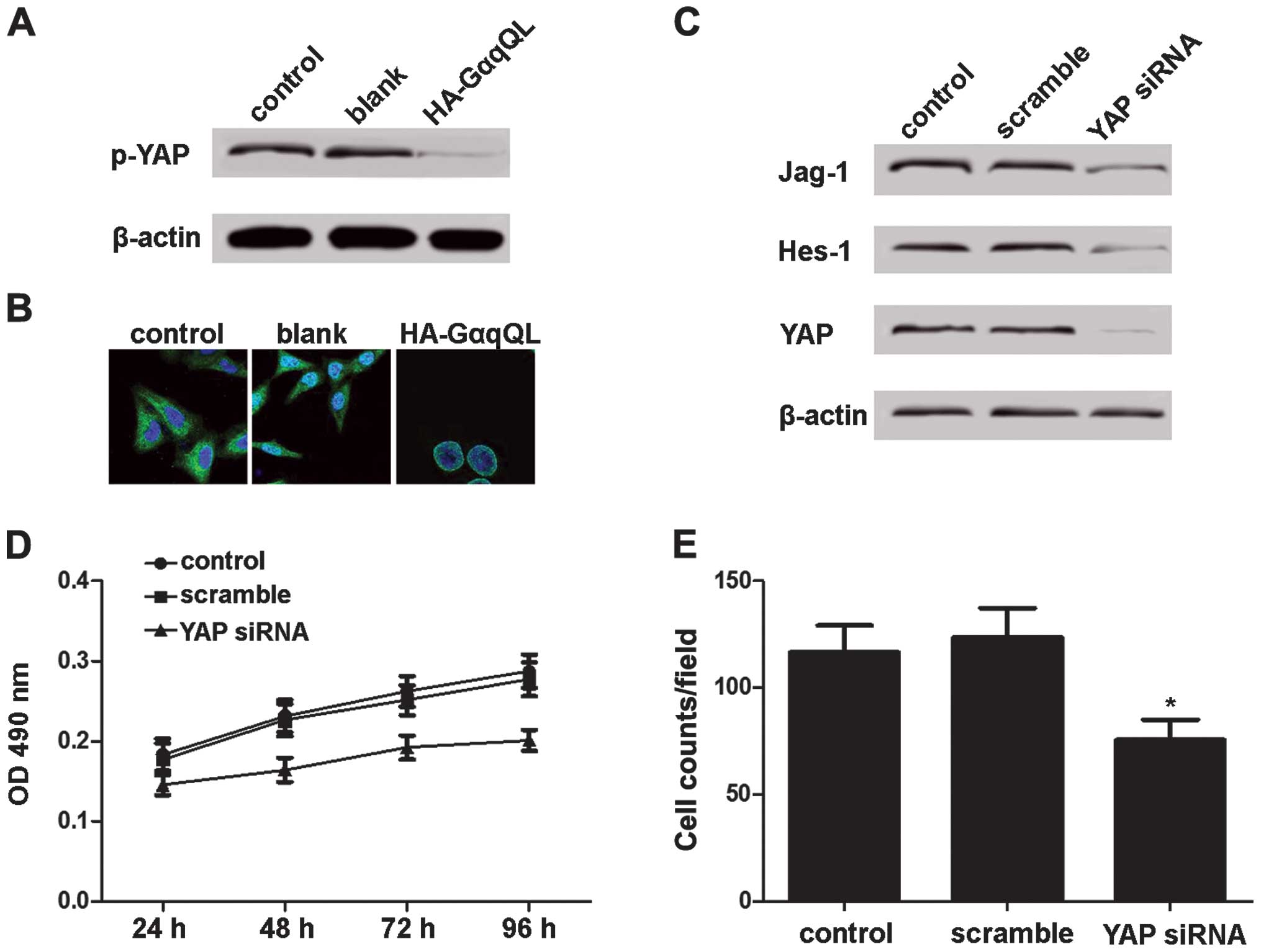
It has been shown that nuclear YAP induces Notch
activation (27). Therefore, we
investigated whether YAP is involved in GNAQ-induced Notch
activation. Mel285 cells were transfected with HA-GαqQL, and the
YAP phosphorylation levels and nuclear translocation were detected.
Compared with the Mel285 cells transfected with empty vectors,
those transfected with HA-GαqQL showed decreased YAP
phosphorylation (Fig. 4A) and
increased YAP nuclear translocation (Fig. 4B). Furthermore, YAP expression in
the 92.1 cells was inhibited, and Jag-1 and Hes-1 expression was
reduced by YAP siRNA (Fig. 4C).
Additionally, the viability and migration of the 92.1 cells was
inhibited by the YAP siRNA transfection (Fig. 4D and E). These results demonstrated
that GNAQ inhibits YAP phosphorylation and promotes YAP
translocation, which mediated Notch activation in uveal melanoma
cells.
GNAQ contributes to the oncogenic
activation of Notch in human uveal melanoma
To confirm the connection between the GNAQ, YAP and
Notch signaling in human uveal melanoma, total RNA was isolated
from 12 fresh-frozen human uveal melanoma samples, and the mRNA
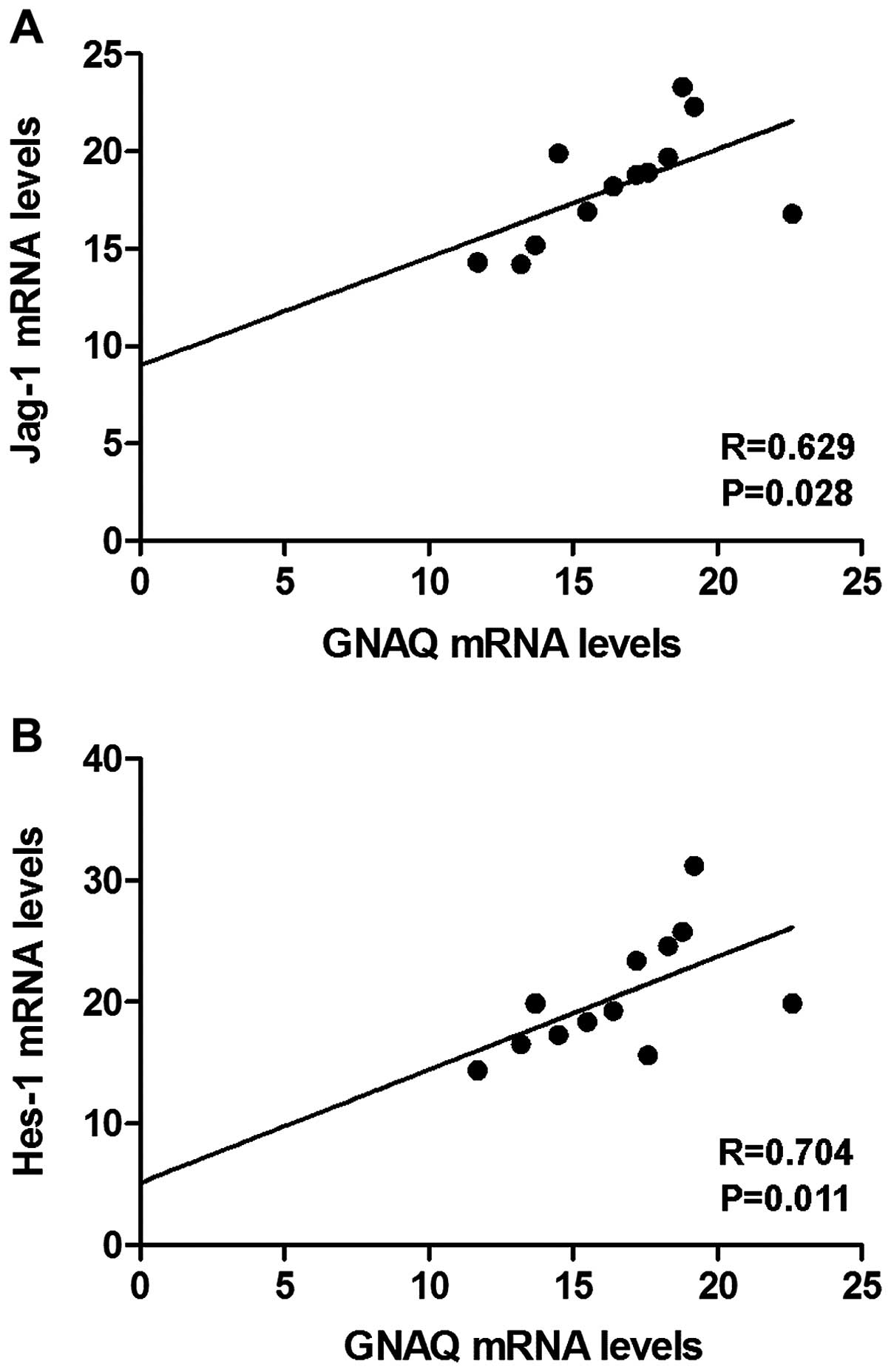
levels of GNAQ, YAP, Jag-1 and Hes-1 were analyzed. Positive
correlations between the GNAQ and Jag-1 mRNA levels and between the
GNAQ and Hes-1 mRNA levels were observed (Fig. 5A and B). However, no correlation was
found between the GNAQ and YAP mRNA levels, which suggested that
GNAQ regulated YAP protein phosphorylation but not its transcript
level.
Discussion
A high incidence of mutations in G protein-coupled
receptors and G proteins has been observed in melanomas (8,28,29).
Notably, GNAQ and GNA11 mutations have been reported in the
majority of uveal melanomas, 83% of blue nevi, 6% of cutaneous
melanomas and 59% of tumors occurring in the meninges (30,31).
The potential role of GTPase-deficient mutants of several Gα
subunits in carcinogenesis has been previously demonstrated
(32,33). The GNAQQ209L mutation
contributes to a loss of GTPase activity and constitutive
activation of GNAQ (34,35). Subsequently, dissociation of GNAQ in
its GTP-bound state results, via Ras and Raf signaling, in MEK1/ERK
activation, which ultimately modulates cell growth and migration
(36). Van Raamsdonk et al
(31) found that blocking the GNAQ
expression in OMM1.3 cells resulted in a significant decrease in
the cell number, loss of anchorage-independent growth and a marked
increase in the sub-G0/G1 population. The present study confirmed
that GNAQ mutation was critical for the viability and migration of
melanoma cells.
Notch activation has been found to be involved in
the viability of cutaneous melanoma. The elevated expression of the
Notch pathway members has been described in melanoma cells compared
with normal melanocytes, and treatment with the tripeptide GSI
caused apoptosis in melanoma cells (37,38).
In a study by Asnaghi et al (39), Jag-2 was introduced into the Mel285
and Mel290 cells, and these cells showed enhanced cell growth and
motility as evaluated by the wound-healing and Transwell invasion
assays. By contrast, transfection of 92.1 and OMM1 cells with Jag-2
short hairpin RNAs indicated that knockdown of the ligand
significantly inhibited cell growth, invasion and migration. Huang
et al (40) reported that
knockdown of Notch1 in uveal melanoma cells with siRNA resulted in
significant cell growth inhibition as well as enhanced apoptosis
and cell cycle arrest in vitro. Treatment with siNotch1
in vivo also significantly inhibited tumor growth and
prolonged the mouse survival rate in a OCM1 xenograft model.
Furthermore, in primary uveal melanoma tissues and uveal melanoma
cells, MRK003 treatment inhibited anchorage-independent cell growth
and invasion, and reduced phosphorylation levels of STAT3 and
ERK1/2 (18). In the present study,
we demonstrated that mutant GNAQ contributed to the activation of
Notch signaling, and inhibition of Notch signaling impeded the
stimulatory effect of GNAQ on melanoma cell viability and
migration.
It has been established that YAP may be involved in
the modulation of tumor cell growth and invasion. Overexpressed YAP
in human hepatocellular carcinoma cell lines and mouse hepatocytes
upregulated Jag-1, resulting in Notch signaling activation and
proliferation promotion (27). Zhou
et al (41) demonstrated
that YAP was overexpressed in human colon cancers and colon
cancer-derived cell lines, where YAP depletion strongly reduces
β-catenin and Notch signaling, and inhibits proliferation and the
survival rate. Notably, YAP has been reported to be regulated by
mutant GNAQ. Feng et al demonstrated that GNAQ stimulates
YAP dephosphorylation and translocation through a Trio-Rho/Rac
signaling circuitry promoting actin polymerization and the
YAP-dependent growth of uveal melanoma cells (42). Moreover, in cell culture and human
tumors, cancer-associated Gq/11 mutants were demonstrated to
activate YAP, which mediated the oncogenic activity of mutant Gq/11
in uveal melanoma development (22). The present findings have
demonstrated that YAP dephosphorylation and nuclear translocation
may play a key role in Notch activation and cell viability and
migration induced by GNAQ. Additionally, the present study provided
evidence that positive correlations between the GNAQ and Jag-1 mRNA
levels and between the GNAQ and Hes-1 mRNA levels were constant in
human uveal melanomas.
In conclusion, results of the present study have
shown the critical role of mutant GNAQ in melanoma cell viability
and migration through the activation of Notch signaling. Of note,
YAP dephosphorylation and nuclear translocation were found to be
essential for the interaction between GNAQ and Notch signaling.
Thus, the present study identified a critical target for uveal
melanoma treatment.
References
|
1
|
Bedikian AY: Metastatic uveal melanoma
therapy: Current options. Int Ophthalmol Clin. 46:151–166. 2006.
View Article : Google Scholar
|
|
2
|
Biswas J, Krishnakumar S and Shanmugam MP:
Uveal melanoma in Asian Indians: A clinicopathological study. Arch
Ophthalmol. 120:522–523. 2002.PubMed/NCBI
|
|
3
|
Sakamoto T, Sakamoto M, Yoshikawa H, Hata
Y, Ishibashi T, Ohnishi Y and Inomata H: Histologic findings and
prognosis of uveal malignant melanoma in Japanese patients. Am J
Ophthalmol. 121:276–283. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuo PK, Puliafito CA, Wang KM, Liu HS and
Wu BF: Uveal melanoma in China. Int Ophthalmol Clin. 22:57–71.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh AD, Turell ME and Topham AK: Uveal
melanoma: Trends in incidence, treatment, and survival.
Ophthalmology. 118:1881–1885. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Collaborative Ocular Melanoma Study Group:
Assessment of metastatic disease status at death in 435 patients
with large choroidal melanoma in the Collaborative Ocular Melanoma
Study (COMS): COMS report no. 15. Arch Ophthalmol. 119:670–676.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gragoudas ES, Egan KM, Seddon JM, Glynn
RJ, Walsh SM, Finn SM, Munzenrider JE and Spar MD: Survival of
patients with metastases from uveal melanoma. Ophthalmology.
98:383–390. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O’Hayre M, Vázquez-Prado J, Kufareva I,
Stawiski EW, Handel TM, Seshagiri S and Gutkind JS: The emerging
mutational landscape of G proteins and G-protein-coupled receptors
in cancer. Nat Rev Cancer. 13:412–424. 2013. View Article : Google Scholar
|
|
9
|
Daniels AB, Lee JE, MacConaill LE,
Palescandolo E, Van Hummelen P, Adams SM, DeAngelis MM, Hahn WC,
Gragoudas ES, Harbour JW, et al: High throughput mass
spectrometry-based mutation profiling of primary uveal melanoma.
Invest Ophthalmol Vis Sci. 53:6991–6996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ambrosini G, Pratilas CA, Qin LX, Tadi M,
Surriga O, Carvajal RD and Schwartz GK: Identification of unique
MEK-dependent genes in GNAQ mutant uveal melanoma involved in cell
growth, tumor cell invasion, and MEK resistance. Clin Cancer Res.
18:3552–3561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Raamsdonk CD, Griewank KG, Crosby MB,
Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green
G, Bouvier N, et al: Mutations in GNA11 in uveal melanoma. N Engl J
Med. 363:2191–2199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mumm JS and Kopan R: Notch signaling: From
the outside in. Dev Biol. 228:151–165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brou C, Logeat F, Gupta N, Bessia C,
LeBail O, Doedens JR, Cumano A, Roux P, Black RA and Israël A: A
novel proteolytic cleavage involved in Notch signaling: The role of
the disintegrin-metalloprotease TACE. Mol Cell. 5:207–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borggrefe T and Oswald F: The Notch
signaling pathway: Transcriptional regulation at Notch target
genes. Cell Mol Life Sci. 66:1631–1646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jarriault S, Le Bail O, Hirsinger E,
Pourquié O, Logeat F, Strong CF, Brou C, Seidah NG and Isral A:
Delta-1 activation of notch-1 signaling results in HES-1
transactivation. Mol Cell Biol. 18:7423–7431. 1998.PubMed/NCBI
|
|
16
|
Liu ZJ, Xiao M, Balint K, Smalley KS,
Brafford P, Qiu R, Pinnix CC, Li X and Herlyn M: Notch1 signaling
promotes primary melanoma progression by activating
mitogen-activated protein kinase/phosphatidylinositol 3-kinase-Akt
pathways and up-regulating N-cadherin expression. Cancer Res.
66:4182–4190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pinnix CC, Lee JT, Liu ZJ, McDaid R,
Balint K, Beverly LJ, Brafford PA, Xiao M, Himes B, Zabierowski SE,
et al: Active Notch1 confers a transformed phenotype to primary
human melanocytes. Cancer Res. 69:5312–5320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Asnaghi L, Ebrahimi KB, Schreck KC, Bar
EE, Coonfield ML, Bell WR, Handa J, Merbs SL, Harbour JW and
Eberhart CG: Notch signaling promotes growth and invasion in uveal
melanoma. Clin Cancer Res. 18:654–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Babchia N, Calipel A, Mouriaux F, Faussat
AM and Mascarelli F: The PI3K/Akt and mTOR/P70S6K signaling
pathways in human uveal melanoma cells: Interaction with B-Raf/ERK.
Invest Ophthalmol Vis Sci. 51:421–429. 2010. View Article : Google Scholar
|
|
20
|
Mende U, Kagen A, Cohen A, Aramburu J,
Schoen FJ and Neer EJ: Transient cardiac expression of
constitutively active Gαq leads to hypertrophy and
dilated cardiomyopathy by calci-neurin-dependent and independent
pathways. Proc Natl Acad Sci USA. 95:13893–13898. 1998. View Article : Google Scholar
|
|
21
|
Marinissen MJ, Servitja JM, Offermanns S,
Simon MI and Gutkind JS: Thrombin protease-activated receptor-1
signals through Gq- and G13-initiated MAPK
cascades regulating c-Jun expression to induce cell transformation.
J Biol Chem. 278:46814–46825. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng
Z, Zhao L, Peyman G, Ouyang H, Jiang W, et al: Mutant Gq/11 promote
uveal melanoma tumorigenesis by activating YAP. Cancer Cell.
25:822–830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng P, Kumar V, Liu H, Youn JI, Fishman
M, Sherman S and Gabrilovich D: Effects of notch signaling on
regulation of myeloid cell differentiation in cancer. Cancer Res.
74:141–152. 2014. View Article : Google Scholar
|
|
24
|
Neradugomma NK, Subramaniam D, Tawfik OW,
Goffin V, Kumar TR, Jensen RA and Anant S: Prolactin signaling
enhances colon cancer stemness by modulating Notch signaling in a
Jak2-STAT3/ERK manner. Carcinogenesis. 35:795–806. 2014. View Article : Google Scholar :
|
|
25
|
Xie M, He CS, Wei SH and Zhang L: Notch-1
contributes to epidermal growth factor receptor tyrosine kinase
inhibitor acquired resistance in non-small cell lung cancer in
vitro and in vivo. Eur J Cancer. 49:3559–3572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lamba S, Felicioni L, Buttitta F, Bleeker
FE, Malatesta S, Corbo V, Scarpa A, Rodolfo M, Knowles M, Frattini
M, et al: Mutational profile of GNAQQ209 in human
tumors. PLoS One. 4:e68332009. View Article : Google Scholar
|
|
27
|
Tschaharganeh DF, Chen X, Latzko P, Malz
M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N, et al:
Yes-associated protein up-regulates Jagged-1 and activates the
Notch pathway in human hepatocellular carcinoma. Gastroenterology.
144:1530–1542.e12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kan Z, Jaiswal BS, Stinson J, Janakiraman
V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J, et al:
Diverse somatic mutation patterns and pathway alterations in human
cancers. Nature. 466:869–873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prickett TD, Wei X, Cardenas-Navia I, Teer
JK, Lin JC, Walia V, Gartner J, Jiang J, Cherukuri PF, Molinolo A,
et al: Exon capture analysis of G protein-coupled receptors
identifies activating mutations in GRM3 in melanoma. Nat Genet.
43:1119–1126. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Küsters-Vandevelde HV, Klaasen A, Küsters
B, Groenen PJ, van Engen-van Grunsven IA, van Dijk MR, Reifenberger
G, Wesseling P and Blokx WA: Activating mutations of the GNAQ gene:
A frequent event in primary melanocytic neoplasms of the central
nervous system. Acta Neuropathol. 119:317–323. 2010. View Article : Google Scholar :
|
|
31
|
Van Raamsdonk CD, Bezrookove V, Green G,
Bauer J, Gaugler L, O’Brien JM, Simpson EM, Barsh GS and Bastian
BC: Frequent somatic mutations of GNAQ in uveal melanoma and blue
naevi. Nature. 457:599–602. 2009. View Article : Google Scholar :
|
|
32
|
Vallar L, Spada A and Giannattasio G:
Altered Gs and adenylate cyclase activity in human
GH-secreting pituitary adenomas. Nature. 330:566–568. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lyons J, Landis CA, Harsh G, Vallar L,
Grünewald K, Feichtinger H, Duh QY, Clark OH, Kawasaki E, Bourne
HR, et al: Two G protein oncogenes in human endocrine tumors.
Science. 249:655–659. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Van Raamsdonk CD, Fitch KR, Fuchs H, de
Angelis MH and Barsh GS: Effects of G-protein mutations on skin
color. Nat Genet. 36:961–968. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Markby DW, Onrust R and Bourne HR:
Separate GTP binding and GTPase activating domains of a G alpha
subunit. Science. 262:1895–1901. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar
|
|
37
|
Hoek K, Rimm DL, Williams KR, Zhao H,
Ariyan S, Lin A, Kluger HM, Berger AJ, Cheng E, Trombetta ES, et
al: Expression profiling reveals novel pathways in the
transformation of melanocytes to melanomas. Cancer Res.
64:5270–5282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Qin JZ, Stennett L, Bacon P, Bodner B,
Hendrix MJ, Seftor RE, Seftor EA, Margaryan NV, Pollock PM, Curtis
A, et al: p53-independent NOXA induction overcomes apoptotic
resistance of malignant melanomas. Mol Cancer Ther. 3:895–902.
2004.PubMed/NCBI
|
|
39
|
Asnaghi L, Handa JT, Merbs SL, Harbour JW
and Eberhart CG: A role for Jag2 in promoting uveal melanoma
dissemination and growth. Invest Ophthalmol Vis Sci. 54:295–306.
2013. View Article : Google Scholar
|
|
40
|
Huang X, Wang L, Zhang H, Wang H, Zhao X,
Qian G, Hu J, Ge S and Fan X: Therapeutic efficacy by targeting
correction of Notch1-induced aberrants in uveal tumors. PLoS One.
7:e443012012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou D, Zhang Y, Wu H, Barry E, Yin Y,
Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD, et al:
Mst1 and Mst2 protein kinases restrain intestinal stem cell
proliferation and colonic tumorigenesis by inhibition of
Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci USA.
108:E1312–E1320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Feng X, Degese MS, Iglesias-Bartolome R,
Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino
G, Sodhi A, et al: Hippo-independent activation of YAP by the GNAQ
uveal melanoma oncogene through a trio-regulated rho GTPase
signaling circuitry. Cancer Cell. 25:831–845. 2014. View Article : Google Scholar : PubMed/NCBI
|