Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common malignancy associated with a high mortality worldwide
(1). To date, surgical resection
and liver transplantation are considered the optimal option for the
treatment of HCC (2). However, not
all HCC patients are suitable for partial hepatectomy or liver
transplantation (3). Therefore, to
develop more effective therapeutic strategies that directly target
key molecules in signaling pathways of tumorigenesis (4) and/or metastasis in HCC is urgently
needed.
Krüppel-like factor 4 (KLF4) is mainly found in
differentiated epithelial cells of the skin and the
gastrointestinal tract (5). KLF4 is
a zinc-finger transcription factor that inhibits cell proliferation
and maintains the differentiation of epithelial cells by regulating
gene expression (6). Moreover, KLF4
expression is also involved in the tumorigenesis and tumor
progression in different types of cancer cells (7). KLF4 has been identified as a
tumor-suppressor gene, since reduction in KLF4 expression has been
reported in esophageal, gastric, colon and lung cancers (8–11).
Conversely, KLF4 may also serve as an oncogene since it was found
to be increased in oral cancer (12). Thus, these findings suggest that
KLF4 has various functions in different type of cancer cells.
Recently, reduction in KLF4 has been found in HCC
samples and was significantly correlated with reduced survival time
of patients (13). Several reports
indicated that KLF4 is involved in the tumor metastasis of HCC and
breast cancer cells through suppression of the expression of
E-cadherin and epithelial-mesenchymal transition (EMT) inducers
such as Slug and Snail (13–15).
Using animal model, it has also been demonstrated that KLF4
inhibits the tumorigenic progression of HCC (13). In addition, KLF4 is positively
regulated by PPARγ via binding directly to the PPAR response
element within the KLF4 promoter in colorectal cancer cells
(16). PPARγ agonist, troglitazone,
has been found to upregulate KLF4 expression, and this KLF4
induction can be prevented by pretreatment with PPARγ antagonist,
GW9662 (16). However, the detailed
mechanisms of KLF4 in the modulation of cell motility and
invasiveness in hepatoma cells remain unclear.
Tissue inhibitors of metalloproteinases (TIMPs)
control the functions and activities of matrix metalloproteinases
(MMPs), which play a crucial role in extracellular matrix
degradation involved in tumor cell invasion, metastasis and
angiogenesis. For example, increased MMP9 and MMP2 associated with
tumor aggressiveness and poor prognosis in HCC (17–20)
are regulated by TIMP-1 and TIMP-2, respectively. Additionally, the
levels of TIMP-1 and TIMP-2 were also used as prognostic factors
for predicting the metastasis of HCC (21,22).
In the present study, we examined the effects of KLF4 on HCC cell
migration and invasion. In addition, the relationship between KLF4
and TIMP-1/TIMP-2 was also investigated.
Materials and methods
Cell culture and reagents
Four HCC cell lines (HepG2, Hep3B, HA22T/VGH and
Mahlavu) and HEK293T were maintained in Dulbecco’s modified Eagle’s
medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10%
fetal bovine serum (FBS; HyClone, Logan, UT, USA), 0.1 mM
non-essential amino acids, 2 mM L-glutamine and 1%
penicillin/streptomycin in a humidified atmosphere containing 5%
CO2 at 37°C. Rosiglitazone, troglitazone and GW9662 were
obtained from Cayman Chemical (Ann Arbor, MI, USA). Recombinant
TIMP-1 protein and myeloperoxidase (MPO) were purchased from Enzo
Life Sciences (Plymouth Meeting, PA, USA) and Millipore (Temecula,
CA, USA), respectively.
Overexpression and knockdown of KLF4
KLF4 was amplified from human cDNA using PCR method
with primers: 5′-GTCTAGAGCCACCATGGCTGTCAGCGACGCGCTG-3′ and
5′-GGGATCCTTAAAAATGCCTCTTCATG-3′. KLF4 was cloned into the
lentiviral expression vector pLV-EF1α-MCS-IRES-Bsd (pLV-Bsd;
Biosettia, San Diego, CA, USA) at the BamHI and NheI
restriction enzyme sites. Construct pLV-Bsd-KLF4 was confirmed by
DNA sequencing. The lentiviral vector pLVO.1-shKLF4 used for KLF4
knockdown was purchased from RNAi core of Academia Sinica (Taiwan).
The oligonucleotide targeting to human KLF4 was 5′-CTGGAC
TTTATTCTCTCCAAT-3′. The lentiviruses were generated by
co-transfecting HEK293T cells with the lentiviral expression
vectors (pLV-Bsd, pLV-Bsd-KLF4 or pLVO.1-shKLF4) and packaging
plasmids (pCMVΔR8.91 and pCMV-VSV-G) using T-Pro NTR II (Ji-Feng
Biotechnology, Taipei, Taiwan). Supernatants containing the
lentivirus were collected 72 h after transfection. HCC cells were
infected with lentivirus in the presence of 8 µg/ml
Polybrene (Sigma, St. Louis, MO, USA). KLF4-overexpressing and
KLF4-knockdown HCC cells were selected by blastidin S and puromycin
(Sigma), respectively.
Proliferation assay
The tetrazolium salt
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
assay was used to determine the effects of KLF4 overexpression on
cell growth. Lenti-Ctrl, Lenti-KLF4, shLuc and shKLF4 cells were
seeded at a density of 5,000 cells/well in 96-well plates and
incubated for 24, 48 and 72 h. MTT reagent (Sigma-Aldrich) was
added into each well of the plates and incubated for another 2 h at
37°C. After incubation, formazan crystals were dissolved in
dimethylsulfoxide (DMSO), and then optical absorbance at a
wavelength of 570 nm was measured by a microplate reader
(SpectraMax 250; Molecular Devices, Sunnyvale, CA, USA).
Wound healing, Transwell migration and
Transwell invasion assays
For the wound-healing assay, the cells were seeded
into each well of the culture insert (Ibidi GmbH, Martinsried,
Germany) and incubated overnight. After cells achieved a confluent
layer, the culture insert was gently removed. Bright field images
were captured after 14 h using an inverted microscope. The changes
in wound area were quantified by ImageJ software.
Cell migration and invasiveness were examined by
Transwell polycarbonate membrane inserts (Corning, Corning, NY,
USA) and Matrigel basement membrane matrix invasion assay (BD
Biosciences, Bedford, MA, USA) according to the manufacturer’s
instructions and Yang et al (23). Five contiguous fields of cells were
counted, and the results were quantified by processing the images
using ImageJ software. Data were normalized to the control and
represent the average fold-change ± SD from three independent
experiments.
Real-time quantitative-polymerase chain
reaction (RT-qPCR) analysis
The target genes were amplified with specific primer
sets (Table I) and detected by SYBR
PCR Master Mix (Applied Biosystems) using ABI PRISM 7900 (Applied
Biosystems, Carlsbad, CA, USA). The alterations in gene expression
were obtained using the ΔΔCt method in which all samples were first
normalized to the level of β-actin in each sample. Relative
normalized units were then compared between the control and
KLF4-overexpressing cells.
 | Table IDNA sequence of the primers for
real-time PCR analysis. |
Table I
DNA sequence of the primers for
real-time PCR analysis.
| Gene | Sequence (5′ →
3′) |
|---|
| E-cadherin | F TGA AGG TGA CAG
AGC CTC TGG AT |
| R TGG GTG AAT TCG
GGC TTG TT |
| KLF4 | F CGA ACC CAC ACA
GGT GAG AA |
| R TAC GGT AGT GCC
TGG TCA GTT C |
| Vimentin | F CCT TGA ACG CAA
AGT GGA ATC |
| R GAC ATG CTG TTC
CTG AAT CTG AG |
| Bmi1 | F ACA TCC GAA GCC
ACA CGC TGC |
| R CGC AGG TTG GAG
CGG TCA GC |
| Snail | F ACA TCC GAA GCC
ACA CGC TGC |
| R CGC AGG TTG GAG
CGG TCA GC |
| MMP2 | F TTG ACG GTA AGG
ACG GAC TC |
| R ACT TGC AGT ACT
CCC CAT CG |
| MMP9 | F TTG ACA GCG ACA
AGA AGT GG |
| R CCC TCA GTG AAG
CGG TAC AT |
| TIMP-1 | F AAG GCT CTG AAA
AGG GCT TC |
| R GAA AGA TGG GAG
TGG GAA CA |
| TIMP-2 | F CCA AGC AGG AGT
TTC TCG AC |
| R GAC CCA TGG GAT
GAG TGT TT |
| β-actin | F TGG CAT TGC CGA
CAG GAT |
| R GCT CAG GAG GAG
CAATGA TCT |
Western blot analysis
Proteins were separated on 10% SDS-PAGE and then
transferred onto a nitrocellulose membrane. After blocking the
membrane in Tris-buffered saline, 0.1% Tween-20 (TBST) buffer with
5% skim milk, primary antibodies: E-cadherin, Snail (Cell
Signaling, Danvers, MA, USA), KLF4 (Santa Cruz Biotechnology, Santa
Cruz, CA, USA), vimentin, β-actin (Sigma), Bmi1 (Upstate
Biotechnology, Lake Placid, NY, USA), MMP2, TIMP-1 (Millipore) and
TIMP-2 (NeoMarkers, Fremont, CA, USA) were used to probe the
proteins on the membrane at 4°C overnight. After incubation with
the horseradish peroxidase-conjugated secondary antibody, the
probed proteins were detected using the enhanced chemiluminescence
system (Millipore) according to the manufacturer’s
instructions.
Enzyme-linked immunosorbent assay
(ELISA)
The concentrations of TIMP-1 and TIMP-2 in the cell
culture supernatants were analyzed using the ELISA kit (R&D
Systems, Minneapolis, MN, USA) according to the manufacturer’s
instructions.
Statistical analysis
The means and standard deviation (SD) were
calculated from three independent experiments. The Student’s t-test
was used for comparing the means of two treatment groups.
Differences were considered to indicate a statistically significant
result when P<0.05.
Results
KLF4 overexpression inhibits the
migratory and invasive abilities in the HCC cells
In order to assess the functional role of KLF4 in
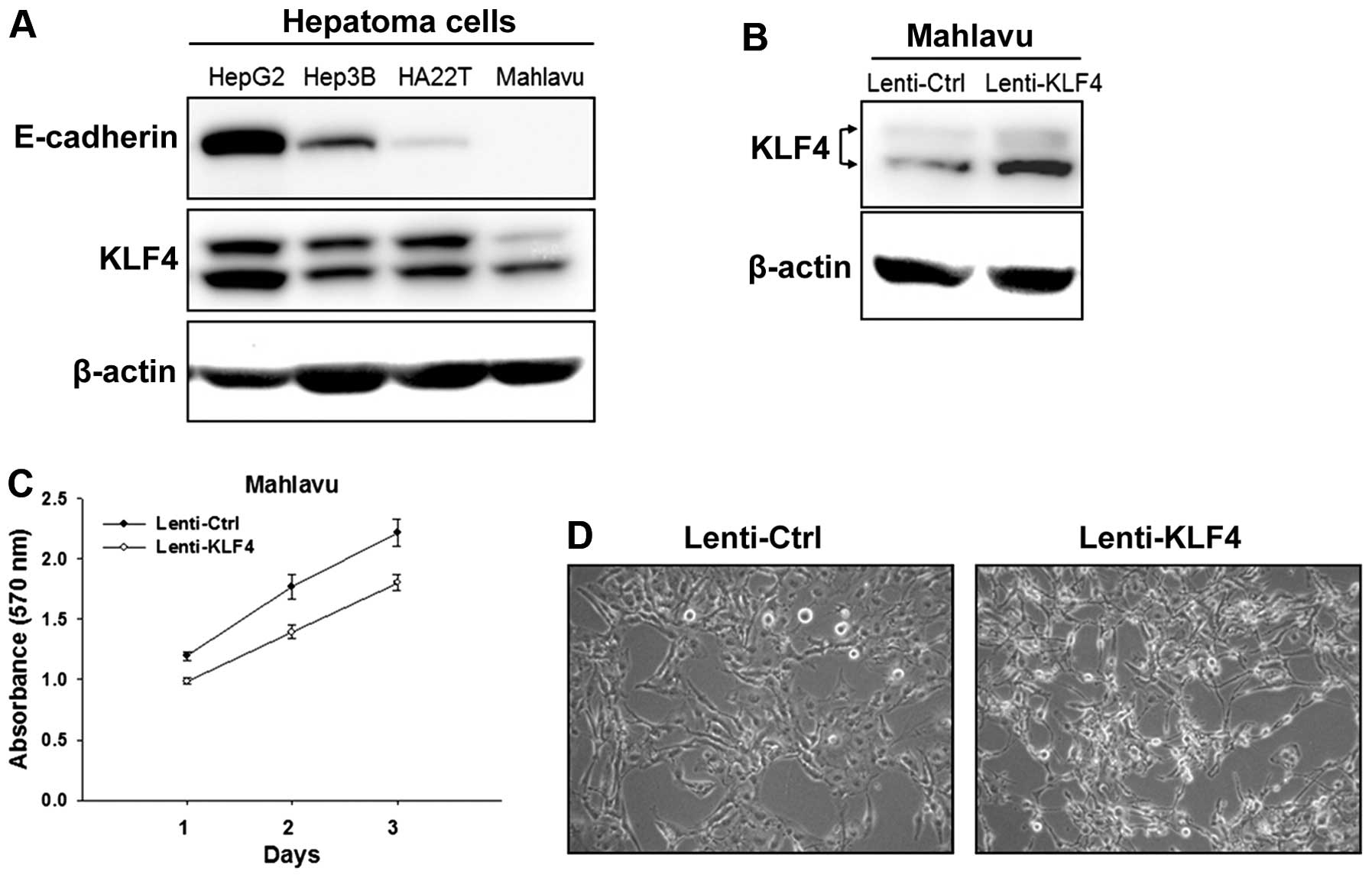
HCC cells, we examined the expression of KLF4 in 4 HCC cell lines.
It was found that HepG2 cells had the highest level of KLF4 and
also the highest level of E-cadherin. In contrast, Hep3B and
HA22T/VGH cells had lower levels of KLF4, and Mahlavu cells had the
lowest level of KLF4 and undetectable E-cadherin (Fig. 1A). We adopted a lentiviral
transduction method to overexpress KLF4 in the Mahlavu cells. Cells
were infected by lentivirus with pLV-Bsd-KLF4 (indicated as
Lenti-KLF4), and overexpression of KLF4 expression was confirmed by
western blot analysis as compared to cells infected by the
lentivirus with the pLV-Bsd vector (indicated as Lenti-Ctrl)
(Fig. 1B). Cell proliferative
ability was examined by the MTT assay, and the results showed that
KLF4 overexpression did not inhibit the growth of the Lenti-KLF4
cells (Fig. 1C). However, KLF4
overexpression led to morphological change in the Lenti-KLF4 cells
which showed a round shape and aggregated form as compared to the
Lenti-Ctrl cells (Fig. 1D).
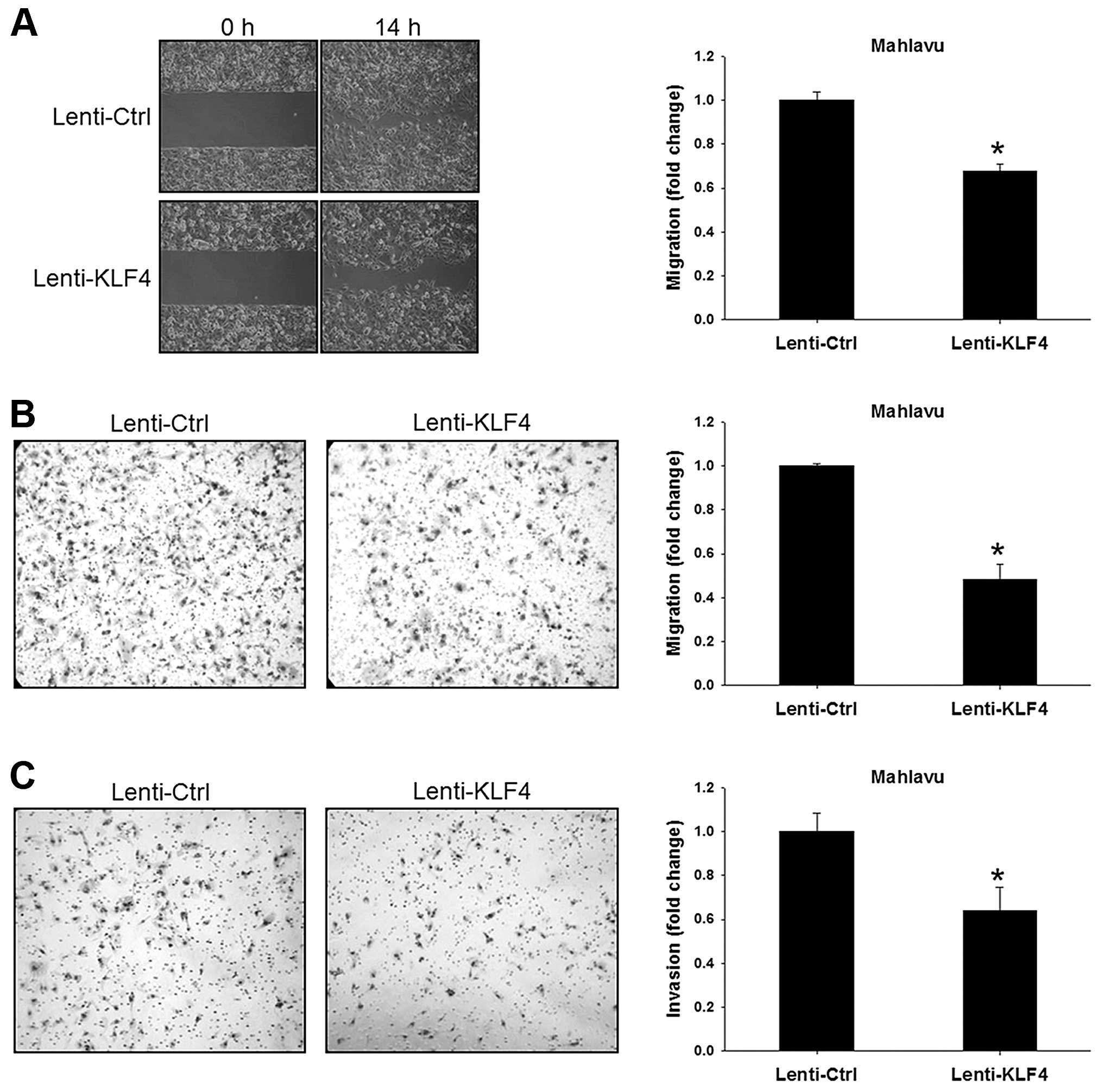
We next used wound healing, Transwell migration and
Transwell invasion assays to evaluate the migratory and invasive
abilities of the Lenti-KLF4 cells. In the wound-healing assay, the
Lenti-Ctrl cells almost filled the gap after a 14-h culture, while
the Lenti-KLF4 cells showed a 30% decrease in the migratory ability
(P<0.001, Fig. 2A). Consistent
results were obtained using the Transwell migration assay showing
that the Lenti-KLF4 cells had a 50% decrease in the migratory
ability (P<0.001, Fig. 2B). In
the Transwell invasion assay, a thin layer of commercial Matrigel
was used as extracellular matrix. Compared to the Lenti-Ctrl cells,
the Lenti-KLF4 cells showed a 35% decrease in the invasive ability
(P<0.001, Fig. 2C). These
results clearly demonstrated that KLF4 over-expression
significantly suppressed the migratory and invasive abilities but
not the growth of HCC cells.
KLF4 overexpression modulates MMPs and
TIMPs
To clarify the interaction between KLF4 and proteins
involved in cell migration, we examined the effects of KLF4
expression on regulation of the expression of MMPs and TIMPs. The
results showed that KLF4 overexpression led to significant
upregulation of both the mRNA and protein levels of TIMP-1, and
TIMP-2 in the Lenti-KLF4 cells as compared to the Lenti-Ctrl cells.
Messenger RNA levels of MMP2 and MMP9 were significantly decreased,
yet the protein expression of MMP2 was slightly increased in the
Lenti-KLF4 cells (Fig. 3A and
B).
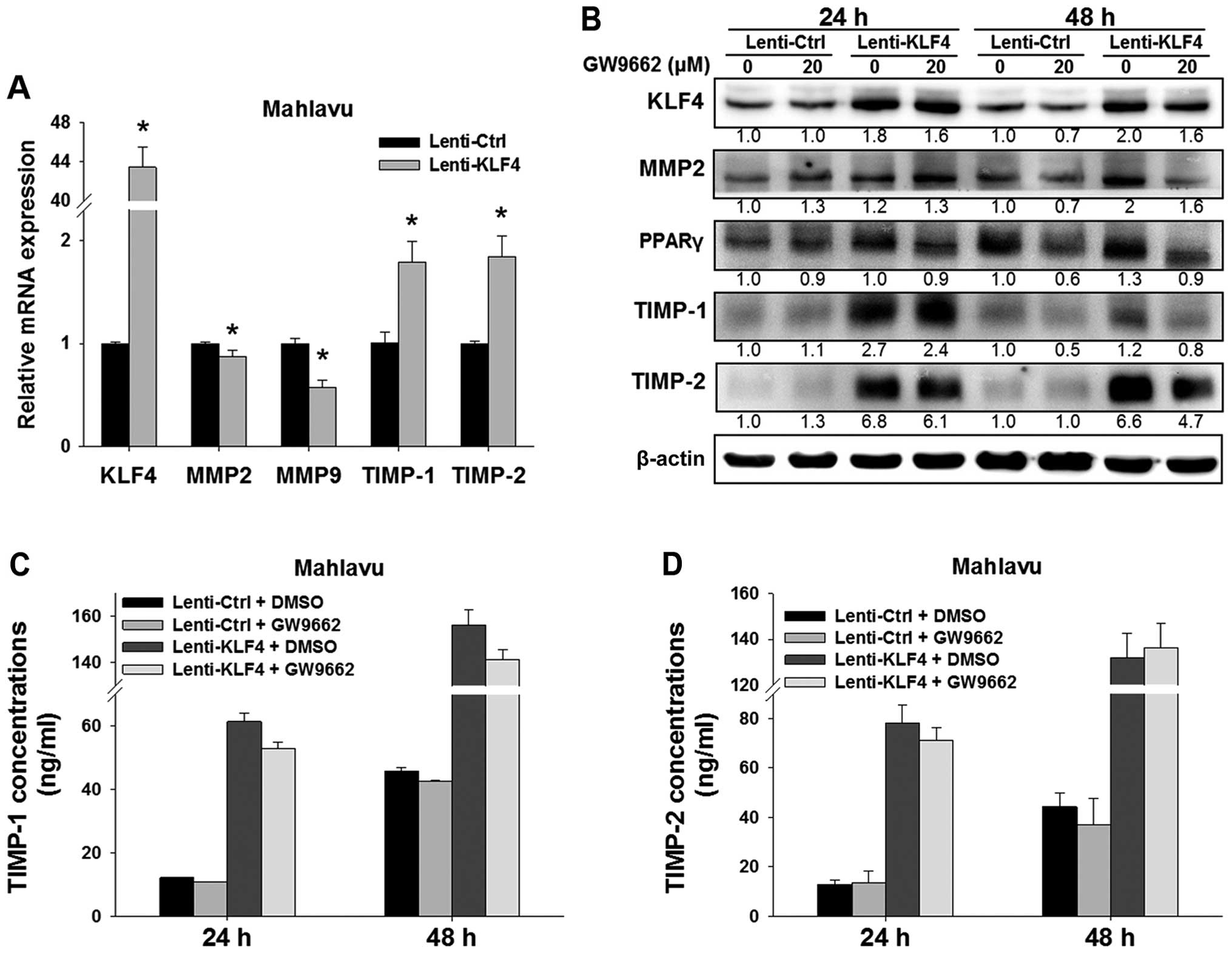 | Figure 3Effects of KLF4 overexpression on
MMP2, MMP9, TIMP-1, and TIMP-2 in HCC cells. (A) Real-time qPCR
analysis was used to analyze the mRNA levels of KLF4,
MMP2, MMP9, TIMP-1 and TIMP-2 in the
Lenti-Ctrl vs. Lenti-KLF4 Mahlavu cells. *P<0.001,
indicates a significant difference from the Lenti-Ctrl cells. (B)
Western blot analysis was used to detect the expression of KLF4,
MMP2, PPARγ, TIMP-1 and TIMP-2 after treatment with/without GW9662.
β-actin was used as a loading control. Relative fold-changes were
indicated as compared to the control at the same time point. (C and
D) After treatment with 20 µM GW9662, ELISA assay was used
to measure the concentrations of secreted TIMP-1 and TIMP-2 in the
cell culture supernatants. Representative data are shown from three
independent experiments. KLF4, Krüppel-like factor 4; MMP, matrix
metalloproteinase. |
To further examine the functional importance of the
PPARγ pathway in relation to KLF4 in modulation of TIMP-1 and
TIMP-2, we used PPARγ antagonist GW9662 to block the PPARγ pathway.
The results showed that GW9662 treatment for 48 h inhibited the
expression of KLF4 in both the Lenti-Ctrl and Lenti-KLF4 cells as
compared to the untreated control (Fig.
3B). Additionally, the expression levels of MMP2, TIMP-1, and
TIMP-2 were also concomitantly downregulated in both the Lenti-Ctrl
and Lenti-KLF4 cells after GW9662 treatment for 48 h.
As newly synthesized TIMPs are secreted and function
in extracellular space, we used ELISA assay to determine the
concentrations of secreted TIMP-1 and TIMP-2 in the cell culture
supernatants. Compared to the Lenti-Ctrl cells, KLF4 overexpression
upregulated the concentrations of secreted TIMP-1 and TIMP-2 in the
Lenti-KLF4 cells (Fig. 3C and D).
However, GW9662 treatment for 48 h had no effect on the levels of
secreted TIMP-1 and TIMP-2 in both the Lenti-Ctrl and Lenti-KLF4
cells.
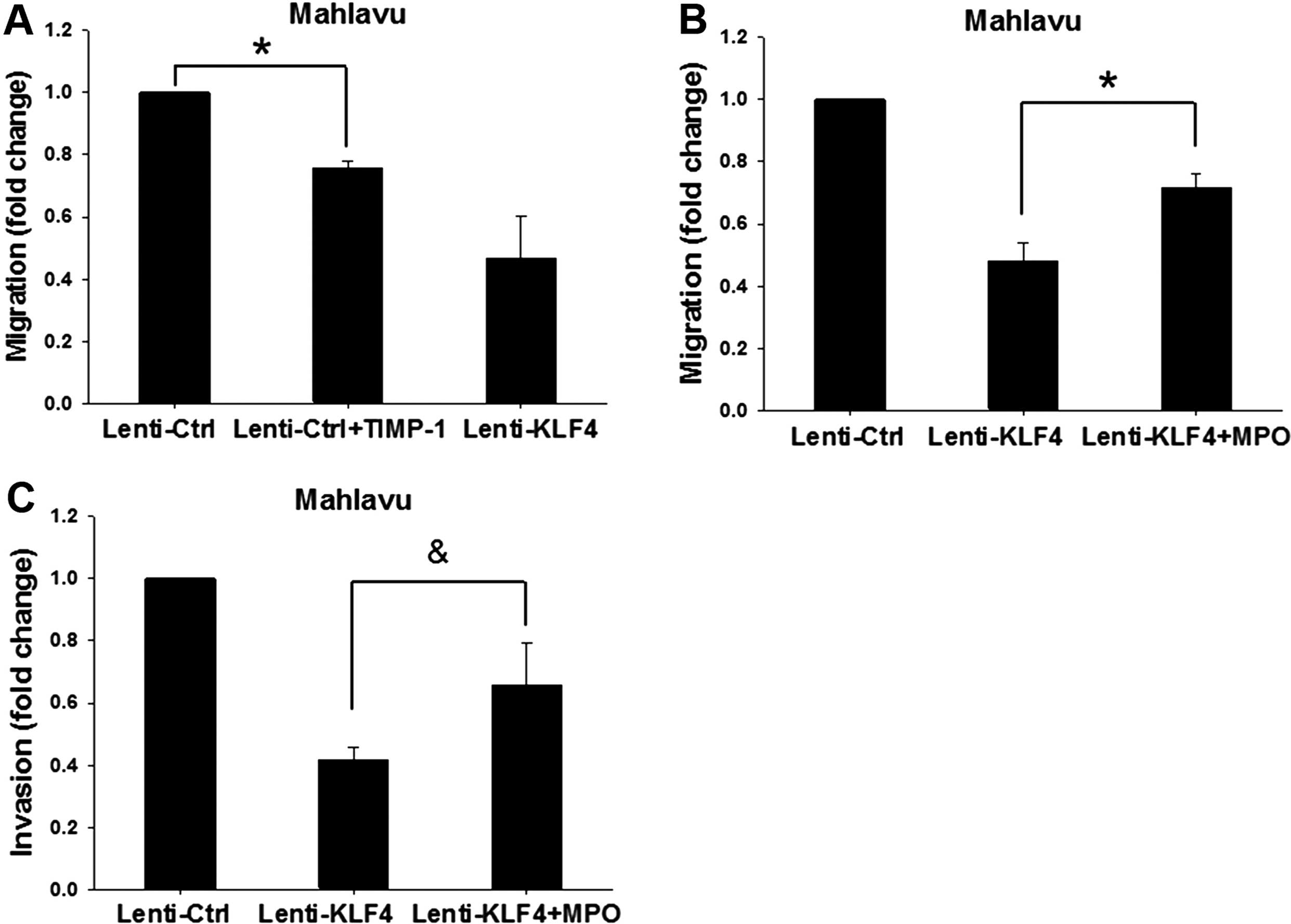
To clarify the role of KLF4-induced TIMP-1/TIMP-2
expression in relation to migration, we found that treatment with
recombinant protein TIMP-1 showed markedly decrease in the
migratory ability of the Lenti-Ctrl cells as compared to the
untreated cells (Fig. 4A). In
addition, we used MPO (50 nM treatment at 37°C for 60 min) to block
the activities of TIMP-1/TIMP-2 in the Lenti-KLF4 cells. The
results showed that the migratory and invasive abilities of the
MPO-treated Lenti-KLF4 cells were significantly increased as
compared to the untreated Lenti-KLF4 cells (Fig. 4B and C). These results together
clearly indicate that KLF4 modulates the expression of TIMP-1 and
TIMP-2, leading to changes in migration and invasion of HCC
cells.
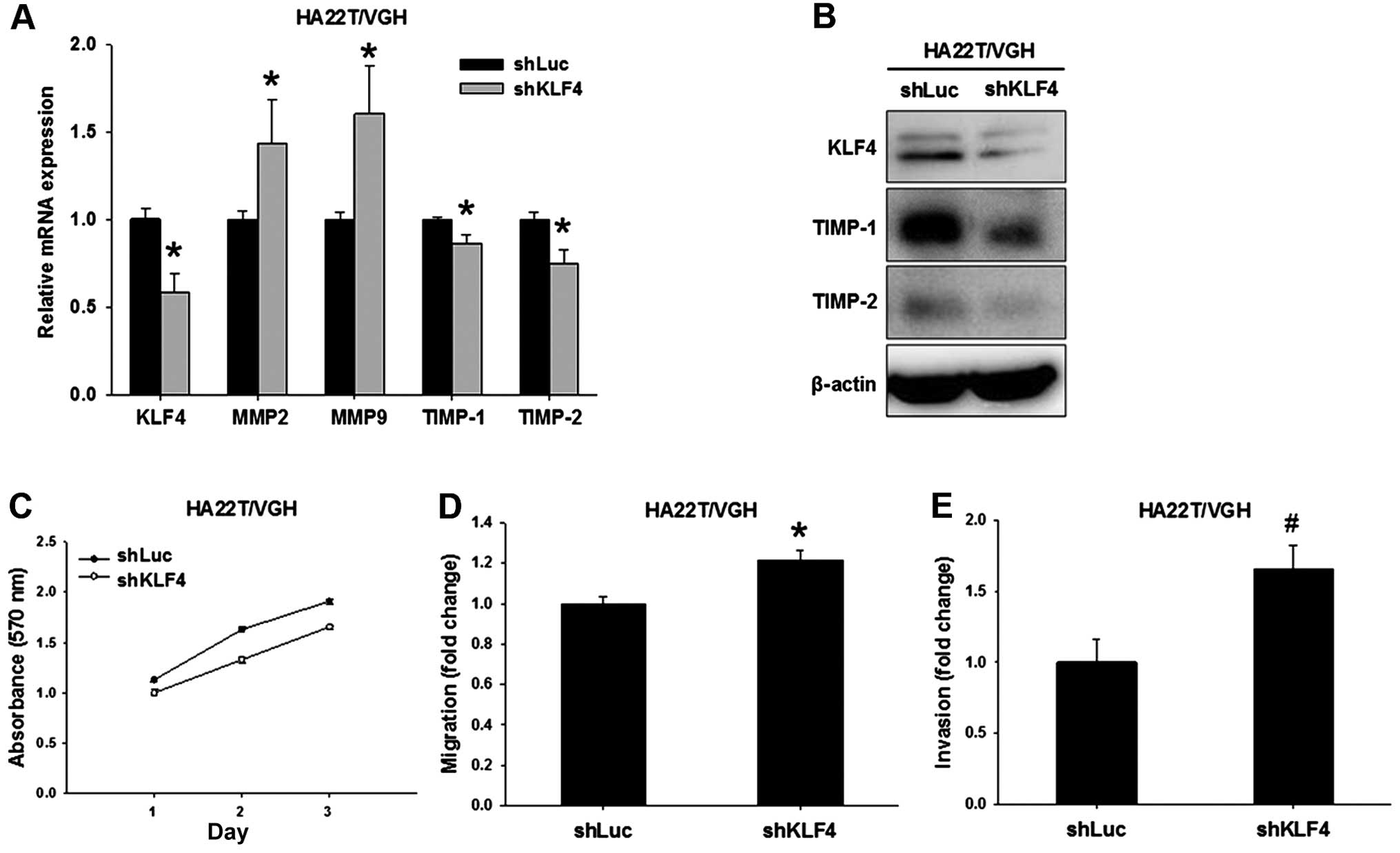
KLF4 silencing inhibits TIMP-1 and TIMP-2
while increasing migration and invasion
In order to further validate the importance of KLF4
in the regulation of cell migration and invasion, we used
lentiviral vector-mediated shRNA to create KLF4-knockdown HA22T/VGH
cells (indicated as shKLF4). Compared to the control (indicated as
shLuc), the mRNA and protein levels of KLF4 were significantly
decreased in the shKLF4 cells (Fig. 5A
and B). KLF4 knockdown led to significant upregulation of MMP2
and MMP9 but downregulation of TIMP-1 and TIMP-2 expression in the
shKLF4 cells (Fig. 5A and B).
We next evaluated the effects of KLF4 knockdown on
cell proliferation, migratory and invasive abilities. In the cell
proliferation assay, the result showed that KLF4 knockdown did not
alter the growth of the shKLF4 cells (Fig. 5C). Compared to the shLuc cells, the
migratory and invasive abilities showed a 1.2-fold (P<0.001,
Fig. 5D) and 1.7-fold (P<0.01,
Fig. 5E) increase in the shKLF4
cells, respectively. These results clearly demonstrated the
critical role of KLF4 in cell migration and invasion by regulating
TIMP-1 and TIMP-2 expression.
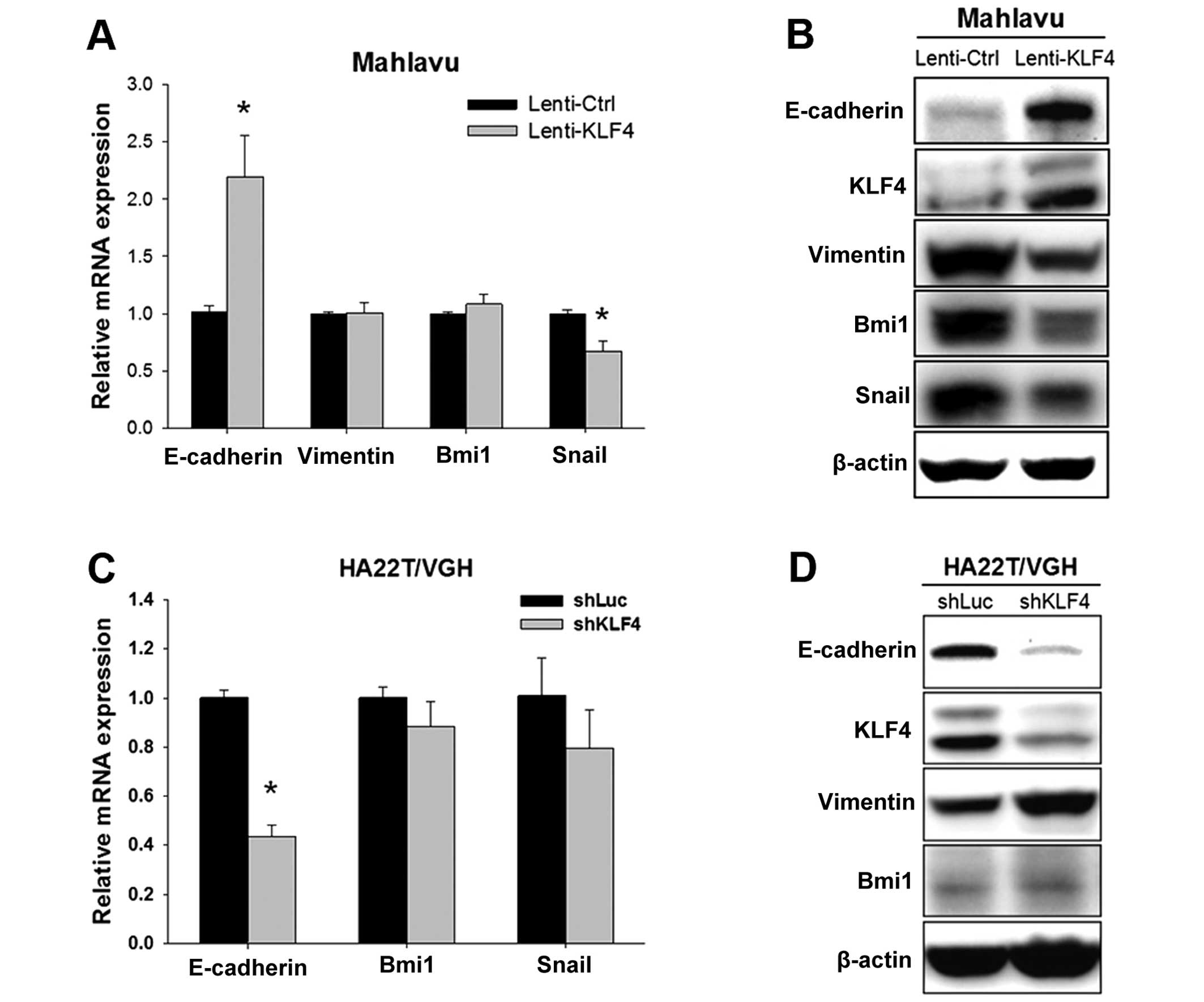
KLF4 regulates E-cadherin and EMT-related
proteins
As EMT has been reported to be highly associated
with cell migration in HCC, we also evaluated the expression levels
of several EMT-related proteins. We found that KLF4 overexpression
in the Mahlavu cells resulted in increased E-cadherin and
decreased Snail mRNA, yet had no effect on the levels of
vimentin and Bmi1 mRNA (Fig. 6A). Western blot results showed that
KLF4 overexpression not only increased the expression of E-cadherin
but also inhibited the protein expression of vimentin, Snail and
Bmi1 (Fig. 6B). Moreover, KLF4
knockdown in the HA22T/VGH cells led to decreased
E-cadherin, while no significant change in the level of
Bmi1 and Snail mRNA was observed (Fig. 6C). Consistent with KLF4
overexpression, KLF4 knockdown resulted in decreased E-cadherin and
increased vimentin protein expression (Fig. 6D). Taken together, these results
suggest that KLF4 also plays an important role in regulating
E-cadherin and EMT-related proteins to modulate cell migration
ability.
Discussion
It has been reported that KLF4 demonstrates
functions to inhibit migration and invasion in several types of
cancer (14,24–26);
however, the detailed molecular mechanisms in HCC remain unclear.
The present study showed that KLF4 overexpression inhibited the
migratory and invasive abilities of highly metastatic Mahlavu cells
with elevated expression levels of TIMP-1/TIMP-2 (Figs. 2 and 3). Knockdown of KLF4 in the HA22T/VGH
cells by shRNA also correlated with increased migration/invasion
and reduced TIMP-1/TIMP-2 levels (Fig.
5). Using TIMP inactivator MPO (27), the effects of KLF4 on
migration/invasion were partially blocked, suggesting that KLF4 can
inhibit cell migration and invasion via upregulation of
TIMP-1/TIMP-2 expression (Fig. 4B and
C). It has been reported that inhibition of TIMP-1 enhanced the
migration of microvascular endothelial cells (28); TIMP-2 overexpression also
significantly inhibited migration/invasion of ras-transformed
breast epithelial cells (29). It
is notable that the expression levels of MMP2, TIMP-1 and TIMP-2
were increased simultaneously in the Lenti-KLF4 cells; however, the
increased fold-change of TIMP-1 and TIMP-2 was higher than that of
MMP2 (Fig. 3B), consequently
resulting in migration/invasion inhibition (Fig. 2). Consistent with our findings, Wang
et al also indicated that KLF4 directly regulates TIMP-2
expression and inhibits migration/invasion in prostate cancer cells
(30). Taken together, we
demonstrated for the first time that KLF4 suppresses HCC cell
migration/invasion through upregulation of TIMP-1 and TIMP-2
expression.
KLF4 has also been reported to inhibit EMT through
regulation of E-cadherin expression in breast cancer cells
(14). EMT, a process defined by
acquisition of mesenchymal phenotype with reduced cell adhesion and
increased mobility, plays a key role not only in development but
also in malignant tumor progression and metastasis (31). Reduction or loss of E-cadherin
expression is considered as an early and critical step to disrupt
intercellular contacts and induce the EMT process (32). Moreover, in various types of
cancers, the expression of E-cadherin was obviously repressed
through the direct binding of transcription factors Snail or Bmi1
on the E-cadherin promoter (33,34).
Li et al found that overexpressed KLF4 induced E-cadherin
and reduced Snail in HepG2 and SK-Hep1 cells (13). Consistent with our findings, our
results showed that KLF4 overexpression led to an increase in
E-cadherin and concomitant decrease in Snail and Bmi1 in Mahlavu
cells (Fig. 6B). On the other hand,
it has been reported that TIMP-2 upregulates E-cadherin expression
in lung cancer cells (35). Thus,
it is most likely that KLF4 not only directly upregulates
E-cadherin expression through the transcription level but also
indirectly induces TIMP-2 to increase the expression of E-cadherin
in HCC cells. These data suggest that KLF4-induced TIMP-1 and
TIMP-2 may be the most important factors to regulate HCC
migration/invasion in HCC cells.
Vimentin, an intermediate filament, is a marker of
mesenchymal cells and has been linked to aggressive tumors. It has
been reported that increased vimentin is significantly correlated
with the metastasis of HCC (36).
Reduction in vimentin by the PPARγ antagonist GW9662 inhibited the
migration and invasion of HCC cells (37). This corresponded well with our
observation that GW9662 inhibited the expression of KLF4 and
TIMP1/TIMP2 (Fig. 3B). Moreover,
consistent with our findings, downregulation of vimentin by KLF4
overexpression (Fig. 6B) inhibited
the migration and invasion of Mahlavu cells. Moreover, upregulation
of vimentin by KLF4 knockdown promoted the migration and invasion
of HA22T/VGH HCC cells (Fig. 5).
Taken together, our results suggest that the overexpression of
vimentin, as a result of loss of KLF4, significantly contributes to
HCC progression.
Our findings provide important insights into the
mechanism of the KLF4-TIMP-1/TIMP-2 signaling pathway in HCC
progression, which inhibits the migration, invasion and metastasis
of HCC cells. These unique findings strongly suggest that KLF4 may
serve as a potential molecular target in cancer therapy for HCC
patients.
Acknowledgments
We thank Dr Shih-Hwa Chiou for providing the
lentiviral expression vector and packaging plasmids. The present
study was supported by grant NSC100-2320-B-075-003,
NSC101-2320-B-075-003, DOH101-TD-C-111-007 and DOH102-TD-C-111-007
from the National Science Council and Ministry of Health and
Welfare, Taiwan, respectively.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang ZY: Hepatocellular carcinoma - cause,
treatment and metastasis. World J Gastroenterol. 7:445–454.
2001.
|
|
3
|
Thomas MB and Abbruzzese JL: Opportunities
for targeted therapies in hepatocellular carcinoma. J Clin Oncol.
23:8093–8108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whittaker S, Marais R and Zhu AX: The role
of signaling pathways in the development and treatment of
hepatocellular carcinoma. Oncogene. 29:4989–5005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garrett-Sinha LA, Eberspaecher H, Seldin
MF and de Crombrugghe B: A gene for a novel zinc-finger protein
expressed in differentiated epithelial cells and transiently in
certain mesenchymal cells. J Biol Chem. 271:31384–31390. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Whitney EM, Gao SY and Yang VW:
Transcriptional profiling of Krüppel-like factor 4 reveals a
function in cell cycle regulation and epithelial differentiation. J
Mol Biol. 326:665–677. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsu HT, Sung MT, Lee CC, Chang YF and Chi
CW: The role of gut-enriched Krüppel-like factor (GKLF)/KLF4 in
gastrointestinal tract-related cancers. J Cancer Res Pract.
27:191–199. 2011.
|
|
8
|
Zhao W, Hisamuddin IM, Nandan MO, Babbin
BA, Lamb NE and Yang VW: Identification of Krüppel-like factor 4 as
a potential tumor suppressor gene in colorectal cancer. Oncogene.
23:395–402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei D, Gong W, Kanai M, Schlunk C, Wang L,
Yao JC, Wu TT, Huang S and Xie K: Drastic down-regulation of
Krüppel-like factor 4 expression is critical in human gastric
cancer development and progression. Cancer Res. 65:2746–2754. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang N, Liu ZH, Ding F, Wang XQ, Zhou CN
and Wu M: Down-regulation of gut-enriched Kruppel-like factor
expression in esophageal cancer. World J Gastroenterol. 8:966–970.
2002.PubMed/NCBI
|
|
11
|
Hu W, Hofstetter WL, Li H, Zhou Y, He Y,
Pataer A, Wang L, Xie K, Swisher SG and Fang B: Putative
tumor-suppressive function of Kruppel-like factor 4 in primary lung
carcinoma. Clin Cancer Res. 15:5688–5695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foster KW, Ren S, Louro ID, Lobo-Ruppert
SM, McKie-Bell P, Grizzle W, Hayes MR, Broker TR, Chow LT and
Ruppert JM: Oncogene expression cloning by retroviral transduction
of adenovirus E1A-immortalized rat kidney RK3E cells:
Transformation of a host with epithelial features by c-MYC and the
zinc finger protein GKLF. Cell Growth Differ. 10:423–434.
1999.PubMed/NCBI
|
|
13
|
Li Q, Gao Y, Jia Z, Mishra L, Guo K, Li Z,
Le X, Wei D, Huang S and Xie K: Dysregulated Krüppel-like factor 4
and vitamin D receptor signaling contribute to progression of
hepatocellular carcinoma. Gastroenterology. 143:799–810.e1-2. 2012.
View Article : Google Scholar
|
|
14
|
Yori JL, Johnson E, Zhou G, Jain MK and
Keri RA: Kruppel-like factor 4 inhibits epithelial-to-mesenchymal
transition through regulation of E-cadherin gene expression. J Biol
Chem. 285:16854–16863. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin ZS, Chu HC, Yen YC, Lewis BC and Chen
YW: Krüppel-like factor 4, a tumor suppressor in hepatocellular
carcinoma cells reverts epithelial-mesenchymal transition by
suppressing slug expression. PLoS One. 7:e435932012. View Article : Google Scholar
|
|
16
|
Li S, Zhou Q, He H, Zhao Y and Liu Z:
Peroxisome proliferator-activated receptor γ agonists induce cell
cycle arrest through transcriptional regulation of Kruppel-like
factor 4 (KLF4). J Biol Chem. 288:4076–4084. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bauvois B: New facets of matrix
metalloproteinases MMP-2 and MMP-9 as cell surface transducers:
Outside-in signaling and relationship to tumor progression. Biochim
Biophys Acta. 1825:29–36. 2012.
|
|
18
|
Nart D, Yaman B, Yilmaz F, Zeytunlu M,
Karasu Z and Kiliç M: Expression of matrix metalloproteinase-9 in
predicting prognosis of hepatocellular carcinoma after liver
transplantation. Liver Transpl. 16:621–630. 2010.PubMed/NCBI
|
|
19
|
Cui J, Dong BW, Liang P, Yu XL and Yu DJ:
Effect of c-myc, Ki-67, MMP-2 and VEGF expression on prognosis of
hepatocellular carcinoma patients undergoing tumor resection. World
J Gastroenterol. 10:1533–1536. 2004.PubMed/NCBI
|
|
20
|
Sakamoto Y, Mafune K, Mori M, Shiraishi T,
Imamura H, Mori M, Takayama T and Makuuchi M: Overexpression of
MMP-9 correlates with growth of small hepatocellular carcinoma. Int
J Oncol. 17:237–243. 2000.PubMed/NCBI
|
|
21
|
Gao ZH, Tretiakova MS, Liu WH, Gong C,
Farris PD and Hart J: Association of E-cadherin, matrix
metalloproteinases, and tissue inhibitors of metalloproteinases
with the progression and metastasis of hepatocellular carcinoma.
Mod Pathol. 19:533–540. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giannelli G, Bergamini C, Marinosci F,
Fransvea E, Quaranta M, Lupo L, Schiraldi O and Antonaci S:
Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular
carcinoma. Int J Cancer. 97:425–431. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang MH, Chen CL, Chau GY, Chiou SH, Su
CW, Chou TY, Peng WL and Wu JC: Comprehensive analysis of the
independent effect of twist and snail in promoting metastasis of
hepatocellular carcinoma. Hepatology. 50:1464–1474. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei D, Kanai M, Jia Z, Le X and Xie K:
Kruppel-like factor 4 induces p27Kip1 expression in and
suppresses the growth and metastasis of human pancreatic cancer
cells. Cancer Res. 68:4631–4639. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dang DT, Chen X, Feng J, Torbenson M, Dang
LH and Yang VW: Overexpression of Krüppel-like factor 4 in the
human colon cancer cell line RKO leads to reduced tumorigenicity.
Oncogene. 22:3424–3430. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, Wang J, Xiao W, Xia D, Lang B, Yu G,
Guo X, Guan W, Wang Z, Hu Z, et al: Epigenetic alterations of
Krüppel-like factor 4 and its tumor suppressor function in renal
cell carcinoma. Carcinogenesis. 34:2262–2270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Rosen H, Madtes DK, Shao B, Martin
TR, Heinecke JW and Fu X: Myeloperoxidase inactivates TIMP-1 by
oxidizing its N-terminal cysteine residue: An oxidative mechanism
for regulating proteolysis during inflammation. J Biol Chem.
282:31826–31834. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reed MJ, Koike T, Sadoun E, Sage EH and
Puolakkainen P: Inhibition of TIMP1 enhances angiogenesis in vivo
and cell migration in vitro. Microvasc Res. 65:9–17. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahn SM, Jeong SJ, Kim YS, Sohn Y and Moon
A: Retroviral delivery of TIMP-2 inhibits H-ras-induced migration
and invasion in MCF10A human breast epithelial cells. Cancer Lett.
207:49–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Place RF, Huang V, Wang X, Noonan
EJ, Magyar CE, Huang J and Li LC: Prognostic value and function of
KLF4 in prostate cancer: RNAa and vector-mediated overexpression
identify KLF4 as an inhibitor of tumor cell growth and migration.
Cancer Res. 70:10182–10191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu
LJ, Kong QL, Xu LH, Zhang X, Liu WL, et al: The polycomb group
protein Bmi-1 represses the tumor suppressor PTEN and induces
epithelial-mesenchymal transition in human nasopharyngeal
epithelial cells. J Clin Invest. 119:3626–3636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bourboulia D, Han H, Jensen-Taubman S,
Gavil N, Isaac B, Wei B, Neckers L and Stetler-Stevenson WG: TIMP-2
modulates cancer cell transcriptional profile and enhances
E-cadherin/beta-catenin complex expression in A549 lung cancer
cells. Oncotarget. 4:166–176. 2013.PubMed/NCBI
|
|
36
|
Hu L, Lau SH, Tzang CH, Wen JM, Wang W,
Xie D, Huang M, Wang Y, Wu MC, Huang JF, et al: Association of
Vimentin overexpression and hepatocellular carcinoma metastasis.
Oncogene. 23:298–302. 2004.
|
|
37
|
Kim KR, Choi HN, Lee HJ, Baek HA, Park HS,
Jang KY, Chung MJ and Moon WS: A peroxisome proliferator-activated
receptor γ antagonist induces vimentin cleavage and inhibits
invasion in high-grade hepatocellular carcinoma. Oncol Rep.
18:825–832. 2007.PubMed/NCBI
|