Introduction
Although much progress has been made in early
diagnosis, surgery, chemotherapy and targeted drugs, gastric cancer
remains the second most common cause of cancer-associated mortality
worldwide (1,2). Metastasis is a major clinical obstacle
for the treatment of gastric cancer and therefore, a better
understanding of the gene regulation involved in the development of
metastasis may lead to therapeutic improvements for gastric cancer
patients.
Metastasis is a multistep process including the
dissociation of cancer cells from primary sites, survival in the
vascular system, and proliferation in distant target organs
(3). As a barrier to metastasis,
cells normally undergo an apoptotic process known as ‘anoikis’, a
form of cell death due to loss of contact with the extracellular
matrix or neighboring cells (3–5).
However, a subset of cancer cells acquires the ability to resist
anoikis and thus survives after detachment from the primary sites
and travels through the circulatory and lymphatic systems to
disseminate throughout the body (3–5).
Recently, identification of the factors and mechanisms that control
anoikis has become a high priority in cancer cell biology and
developmental therapeutics.
Deleted in breast cancer 1 (DBC1) is a nuclear
protein encoded by a gene on 8p21 that was originally believed to
reside within a deleted region in breast cancer, a deletion
assignment that was subsequently found to be inaccurate (6,7). DBC1
overexpression has been observed in colorectal, esophageal and
breast cancer, where its overexpression correlates, in some cases,
with poor prognosis (8–11). Currently, the molecular and cell
functions of DBC1 are being extensively investigated to reveal its
precise physiological role. The endogenous DBC1 is a nuclear
protein and the amino-terminus of DBC1 has been shown to be a
protein-interaction surface. Additionally, DBC1 serves as a
transcriptional factor that represses the transcriptional
activation function, such as SIRT1, BRCA1 and estrogen receptor β
(12–15). However, DBC1 was recently reported
to interact with IKK-β, stimulate its kinase activity, and thus
promote NF-κB transcriptional activity through the phosphorylation
of relA serine-536, by which, DBC1 suppressed anoikis in normal
epithelial and breast cancer cell lines (7).
In this study, we performed immunohistochemical
staining to examine the prevalence and prognostic impact of DBC1
expression in gastric cancer patients. Moreover, we investigated
the possible role and mechanism of DBC1 in the regulation of
anoikis in gastric cancer cells and demonstrated that DBC1 promoted
anoikis resistance of gastric cancer cells by regulating NF-κB
activity.
Materials and methods
Patients and samples
A total of 142 cases of gastric adenocarcinoma
patients who had radical gastrectomy at the 82nd hospital of the
PLA between January 2008 and December 2009 were included in the
present study. None of the patients received chemotherapy prior to
surgery and were followed-up by a review of medical records and
telephone calls. Data on gender, age, histological type, TNM stage,
metastatic status and the Lauren classification were collected from
the medical record library of the hospital. This study was approved
by the Ethics Committee of the 82nd hospital of the PLA, and
informed consent was obtained from the patients in accordance with
the Declaration of Helsinki.
Immunohistochemical staining
Immunohistochemical staining was carried out using a
rabbit SP immunostaining kit (Zhongshan Goldenbridge Biotechnology,
Beijing, China). Sections were dewaxed in xylene and dehydrated
through a gradient concentration of alcohol. Then, sections were
treated in a microwave for 10 min for antigen retrieval. After
blocking the endogenous peroxidase and non-specific staining with
0.3% (v/v) hydrogen peroxide and normal goat serum, the sections
were incubated with anti-DBC1 antibody (1:100 dilution, Bethyl Lab,
Montgomery, TX, USA) overnight at 4°C. After washing with PBS, the
sections were incubated with horseradish peroxidase
(HRP)-conjugated biotinylated IgG for 30 min, followed by washing
with phosphate-buffered saline (PBS). The sections were visualized
by diaminobenzidine (DAB) solution and counterstained with
hematoxylin (both from Zhongshan Goldenbridge Biotechnology). The
sections incubated with rabbit IgG instead of primary antibody were
used as negative controls.
Staining evaluation
Immunohistochemical staining was examined by two
independent pathologists who were blinded to the
clinicopathological information. Each case was evaluated to
estimate the intensity of cell staining and the percentage of
positive tumor cells. The intensity of cell staining was graded
according to the following scale: 0 (negative), 1 (weak), 2
(moderate) and 3 (strong). The extent of staining was evaluated by
the percentage of positive tumor cells: 0 (negative), 1 (1–25%), 2
(26–50%), 3 (51–75%) and 4 (76–100%). For each sample, the score
for intensity was multiplied by the score for extent of staining to
provide a final score. Therefore, the expression of DBC1 was
defined as: 0 (negative, −), 1–4 (low expression, +), 5–8 (moderate
expression, ++), and 9–12 (high expression, +++). For multiple
lymph node metastases in a single case, an average
immunohistochemical score was calculated to define the expression
of DBC1 in metastatic lymph nodes.
Cell culture and transfection
Human MKN45 and MGC803 gastric cancer cell lines
were cultured in modified Eagle’s medium (MEM) supplemented with
10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml
streptomycin. The cells were maintained at 37°C in a humidified 5%
CO2 incubator. For transient transfection, the gastric
cancer cells were transfected with lentivirus-mediated DBC1
(Lent-DBC1) or DBC1 shRNA (Lent-sh-DBC1) at a multiplicity of
infection of 10. The cells transfected with lentivirus vector
encoding the green fluorescent protein were used as controls.
Anoikis analysis
After a 48-h transfection, the gastric cancer cells
were collected and continuously cultured in plates pre-coated with
Poly-HEMA for 48 h (16,17). Subsequently, the cells were stained
with Annexin V/PI and analyzed by flow cytometry.
Luciferase analysis
The gastric cancer cells were seeded in 6-well
plates at a concentration of 1.0×105/well. After 24 h,
the cells were infected with lentivirus and treated with Bay
11-7082 (10 µM) or PDTC (50 µM) 24 h later. After
another 24 h, cells were co-transfected with 1 µg of NF-κB
luciferase reporter pNF-κB-luc WT and 50 ng of Renilla
luciferase plasmid phRL-TK (Promega, Madison, WI, USA) using
Lipofectamine 2000 transfection reagent (Invitrogen, USA). Reporter
activities were analyzed using the Promega dual luciferase assay
kit (Promega) according to the manufacturer’s instructions. The
luciferase activity was normalized to the Renilla luciferase
activity.
Western blot analysis
Total cell lysate was prepared in 1X SDS buffer.
Proteins were separated by SDS-PAGE and transferred onto PDVF
membranes. The membranes were then blotted with antibodies to DBC1
(Bethyl Lab), c-FLIP, bcl-xl and β-actin (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). Antigen-antibody complexes were
visualized with enhanced chemiluminescence (Amersham Pharmacia
Biotech, Piscataway, NJ, USA).
RT-qPCR analysis
The TaqMan stem-loop RT-PCR method was used to
assess the expression of c-FLIP, bcl-xl and GAPDH with kits from
Applied Biosystems (Foster City, USA). For relative expression
levels, the 2(−ΔCt) method was used as previously described
(18). Experiments were carried out
in triplicate for each data point, and a data analysis was
performed using Bio-Rad IQ software (Hercules, CA, USA).
Statistical analysis
Data are presented as means ± SEM. The Chi-square
test was used to compare the differences in the DBC1 expression
level with various clinicopathological parameters in gastric
cancer. Survival curves were plotted by the Kaplan-Meier method and
the log-rank test was carried out to compare differences in
survival. Statistical analyses were performed using SPSS 17.0
software (Chicago, IL, USA). P<0.05 was considered statistically
significant.
Results
Increased expression of DBC1 is
associated with lymph node metastasis in gastric
adenocarcinoma
Immunohistochemistry was performed to detect the
expression of DBC1 in 142 cases of gastric adenocarcinoma tissues
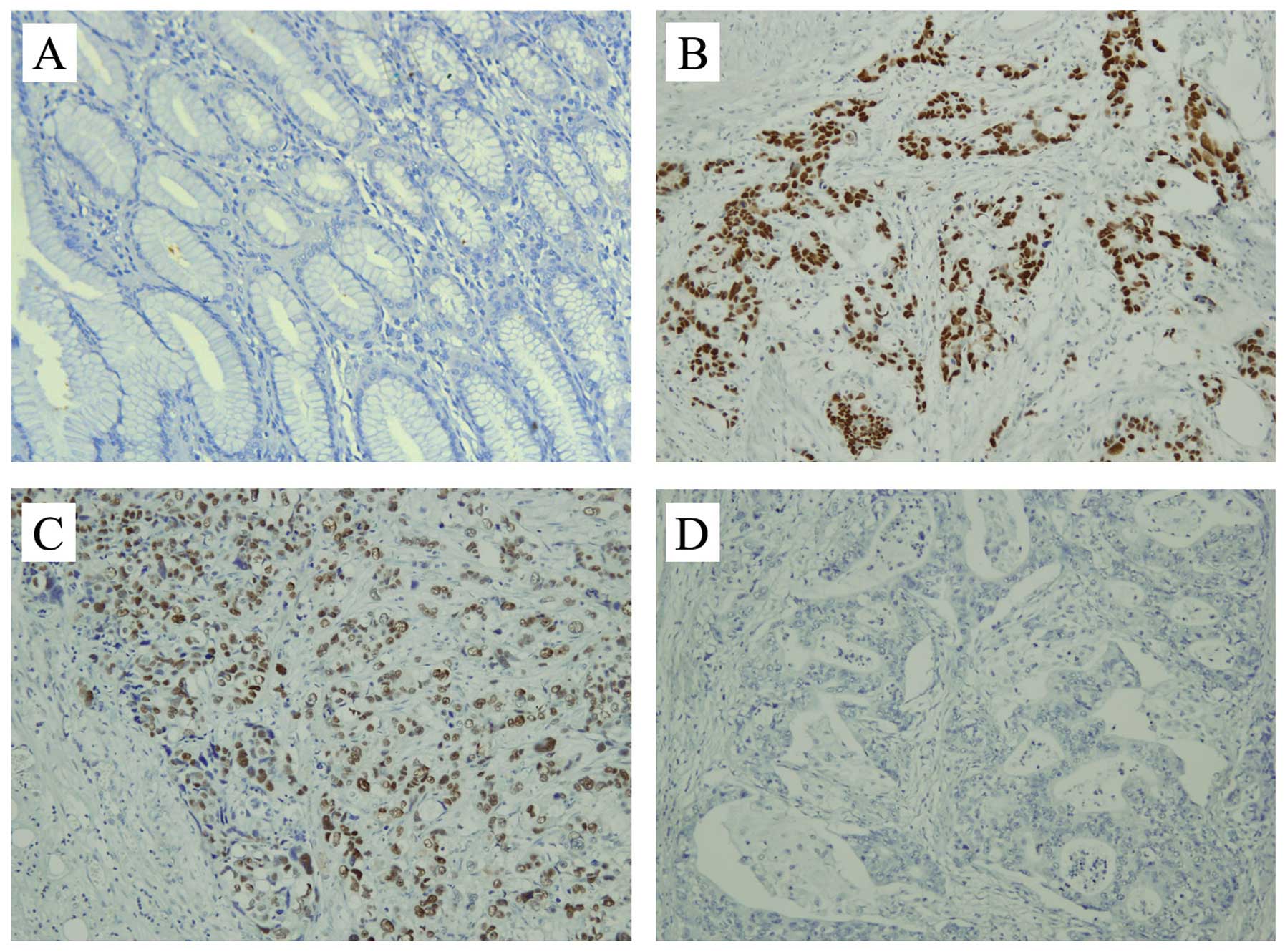
and the corresponding adjacent non-tumor tissues. DBC1 was detected
primarily in the nuclei of cancer cells (Fig. 1). High expression (+++), moderate
expression (++), low expression (+), and negative expression (−) of
DBC1 were identified in 13, 32, 47 and 50 samples of the
adenocarcinoma tissues, respectively, while in the adjacent
non-tumor tissues, only a few cells with weak staining for DBC1
were observed in two cases. We also assessed the potential
relationship between DBC1 expression and clinical characteristics
of gastric adenocarcinomas. As shown in Table I, the expression of DBC1 was
significantly correlated with TNM stage (P<0.05) and lymph node
metastasis (P<0.05). However, there was no significant
correlation between DBC1 expression and age, gender, distant
metastasis, histological grade and the Lauren classification
(P>0.05).
 | Table ICorrelation between DBC1 expression
and clinical characteristics of gastric cancer. |
Table I
Correlation between DBC1 expression
and clinical characteristics of gastric cancer.
| Characteristics | No. of patients | DBC1 staining
| P-value |
|---|
| − | + | ++ | +++ |
|---|
| Age (years) | | | | | | 0.710 |
| ≥60 | 90 | 31 | 32 | 18 | 9 | |
| <60 | 52 | 19 | 15 | 14 | 4 | |
| Gender | | | | | | 0.944 |
| Female | 51 | 17 | 18 | 12 | 4 | |
| Male | 91 | 33 | 29 | 20 | 9 | |
| TNM stage | | | | | | 0.021a |
| I and II | 69 | 32 | 22 | 12 | 3 | |
| III and IV | 73 | 18 | 25 | 20 | 10 | |
| LN metastasis | | | | | | 0.017a |
| Absence | 42 | 23 | 10 | 7 | 2 | |
| Presence | 100 | 27 | 37 | 25 | 11 | |
| Distant
metastasis | | | | | | 0.363 |
| Absence | 139 | 50 | 46 | 31 | 12 | |
| Presence | 3 | 0 | 1 | 1 | 1 | |
| Histological
grade | | | | | | 0.279 |
|
Well-differentiated | 9 | 4 | 2 | 2 | 1 | |
| Moderately
differentiated | 81 | 33 | 29 | 14 | 5 | |
| Poorly
differentiated | 52 | 13 | 16 | 16 | 7 | |
| Lauren
classification | | | | | | 0.987 |
| Intestinal | 60 | 21 | 20 | 14 | 5 | |
| Diffuse | 70 | 26 | 22 | 15 | 7 | |
| Mixed | 12 | 3 | 5 | 3 | 1 | |
Given that DBC1 expression was significantly
correlated with lymph node metastasis, we also detected the
expression of DBC1 in lymph node metastasis and found that the
expression level of DBC1 in the metastatic lymph nodes was
concomitant with that in the primary gastric adenocarcinoma tissues
(Linear correlation analysis for immunohistochemical scores,
P<0.05). Moreover, in cases identified as low DBC1 expression,
we found that the percentage of DBC1-positively expressed cancer
cells in metastatic lymph nodes was markedly higher than that for
the primary site (P<0.05, Fig.
1C).
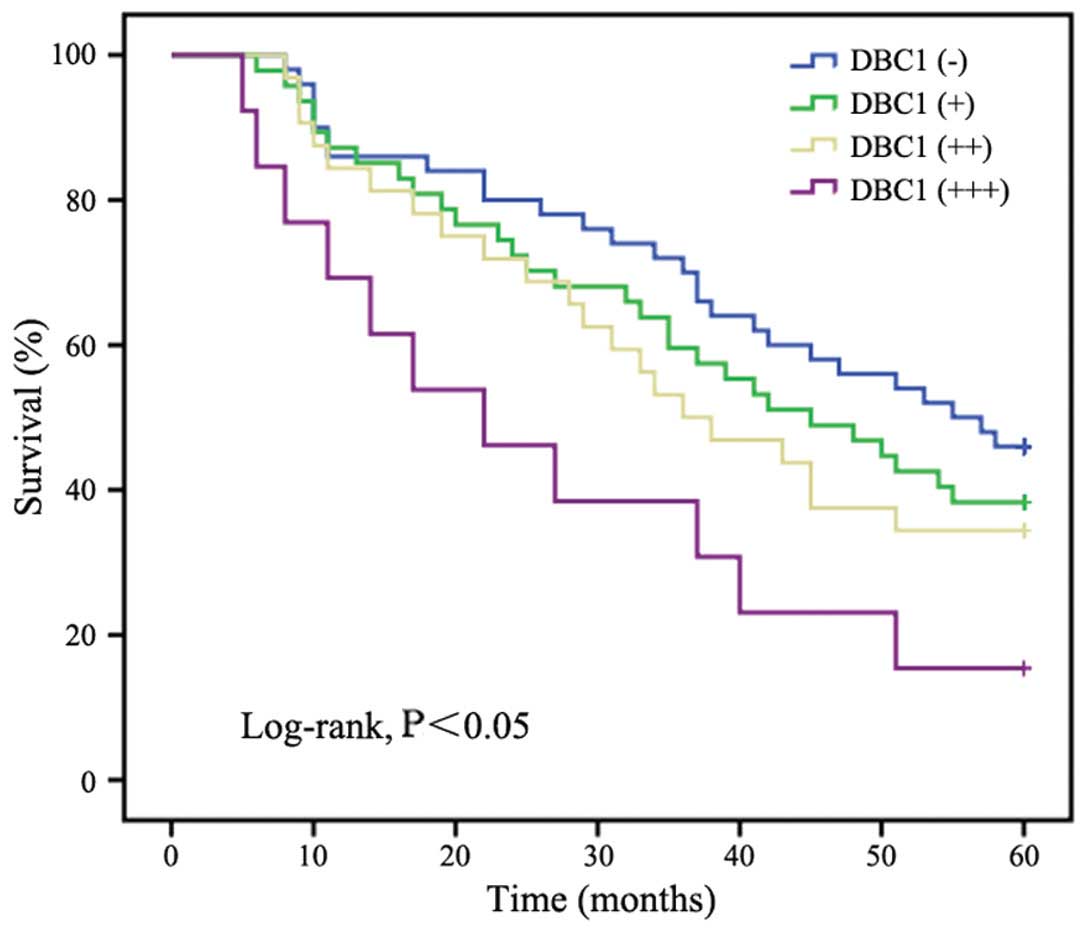
The relationship between DBC1 expression and
survival was also analyzed. The mean survival times in patients
with high expression (+++), moderate expression (++), low
expression (+) and negative expression (−) of DBC1 were 27.5, 38.0,
40.7 and 44.6 months, respectively. The Kaplan-Meier analysis
(Fig. 2) revealed that DBC1
expression was significantly correlated with a shorter overall
survival time (log-rank, Chi-square=8.551, P<0.05).
DBC1 expression correlates with the
ability of gastric cancer cells to resist anoikis
Resistance to anoikis has been considered a hallmark
of metastatic cancer cells, as it is required for
anchorage-independent growth during tumor dissemination. Previous
studies have identified that DBC1 is an important co-factor for the
control of the IKK-β/NF-κB signaling pathway that regulates anoikis
(7). Therefore, we hypothesized
that DBC1 is involved in the metastasis of gastric cancer cells by
mediating resistance to anoikis via IKK-β/NF-κB signal. We
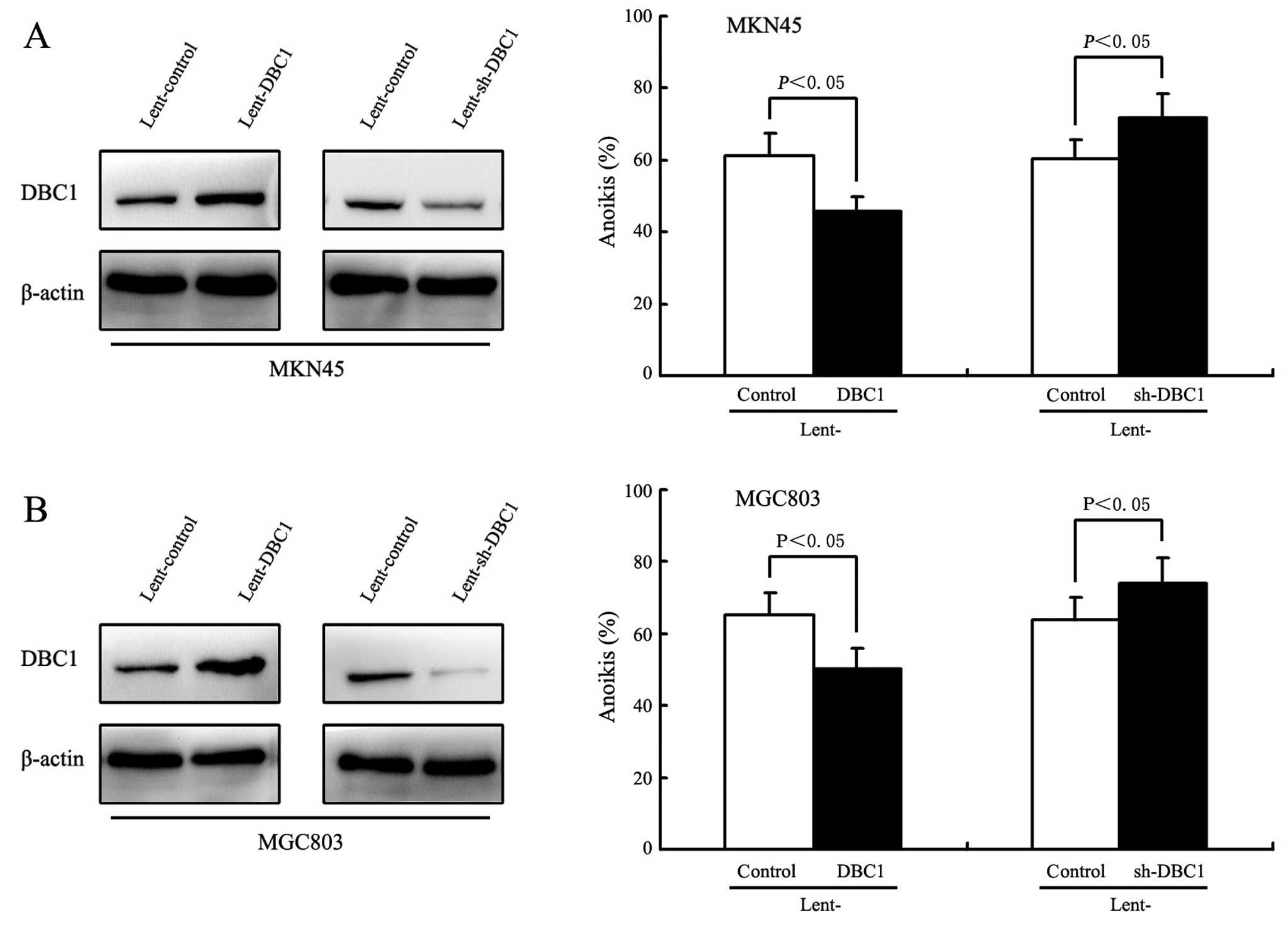
initially examined the effect of DBC1 expression on anoikis in
gastric cancer cells. To achieve this, lentivirus-mediated DBC1 or
DBC1 shRNA were transfected into MKN45 and MGC803 cells. As shown
by western blot analysis in Fig.
3A, DBC1 protein levels in the MKN45 and MGC803 cells following
transfection with lentivirus-mediated DBC1 were significantly
increased compared with that of controls. As expected, in the MKN45
and MGC803 cells, the DBC1 upregulation resulted in a significant
inhibition of the cell detachment-induced anoikis (P<0.05,
Fig. 3B). Concurrently, the
specific down-regulation of DBC1 expression by lentivirus-mediated
DBC1 shRNA induced an increased sensitivity to the anoikis in MKN45
and MGC803 cells (P<0.05, Fig. 3A
and B).
IKK-β/NF-κB signaling pathway is involved
in the regulation of anoikis by DBC1 in gastric cancer cells
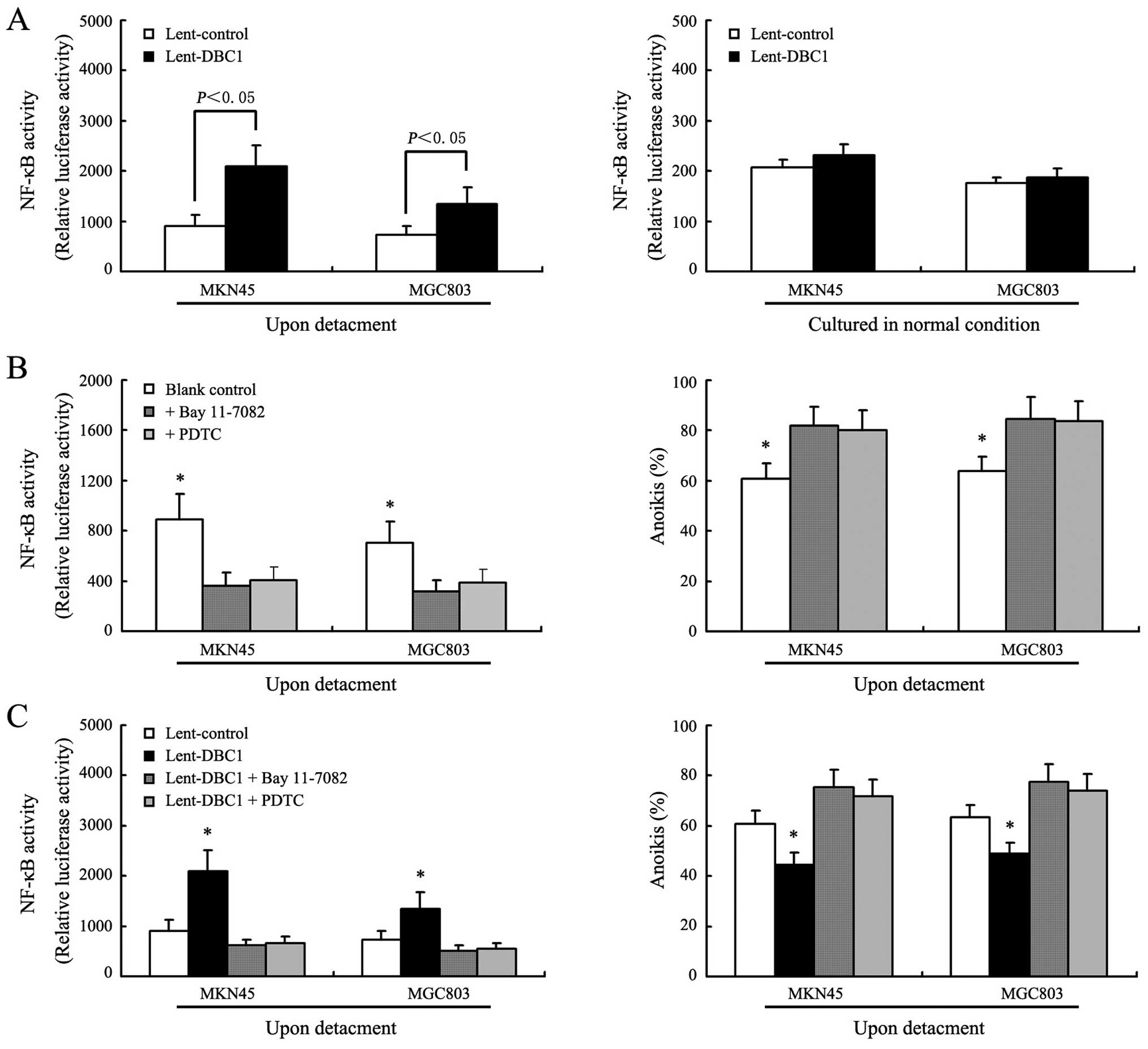
In this study, Bay 11-7082, a highly specific
inhibitor of IKK-β activity, and PDTC, a specific inhibitor of
NF-κB activity, were used to assess the role of the IKK-β/NF-κB
signaling pathway in the regulation of anoikis by DBC1 (19,20).
Fig. 4A shows that in DBC1
upregulated gastric cancer cells (MKN45 and MGC803), the levels of
NF-κB activation following detachment were significantly higher
than that of the control cells (P<0.05). However, we also
observed that in gastric cancer cells cultured in normal cell
plates, DBC1 upregulation did not obviously influence the
activation of NF-κB (P>0.05). Fig.
4B shows that NF-κB activation was significantly inhibited in
gastric cancer cells following detachment after exposure of Bay
11-7082 or PDTC, and coincidently, the anoikis rates of gastric
cancer cells were significantly increased. Fig. 4C shows that either Bay 11-7082 or
PDTC reversed the increased anoikis resistance in DBC1-upregulated
gastric cancer cells, which indicated that DBC1 contributed to the
anoikis resistance by activating the IKK-β/NF-κB signaling
pathway.
DBC1 promotes anoikis resistance by
regulating NF-κB-mediated transcription
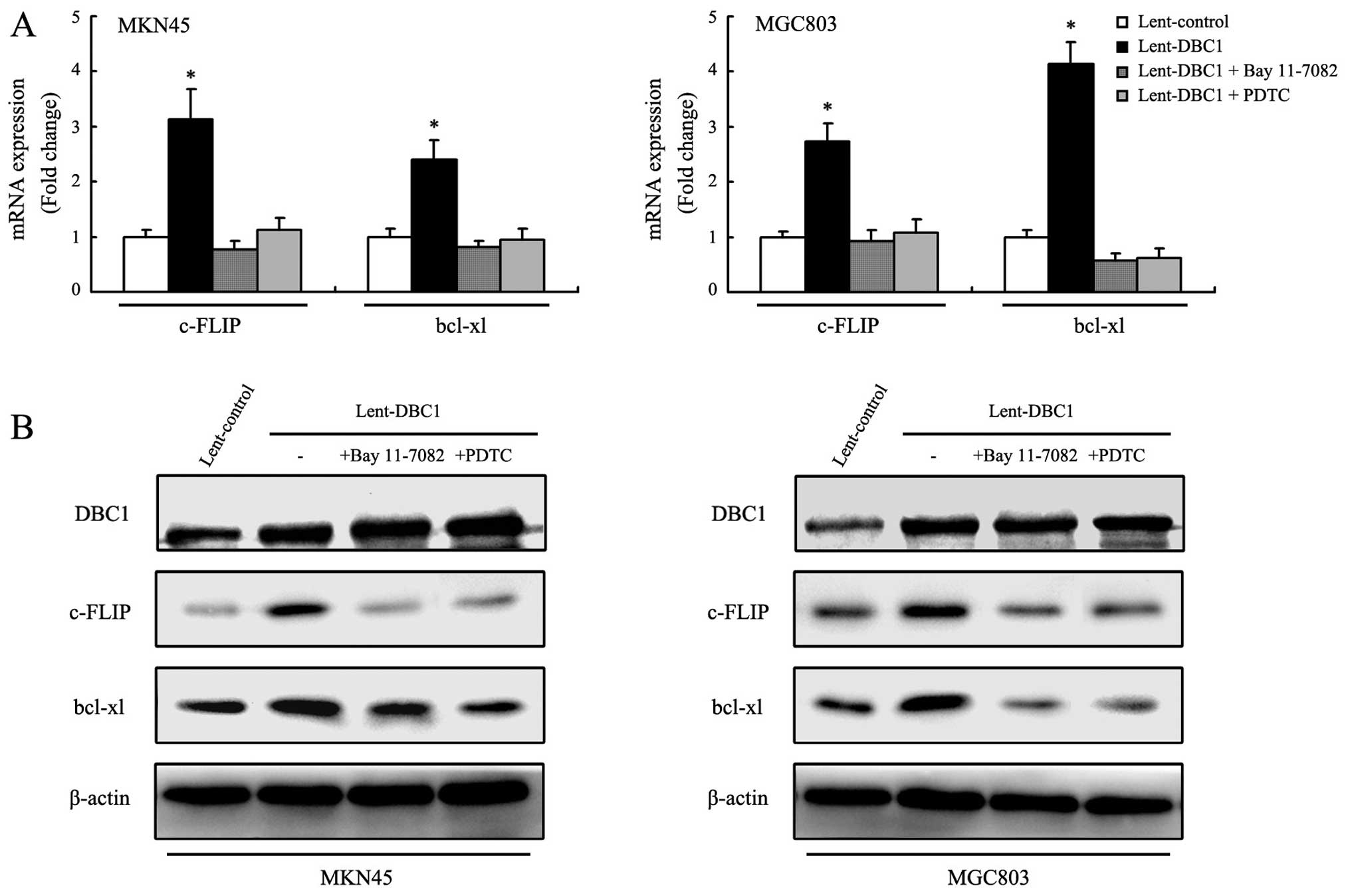
Among NF-κB target genes, c-FLIP and bcl-xl are
particularly noted for their ability to regulate anoikis (7,21,22).
We examined the mRNA and protein expression changes of c-FLIP and
bcl-xl in DBC1 upregulated gastric cancer cells (MKN45 and MGC803).
As shown in Fig. 5A and B, the mRNA
and protein expression levels of c-FLIP and bcl-xl were
significantly increased in DBC1 upregulated gastric cancer cells
following detachment. We also examined the effects of Bay 11-7082
and PDTC on the expression of c-FLIP and bcl-xl. As expected, after
exposure of Bay 11-7082 or PDTC, the improved mRNA and protein
expression of c-FLIP and bcl-xl were reversed in the DBC1
upregulated gastric cancer cells, further indicating that DBC1
promotes anoikis resistance by regulating NF-κB-mediated
transcription.
Discussion
DBC1 was first identified in 2002 by Hamaguchi et
al (23). As a new
transcriptional co-activator, DBC1 exhibits its function by
modulating the activities of various proteins. Currently, the role
of DBC1 in cell survival remains controversial. On the one hand,
DBC1 has been revealed to be able to directly bind to the catalytic
domain of SIRT1 and decrease the deacetylase activity of SIRT1,
thus inhibiting SIRT1-dependent cell survival (12,13).
On the other hand, DBC1 mediates endocrine-resistant breast cancer
cell survival. It promotes estrogen-independent proliferation and
inhibits estrogen-independent apoptosis in estrogen receptor α
positive breast cancer cells (24,25).
DBC1 also represses the transcription of BRCA1 and inhibits its
tumor-suppressor activity (14).
Several studies also reported that DBC1 modulates cell growth by
regulating the transcriptional activity of retinoic acid receptor α
and androgen receptor in breast and prostate cancer cells (26–28).
The abovementioned findings indicated that DBC1 may act as either a
tumor promoter or a tumor suppressor in tumorigenesis, depending on
the cell context and type of tumor.
Although DBC1 was initially termed as deleted in
breast cancer 1, it was subsequenlty found to be overexpressed in
breast cancer tissues in comparison with corresponding matched
normal tissues, and that overexpression was correlated with
clinicopathological factors, especially metastasis, and poor
prognosis (10,11). DBC1 was then found to be
overexpressed in various human cancers including esophageal cancer,
colorectal cancer, soft tissue sarcomas and lymphoma (8,9,29,30).
With regards to gastric cancer, Kang et al and Cha et
al showed that DBC1 was overexpressed in gastric cancer tissues
and could be used as a prognostic indicator for gastric cancer
patients (31,32). In the present study, we found that
DBC1 was significantly upregulated in gastric adenocarcinoma
patients and this expression was significantly correlated with TNM
stage and lymph node metastasis. Our results also support that DBC1
is a prognostic factor that is associated with poor prognosis in
gastric cancer. In addition, we examined the expression of DBC1 in
metastatic lymph nodes and found a higher expression that in the
primary gastric cancer tissues, further indicating that DBC1 may
play an important role in gastric cancer metastasis.
Anoikis, a special form of apoptosis occurring when
cells detach from the extracellular matrix, is a critical mechanism
in maintaining tissue homeostasis and development.
Anoikis-resistance has been considered as a hallmark of metastatic
cancer cells, especially because the anchorage-independent growth
of cancer cells is a classic characteristic of different types of
human malignancies (33). Among the
signaling and transcription factor pathways involved in regulating
anoikis, inflammatory-response transcription factor NF-κB is
notable because it links anoikis with inflammatory signaling
between and within cells (34,35).
Recently, Park et al reported that DBC1 suppresses anoikis
in both normal epithelial and cancer cells (breast cancer MCF10a
cells) by regulating the IKK-β/NF-κB signaling pathway. DBC1 may be
conceptualized as a co-factor for IKK-β that stimulates its kinase
activity on relA (s536), promoting the transcriptional activation
of NF-κB target genes such as c-FLIP and bcl-xl, which enhance
anoikis resistance (7). Using gene
transfection assays, we showed that DBC1 expression correlates with
the ability of anoikis resistance in gastric cancer cells.
Furthermore, by using Bay 11-7082 and PDTC, we demonstrated that
the IKK-β/NF-κB signaling pathway is involved in the regulation of
anoikis resistance by DBC1 in gastric cancer cells. Combined with
the immunohistochemical results, we suggest that there is a subset
of gastric cancer cells with a high DBC1 expression that can
survive the detachment process for enhanced NF-κB activation,
subsequently leading to metastasis.
In summary, our study has demonstrated that DBC1 is
overexpressed in gastric cancer and associated with poor prognosis.
Our study also provides experimental evidence that DBC1 promotes
anoikis resistance in gastric cancer cells by regulating NF-κB
activity, raising the possibility of using DBC1 as a new
therapeutic target for preventing metastasis.
References
|
1
|
Lin JT: Screening of gastric cancer: Who,
when, and how. Clin Gastroenterol Hepatol. 12:135–138. 2014.
View Article : Google Scholar
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
3
|
Kim YN, Koo KH, Sung JY, Yun UJ and Kim H:
Anoikis resistance: An essential prerequisite for tumor metastasis.
Int J Cell Biol. 2012:3068792012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan K, Goldstein D, Crowe P and Yang JL:
Uncovering a key to the process of metastasis in human cancers: A
review of critical regulators of anoikis. J Cancer Res Clin Oncol.
139:1795–1805. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paoli P, Giannoni E and Chiarugi P:
Anoikis molecular pathways and its role in cancer progression.
Biochim Biophys Acta. 1833:3481–3498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zannini L, Buscemi G, Kim JE, Fontanella E
and Delia D: DBC1 phosphorylation by ATM/ATR inhibits SIRT1
deacetylase in response to DNA damage. J Mol Cell Biol. 4:294–303.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park SH, Riley P IV and Frisch SM:
Regulation of anoikis by deleted in breast cancer-1 (DBC1) through
NF-κB. Apoptosis. 18:949–962. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Gu Y, Sha S, Kong X, Zhu H, Xu B,
Li Y and Wu K: DBC1 is overexpressed and associated with poor
prognosis in colorectal cancer. Int J Clin Oncol. 19:106–112. 2014.
View Article : Google Scholar
|
|
9
|
Kim SH, Kim JH, Yu EJ, Lee KW and Park CK:
The over-expression of DBC1 in esophageal squamous cell carcinoma
correlates with poor prognosis. Histol Histopathol. 27:49–58.
2012.
|
|
10
|
Lee H, Kim KR, Noh SJ, Park HS, Kwon KS,
Park BH, Jung SH, Youn HJ, Lee BK, Chung MJ, et al: Expression of
DBC1 and SIRT1 is associated with poor prognosis for breast
carcinoma. Hum Pathol. 42:204–213. 2011. View Article : Google Scholar
|
|
11
|
Hiraike H, Wada-Hiraike O, Nakagawa S,
Saji S, Maeda D, Miyamoto Y, Sone K, Tanikawa M, Oda K, Nakagawa K,
et al: Expression of DBC1 is associated with nuclear grade and HER2
expression in breast cancer. Exp Ther Med. 2:1105–1109. 2011.
|
|
12
|
Zhao W, Kruse JP, Tang Y, Jung SY, Qin J
and Gu W: Negative regulation of the deacetylase SIRT1 by DBC1.
Nature. 451:587–590. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JE, Chen J and Lou Z: DBC1 is a
negative regulator of SIRT1. Nature. 451:583–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hiraike H, Wada-Hiraike O, Nakagawa S,
Koyama S, Miyamoto Y, Sone K, Tanikawa M, Tsuruga T, Nagasaka K,
Matsumoto Y, et al: Identification of DBC1 as a transcriptional
repressor for BRCA1. Br J Cancer. 102:1061–1067. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koyama S, Wada-Hiraike O, Nakagawa S,
Tanikawa M, Hiraike H, Miyamoto Y, Sone K, Oda K, Fukuhara H,
Nakagawa K, et al: Repression of estrogen receptor beta function by
putative tumor suppressor DBC1. Biochem Biophys Res Commun.
392:357–362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schafer ZT, Grassian AR, Song L, Jiang Z,
Gerhart-Hines Z, Irie HY, Gao S, Puigserver P and Brugge JS:
Antioxidant and oncogene rescue of metabolic defects caused by loss
of matrix attachment. Nature. 461:109–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang YF, Zhang AR, Zhang BC, Rao ZG, Gao
JF, Lv MH, Wu YY, Wang SM, Wang RQ and Fang DC: MiR-26a regulates
cell cycle and anoikis of human esophageal adenocarcinoma cells
through Rb1-E2F1 signaling pathway. Mol Biol Rep. 40:1711–1720.
2013. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Rauert-Wunderlich H, Siegmund D, Maier E,
Giner T, Bargou RC, Wajant H and Stühmer T: The IKK inhibitor Bay
11-7082 induces cell death independent from inhibition of
activation of NFκB transcription factors. PLoS One. 8:e592922013.
View Article : Google Scholar
|
|
20
|
Liu SF, Ye X and Malik AB: Inhibition of
NF-kappaB activation by pyrrolidine dithiocarbamate prevents in
vivo expression of proinflammatory genes. Circulation.
100:1330–1337. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oztürk S, Schleich K and Lavrik IN:
Cellular FLICE-like inhibitory proteins (c-FLIPs): Fine-tuners of
life and death decisions. Exp Cell Res. 318:1324–1331. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luqman S and Pezzuto JM: NFkappaB: A
promising target for natural products in cancer chemoprevention.
Phytother Res. 24:949–963. 2010.PubMed/NCBI
|
|
23
|
Hamaguchi M, Meth JL, von Klitzing C, Wei
W, Esposito D, Rodgers L, Walsh T, Welcsh P, King MC and Wigler MH:
DBC2, a candidate for a tumor suppressor gene involved in breast
cancer. Proc Natl Acad Sci USA. 99:13647–13652. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trauernicht AM, Kim SJ, Kim NH, Clarke R
and Boyer TG: DBC-1 mediates endocrine resistant breast cancer cell
survival. Cell Cycle. 9:1218–1219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trauernicht AM, Kim SJ, Kim NH and Boyer
TG: Modulation of estrogen receptor alpha protein level and
survival function by DBC-1. Mol Endocrinol. 21:1526–1536. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim JE and Sung S: Deleted in breast
cancer 1 (DBC1) is a dynamically regulated protein. Neoplasma.
57:365–368. 2010.PubMed/NCBI
|
|
27
|
Garapaty S, Xu CF, Trojer P, Mahajan MA,
Neubert TA and Samuels HH: Identification and characterization of a
novel nuclear protein complex involved in nuclear hormone
receptor-mediated gene regulation. J Biol Chem. 284:7542–7552.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fu J, Jiang J, Li J, Wang S, Shi G, Feng
Q, White E, Qin J and Wong J: Deleted in breast cancer 1, a novel
androgen receptor (AR) coactivator that promotes AR DNA-binding
activity. J Biol Chem. 284:6832–6840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S,
Yu TK, Kim KM, Park HS, Lee JH, Moon WS, et al: Expression of SIRT1
and DBC1 is associated with poor prognosis of soft tissue sarcomas.
PLoS One. 8:e747382013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park HS, Bae JS, Noh SJ, Kim KM, Lee H,
Moon WS, Chung MJ, Kang MJ, Lee DG and Jang KY: Expression of DBC1
and Androgen Receptor Predict Poor Prognosis in Diffuse Large B
Cell Lymphoma. Transl Oncol. 6:370–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang Y, Jung WY, Lee H, Lee E, Kim A and
Kim BH: Expression of SIRT1 and DBC1 in gastric adenocarcinoma.
Korean J Pathol. 46:523–531. 2012. View Article : Google Scholar
|
|
32
|
Cha EJ, Noh SJ, Kwon KS, Kim CY, Park BH,
Park HS, Lee H, Chung MJ, Kang MJ, Lee DG, et al: Expression of
DBC1 and SIRT1 is associated with poor prognosis of gastric
carcinoma. Clin Cancer Res. 15:4453–4459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guadamillas MC, Cerezo A and Del Pozo MA:
Overcoming anoikis-pathways to anchorage-independent growth in
cancer. J Cell Sci. 124:3189–3197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aggarwal BB and Sung B: NF-κB in cancer: A
matter of life and death. Cancer Discov. 1:469–471. 2011.
View Article : Google Scholar
|
|
35
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|