Introduction
Breast cancer is a heterogeneous disease influenced
by genetic and environmental factors (1). Tumour metastasis is regulated by a set
of non-randomized events, starting from loss of cancer cells
adhesion at the primary site, local invasion, intravasation,
survival in circulation, extravasation and colonisation at distant
sites. The nervous and vascular system share several anatomical and
developmental similarities as these systems are combined in
neurovascular bundles and in peripheral tissues. Notably, these
shared developmental links also assist scientists to speculate the
involvement of certain molecules controlling growth and migration
of nerves in cancer cell proliferation, differentiation and
dissemination (2,3). Apart from acting as axonal guidance
cues, the involvement of semaphorins and plexins in altering the
cytoskeleton, organization of actin filaments and microtubule
networks in non-neural systems has also been observed (4,5).
Semaphorins are classified as secreted,
transmembrane or glycosylphosphatidylinositol-linked proteins
containing a phylogenetically conserved extracellular ‘sema’
domain. These molecules are further delineated into eight classes,
of which classes 3–7 are present in vertebrates (6). Plexins are transmembrane protein
receptors for semaphorins grouped further under A to D categories.
Previously, the two families were initially identified as axon
guidance cues in the nervous system and were later found to be
involved in other systems including vascular, reproductive and
immune systems (4,7–9).
Aberrant expression of these molecules was also
associated with different diseases. For example, mutations of
plexin-A2 and Sema3D have been observed in schizophrenia and
anxiety (10,11). Similarly, involvement of semaphorin
4D (Sema4D) and plexin-B1 in the perineural invasion of tumour
cells and angiogenesis has been established (12). Sema4D also termed CD100, constitutes
863 amino acids containing a transmembrane, an immunoglobulin
(Ig)-like and a sema domain (13).
Apart from their role in axonal guidance, Sema4D and plexin-B1
interactions have also been found to be responsible for T-cell
proliferation (14) and B-cell
survival and aggregation (15). A
higher expression of Sema4D has been observed in T cells while its
lowest level is evident in mature B cells, macrophages and
dendritic cells (6). P1exin-B1, a
transmembrane protein is present on plasma membrane in the majority
of cell types and acts as a binding receptor for Sema4D (6,17).
Plexin-B2 and -B3 are the binding receptors for Sema4C and Sema5A,
respectively (18–20). Altered expression patterns of these
molecules have been observed in different types of tumours, as well
as various cancer cell lines. Cancer cell proliferation and
tumour-related angiogenesis may vary to a greater extent under the
influence of these molecules.
Increased Sema4D and plexin-B1 expression in
pancreatic ductal adenocarcinoma patients is correlated with lymph
node involvement and metastasis (21). Similarly, in soft tissue sarcoma
patients the elevated expression of Sema4D is associated with an
increased mitotic division of cancer cells. Higher Sema4D levels
are correlated with poorer overall and disease-free survival
(22). Mammary cancer cell
proliferative, angiogenic and metastatic abilities were well
compromised under Sema4D knockout. A role for Sema4D as an oncogene
responsible for invasiveness, metastasis and angiogenesis
progression in mammary cell lines (66cl4, 4T1 and 168FARN) was also
established (23). However, a
significant effect of Sema4D as a guardian against metastatic
progression has also been observed in a mammary tumour cohort
(24). Contrasting findings
regarding the expression profile of Sema4D and the plexin-B family
in relation to different types of tumours have also been reported.
For example, an increased Sema4D and plexin-B1 expression has also
been observed in prostate (25–27),
cervical (28), and breast and
ovarian cancers (29). A combined
effect of increased plexin-B1 along with c-Met was associated with
poor cell differentiation and higher lymph node metastasis. The
co-expression of the two proteins was correlated with unfavourable
outcomes according to a study of 50 breast and ovarian neoplasms
using immunohistochemistry and immunofluorescence staining
(29). The interactions among
Sema4D, plexin-B1 and Met were also responsible for triggering
tumour invasive growth and metastasis (25). However, in renal and breast cancer
patients, a reduced expression of plexin-B1 was observed in
relation to disease progression (30–32).
Plexin-B2 also shares a structural homology with plexin-B1 and its
interaction with Sema4D has also been established (33).
In the present study, the expression profile of
these molecules (Sema4D, plexin-B1, -B2 and -B3) was examined in a
breast cancer cohort. The aim of the present study was to provide a
thorough insight into the expression profiles of these molecules
and their association with breast cancer progression, disease
stage, cell differentiation, nodular involvement, local recurrence
and bone metastasis.
Materials and methods
Collection of breast cancer
specimens
Mammary tissue samples (n=169) were collected
immediately after surgery and stored at −80°C until further use,
with prior approval from the local Ethics Committee. Breast cancer
tissues (n=147) and background normal breast tissues (n=22) were
verified by a consultant pathologist. A routine follow-up was
carried out after surgery with a median follow-up period of 120
months. A higher incidence of ductal carcinoma tissues was observed
in this cohort. Grading along with Nottingham prognostic index
(NPI) values were evaluated by independent histologists aided with
clinical and laboratory reports. Data regarding cohort are provided
in Table I.
 | Table IExpression of Sema4D, plexin-B in
breast cancer cohort. |
Table I
Expression of Sema4D, plexin-B in
breast cancer cohort.
| Clinicopathological
status | No. | Transcripts
(copies/μl, mean ± SD)
|
|---|
| Sema4D | Plexin-B1 | Plexin-B2 | Plexin-B3 |
|---|
| Tissue samples | | | | | |
| Normal | 22 | 29.4±22.4 | 3235±1906 | 167±123 | 0.701±0.292 |
| Tumour | 147 | 24.82±4.79 | 2548±976 | 178.2±42 | 1.910±0.424 |
| Tumour grade | | | | | |
| 1 | 21 | 18.19±6.31 | 12111±5204 | 137.8±67.5 | 0.316±0.137 |
| 2 | 43 | 18.82±6.73 | 664±530a | 149.5±38.7 | 2.624±0.989a |
| 3 | 58 | 31.79±8.58 | 602±262a | 220.2±81.4 | 2.023±0.520a |
| TNM staging | | | | | |
| I | 70 | 29.77±6.99 | 4544±1793 | 179.7±35.8 | 2.041±0.649 |
| II | 40 | 15.99±5.22 | 376±200a | 104.2±32.4 | 2.018±0.746 |
| III | 7 | 11.44±5.84 | 66.9±32.2a | 80.7±64.5 | 0.806±0.224 |
| IV | 4 | 12.8±12.2 | 14.44±8.56a | 28±18.0a | 0.807±0.694 |
| NPI (score) | | | | | |
| 1 (<3.4) | 66 | 20.40±6.63 | 2708±1678 | 107.2±27.8 | 1.801±0.603 |
| 2 (3.4–5.4) | 38 | 28.68±6.56 | 2861±1489 | 201.9±48.5 | 1.451±0.600 |
| 3 (>5.4) | 16 | 38.0±20.3 | 1903±1311 | 439±275 | 3.80±1.64 |
| Clinical
outcomes | | | | | |
| Disease-free | 90 | 28.11±6.27 | 3262±1358 | 200.1±57.6 | 1.737±0.438 |
| With
metastases | 7 | 3.77±2.86a | 714±681 | 62.8±48.2 | 7.08±4.31 |
| With local
recurrence | 5 | 3.12±2.14a | 162±102a | 16.5±10.6a | 0.951±0.761 |
| Died of breast
cancer | 16 | 26.6±10.3 | 645±463 | 261.4±72.7 | 1.613±0.670 |
| Poor
prognosis | 28 | 16.44±6.19 | 565±301 | 160.8±46.0 | 2.96±1.26 |
Tissue processing and extraction of RNA
and generation of cDNA
Approximately 20 sections from each tissue sample
were homogenised in an RNA extraction solution using a hand held
homogeniser for RNA isolation. RNA quantification was carried out
using a UV spectrophotometer (WPA UV 1101; Biotech Photometer,
Cambridge, UK). Reverse transcription (RT) was performed from 1
μg of total RNA using a Reverse Transcription kit (AbGene
Laboratories, Essex, UK).
Conventional PCR
The quality of generated cDNA was verified using
GAPDH primers (Table II). Reaction
conditions started with an initial denaturation of 5 min at 94°C
followed by 35 cycles of 10 sec at 94°C, 30 sec at 55°C for
annealing and 30 sec at 72°C, with a final elongation of 72°C for
10 min. Thermal cyclers used in this regard were obtained from
Perkin-Elmer, Surrey, UK. Amplified products were then separated on
a 2% agarose gel and visualized under ultraviolet light following
ethidium bromide staining.
 | Table IIPrimer sequences used in the present
study. |
Table II
Primer sequences used in the present
study.
| Gene | Sense primers
(5′-3′) | Antisense primers
(5′-3′) |
|---|
| Sema4D |
ctcagcagggaacaagact |
actgaacctgaccgtacactccagctctgcatcatc |
| Plexin-B1 |
gaggtggcctacatcgag |
actgaacctgaccgtacagtggtctgagccacagg |
| Plexin-B2 |
gaagacaccatccacatc |
actgaacctgaccgtacaatgcacgtcaaagatgaag |
| Plexin-B3 |
ctcaacctgggcatcag |
actgaacctgaccgtacaggctcgcagtacaggtg |
| GAPDH |
ggctgcttttaactctggta |
gactgtggtcatgagtcctt |
| GAPDH (q-PCR) |
ctgagtacgtcgtggagtc |
actgaacctgaccgtacagagatgatgacccttttg |
Quantitative PCR
This study was based on the Ampliflour™ technology
which was performed using the StepOne™ system (Applied Biosystems,
Foster City, CA, USA). The methodology for this study has
previously been optimized to quantify the transcript copy number in
the mammary carcinoma specimens, as previously reported (34). Briefly, a set of standards along
with the negative controls were included in this study. Beacon
Designer software (Premier Biosoft International, Palo Alto, CA,
USA) was used to design the primer pairs provided in Table II. A short stretch of sequence,
complementary to universal Z probe, was also incorporated at the
5′-end of each reverse primer (InterGen, Inc., Purchase, NY, USA).
The reagents used for this reaction included 2X concentrated
hot-start Q-Master mix (AbGene Laboratories), 10 pmol of specific
forward primer, 1 pmol of reverse primer, 10 pmol of FAM-tagged
universal probe and cDNA. The qPCR conditions were 95°C for 15 min,
followed by 60 cycles at 95°C for 20 sec, 55°C for 30 sec and 72°C
for 20 sec. qPCR for GAPDH was also performed on the same samples
to normalise for any residual differences in the initial
quantification of cDNA, as previously reported (34,35).
Effect of SERMs on cancer cell lines
The effect of oestrogen receptors on the expression
of Sema4D and plexin-B1 was studied using MCF-7 and MDA-MB-231
breast cancer cell lines. These cell lines were purchased from the
European Collection of Animal Cell Culture (ECACC; Salisbury, UK).
The lines were routinely maintained in Dulbecco’s modified Eagle’s
medium (DMEM)/F12 medium with 10% foetal calf serum (FCS).
Selective oestrogen receptor modulators (SERMs) used in the present
study included an agonist and antagonist for ERα and ERβ receptors.
PPT (agonist) for ERα receptor while ERβ041 (agonist) for ERβ
receptor were purchased from Tocris Biosciences (Bristol, UK). The
SERMs were dissolved in a DMEM at 4X concentration in reference to
their respective IC50 value.
A duplicate set of 6-well plates was used for this
study. The cells (5×105) from each cell line were seeded
in the well separately. The plates were incubated at 37°C in a
normal DMEM/F12 medium for a minimal of 24 h. The cells were later
exposed to serum starvation for170 1 h duration. These wells were
then exposed to a variable concentration of SERMs. After 4–5 h of
treatment, the cells were lysed in total RNA isolation reagents for
RNA isolation.
Statistical analysis
Statistical analysis was carried out using the
Minitab statistical software package (version 14). Non-normally
distributed data were assessed using the Mann-Whitney test (IQR),
whereas the Student’s t-test (mean ± SD) was used for normally
distributed data where appropriate. P<0.05 was defined as
statistically significant. A Kaplan-Meier survival analysis was
carried out using SPSS statistical software (version 12; SPSS,
Inc.).
Results
Aberrant expression of Sema4D and the
plexin-B family in breast cancer
Transcript levels of Sema4D and plexin-B2 in the
breast tumours were similar to their expression in the normal
background tissues. Plexin-B1 appeared to be expressed at
relatively lower levels in the tumours. Notably, plexin-B3 was
upregulated in the tumours, p=0.02 when compared to the control
(Table I).
Correlation of Sema4D and the plexin-B
family with differentiation of breast cancer cells
Transcript levels of Sema4D did not show any
significant variations among well, moderately and poorly
differentiated tumour tissues (Table
I). The expression of plexin-B1 was markedly reduced when
compared among well (grade 1) and both moderate (grade 2) and
poorly differentiated (grade 3) tumour tissues, respectively
(Table I). No significant
correlation of plexin-B2 with tumour grading was observed. A
pronounced increase of plexin-B3 expression was observed in
moderately and poorly differentiated tumours in comparison to
well-differentiated tumours. This increase of plexin-B3 was also
statistically significant among grade 1 vs. 2 p=0.026; and grade 1
vs. 3 p=0.0024, respectively (Table
I).
Expressional variations among different
breast cancer types
Increased expression of Sema4D and plexin-B1 was
observed in ductal (n=87) when compared with lobular (n=12), muscin
(n=4), medullary (n=2), tubular (n=1) and other types of breast
cancer patients (n=7). A significant correlation of the expression
of Sema4D and plexin-B1 among ductal versus all previously
mentioned types, excluding lobular cancer, was also established in
the cohort (p<0.001). Similarly, plexin-B2 and -B3 molecules
showed the highest expression in ductal cancer patients when
compared with other types of breast cancers. A significant
correlation of the plexin-B2 and -B3 expression levels among ductal
versus all earlier mentioned types, excluding lobular cancer, was
also established in the cohort (p<0.001).
Correlation of tumor-node-metastasis
(TNM) staging
Transcript levels of Sema4D, plexin-B1, -B2 and -B3
tended to be reduced in the tumours at more advanced stages
according to the grouping of TNM stages. Significant associations
were observed in levels of plexin-B1 and -B2. The highest
transcript levels of plexin-B1 were evident in tumours at an
extremely early stage (TNM1), with its expression level being
reduced during disease progression. The lowest expression of
plexin-B1 was seen in the most advanced diseases. Similarly, the
lowest expression levels of plexin-B2 were evident in the tumours
with metastases (Table I).
Relationship with the clinical outcome of
breast cancer
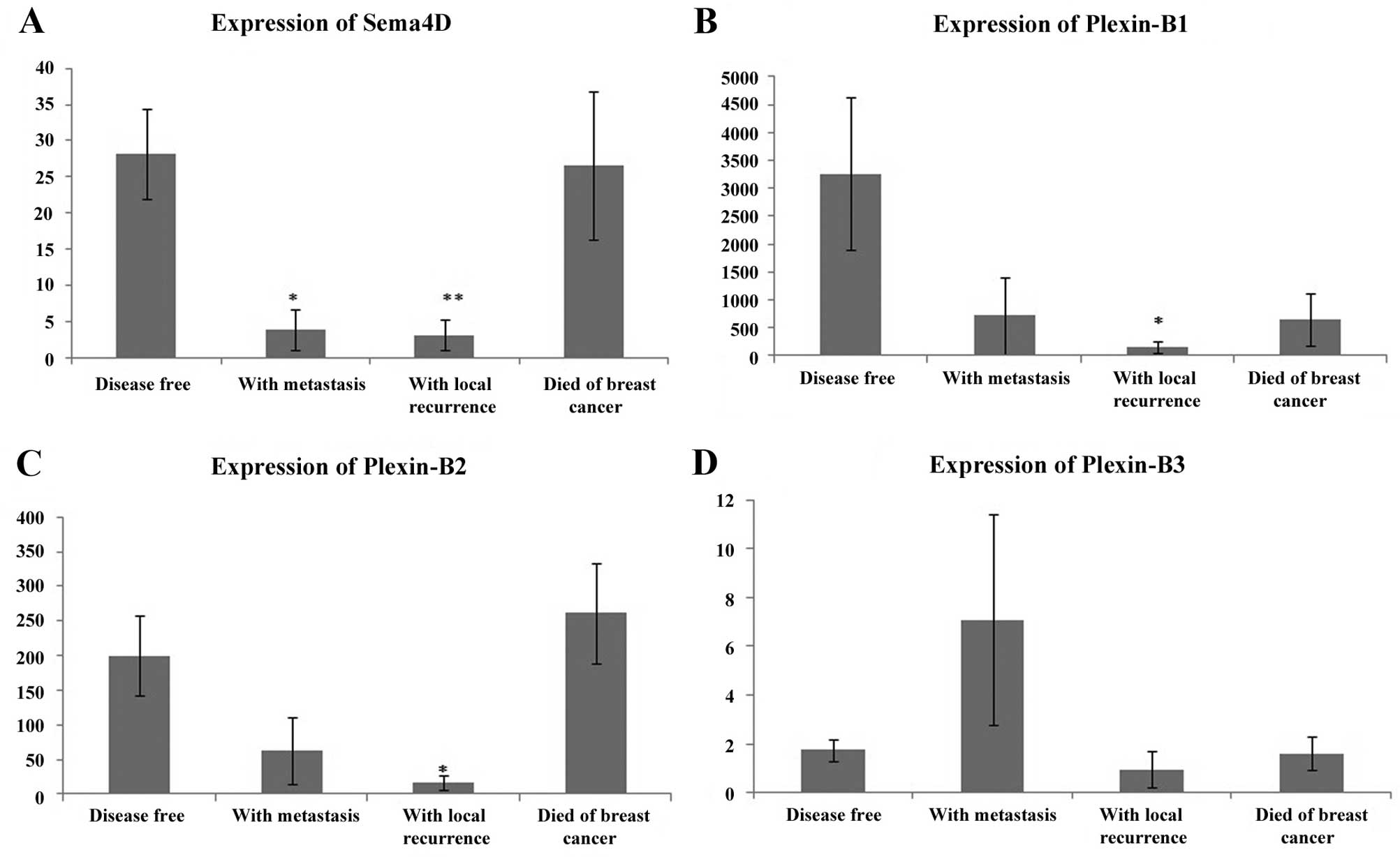
A reduced expression of Sema4D was strongly
correlated with distant metastasis (p=0.0008) and local recurrence
(p=0.0003) in comparison to its expression in disease-free patients
(Table I). The expression of
plexin-B1 downregulation was significantly associated with
disease-free survival. No significant association of the plexin-B2
and -B3 expression profiles with the clinical outcome and
disease-free survival were observed in the breast cohort. The
reduced expression of plexin-B2 was significantly correlated with
local recurrence in comparison to the disease-free one (p=0.002)
(Fig. 1).
Expression of Sema4D and the plexin-B
family with overall survival
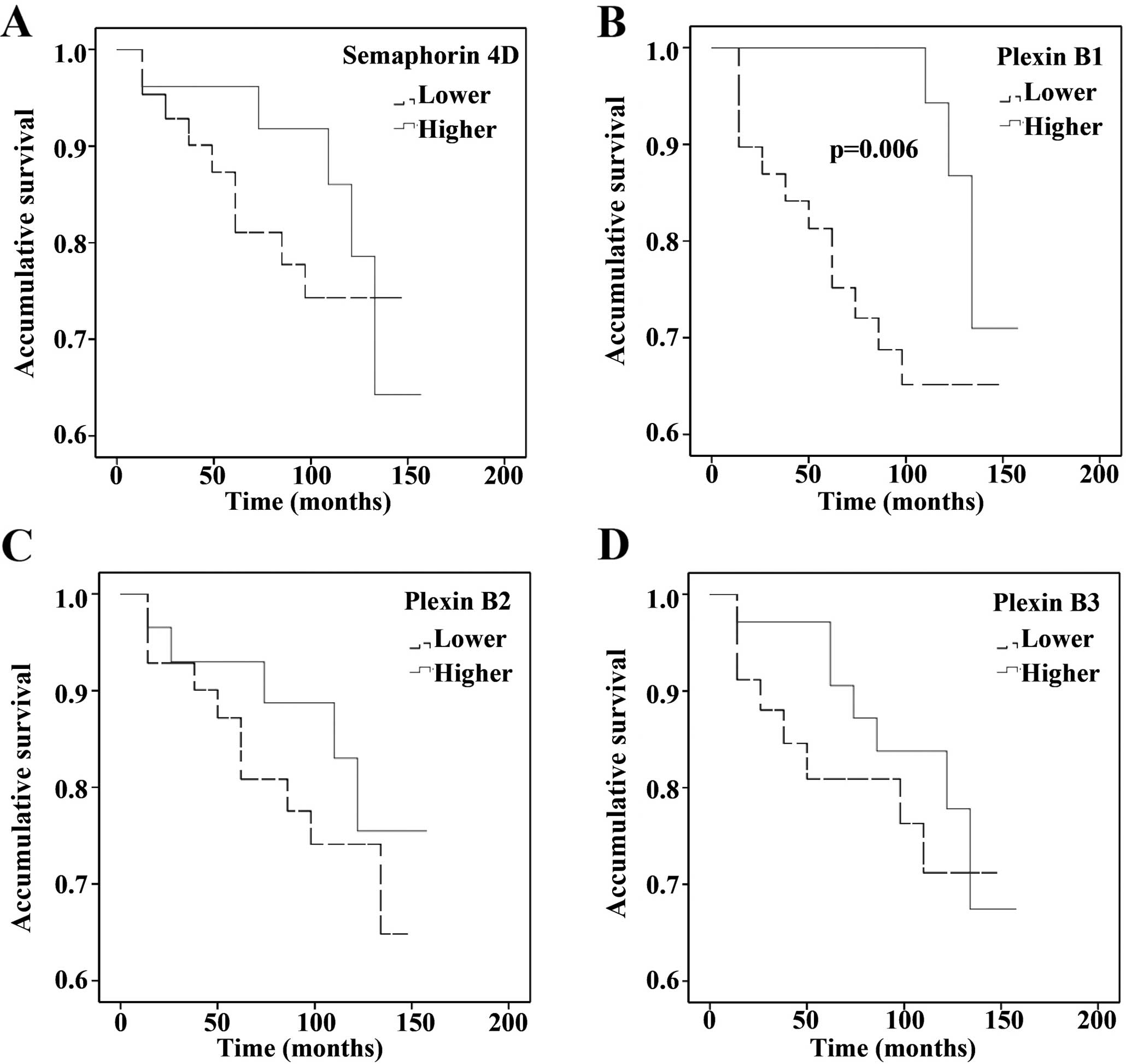
The effect of the altered expression pattern of
these molecules on patient survival was also carried out using the
Kaplan-Meier survival curve (Fig.
2). Patients with an increased expression of Sema4D showed an
increased disease-free survival when compared with patients stating
reduced or almost negative Sema4D (p=0.033). However, the
plexin-B1-reduced expression was significantly associated with
worst outcome in the cohort (p=0.006). No significant relationship
of plexin-B2 and -B3 with overall survival was observed.
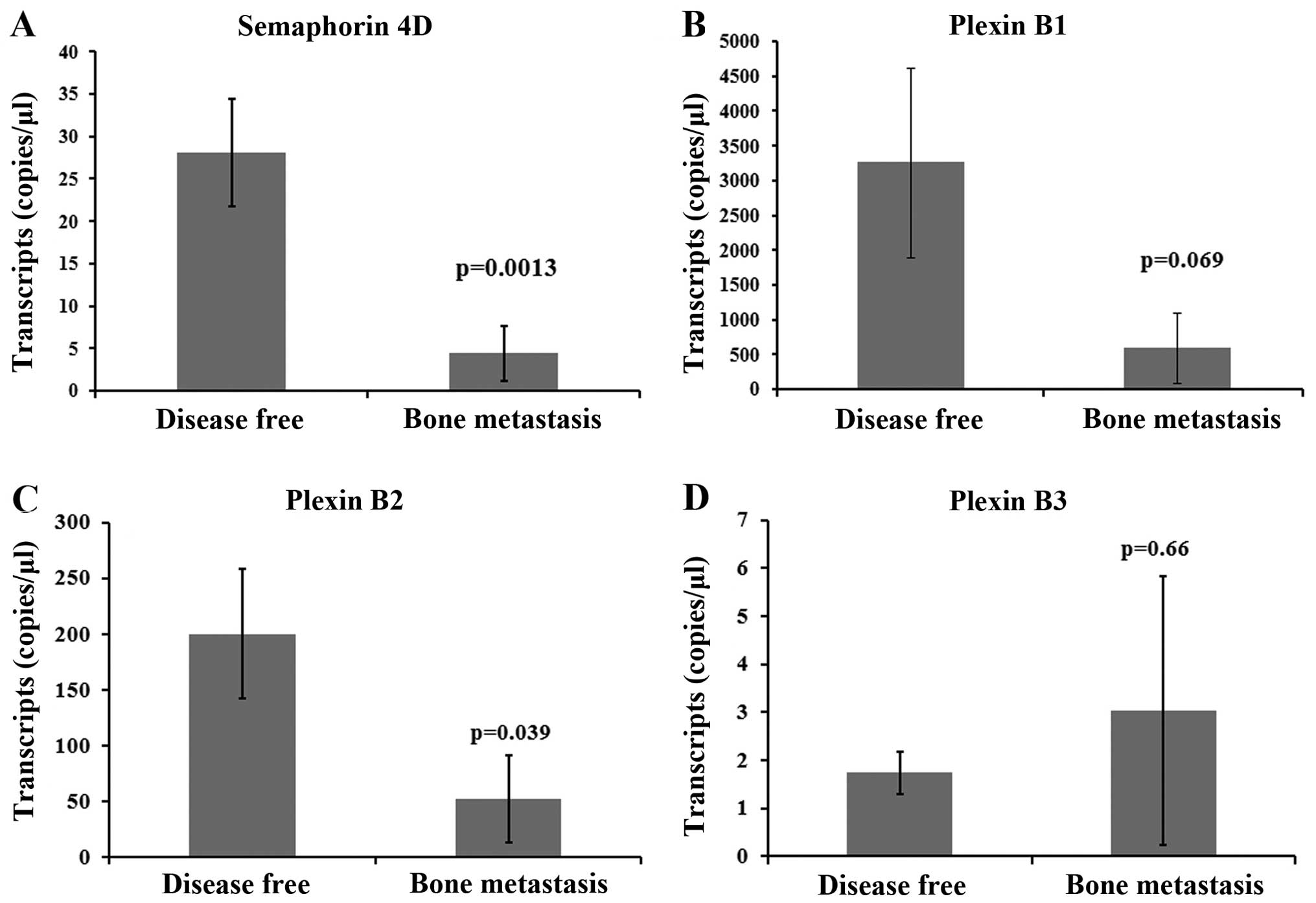
Effect of Sema4D and the plexin-B family
on bone metastasis
A significant correlation of the Sema4D expression
with bone metastasis was observed in the cohort (p=0.0013, Fig. 3A). Patients with bone metastasis
showed a decreased Sema4D expression when compared with
disease-free patients. However, no significant association between
plexin-B1 and bone metastasis was seen, although the levels were
much lower in patients with bone metastasis compared to
disease-free patients (p=0.069, Fig.
3B). Of note, a reduction in plexin-B2 was significantly
correlated with bone metastasis (p=0.039, Fig. 3C). Plexin-B3 upregulation was
observed in patients with bone metastasis, but was not found to be
statistically significant (p=0.66, Fig.
3D).
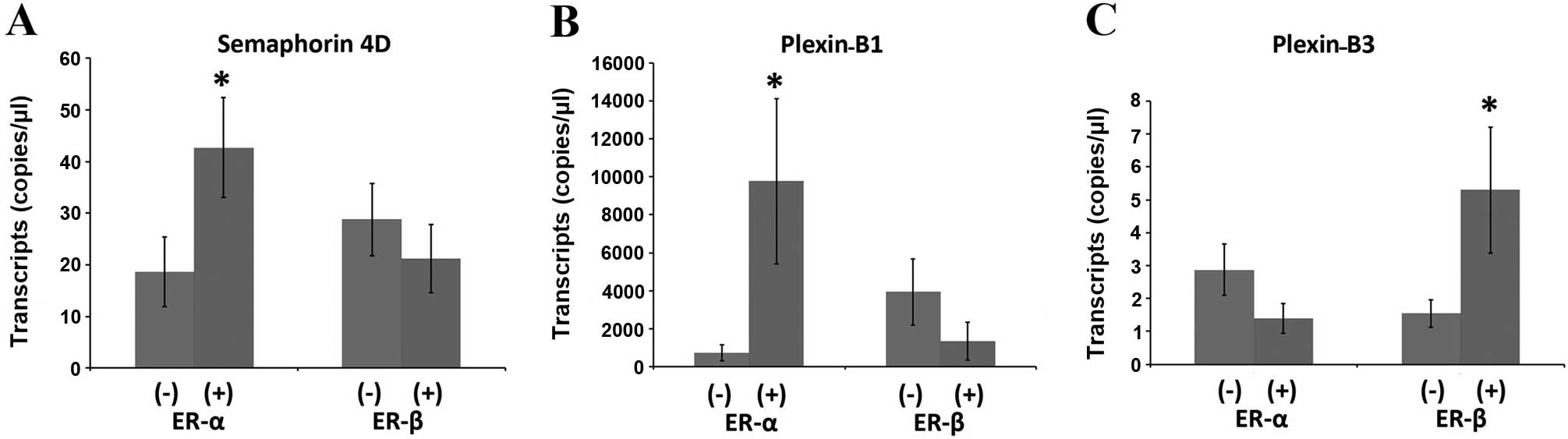
Relationship with oestrogen receptor
A significant increase in the Sema4D expression in
ERα-positive ductal breast cancer patients (n=57) compared with
ERα-negative ductal carcinoma patients (n=23) (p=0.044) was
observed in the clinical cohort. Higher levels of plexin-B1 were
also observed in the ERα-positive patients as compared to the
ERα-negative patients (p=0.05). Although the expression of Sema4D
and plexin-B1 appeared to be lower in the ERβ-positive tumours, no
statistical difference was observed in the analysis. By contrast,
the expression profiles of Sema4D and plexin-B1 and the expression
levels of plexin-B2 were similar in the tumours of different ER
status. Among the four genes, the plexin-B3 expression appeared to
be inversely linked to ERα status, and its expression was
upregulated in the ERβ-positive tumours (Fig. 4).
Regulation of Sema4D and plexin-B1 by
SERMs
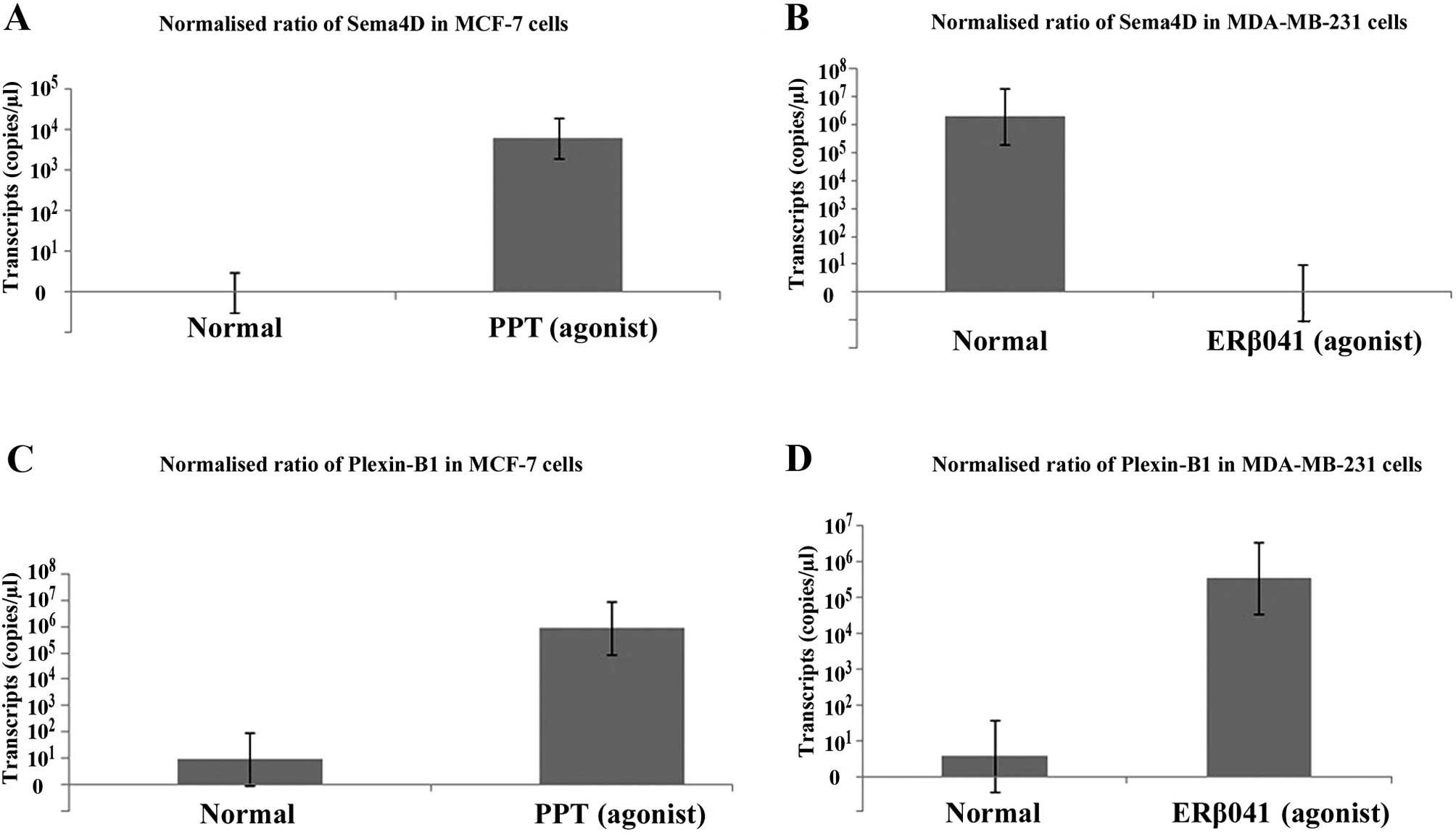
The expression of Sema4D and plexin-B1 was
determined in MCF-7 and MDA-MB-231 cells following exposure to
different SERMs. Increased transcription of Sema4D was observed in
the MCF-7 cells treated with PPT (ERα agonist) compared to the
control (Fig. 5A). Notably, the
inverse correlation of Sema4D expression with ERβ041 (ERβ agonist)
treatment was observed in the MDA-MB-231 cells (Fig. 5B). This finding was also in
agreement with the correlation between the Sema4D and ER receptors
observed in the breast cancer cohort. Similarly, the transcription
of plexin-B1 was upregulated following exposure of the PPT
(agonist)-treated MCF-7 cells when compared to the controls
(Fig. 5C). Plexin-B1 transcription
was also upregulated by the ERβ receptor agonist in the MDA-MB-231
cells (Fig. 5D). Although these
findings were in agreement with cohort observations, this area
requires further research to explore the oestrogen-regulated
signaling pathways and its effects of semaphorin signalling.
Discussion
The dual role of semaphorins and plexins as oncogene
or tumour-suppressor molecules has been previously reported in the
literature. As in melanoma, the downregulation of plexin-B1 was
strongly associated with cancer progression (36,37),
while increased levels of this molecule have been observed in
ovarian, prostate and breast cancer (38). In the present study, no significant
association of the Sema4D, plexin-B1 and -B2 aberrant expression
among normal and diseased patients was observed in the cohort. This
result is similar to the findings by Yang et al, which show
no significant difference in the plexin-B2 protein expression
between breast carcinoma and epithelial cells of normal breast
tissue (39). In the present study,
upregulation of plexin-B3 was identified in breast cancer compared
to the normal controls. These findings are also in concordance with
the study on gastric cancer patients, the results of which showed
an increased expression of plexin-B3 and its ligand (Sema5A) in
gastric cancer (40).
The reduced expression of Sema4D did not show any
significant relationship with tumour cell differentiation in the
current cohort. However, the cognate receptor (plexin-B1) showed a
strong inverse correlation with tumour cell differentiation. This
result is contrary to previous observations reported on breast and
ovarian cancer samples. In a previous study, the co-expression of
plexin-B1 and Met was associated with worse grading and higher
incidences of lymph node metastases (29). These variations are due to i)
involvement and influence of other factors interacting with
semaphorins and their cognate receptors and ii) heterogeneous
tumour orientation. The role of plexin-B1 as a tumour suppressor
was observed in melanoma and melanocytes, where B-Raf/MKK/ERK
stimulation led to its suppression and tumour progression (36). Thus, plexin-B1-interacting molecules
are also noteworthy. In another study, the mammary tumours showing
the co-expression of plexin-B2 and her-2 were characterised as
worse staging with higher incidences of lymph node metastases than
those that express plexin-B2 alone. However, no significant
association of plexin-B2 alone with the TNM stage and grade was
observed in these tissues (39). A
direct correlation of plexin-B3 with advanced tumour grading in
breast cancer cohort was consistent with previous findings. In
gastric cancer, a gradual increase in the expression of both
receptor and ligand (plexin-B3 and Sema5A) from non-neoplastic
mucosa, primary gastric and metastasis was reported (40).
The reduced expression of Sema4D was strongly
associated with an unfavourable outcome when compared with the
disease-free patients. A study conducted on invasive ductal breast
cancers using microarray and qPCR data revealed that 888 genes were
significantly (p≤0.05) differentially expressed between grade I and
II tumours. A potentially protective effect of Sema4D, Sema4F and
plexin-A2 on benign tumours towards growth and metastatic
suppression has been reported (24). Those findings suggest Sema4D
putative involvement as a clinical prognostic marker. However,
apart from the aforementioned study a completely contrasting
feature of Sema4D (acting as oncogene molecule) has also been
reported (38,41). As in ovarian cancer, the
overexpression of Sema4D together with HIF-1α and VEGF were
associated with poor prognosis. An increased expression of Sema4D
was also associated with histological grading, stages and lymph
node metastasis (41). An increased
expression of Sema4D between the cytoplasm and cell surface has
also been reported in head and neck squamous cell carcinoma, oral,
prostate, breast and lung cancer tissues (38). These disparities in findings are
also adequately addressed in the literature. One of the main
contributing factors in this regard is that the correlations of
these molecules fluctuate strongly when measured over different
subsets of patients (42). Loss of
plexin-B1 is significantly associated with a worse outcome in
patients and the present study was also in concordance with
previously published studies (31).
A higher expression of plexin-B1 in ER-positive was correlated with
the disease-free and overall survival (43).
The expression of Sema4D and plexin-B2 was
significantly associated with bone metastasis. Patients having a
reduced level of Sema4D and plexin-B2 showed an increased tendency
towards bone metastastic progression. This area requires further
research to investigate the factors responsible for modulating
these effects on bone marrows.
These findings collectively suggest aberrant
expression and dysfunctions of these molecules occurring in certain
malignancies, including breast cancer, as shown by results of the
present study. Conflicting findings from different studies also
suggest these molecules may play more complicated roles in cancer
regarding the type of cancer, and some other non-clarified
subgroups of a particular cancer. For example, in breast cancer the
ERs status may be involved in such differences. In the present
cohort, a significant increase in Sema4D and plexin-B1 was
identified in relation to the ERα-positive tumour patients as
compared to the ERα-negative ones. A similar trend regarding the
expression of Sema4D and plexin-B1 was also observed in the MCF-7
and MD-MB-231 cancer cell lines following exposure to ERα and ERβ,
respectively. These findings are in concordance with previously
published studies where increased plexin-B1 levels in ER-positive
are correlated with increased disease-free and overall survival
(43). A direct expressional
correlation of plexin-B1 with ER status was observed in only those
cancer cells showing stem cell-like expression status, where
proliferative activity was coupled with ER status (31). Thus, the regulation of Sema4D and
plexin-B1 influenced by oestrogen receptors is an important domain
to determine future therapeutic strategies.
In conclusion, the Sema4D and plexin-B1 reduced
levels are associated with breast cancer progression and a poor
outcome. Increased plexin-B3 is also a contributory factor for bone
metastasis. Oestrogen receptors regulate the expressional profiling
of semaphorins and plexins. Involvement of these molecules in bone
metastasis and ERs require further investigations to provide a
better understanding of the diseases and opportunities to improve
personalised treatment according to the molecular signature.
Acknowledgments
We would like to thank the participants of the
present study, as well as the Cancer Research Wales and the higher
Education Commission of Pakistan (FAM) for providing funds for the
present study.
References
|
1
|
Singletary SE: Rating the risk factors for
breast cancer. Ann Surg. 237:474–482. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Autiero M, Waltenberger J, Communi D,
Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M,
Bono F, et al: Role of PlGF in the intra- and intermolecular cross
talk between the VEGF receptors Flt1 and Flk1. Nat Med. 9:936–943.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou Y, Gunput RA and Pasterkamp RJ:
Semaphorin signaling: Progress made and promises ahead. Trends
Biochem Sci. 33:161–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perälä N, Sariola H and Immonen T: More
than nervous: The emerging roles of plexins. Differentiation.
83:77–91. 2012. View Article : Google Scholar
|
|
6
|
Tamagnone L, Artigiani S, Chen H, He Z,
Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, et al:
Plexins are a large family of receptors for transmembrane,
secreted, and GPI-anchored semaphorins in vertebrates. Cell.
99:71–80. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Regev A, Goldman S and Shalev E:
Semaphorin-4D (Sema-4D), the Plexin-B1 ligand, is involved in mouse
ovary follicular develop ment. Reprod Biol Endocrinol. 5:122007.
View Article : Google Scholar
|
|
8
|
Pasterkamp RJ and Giger RJ: Semaphorin
function in neural plasticity and disease. Curr Opin Neurobiol.
19:263–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mizui M, Kumanogoh A and Kikutani H:
Immune semaphorins: Novel features of neural guidance molecules. J
Clin Immunol. 29:1–11. 2009. View Article : Google Scholar
|
|
10
|
Wray NR, James MR, Mah SP, Nelson M,
Andrews G, Sullivan PF, Montgomery GW, Birley AJ, Braun A and
Martin NG: Anxiety and comorbid measures associated with PLXNA2.
Arch Gen Psychiatry. 64:318–326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujii T, Uchiyama H, Yamamoto N, Hori H,
Tatsumi M, Ishikawa M, Arima K, Higuchi T and Kunugi H: Possible
association of the semaphorin 3D gene (SEMA3D) with schizophrenia.
J Psychiatr Res. 45:47–53. 2011. View Article : Google Scholar
|
|
12
|
Negishi-Koga T, Shinohara M, Komatsu N,
Bito H, Kodama T, Friedel RH and Takayanagi H: Suppression of bone
formation by osteoclastic expression of semaphorin 4D. Nat Med.
17:1473–1480. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hall KT, Boumsell L, Schultze JL,
Boussiotis VA, Dorfman DM, Cardoso AA, Bensussan A, Nadler LM and
Freeman GJ: Human CD100, a novel leukocyte semaphorin that promotes
B-cell aggregation and differentiation. Proc Natl Acad Sci USA.
93:11780–11785. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishida I, Kumanogoh A, Suzuki K, Akahani
S, Noda K and Kikutani H: Involvement of CD100, a lymphocyte
semaphorin, in the activation of the human immune system via CD72:
Implications for the regulation of immune and inflammatory
responses. Int Immunol. 15:1027–1034. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deaglio S, Vaisitti T, Bergui L, Bonello
L, Horenstein AL, Tamagnone L, Boumsell L and Malavasi F: CD38 and
CD100 lead a network of surface receptors relaying positive signals
for B-CLL growth and survival. Blood. 105:3042–3050. 2005.
View Article : Google Scholar
|
|
16
|
Bougeret C, Mansur IG, Dastot H, Schmid M,
Mahouy G, Bensussan A and Boumsell L: Increased surface expression
of a newly identified 150-kDa dimer early after human T lymphocyte
activation. J Immunol. 148:318–323. 1992.PubMed/NCBI
|
|
17
|
Ch’ng ES and Kumanogoh A: Roles of Sema4D
and Plexin-B1 in tumor progression. Mol Cancer. 9:2512010.
View Article : Google Scholar
|
|
18
|
Nagase T, Ishikawa K, Nakajima D, Ohira M,
Seki N, Miyajima N, Tanaka A, Kotani H, Nomura N and Ohara O:
Prediction of the coding sequences of unidentified human genes.
VII. The complete sequences of 100 new cDNA clones from brain which
can code for large proteins in vitro. DNA Res. 4:141–150. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang BS, Ahmad M, Deng AY and Leenen FH:
Neuronal responsiveness to central Na+ in 2 congenic
strains of Dahl saltsensitive rats. Hypertension. 49:1315–1320.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Artigiani S, Conrotto P, Fazzari P,
Gilestro GF, Barberis D, Giordano S, Comoglio PM and Tamagnone L:
Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep.
5:710–714. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kato S, Kubota K, Shimamura T, Shinohara
Y, Kobayashi N, Watanabe S, Yoneda M, Inamori M, Nakamura F,
Ishiguro H, et al: Semaphorin 4D, a lymphocyte semaphorin, enhances
tumor cell motility through binding its receptor, plexinB1, in
pancreatic cancer. Cancer Sci. 102:2029–2037. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ch’ng E, Tomita Y, Zhang B, He J, Hoshida
Y, Qiu Y, Morii E, Nakamichi I, Hamada K, Ueda T, et al: Prognostic
significance of CD100 expression in soft tissue sarcoma. Cancer.
110:164–172. 2007. View Article : Google Scholar
|
|
23
|
Sierra JR, Corso S, Caione L, Cepero V,
Conrotto P, Cignetti A, Piacibello W, Kumanogoh A, Kikutani H,
Comoglio PM, et al: Tumor angiogenesis and progression are enhanced
by Sema4D produced by tumor-associated macrophages. J Exp Med.
205:1673–1685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gabrovska PN, Smith RA, Tiang T, Weinstein
SR, Haupt LM and Griffiths LR: Semaphorin-plexin signalling genes
associated with human breast tumourigenesis. Gene. 489:63–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Conrotto P, Corso S, Gamberini S, Comoglio
PM and Giordano S: Interplay between scatter factor receptors and B
plexins controls invasive growth. Oncogene. 23:5131–5137. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Conrotto P, Valdembri D, Corso S, Serini
G, Tamagnone L, Comoglio PM, Bussolino F and Giordano S: Sema4D
induces angiogenesis through Met recruitment by Plexin B1. Blood.
105:4321–4329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wong OG, Nitkunan T, Oinuma I, Zhou C,
Blanc V, Brown RS, Bott SR, Nariculam J, Box G, Munson P, et al:
Plexin-B1 mutations in prostate cancer. Proc Natl Acad Sci USA.
104:19040–19045. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qiang R, Wang F, Shi LY, Liu M, Chen S,
Wan HY, Li YX, Li X, Gao SY, Sun BC, et al: Plexin-B1 is a target
of miR-214 in cervical cancer and promotes the growth and invasion
of HeLa cells. Int J Biochem Cell Biol. 43:632–641. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Valente G, Nicotra G, Arrondini M, Castino
R, Capparuccia L, Prat M, Kerim S, Tamagnone L and Isidoro C:
Co-expression of plexin-B1 and Met in human breast and ovary
tumours enhances the risk of progression. Cell Oncol. 31:423–436.
2009.PubMed/NCBI
|
|
30
|
Gómez Román JJ, Garay GO, Saenz P,
Escuredo K, Sanz Ibayondo C, Gutkind S, Junquera C, Simón L,
Martínez A, Fernández Luna JL, et al: Plexin B1 is downregulated in
renal cell carcinomas and modulates cell growth. Transl Res.
151:134–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rody A, Holtrich U, Gaetje R, Gehrmann M,
Engels K, von Minckwitz G, Loibl S, Diallo-Danebrock R, Ruckhäberle
E, Metzler D, et al: Poor outcome in estrogen receptor-positive
breast cancers predicted by loss of plexin B1. Clin Cancer Res.
13:1115–1122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rody A, Karn T, Ruckhäberle E, Hanker L,
Metzler D, Müller V, Solbach C, Ahr A, Gätje R, Holtrich U, et al:
Loss of Plexin B1 is highly prognostic in low proliferating ER
positive breast cancers-results of a large scale microarray
analysis. Eur J Cancer. 45:405–413. 2009. View Article : Google Scholar
|
|
33
|
Zielonka M, Xia J, Friedel RH, Offermanns
S and Worzfeld T: A systematic expression analysis implicates
Plexin-B2 and its ligand Sema4C in the regulation of the vascular
and endocrine system. Exp Cell Res. 316:2477–2486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang WG, Douglas-Jones A and Mansel RE:
Levels of expression of lipoxygenases and cyclooxygenase-2 in human
breast cancer. Prostaglandins Leukot Essent Fatty Acids.
69:275–281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Malik FA, Sanders AJ, Jones AD, Mansel RE
and Jiang WG: Transcriptional and translational modulation of KAI1
expression in ductal carcinoma of the breast and the prognostic
significance. Int J Mol Med. 23:273–278. 2009.PubMed/NCBI
|
|
36
|
Argast GM, Croy CH, Couts KL, Zhang Z,
Litman E, Chan DC and Ahn NG: Plexin B1 is repressed by oncogenic
B-Raf signaling and functions as a tumor suppressor in melanoma
cells. Oncogene. 28:2697–2709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stevens L, McClelland L, Fricke A,
Williamson M, Kuo I and Scott G: Plexin B1 suppresses c-Met in
melanoma: A role for plexin B1 as a tumor-suppressor protein
through regulation of c-Met. J Invest Dermatol. 130:1636–1645.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Basile JR, Castilho RM, Williams VP and
Gutkind JS: Semaphorin 4D provides a link between axon guidance
processes and tumor-induced angiogenesis. Proc Natl Acad Sci USA.
103:9017–9022. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ye SM, Han M, Kan CY, Yang LL, Yang J, Ma
QF and Wang SX: Expression and clinical significance of Sema4C in
esophageal cancer, gastric cancer and rectal cancer. Zhonghua Yi
Xue Za Zhi. 92:1954–1958. 2012.In Chinese. PubMed/NCBI
|
|
40
|
Pan GQ, Ren HZ, Zhang SF, Wang XM and Wen
JF: Expression of semaphorin 5A and its receptor plexin B3
contributes to invasion and metastasis of gastric carcinoma. World
J Gastroenterol. 15:2800–2804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen Y, Zhang L, Pan Y, Ren X and Hao Q:
Over-expression of semaphorin4D, hypoxia-inducible factor-1α and
vascular endothelial growth factor is related to poor prognosis in
ovarian epithelial cancer. Int J Mol Sci. 13:13264–13274. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ein-Dor L, Kela I, Getz G, Givol D and
Domany E: Outcome signature genes in breast cancer: Is there a
unique set? Bioinformatics. 21:171–178. 2005. View Article : Google Scholar
|
|
43
|
van de Vijver MJ, He YD, van’t Veer LJ,
Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C,
Marton MJ, et al: A gene-expression signature as a predictor of
survival in breast cancer. N Engl J Med. 347:1999–2009. 2002.
View Article : Google Scholar : PubMed/NCBI
|