Introduction
microRNAs (miRNAs) are small non-coding RNAs that
regulate gene expression post-transcriptionally by binding to
complementary sequences in the 3′-untranslated region of messenger
RNAs (mRNAs) (1). Approximately 30%
of human genes are under miRNA regulation. Not only does each miRNA
regulate many genes, but also each gene is regulated by many miRNAs
(2,3). Many miRNAs are highly conserved among
species and play critical roles in a variety of biological
processes, including development, differentiation, proliferation,
apoptosis and stem cell maintenance (1,4,5).
Consistent with their roles in these processes, many studies have
shown widespread alteration of miRNA expression patterns in cancer
(6,7). Indeed, downregulation of a subset of
miRNAs is a commonly observed feature noted in cancer, suggesting
that these molecules may act as tumor suppressors (8). Many other recent studies show that
dysfunction of miRNAs is related to chronic lymphocytic leukemia
and several solid tumors, including colon, lung, breast, gastric
and liver cancers (1,9–12).
Furthermore, disruption of miRNA biogenesis in various types of
cancer has been implicated in a variety of mechanisms, such as
genomic deletion, mutation, epigenetic silencing and miRNA
processing defects (13–16).
Gastric cancer is one of the most common causes of
death from cancer among men and women worldwide (17). To date, we and many other
researchers have identified a wide variety of tumor-suppressor
genes and other tumor-related genes that are silenced by abnormal
DNA methylation in gastric cancer (18,19).
In addition, it was recently shown that epigenetic mechanisms are
involved in the alteration of several miRNA genes in gastric cancer
(18,20), although little is known concerning
the dysregulation of miRNAs in this disease. Previously, several
novel DNA methylation-regulated miRNAs were classified in colon
cancer cells by combining genome-wide DNA methylation and miRNA
expression analyses (21). In the
present study, we aimed to test the epigenetic silencing of three
miRNAs (MIR219.2, MIR663B and MIR1237) in
gastric cancer and evaluate the phenotypic impact of such
epigenetic silencing on gastric cancer.
In short, we found MIR219.2, MIR663B
and MIR1237 to harbor dense DNA methylation in gastric
cancer cell lines and that such aberrant DNA hypermethylation
correlated with their transcriptional silencing as well as
re-expression after 5-aza-2′-deoxycytidine (5-aza-dC) treatment. We
also determined that ectopic expression of MIR219.2,
MIR663B and MIR1237 resulted in important biological
roles, such as a decrease in cell proliferation, migration and
invasion, suggesting that these miRNAs have a tumor-suppressive
role in gastric cancer by decreasing epithelial-to-mesenchymal
transition (EMT)-associated factors. In addition, we retrieved
several target genes regulated by MIR219.2, MIR663B
and MIR1237 using TargetScan and miRBase databases. Finally,
we identified the candidate target genes regulated by
MIR219.2, MIR663B and MIR1237, suggesting that
epigenetic silencing of these miRNAs may be responsible for some
important tumor-suppressive characteristics of gastric cancer
cells.
Materials and methods
Cell culture and 5-aza-dC treatments
Five human gastric cancer cell lines (AGS, AZ521,
KATO III, NCI-N87 and SNU-1) were used in this study and were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA) and Korea Research Institute of Bioscience and
Biotechnology BioResource Center in Korea. The AGS cell line was
cultured using Ham’s F-12, AZ521 was maintained in MEM medium, KATO
III was grown in Iscove’s modified Dulbecco’s medium (IMDM), and
NCI-N87 and SNU-1 were propagated in RPMI-1640 medium (all from
WelGene, Daegu, Korea). All cell culture media were supplemented
with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA) and 1%
antibiotic-antimycotic (Gibco, Grand Island, NY, USA). All cell
lines were incubated at 37°C with 20% O2 and 5%
CO2. To investigate the effect of 5-aza-dC treatments,
cells were treated with 5 μM 5-aza-dC (Sigma, St. Louis, MO,
USA) for 72 h.
Tissue samples
The biospecimens and data used in this study were
provided by the Inje Biobank of Inje University Busan Paik
Hospital, a member of the Korea Biobank Network.
DNA methylation analyses
Primer pairs for methylation analysis were
preferentially designed near the putative transcription start site
(TSS) in the 5′ CpG islands of the genes. All methylation-specific
primers were designed by MethPrimer (http://www.urogene.org/methprimer). Methylation
analyses, including methylation-specific PCR (MSP) and bisulfite
sequencing analysis, were performed as previously described
(18).
Quantitative real-time RT-PCR
(qRT-PCR)
Total RNA was isolated from human gastric cancer
cell lines using TRI-Solution (Bioscience Technology, Kyungsan,
Korea) following the manufacturer’s instructions. RNA
quantity was measured using a NanoDrop 2000/2000c instrument
(Thermo Scientific, Rockford, IL, USA), and 1 μg of RNA was
reverse-transcribed into cDNA using the iScript cDNA Synthesis kit
(Bio-Rad, Hercules, CA, USA). For expression studies using RT-PCR,
primers were designed using the Open Access Primer3 program
(http://frodo.wi.mit.edu/primer3).
Quantitative RT-PCR was performed on a C1000 Thermal Cycler
(BioRad) using the PCR primers we specifically designed. The
expression levels of pri-miRNAs and target genes were normalized to
β-actin levels. ΔΔCt method was used for the relative
quantification of expression.
miRNA transfection
To ectopically express MIR219.2,
MIR663B and MIR1237 in the AGS gastric cancer cell
line, cells were transfected with 20 nM hsa-miR-219a-2-3p
(MSY0004675), hsa-miR-663b (MSY0005867) and hsa-miR-1237
(MSY0005592) miScript mimics, or AllStars Negative Control siRNA
(1027281) (all from Qiagen, GmbH, Hilden, Germany) using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the
manufacturer’s instructions.
Cell proliferation and viability
assays
Cell proliferation was analyzed using the MTT assay.
At 24 h after transfection of miRNA mimics or the negative control,
AGS cells (2×105 cells/well) were re-plated in 6-well
plates and incubated at 37°C. After 72 h, the cells were washed
twice with phosphate-buffered saline (PBS) and 5 mg/ml MTT in PBS
was added to each well for 4 h. After removing the MTT solution, a
solubilization solution (DMSO/EtOH, 1:1 ratio) was added to each
well to dissolve the formazan crystals. The absorbance at 570 nm
was measured using a Paradigm microplate reader (Beckman Coulter,
Fullerton, CA, USA). After re-plating (5×103 cells/well)
in 6-well plates, cell numbers were counted at 24, 48, 72 and 96
h.
Wound-healing assay
Mimic or control siRNA-transfected AGS cells were
plated overnight to achieve a subconfluent cell monolayer in 6-well
plates. Then, a scratch was made in the cell layer with a sterile
200-μl pipette tip, and cultures were washed twice with
serum-free medium to remove floating cells. Cells were incubated in
culture medium. After 16 h, wound healing was visualized by
comparing images using a QImaging QIClick camera system mounted on
a phase-contrast Nikon TS100 microscope (Nikon, Melville, NY, USA).
The distance traveled by the cells was determined by measuring the
wound width at 16 h and subtracting it from the wound width at 0
h.
Migration and invasion assays
Cell migration was determined using Transwell plates
(24-well, 8-μm pore size; Corning Costar, Rochester, NY,
USA) and the invasion assay was carried out using a Matrigel-coated
invasion chamber (24-well, 8-μm pore size; Corning Costar).
The upper chamber contained cells in specific medium with 1% FBS,
and the lower chamber contained medium with 10% FBS. Cells were
incubated for 16 h at 37°C with 20% O2 and 5%
CO2. Non-migratory or non-invasive cells were scraped
off the upper membrane with a cotton swab. Migratory or invasive
cells remaining on the bottom membrane were counted after staining
with Giemsa (Sigma). Images were captured using a QIcam image
camera system mounted on a Nikon Eclipse 80i microscope
(Nikon).
Western blot analysis
Total cell lysates (20 μg) were separated by
SDS-PAGE and transferred to PVDF membranes (GE Healthcare Life
Sciences, Piscataway, NJ, USA). The membranes were blocked with 5%
milk dissolved in Tris-buffered saline containing 0.02% Tween-20
and incubated overnight at 4°C with specific primary antibodies.
The membranes were subsequently incubated with specific horseradish
peroxidase-conjugated secondary antibodies. Protein bands were
visualized using a Fusion FX5 system (Vilber Lourmat, Eberhardzell,
Germany). The following primary antibodies were used:
anti-E-cadherin, anti-N-cadherin, anti-Slug and anti-vimentin (all
from Santa Cruz Biotechnology, Santa Cruz, CA, USA) and
anti-β-actin (Sigma) antibodies.
Bioinformatic analysis of miRNA target
genes
The miRNA target genes were collected from miRBase
(http://www.miRbase.org) and TargetScan Human
Release 6.2 (http://www.targetscan.org). Sequences of miRNAs were
aligned using the BioEdit program (http://www.mbio.ncsu.edu/bioedit/bioedit.html), and,
using the University of California Santa Cruz (UCSC) Genome Browser
(http://genome.ucsc.edu) and BLAT search, genome
locations and matches with CpG islands were analyzed. To identify
the functions and signaling pathways of the target genes, the
super-pathway categories within the GeneCards database (http://www.genecards.org/cgi-bin), which included
Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.kegg.jp/kegg/pathway.html),
were used. Gene interaction analyses of miRNA target genes were
performed using the GeneMANIA web tool (http://www.genemania.org) and visualized by Cytoscape
ver. 3.0.2. Output of the GeneMANIA search followed query-dependent
weighting options.
Statistical analyses
Quantified data are expressed as the mean ± standard
deviation (SD) values. Significance testing was conducted via
Student’s t-test.
Results
Expression of MIR219.2, MIR663B and
MIR1237 are regulated by DNA hypermethylation in gastric cancer
cell lines
To investigate whether pri-MIR219.2,
pri-MIR663B and pri-MIR1237 were regulated by DNA
methylation in gastric cancer, we treated AGS, AZ521, N87, KATO III
and SNU-1 gastric cancer cells with the demethylating agent
5-aza-dC to determine whether these miRNAs are re-expressed after
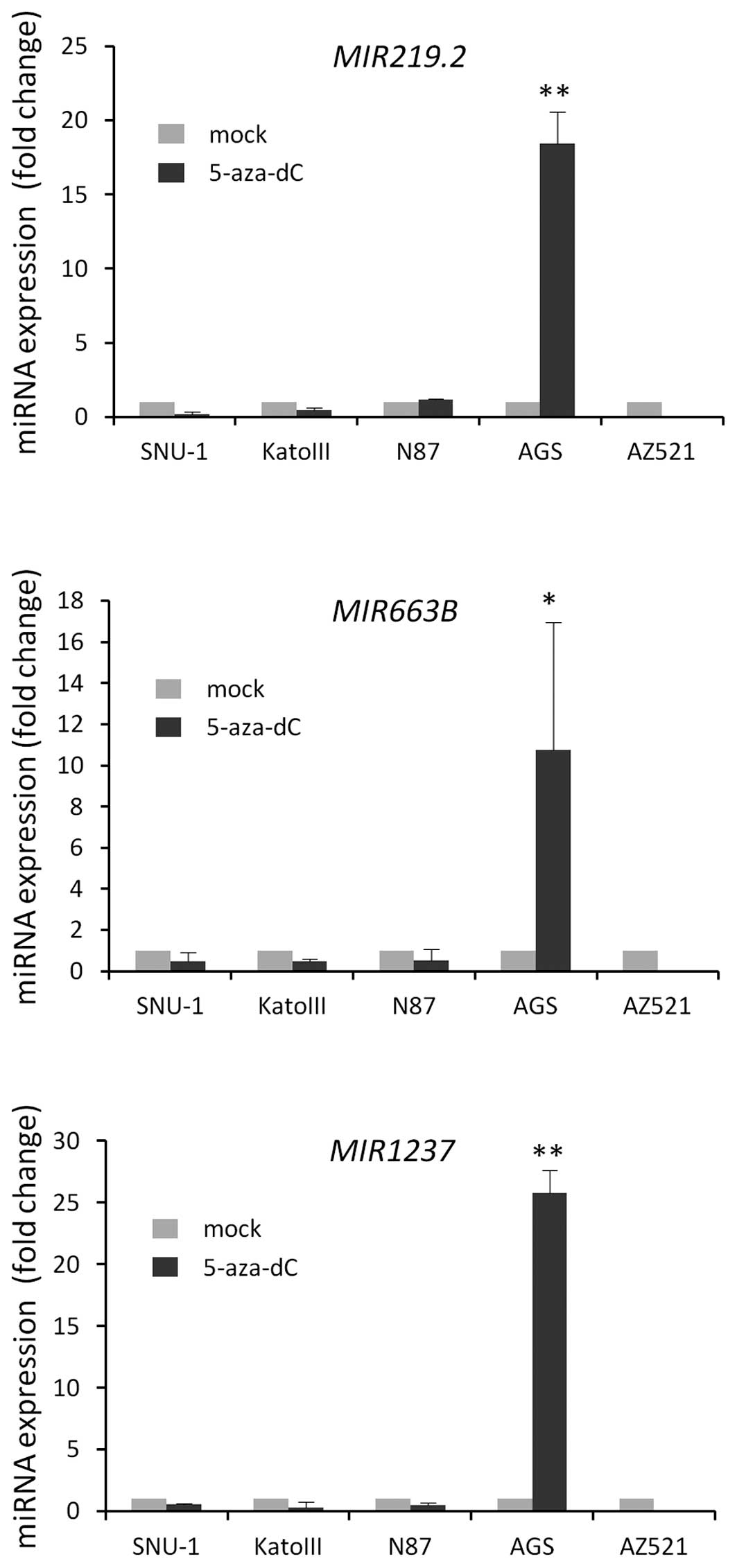
5-aza-dC treatment by qRT-PCR.
Notably, we observed the re-expression of three
miRNAs after 5-aza-dC treatment in only AGS cells; we did not
observe any significant re-expression of these miRNAs after
5-aza-dC treatment in the other cell lines that were tested
(Fig. 1). To verify whether these
miRNAs are upregulated by DNA methylation changes, we assessed the
level of DNA methylation in the proximal region of these mRNAs by
MSP in gastric cancer cell lines and bisulfite genomic sequencing
in AGS cells.
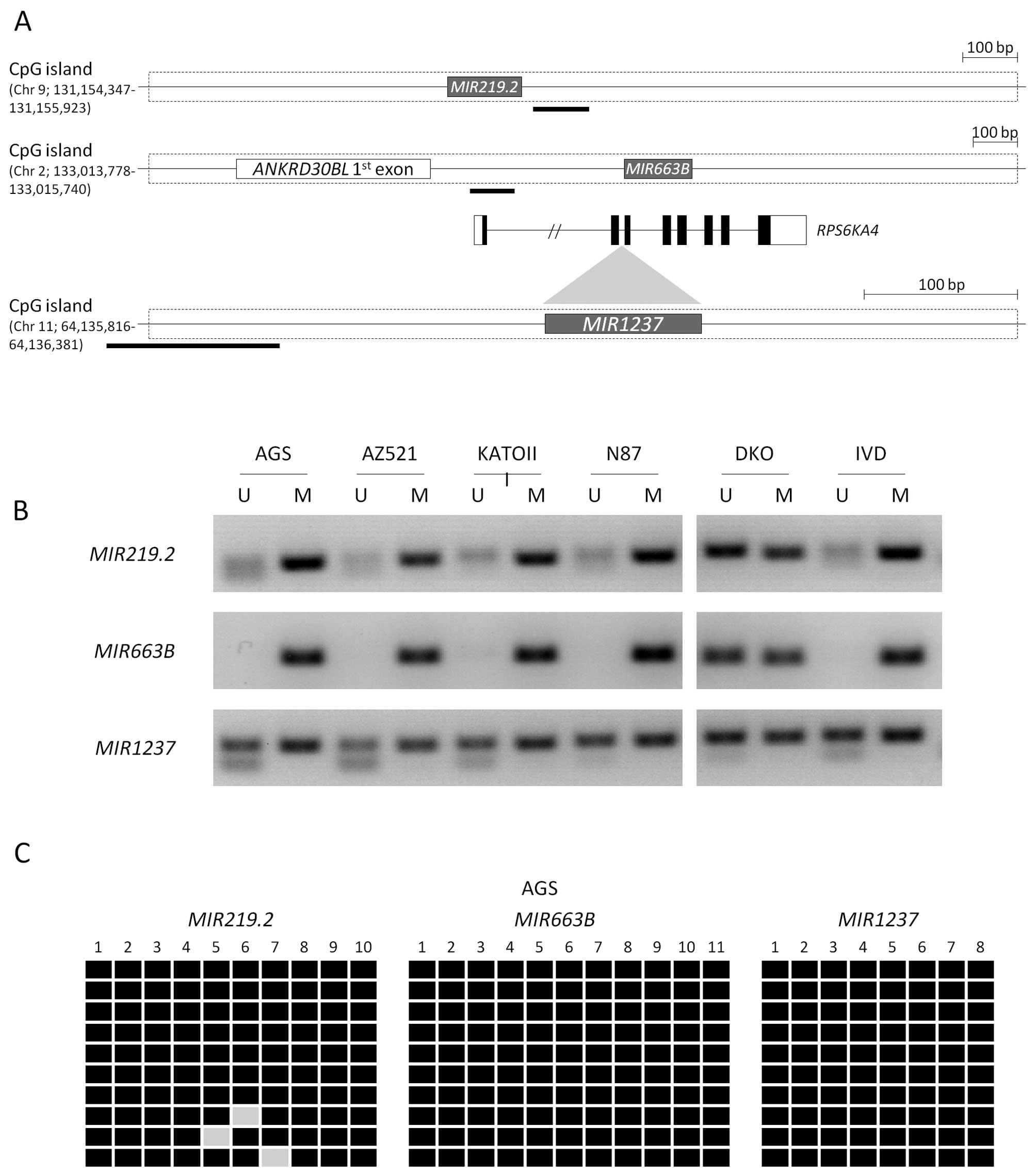
Using specific primers in their CpG island region
for methylation analyses (Fig. 2A),
we evaluated the methylation patterns in five different gastric
cancer cell lines. MIR219.2 and MIR663B were
completely methylated in most of the gastric cancer cell lines that
were tested. MIR1237 was mostly methylated with a partial
methylation pattern compared with the other miRNAs (Fig. 2B). However, we confirmed that these
miRNAs are mostly methylated in gastric cancer cell lines,
suggesting that these data correlate with the re-expression of
miRNAs by 5-aza-dC treatment. We also confirmed the methylation
status in the AGS cell line by bisulfite sequencing analysis,
showing that most CpG sites in these miRNAs were methylated
(Fig. 2C). To further detect
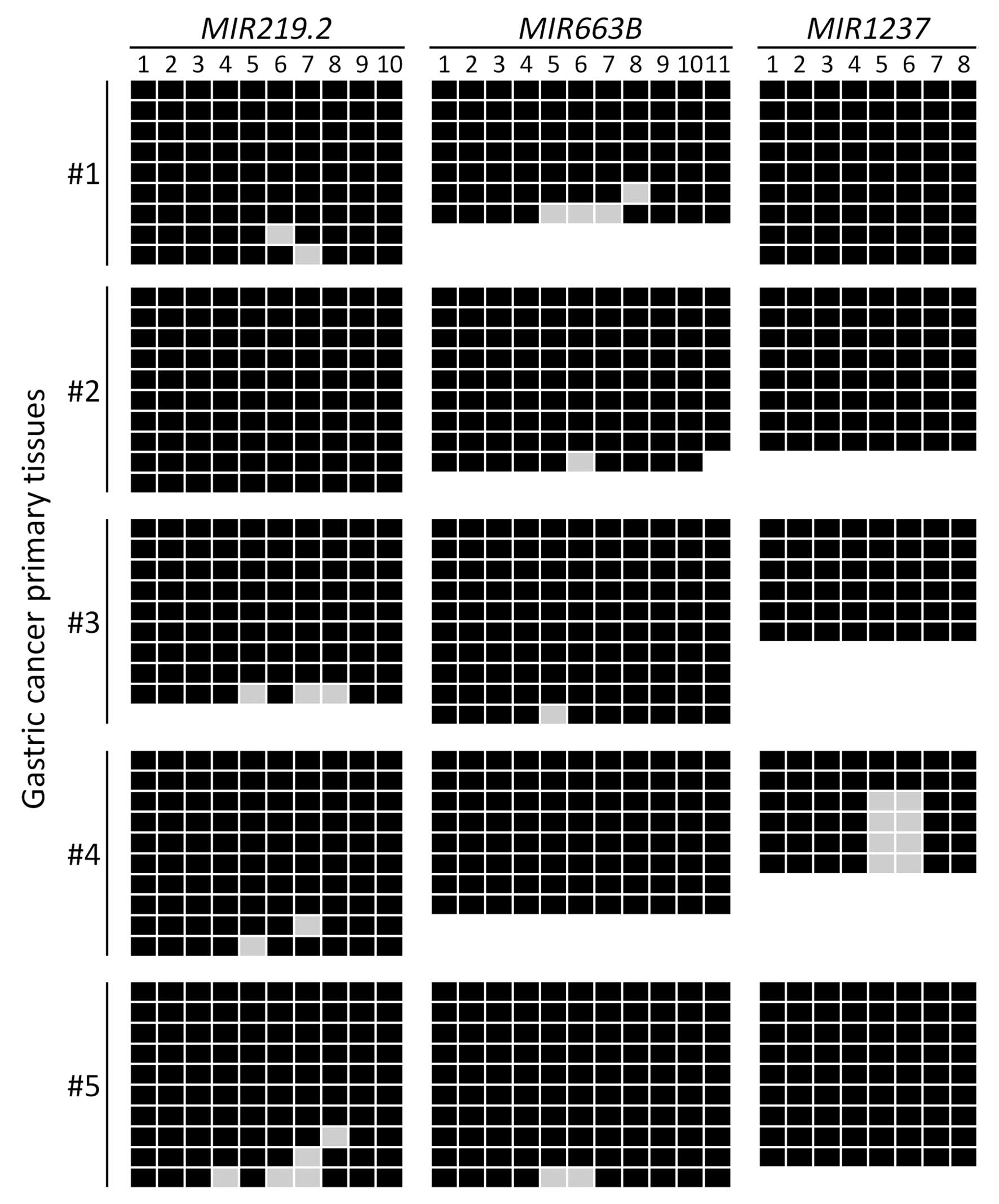
whether MIR219.2, MIR663B and MIR1237 are
methylated in gastric cancer patient tissues, we examined the
methylation status of these miRNAs in five gastric cancer primary
tissues using bisulfite sequencing analysis. We observed that
MIR219.2, MIR663B and MIR1237 were highly
methylated in the gastric cancer primary tissues with 95%
(MIR219.2 and MIR663B) and 83% (MIR1237) of
the analyzed CpG sites methylated (Fig.
3). These results suggest that MIR219.2, MIR663B
and MIR1237 are densely methylated in gastric cancer cells
correlated with transcriptional silencing as well as they are also
highly methylated in cancer specimens.
Functional analysis of MIR219.2, MIR663B
and MIR1237 in gastric cancer cells
We next tested the biological functions of these
epigenetically regulated miRNAs to understand the relevance of
their DNA methylation in gastric cancer cells. To determine whether
expression of these epigenetically silenced miRNAs would affect
cancer cell growth and migration, we transfected AGS cells with
miRNA mimics of MIR219.2, MIR663B, MIR1237 and
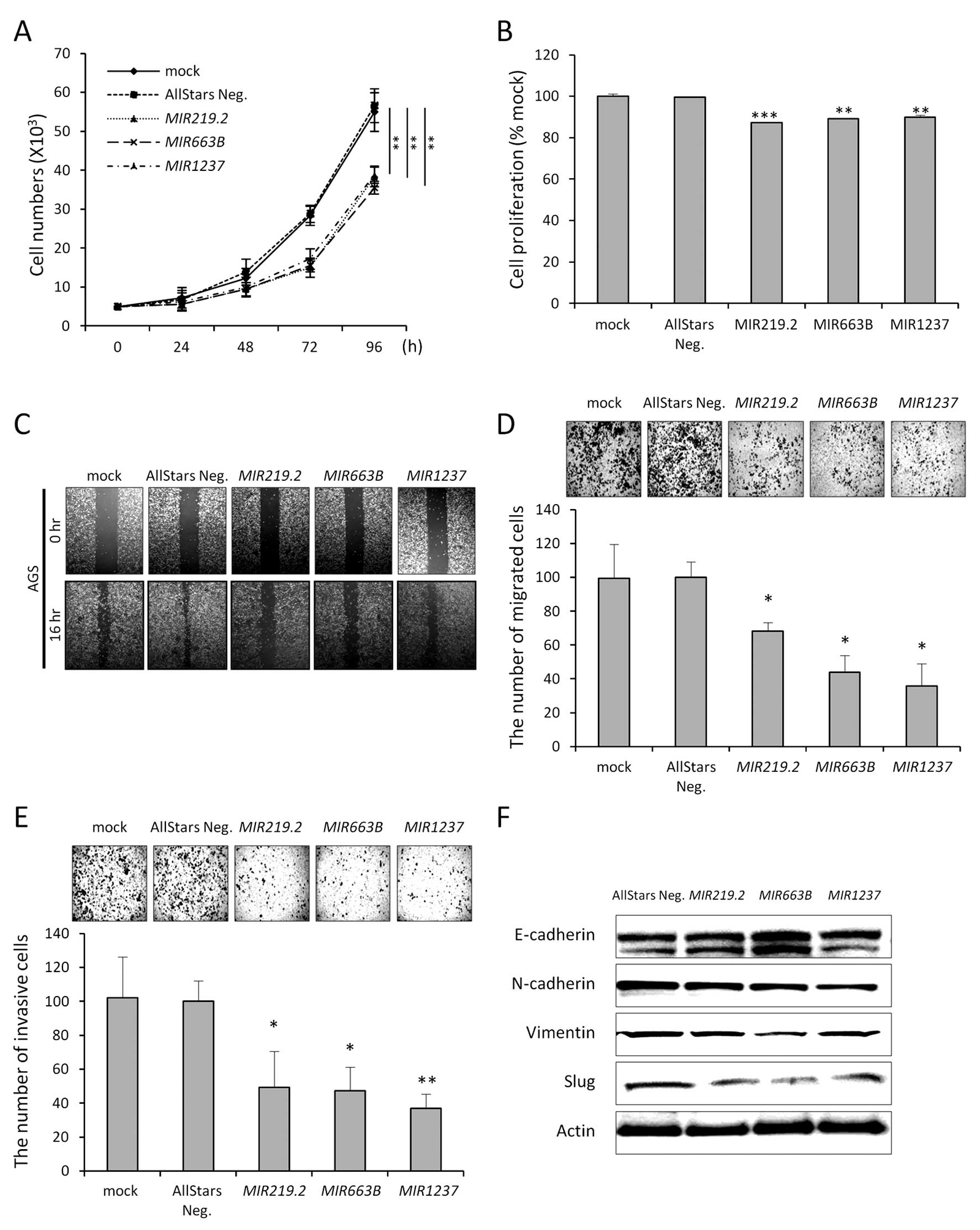
a non-targeting negative control. Growth curve analysis showed that
AGS cell growth was inhibited by ~63% (MIR219.2), 68
(MIR663B) and 69% (MIR1237) compared to the control
(Fig. 4A). Along with growth data,
MTT assay demonstrated a 13, 11 and 10% decrease in proliferation
following MIR219.2, MIR663B and MIR1237 mimic
transfection compare to the control, respectively (Fig. 4B).
Based on the growth curve, we postulated that
ectopic expression of MIR219.2, MIR663B and
MIR1237 would impact cell migration or invasion. In the
wound-healing assays, AGS cells transfected with each miRNA mimic
showed less wound closure compared with the mock-treated or control
cells (Fig. 4C). These results were
further confirmed using Transwell migration and invasion assays
(Fig. 4D and E). These data
indicate that ectopic expression of these miRNAs could affect
actual migration- or invasion-associated molecules in cancer. Tumor
cell invasion is involved with the loss of cell-cell interaction
together with the acquisition of migratory properties and is often
associated with EMT (22).
Therefore, we next examined whether ectopic
expression of these miRNAs suppresses the migratory and invasive
properties of gastric cancer cells through disturbing EMT. Ectopic
expression of miRNAs in the AGS cells decreased mesenchymal cell
markers, such as N-cadherin and vimentin, while increasing
epithelial cell marker E-cadherin in the AGS cells. These EMT
markers are directly regulated by EMT transcription factors, such
as Slug. Notably, Slug expression was markedly decreased by ectopic
expression of MIR219.2, MIR663B and MIR1237
(Fig. 4F). These data are
consistent with the migration and invasion data and strongly
support the hypothesis that miRNAs could have a tumor-suppressive
effect in gastric cancer by suppressing mesenchymal traits of
cancer.
Identification of target genes regulated
by MIR219.2, MIR663B and MIR1237
miRNAs modulate gene expression by interacting with
their target mRNAs, resulting in mRNA degradation or translational
repression. To help us identify potential target genes of
MIR219.2, MIR663B and MIR1237 in gastric
cancer, target genes were predicted using TargetScan Human 6.2 and
miRBase. For MIR219.2, MIR663B and MIR1237,
156, 136 and 299 target genes were predicted, respectively. Since
many target genes were identified, we selected several common genes
for the three miRNAs (Table I).
Therefore, to validate whether these candidate target genes are
regulated by miRNAs, we analyzed the mRNA levels of these target
genes using qRT-PCR in the AGS cells upon transfection of the miRNA
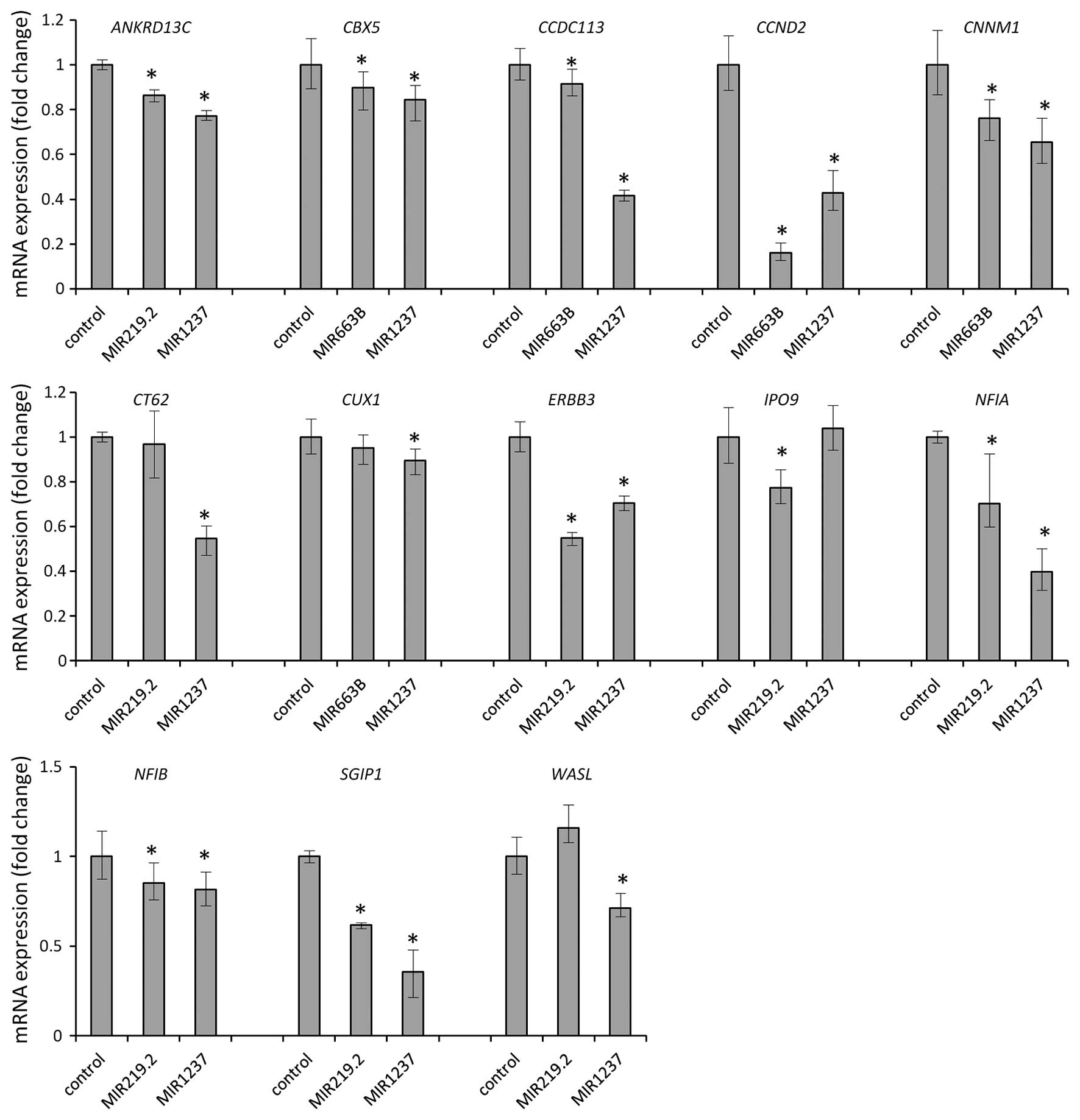
mimics. We found that MIR219.2, MIR663B and
MIR1237 mimics consistently depleted mRNA levels of
ANKRD13C, CBX5, CCDC113, CCND2,
CNNM1, CT62, CUX1, ERBB3, IPO9,
NFIA, NFIB, SGIP1 and WASL (Fig. 5). After we validate this, we
identified 13 candidates as bona fide targets of MIR219.2,
MIR663B and MIR1247 in gastric cancer. All 13
candidate genes were downregulated by ectopic expression of
MIR219.2, MIR663B and MIR1247 in the AGS cells
which suggest that expression of the target genes we identified is
regulated by these miRNAs.
 | Table ITarget gene list for MIR219-2,
MIR663B and MIR1237. |
Table I
Target gene list for MIR219-2,
MIR663B and MIR1237.
| Gene | miRNA
| Accession no. | Function |
|---|
| 219.2 | 663B | 1237 |
|---|
|
ANKRD13C | V | | V | NM_030816.4 | Ankyrin repeat
domain 13C |
|
C20orf112 | | V | V | NM_001256798.1;
NM_080616.4 | Chromosome 20 open
reading frame 112 |
| CBX5 | | V | V | NM_001127321.1;
NM_001127322.1; NM_012117.2 | Chromobox homolog
5 |
| CCDC113 | | V | V | NM_001142302.1;
NM_014157.3 | Coiled-coil domain
containing 113 |
| CCND2 | | V | V | NM_001759.3 | Cyclin D2 |
| CLCN5 | | V | V | NM_000084.4;
NM_001127898.3; NM_001127899.3; NM_001272102.1; NM_001282163.1 | Chloride channel,
voltage-sensitive 5 |
| CNNM1 | | V | V | NM_020348.2 | Cyclin M1 |
| CT62 | V | | V | NM_001102658.1 | Cancer/Testis
antigen 62 |
| CUX1 | | V | V | NM_001202543.1;
NM_001202544.1; NM_001202545.1; NM_001202546.1; NM_001913.3;
NM_181500.2; NM_181552.3 | Cut-like homeobox
1 |
| DCC | V | | V | NM_005215.3 | Deleted in
colorectal carcinoma |
| DCX | V | V | | NM_000555.3;
NM_001195553.1; NM_178151.2; NM_178152.2; NM_178153.2 | Doublecortin |
| ERBB3 | V | | V | NM_001005915.1;
NM_001982.3 | V-Erb-B2 avian
erythroblastic leukemia viral oncogene homolog 3 |
| IPO9 | V | | V | NM_018085.4 | Importin 9 |
| NFIA | V | | V | NM_001134673.3;
NM_001145511.1; NM_001145512.1; NM_005595.4 | Nuclear factor
I/A |
| NFIB | V | | V | NM_001190737.1;
NM_001190738.1; NM_001282787.1; NM_005596.3 | Nuclear factor
I/B |
| NPEPPS | V | | V | NM_006310.3 | Aminopeptidase
puromycin sensitive |
| PMEPA1 | V | | V | NM_001255976.1;
NM_020182.4; NM_199169.2; NM_199170.2; NM_199171.2 | Prostate
transmembrane protein, androgen induced 1 |
| PTPRT | V | | V | NM_007050.5;
NM_133170.3 | Protein tyrosine
phosphatase, receptor type, T |
| PURB | V | | V | NM_033224.4 | Purine-rich element
binding protein B |
| RC3H1 | V | | V | NM_172071.2 | Ring finger and
CCCH-type domains 1 |
| SERP1 | V | | V | NM_014445.3 | Stress-associated
endoplasmic reticulum protein 1 |
| SGIP1 | V | | V | NM_032291.2 | SH3-domain
GRB2-like (endophilin) interacting protein 1 |
| SMC1A | | V | V | NM_001281463.1;
NM_006306.3 | Structural
maintenance of chromosomes 1A |
| STAT5B | | V | V | NM_012448.3 | Signal transducer
and activator of transcription 5B |
| USP47 | V | V | | NM_001282659.1;
NM_017944.3 | Ubiquitin-specific
peptidase 47 |
| WASL | V | | V | NM_003941.3 | Wiskott-Aldrich
syndrome-like |
| YOD1 | V | | V | NM_001276320.1;
NM_018566.3 | YOD1 OTU
deubiquinating enzyme 1 homolog |
Discussion
miRNAs are important regulatory molecules that
modulate gene expression in both developmental and disease
processes. Their regulation is, therefore, a critical component in
understanding each biological context (23). Dysregulation of miRNA expression is
commonly observed in a wide variety of cancers, and epigenetic
mechanisms have been shown to be key mediators underlying the
downregulation of miRNA expression. To screen for epigenetically
silenced miRNA genes in gastric cancer, we first performed a
microarray analysis to identify miRNAs upregulated by demethylation
and histone deacetylase inhibition. There are several miRNAs that
have been found to be regulated by DNA hypermethylation and could
have tumor-suppressive roles in various cancers.
In recent years, accumulated evidence that the
transcription of a handful of miRNAs in cancer is regulated by DNA
methylation has been reported. For example, MIR127 has been
found to be activated by epigenetic drug treatment in cancer cells
(15). In addition, methylation of
MIR9 and MIR148a has been observed in human
metastatic cancer cell lines (24),
and MIR203 is epigenetically silenced in hematopoietic
malignancies, which leads to enhanced expression of ABL1 and
BCR-ABL1 (25). Recently,
MIR34a reportedly acts as a tumor suppressor in colon cancer
and neuroblastoma (25,26). All three MIR34 family members
are directly regulated by p53, and ectopic expression of
MIR34s induces cell cycle arrest and/or apoptosis in human
cancer cells (5,27,28).
Conversely, expression of MIR34s is frequently
down-regulated in human malignancies, including lung, colon and
ovarian cancers (26-29). Very recently we also first reported
that transcription of MIR941, MIR1247 and their
target genes are regulated by DNA methylation in gastric cancers
(18). In addition, Lujambio et
al (24) identified
MIR34b/c methylation by screening cell lines derived from
metastatic colon cancer, melanoma, and head and neck cancer, and
Corney et al (28) recently
reported downregulation of MIR34b/c in metastatic ovarian
cancer, which suggests that inactivation of MIR34b/c may be
associated with cancer metastasis.
In the present study, ectopic expression of
MIR219.2, MIR663B and MIR1237 reduced cell
growth, proliferation, migration and invasion, suggesting that
these miRNAs have tumor-suppressive effects in gastric cancer
cells. Moreover, we support more strongly a tumor-suppressive role
as evidenced by decreased mesenchymal phenotypes using mesenchymal
cell markers, such as N-cadherin and vimentin.
We predicted target genes using the TargetScan and
miRBase databases and found 13 candidate genes that were validated
by qRT-PCR among the more than 100 predicted target genes. These 13
genes could be potential targets of MIR219.2, MIR663B
and MIR1237. Although these genes were among the predicted
targets, we were interested in how these genes are associated
within the cellular pathway network. Networks of collected target
genes were analyzed together by GeneMANIA and visualized by
Cytoscape version 3.0.2. Twenty-four target genes were found to be
related to 20 neighbor genes, and there are 170 total interaction
links. In particular, the functions of networks are categorized as
follows: cohesin complex, protein-DNA complex, regulation of anion
transport, chloride channel activity, chromosome condensation,
anion channel activity, chloride transmembrane transporter
activity, excretion, chloride transport and nuclear chromosome
(data not shown). These results support the hypothesis that
selected target genes may play various important roles and are
intimately connected with each other. Therefore, to understand the
possible roles of selected target genes, signal pathways and the
relationship with cancers were predicted using KEGG and GeneCards
databases. These data suggest that target genes of MIR219.2,
MIR663B and MIR1237 are related to each other through
critical cancer pathways; therefore, further study is necessary to
understand the biological interactions between miRNAs and target
genes in cancer.
Among the candidate target genes of MIR219.2
and MIR1237, the receptor tyrosine kinase ERBB3
(epidermal growth factor receptor family) has been reported to be
targeted by several miRNAs (MIR199a/125b, MIR143 and
MIR145) in various types of cancer (30-32).
High levels of ERBB3 are strongly associated with tumor progression
and poor outcome in gastric cancer patients (33,34).
CCND2 (cyclin D2) is known as a proto-oncogene, and
overexpression of CCND2 is closely correlated with
progression in gastric cancer (35,36).
Similar to our study, CCND2 was found to be downregulated by
MIR1, and then cell proliferation, migration and invasion
were reduced in thyroid cancer carcinoma cells (37). In addition, CCND2 is also
targeted by MIR206 and MIR29, which results in the
suppression of cell growth in gastric cancer (38,39).
In summary, we demonstrated that MIR219.2,
MIR663B and MIR1237 are silenced in gastric cancer
cell lines by DNA hypermethylation. Our expression and functional
studies suggest that ectopic expression of these miRNAs has
tumor-suppressive effects in the AGS gastric cancer cell line by
decreasing mesenchymal phenotypes. Moreover, their molecular
targets are associated with multiple biological pathways according
to our bioinformatic analyses. Therefore, our functional data on
MIR219.2, MIR663B and MIR1237 target genes is
a compelling lead, but the detailed relationships between many more
target genes and the three miRNAs will require further study. More
importantly, we demonstrated that such abnormal epigenetic
silencing occurs in gastric cancer and may be useful in developing
new potential therapeutic tools for this disease.
Acknowledgments
This study was supported by the National R&D
Program (nos. 50591-2015) through the Dongnam Institute of
Radiological and Medical Sciences (DIRAMS) funded by the Ministry
of Science, ICT and Future Planning (MSIP) of the Korean
government.
References
|
1
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He L, He X, Lim LP, de Stanchina E, Xuan
Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dalmay T and Edwards DR: MicroRNAs and the
hallmarks of cancer. Oncogene. 25:6170–6175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takamizawa J, Konishi H, Yanagisawa K,
Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y,
et al: Reduced expression of the let-7 microRNAs in human lung
cancers in association with shortened postoperative survival.
Cancer Res. 64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoon SO, Chun SM, Han EH, Choi J, Jang SJ,
Koh SA, Hwang S and Yu E: Deregulated expression of microRNA-221
with the potential for prognostic biomarkers in surgically resected
hepa-tocellular carcinoma. Hum Pathol. 42:1391–1400. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mraz M and Pospisilova S: MicroRNAs in
chronic lymphocytic leukemia: From causality to associations and
back. Expert Rev Hematol. 5:579–581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and downregulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:15524–15529. 2002. View Article : Google Scholar
|
|
14
|
Calin GA, Ferracin M, Cimmino A, Di Leva
G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, et
al: A MicroRNA signature associated with prognosis and progression
in chronic lymphocytic leukemia. N Engl J Med. 353:1793–1801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saito Y, Liang G, Egger G, Friedman JM,
Chuang JC, Coetzee GA and Jones PA: Specific activation of
microRNA-127 with down-regulation of the proto-oncogene BCL6 by
chromatin-modifying drugs in human cancer cells. Cancer Cell.
9:435–443. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakamura T, Canaani E and Croce CM:
Oncogenic All1 fusion proteins target Drosha-mediated microRNA
processing. Proc Natl Acad Sci USA. 104:10980–10985. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001. View Article : Google Scholar
|
|
18
|
Kim JG, Kim TO, Bae JH, Shim JW, Kang MJ,
Yang K, Ting AH and Yi JM: Epigenetically regulated MIR941 and
MIR1247 target gastric cancer cell growth and migration.
Epigenetics. 9:1018–1030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ushijima T, Nakajima T and Maekita T: DNA
methylation as a marker for the past and future. J Gastroenterol.
41:401–407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ando T, Yoshida T, Enomoto S, Asada K,
Tatematsu M, Ichinose M, Sugiyama T and Ushijima T: DNA methylation
of microRNA genes in gastric mucosae of gastric cancer patients:
its possible involvement in the formation of epigenetic field
defect. Int J Cancer. 124:2367–2374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan H, Choi AJ, Lee BH and Ting AH:
Identification and functional analysis of epigenetically silenced
microRNAs in colorectal cancer cells. PLoS One. 6:e206282011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Esquela-Kerscher A and Slack FJ: OncomiRs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lujambio A, Calin GA, Villanueva A, Ropero
S, Sánchez-Cé-spedes M, Blanco D, Montuenga LM, Rossi S, Nicoloso
MS, Faller WJ, et al: A microRNA DNA methylation signature for
human cancer metastasis. Proc Natl Acad Sci USA. 105:13556–13561.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Welch C, Chen Y and Stallings RL:
MicroRNA-34a functions as a potential tumor suppressor by inducing
apoptosis in neuro-blastoma cells. Oncogene. 26:5017–5022. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tazawa H, Tsuchiya N, Izumiya M and
Nakagama H: Tumor-suppressive MIR34a induces senescence-like growth
arrest through modulation of the E2F pathway in human colon cancer
cells. Proc Natl Acad Sci USA. 104:15472–15477. 2007. View Article : Google Scholar
|
|
27
|
Bommer GT, Gerin I, Feng Y, Kaczorowski
AJ, Kuick R, Love RE, Zhai Y, Giordano TJ, Qin ZS, Moore BB, et al:
p53-mediated activation of miRNA34 candidate tumor-suppressor
genes. Curr Biol. 17:1298–1307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Corney DC, Flesken-Nikitin A, Godwin AK,
Wang W and Nikitin AY: microRNA-34b and microRNA-34c are targets of
p53 and cooperate in control of cell proliferation and
adhesion-independent growth. Cancer Res. 67:8433–8438. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Toyota M, Suzuki H, Sasaki Y, Maruyama R,
Imai K, Shinomura Y and Tokino T: Epigenetic silencing of
microRNA-34b/c and B-cell translocation gene 4 is associated with
CpG island meth-ylation in colorectal cancer. Cancer Res.
68:4123–4132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He J, Xu Q, Jing Y, Agani F, Qian X,
Carpenter R, Li Q, Wang XR, Peiper SS, Lu Z, et al: Reactive oxygen
species regulate ERBB2 and ERBB3 expression via miR-199a/125b and
DNA methylation. EMBO Rep. 13:1116–1122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lei H, Zou D, Li Z, Luo M, Dong L, Wang B,
Yin H, Ma Y, Liu C, Wang F, et al: MicroRNA-219–2–3p functions as a
tumor suppressor in gastric cancer and is regulated by DNA
methylation. PLoS One. 8:e603692013. View Article : Google Scholar
|
|
32
|
Yan X, Chen X, Liang H, Deng T, Chen W,
Zhang S, Liu M, Gao X, Liu Y, Zhao C, et al: miR-143 and miR-145
synergistically regulate ERBB3 to suppress cell proliferation and
invasion in breast cancer. Mol Cancer. 13:2202014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hayashi M, Inokuchi M, Takagi Y, Yamada H,
Kojima K, Kumagai J, Kawano T and Sugihara K: High expression of
HER3 is associated with a decreased survival in gastric cancer.
Clin Cancer Res. 14:7843–7849. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Begnami MD, Fukuda E, Fregnani JH,
Nonogaki S, Montagnini AL, da Costa WL Jr and Soares FA: Prognostic
implications of altered human epidermal growth factor receptors
(HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor
outcome. J Clin Oncol. 29:3030–3036. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oshimo Y, Nakayama H, Ito R, Kitadai Y,
Yoshida K, Chayama K and Yasui W: Promoter methylation of cyclin D2
gene in gastric carcinoma. Int J Oncol. 23:1663–1670.
2003.PubMed/NCBI
|
|
36
|
Takano Y, Kato Y, Masuda M, Ohshima Y and
Okayasu I: Cyclin D2, but not cyclin D1, overexpression closely
correlates with gastric cancer progression and prognosis. J Pathol.
189:194–200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leone V, D’Angelo D, Rubio I, de Freitas
PM, Federico A, Colamaio M, Pallante P, Medeiros-Neto G and Fusco
A: MiR-1 is a tumor suppressor in thyroid carcinogenesis targeting
CCND2, CXCR4, and SDF-1alpha. J Clin Endocrinol Metab.
96:E1388–E1398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang L, Liu X, Jin H, Guo X, Xia L, Chen
Z, Bai M, Liu J, Shang X, Wu K, et al: miR-206 inhibits gastric
cancer proliferation in part by repressing cyclinD2. Cancer Lett.
332:94–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gong J, Li J, Wang Y, Liu C, Jia H, Jiang
C, Wang Y, Luo M, Zhao H, Dong L, et al: Characterization of
microRNA-29 family expression and investigation of their
mechanistic roles in gastric cancer. Carcinogenesis. 35:497–506.
2014. View Article : Google Scholar
|