Introduction
Malignant pleural mesothelioma (MPM) are incurable
thoracic malignancy, that has poor prognosis because it is
frequently diagnosed at an advanced stage (1,2). The
worldwide incidence of mesothelioma is expected to increase,
particularly in Europe and Japan (3–5). The
primary cause of MPM is often linked to asbestos exposure, and the
number of patients worldwide is predicted to peak in the next 2
decades (6,7). Investigations for the molecular
pathogenesis of MPM has begun (8–12).
Recent whole-exome sequencing revealed frequent genetic alterations
in BAP1, NF2, CDKN2A and CUL1 in 22 MPMs (13). The latent period between the first
exposure to asbestos and the onset of this disease is ~30 years,
and the first symptom is insidious and may include chest pain and
breathlessness. Many clinical trials including surgery,
radiotherapy, and chemotherapy were reported, but the prognosis of
patients remains poor. Although there was recent progress in
clinical treatment with combination chemotherapies, a curative
therapy for MPM remains unknown; the median survival ranges between
9 and 17 months after diagnosis (14–17).
Combinations of cisplatin and pemetrexed appear to be the best
chemotherapy regimen for MPM. Thus, effective clinical approaches
such as molecular-targeted therapy are needed to treat MPM.
The recently-discovered epithelial NADPH oxidases
(Noxs) mediate critical physiological and pathological processes
including cell signaling, inflammation and mitogenesis by
generating reactive oxygen species (ROS) (18). The Nox enzyme complex was first
described in neutrophils, where it is normally quiescent but
generates a large quantity of ROS upon activation during
phagocytosis and plays a vital role in nonspecific host defense
against ingested pathogens (19,20).
Many non-phagocytic cells contain NADPH oxidases (20). There are 7 identified family members
in the NADPH family: 5 Noxs and 2 dual oxidases (DUOXs) (20). Noxs and the mitochondria are major
sources of cellular ROS (21).
Cancer cells produce ROS that act as signaling molecules to promote
cell survival (22,23). Nox4-mediated ROS inhibit apoptosis
and promote tumor cell growth in pancreatic cancer cells (24,25).
However, our understanding of the roles of the Nox family members
in the development and growth of human cancer is limited (26–30).
We hypothesized that intracellular ROS conferred
anti-apoptotic activity and thus a growth advantage to MPM cells.
In this study, we demonstrated that treatment with diphenylene
iodonium (DPI), a flavoenzyme inhibitor (31) and knockdown of Nox4 suppressed ROS
production in MPM cells, which induced apoptosis, suggesting that
Nox4-generated ROS at least in part, transmits cell survival
signals and provides a useful clinical approach for MPM
treatment.
Materials and methods
Cell culture and materials
Seven MPM cell lines (ACC-MESO-1, ACC-MESO4,
Y-MESO-8A, MSTO-211H, NCI-H28, NCI-H290 and NCI-H2052) and a normal
mesothelial cell line (Met-5A) were kindly provided by Dr Y.
Sekido, Division of Molecular oncology, Aichi Cancer Center
Research Institute. Cells were maintained at 37°C under 5%
CO2 air atmosphere in DMEM culture medium (Sigma, St.
Louis, MO, USA) supplemented with 10% heat-inactivated FBS, 2 mM
L-glutamine, 200 U/ml penicillin and 100 μg/ml streptomycin.
Heparinized peripheral blood was collected from normal individuals
after informed consent was obtained, and PBMCs were separated using
density-gradient centrifugation.
Analysis and quantification of Nox4 mRNA
levels by RT-PCR and real-time PCR
Reverse transcription (RT) was conducted as follows:
8 μl water containing 1 μg total RNA was added to 50
ng random primers (Life Technologies) and incubated at 65°C for 5
min. cDNA was prepared with SuperScript III First-Strand Synthesis
Supermix (Invitrogen, Carsbad, CA, USA) according to the
manufacturer's protocol.
Real-time PCR was performed using SYBR Premix Ex Taq
II (Takara Bio, Otsu, Shiga, Japan), and PCR amplifications were
performed in an ABI PRISM 7500 Sequence Detection System (Applied
Biosystems, Foster City, CA, USA). Briefly, a solution of SYBR
Premix Ex Taq II (10 μl; Takara Bio) containing sense and
antisense primers (10 μM each) was prepared and 2 μl
cDNA was added to a final volume of 20 μl. Conditions for
PCR included 42°C for 5 min, 95°C for 10 sec, and 40 cycles of 95°C
for 5 sec and 60°C for 34 sec. Data were analyzed with Sequencer
Detector version 1.6 software (ABI-PE). The threshold cycle (CT)
during the exponential phase of amplification was determined by
real-time monitoring of fluorescent emission by nuclease activity
of Taq polymerase. β-actin was used as an internal control.
Relative transcripts were determined by the following formula:
1/2(CTtarget − CTcontrol) (32). Specific primers for Noxs 1-5
and β-actin were synthesized (Star Oligo Rikaken, Nagoya,
Japan). PCR primer pairs were as follows: Nox1, sense
5′-AGCGTCTGCTCTCTGCTTGAA-3′ and antisense
5′-GGCTGCAAAATGAGCAGGT-3′; Nox2, sense
5′-TGCCTTTGAGTGGTTTGCAGAT-3′ and antisense
5′-ATTGGCCTGAGACTCATCCCA-3′; Nox3, sense
5′-GAACCCTCGGCTTGGAAAT-3′ and antisense
5′-TGGCTTACCACCTTGGTAATGA-3′; Nox4, sense
5′-CCCTCACAATGTGTCCAACTGA-3′ and antisense
5′-GGCAGAATTTCGGAGTCTTGAC-3′; Nox5, sense
5′-AAGAGTCAAAGGTCGTCCAAGG-3′ and antisense
5′-GCTTTCTTTTCTGGTGCCTGT-3′; β-actin, sense
5′-GATGACCCAGATCATGTTTGAGACC-3′ and antisense
5′-CGGTGAGGATCTTCATGAGGTAGT-3′.
Cell viability assay
The viability of the cells transfected with
Nox4 siRNAs or treated with NAC, DPI, or specific inhibitors
for protein kinases was determined using the MTT assay. MPM cells
(1×103) were incubated with each reagent at each
concentration in triplicate in 96-well culture plates at 37°C in
humidified air with 5% CO2. Three wells contained MPM
cells in drug-free medium to determine the control cell survival
and the percentage of cells after culture. Three wells contained
medium only to blank the spectrophotometer. After 2 days, 10
μl (5 mg/ml) MTT salt was added for 6 h. Formazan production
was quantitated using a spectrophotometer at 562 nm. The optical
density (OD) is linearly related to the cell number. Cell survival
(CS) was calculated at each drug concentration by the equation CS =
(OD treated well/mean OD control wells) × 100%.
Flow cytometric analysis of
apoptosis
To analyze apoptosis, the externalization of
phosphatidylserine was measured by flow cytometric staining with
FITC-conjugated Annexin V (BD Pharmingen). Cells in 6-well plates
(2×105 cells per well) were treated for 48 h, washed,
resuspended in 100 μl Annexin-binding buffer, and stained
with 5 μl Annexin V-FITC and propidium iodide for 20 min.
Flow cytometric analysis was performed using FACSCalibur (BD
Biosciences) and Cell Quest Pro Version 4.0.2 (BD Biosciences)
software. Cells that were positively stained with Annexin V were
counted as apoptotic populations.
Measurement of intracellular ROS
production
Cells (2×105 per well) were seeded in
6-well plates and treated with 10 μM DPI for 48 h or
transfected Nox4 siRNAs. Then, cells were incubated with 2.5
μM of 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA;
Molecular Probes, Eugene, OR, USA) for 30 min at 37°C in the dark,
washed with Hank's buffer, and fixed in 1% paraformaldehyde. The
fluorescence intensity was measured using FACS, with the excitation
source at 488 nm and an emission wavelength of 580 nm. An analysis
was performed with the software program BD FACStationt System Data
Management System (Becton-Dickinson). Background fluorescence from
the blank was subtracted from each reading.
Transfection and immunoblotting
Cells were transfected with Nox4 siRNAs or
scramble siRNAs utilizing Lipofectamine 2000 (Invitrogen) according
to the manufacturer's protocol. siRNAs were designed from the human
Nox4 cDNA sequences as follows (Integrated DNA Technologies,
Coralville, IA, USA): 5′-GCUGAAGUAUCAAACUAUUUAGAT-3′ and
5′-AUCUAAAUUAGUUUGAUACUUCAGCAG-3′ for Nox4RNAi-1, and
5′-GAAUUACAGUGAAGACUUUGUUGAA-3′ and
5′-UUCAACAAAGUCUUCACUGUAAUUCAC-3′ for Nox4RNAi-2. Universal
scrambled siRNA sequences, which have no significant homology to
mouse, rat, or human genome databases, were used as controls
(Invitrogen).
For western blot analysis, equal amounts of reduced
proteins (20 μg) were loaded on 10% Bis-Tris-buffered
polyacrylamide gels. After gel electrophoresis, proteins were
transferred to PVDF membranes (Invitrogen) by electroblotting. The
membranes were preincubated for 1 h in 5% low-fat dried milk in TBS
and 0.1% Tween-20 (TBS-T) to block non-specific binding sites.
After washing with TBS-T, membranes were incubated overnight with a
1:1,000 dilution of primary antibody in TBS-T containing 5% BSA at
4°C (Cell Signaling Technology), and probed with horseradish
peroxidase-conjugated secondary antibody (1:2,000 dilution) for 1 h
at room temperature. The bound antibodies were visualized with the
ECL reaction (GE Healthcare).
Statistical analysis
Data were analyzed by the Welch t-test, Fisher's
exact-test, or ANOVA using Statview software (SAS, Cary, NC, USA),
and P-values at <0.05 were considered to be statistically
significant.
Results
Inhibition of cell growth, suppression of
ROS generation, and induction of apoptosis by antioxidants
The flavoenzyme inhibitor, DPI inhibits
membrane-bound, flavoprotein-containing Noxs. We examined whether
the antioxidant NAC and the flavoenzyme inhibitor DPI, affected the
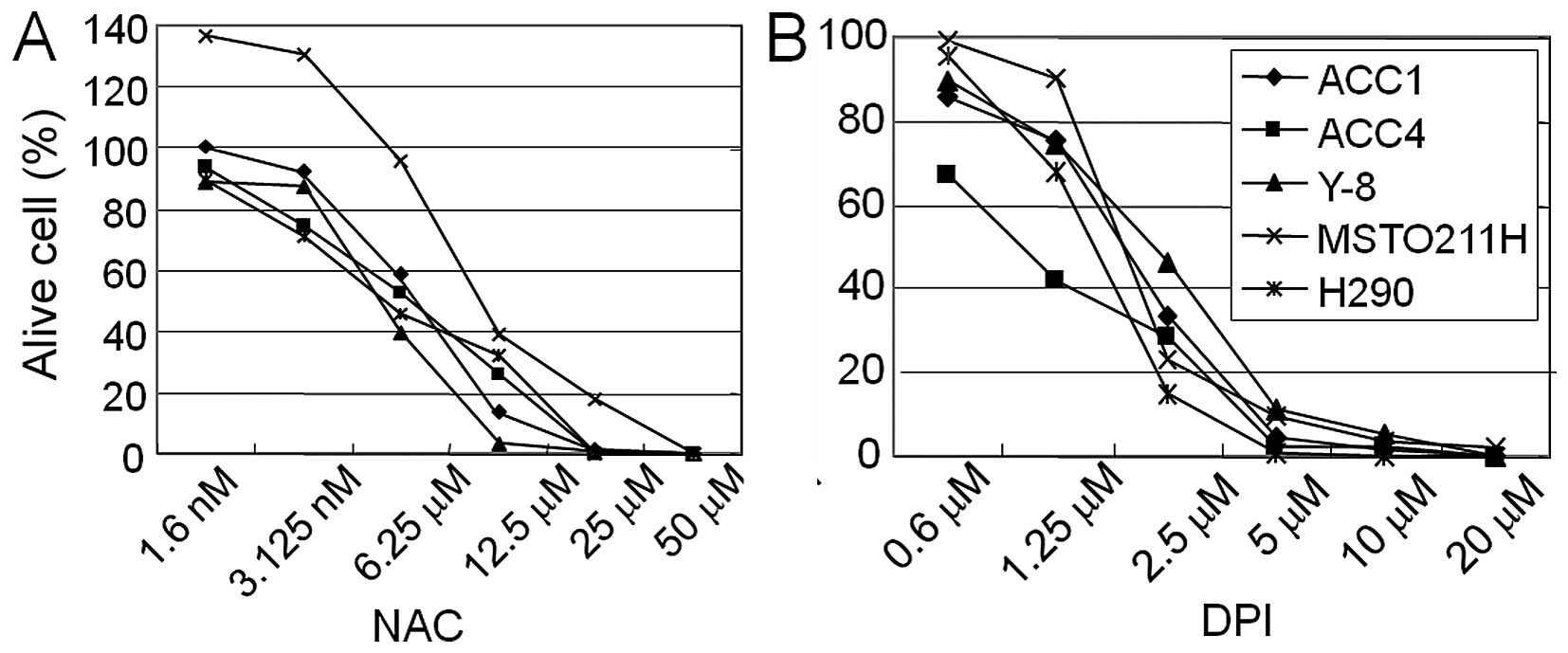
cell viability of mesothelioma cell lines. Five MPM cell lines
(ACC1-MESO, ACC4-MESO, Y8-MESO, MSTO-211H and H290) were treated
with various concentrations of NAC or DPI for 48 h. Both NAC and
DPI treatments inhibited the cell viability in a dose-dependent
manner (Fig. 1). The
IC50 values for DPI were as follows: 2.1 μM
(ACC1-MESO), 0.8 μM (ACC4-MESO), 2.5 μM (Y8-MESO),
2.2 μM (MSTO-211H) and 2.2 μM (H290).
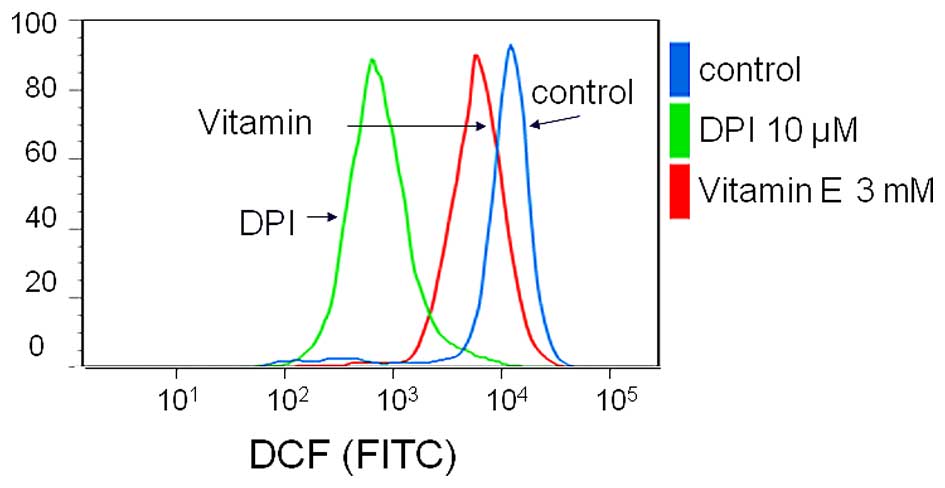
To verify that antioxidants affect ROS generation,
we evaluated ROS production using flow cytometry. With vitamin E
and DPI treatment, DCF fluorescence intensity, 2.1×104
in untreated cells was reduced to 0.7×104 and
0.1×104, respectively (Fig.
2). Thus, MPM cells regularly generated ROS, and both vitamin E
and DPI treatment suppressed ROS generation.
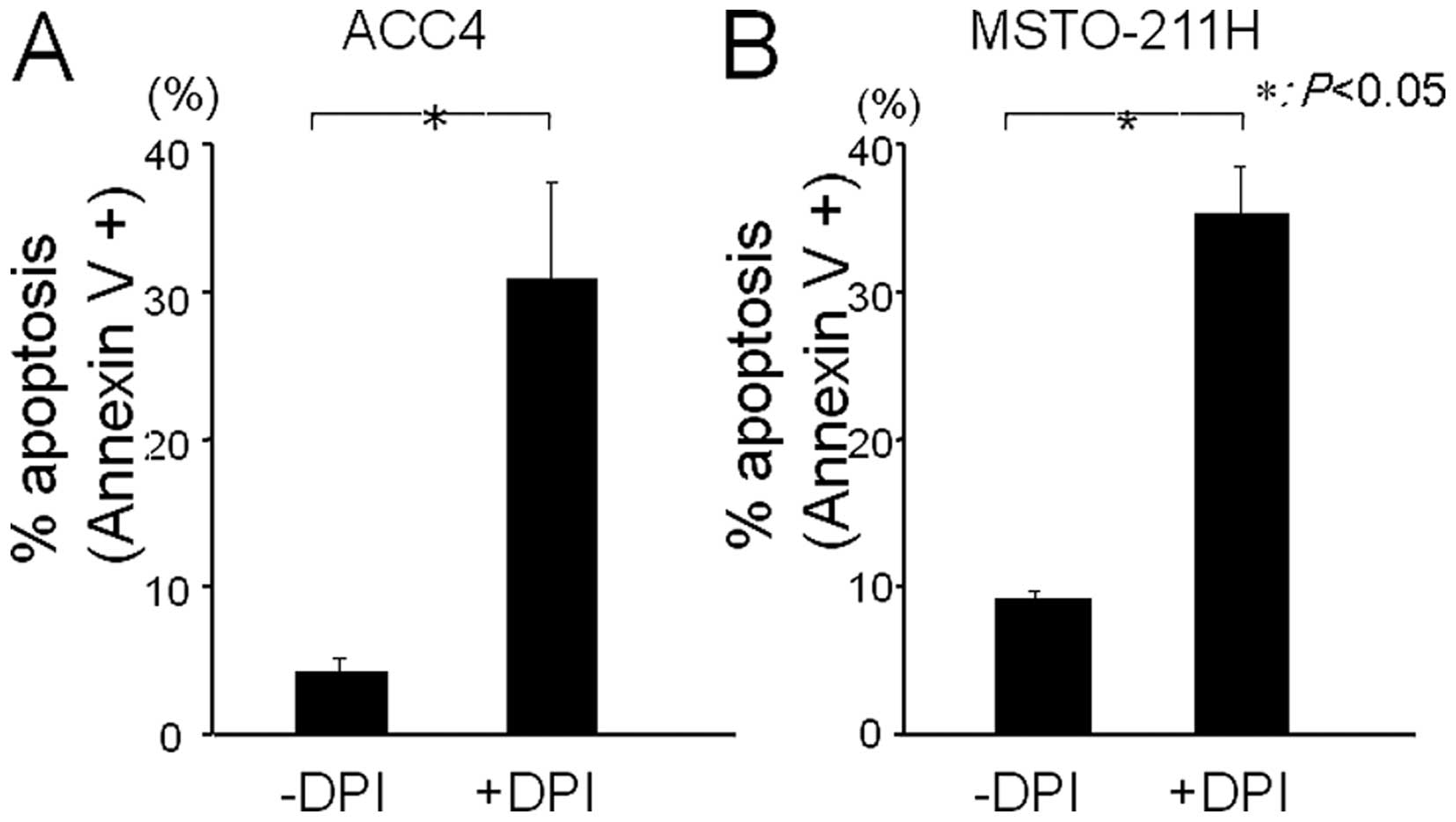
We further examined the effect of DPI on the
induction of apoptosis of MPM cells using Annexin V assay. DPI
treatment significantly induced apoptosis (27 and 26%) in ACC1 and
MSTO-221H cells, respectively (Fig.
3). Our results strongly suggest that depletion of ROS leads to
the apoptosis of MPM cells.
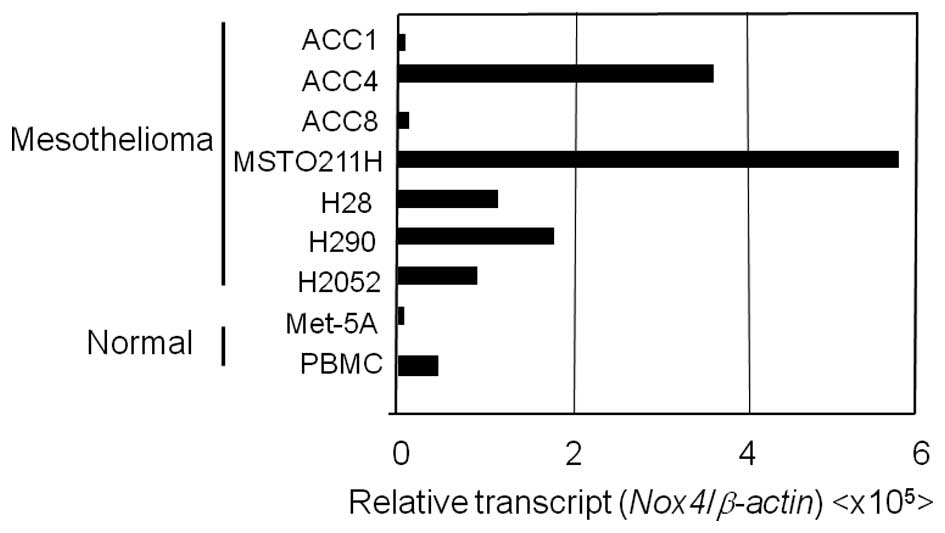
Expression and quantification of Nox 1-5
mRNAs in MPM cell lines
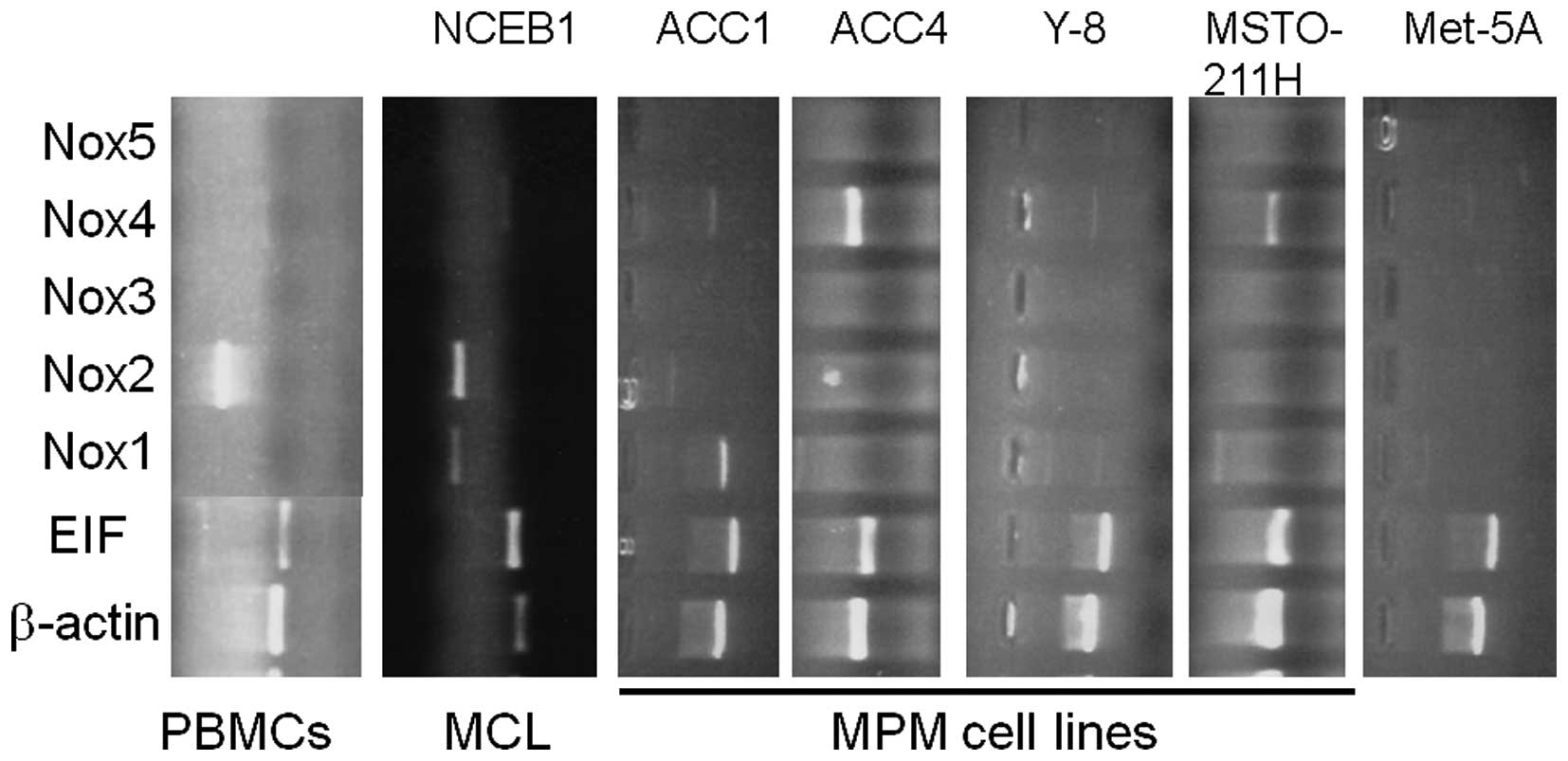
The Nox family members produce ROS that are though
to be pivotal for cell proliferative signaling. To examine the role
of the Nox family in proliferation of MPM cells, we analyzed the
mRNA expression of Nox family members in 7 MPM cell lines and a
non-malignant mesothelial cell line (Met-5A) (Fig. 4). Nox4 mRNA was expressed in
all of the examined MPM cell lines, whereas little or no
Nox2, Nox3 and Nox5 mRNAs were detected
(Fig. 4). In the ACC-MESO4 cell
line, Nox1 mRNA expression was readily detected.
Subsequently, the expression levels of Noxs
1-5 relative to β-actin were measured by quantitative real-time
RT-PCR (Fig. 5). Expression was
arbitrarily graded as low (Nox copy number/β-actin copy number
<500×10−8), intermediate (ratio >500 but
<2,000×10−8) or high (ratio
>2,000×10−8). Nox genes with expression ratios
>500×10−8 were routinely visible by RT-PCR analysis
using ≥40 μg total RNA. High- or intermediate-level
Nox4 mRNA expression was observed in all examined MPM cell
lines. Especially, 2 ACC-MESO4 and MSTO-211H cell lines expressed
Nox4 mRNA at a high level. For comparison, we examined
expression of the Nox family in several lymphoma/leukemia cell
lines; mantle lymphoma cells and Jurkat leukemia cells expressed
low levels of Nox2 mRNA (Fig.
5). High levels of Nox2 were detected in human PBMCs
(Fig. 5) and the Jurkat cell line
(data not shown).
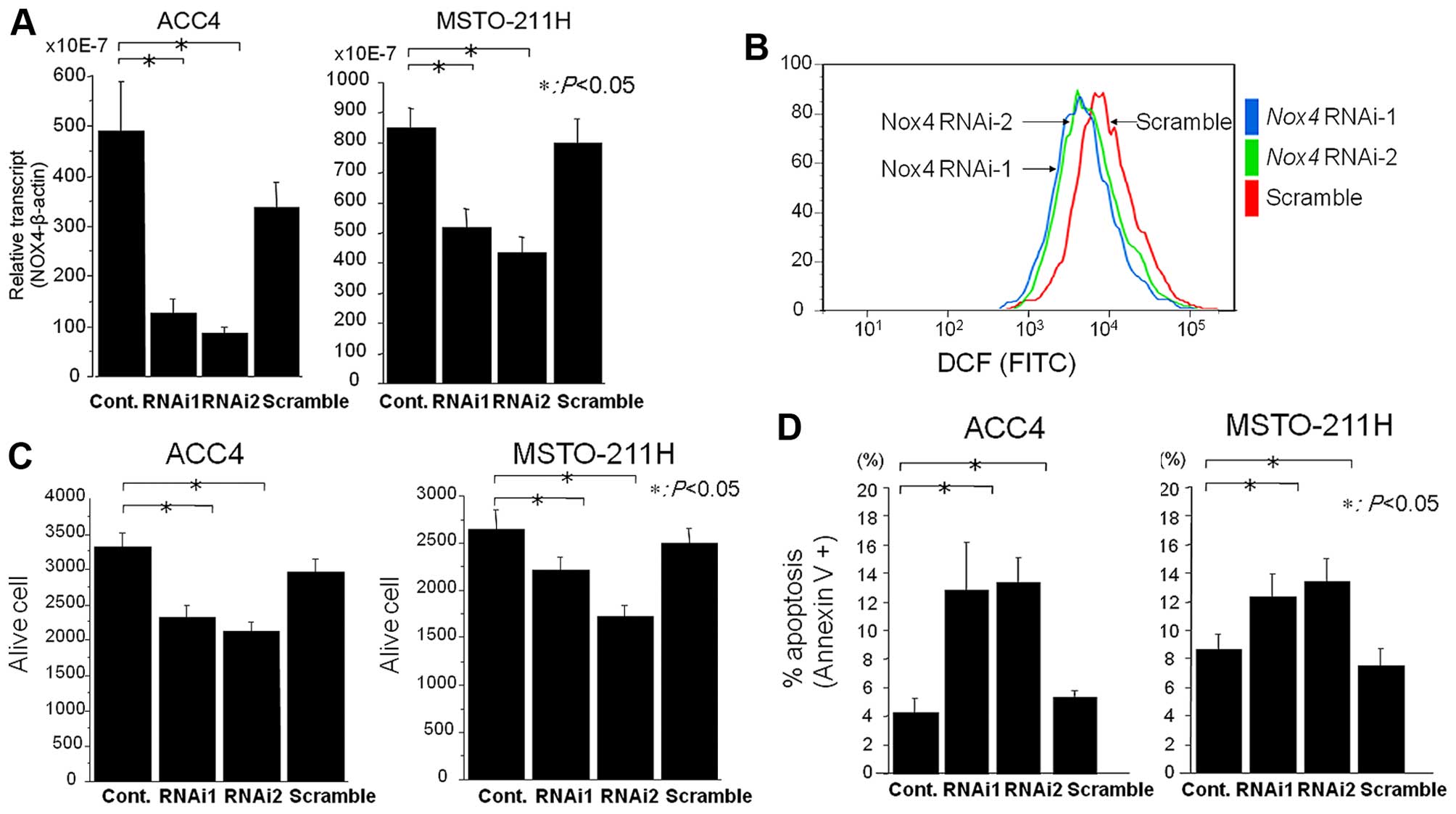
Nox4 mediates ROS production in MPM
cells
We utilized an RNA interference approach to verify
whether Nox4 mediates ROS production in MPM cells. The expression
of endogenous Nox4 mRNAs in ACC-MESO4 and MSTO-211H cells
was significantly suppressed upon transfection of Nox4
siRNAs, (Fig. 6A). Intracellular
ROS production was evaluated by flow cytometry. The transfection of
Nox4 siRNAs significantly suppressed ROS levels compared to
controls (Fig. 6B), indicating that
Nox4, at least in part, is responsible for intracellular ROS
generation. However, the ROS generation was not completely
inhibited by transfection of Nox4 siRNAs, suggesting the
possibility that other Nox members and mitochondria sources may
also contribute to ROS generation.
Suppression of ROS generation by Nox4
siRNAs induces apoptosis
To explore whether Nox4-generated ROS regulate cell
survival, we examined the effect of Nox4 siRNAs on cell
viability and apoptosis. The knockdown of Nox4 significantly
reduced the cell viability of ACC-MESO4 and MSTO-211H cells by 30%
(Fig. 6C). To verify whether the
inhibitory effect of Nox4 siRNAs is associated with
apoptosis, an Annexin V assay was performed. The transfection of
Nox4 siRNAs induced apoptosis by 13% in MPM cells (Fig. 6D). Thus, Nox4 siRNAs
suppressed ROS production, and the depletion of ROS by Nox4
siRNAs and DPI treatment induced apoptosis in MPM cells.
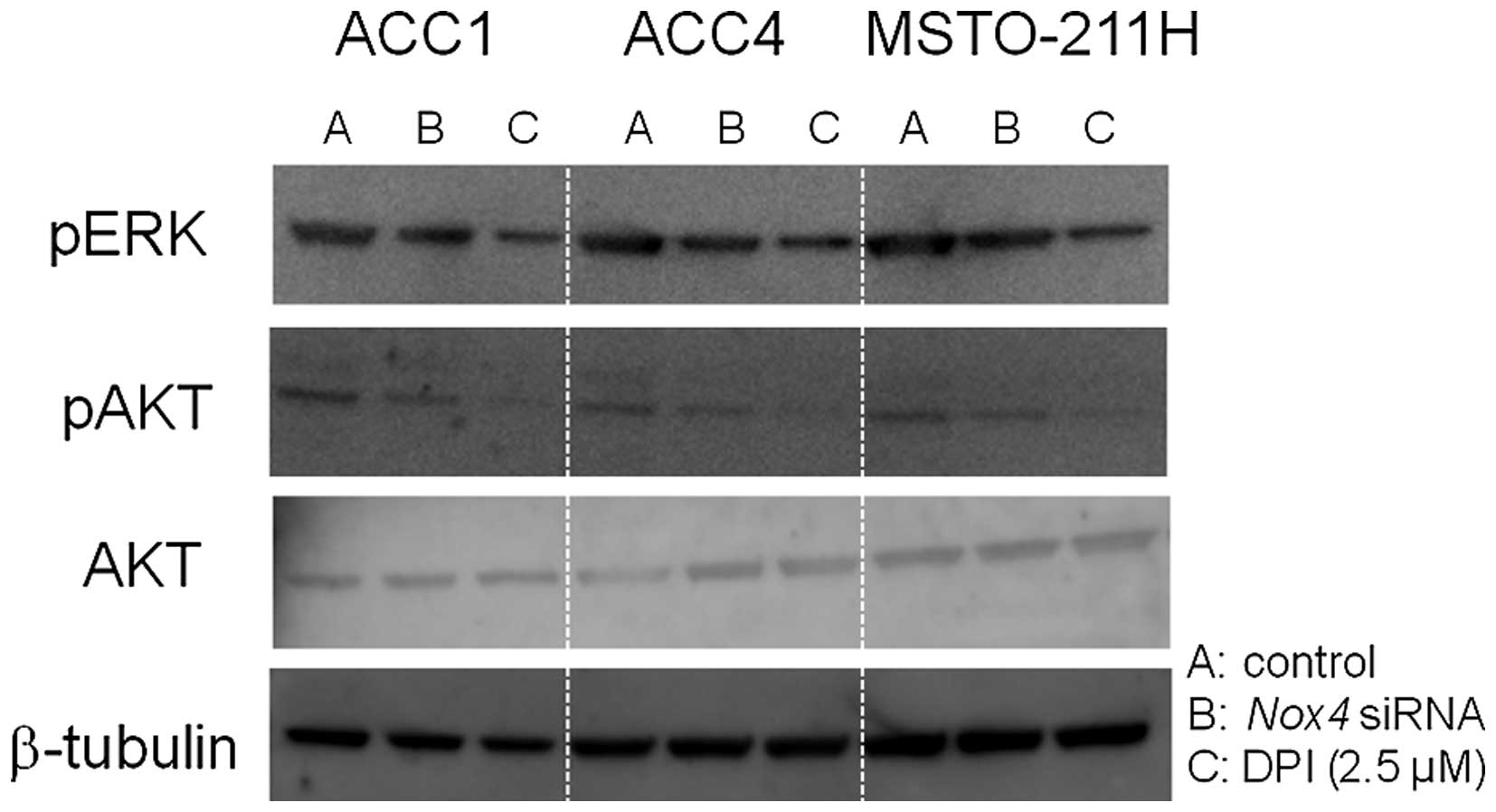
The role of protein kinases in cell
survival signaling in MPM cells
Both PI3K/AKT and MEK/ERK1/2 signaling cascades have
important roles in cell proliferation, but they also mediate
apoptosis (33). Western blot
analysis showed that ACC1, ACC4, and MSTO-211H cell lines expressed
phospho-AKT and phospho-ERK (Fig.
7). Nox4 siRNAs transfection and DPI treatment
attenuated the phosphorylation of AKT and ERK. Given that
transfection of Nox4 siRNAs and DPI treatment induced
apoptosis in MPM cells, the inactivation of PI3K/AKT and MEK/ERK1/2
signaling cascades likely plays an important role in the induction
of apoptosis.
Discussion
It is well established that the development of MPM
is associated with asbestos exposure (34,35).
Chronic inflammation accelerates the development and progression of
malignant mesothelioma, possibly because of cytokine release and
ROS generation. The inflammation that infiltrate into tissue areas
containing asbestos deposits consists largely of phagocytic
macrophages that internalize asbestos and release numerous
cytokines and mutagenic ROS.
In this study, we first examined the role of ROS in
cell proliferation. Although ROS are thought to cause
stress-induced apoptosis, ROS often provide cancer cells with a
survival advantage. In fact, we showed that suppression of ROS
levels by NAC and DPI treatment reduced the viability of MPM cells.
Similar results were observed in other cancer cells including
pancreatic cells. Second, we examined whether the Nox4-mediated
generation of intracellular ROS conferred anti-apoptotic activity
and thus a growth advantage to MPM cells. To this end, we analyzed
the expression levels of Nox genes in 7 MPM cell lines, and
a normal mesothelial cell line. RT-PCR analysis revealed that
Nox4 mRNA was expressed in all MPM cell lines, whereas
little or no Nox2, Nox3 and Nox5 mRNAs were
detected. Quantitative real-time RT-PCR also revealed that a high
or intermediate level of Nox4 mRNA expression was observed
in all MPM cell lines; high-level expression was detected in
ACC-MESO4 and MSTO-211H cells compared to the normal mesothelial
cell line Met-5A (P<0.01).
The siRNAs targeting Nox4 in 2 MPM cell lines
reduced intracellular ROS generation by 50%, and cell viability by
30%. The depletion of ROS by DPI treatment or knockdown of Nox4
induced apoptosis. Collectively, our findings suggest that ROS
generated by Nox4, at least in part, transmit cell survival signals
and their depletion leads to apoptosis. High-level Nox1 mRNA
expression was observed in colorectal cancer cell lines. Growth
inhibition profiling of DPI revealed a modest positive correlation
with Nox1 levels (31).
Exposure of HT-29 colon cancer cells, which expresses Nox1,
to DPI had inhibitory effects on the steady-state ROS levels, and
decreased STAT, ERK1/2, and AKT signaling activity (31). Nox4 overexpression is also reported
in primary breast, ovarian, prostate, melanoma, and glioblastoma
cancer cell lines (36–38). Nox4 expression was intermediate to
high in 2 of the 4 tested ovarian cancer cell lines. Notably, in
A2780/DDP cells, high level acquired resistance to cisplatin was
associated with a marked decrease in the expression level of Nox4.
Recently, Nox4 was shown to be an oncoprotein localized to
mitochondria (39). Together with
these studies, our study suggests that Nox4 may act as an
oncogene, and Nox4-related signaling molecules may be good
candidates for molecular-targeted therapy for MPM. Given a possible
role of Nox4 in tumorigenesis, it will be of particular interest to
investigate the correlation of Nox4 expression in primary
mesotheliomas and prognosis in the patients.
AKT (protein kinase B) is a regulator of cell
survival in response to a growth factor. AKT is activated through
its phosphorylation, and it inhibits apoptosis-inducing proteins,
thereby promoting cell survival. The ERK pathway is mostly
activated by growth factors and mediates cell proliferation, but it
also mediates apoptosis by stress stimuli (33). To examine the role of AKT and ERK in
the apoptosis of MPM cells, we evaluated the phosphorylation state
of AKT and ERK. Both DPI treatment and knockdown of Nox4 attenuated
their phosphorylation levels, suggesting that AKT and ERK play a
role in cell survival signals in MPM cells. Consistent with our
results, the PI3K-AKT pathway was reported to be activated in human
malignant mesothelioma (40). In
addition, both selective inhibitors for PI3K/AKT and MEK/ERK1/2
were effective in downregulating the expression of prometastasis
phenotypes of MPM cells (41).
In conclusion, we demonstrated that Nox4-mediated
ROS generation, at least in part, transmits cell survival signals,
and ROS depletion by the knockdown of Nox4 and DPI treatment leads
to apoptosis in a subset of MPM cells. Our study raises the
possibility of the Nox4-ROS-AKT signaling pathway as a novel
therapeutic target for MPM. Antioxidant treatment targeted to this
signaling pathway has the potential to enhance the therapeutic
index of cisplatin-based therapy. Further studies are warranted to
contribute to the knowledge required to ultimately develop targeted
therapies for MPM.
Abbreviations:
|
DCF
|
dichlorofluorescin
|
|
Duox
|
dual oxidase
|
|
DCFH-DA
|
2′,7′-dichlorodihydrofluorescein
diacetate
|
|
DPI
|
diphenylene iodonium
|
|
MPM
|
malignant pleural mesothelioma
|
|
NAC
|
N-acetylcysteine
|
|
Nox
|
NADPH oxidase
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
ROS
|
reactive oxygen species
|
Acknowledgments
This study was supported by grant AI 52213 from the
Aichi Cancer Center to Y.M. and a grant of Strategic Research
Foundation Grant-Aided Project for Private Universities from the
Ministry of Education, Culture, Sports, Science and Technology,
Japan (MEXT) to Y.H. The authors thank Dr Yoshitaka Sekido
(Division of Molecular Oncology Aichi Cancer Center Research
Institute) for providing MPM cell lines.
References
|
1
|
Robinson BW and Lake RA: Advances in
malignant mesothelioma. N Engl J Med. 353:1591–1603. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Campbell NP and Kindler HL: Update on
malignant pleural mesothelioma. Semin Respir Crit Care Med.
32:102–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peto J, Decarli A, La Vecchia C, Levi F
and Negri E: The European mesothelioma epidemic. Br J Cancer.
79:666–672. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murayama T: Epidemic of asbestos related
diseases. Proceedings of the global Asbestos Congress (Tokyo); pp.
172004
|
|
5
|
Stewart DJ, Martin-Ucar A, Pilling JE,
Edwards JG, O'Byrne KJ and Waller DA: The effect of extent of local
resection on patterns of disease progression in malignant pleural
mesothelioma. Ann Thorac Surg. 78:245–252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takahashi K: Emerging health effects of
asbestos in Asia. Proceedings of the Global Asbestos Congress
(Tokyo); pp. 22004
|
|
7
|
Murayama T, Takahashi K, Natori Y and
Kurumatani N: Estimation of future mortality from pleural malignant
mesothelioma in Japan based on an age-cohort model. Am J Ind Med.
49:1–7. 2006. View Article : Google Scholar
|
|
8
|
Rascoe PA, Jupiter D, Cao X, Littlejohn JE
and Smythe WR: Molecular pathogenesis of malignant mesothelioma.
Expert Rev Mol Med. 14:e122012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murakami H, Mizuno T, Taniguchi T, Fujii
M, Ishiguro F, Fukui T, Akatsuka S, Horio Y, Hida T, Kondo Y, et
al: LATS2 is a tumor suppressor gene of malignant mesothelioma.
Cancer Res. 71:873–883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujii M, Toyoda T, Nakanishi H, Yatabe Y,
Sato A, Matsudaira Y, Ito H, Murakami H, Kondo Y, Kondo E, et al:
TGF-β synergizes with defects in the Hippo pathway to stimulate
human malignant mesothelioma growth. J Exp Med. 209:479–494. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi Y, Moura U, Opitz I, Soltermann A,
Rehrauer H, Thies S, Weder W, Stahel RA and Felley-Bosco E: Role of
hedgehog signaling in malignant pleural mesothelioma. Clin Cancer
Res. 18:4646–4656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Testa JR, Cheung M, Pei J, Below JE, Tan
Y, Sementino E, Cox NJ, Dogan AU, Pass HI, Trusa S, et al: Germline
BAP1 mutations predispose to malignant mesothelioma. Nat Genet.
43:1022–1025. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo G, Chmielecki J, Goparaju C, Heguy A,
Dolgalev I, Carbone M, Seepo S, Meyerson M and Pass HI: Whole-exome
sequencing reveals frequent genetic alterations in BAP1, NF2,
CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res.
75:264–269. 2015. View Article : Google Scholar
|
|
14
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S,
Manegold C, et al: Phase III study of pemetrexed in combination
with cisplatin versus cisplatin alone in patients with malignant
pleural mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsao AS, Wistuba I, Roth JA and Kindler
HL: Malignant pleural mesothelioma. J Clin Oncol. 27:2081–2090.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stahel RA and Weder W: Improving the
outcome in malignant pleural mesothelioma: Nonaggressive or
aggressive approach? Curr Opin Oncol. 21:124–130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kindler HL, Karrison TG, Gandara DR, Lu C,
Krug LM, Stevenson JP, Jänne PA, Quinn DI, Koczywas MN, Brahmer JR,
et al: Multicenter, double-blind, placebo-controlled, randomized
phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo
in patients with malignant mesothelioma. J Clin Oncol.
30:2509–2515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brar SS, Kennedy TP, Sturrock AB,
Huecksteadt TP, Quinn MT, Whorton AR and Hoidal JR: An NAD(P)H
oxidase regulates growth and transcription in melanoma cells. Am J
Physiol Cell Physiol. 282:C1212–C1224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lambeth JD: NOX enzymes and the biology of
reactive oxygen. Nat Rev Immunol. 4:181–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dworakowski R, Anilkumar N, Zhang M and
Shah AM: Redox signalling involving NADPH oxidase-derived reactive
oxygen species. Biochem Soc Trans. 34:960–964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen K, Craige SE and Keaney JF Jr:
Downstream targets and intracellular compartmentalization in Nox
signaling. Antioxid Redox Signal. 11:2467–2480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Storz P: Reactive oxygen species in tumor
progression. Front Biosci. 10:1881–1896. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Szatrowski TP and Nathan CF: Production of
large amounts of hydrogen peroxide by human tumor cells. Cancer
Res. 51:794–798. 1991.PubMed/NCBI
|
|
24
|
Mochizuki T, Furuta S, Mitsushita J, Shang
WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayama J, Konagai A,
et al: Inhibition of NADPH oxidase 4 activates apoptosis via the
AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic
cancer PANC-1 cells. Oncogene. 25:3699–3707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vaquero EC, Edderkaoui M, Pandol SJ,
Gukovsky I and Gukovskaya AS: Reactive oxygen species produced by
NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J
Biol Chem. 279:34643–34654. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng G, Cao Z, Xu X, van Meir EG and
Lambeth JD: Homologs of gp91phox: Cloning and tissue expression of
Nox3, Nox4, and Nox5. Gene. 269:131–140. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Donkó A, Péterfi Z, Sum A, Leto T and
Geiszt M: Dual oxidases. Philos Trans R Soc Lond B Biol Sci.
360:2301–2308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geiszt M, Witta J, Baffi J, Lekstrom K and
Leto TL: Dual oxidases represent novel hydrogen peroxide sources
supporting mucosal surface host defense. FASEB J. 17:1502–1504.
2003.PubMed/NCBI
|
|
29
|
Juhasz A, Ge Y, Markel S, Chiu A,
Matsumoto L, van Balgooy J, Roy K and Doroshow JH: Expression of
NADPH oxidase homologues and accessory genes in human cancer cell
lines, tumours and adjacent normal tissues. Free Radic Res.
43:523–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu Y, Antony S, Juhasz A, Lu J, Ge Y,
Jiang G, Roy K and Doroshow JH: Up-regulation and sustained
activation of Stat1 are essential for interferon-gamma
(IFN-gamma)-induced dual oxidase 2 (Duox2) and dual oxidase A2
(DuoxA2) expression in human pancreatic cancer cell lines. J Biol
Chem. 286:12245–12256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Doroshow JH, Juhasz A, Ge Y, Holbeck S, Lu
J, Antony S, Wu Y, Jiang G and Roy K: Antiproliferative mechanisms
of action of the flavin dehydrogenase inhibitors diphenylene
iodonium and di-2-thienyliodonium based on molecular profiling of
the NCI-60 human tumor cell panel. Biochem Pharmacol. 83:1195–1207.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miura Y, Thoburn CJ, Bright EC, Phelps ML,
Shin T, Matsui EC, Matsui WH, Arai S, Fuchs EJ, Vogelsang GB, et
al: Association of Foxp3 regulatory gene expression with
graft-versus-host disease. Blood. 104:2187–2193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee YJ, Cho HN, Soh JW, Jhon GJ, Cho CK,
Chung HY, Bae S, Lee SJ and Lee YS: Oxidative stress-induced
apoptosis is mediated by ERK1/2 phosphorylation. Exp Cell Res.
291:251–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carbone M and Yang H: Molecular pathways:
Targeting mechanisms of asbestos and erionite carcinogenesis in
mesothelioma. Clin Cancer Res. 18:598–604. 2012. View Article : Google Scholar :
|
|
35
|
Carbone M, Ly BH, Dodson RF, Pagano I,
Morris PT, Dogan UA, Gazdar AF, Pass HI and Yang H: Malignant
mesothelioma: Facts, myths, and hypotheses. J Cell Physiol.
227:44–58. 2012. View Article : Google Scholar
|
|
36
|
Ushio-Fukai M and Nakamura Y: Reactive
oxygen species and angiogenesis: NADPH oxidase as target for cancer
therapy. Cancer Lett. 266:37–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shono T, Yokoyama N, Uesaka T, Kuroda J,
Takeya R, Yamasaki T, Amano T, Mizoguchi M, Suzuki SO, Niiro H, et
al: Enhanced expression of NADPH oxidase Nox4 in human gliomas and
its roles in cell proliferation and survival. Int J Cancer.
123:787–792. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamaura M, Mitsushita J, Furuta S, Kiniwa
Y, Ashida A, Goto Y, Shang WH, Kubodera M, Kato M, Takata M, et al:
NADPH oxidase 4 contributes to transformation phenotype of melanoma
cells by regulating G2-M cell cycle progression. Cancer Res.
69:2647–2654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Graham KA, Kulawiec M, Owens KM, Li X,
Desouki MM, Chandra D and Singh KK: NADPH oxidase 4 is an
oncoprotein localized to mitochondria. Cancer Biol Ther.
10:223–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Suzuki Y, Murakami H, Kawaguchi K,
Tanigushi T, Fujii M, Shinjo K, Kondo Y, Osada H, Shimokata K,
Horio Y, et al: Activation of the PI3K-AKT pathway in human
malignant mesothelioma cells. Mol Med Rep. 2:181–188.
2009.PubMed/NCBI
|
|
41
|
Cole GW Jr, Alleva AM, Zuo JT, Sehgal SS,
Yeow WS, Schrump DS and Nguyen DM: Suppression of pro-metastasis
phenotypes expression in malignant pleural mesothelioma by the PI3K
inhibitor LY294002 or the MEK inhibitor UO126. Anticancer Res.
26A:809–821. 2006.
|