Introduction
The NOTCH signaling pathway has been shown to have
dual roles in development and carcinogenesis in multiple organs
(1). In mammals, four NOTCH
family genes, NOTCH1–4 have been described, each of which
encodes a transmembrane receptor comprised of intracellular and
extracellular domains. When a NOTCH ligand, e.g., a JAG or a DLL
family protein, binds to the receptor, the intracellular domain is
cleaved by the γ-secretase and translocated into the nucleus as an
activated transcription factor for NOTCH target genes, including
HES genes (1).
The relationship between NOTCH signaling and human
hepatocarcinogenesis is still controversial. Both negative
(2,3) and positive (4,5)
correlations have been proposed. However, most studies have
evaluated the effects of NOTCH through activating NOTCH1 or
downstream NOTCH effectors common for the NOTCH family. Since
NOTCH2, not NOTCH1 is essential for normal hepatic development in
mice (6), more specific studies on
human NOTCH2 are needed to support the established notion that
cancer cells mimic immature features of their fetal
counterparts.
To our knowledge, there is only one study on NOTCH2
expression in human hepatocellular carcinomas (HCCs); in this
study, no nuclear localization of NOTCH2 protein was observed by
immunohistochemistry analysis in any of the examined tumors
(7). In the present study, we
sought to investigate NOTCH2 signaling in human HCCs using tissue
microarrays and cell lines. Contradictory to the previous study
(7), our data supported that NOTCH2
had important roles in terms of aggressiveness and morphologic
transformation of HCC cells.
Materials and methods
Tissue microarray and
immunohistochemistry
We used tissue microarrays for human primary and
metastatic HCCs (SuperBioChips Laboratories, Seoul, Korea).
Immunohistochemical staining was performed as previously described
(8), using primary antibodies
against human activated NOTCH2 (ab52302), activated NOTCH1 (ab8925)
(both from Abcam, Cambridge, UK), α-fetoprotein (AFP), cytokeratin
19 (CK19) (both from Dako, Glostrup, Denmark), and EpCAM (ab187270;
Abcam). The anti-NOTCH antibodies have been shown to react only
with the activated forms of the intracellular domain after cleavage
by γ-secretase (1). Clinical
staging or histopathological grading of differentiation of the
primary HCCs were performed according to the American Joint
Committee on Cancer (AJCC) Cancer Staging Manual (9), or the General Rules for the Clinical
and Pathological Study of Primary Liver Cancer (10), respectively. For quantification of
the nuclear/cytoplasmic (N/C) ratio and nuclear density, the HCC
tissues on the tissue microarray were photographed and analyzed
using ImageJ software (http://rsb.info.nih.gov/ij/).
Cell lines and transfection
Six human HCC cell lines, Huh7, Hep3B, HepG2, HLE,
HLF and PLC/5 were used for analyses. For transient knockdown of
NOTCH2, we used anti-NOTCH2 siRNA (OriGene,
Rockville, MD, USA). A total of 5×104 cells were
inoculated into each well of a 6-well tissue culture plates and
transfected with 5 µM siRNA using the X-tremeGENE siRNA
Transfection reagent (Roche, Basel, Switzerland). The cells were
harvested 48 h after transfection, and protein and total RNA were
collected for analysis. Universal scrambled negative control siRNA
was used as a negative control (OriGene).
For stable knockdown of NOTCH2, the pRS
plasmid vector harboring an shRNA targeting NOTCH2 and the
puromycin-resistance gene were employed (OriGene). The same plasmid
with a scrambled sequence was used as the negative control. For
stable overexpression of NOTCH2, the pEF-BOS-neo SE plasmid
vector with an expression construct of a partial NOTCH2 cDNA
sequence for the intracellular region and the G418-resistance gene
(11,12) was obtained from Riken DNA Bank
(Tsukuba, Japan). Stable transfectants were selected for 2 weeks
with 2.0 µg/ml puromycin (Sigma, St. Louis, MO, USA) or 1
mg/ml G418 (Roche) and clonal cell lines were established.
Western blot analysis
Monolayer cell cultures at 80% confluence were
analyzed by western blotting as previously described (8), using primary antibodies against human
activated NOTCH2 (ab72803; Abcam), E-cadherin (Dako) or β-actin
(Sigma).
Semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from cultured cells at 80%
confluence using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and
subsequent RT-PCR was performed as previously described (13). First-strand cDNAs were synthesized
with oligo(dT) primer and the SuperScript III First-Strand
Synthesis system (Invitrogen). Each single-stranded cDNA was
diluted for subsequent PCR amplification. The standard PCR
procedure was carried out in 15 µl of PCR buffer. PCR
conditions were: initial denaturation at 94°C for 7 min, following
by appropriate cycles of 94°C for 30 sec, 55°C for 1 min, and 72°C
for 1 min and a final extension step of 72°C for 10 min. Each PCR
product (15 µl) was run on native 7% polyacrylamide gels,
stained with ethidium bromide and visualized by ultraviolet
illumination. Staining intensity was quantified using ImageJ
software. The screened gene panel included human NOTCH2,
HES1, E-cadherin, albumin (ALB),
JAG1, JAG2, DLL1, DLL3 and DLL4;
GAPDH was screened as the control gene (Table I). The cycle number for each gene
was determined as optimal when the amplification was within the
linear range.
 | Table IList of genes analyzed by
semi-quantitative RT-PCR. |
Table I
List of genes analyzed by
semi-quantitative RT-PCR.
| Gene | Primer sequence (5′
to 3′) | Product size
(bp) | PCR cycle no. |
|---|
| NOTCH2 |
AAGCAGAGTCCCAGTGCCTA
CAGGGGGCACTGACAGTAAT | 173 | 36 |
| JAG1 |
GACTCATCAGCCGTGTCTCA
TGGGGAACACTCACACTCAA | 190 | 26 |
| JAG2 |
AGGTGGAGACGGTTGTTACG
TTGCACTGGTAGAGCACGTC | 250 | 41 |
| DLL1 |
TGTGCCTCAAGCAACTACCAG
TTCTGTTGCGAGGTCATCAG | 230 | 39 |
| DLL3 |
GAGACACCCAGGTCCTTTGA
CAGTGGCAGATGTAGGCAGA | 152 | 38 |
| DLL4 |
TGCAGGAGTTCATCAACGAG
GAAATTGAAGGGCAGTTGGA | 227 | 38 |
| HES1 |
CGGACATTCTGGAAATGACA
CATTGATCTGGGTCATGCAG | 222 | 45 |
|
E-cadherin |
GCTGGAGATTAATCCGGACA
ACCTGAGGCTTTGGATTCCT | 237 | 38 |
| ALB |
TGCTTGAATGTGCTGATGACAGGG
AAGGCAAGTCAGCAGGCATCTCATC | 161 | 40 |
| GAPDH |
ACCACAGTCCATGCCATCAC
TCCACCACCCTGTTGCTGTA | 452 | 27 |
Transmembrane invasion and migration
assays
In vitro invasion or migration assays were
performed using BioCoat Matrigel invasion chambers in 24-well
plates or those without Matrigel (Becton-Dickinson, Franklin Lakes,
NJ, USA), respectively (13).
Suspensions of 2.5×104 cells in 0.5 ml of serum-free
Dulbecco's modified Eagle's medium (DMEM) were applied onto
Matrigel-coated, 8-µm pore-sized polycarbonate membranes at
the bottom of each upper chamber, which was inserted into a lower
well containing 0.75 ml of DMEM with 10% fetal bovine serum as the
chemoattractant. After 22 h of incubation, cells remaining on the
upper surface of the membrane were removed with a cotton swab, and
those that had invaded through to the bottom chamber were fixed
with 4% paraformaldehyde and stained with crystal violet. Cells
were observed under a light microscope, and four randomly selected
fields were photographed at a magnification of ×100. Invasion
efficiency was expressed as the mean number of cells per
photographed microscopic field. Each experiment was performed in
quadruplicate. The protocol for the migration assay was the same as
that for the invasion assay, except that a membrane without
Matrigel coating was used (BioCoat control culture inserts;
Becton-Dickinson).
In vivo tumorigenic assay
The animal experiments were approved by the Kochi
University Animal Experiment Committee (permit no. G-00058).
Immunodeficient male NOD. CB17-PrkdcScid/J (NOD
SCID) mice (ages 8–10 weeks) were purchased from Charles River
Laboratories Japan (Yokohama, Japan). Cultured cells were suspended
in phosphate-buffered saline and 1×106 cells were
transplanted into the subcutis of each mouse at the interscapular
region. After 6 weeks, mice were sacrificed by cervical
decapitation. Developed tumors were excised, weighed and fixed in
10% buffered formalin for histology.
Statistical analysis
For comparison of two independent mean values we
employed the t-test. For the analysis of contingency tables,
Fisher's exact test was applied. Differences were considered
significant at P-values <0.05.
Results
Immunohistochemical analysis of NOTCH2
and NOTCH1
A total of 74 primary human HCCs and 9
non-neoplastic liver tissues, each adjacent to an independent
primary HCC were immunohistochemically analyzed for activated
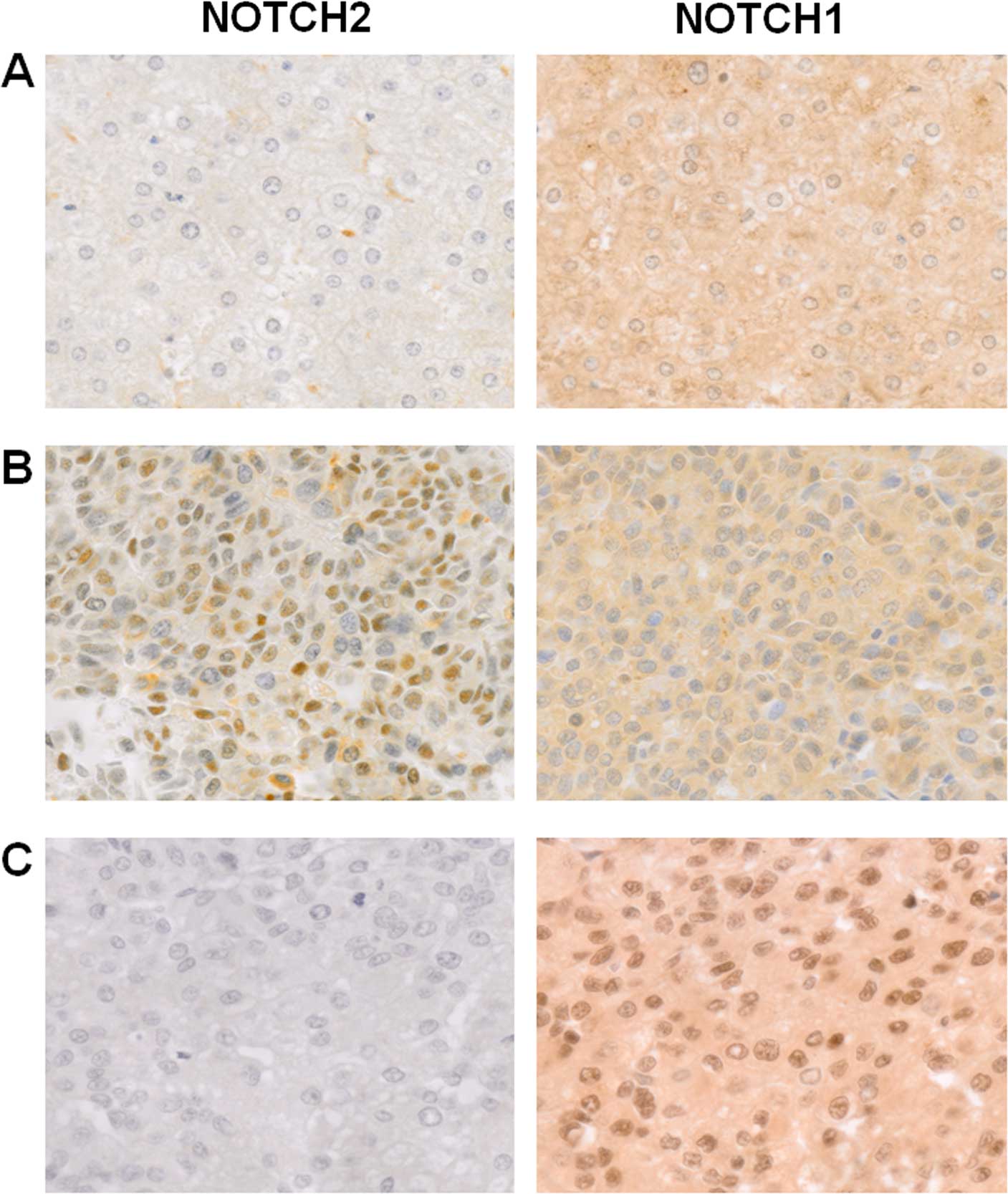
intracellular domains of NOTCH2 and NOTCH1 (Fig. 1). While hepatocytes in the
non-neoplastic livers showed weak cytoplasmic staining for both
NOTCH2 and NOTCH1, the degree of staining for NOTCH2 was less
intense under our reaction conditions (Fig. 1A). None of the non-neoplastic liver
tissues exhibited a nuclear-predominant staining pattern for either
NOTCH2 or NOTCH1 in hepatocytes. Since NOTCH signaling operates
through nuclear localization of the activated intracellular domain
(1), a tumor was judged positive
only when >10% of the cells exhibited distinct
nuclear-predominant staining (Fig. 1B
and C). This cut-off criterion was set according to a
preliminary test for reproducibility of the judgment between two
observers. On average, NOTCH2-positive tumors were in significantly
more advanced clinical stages than NOTCH2-negative tumors (Table II). A similar trend was observed
for NOTCH1-positive tumors, although this result did not reach
statistical significance. In addition, all NOTCH2-positive cases
were observed in men, and this difference was statistically
significant. There was no significant correlation between the
histopathological grade of differentiation and positive staining
for either NOTCH2 or NOTCH1.
 | Table IIClinicopathological features of 74
primary HCCs based on nuclear-predominant immunohistochemical
staining for NOTCH2 or NOTCH1. |
Table II
Clinicopathological features of 74
primary HCCs based on nuclear-predominant immunohistochemical
staining for NOTCH2 or NOTCH1.
| NOTCH2
| NOTCH1
|
|---|
| Positive
casesa | Negative
casesb | P-value | Positive
casesa | Negative
casesb | P-value |
|---|
| Analyzed cases 74
primary HCCs | 14 (19%) | 60 (81%) | NA | 18 (24%) | 56 (76%) | NA |
| Age, mean
(years) | 56.7 | 55.0 | NS | 54.1 | 55.7 | NS |
| Gender |
| Male/female | 14/0 | 44/16 | 0.031 | 15/3 | 43/13 | NS |
| Clinical stage
(score)c |
| I (1)/II (2)/III
(3)/IV (4) | 1/4/7/2 | 23/17/18/2 | | 3/6/8/1 | 21/15/17/3 | |
| Mean score | 2.71 | 1.98 | 0.004 | 2.39 | 2.04 | NS |
| Histological grade
(score)d |
| well (1)/mod
(2)/poor (3) | 1/10/1 | 10/27/7 | | 2/10/1 | 9/27/7 | |
| Mean score | 2.00 | 1.93 | NS | 1.92 | 1.95 | NS |
We next analyzed metastatic HCC tissues in various
organs obtained from an independent set of 18 patients. The
NOTCH2-positive rate was significantly higher in metastatic HCCs,
and the same conclusion was reached with NOTCH1 (Table III). In primary HCCs,
NOTCH2-positive HCCs showed no significant difference in NOTCH1
positivity compared with the NOTCH2-negative HCCs. However, in
metastatic HCCs, >90% of NOTCH2-positive tumors were
simultaneously positive for NOTCH1 (Table IV).
 | Table IIIComparison of independent sets of
primary and metastatic HCCs analyzed for nuclear NOTCH2 and NOTCH1
using immunohistochemistry. |
Table III
Comparison of independent sets of
primary and metastatic HCCs analyzed for nuclear NOTCH2 and NOTCH1
using immunohistochemistry.
| HCC | Total cases | NOTCH2
| NOTCH1
|
|---|
| Positive
casesa (%) | Negative
casesb (%) | P-value | Positive
casesa (%) | Negative
casesb (%) | P-value |
|---|
| Primary | 74 | 14 (19) | 60 (81) | | 18 (24) | 56 (76) | |
| Metastatic | 18 | 11 (61) | 7 (39) | 0.001 | 12 (67) | 6 (33) | 0.001 |
 | Table IVCorrelation between
immunohistochemical staining for nuclear NOTCH2 and NOTCH1 in
primary or metastatic HCCs. |
Table IV
Correlation between
immunohistochemical staining for nuclear NOTCH2 and NOTCH1 in
primary or metastatic HCCs.
| NOTCH1
|
|---|
Primary HCC cases
| Metastatic HCC
cases
|
|---|
| Positivea (%) | Negativeb (%) | P-value | Positivea (%) | Negativeb (%) | P-value |
|---|
| NOTCH2 |
| Positivea | 2 (14) | 12 (86) | | 10 (91) | 1 (9) | |
| Negativeb | 16 (27) | 44 (73) | NS | 2 (29) | 5 (71) | 0.013 |
During the immunohistochemical analysis, we noticed
that cells in NOTCH2-positive HCCs tended to be small in size and
high in N/C ratio (Fig. 1),
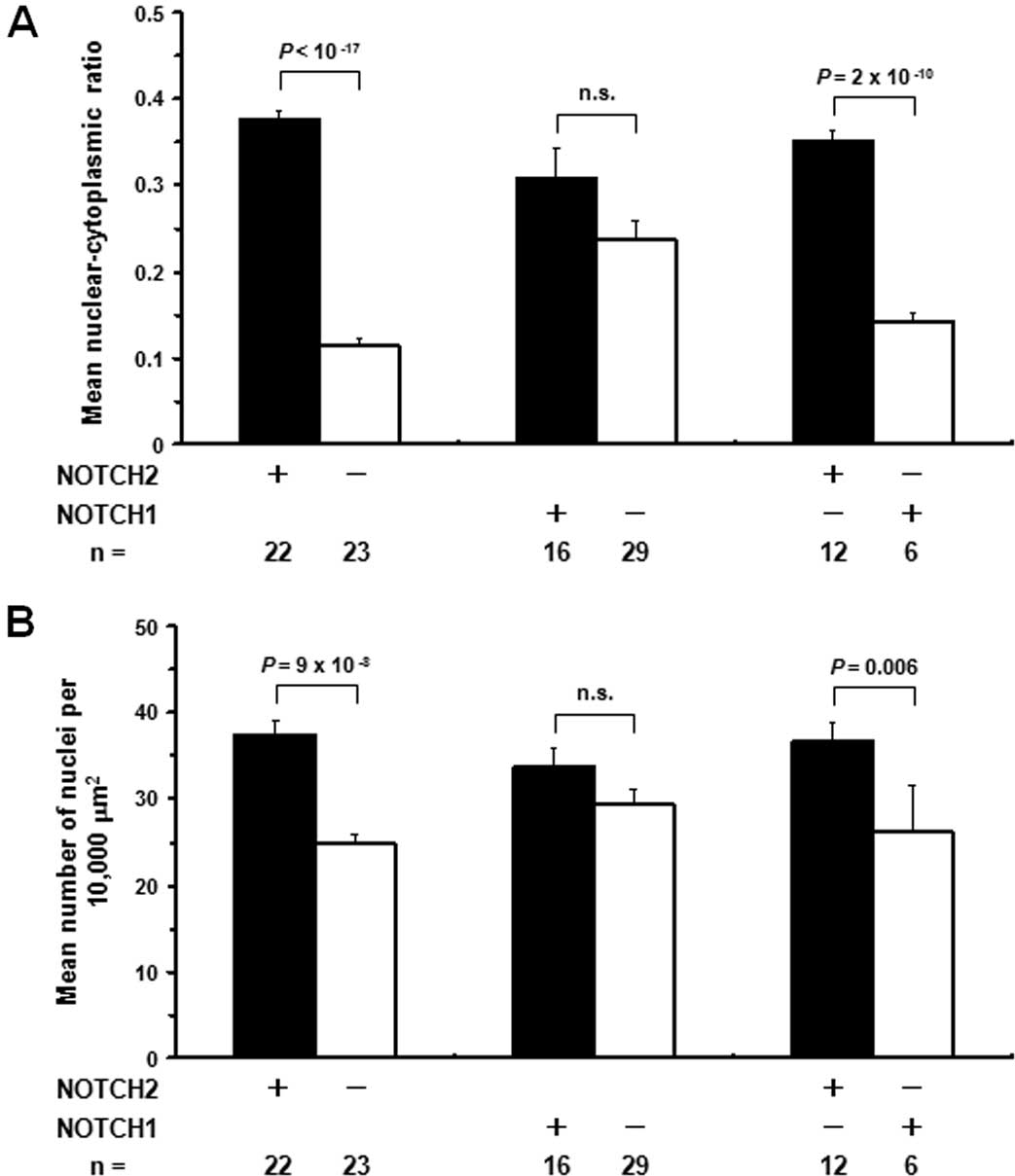
reminiscent of immature hepatoblasts. Thus, we quantified N/C
ratios and nuclear densities in primary and metastatic HCC cases
(Fig. 2). Among the 25
NOTCH2-positive HCCs on the tissue microarray, three cases were
excluded from the analysis due to their low tumor cell content on
the array. As controls, 23 NOTCH2-negative HCCs were randomly
selected and simultaneously analyzed, and both of the parameters
were significantly higher for NOTCH2-positive HCCs compared with
NOTCH2-negative HCCs. Such features were not evident for
NOTCH1-positive HCCs. Furthermore, tumors positive for only NOTCH2
showed significantly higher mean N/C ratios and nuclear densities
than those positive for only NOTCH1.
Expression of hepatoblast markers in
NOTCH2-positive HCCs
Since the NOTCH2 signaling was associated with
immature morphology of HCC cells, we immunohistochemically analyzed
expression of 3 representative hepatoblast markers, AFP, CK19 and
EpCAM (14), with 14
NOTCH2-positive and 44 NOTCH2-negative primary HCCs (Table V). A tumor was judged positive when
>10% of the cells exhibited distinct staining in order to keep
the reproducibility of the judgment between two observers. Compared
with the NOTCH2-negative HCCs, the NOTCH2-positive HCCs were more
frequently positive for AFP and also larger in the mean
multiplicity of positive hepatoblast markers per case.
 | Table VExpression of representative
hepatoblast markers, AFP, CK19 and EpCAM, in NOTCH2-positive and
-negative primary HCCs. |
Table V
Expression of representative
hepatoblast markers, AFP, CK19 and EpCAM, in NOTCH2-positive and
-negative primary HCCs.
| Total cases | Positive
casesa
| Mean multiplicity
of positive markers/case |
|---|
| AFP | EpCAM | CK19 |
|---|
| NOTCH2 |
| Positive | 14 | 7 (50%) | 10 (71%) | 3 (21%) | 1.43 |
| Negative | 44 | 9 (20%) | 19 (43%) | 5 (11%) | 0.75 |
| P-value | | 0.043 | NS | NS | 0.024 |
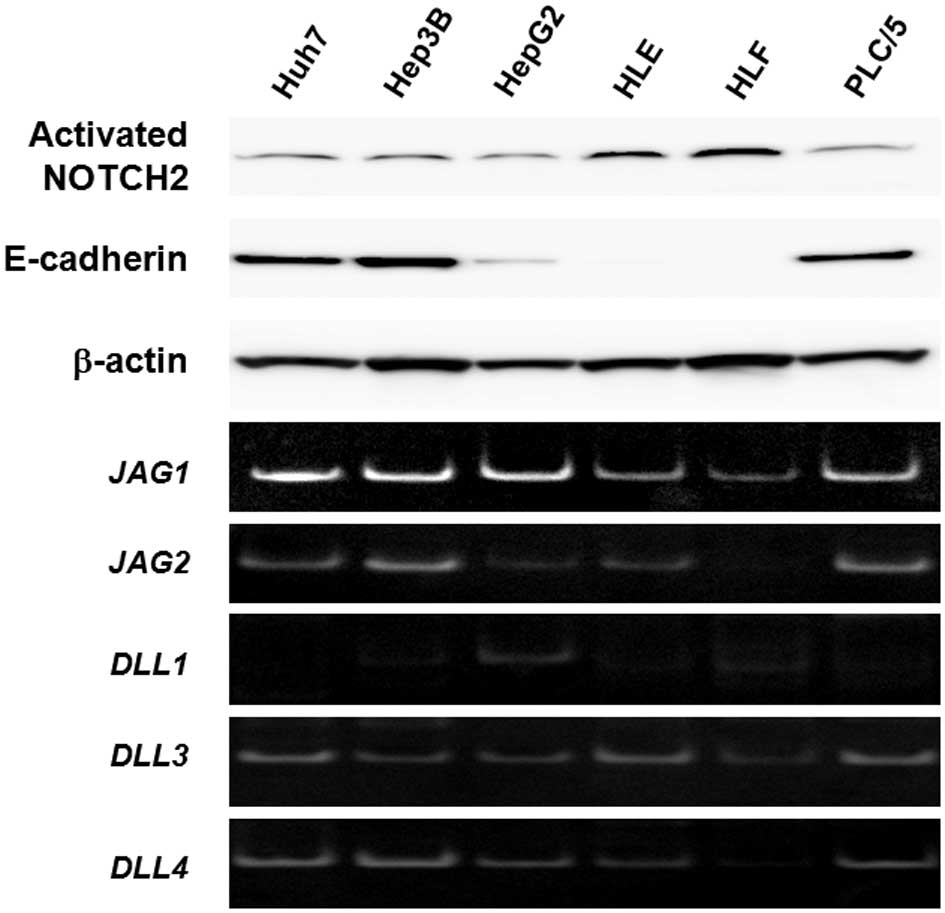
NOTCH2 expression in human HCC cell
lines
To determine whether NOTCH2 actually controlled
aggressive behavior and immature cellular morphology of HCC cells,
we examined NOTCH2 expression in six human HCC cell lines by
western blot analysis (Fig. 3).
Anti-NOTCH2 antibodies specifically recognizing the activated
NOTCH2 intracellular domain demonstrated that this domain was
detectable in all cell lines analyzed. However, the expression
levels varied. For example, two HCC cell lines, HLE and HLF,
expressed relatively high levels of NOTCH2, accompanied by loss of
E-cadherin expression as a marker protein for differentiated
epithelium. The other cell lines tested were positive for
E-cadherin and exhibited relatively lower NOTCH2 expression.
Consequently, there was an apparent correlation between cellular
anaplasia and NOTCH2 activation in cultured HCC cells. RT-PCR
analyses of NOTCH ligand genes, i.e., JAG1, JAG2,
DLL1, DLL3 and DLL4 (Fig. 3), indicated that each cell line
expressed all of these genes; however, the expression levels of
these targets varied between the cell lines.
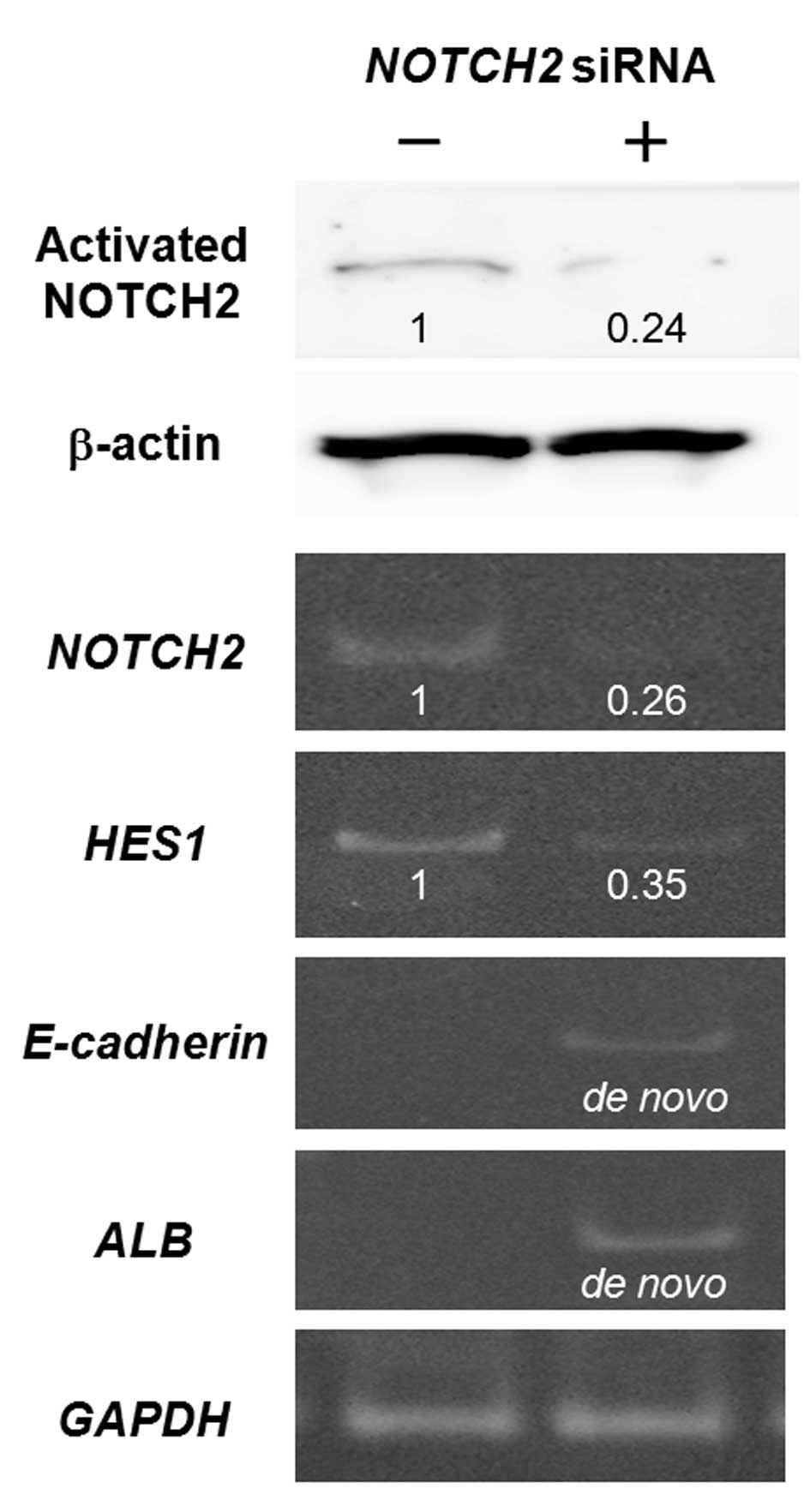
Knockdown of NOTCH2 expression in HLF
cells
To further clarify the role of NOTCH2 signaling, we
used the HLF cell line with a high NOTCH2 expression, for gene
knockdown experiments. We first transiently transfected the cells
with siRNA targeting NOTCH2 (Fig. 4). HLF cells were revealed to be
anaplastic HCC cells that do not express detectable amounts of mRNA
for E-cadherin or ALB, a mature hepatocyte marker.
However, 48 h after transfection, expression of E-cadherin
and ALB, mRNAs was induced, concurrent with the reductions
in mRNA/protein for NOTCH2 and mRNA for HES1, a
representative NOTCH target gene (1).
We then attempted to generate an HLF clone with
reduced levels of NOTCH2 by stable transfection of
NOTCH2 shRNA. Western blotting revealed that NOTCH2
expression was most effectively inhibited in a clone, termed
HLF-kd, which was further characterized in comparison with a clone
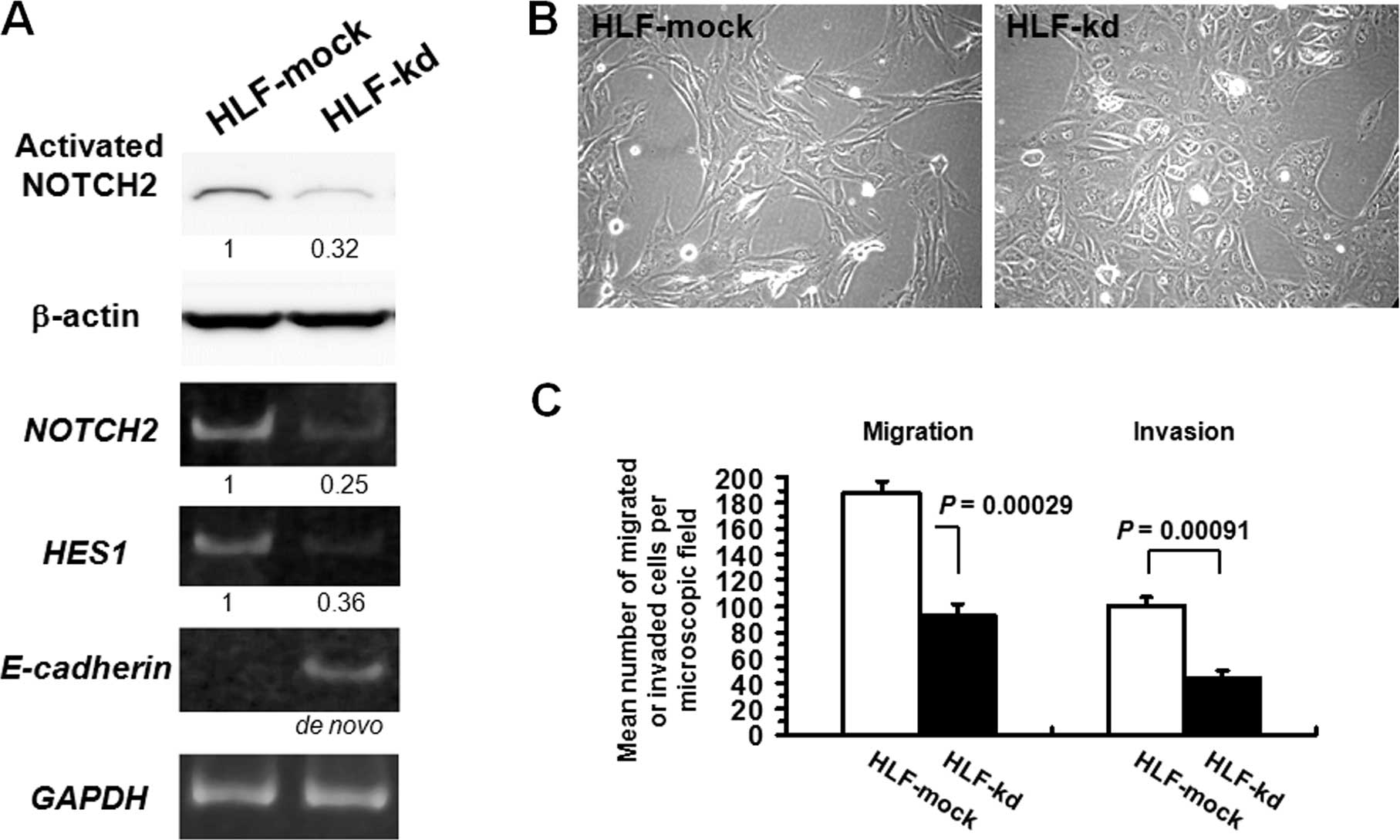
mock-trans-fected with vector only, HLF-mock (Fig. 5A). HLF-kd cells showed de
novo expression of E-cadherin. The NOTCH target gene
HES1 exhibited decreased mRNA expression, confirming that
NOTCH2 signaling was inhibited in HLF-kd cells. Morphologically,
while HLF-mock cells as well as parental HLF cells were spindle in
shape, HLF-kd cells were polygonal and more epithelial-like
(Fig. 5B). We next evaluated the
effects of NOTCH2 knockdown on migration and invasion in HLF
cells in vitro (Fig. 5C).
HLF-kd cells exhibited significantly reduced migratory potential
and invasiveness compared with control HLF-mock cells.
Overexpression of NOTCH2 intracellular
domain in Huh7 cells
Among the six HCC cell lines tested, three,
including Huh7, retained high E-cadherin expression with relatively
low NOTCH2 expression levels (Fig.
3). In order to clarify the effects of induced overexpression
of NOTCH2, we transfected Huh7 cells with an expression construct
for the NOTCH2 intracellular domain. One clone with the highest
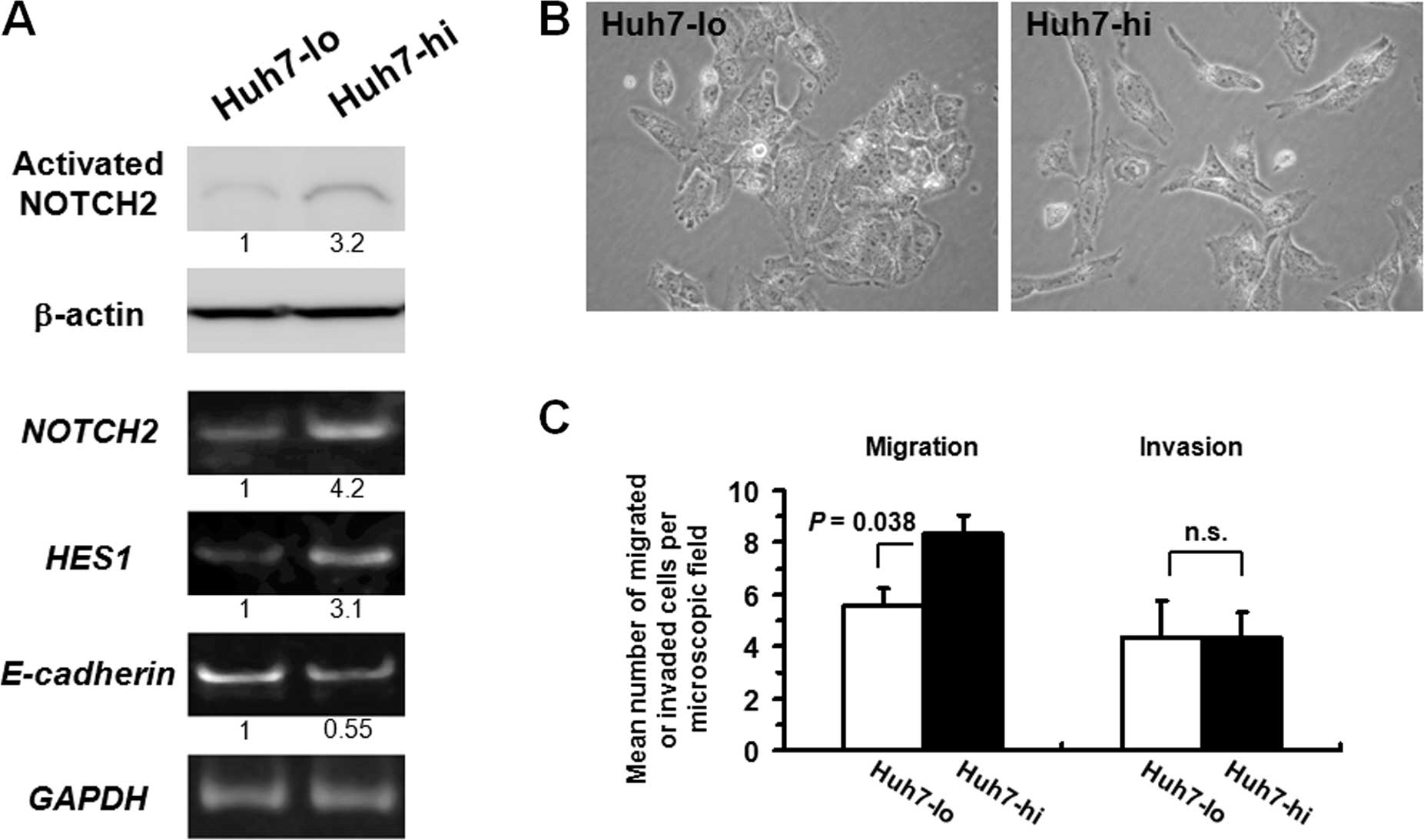
expression of NOTCH2 was isolated (Huh7-hi), as was the clone with
the lowest NOTCH2 expression (Huh7-lo; Fig. 6A). Compared with Huh7-lo cells,
Huh7-hi cells exhibited an increase in HES1 mRNA and a
modest decrease in E-cadherin mRNA. Morphologically, while
Huh7-lo cells were typically epithelial-like, represented by their
polygonal shape and tight adhesion, Huh7-hi cells were more
elongated and scattered (Fig. 6B).
In vitro migration and invasion assays revealed that Huh7-hi
cells acquired a modest increase in migratory potential, but no
apparent enhancement in invasiveness compared with Huh7-lo cells
(Fig. 6C). Both Huh7-hi and -lo
cells exhibited only <5% of the migratory or invasive activity
relative to HLF-mock cells, indicating that the intrinsic migratory
and invasive potentials of Huh7 cells were limited.
Effects of NOTCH2 expression on in vivo
tumorigenicity and morphology of HCC cell lines in vivo
The results of in vitro experiments indicated
that NOTCH2 signaling causes immature morphology and biological
aggressiveness in human HCC cells. We thus performed in vivo
tumorigenicity assays using immunodeficient NOD SCID mice and HCC
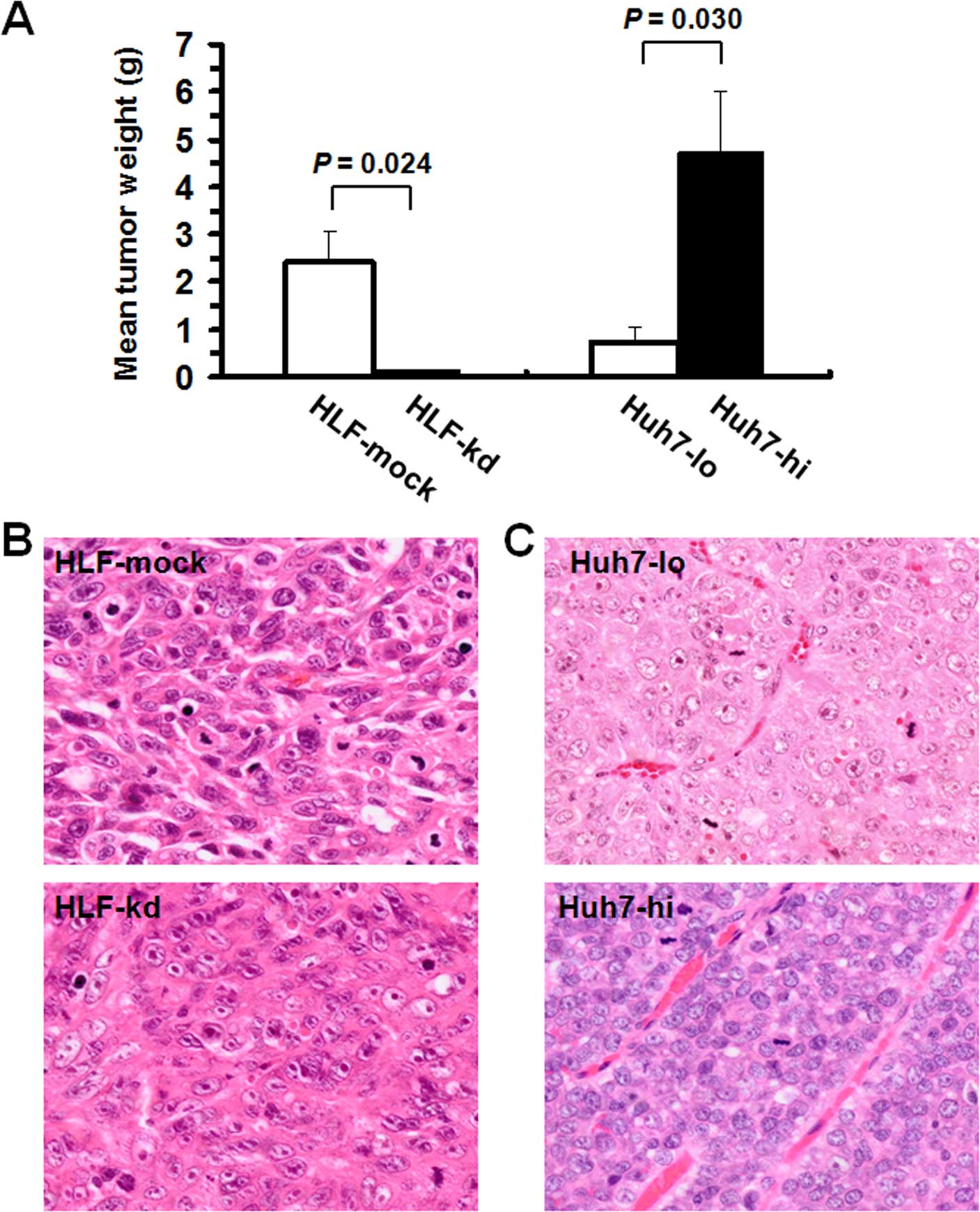
cells with stable modifications to NOTCH2 expression. HLF-kd-based
tumors exhibited significantly lower mean tumor weights than
control HLF-mock-based tumors (Fig.
7A). Histologically, tumors arising from HLF-mock cells were
highly anaplastic, with hyperchromatic nuclei, and exhibited
increased cellularity and N/C ratios (Fig. 7B). On the contrary, tumors arising
from HLF-kd cells exhibited more hepatocyte-like cellular
morphologies, with relatively abundant, eosinophilic cytoplasms and
vesicular nuclei with prominent nucleoli. The same experiments were
performed with Huh7-hi and Huh7-lo cells. Huh7-hi-based tumors
exhibited significantly higher mean tumor weights than tumors
arising from Huh7-lo cells (Fig.
7A). Tumors arising from Huh7-hi or Huh7-lo cells were
histopathologically diagnosed as moderately to poorly
differentiated, trabecular HCCs (Fig.
7C). However, relative to the tumors arising from Huh7-lo
cells, those arising from Huh7-hi cells exhibited a more immature
morphology characterized by high cellularity and N/C ratio.
Discussion
Our results indicated that, contradictory to the
results of a previous study (7),
activation of NOTCH2 signaling is not a rare event in clinically
advanced or metastatic HCCs. We further provided evidence that
NOTCH2 signaling induces morphological immaturity in HCC cells,
while NOTCH1 activation did not seem to be tightly linked to this
phenotype. The immature morphology of individual HCC cells was not
necessarily associated with poor grade of differentiation, since
NOTCH2 signaling did not appear to affect the structural
atypism.
Unlike HCCs, most human hepatoblastomas have been
shown to be positive for nuclear NOTCH2 using immunohistochemistry
analysis (15). Hepatoblastoma
cells, particularly of embryonal type, are morphologically
immature, exhibiting small sizes and high N/C ratios. Thus,
NOTCH2-positive HCC cells share some morphological characteristics
with hepatoblastoma cells. In fact, NOTCH2-positive HCCs more
frequently expressed representative hepatoblast markers, AFP, CK19
and EpCAM, than NOTCH2-negative HCCs. However, in contrast to
typical hepatoblastomas, half of the NOTCH2-positive HCCs were
immunohistochemically negative for AFP, implying that
NOTCH2-positive HCC cells may not be simply regarded as equivalent
to hepatoblastoma cells.
In mouse models, constitutive activation of either
NOTCH1 or NOTCH2 specifically in the liver has been shown to
promote hepatocellular tumor development (5,16). In
addition, recent studies using cultured human HCC cells revealed
that NOTCH1 activation augments the migration and invasiveness of
HCC cells in vitro (17,18).
These observations, along with our present results, indicate that
both NOTCH1 and NOTCH2 may be capable of promoting human
hepatocarcinogenesis. In particular, our data on primary and
metastatic HCCs suggested that cooperation between NOTCH2 and
NOTCH1 signaling may confer a selective advantage for metastasis in
HCC cells. However, we found that only NOTCH2 was significantly
associated with advanced clinical staging in primary HCCs.
Moreover, some earlier studies proposed that NOTCH1 signaling may
inhibit human hepatocarcinogenesis through induction of apoptosis
in HCC cells (6,7). Therefore, NOTCH2 rather than NOTCH1
may be more important for the promotion of
hepatocarcinogenesis.
The correlation between activated NOTCH2 signaling
and advanced clinical stage or metastasis suggested the potential
usefulness of NOTCH2 as a predictor of prognosis in patients with
HCC. This idea was also supported by our in vitro migration
and invasion assay results or the results of our in vivo
tumorigenicity assay using human HCC cell lines. Notably, all
NOTCH2-positive primary HCC patients in the present study were men,
which probably reflected the poorer prognosis of male HCC patients
than female HCC patients (19).
Acknowledgments
We thank Dr Tasuku Honjo and Dr Shigekazu Nagata for
their distribution of the expression vector plasmid for the NOTCH2
intracellular domain through Riken DNA Bank. This study was
supported in part by grant from the Grants-in-Aid for Scientific
Research from the Japan Society for the Promotion of Science.
References
|
1
|
Tien AC, Rajan A and Bellen HJ: A Notch
updated. J Cell Biol. 184:621–629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kureishy N, Sapountzi V, Prag S, Anilkumar
N and Adams JC: Fascins, and their roles in cell structure and
function. BioEssays. 24:350–361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang C, Qi R, Li N, Wang Z, An H, Zhang Q,
Yu Y and Cao X: Notch1 signaling sensitizes tumor necrosis
factor-related apoptosis-inducing ligand-induced apoptosis in human
hepatocellular carcinoma cells by inhibiting Akt/Hdm2-mediated p53
degradation and up-regulating p53-dependent DR5 expression. J Biol
Chem. 284:16183–16190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang XQ, Zhang W, Lui EL, Zhu Y, Lu P, Yu
X, Sun J, Yang S, Poon RT and Fan ST: Notch1-Snail1-E-cadherin
pathway in metastatic hepatocellular carcinoma. Int J Cancer.
131:E163–E172. 2012. View Article : Google Scholar
|
|
5
|
Villanueva A, Alsinet C, Yanger K, Hoshida
Y, Zong Y, Toffanin S, Rodriguez-Carunchio L, Solé M, Thung S,
Stanger BZ, et al: Notch signaling is activated in human
hepatocellular carcinoma and induces tumor formation in mice.
Gastroenterology. 143:1660–1669.e7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geisler F, Nagl F, Mazur PK, Lee M,
Zimber-Strobl U, Strobl LJ, Radtke F, Schmid RM and Siveke JT:
Liver-specific inactivation of Notch2, but not Notch1, compromises
intrahepatic bile duct development in mice. Hepatology. 48:607–616.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao J, Song Z, Chen Y, Xia L, Wang J, Fan
R, Du R, Zhang F, Hong L, Song J, et al: Deregulated expression of
Notch receptors in human hepatocellular carcinoma. Dig Liver Dis.
40:114–121. 2008. View Article : Google Scholar
|
|
8
|
Yamasaki K, Hayashi Y, Okamoto S, Osanai M
and Lee GH: Insulin-independent promotion of chemically induced
hepatocellular tumor development in genetically diabetic mice.
Cancer Sci. 101:65–72. 2010. View Article : Google Scholar
|
|
9
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York: 2010
|
|
10
|
Liver Cancer Study Group of Japan: The
Clinical and Pathological Study of Primary Liver Cancer. 5th
edition. Kanehara Press; Tokyo: 2008
|
|
11
|
Mizushima S and Nagata S: pEF-BOS, a
powerful mammalian expression vector. Nucleic Acids Res.
18:53221990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kato H, Sakai T, Tamura K, Minoguchi S,
Shirayoshi Y, Hamada Y, Tsujimoto Y and Honjo T: Functional
conservation of mouse Notch receptor family members. FEBS Lett.
395:221–224. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hayashi Y, Osanai M and Lee GH: Fascin-1
expression correlates with repression of E-cadherin expression in
hepatocellular carcinoma cells and augments their invasiveness in
combination with matrix metalloproteinases. Cancer Sci.
102:1228–1235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Theise N, Chua M and Reid LM: The
stem cell niche of human livers: Symmetry between development and
regeneration. Hepatology. 48:1598–1607. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Litten JB, Chen TT, Schultz R, Herman K,
Comstock J, Schiffman J, Tomlinson GE and Rakheja D: Activated
NOTCH2 is overexpressed in hepatoblastomas: An immunohistochemical
study. Pediatr Dev Pathol. 14:378–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dill MT, Tornillo L, Fritzius T,
Terracciano L, Semela D, Bettler B, Heim MH and Tchorz JS:
Constitutive Notch2 signaling induces hepatic tumors in mice.
Hepatology. 57:1607–1619. 2013. View Article : Google Scholar
|
|
17
|
Zhou L, Wang DS, Li QJ, Sun W, Zhang Y and
Dou KF: The down-regulation of Notch1 inhibits the invasion and
migration of hepatocellular carcinoma cells by inactivating the
cyclo-oxygenase-2/Snail/E-cadherin pathway in vitro. Dig Dis Sci.
58:1016–1025. 2013. View Article : Google Scholar
|
|
18
|
Zhou L, Zhang N, Song W, You N, Li Q, Sun
W, Zhang Y, Wang D and Dou K: The significance of Notch1 compared
with Notch3 in high metastasis and poor overall survival in
hepatocellular carcinoma. PLoS One. 8:e573822013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ng IO, Ng MM, Lai EC and Fan ST: Better
survival in female patients with hepatocellular carcinoma. Possible
causes from a pathologic approach. Cancer. 75:18–22. 1995.
View Article : Google Scholar : PubMed/NCBI
|