Introduction
Hepatitis B virus (HBV) infection is a major global
health issue, and more than 350 million individuals are infected by
HBV worldwide (1). Approximately
10–15% of pregnant women in China are carriers of the HBV surface
antigen (HBsAg) and HBV e antigen (HBeAg) and 5–15% of their babies
are infected by intrauterine transmission (2,3). The
mechanism of intrauterine HBV infection is not completely
understood; transplacental leakage of maternal blood has been
suggested as a possible mechanism (4). Trophoblasts, specialized cells of the
placenta, mediate the contact between two genetically different
individuals, the embryo and the mother, by establishing a transient
embryonic organ, the placenta (5).
Impaired trophoblast function may result in a range of adverse
pregnancy outcomes, such as spontaneous abortion, intrauterine
infection and stillbirth (6).
Among the HBV gene products, the hepatitis B virus X
protein (HBx) is a multifunctional viral regulator and is
considered as one of the most important determinants involved in
viral pathogenesis and carcinogenesis (7). HBx, which consists of 154 amino acids,
contains four regions important for transcriptional regulation,
cell cycle control, cell adhesion and modulation of cytoplasmic
signal transduction pathways (8).
Different regions of HBx may play different roles in the
pathological process. For example, HBxΔ127 (deletion from 382 to
401 bp) was able to upregulate transcriptional activities of
nuclear factor-κB, survivin and human telomerase reverse
transcriptase, as well as the expression levels of c-Myc and
proliferating cell nuclear antigen in hepatoma cells (9).
To our knowledge, the role of HBx in trophoblasts
remains unclear. In the present study, we constructed two HBx
mutants, including N-terminal and C-terminal mutants, and analyzed
the biological activities of HTR-8/SVneo cells with those truncated
mutants of the HBx gene. Finally, we identified the underlying
mechanism involving the promotion of cell growth and invasion
mediated by the HBx mutants.
Materials and methods
Cell lines and culture conditions
The trophoblast cell line HTR-8/SVneo, stored in our
laboratory, was maintained in RPMI-1640 medium which was
supplemented with 10% heat-inactivated FBS, 2 mM glutamine,
penicillin (100 U/ml) and streptomycin (100 mg/ml) at 37°C in 5%
CO2.
Plasmid construction and
transfection
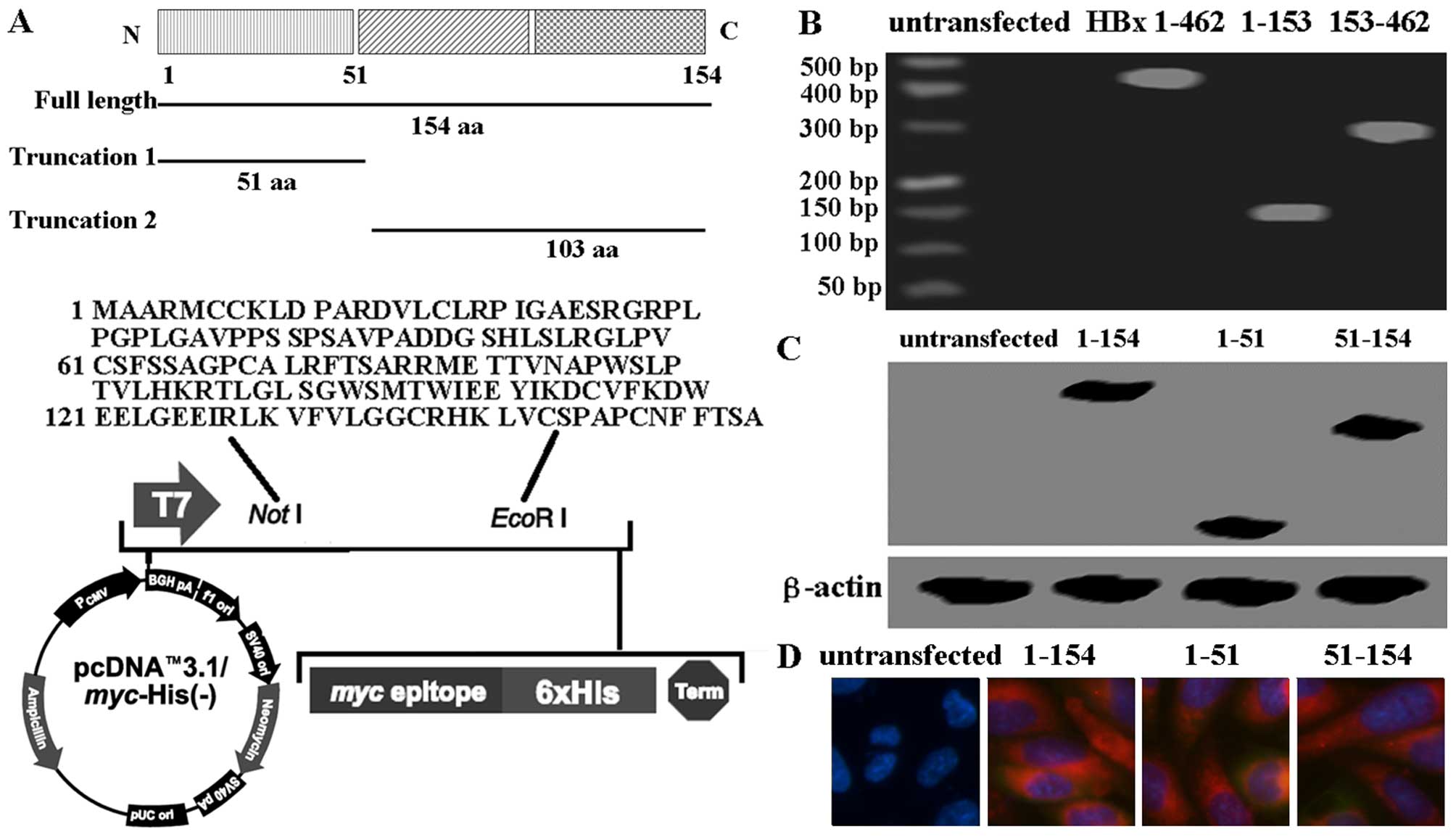
The ideograph of HBx protein and its truncated form
is shown in Fig. 1A. The plasmid
pcDNA3.1-HBx was kindly provided by Mr. Jin-Cheng Li (China Medical
University, Shenyang, China). For the preparation of truncated HBx
proteins, the fragments were respectively amplified from full
length HBx by retro-transcription PCR (RT-PCR) where restriction
sites were added to PCR primers (Table
I) and cloned into pcDNA3.1. The sequence of the successful
clone was confirmed by DNA sequencing. Transfection of plasmids to
the HTR-8/SVneo cells was performed using Lipofectamine 2000
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer's
instructions. Briefly, the cells were plated in RPMI-1640 medium
with 10% FBS and cultured until they achieved 70–80% confluency.
Culture medium was then replaced with low-serum media (containing
0.5% FBS), and then the cells were transfected with 5
µg/well of the plasmid. Forty-eight hours after
transfection, the pcDNA3.1-transfected (mock),
pcDNA3.1-HBx-trans-fected (intact), pcDNA3.1-HBx-ΔC-transfected
(T1), and pcDNA3.1-HBx-ΔN-transfected (T2) HTR-8/SVneo cells were
used in the subsequent studies.
 | Table IPrimers used to generate intact and
truncated forms of HBx. |
Table I
Primers used to generate intact and
truncated forms of HBx.
| Region of HBx
amplified | Primers (5′-3′) | Product (bp) |
|---|
| aa 1–51 (Intact) | F:
GCGGCCGCATTGCGGCCGCATTAGGCAG | 153 |
| R: GAATTCAACCATGGCTGCTAGGCTGTGC | |
| aa 1–17 (Truncated
1) | F:
GCGGCCGCTTACCCGTGGTCGGTCGGT | 51 |
| R: GAATTCAACCATGGCTGCTAGGCTGTGC | |
| aa 18–51 (Truncated
2) | F:
GCGGCCGCATTAGGCAGAGGTGAAAAAGT | 102 |
| R: GAATTCACTATGGCGCACCTCTCTTTACGCG | |
Retro-transcription PCR and quantitative
real-time PCR
The expression of the relevant HBx in the
established cell lines was verified by RT-PCR. Total RNA was
extracted from the cell lines (the cells transfected with intact
HBx, truncated 1 and 2) using TRIzol reagent (Invitrogen) according
to the manufacturer's instructions. First-strand cDNAs were
generated in reverse transcriptase reactions containing total RNA,
poly(dT) oligonucleotides, and SuperScript II reverse transcriptase
(Invitrogen). cDNAs were then subjected to RT-PCR analysis. The
primers used to generate the intact and truncated forms of HBx are
shown in Table I. The mRNA levels
of inflammatory factors were detected using the primers that are
listed in Table II. GAPDH
was used as an internal control. Amplification of the primers as
described above was performed with 1 cycle at 95°C for 10 min and
40 cycles of 95°C for 15 sec and 60°C for 60 sec.
 | Table IIPrimers used to detect the mRNA
levels of inflammatory factors. |
Table II
Primers used to detect the mRNA
levels of inflammatory factors.
| Gene | Sequence (5′–3′;
forward/reverse) |
|---|
| IL-1α |
AGAAGAGACGGTTGAGTTTAAGCCAATCCA/ATCCAGTCGTGGAAAATCGAAGGACTC |
| IL-1β |
CAGGGACAGGATATGGAGCAACAA/CATCTTTCAACACGCAGGACAGGT |
| TNF-α |
AGGCCAAGCCCTGGTATGAGC/CACAGGGCAATGATCCCAAAGTAG |
| IL-6 |
CACCCCTGACCCAACCACAAAT/TCCTTAAAGCTGCGCAGAATGAGA |
| IL-10 |
CCGCCTCAGCCTCCCAAAGT/CCCTAACCTCATTCCCCAACCAC |
| IFN-γ |
TAGCAACAAAAAGAAACGAGATGACT/GATTTTGTCCCTTCGCTTTTTCC/ |
| GAPDH |
TGGTATCGTGGAAGGACTCATGAC/ATGCCAGTGAGCTTCCCGTTCAGC |
TGF-β1 treatment
Recombinant human transforming growth factor-β1
(TGF-β1) was obtained from R&D Systems (Minneapolis, MN, USA).
The effect of TGF-β1 on the untransfected HTR-8/SVneo and
pcDNA3.1-HBx-transfected cells was determined by adding recombinant
TGF-β1 (5 ng/ml) to cell monolayers at 70% confluency. Cells were
incubated for an additional 24 h and used in the subsequent
studies.
Cell growth assay
Viability of the transfected cells was determined
using the 3-(4,5-dimethylthiazolyl)-2,5-di-phenyltetrazolium
bromide (MTT) assay (Sigma-Aldrich, Carlsbad, CA, USA). The cells
were plated in 96-well plates (1,500 cells/well) and incubated
under normal culture conditions. After 24 h, the cells were treated
with 0.5 mg/ml MTT for 4 h and lysed with dimethyl sulfoxide
(DMSO). Absorbance rates were measured at 550–560 nm using a
microplate reader (Bio-Rad, Hercules, CA, USA).
Apoptosis detection
Cells were trypsinized, washed twice with cold PBS,
and resuspended in 200 µl binding buffer. Annexin V-FITC was
added to a final concentration of 0.5 µg/ml, according to
the manufacturer's instructions (Keygen, Nanjing, China). After 20
min of incubation at room temperature in the dark, 400 µl of
binding buffer containing propidium iodide (PI, 50
µg/µl) was added, and samples were immediately
analyzed on a FACSCalibur flow cytometer (Becton-Dickinson Medical
Devices, Shanghai, China).
Invasion assays
Transwell chamber (8-µm pore size
polycarbonate membrane; Cell Biolabs, San Diego, CA, USA) Matrigel
invasion assays were performed as previously described (10). Cells (30,000) were placed in the
upper chamber and allowed to invade for 24 h. Twenty fields of
cells were acquired at ×10 magnification and quantified. Relative
invasion was expressed as a ratio to the control cells.
Western blotting
Cells and tissues were washed and lysed and an equal
amount of proteins to ensure equal protein loading was subjected to
SDS-PAGE and then blotted onto PVDF membranes (GE Healthcare Corp.,
Piscataway, NJ, USA). The membranes were blocked in 5% milk-TBST
and then probed with the primary antibody. TGF-β1 RI (sc-398),
p-Smad3 (sc-130218), Smad3 (sc-101154), p-Smad2 (sc-135644), Smad2
(sc-6200), E-cadherin (sc-7870), vimentin (sc-6260), N-cadherin
(sc-7939) and β-actin (sc-47778) were purchased from Santa Cruz
Biotechnology (Shanghai, China). Anti-HBx (Abcam, Cambridge, UK)
was used to identify the results of the transfection. β-actin was
used as an internal control. The secondary antibody was anti-mouse
IgG, anti-rabbit IgG, or anti-goat IgG (determined by primary
antibodies) at a dilution of 1:1,000–2,000 (Amersham Biosciences,
Needham, MA, USA). Then the results were detected by enhanced
chemiluminescence (Amersham Pharmacia, Piscataway, NJ, USA).
Phyre database was used to generate a
predicted structural model
The protein sequence of HBX was obtained from Pubmed
(http://www.ncbi.nlm.nih.gov/protein/CAA49453.1) and
submitted to Protein Homology/analogY Recognition Engine (Phyre
version 2). Based on the homology sequence in the Phyre server, the
three-dimensional structure of ING2 protein was predicted.
Physicochemical profiles of HBX
Physicochemical profiles, such as titration curve,
hydrophobicity, antigenicity, fexibility, and solvent
accessibility, were analyzed using Antheprot 5.0 software.
Statistical analysis
Statistical analyses were carried out using GraphPad
Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). Data are
expressed as mean ± standard deviation of three independent
experiments performed in triplicate. Data comparisons in relation
to the control were performed by one-way ANOVA. Differences were
considered statistically significant if a p-value <0.05 was
achieved.
Results
mRNA and protein levels of different HBx
fragments evaluated in the cell lines
After transfection, the mRNA and protein levels of
the different HBx fragments were detected using western blot
analysis, RT-PCR and immunofluorescence. As shown in Fig. 1B and C, the levels of HBx protein
and mRNA in the HTR-8/SVneo cells following transfection were
higher than levels in the cells without transfection. Furthermore,
intact HBx protein and truncated HBx fragments were localized in
the cytoplasm by immunofluorescence assay (Fig. 1D). The results showed that intact
HBx protein and the truncated HBx fragments were successfully
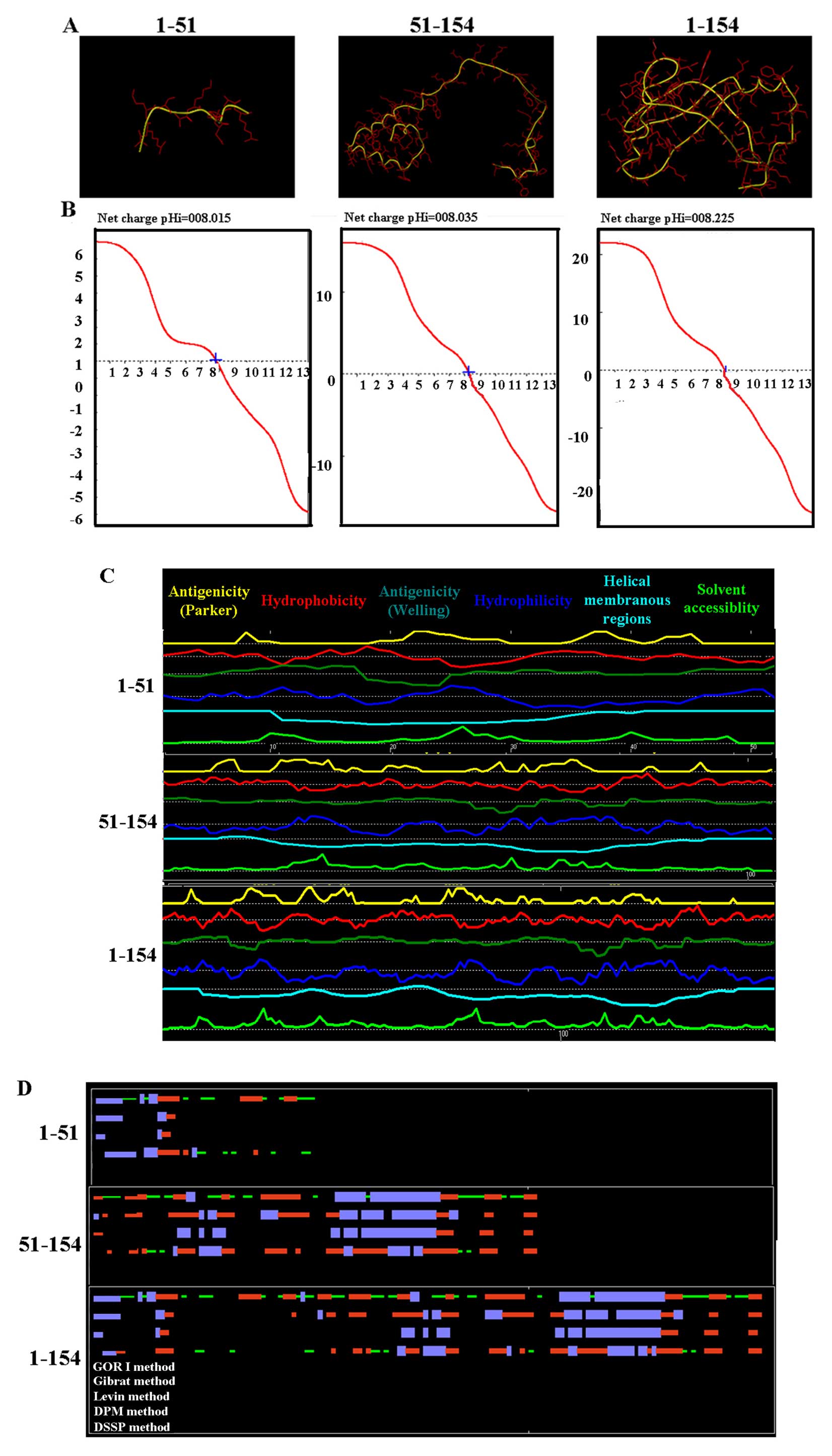
transfected into the HTR-8/SVneo cells. Furthermore, 3D structures
of intact HBx protein and truncated HBx fragments were predicated
using Phyre version 2 (Fig. 2A).
Titration curve, hydrophobicity, antigenicity, fexibility and
solvent accessibility of the different HBx fragments did not have
significant difference as determined using Antheprot 5.0 software
(Fig. 2B and C). 2D structures of
intact HBx protein and truncated HBx fragments were also confirmed
(Fig. 2D).
The roles of different HBx fragments in
HTR-8/SVneo cells
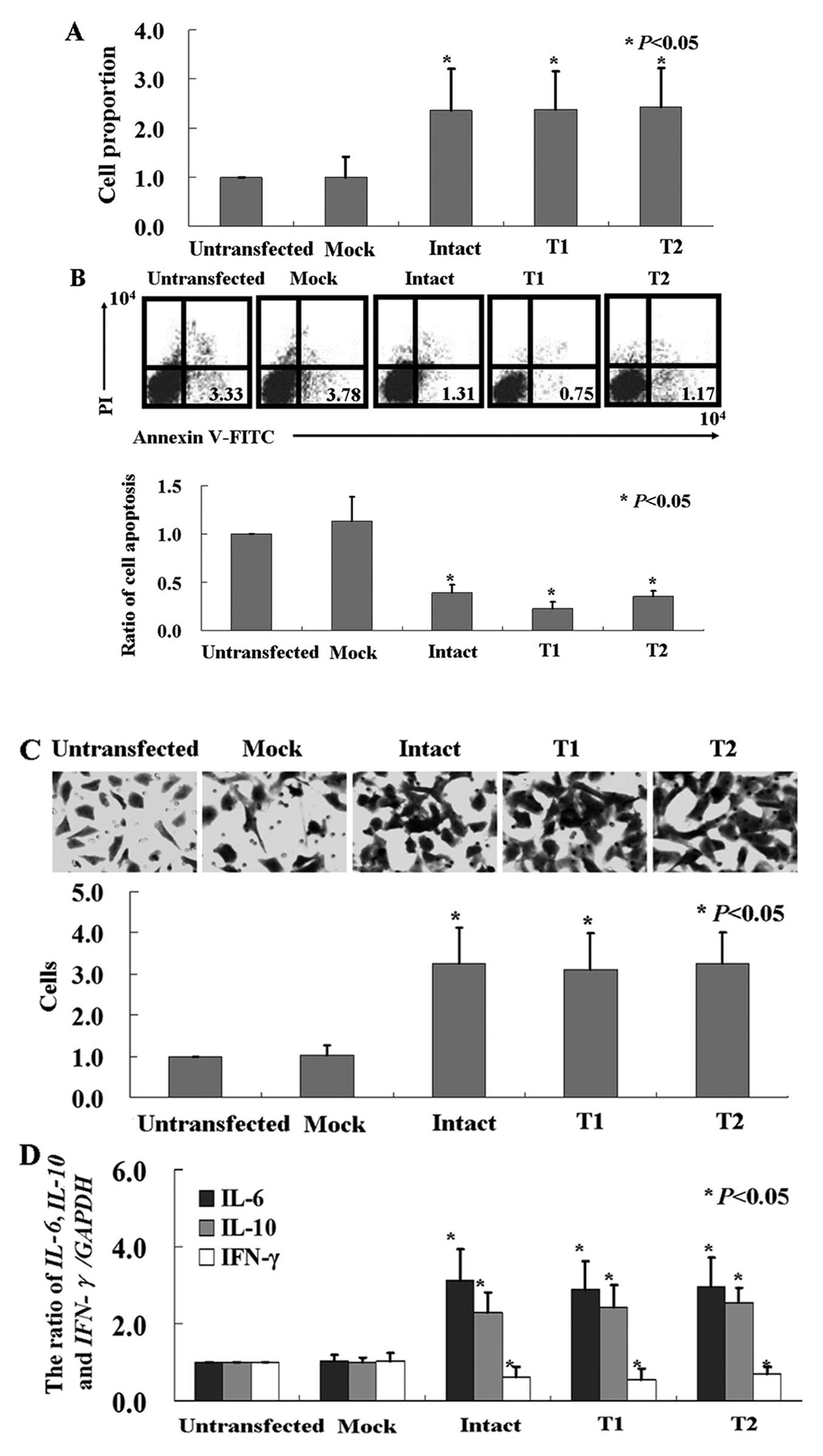
Cell viability was monitored using an MTT assay, and
Fig. 3A shows that the
proliferation rate of the HTR-8/SVneo cells was induced by both
intact and truncated HBx (P<0.05). We utilized Annexin V-FITC
and PI double staining to detect the apoptotic cells. Our results
showed that the apoptotic ratio of the cells after transfection
with the intact or truncated HBx was significantly decreased
(Fig. 3B, P<0.05). We determined
whether there were any changes in the invasive ability in the
HBx-expressing cells using the Transwell assay. We found that
significantly more HBx-expressing cells migrated to the lower
membrane compared with the control cells (Fig. 3C, P<0.05). The mRNA levels of
inflammatory factors IL-6 and IL-10 in the
HBx-expressing cells were significantly higher than levels in the
control cells, while IFN-γ was lower (Fig. 3D, P<0.05). In contrast, slightly
increased levels of IL-1α, IL-1β and TNF-α
were detected in the HTR-8/SVneo cells after HBx treatment (data
not shown).
HBx fragments activate the Smad signaling
pathway and induce changes in epithelial-mesenchymal transition
(EMT)
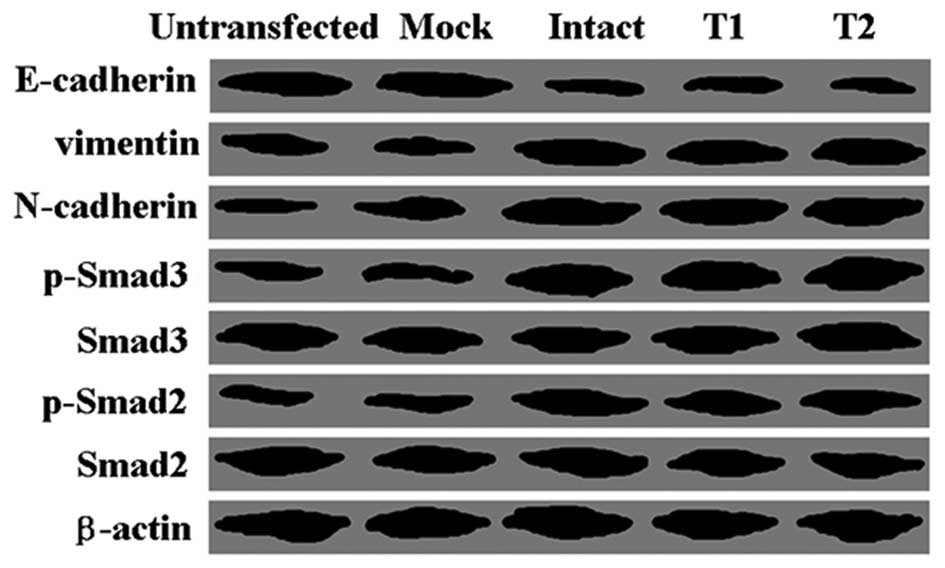
Furthermore, we carried out western blot analysis to
identify the mechanism of apoptosis induced by HBx. We found that
the levels of phospho-Smad3 and phospho-Smad2 in the transfected
cells were higher than the levels in the untransfected cells, while
levels of total Smad3 and Smad2 were not altered (Fig. 4). Compared with the untransfected
cells, HBx-transfected cells showed a lower expression level of
epithelial marker E-cadherin, while higher expression levels of
mesenchymal markers vimentin and N-cadherin were noted (Fig. 4).
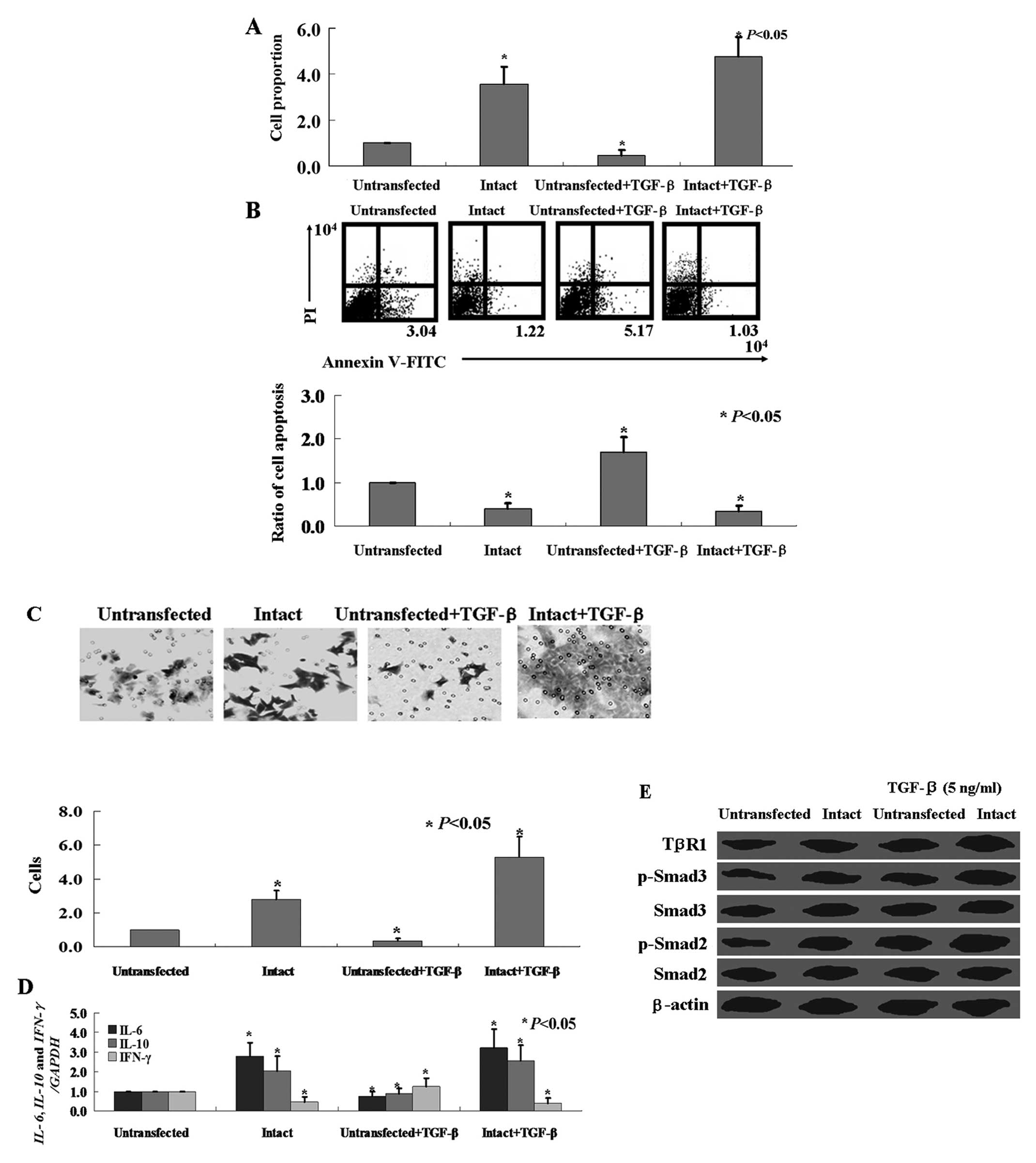
TGF-β1 decreases HTR-8/SVneo cell
proliferation and invasion, while increases HBx-transfected
HTR-8/SVneo cell proliferation and invasion
TGF-β1 was used to further clarify the effect of the
Smad signaling pathway on HTR-8/SVneo cells. HTR-8/SVneo cells were
treated with recombinant human TGF-β1 (5 ng/ml). The proliferation
rate of the HTR-8/SVneo cells was inhibited by recombinant human
TGF-β1 (Fig. 5A, P<0.05).
Annexin V-FITC and PI double staining showed that recombinant human
TGF-β1 induced apoptosis in the HTR-8/SVneo cells (Fig. 5B, P<0.05). Matrigel invasion
assay showed that treatment with 5 ng/ml of TGF-β1 significantly
decreased HTR-8/SVneo cell invasion (Fig. 5C, P<0.05). The mRNA levels of
inflammatory factors IL-6 and IL-10 in the
HTR-8/SVneo cells treated with TGF-β1 were lower than levels in the
control cells (Fig. 5D, P<0.05).
Western blot analysis showed that TGF-β1 treatment induced TGF-β1
RI expression, and Smad2 and Smad3 phosphorylation in the
HTR-8/SVneo cells (Fig. 5E).
Although TGF-β1 also induced Smad2 and Smad3 phosphorylation in the
HBx-transfected HTR-8/SVneo cells (Fig.
5E), opposite effects of TGF-β1 on the HBx-transfected
HTR-8/SVneo cells were observed when compared with the
untransfected cells (Fig. 5).
TGF-β1 increased proliferation (Fig.
5A, P<0.05) and invasion (Fig.
5C, P<0.05), inhibited apoptosis (Fig. 5B, P<0.05), and induced the
inflammatory response (Fig. 5D,
P<0.05) in the HBx-transfected HTR-8/SVneo cells.
Discussion
Mother-to-infant transmission of HBV can occur as an
intrauterine, intrapartum or postpartum infection (4). Intrauterine infection by HBV is mainly
transmitted through the placenta. HBV can reach the placenta though
maternal blood and infect placental trophoblast cells and then
survive and replicate in the cells, and functional protein HBxAg is
produced (11).
In the present study, we confirmed that HBx
inhibited apoptosis and increased invasive ability in the
HTR-8/SVneo cells. Bai et al (11) found that the apoptosis index of
HBV-infected high replication cells was lower than that of
uninfected ones. Liu et al (12) found that HBx could promote hepatoma
cell invasion and metastasis. The HBx genome consists of an
N-terminal negative regulatory domain and a C-terminal
transactivation domain (13). The
HBx fragment at the C-terminal can strongly enhance the
proliferation and growth of liver cells (14). However, Chau et al (15) found that the first 50 aa
NH2 region of HBx has the ability to resist cell death
stimulation. Notably, we found that two truncated forms of HBx
(N-terminal or C-terminal mutants) had similar effects as intact
HBx on the HTR-8/SVneo cells. Based on our present data, we could
not explain these contradictory results of HBx in different cells
as HBx has been reported to be both pro-apoptotic and
anti-apoptotic. We will investigate the contradiction in future
research.
The trophoblast is a cell type with endocrine
functions that also secretes various cytokines throughout the
gestation period (16). IL-10 was
found to be a critical molecule for successful pregnancy outcome in
both human and mouse pregnancy models (17). Lack of IL-10 expression in
trophoblasts may contribute to an increased inflammatory response
in the placenta (18). Regulation
of IL-6 secretion during pregnancy is essential for maintaining
normal gestation. Elevated IL-6 is observed during recurrent
miscarriage, pre-eclampsia and preterm delivery (19). Moreover, IFN-γ can be deleterious to
pregnancy, as determined by in vivo animal model studies
(20). Hu et al (21) provided direct evidence that IFN-γ
can influence extravillous cytotrophoblast (EVT) outgrowth and
migration. In the present study, we also found upregulation of
IL-6 and IL-10 mRNA levels and downregulation of the
IFN-γ mRNA level in the HTR-8/SVneo cells following HBx
transfection.
EMT is a process whereby epithelial cells change to
a mesenchymal phenotype, and this process is important in the
progression of human carcinomas to a more invasive, metastatic
capacity (22). We confirmed that
HBx-induced invasion of HTR-8/SVneo cells involves EMT.
Furthermore, we found that the Smad signaling pathway was activated
by HBx in the HTR-8/SVneo cells. The Smad family of proteins
(Smad1–8) are classified according to their different functions;
Smad1, 2, 3, 5 and 8 are 'receptor-regulated SMADs', Smad4 is
termed the 'common-mediator SMAD', and Smad6 and 7 are 'inhibitory
SMADs' (23). These proteins
regulate numerous cellular processes, such as cell proliferation,
differentiation, apoptosis and control of developmental fate
(24). Robust activation of Smad2
and Smad3 is through phosphorylation of C-terminal regulatory
residues (23). In the present
study, we confirmed that p-Smad2 and p-Smad3 were significantly
upregulated in the HTR-8/SVneo cells following HBx transfection. To
confirm the effect of the Smad signaling pathway on HTR-8/SVneo
cells, TGF-β1 was used as a control. TGF-β1 activates TGF-β
receptor I (TβRI) and TβRII, which results in the phosphorylation
of receptor-regulated SMAD2/3 proteins (25). In the present study, we found that
TGF-β1 treatment induced proliferation and invasion, and inhibited
apoptosis in the HTR-8/SVneo cells via activation of the Smad
signaling pathway. Cheng et al (26) also found that TGF-β1 induced the
downregulation of VE-cadherin and decreased cell invasion in human
trophoblast cells. Notably, we found that activation of the Smad
signaling pathway had opposite roles in the HTR-8/SVneo cells with
HBx transfection. HBx plays critical roles in the pathogenesis of
hepatocellular carcinoma development, based on its tumorigenic
activity in vitro and in vivo (27). According to the results of previous
studies, we concluded that HTR-8/SVneo cells with HBx transfection
also underwent cancerous transformation. More evidence was provided
by Murata et al (28). They
found that HBx shifts TGF-β signaling from tumor suppression to
oncogenesis in early chronic hepatitis B. However, the mechanism in
HTR-8/SVneo cells should be validated in future studies.
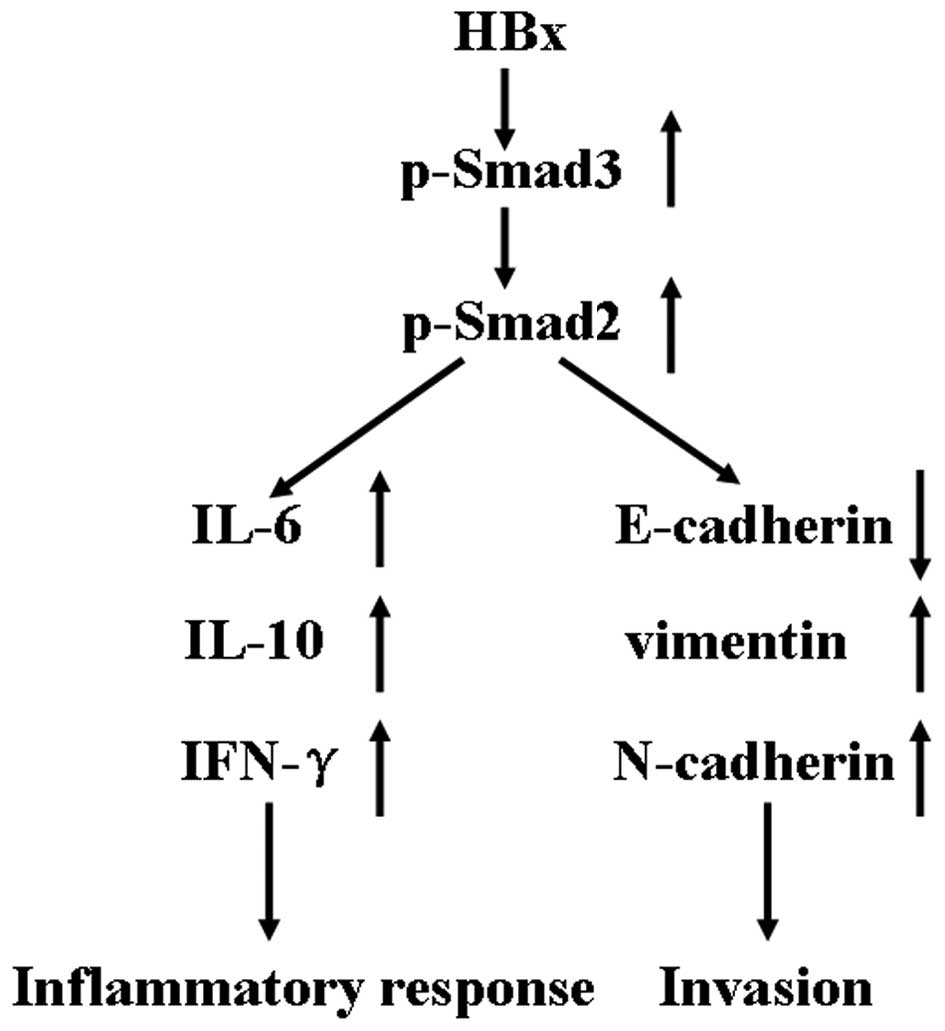
Taken together, in all our experiments, we
elucidated the biological effects of different HBx fragments on
HTR-8/SVneo cells. However, there was no difference among these
fragments. We demonstrated that HBx activates the Smad signaling
pathway in HTR-8/SVneo cells. After the signaling pathway was
activated, a lower apoptotic ratio, higher cell motility, and an
enhanced inflammatory response were observed in the HTR-8/SVneo
cells (Fig. 6). In further studies,
we will truncate other domains of HBx and detect their
activities.
Acknowledgments
We thank Mr. Jin-Cheng Li (China Medical University,
Shenyang, China) for providing the plasmid pcDNA3.1-HBx.
References
|
1
|
Lok AS: Chronic hepatitis B. N Engl J Med.
346:1682–1683. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu DZ, Yan YP, Choi BC, Xu JQ, Men K,
Zhang JX, Liu ZH and Wang FS: Risk factors and mechanism of
transplacental transmission of hepatitis B virus: A case-control
study. J Med Virol. 67:20–26. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang SL, Yue YF, Bai GQ, Shi L and Jiang
H: Mechanism of intrauterine infection of hepatitis B virus. World
J Gastroenterol. 10:437–438. 2004.PubMed/NCBI
|
|
4
|
Ohto H, Lin HH, Kawana T, Etoh T and
Tohyama H: Intrauterine transmission of hepatitis B virus is
closely related to placental leakage. J Med Virol. 21:1–6. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Veerbeek JH, Nikkels PG, Torrance HL,
Gravesteijn J, Post Uiterweer ED, Derks JB, Koenen SV, Visser GH,
Van Rijn BB and Franx A: Placental pathology in early intrauterine
growth restriction associated with maternal hypertension. Placenta.
35:696–701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lunghi L, Ferretti ME, Medici S, Biondi C
and Vesce F: Control of human trophoblast function. Reprod Biol
Endocrinol. 5:6–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bouchard MJ and Schneider RJ: The
enigmatic X gene of hepatitis B virus. J Virol. 78:12725–12734.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang H, Oishi N, Kaneko S and Murakami S:
Molecular functions and biological roles of hepatitis B virus x
protein. Cancer Sci. 97:977–983. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Shan CL, Li N, Zhang X, Zhang XZ,
Xu FQ, Zhang S, Qiu LY, Ye LH and Zhang XD: Identification of a
natural mutant of HBV X protein truncated 27 amino acids at the
COOH terminal and its effect on liver cell proliferation. Acta
Pharmacol Sin. 29:473–480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu S, Cui H, Li Q, Zhang L, Na Q and Liu
C: RhoGDI2 is expressed in human trophoblasts and involved in their
migration by inhibiting the activation of RAC1. Biol Reprod.
90:882014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bai G, Wang Y, Zhang L, Tang Y and Fu F:
The study on the role of hepatitis B virus X protein and apoptosis
in HBV intrauterine infection. Arch Gynecol Obstet. 285:943–949.
2012. View Article : Google Scholar
|
|
12
|
Liu H, Xu L, He H, Zhu Y, Liu J, Wang S,
Chen L, Wu Q, Xu J and Gu J: Hepatitis B virus X protein promotes
hepatoma cell invasion and metastasis by stabilizing Snail protein.
Cancer Sci. 103:2072–2081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo N, Cai Y, Zhang J, Tang W, Slagle BL,
Wu X and He S: The C-terminal region of the hepatitis B virus X
protein is required for its stimulation of HBV replication in
primary mouse hepatocytes. Virus Res. 165:170–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Q, Zhang W, Liu Q and Zhang X, Lv N,
Ye L and Zhang X: A mutant of hepatitis B virus X protein
(HBxDelta127) promotes cell growth through a positive feedback loop
involving 5-lipoxygenase and fatty acid synthase. Neoplasia.
12:103–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chau DK, Chen GG, Zhang H, Leung BC, Chun
S and Lai PB: Differential functions of C- and N-terminal hepatitis
B x protein in liver cells treated with doxorubicin in normoxic or
hypoxic condition. PLoS One. 7:e501182012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noyola-Martínez N, Díaz L, Avila E,
Halhali A, Larrea F and Barrera D: Calcitriol downregulates TNF-α
and IL-6 expression in cultured placental cells from preeclamptic
women. Cytokine. 61:245–250. 2013. View Article : Google Scholar
|
|
17
|
Sharma S, Stabila J, Pietras L, Singh AR,
McGonnigal B, Ernerudh J, Matthiesen L and Padbury JF:
Haplotype-dependent differential activation of the human IL-10 gene
promoter in macrophages and trophoblasts: Implications for
placental IL-10 deficiency and pregnancy complications. Am J Reprod
Immunol. 64:179–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong Q, Fan R, Zhao S and Wang Y:
Over-expression of SOCS-3 gene promotes IL-10 production by JEG-3
trophoblast cells. Placenta. 30:11–14. 2009. View Article : Google Scholar
|
|
19
|
Prins JR, Gomez-Lopez N and Robertson SA:
Interleukin-6 in pregnancy and gestational disorders. J Reprod
Immunol. 95:1–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Z, Chen Y, Yang Y and Peng JP: The
effect on MHC class II expression and apoptosis in placenta by
IFNgamma administration. Contraception. 65:177–184. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu Y, Tan R, MacCalman CD, Eastabrook G,
Park SH, Dutz JP and von Dadelszen P: IFN-gamma-mediated
extravillous trophoblast outgrowth inhibition in first trimester
explant culture: A role for insulin-like growth factors. Mol Hum
Reprod. 14:281–289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomson S, Petti F, Sujka-Kwok I, Mercado
P, Bean J, Monaghan M, Seymour SL, Argast GM, Epstein DM and Haley
JD: A systems view of epithelial-mesenchymal transition signaling
states. Clin Exp Metastasis. 28:137–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmierer B and Hill CS: TGFbeta-SMAD
signal transduction: Molecular specificity and functional
flexibility. Nat Rev Mol Cell Biol. 8:970–982. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weiss A and Attisano L: The TGFbeta
superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol.
2:47–63. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Busnadiego O, González-Santamaría J,
Lagares D, Guinea-Viniegra J, Pichol-Thievend C, Muller L and
Rodríguez-Pascual F: LOXL4 is induced by transforming growth factor
β1 through Smad and JunB/Fra2 and contributes to vascular matrix
remodeling. Mol Cell Biol. 33:2388–2401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng JC, Chang HM and Leung PC:
Transforming growth factor-β1 inhibits trophoblast cell invasion by
inducing Snail-mediated down-regulation of vascular
endothelial-cadherin protein. J Biol Chem. 288:33181–33192. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu Z, Yen TS, Wu L, Madden CR, Tan W,
Slagle BL and Ou JH: Enhancement of hepatitis B virus replication
by its X protein in transgenic mice. J Virol. 76:2579–2584. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murata M, Matsuzaki K, Yoshida K, Sekimoto
G, Tahashi Y, Mori S, Uemura Y, Sakaida N, Fujisawa J, Seki T, et
al: Hepatitis B virus X protein shifts human hepatic transforming
growth factor (TGF)-beta signaling from tumor suppression to
oncogenesis in early chronic hepatitis B. Hepatology. 49:1203–1217.
2009. View Article : Google Scholar : PubMed/NCBI
|