Introduction
Cholangiocarcinoma is the most common biliary
malignancy and the second most common hepatic malignancy after
hepatocellular carcinoma (HCC) (1).
Cholangiocarcinoma accounts for 3% of all gastrointestinal tumors,
and the overall incidence of cholangiocarcinoma appears to have
increased over the past 3 decades (2). Hepatobiliary cancers are associated
with a poor prognosis since many patients with biliary tract
cancers present with advanced disease. The percentage of patients
who survive 5 years after diagnosis has not increased during this
time period, remaining at 10% (3,4).
Understanding of cholangiocarcinoma biology, the oncogenic
landscape of this disease, and its complex interaction with the
tumor micro-environment could lead to improved therapies and
increased patient survival (5).
Galectin-9 (Gal-9) is a tandem-repeat-type galectin
with two carbohydrate-recognition domains (CRDs) and was first
identified as an eosinophil chemoattractant and activation factor
(6–9). Similar to other galectins, Gal-9
regulates various cellular functions, such as cell aggregation and
adhesion, and apoptosis of tumor cells (9,10).
Gal-9 enhances antitumor immunity by the initial CRD-independent
maturation of dendritic cells and subsequent induction of
Th1-mediated antitumor immunity (11). In addition, treatment with
recombinant Gal-9 prolonged survival in a murine melanoma model,
not only increasing the numbers of CD8 cytotoxic T cells (CTLs) but
also those of natural killer (NK) cells and macrophages (12).
Recent studies have uncovered additional mechanisms
by which T cell immunoglobulin mucin-3 (Tim-3), a receptor for
Gal-9, negatively regulates T cell responses by promoting
CD8+ T cell exhaustion and inducing expansion of
myeloid-derived suppressor cells (13,14).
Most studies that investigated the mechanism of T
cell death by Gal-9 were performed using leukemic T cell lines.
Particularly, recombinant Gal-9 induced apoptosis, which depends on
the presence of a functional CRD, in various T cell leukemic cell
lines in a dose-dependent manner (15,16).
Additionally, several in vitro and in vivo studies
have indicated that Gal-9 inhibits the growth of multiple myeloma
(17) and chronic myeloid leukemia
(18). In hematologic malignancies,
Gal-9 suppresses cell proliferation and tumor growth in
vitro and in vivo. However, in solid malignancies, the
antitumor effect of Gal-9 remains unknown.
Less is known concerning the antitumor effects of
Gal-9 on cholangiocarcinoma cells and on the microRNAs (miRNAs)
associated with these effects. Therefore, the present study aimed
to evaluate the effects of Gal-9 on the growth of
cholangiocarcinoma cell lines, its mechanism of action, and the
miRNAs associated with the antitumor effect of Gal-9.
Materials and methods
Reagents and antibodies
Recombinant mutant forms of human Gal-9 lacking the
linker peptides were expressed and purified as previously described
(19). Cell Counting Kit-8 (CCK-8)
was purchased from Dojindo Laboratories (Kumamoto, Japan), and all
other chemicals were obtained from Sigma Chemical (Tokyo,
Japan).
Following antibodies were used: anti-β-actin
monoclonal antibody (A5441, used at a dilution of 1:3,000;
Sigma-Aldrich, St. Louis, MO, USA); cyclin D1 (RB-9041, used at
1:1,000); cyclin E (used at 1:1,000) (both from Thermo Fisher
Scientific, Waltham, MA, USA); Cdk6 (sc-177, used at 1:1,000); Cdk4
(sc-749, used at 1:1,000); Cdk2 (sc-163, used at 1:2,000) (all from
Santa Cruz Biotechnology, Santa Cruz, CA, USA); phosphorylated
retinoblastoma protein (Rb; no. 558385, used at 1:1,000; BD
Pharmingen); Rb (#9309, used at 1:1,000; Cell Signaling
Technology); and secondary horseradish peroxidase (HRP)-linked
anti-mouse and anti-rabbit IgG antibodies (used at 1:2,000; GE
Healthcare, UK).
Cell lines and culture
The two human cholangiocarcinoma TFK-1 and HuH-28
cell lines were obtained from the Japanese Cancer Research
Resources Bank (Osaka, Japan) and passaged at our laboratory for
<6 months. The cell lines were authenticated by the Cell Bank
using short tandem repeat PCR. TFK-1 was grown in RPMI-1640 and
HuH-28 was grown in MEM, with both media types supplemented with
10% fetal bovine serum (FBS) and 100 mg/l of
penicillin-streptomycin in a humidified atmosphere with 5% of
CO2 at 37°C.
Cell proliferation assay
Cell proliferation assays were conducted in TFK-1
and HuH-28 cells. Each cell line (5×103) was seeded into
96-well plates and cultured in 100 μl of culture medium
supplemented with 10% FBS. After 24 h, the cells were treated with
0.03, 0.1 or 0.3 μM Gal-9 and then cultured for an
additional 48 h. CCK-8 reagent (10 μl) was added to each
well, and the plates were incubated at 37°C for 3 h. The absorbance
of each well was measured at 450 nm using an auto-microplate
reader.
ELISA for apoptosis
M30 Apoptosense enzyme-linked immunosorbent assay
(ELISA) kits obtained from PEVIVA AB (Bromma, Sweden) was used to
evaluate caspase-cleaved keratin 18 (CCK18) levels (20). Cell line cells (5×103
cells) were seeded into 96-well plates and cultured in 100
μl of culture medium for 24 h. The cells were then treated
with 0.3 μM Gal-9, and the assays were performed according
to the manufacturer's instructions. The amount of antigen in
controls and samples were calculated by interpolation to a standard
curve.
Flow cytometry
To evaluate the mechanism of growth inhibition by
Gal-9, cell cycle profiles were analyzed after treatment with
Gal-9. TFK-1 cells (1.0×106 cells in a 100-mm dish) were
treated with or without 0.3 μM Gal-9 for 24 h. The cell
cycle distribution was analyzed by measuring the amount of
propidium iodide (PI)-labeled DNA in ethanol-fixed cells. Fixed
cells were washed with phosphate-buffered saline (PBS) and stored
at −20°C until analysis by flow cytometry. On the day of analysis,
the cells were washed with cold PBS, suspended in 100 μl of
PBS plus 10 μl of RNase A (250 μg/ml) and incubated
for 30 min. To each suspension was added 110 μl of PI stain
(100 μg/ml), and the cells were incubated at 4°C for at
least 30 min prior to analysis. Flow cytometry was performed using
a Cytomics FC500 flow cytometer (Beckman Coulter) with an argon
laser (488 nm). The percentages of cells in different phases of the
cell cycle were analyzed by FlowJo software (TreeStar, Ashland, OR,
USA). All experiments were performed in triplicate to assess
consistency of response.
Gel electrophoresis and western
blotting
TFK-1 cells (1.0×106/dish) were seeded in
100-mm culture dishes and cultured for 24 h; Gal-9 was added, and
the cells were further cultured for 48 h. The cells were washed
twice in PBS and lysed using a protease inhibitor cocktail
(ProPrep, 'Complete' protease inhibitor mixture; iNtRON
Biotechnology, Sungnam, Korea). Protein concentration was
quantified using the NanoDrop 2000 fluorospectrometer (Thermo
Scientific Corp., USA). Whole-cell lysates (1–10 μg) were
resolved by SDS-polyacrylamide gel electrophoresis on 10%
Tris-glycine gradient gels by SDS-PAGE (21), and the proteins were transferred to
nitrocellulose membranes. After blocking, the membranes were
incubated with primary antibodies and then incubated with
HRP-conjugated secondary antibodies (22). Immunoreactive proteins were
visualized with an enhanced chemiluminescence detection system
(Perkin-Elmer Co., Waltham, MA, USA) on X-ray film.
Xenograft model analysis
Animal experiments were performed according to the
guidelines of the Committee on Experimental Animals of Kagawa
University, Kagawa, Japan.
Eighteen female athymic mice (BALB/c-nu/nu;
6-weeks old; 20–25 g) were purchased from Japan SLC Inc. (Shizuoka,
Japan). The animals were maintained under specific pathogen-free
conditions using a laminar airflow rack. The mice had continuous
free access to sterilized (γ-irradiated) food (CL-2; CLEA Japan
Inc.) and autoclaved water.
Each mouse was subcutaneously inoculated with TFK-1
cells (5×106 cells/animal) in the flank. Two weeks
later, the xenografts were identifiable as masses of maximal
diameter >6 mm. We randomly assigned the animals to 3 groups.
These groups were treated with 30 μg of Gal-9 (n=6), 90
μg of Gal-9 (n=6) or control (PBS only; n=6).
The Gal-9-treated groups were injected
subcutaneously with Gal-9 (30 or 90 μg) 3 times a week. The
control group was administered PBS alone. Tumor growth was
monitored daily by the same investigators (K. Kobayashi and T.
Masaki), and tumor size was measured weekly by measuring the 2
largest perpendicular dimensions. Tumor volume was calculated as
follows: tumor volume (mm3) = [tumor length (mm) x tumor
width (mm)2]/2 (23).
All animals were sacrificed 7 weeks after treatment, with all
animals remaining alive during this period.
Antibody arrays of apoptosis-related
proteins
The Human apoptosis antibody array kit (R&D
Systems, Minneapolis, MN, USA) was used according to the
manufacturer's instructions.
Antibody arrays of phosphorylated
receptor tyrosine kinases (p-RTKs)
Human p-RTKs were assayed using Human p-RTK array
kits (R&D Systems) according to the manufacturer's
instructions. Briefly, p-RTK array membranes were blocked with 5%
BSA/TBS (0.01 M Tris-HCl, pH 7.6) for 1 h and incubated with 2 ml
of lysate prepared from cell lines after normalization so that the
amounts of protein were equal. After 3 washes for 10 min each with
TBS plus 0.1% v/v Tween-20 and 2 washes for 10 min with TBS alone
to remove unbound materials, the membranes were incubated with
anti-phospho-tyrosine-HRP antibody for 2 h at room temperature. The
unbound HRP antibody was washed out with TBS plus 0.1% Tween-20.
Finally, each array membrane was exposed to X-ray film using a
chemiluminescence detection system (Perkin-Elmer Co.).
Angiogenic profile analysis using an
antibody array
The RayBio Human Angiogenesis Antibody Array
(RayBiotech Inc.) was used according to the manufacturer's
protocol. This method is a dot-based assay enabling detection and
comparison of 20 angiogenesis-specific cytokines. Each array
membrane was exposed to X-ray film using a chemiluminescence
detection system.
Analysis of miRNA arrays
Total RNA was extracted from tumor samples using
RNeasy Mini kits (Qiagen) according to the manufacturer's
instructions. RNA samples typically showed
A260/280 ratios between 1.9 and 2.1, using an
Agilent 2100 Bioanalyzer (Agilent Technologies).
After RNA measurement with an RNA 6000 Nano kit
(Agilent Technologies), the samples were labeled using a
miRCURYHy3/Hy5 Power Labeling kit and were hybridized to a human
miRNA Oligo chip (v.19.0; Toray Industries). The chips were scanned
with a 3D-Gene Scanner 3000, and the results were analyzed by
3D-Gene extraction version 1.2 software (both from Toray
Industries). Differences in miRNA expression between Gal-9-treated
and control samples were assessed by analyzing the raw data using
GeneSpring GX v10.0 (Agilent Technologies). The samples were first
normalized relative to 28S RNA and baseline corrected to the median
of all samples.
Replicate data were consolidated into 2 groups
(those from Gal-9-treated cells and those from control cells) and
were organized using the hierarchical clustering and ANOVA
functions in GeneSpring software. Hierarchical clustering was
performed using the clustering function (condition tree) and
Euclidean correlation as a distance metric. Two-way ANOVA analysis
and asymptotic P-value computation without any error correction on
the samples were performed to determine the miRNAs varying most
prominently across the groups. The P-value cut-off was set to 0.05.
Only changes >50% for at least one of the time points for each
sample were considered significant. All analyzed data were scaled
by global normalization. The significance of differentially
expressed miRNAs was analyzed by the Student's t-test.
Statistical analyses
All statistical analyses were performed using
computer-assisted JMP 9.0 (SAS Institute, Cary, NC, USA). Paired
analysis between groups used t-tests. A P-value <0.05 was
considered significant.
Results
Gal-9 suppresses the proliferation of
human cholangiocarcinoma cells
To evaluate the effect of the growth activity of
Gal-9 on human cholangiocarcinoma cells in vitro, we
examined the effect of Gal-9 on proliferation in two
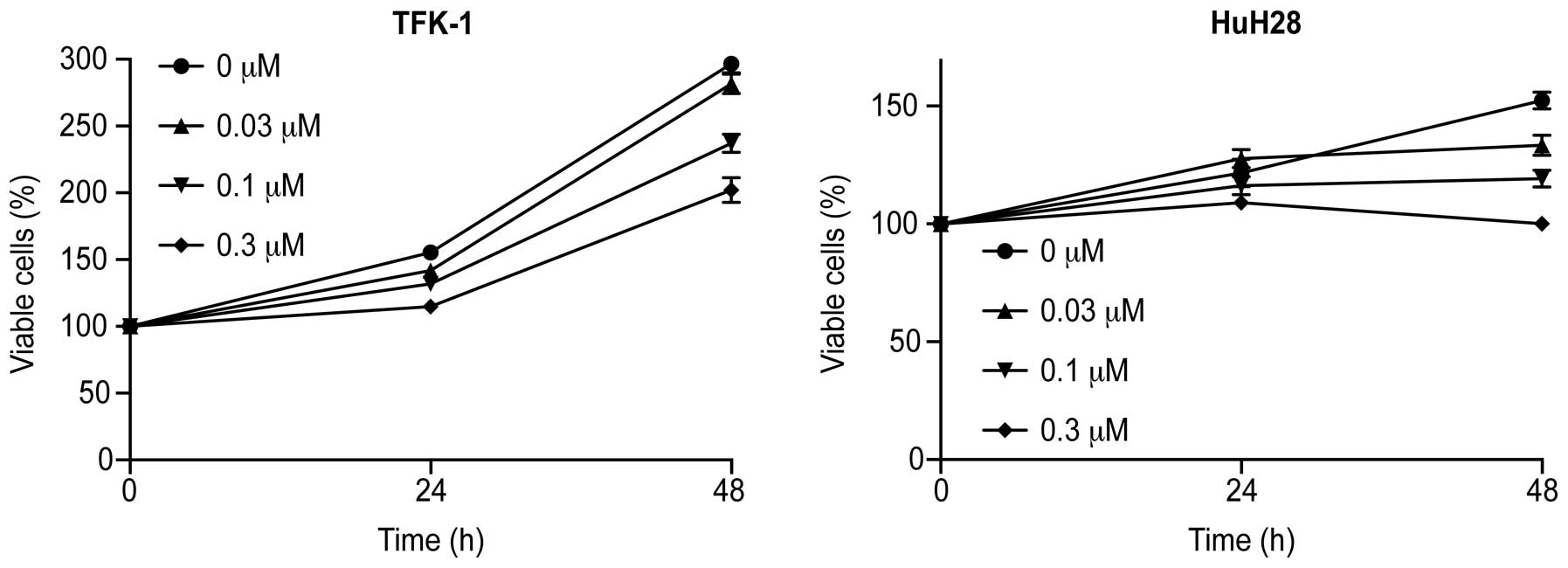
cholangiocarcinoma cell lines, TFK-1 and HuH-28. The cells were
grown in 10% FBS and treated with 0, 0.03, 0.1 or 0.3 μM
Gal-9 for 48 h. Gal-9 led to a dose-dependent and strong inhibition
of cell proliferation in the two cholangiocarcinoma cell lines
(Fig. 1).
Gal-9 induces apoptosis of
cholangiocarcinoma cells
To clarify the mechanism of the growth inhibitory
effect of Gal-9, we first examined the induction of apoptosis by
Gal-9.
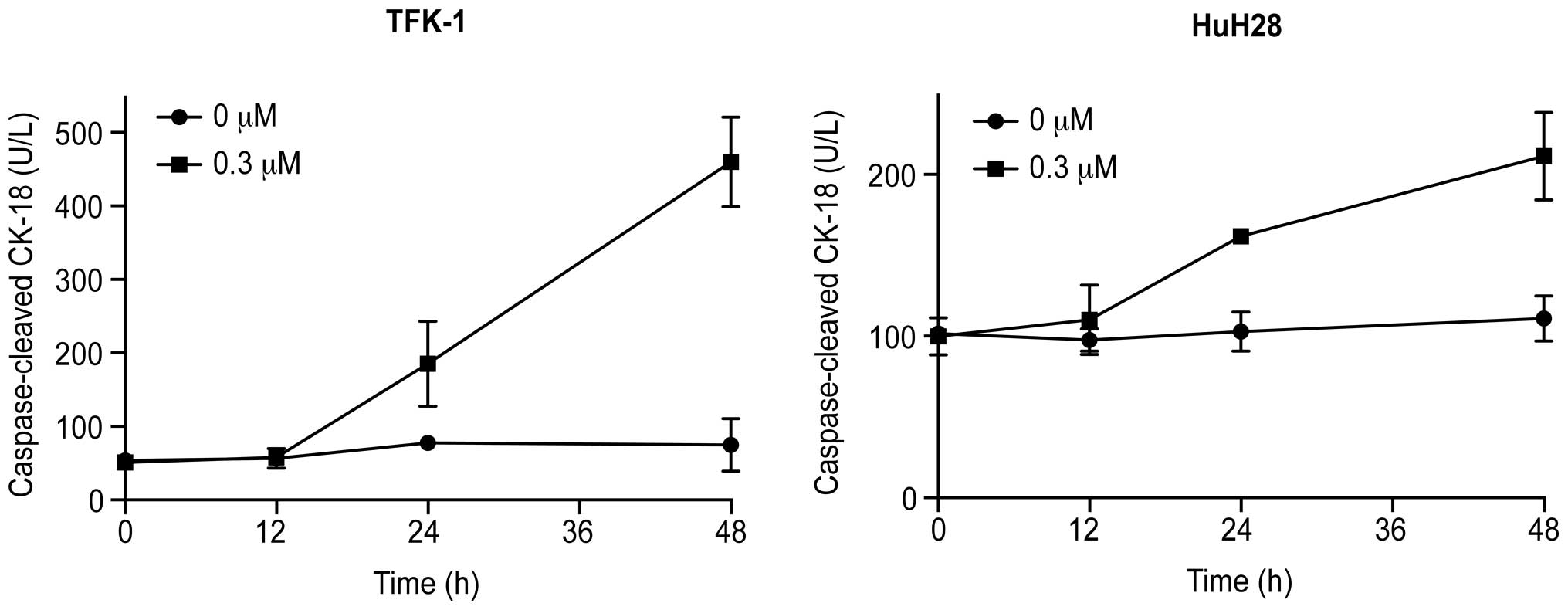
CCK18 expression was determined by ELISA to confirm
whether apoptosis is involved in Gal-9-induced cell death. Gal-9
increased the levels of CCK18 in the two CCA cell lines (Fig. 2).
No or little effects of Gal-9 on the cell
cycle in TFK-1 cells
It became intriguing to clarify whether effects of
Gal-9 on cell cycle are simultaneously involved in the suppression
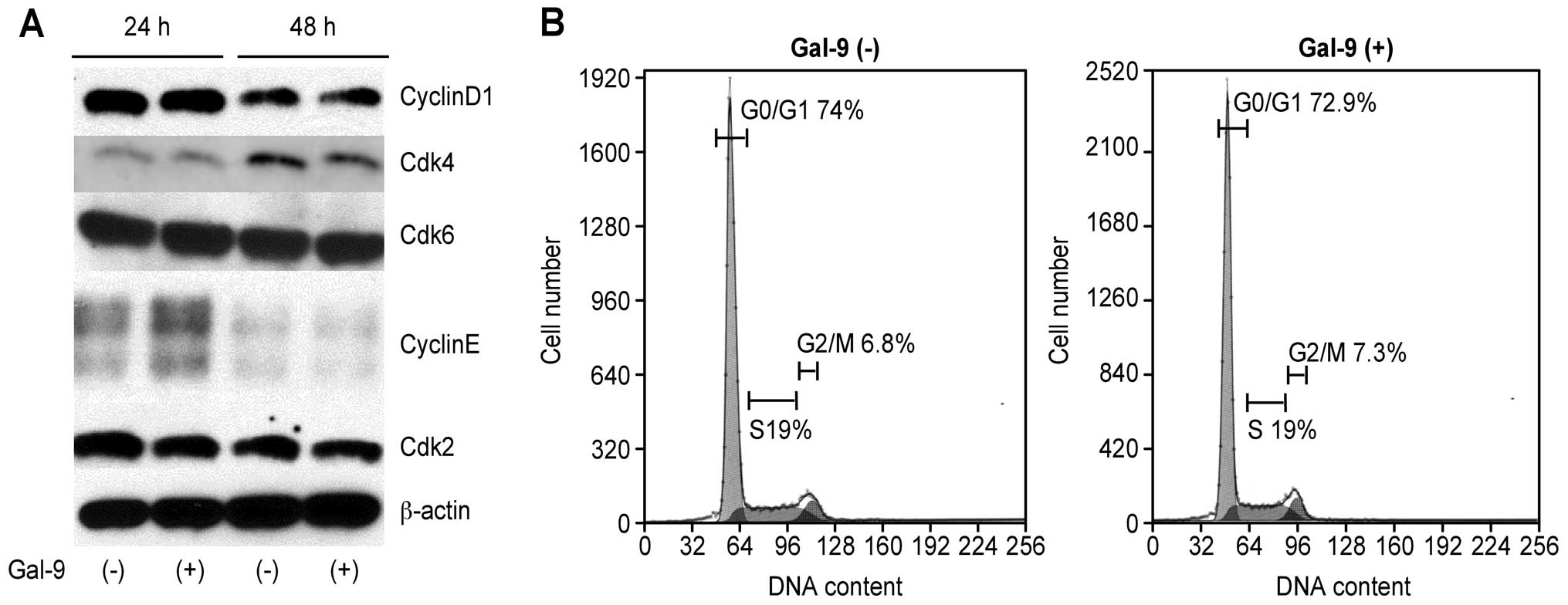
of cholangiocarcinoma cells. The effects of Gal-9 on the expression
of various cell cycle-related molecules in TFK-1 cells were
evaluated by western blotting. Cells were treated 0 or with 0.3
μM Gal-9 for 24–48 h. Assays of the expression of other
proteins associated with the G0 to G1
transition showed that the Cdk6, catalytic subunit of cyclin D1,
Cdk2 and Cdk4 were not changed at 24 and 48 h after the addition of
0.3 μM Gal-9 (Fig. 3A).
Next, we performed flow cytometric analysis of the
cell cycle to evaluate the contribution of cell cycle arrest to the
suppression of CCA cell lines by Gal-9. TFK-1 cells treated with
0.3 μM Gal-9 showed no change in the cell cycle profile
(Fig. 3B). These results suggested
that Gal-9 suppresses cholangiocarcinoma cell growth through the
tumor cell apoptosis but not through cell cycle arrest.
Gal-9 suppresses tumor cell growth in
vivo
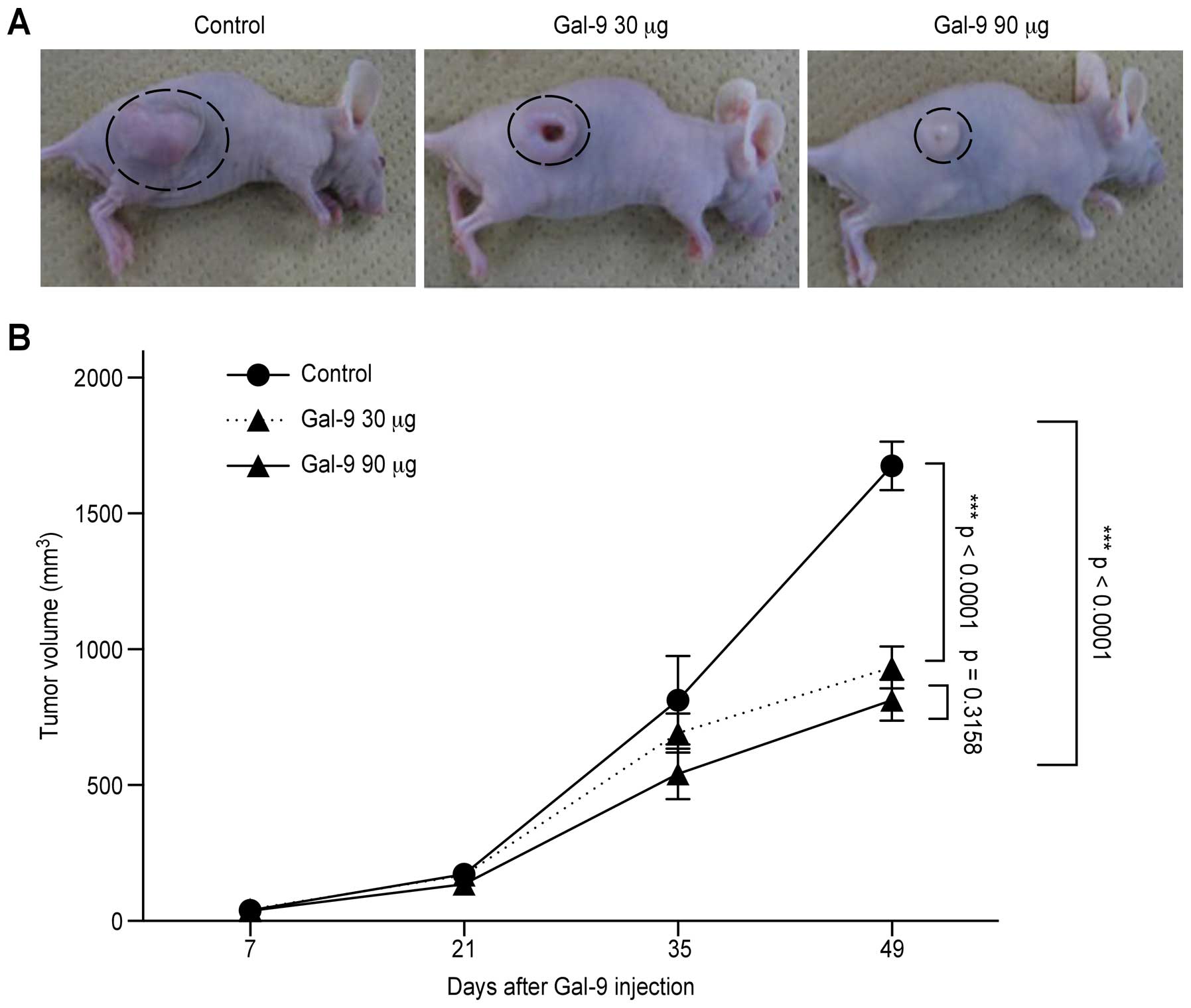
Next experiments were performed to clarify whether
Gal-9 also exhibits tumor growth suppressive activity in
vivo. Nude mice were injected subcutaneously with TFK-1 cells,
followed by the subcutaneous injection of Gal-9. Gal-9
significantly suppressed the tumor growth of TFK-1 cells compared
with untreated control mice (Fig. 4A
and B). Throughout the in vivo study, Gal-9 had no
apparent effects on these mice and did not affect their weights.
All animals survived throughout the experiment.
Effects of Gal-9 on apoptosis-associated
proteins in TFK-1 cells
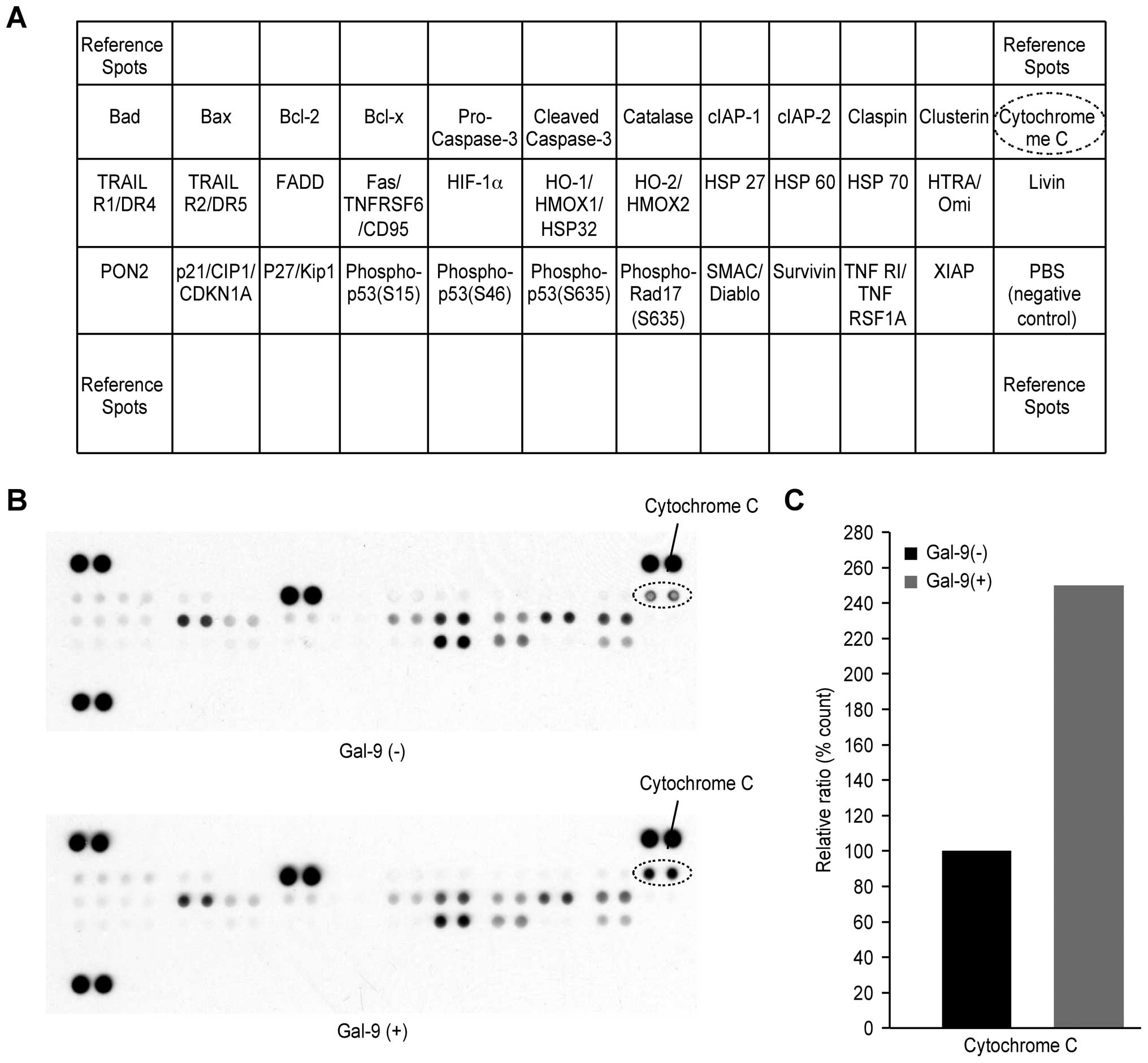
We used an apoptosis array system to identify the
apoptosis-associated proteins associated with the antitumor effect
of Gal-9. Using an antibody array enabled the expression of 35
apoptosis-associated proteins to be screened in TFK-1 cells in the
presence and absence of Gal-9 (Fig.
5A). Gal-9 increased the levels of expression of cytochrome
c (Fig. 5B). Densitometry
analysis showed that the ratio of cytochrome c spots of
Gal-9-treated to untreated cells was 249.9% (Fig. 5C). Expression levels of the
apoptosis-associated proteins other than cytochrome c were
not affected by Gal-9.
Effects of Gal-9 on p-RTKs in TFK-1
cells
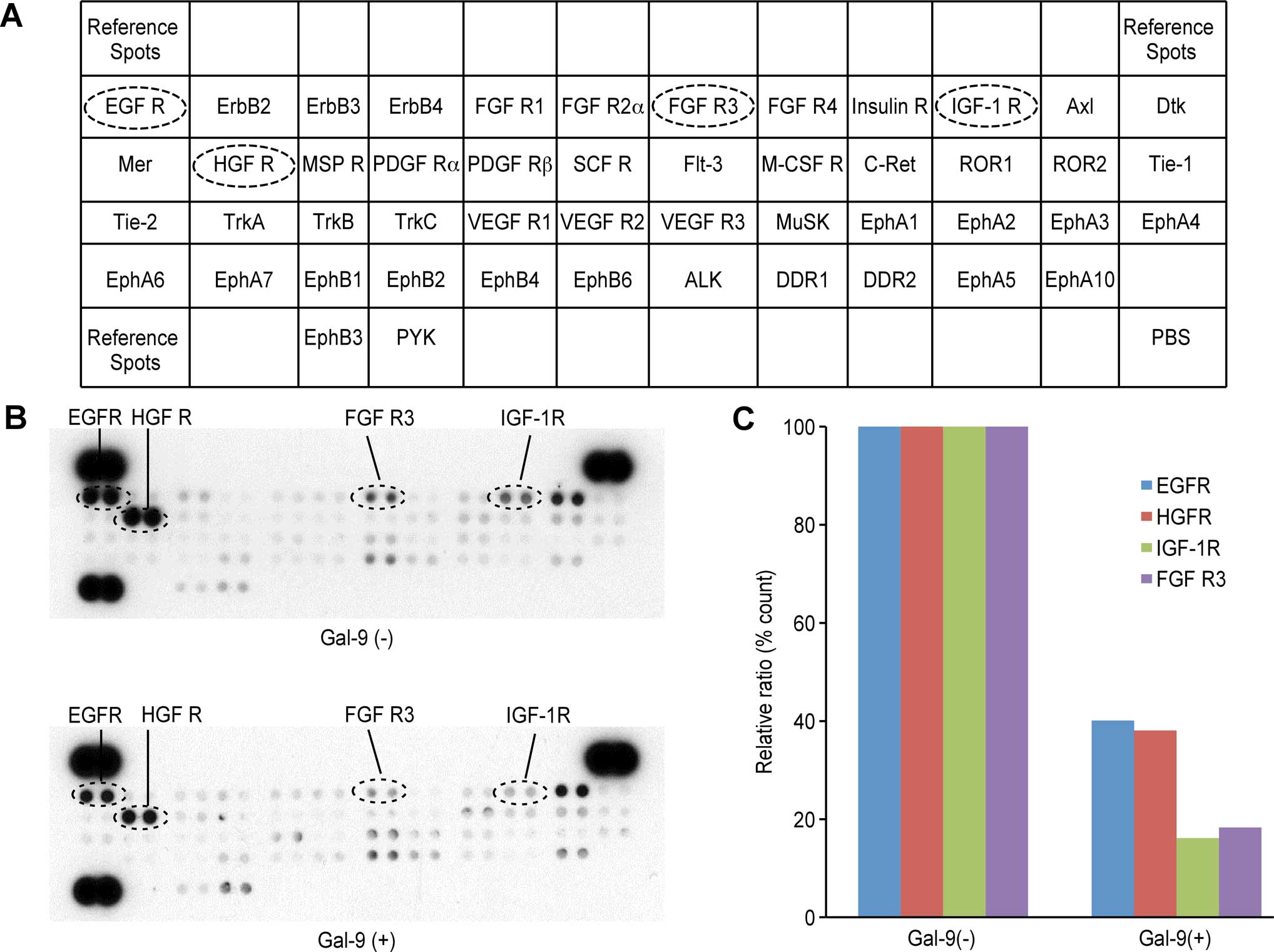
We used a p-RTK array system to identify the key
RTKs associated with the antitumor effect of Gal-9. Using an
antibody array enabled the expression of 49 activated RTKs to be
screened in TFK-1 cells in the presence and absence of Gal-9
(Fig. 6A). Gal-9 reduced the levels
of expression of phosphorylated epidermal growth factor receptor
(p-EGFR) and phosphorylated insulin-like growth factor-1 receptor
(p-IGF-1R), as well as reduced the expression of hepatocyte growth
factor receptor (HGFR) and fibroblast growth factor receptor 3
(FGFR3; Fig. 6B). Densitometry
analysis showed that the ratio of p-EGFR, p-HGFR, p-IGF-1R and
p-FGF R3 spots of Gal-9-treated to untreated cells was 40.1, 38.0,
16.1 and 18.3%, respectively (Fig.
6C).
Effects of Gal-9 on angiogenesis in TFK-1
cells
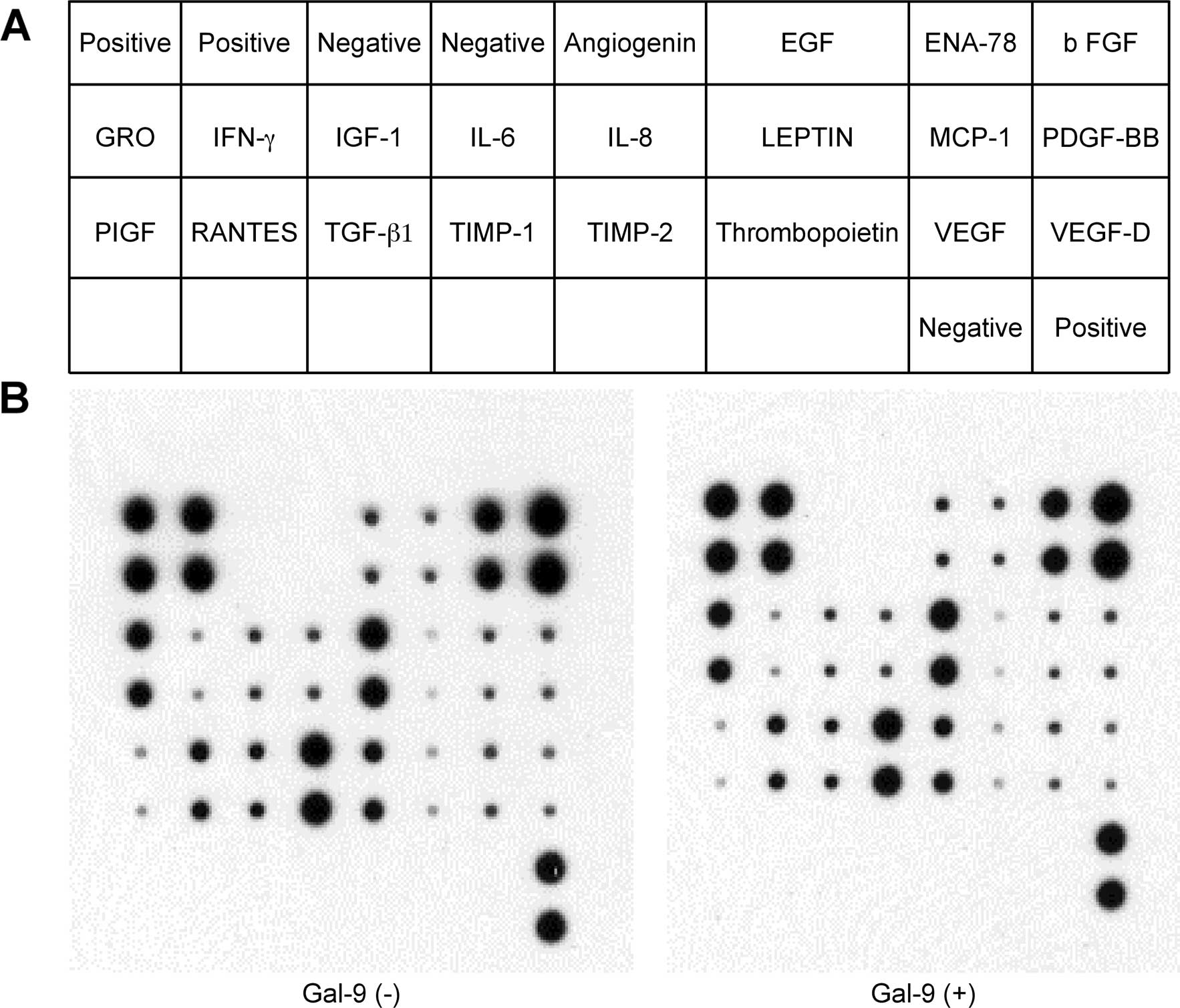
We used an angiogenesis array system to identify the
key angiogenesis-related molecules associated with the antitumor
activity of Gal-9 on TFK-1 cells (Fig.
7A). The 20 angiogenesis molecules screened showed no change by
treatment with Gal-9 (Fig. 7B).
Effects of Gal-9 on miRNA expression in
the tumorous tissues in vivo
Using a custom microarray platform, we analyzed the
level of 2555 miRNA probe in tumorous tissues in vivo that
were treated with and without Gal-9.
In a tumor xenograft model, in the Gal-9 group,
there were 22 upregulated and 27 downregulated miRNAs of the 985
miRNAs (GEO, accession no. GSE30289; Table I).
 | Table IStatistical results and chromosomal
location of miRNAs in tumorous tissues treated with and without
galectin-9. |
Table I
Statistical results and chromosomal
location of miRNAs in tumorous tissues treated with and without
galectin-9.
| miRNA | Fold
(treated/untreated), mean ± SD | P-value | Chromosomal
localization |
|---|
| Upregulated | | | |
|
hsa-miR-4722-5p | 2.56±0.40 | 0.0418 | 16 |
| hsa-miR-198 | 2.26±0.30 | 0.0267 | 3q13.33 |
|
hsa-miR-29b-2-5p | 1.87±0.52 | 0.0197 | 1q32.2 |
| hsa-miR-4430 | 1.81±0.39 | 0.0310 | 2 |
| hsa-miR-5739 | 1.76±0.63 | 0.0472 | 22 |
| hsa-miR-1275 | 1.57±0.30 | 0.0495 | 6 |
| hsa-miR-760 | 1.57±0.40 | 0.0302 | 1p22.1 |
| hsa-miR-4792 | 1.56±0.24 | 0.0131 | 3 |
| hsa-miR-1246 | 1.51±0.40 | 0.0321 | 2q31.1 |
| hsa-miR-4449 | 1.50±0.08 | 0.0483 | 4 |
| hsa-miR-4442 | 1.49±0.31 | 0.0306 | 3 |
|
hsa-miR-199b-5p | 1.39±0.18 | 0.0053 | 9q34.11 |
|
hsa-miR-4655-5p | 1.39±0.15 | 0.0058 | 7 |
| hsa-miR-4651 | 1.37±0.26 | 0.0225 | 7 |
| hsa-miR-6124 | 1.37±0.34 | 0.0002 | 11 |
|
hsa-miR-1185-2-3p | 1.33±0.19 | 0.0411 | 14 |
|
hsa-miR-1285-3p | 1.31±0.36 | 0.0498 | |
|
hsa-miR-92b-5p | 1.30±0.11 | 0.0401 | 1q22 |
| hsa-miR-1908 | 1.29±0.17 | 0.0402 | 11 |
| hsa-miR-4327 | 1.28±0.19 | 0.0389 | 21 |
| hsa-miR-6075 | 1.27±0.11 | 0.0413 | 5 |
| hsa-miR-4516 | 1.17±0.13 | 0.0270 | 16 |
| Downregulated | | | |
|
hsa-miR-513a-5p | 0.35±0.02 | 0.0181 | |
|
hsa-miR-181a-3p | 0.10±0.05 | 0.0495 | |
| hsa-miR-93-3p | 0.23±0.46 | 0.0457 | 7q22.1 |
| hsa-miR-5096 | 0.17±0.02 | 0.0219 | 4 |
|
hsa-miR-27a-5p | 0.20±0.04 | 0.0380 | 19p13.13 |
|
hsa-miR-16-2-3p | 0.20±0.05 | 0.0477 | 3q25.33 |
|
hsa-miR-181a-5p | 0.11±0.02 | 0.0239 | |
| hsa-miR-3135b | 0.12±0.03 | 0.0334 | 6 |
|
hsa-miR-92a-3p | 0.09±0.03 | 0.0276 | |
| hsa-miR-4443 | 0.11±0.01 | 0.0072 | 3 |
|
hsa-miR-18b-5p | 0.06±0.01 | 0.0141 | Xq26.2 |
| hsa-miR-25-3p | 0.01±0.02 | 0.0153 | 7q22.1 |
| hsa-miR-9-5p | 0.27±0.03 | 0.0263 | |
| hsa-miR-28-3p | 0.12±0.00 | 0.0007 | |
|
hsa-miR-4655-3p | 0.10±0.01 | 0.0080 | 7 |
|
hsa-miR-27a-3p | 0.04±0.00 | 0.0006 | 19p13.13 |
|
hsa-miR-361-5p | 0.09±0.00 | 0.0011 | Xq21.2 |
|
hsa-miR-148b-3p | 0.08±0.04 | 0.0376 | 12q13.13 |
|
hsa-miR-30e-5p | 0.02±0.01 | 0.0093 | 1p34.2 |
|
hsa-miR-146b-5p | 0.14±0.03 | 0.0295 | 10q24.32 |
| hsa-miR-17-3p | 0.09±0.01 | 0.0118 | 13q31.3 |
|
hsa-miR-103a-3p | 0.05±0.05 | 0.0466 | |
|
hsa-miR-324-5p | 0.09±0.00 | 0.0030 | 17p13.1 |
|
hsa-miR-30c-5p | 0.05±0.04 | 0.0381 | |
|
hsa-miR-29b-3p | 0.09±0.02 | 0.0197 | |
|
hsa-miR-151a-3p | 0.18±0.04 | 0.0378 | 8q24.3 |
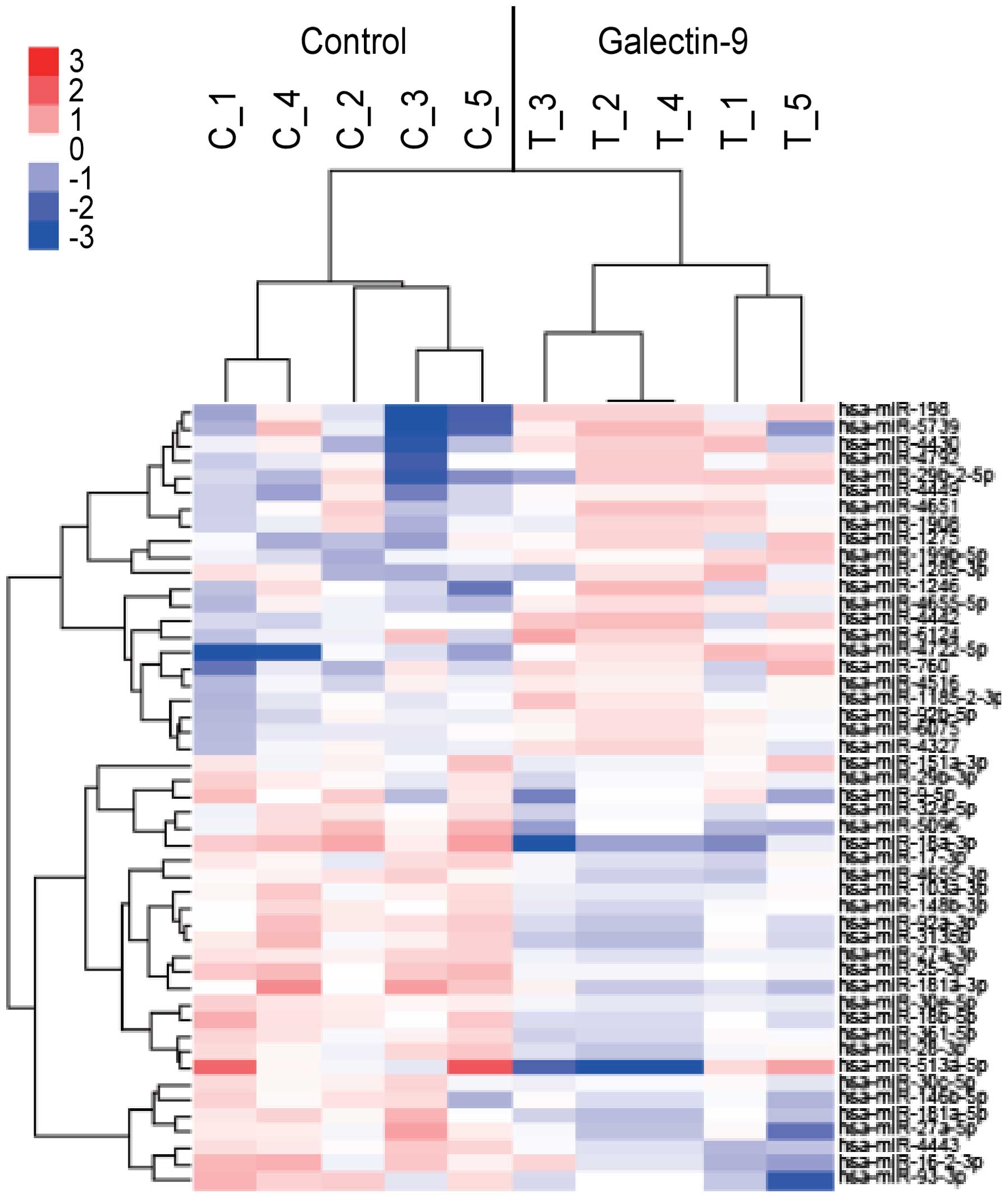
Unsupervised hierarchical clustering analysis using
Pearson's correlation showed that tumorous tissues in vivo
treated with Gal-9 clustered together, and separately from
untreated cell lines (Fig. 8). We
found that miR-198 was upregulated in tumor tissues treated with
Gal-9.
Discussion
Our present results revealed that galectin-9 (Gal-9)
suppresses cell proliferation and tumor growth of human
cholangiocarcinoma cell lines in vitro and in vivo.
The antitumor effect of Gal-9 in T cell hemostasis, cell
aggregation, and metastasis is well known (13,14).
Previous findings suggested that Gal-9 inhibits the proliferation
of hematologic malignancies, such as multiple myeloma (17) and chronic myeloid leukemia (18) and significantly retards the tumor
growth of myeloma xenografts in mice (17). Cell surface-associated Gal-9
triggered the aggregation of melanoma cells, indicative of
Gal-9-mediated cellular adhesion and inhibition of cell detachment
(24). In hematologic malignancies,
Gal-9 suppresses cell proliferation and tumor growth in
vitro and in vivo. On the other hand, in solid
malignancies, breast cancer cell lines with high levels of
endogenous Gal-9 had a strong tendency to aggregate, whereas cells
with low levels of Gal-9 did not (25). Importantly, ectopic expression of
endogenous Gal-9, as well as treatment with recombinant Gal-9,
triggered the formation of tight cellular clusters (24,25).
Therefore, Gal-9 directly suppresses cell proliferation and tumor
growth, and has therapeutic potential for several solid tumors.
Gal-9 increases Tim-3+ dendritic cells
and CD8+ T cells and enhances antitumor immunity via
Gal-9-Tim-3 interactions. Nagahara et al reported that
mature CD11+ dendritic cells became Tim-3-positive;
however, Tim-3-dependent interaction with CD8+ T-cells
triggers the production of IFN-γ (26). In line with these in vitro
findings, splenocytes of sarcoma-bearing mice that were treated
with Gal-9 contained a higher number of
Tim3+CD8+ cytotoxic T cells (CTLs). These
CTLs have higher granzyme B and perforin expression levels,
indicative of higher cytotoxic activity (26). We used a xenograft model, resulting
in an inhibited immune system due to a greatly reduced number of T
cells. Whether Gal-9 suppresses cholangiocarcinoma tumor growth
through not depending Gal-9/Tim-3 pathway still needs to be
identified in our further study.
Recombinant Gal-9 induces apoptosis and cell death
through apoptotic signaling pathways (17,18).
Apoptotic signaling was caspase-dependent and induced by the
activation of the MAP kinases JNK and p38 in multiple myeloma cells
(17). In addition, Gal-9 induced
the proapoptotic Bcl-2 family member Noxa via activating
transcription factor 3, leading to death in chronic myeloma cells
(18). Various hematologic
malignancies are sensitive to apoptotic elimination by recombinant
Gal-9. Cleavage of cytokeratin 18 (CK18) occurs as an early event
during apoptosis following activation of apoptosis executioners,
particularly effector caspases, yet remains intact during other
types of cell death, such as autophagy or necrosis (27). Therefore, several studies have made
use of this phenomenon to detect cellular apoptosis at its early
phase (28–30). Our data have suggested that Gal-9
increased the levels of CCK18 in two cholangiocarcinoma cell lines.
Additionally, we found that the expression of cytochrome c
increased in Gal-9 treated cholangiocarcinoma cell lines using
apoptosis array analysis. Cytochrome c released from damaged
mitochondria is a very early event in the intrinsic apoptosis
pathway and contributes to caspase-9 activation. These data
indicate that Gal-9 induces apoptosis of cholangio-carcinoma cell
lines in the intrinsic apoptosis pathway through caspase-dependent
or -independent pathways.
The expression levels of cell cycle-related proteins
(Cdk2, Cdk4, Cdk6, cyclin D1 and cyclin E) did not change 24 and 48
h after the addition of Gal-9. Additionally, flow cytometric
analysis showed that Gal-9 did not affect the G0 to
G1 transition in cholangiocarcinoma cells in
vitro. These data suggest that the antitumor effect of Gal-9
may not be related to the reduction of various cell cycle-related
proteins, particularly cyclin D1. Additionally, the 20 screened
angiogenesis molecules showed no change by treatment with Gal-9 in
human cholangiocarcinoma cell lines.
In contrast, according to the pRTK array, Gal-9
reduced the levels of expression of phosphorylated epidermal growth
factor receptor (p-EGFR) and phosphorylated insulin-like growth
factor-1 receptor (p-IGF-1R), as well as reduced the expression of
HGFR and FGFR3.
miRNAs associated with the antitumor effects of
Gal-9 were assessed using miRNA expression arrays. Cluster analyses
clearly showed that Gal-9 treatment affected the extent of miRNA
expression in tumor tissues. We identified 49 miRNAs that were
differentially expressed in a cluster. These miRNAs are meaningful
candidates to gauge the effectiveness of Gal-9 treatment and to
provide clues to the molecular basis of the anticancer effects of
Gal-9, particularly those mediated by miRNAs.
Particularly, miR-198 is downregulated in
hepatocellular carcinoma (HCC) compared with the normal liver
(31), and it plays a tumor
suppressor role by negatively regulating the HGF/c-Met pathway
(32). miR-198 inhibits cell
proliferation and tumor growth in lung cancer cellular
proliferation and induces apoptosis through targeting FGFR1 in lung
cancer cells (33). Reduced miR-198
expression is associated with poor prognosis, and high miR-198
predicts better prognosis and increased survival in pancreatic
cancer (34). Therefore, the
possibility cannot be excluded that miR-198 is closely associated
with induction of apoptosis by Gal-9.
In conclusion, our results revealed that Gal-9
inhibits human cholangiocarcinoma cell proliferation and tumor
growth, possibly by inducing apoptosis to produce cytochrome
c, which is an apoptosis-related molecule through the
alteration of miRNAs. These findings suggest that Gal-9 may be a
candidate as a new therapeutic target in the treatment of
cholangiocarcinoma.
Abbreviations:
|
Gal-9
|
galectin-9
|
|
CRDs
|
carbohydrate-recognition domains
|
|
CTLs
|
cytotoxic T cells
|
|
miRNAs
|
microRNAs
|
|
CCK-8
|
Cell Counting Kit-8
|
|
phospho-RTK
|
phosphorylated receptor tyrosine
kinases
|
|
EGFR
|
epidermal growth factor receptor
|
|
IGF-1R
|
insulin-like growth factor-1
receptor
|
|
HGFR
|
hepatocyte growth factor receptor
|
|
FGFR3
|
fibroblast growth factor receptor
3
|
Acknowledgments
We thank Ms. Noriko Murao and Ms. Kana Ogawa for
providing technical assistance.
References
|
1
|
Welzel TM, McGlynn KA, Hsing AW, O'Brien
TR and Pfeiffer RM: Impact of classification of hilar
cholangiocar-cinomas (Klatskin tumors) on the incidence of intra-
and extrahepatic cholangiocarcinoma in the United States. J Natl
Cancer Inst. 98:873–875. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khan SA, Davidson BR, Goldin RD, Heaton N,
Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD,
Thillainayagam AV, et al British Society of Gastroenterology:
Guidelines for the diagnosis and treatment of cholangiocarcinoma:
An update. Gut. 61:1657–1669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Everhart JE and Ruhl CE: Burden of
digestive diseases in the United States Part III: Liver, biliary
tract, and pancreas. Gastroenterology. 136:1134–1144. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tyson GL and El-Serag HB: Risk factors for
cholangiocarcinoma. Hepatology. 54:173–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Razumilava N and Gores GJ:
Cholangiocarcinoma. Lancet. 383:2168–2179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsumoto R, Matsumoto H, Seki M, Hata M,
Asano Y, Kanegasaki S, Stevens RL and Hirashima M: Human ecalectin,
a variant of human galectin-9, is a novel eosinophil
chemoattractant produced by T lymphocytes. J Biol Chem.
273:16976–16984. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsushita N, Nishi N, Seki M, Matsumoto
R, Kuwabara I, Liu FT, Hata Y, Nakamura T and Hirashima M:
Requirement of divalent galactoside-binding activity of
ecalectin/galectin-9 for eosinophil chemoattraction. J Biol Chem.
275:8355–8360. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsumoto R, Hirashima M, Kita H and
Gleich GJ: Biological activities of ecalectin: A novel
eosinophil-activating factor. J Immunol. 168:1961–1967. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saita N, Goto E, Yamamoto T, Cho I,
Tsumori K, Kohrogi H, Maruo K, Ono T, Takeya M, Kashio Y, et al:
Association of galectin-9 with eosinophil apoptosis. Int Arch
Allergy Immunol. 128:42–50. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Asakura H, Kashio Y, Nakamura K, Seki M,
Dai S, Shirato Y, Abedin MJ, Yoshida N, Nishi N, Imaizumi T, et al:
Selective eosinophil adhesion to fibroblast via IFN-gamma-induced
galectin-9. J Immunol. 169:5912–5918. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai SY, Nakagawa R, Itoh A, Murakami H,
Kashio Y, Abe H, Katoh S, Kontani K, Kihara M, Zhang SL, et al:
Galectin-9 induces maturation of human monocyte-derived dendritic
cells. J Immunol. 175:2974–2981. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nobumoto A, Oomizu S, Arikawa T, Katoh S,
Nagahara K, Miyake M, Nishi N, Takeshita K, Niki T, Yamauchi A, et
al: Galectin-9 expands unique macrophages exhibiting plasma-cytoid
dendritic cell-like phenotypes that activate NK cells in
tumor-bearing mice. Clin Immunol. 130:322–330. 2009. View Article : Google Scholar
|
|
13
|
Wiersma VR, de Bruyn M, Helfrich W and
Bremer E: Therapeutic potential of Galectin-9 in human disease. Med
Res Rev. 33(Suppl 1): E102–E126. 2013. View Article : Google Scholar
|
|
14
|
Fujihara S, Mori H, Kobara H, Rafiq K,
Niki T, Hirashima M and Masaki T: Galectin-9 in cancer therapy.
Recent Pat Endocr Metab Immune Drug Discov. 7:130–137. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kashio Y, Nakamura K, Abedin MJ, Seki M,
Nishi N, Yoshida N, Nakamura T and Hirashima M: Galectin-9 induces
apoptosis through the calcium-calpain-caspase-1 pathway. J Immunol.
170:3631–3636. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu LH, Nakagawa R, Kashio Y, Ito A, Shoji
H, Nishi N, Hirashima M, Yamauchi A and Nakamura T:
Characterization of galectin-9-induced death of Jurkat T cells. J
Biochem. 141:157–172. 2007. View Article : Google Scholar
|
|
17
|
Kobayashi T, Kuroda J, Ashihara E, Oomizu
S, Terui Y, Taniyama A, Adachi S, Takagi T, Yamamoto M, Sasaki N,
et al: Galectin-9 exhibits anti-myeloma activity through JNK and
p38 MAP kinase pathways. Leukemia. 24:843–850. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuroda J, Yamamoto M, Nagoshi H, Kobayashi
T, Sasaki N, Shimura Y, Horiike S, Kimura S, Yamauchi A, Hirashima
M, et al: Targeting activating transcription factor 3 by Galectin-9
induces apoptosis and overcomes various types of treatment
resistance in chronic myelogenous leukemia. Mol Cancer Res.
8:994–1001. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishi N, Itoh A, Fujiyama A, Yoshida N,
Araya S, Hirashima M, Shoji H and Nakamura T: Development of highly
stable galectins: Truncation of the linker peptide confers
protease-resistance on tandem-repeat type galectins. FEBS Lett.
579:2058–2064. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schutte B, Henfling M, Kölgen W, Bouman M,
Meex S, Leers MP, Nap M, Björklund V, Björklund P, Björklund B, et
al: Keratin 8/18 breakdown and reorganization during apoptosis. Exp
Cell Res. 297:11–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Towbin H, Staehelin T and Gordon J:
Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: Procedure and some applications. Proc Natl
Acad Sci USA. 76:4350–4354. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Incalci M, Colombo T, Ubezio P,
Nicoletti I, Giavazzi R, Erba E, Ferrarese L, Meco D, Riccardi R,
Sessa C, et al: The combination of yondelis and cisplatin is
synergistic against human tumor xenografts. Eur J Cancer.
39:1920–1926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kageshita T, Kashio Y, Yamauchi A, Seki M,
Abedin MJ, Nishi N, Shoji H, Nakamura T, Ono T and Hirashima M:
Possible role of galectin-9 in cell aggregation and apoptosis of
human melanoma cell lines and its clinical significance. Int J
Cancer. 99:809–816. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Irie A, Yamauchi A, Kontani K, Kihara M,
Liu D, Shirato Y, Seki M, Nishi N, Nakamura T, Yokomise H, et al:
Galectin-9 as a prognostic factor with antimetastatic potential in
breast cancer. Clin Cancer Res. 11:2962–2968. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagahara K, Arikawa T, Oomizu S, Kontani
K, Nobumoto A, Tateno H, Watanabe K, Niki T, Katoh S, Miyake M, et
al: Galectin-9 increases Tim-3+ dendritic cells and
CD8+ T cells and enhances antitumor immunity via
galectin-9-Tim-3 interactions. J Immunol. 181:7660–7669. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kramer G, Erdal H, Mertens HJ, Nap M,
Mauermann J, Steiner G, Marberger M, Bivén K, Shoshan MC and Linder
S: Differentiation between cell death modes using measurements of
different soluble forms of extracellular cytokeratin 18. Cancer
Res. 64:1751–1756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Linder S: Cytokeratin markers come of age.
Tumour Biol. 28:189–195. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cummings J, Ranson M, Butt F, Moore D and
Dive C: Qualification of M30 and M65 ELISAs as surrogate biomarkers
of cell death: Long term antigen stability in cancer patient
plasma. Cancer Chemother Pharmacol. 60:921–924. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scott LC, Evans TR, Cassidy J, Harden S,
Paul J, Ullah R, O'Brien V and Brown R: Cytokeratin 18 in plasma of
patients with gastrointestinal adenocarcinoma as a biomarker of
tumour response. Br J Cancer. 101:410–417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Varnholt H: The role of microRNAs in
primary liver cancer. Ann Hepatol. 7:104–113. 2008.PubMed/NCBI
|
|
32
|
Tan S, Li R, Ding K, Lobie PE and Zhu T:
miR-198 inhibits migration and invasion of hepatocellular carcinoma
cells by targeting the HGF/c-MET pathway. FEBS Lett. 585:2229–2234.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J, Zhao H, Xin Y and Fan L:
MicroRNA-198 inhibits proliferation and induces apoptosis of lung
cancer cells via targeting FGFR1. J Cell Biochem. 115:987–995.
2014. View Article : Google Scholar
|
|
34
|
Marin-Muller C, Li D, Bharadwaj U, Li M,
Chen C, Hodges SE, Fisher WE, Mo Q, Hung MC and Yao Q: A
tumorigenic factor interactome connected through tumor suppressor
microRNA-198 in human pancreatic cancer. Clin Cancer Res.
19:5901–5913. 2013. View Article : Google Scholar : PubMed/NCBI
|