Introduction
Malignant mesothelioma is an aggressive tumor
arising from mesothelial cells of serous membranes, including the
pleura, peritoneum and pericardium (1–3).
Mesothelioma is highly resistant to most chemotherapeutic drugs and
radiation therapy (3), and surgical
therapy generally show limited efficacy (3–5). So
far a combination of cisplatin and pemetrexed appears to be the
best chemotherapy regimen for mesothelioma, but the median survival
of patients with mesothelioma remains at <12 months (5). Thus, new approaches for the
mesothelioma treatment are urgently required.
Anoikis, a Greek word meaning 'homelessness', is
defined as the subset of apoptosis triggered by cell-cell or
cell-extracellular matrix (ECM) detachments (6,7).
Apoptosis is programmed cell death in which caspases relay messages
through so-called initiator caspases to effector caspases that
medicate apoptotic processes, such as externalization of
phosphatidylserine, membrane blebbing and nuclear fragmentation
(8). These apoptotic features are
all observed during anoikis (9).
Anoikis plays a crucial role in defense mechanisms by preventing
the re-adhesion of detached cells to incorrect locations and their
unregulated growth (10,11). Anoikis resistance is emerging as a
hallmark of cancer cells and contributes to invasion and metastasis
formation of many types of tumors including mesothelioma (12). Three-dimensional tissue culture
methods, including the formation of multicellular aggregates
(spheroid) using suspension culture, have been adopted with the
superiority over conventional monolayer culture to mimic the tumor
behavior in vivo (13).
Spheroid formation has been reported to be involved in anoikis
resistance (13,14).
Src family kinases (SFK) are non-receptor and
cytoplasmic tyrosine kinases that have a pivotal role in cell
adhesion, proliferation, survival and apoptosis. Among the SFK,
Src, Yes and Fyn show ubiquitous expression, whereas others
including Lyn, exhibit more restricted tissue localization
(15,16). There have been many studies showing
that Src protein level or its kinase activity is increased in a
variety of human tumors (16). Our
previous study clarified that human mesothelioma cells express Lyn
in addition to Src, Yes and Fyn (17).
Mesothelioma cells form spheroid-like cell
aggregates in pleural and peritoneal effusions (14). Spheroid formation in other tumor
types such as osteosarcoma appears to play a role in
chemoresistance (18), but whether
the aggregates of mesothelioma cells are associated with
chemoresistance remains unclear. In this study, we examined the
association between anoikis and chemoresistance in the human
mesothelioma cell line NCI-H2052. We found that suspension cultures
induced spheroid formation, resulting in the development of
resistance to anoikis and to cisplatin. Further clarification of
cell growth-related signal transduction revealed that suspension
culture induces SFK activation and that inhibition of the SFK
activation abolishes anoikis resistance, which in turn facilitates
cisplatin-induced apoptosis in NCI-H2052 cells.
Materials and methods
Cell lines and reagents
A non-malignant transformed human pleural
mesothelial cell line, Met5A and two human mesothelioma cell lines,
NCI-H28 and NCI-H2052, were obtained from the American Type Culture
Collection (Rockville, MD, USA). Conventional tissue-culture dishes
(Becton-Dickinson Labware, Franklin Lakes, NJ, USA) for monolayer
cultures and non-adherent dishes with ultra-low cell binding
capacity (HydroCell, Cellseed Inc., Tokyo, Japan) for suspension
cultures were used. Cells were cultured in culture medium composed
of RPMI-1640 medium (Sigma, St. Louis, MO, USA) supplemented with
10% fetal bovine serum (Moregate, Brisbane, Australia), 5
µg/ml penicillin, 5 µg/ml streptomycin, and 10
µg/ml neomycin. Cells were incubated at 37°C under
95% air and 5% CO2. Cisplatin (LKT Lab., Saint Paul, MN,
USA) and dasatinib (Biovision, Mountain View, CA, USA) were
prepared in dimethylsulfoxide (DMSO) using the following stock
solutions: 125 mM cisplatin and 5 mM dasatinib. DMSO was used as a
vehicle control as appropriate. Other chemicals were purchased from
Sigma.
Cell proliferation analysis
Cell proliferation was analyzed as described earlier
with slight modifications (19).
Briefly, NCI-H2052 cells were seeded at 1×105 per 5 ml
in 60-mm dishes. The cells were incubated for 24–96 h and harvested
by trypsinization. Cell numbers were measured with a Coulter
Counter Z1 (Coulter Japan, Tokyo, Japan).
Morphological observation
Morphological observation was performed as described
(20). Briefly, NCI-H2052 cells
were incubated for 72 h as described above. For suspension
cultures, the cells were spun down and the cell culture supernatant
was aspirated. The cells were observed under a light microscope
(Nicon, Tokyo, Japan) for phase contrast images.
Treatment with cisplatin and
dasatinib
In experiments using cisplatin, cells were seeded at
2×105 per 9 ml culture medium in 100-mm dishes or at
2×103 per 90 µl culture medium in each well of a
96-well plate, and cultured for 24 h. After the incubation, 1 ml
and 10 µl of fresh culture medium containing cisplatin or
vehicle (DMSO) were added to the dishes and the wells of the plate,
respectively, and incubation was continued for an additional 24–72
h. In experiments using dasatinib, cells were seeded at
2×105 per 10 ml culture medium containing dasatinib or
DMSO in 100-mm dishes, and then cultured for 24–96 h. The final
concentration of dasatinib was chosen based on a prior study
(17). In the combination
experiments of cispl-atin and dasatinib, cells were seeded at
2×105 per 9 ml culture medium containing dasatinib or
DMSO in 100-mm dishes, and cultured for 24 h. After the incubation,
cisplatin or DMSO was added to 1 ml of fresh culture medium
supplemented with dasatinib or DMSO, the medium was then added to
the dishes, and incubation was continued for an additional 72
h.
Cell viability analysis
Cell viability analysis was performed as described
(21). Briefly, cells were seeded
in each well of a 96-well plate (Becton-Dickinson Labware) as
described above. Cell viability was analyzed by a colorimetric
assay using Cell Counting Kit-8 (CCK-8) (Dojin Chemical Institute,
Kumamoto, Japan) according to the manufacturer's protocol. Color
intensity was quantified by a microplate reader (SPECTRAmax
PLUS384, Molecular Devices, Sunnyvale, CA, USA).
Flow cytometric analysis of
apoptosis
Apoptosis was analyzed by flow cytometry using an
Annexin V (Ax)-FITC Kit (Medical and Biological Laboratories,
Nagoya, Japan) as described (21).
Briefly, 1×105 cells treated with cisplatin or dasatinib
were trypsinized, washed with phosphate-buffered saline (PBS) and
then labeled with Ax-FITC and propidium iodide (PI). Fluorescence
intensity was measured using a Cytomics FC 500 flow cytometer and
CXP software (Beckman Coulter, Fullerton, CA, USA).
Western blotting and antibodies
Western blotting was performed as described
(21). All antibodies used were
purchased from Cell Signaling Technology (Beverly, MA, USA). All
western blot analyses were performed three times and representative
data are shown.
Statistical analysis
All data are presented as the means ± standard
errors (SEs) of 3–4 independent experiments. Comparisons between
two groups were performed using Student's unpaired t-test
(*p<0.05, **p<0.005).
Results
NCI-H2052 cells form spheroids and
develop anoikis resistance in suspension cultures
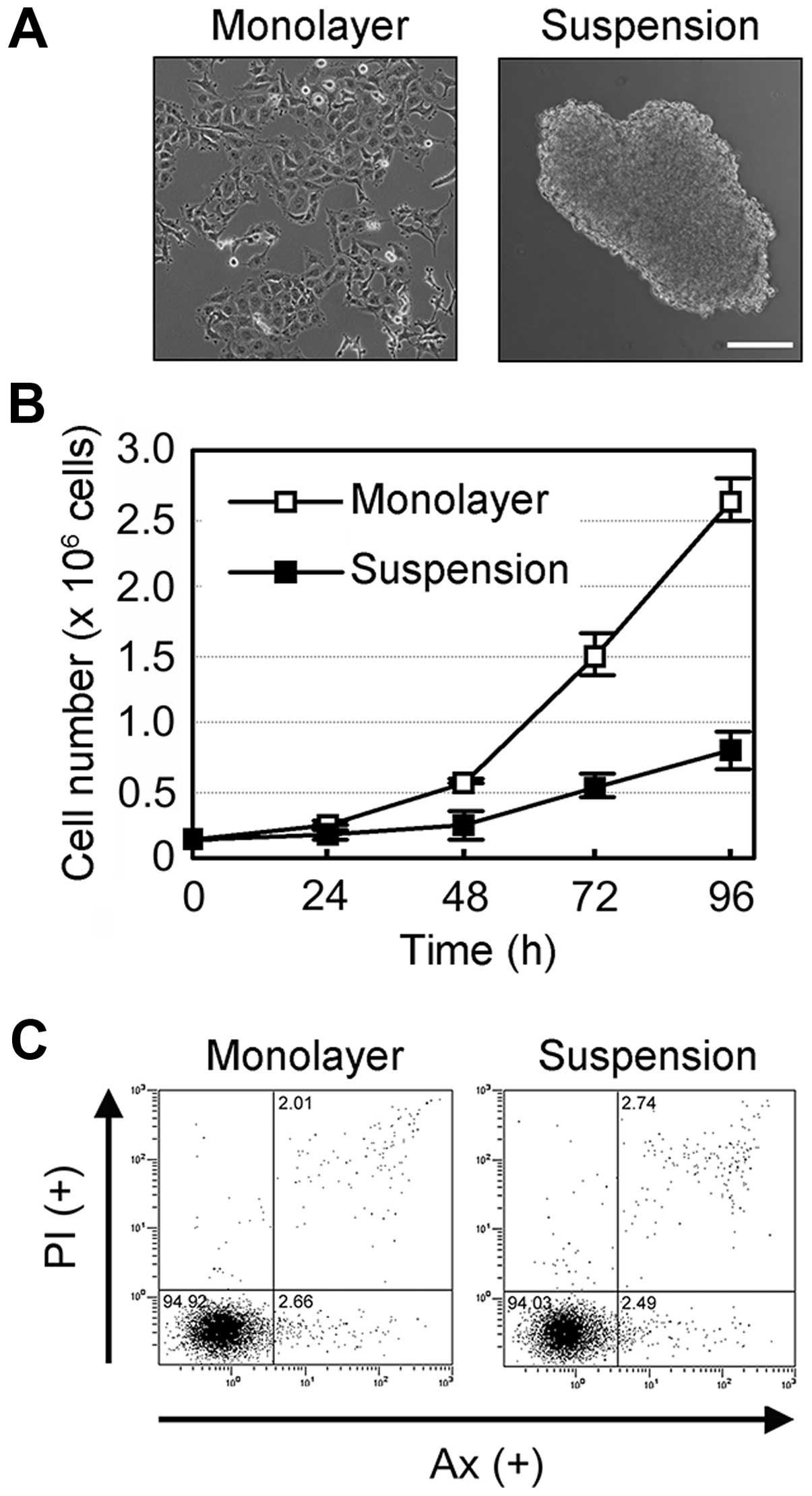
The human mesothelioma NCI-H2052 cells adhered to
the tissue-culture dish in conventional monolayer cultures
(Fig. 1A, left panel), but were
found to form multicellular aggregates (spheroids) in suspension
cultures using a non-adherent dish with ultra-low cell binding
capacity (HydroCell) after a 24-h incubation period (Fig. 1A, right panel). Anoikis, a subset of
apoptosis, is known to be induced by cell-cell or cell-ECM
detachment and has been reported to be induced in suspension
cultures of mesothelial cells (14). NCI-H2052 cells in suspension culture
proliferated in a time-dependent manner, although the proliferation
rate was markedly lower in suspension cultures than in monolayer
cultures (Fig. 1B). Furthermore,
flow cytometric analysis using double staining with Annexin V (Ax)
and propidium iodide (PI) revealed that the suspension cultures as
well as monolayer cultures showed little apoptosis in NCI-H2052
cells (Fig. 1C). These results
suggest that suspension culture induces spheroid formation,
resulting in the development of anoikis resistance in NCI-H2052
cells.
SFK activation induced by suspension
culture is responsible for anoikis resistance in NCI-H2052
cells
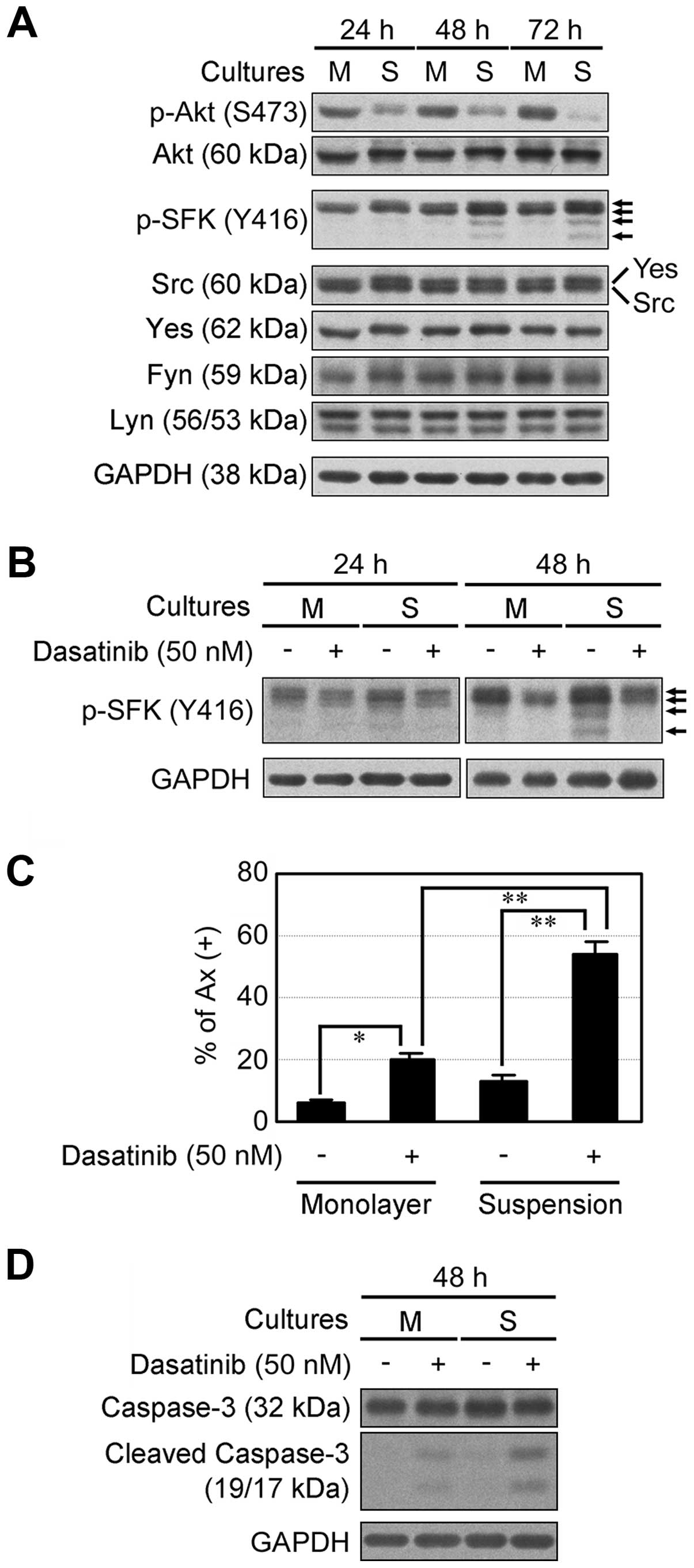
We examined cell growth-related signal transduction
in NCI-H2052 cells in monolayer and suspension cultures. Compared
to monolayer cultures, suspension cultures suppressed Akt
phosphorylation after a 24-h incubation, but induced SFK
phosphorylation after a 48-h incubation in a time-dependent manner
(Fig. 2A). We then investigated the
effects of dasatinib, an inhibitor of multi-tyrosine kinases
including SFK, on NCI-H2052 cells in monolayer and suspension
cultures. Dasatinib inhibited SFK phosphorylation in monolayer and
suspension cultures (Fig. 2B).
Dasatinib slightly induced apoptosis in monolayer cultures, but
apoptosis was markedly induced by dasatinib in suspension cultures
(Fig. 2C). In addition, dasatinib
markedly induced caspase-3 cleavage in suspension cultures
(Fig. 2D). Thus, inhibition of SFK
activation by dasatinib abolished anoikis resistance induced by
spheroid formation. Collectively, these results suggest that SFK
activation induced by spheroid formation is responsible for anoikis
resistance in NCI-H2052 cells.
Spheroid formation in suspension cultures
develops resistance to cisplatin in NCI-H2052 cells
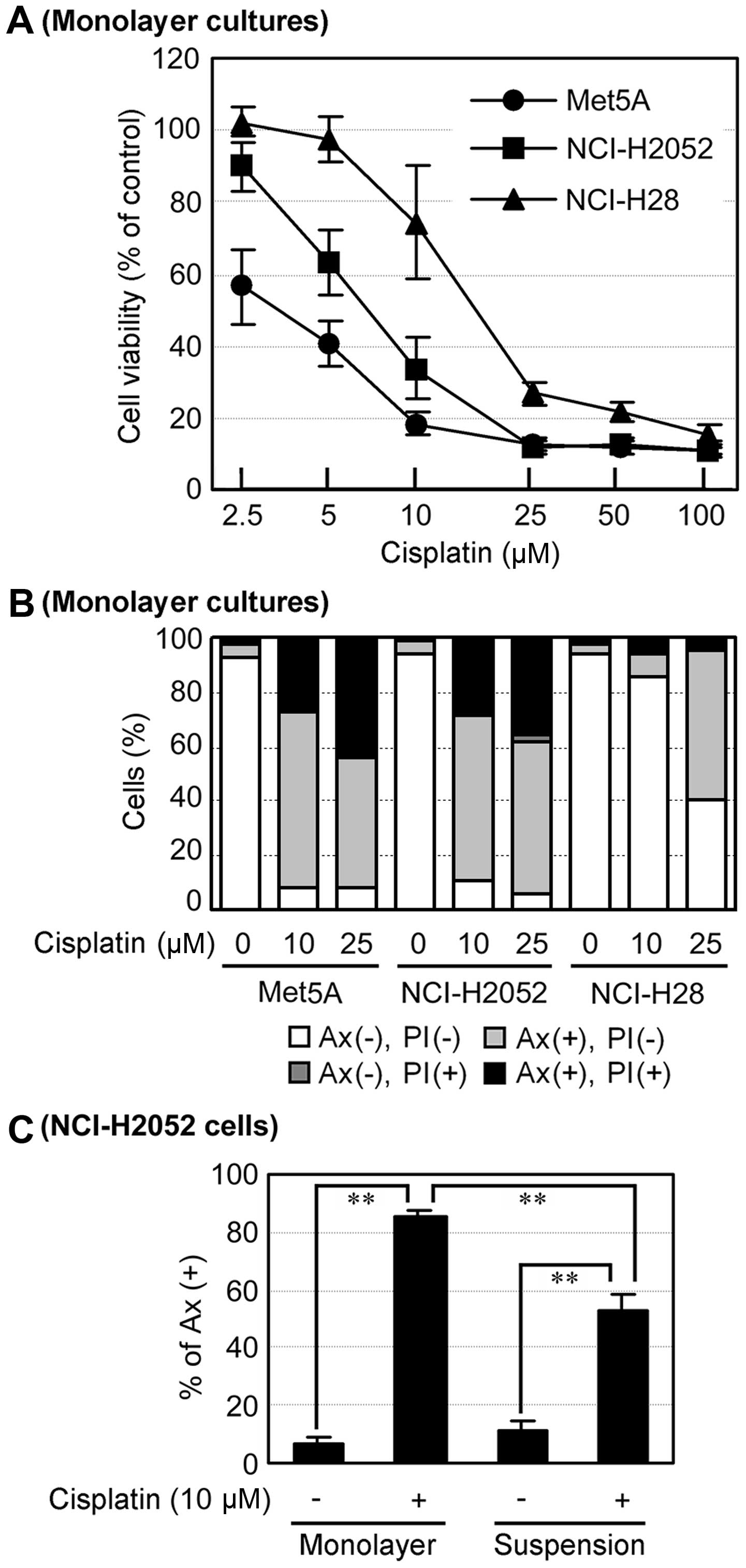
Subsequently, we investigated the effects of
cisplatin on the human mesothelial cell line Met5A, and the human
mesothelioma cell lines NCI-H2052 and NCI-H28. In monolayer
cultures, cisplatin reduced cell viability in Met5A and NCI-H2052
cells to a much higher degree than in NCI-H28 cells (Fig. 3A). In addition, cisplatin induced
apoptosis in these cell lines in a concentration-dependent manner,
but 10 µM cisplatin did not induce apoptosis in NCI-H28
cells compared to Met5A and NCI-H2052 cells (Fig. 3B). These results suggest that
NCI-H2052 cells as well as Met5A cells are more sensitive to
cisplatin than NCI-H28 cells in monolayer cultures. Intriguingly,
suspension cultures suppressed cisplatin-induced apoptosis compared
to mono-layer cultures in NCI-H2052 cells (Fig. 3C). These results suggest that
spheroid formation in suspension culture induces cisplatin
resistance in NCI-H2052 cells.
Dasatinib facilitates cisplatin-induced
apoptosis in suspension culture in NCI-H2052 cells
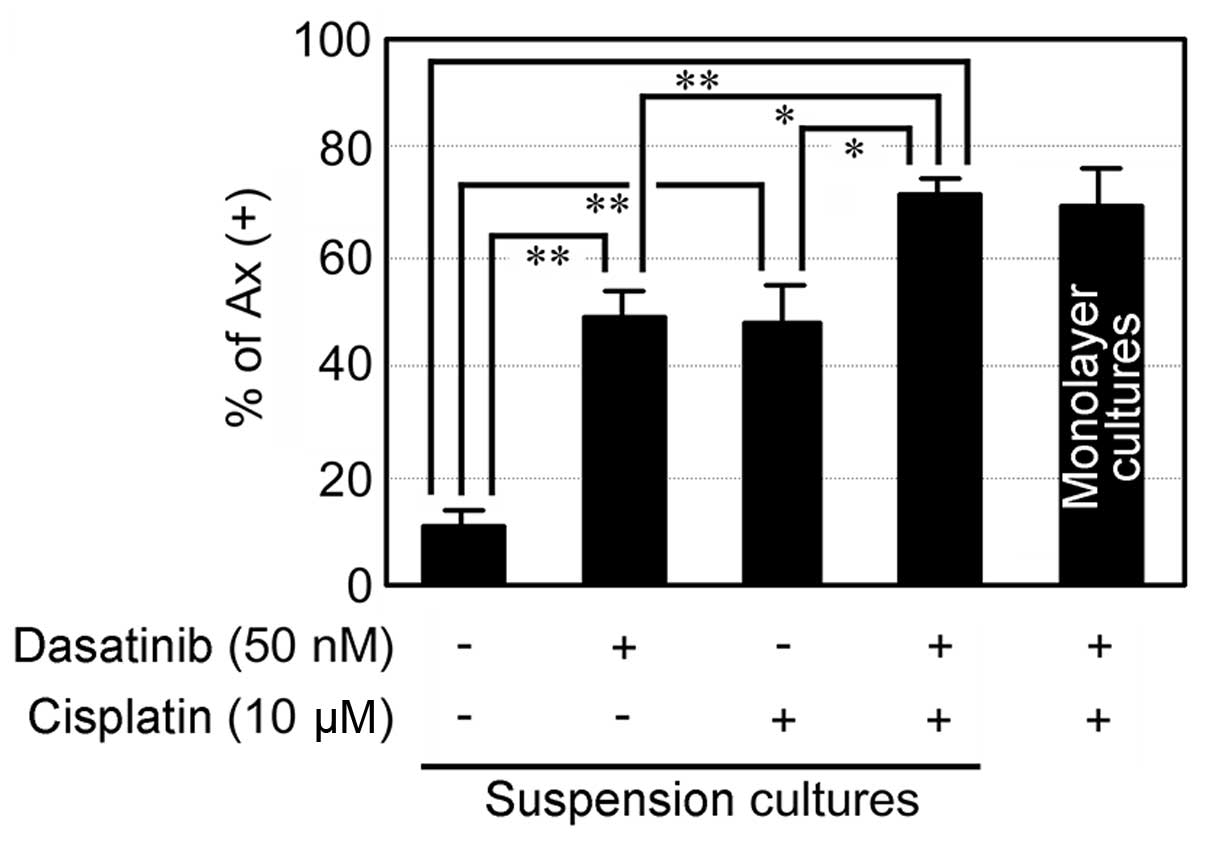
To further investigate whether dasatinib facilitates
cisplatin-induced apoptosis in suspension culture, we treated
NCI-H2052 cells with dasatinib together with cisplatin. Treatment
with the combination of dasatinib and cisplatin induced apoptosis
more significantly than that with either alone in suspension
cultures (Fig. 4). In addition, the
degree of apoptosis induced by the combination of dasatinib and
cisplatin in suspension cultures was comparable to that in
monolayer cultures. The results suggest that dasatinib abolishes
anoikis resistance, leading to facilitation of cisplatin-induced
apoptosis in spheroid-forming NCI-H2052 cells.
Discussion
As most tissues have three-dimensional structures
composed of multiple types of cells, suspension culture that
induces spheroid formation has been increasingly used in the field
of cancer research to mimic the in vivo condition. In the
present study, we adopted a suspension culture system by using a
non-adherent dish with ultra-low cell binding capacity, which
enabled NCI-H2052, a mesothelioma cell line, to form spheroids
(Fig. 1). We further used this
culture system to analyze the sensitivity of these mesothelioma
cells to anoikis and drug-induced apoptosis.
Anoikis is the subset of apoptosis triggered by the
detachment of cells from other cells or the ECM, and anoikis
resistance is associated with cancer metastasis. We found that
NCI-H2052 cells in suspension cultures formed spheroids, which led
to the induction of anoikis resistance (Fig. 1). Mesothelioma cells form
spheroid-like cell aggregates in pleural and peritoneal effusions,
and mesothelioma cell lines, but not the non-malignant mesothelial
cell line Met5A, resist anoikis as multicellular aggregates in
suspension culture (14). Some
tumors, including lung and ovarian cancers, have shown a similar
correlation between spheroid formation and anoikis resistance
(22,23). These results suggest that spheroid
formation of malignant tumors favors the induction of anoikis
resistance.
Akt is a serine/threonine kinase that plays a
crucial role in multiple cellular processes such as cell
proliferation, survival and apoptosis, and especially inactivates
pro-apoptotic factors, leading to cell survival (24). In anoikis resistant osteosarcoma
cells, Akt activity was upregulated in suspension cultures
(25). However, in the present
study, Akt activity in suspension cultures was lower than that in
monolayer cultures (Fig. 2).
Similarly, Barbone et al reported that Akt activity is
downregulated in suspension cultures in mesothelioma cells
(26). Presently, we have no
rational explanation for this difference, but given the
multifactorial nature of cancer, it is possible that Akt
downregulation in anoikis resistance may be mesothelioma-specific.
In addition, this Akt downregulation may be associated with the
observed decrease in growth rate of NCI-H2052 cells in suspension
cultures.
Malignant mesothelioma is refractory to conventional
chemotherapy, which is related to resistance to the apoptosis
induced by chemotherapeutic drugs. It has been reported that
spheroid cells in osteosarcoma and ovarian cancer are more
resistant to chemotherapeutic drugs than their parental cells
(18,27). In the present study,
spheroid-forming NCI-H2052 cells developed cisplatin resistance
(Fig. 3). Thus, it seems to be a
general phenomenon that spheroid formation of malignant tumors
induces chemoresistance.
v-Src transformation in MDCK cells induces anoikis
resistance (6), but how SFK
including endogenous Src (c-Src) are involved in anoikis resistance
remains unclear in malignant mesothelioma. It has been reported
that suspension culture induces Src activation in anoikis resistant
osteosarcoma cells (25).
Similarly, we found that suspension cultures induced SFK activation
and anoikis resistance in mesothelioma cells (Fig. 2). Our previous study also showed
that the Fyn-expressing mesothelioma NCI-H2052 cells, are more
insensitive to SFK inhibitors including dasatinib than
Fyn-deficient mesothelioma cells, NCI-H28, in monolayer cultures
(17). However, we observed that
the rate of NCI-H2052 cell apoptosis induced by dasatinib in
suspension cultures was more prominent than that in monolayer
cultures (Fig. 2). These results
suggest that inhibition of SFK activation abolishes the anoikis
resistance of spheroid-forming mesothelioma cells. Furthermore, the
present study shows for the first time that inhibition of SFK
activation in spheroid cultures of a mesothelioma cell line
facilitates cisplatin-induced apoptosis (Fig. 4). Phase II clinical trials have
shown that dasatinib alone has been ineffective in unselected
mesothelioma patients (28), but
our study suggests that combination therapy using dasatinib and
cisplatin may be more effective than dasatinib alone in the
treatment of mesothelioma.
In the present study, spheroid formation in
suspension cultures was observed after a 24-h incubation period
(data not shown), but these suspension cultures activated SFK only
after a 48-h incubation period (Fig.
3), suggesting that spheroid formation preceded SFK activation.
In addition, we observed that dasatinib treatment could partially
attenuate spheroid aggregation (data not shown). However, it is
still uncertain whether SFK are directly involved in spheroid
formation. Further studies are needed to clarify the precise
relationship between spheroid formation and SFK activation.
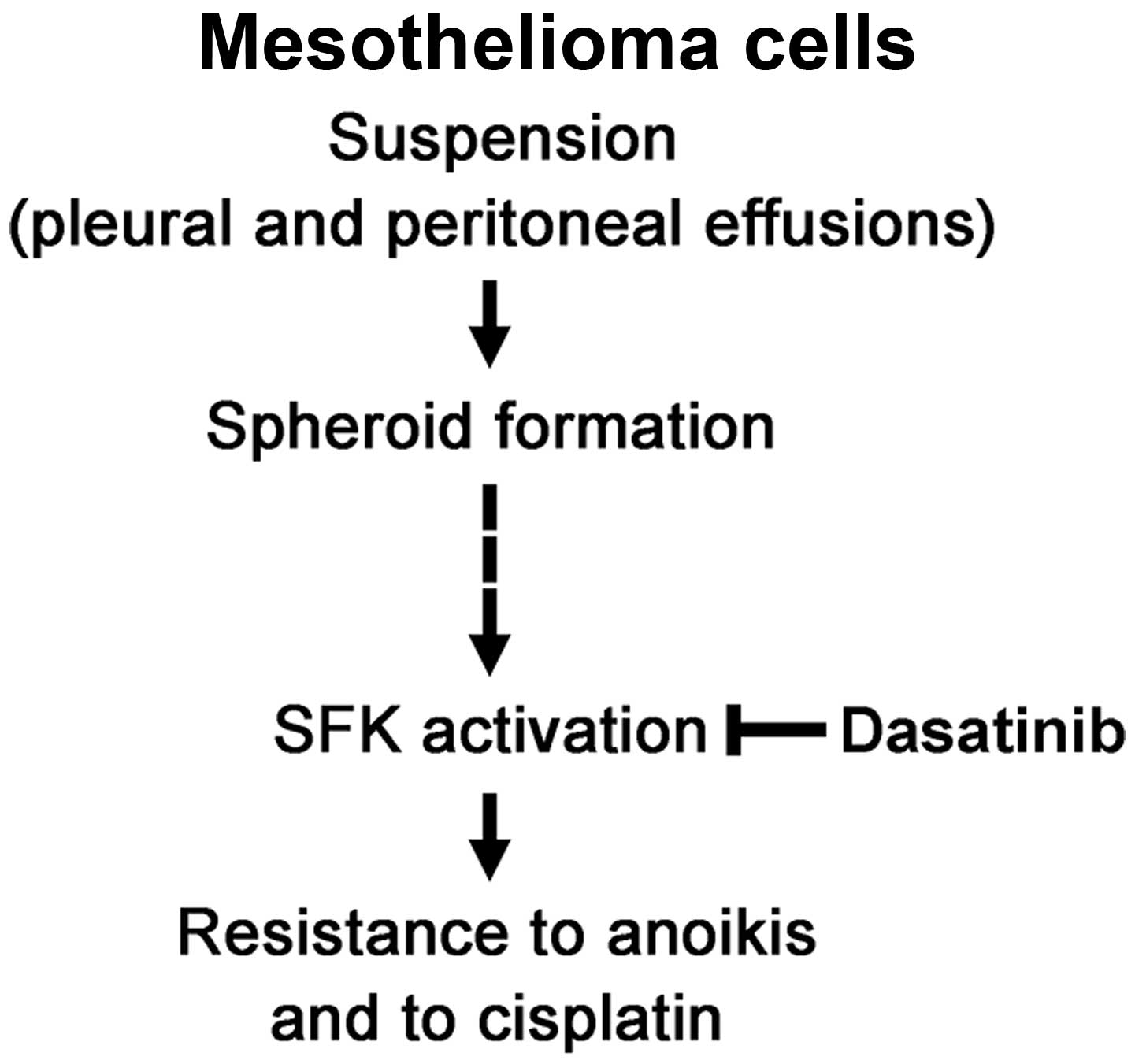
In conclusion, mesothelioma cells form spheroids in
suspension culture that induces SFK activation, resulting in
developing resistance to anoikis and to cisplatin (Fig. 5). This study also suggests that the
combination of dasatinib and cisplatin is potentially useful for
treatment of malignant mesothelioma.
Acknowledgments
This study was supported by KAKENHI to Y. Fujimori,
Grant-in-Aid for Researchers, Hyogo College of Medicine, 2013 and
MEXT-Supported Program for the Strategic Research Foundation at
Private Universities, 2012–2015.
References
|
1
|
Robinson BW, Musk AW and Lake RA:
Malignant mesothelioma. Lancet. 366:397–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsao AS, Wistuba I, Roth JA and Kindler
HL: Malignant pleural mesothelioma. J Clin Oncol. 27:2081–2090.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vogelzang NJ: Chemotherapy for malignant
pleural mesothelioma. Lancet. 371:1640–1642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goudar RK: New therapeutic options for
mesothelioma. Curr Oncol Rep. 7:260–265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vogelzang NJ, Rusthoven JJ, Symanowski J,
Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S,
Manegold C, et al: Phase III study of pemetrexed in combination
with cisplatin versus cisplatin alone in patients with malignant
pleural mesothelioma. J Clin Oncol. 21:2636–2644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frisch SM and Francis H: Disruption of
epithelial cell-matrix interactions induces apoptosis. J Cell Biol.
124:619–626. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meredith JE Jr, Fazeli B and Schwartz MA:
The extracellular matrix as a cell survival factor. Mol Biol Cell.
4:953–961. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valentijn AJ, Zouq N and Gilmore AP:
Anoikis. Biochem Soc Trans. 32:421–425. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Ridder L, Cornelissen M and de Ridder
D: Autologous spheroid culture: A screening tool for human brain
tumour invasion. Crit Rev Oncol Hematol. 36:107–122. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simpson CD, Anyiwe K and Schimmer AD:
Anoikis resistance and tumor metastasis. Cancer Lett. 272:177–185.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong X and Rescorla FJ: Cell surface
adhesion molecules and adhesion-initiated signaling: Understanding
of anoikis resistance mechanisms and therapeutic opportunities.
Cell Signal. 24:393–401. 2012. View Article : Google Scholar
|
|
13
|
Mueller-Klieser W: Three-dimensional cell
cultures: From molecular mechanisms to clinical applications. Am J
Physiol. 273:C1109–C1123. 1997.PubMed/NCBI
|
|
14
|
Daubriac J, Fleury-Feith J, Kheuang L,
Galipon J, Saint-Albin A, Renier A, Giovannini M, Galateau-Sallé F
and Jaurand MC: Malignant pleural mesothelioma cells resist anoikis
as quiescent pluricellular aggregates. Cell Death Differ.
16:1146–1155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thomas SM and Brugge JS: Cellular
functions regulated by Src family kinases. Annu Rev Cell Dev Biol.
13:513–609. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yeatman TJ: A renaissance for SRC. Nat
Revc Cancer. 4:470–480. 2004. View
Article : Google Scholar
|
|
17
|
Eguchi R, Kubo S, Takeda H, Ohta T, Tabata
C, Ogawa H, Nakano T and Fujimori Y: Deficiency of Fyn protein is
prerequisite for apoptosis induced by Src family kinase inhibitors
in human mesothelioma cells. Carcinogenesis. 33:969–975. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arai K, Sakamoto R, Kubota D and Kondo T:
Proteomic approach toward molecular backgrounds of drug resistance
of osteosarcoma cells in spheroid culture system. Proteomics.
13:2351–2360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eguchi R, Kubo S, Ohta T, Kunimasa K,
Okada M, Tamaki H, Kaji K, Wakabayashi I, Fujimori Y and Ogawa H:
FK506 induces endothelial dysfunction through attenuation of Akt
and ERK1/2 independently of calcineurin inhibition and the caspase
pathway. Cell Signal. 25:1731–1738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eguchi R, Fujimori Y, Ohta T, Kunimasa K
and Nakano T: Calpain is involved in cisplatin-induced endothelial
injury in an in vitro three-dimensional blood vessel model. Int J
Oncol. 37:1289–1296. 2010.PubMed/NCBI
|
|
21
|
Eguchi R, Fujimori Y, Takeda H, Tabata C,
Ohta T, Kuribayashi K, Fukuoka K and Nakano T: Arsenic trioxide
induces apoptosis through JNK and ERK in human mesothelioma cells.
J Cell Physiol. 226:762–768. 2011. View Article : Google Scholar
|
|
22
|
Carduner L, Picot CR, Leroy-Dudal J, Blay
L, Kellouche S and Carreiras F: Cell cycle arrest or survival
signaling through αv integrins, activation of PKC and ERK1/2 lead
to anoikis resistance of ovarian cancer spheroids. Exp Cell Res.
320:329–342. 2014. View Article : Google Scholar
|
|
23
|
McCarroll JA, Gan PP, Erlich RB, Liu M,
Dwarte T, Sagnella SS, Akerfeldt MC, Yang L, Parker AL, Chang MH,
et al: TUBB3/βIII-tubulin acts through the PTEN/AKT signaling axis
to promote tumorigenesis and anoikis resistance in non-small cell
lung cancer. Cancer Res. 75:415–425. 2015. View Article : Google Scholar
|
|
24
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: A play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Diaz-Montero CM, Wygant JN and McIntyre
BW: PI3-K/Akt-mediated anoikis resistance of human osteosarcoma
cells requires Src activation. Eur J Cancer. 42:1491–1500. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barbone D, Yang TM, Morgan JR, Gaudino G
and Broaddus VC: Mammalian target of rapamycin contributes to the
acquired apoptotic resistance of human mesothelioma multicellular
spheroids. J Biol Chem. 283:13021–13030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao J, Qian F, Tchabo N,
Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB,
Morrison CD, et al: Ovarian cancer spheroid cells with stem
cell-like properties contribute to tumor generation, metastasis and
chemotherapy resistance through hypoxia-resistant metabolism. PLoS
One. 9:e849412014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dudek AZ, Pang H, Kratzke RA, Otterson GA,
Hodgson L, Vokes EE and Kindler HL; Cancer and Leukemia Group B:
Phase II study of dasatinib in patients with previously treated
malignant mesothelioma (cancer and leukemia group B 30601): a brief
report. J Thorac Oncol. 7:755–759. 2012. View Article : Google Scholar : PubMed/NCBI
|