Introduction
Lung cancer is characterized by uncontrolled cell
growth in tissues of the lung, trachea, or bronchi. This malignancy
is currently the leading cause of cancer-related mortality in the
world (1,2). In Korea, the incidence of lung cancer
increases gradually with age (3).
Recently, Jung et al (1)
reported that lung cancer is the fourth most prevalent type of
malignancy, and the most common cause of cancer-related mortalities
in Korea (1). Most (~85%) patients
with lung cancer have the non-small cell lung cancer (NSCLC)
subtype, and the majority of these patients have advanced disease
(defined as stage IIIB or IV at the time of diagnosis) (4). The rapid developments in
oncogene-directed targeted therapies have significantly changed the
treatment paradigm of NSCLC during the last decade. However, tumors
exposed to targeted therapies may develop acquired resistance
against these treatments over time. Therefore, the development of
novel agents to combat NSCLC, and associated resistance, is
imperative.
The
(±)-(R*)-5-methoxy-11-methyl-2-((R*)-2-methyloxiran-2-yl)-1,2-dihydrobenzofuro[4,5-b][1,8]naphthyridin-6(11H)-one,
MHY-449, is a novel synthetic compound containing xanthone and
acridone components, derived from psorospermin and acronycine
templates, respectively (5).
MHY-449 has been shown to induce apoptosis in various human cancer
cell lines, including breast (5),
colon (6) and prostate (5,7). While
its precise mechanism of anticancer activity has not been fully
elucidated, we have previously reported that MHY-449 induces G2/M
phase cell cycle arrest (5,6), inhibits Akt/forkhead box O1 (7), activates caspases (6) and extracellular signal-regulated
kinase (ERK) (7), modulates cell
cycle regulatory proteins (6), and
downregulates the anti-apoptotic protein Bcl-2 (6,7). These
anticancer activities make MHY-449 an attractive candidate for
potential pharmaceutical development. In this study, we evaluated
the effect of MHY-449 on NSCLC cells in vitro. The results
showed that MHY-449 exhibits a potent anticancer effect in NSCLC
cells, attributable to its ability to induce apoptosis. It was also
found that Akt is essential to MHY-449-induced apoptosis in these
cells.
Materials and methods
Chemicals
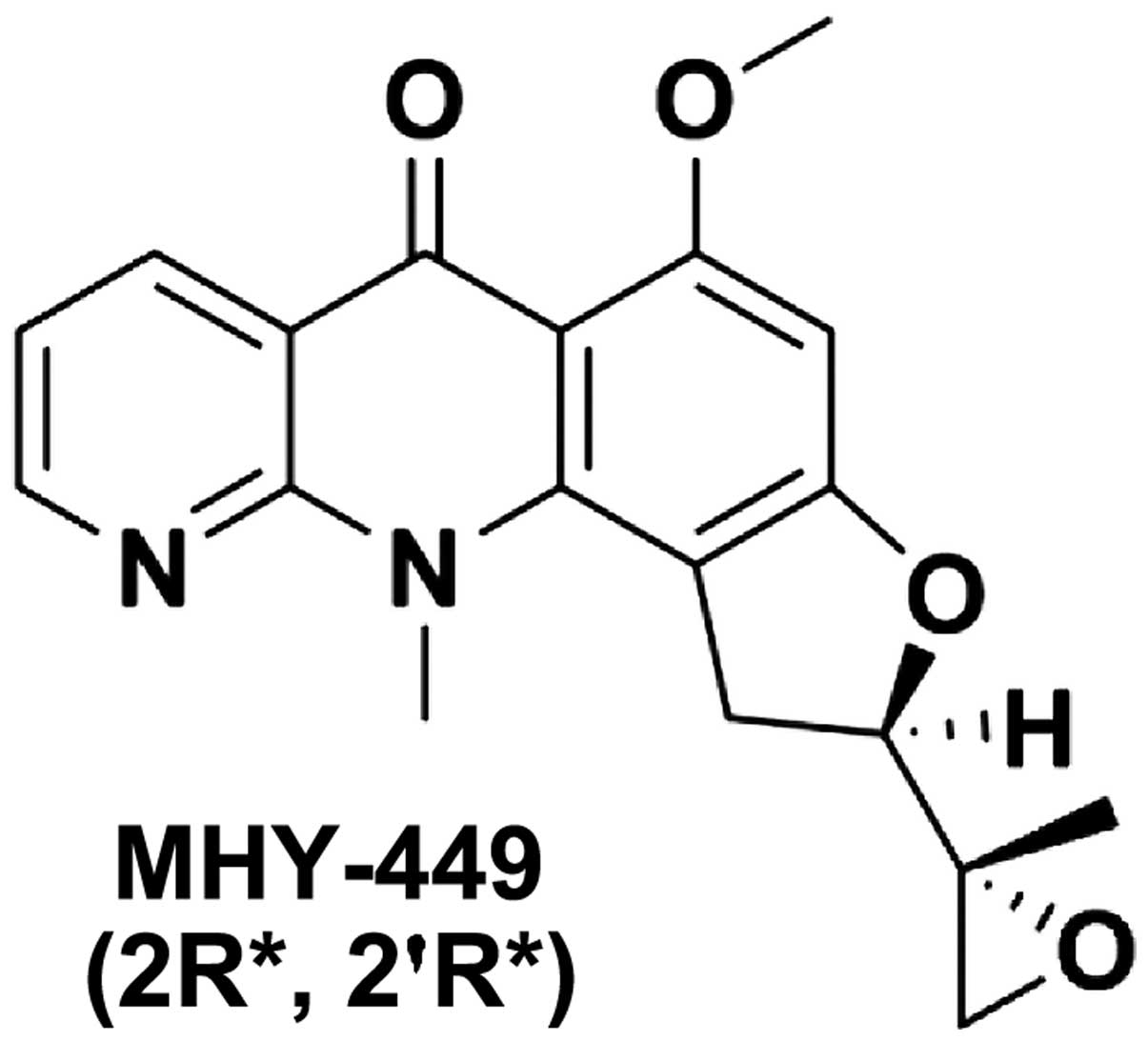
The simplified code name and chemical structure of
MHY-449 used in this study are shown in Fig. 1. The methods used for the design and
synthesis of this compound have been previously described (ref?).
MHY-449 was dissolved in dimethyl sulfoxide (DMSO) to yield a 10-mM
stock solution and stored at −20°C until use. The maximal
concentration of DMSO did not exceed 0.1% (v/v) in the treatment
range, where there was no influence on cell growth. DMSO and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
were obtained from Amresco LLC (Solon, OH, USA). Antibodies
specific for pro-caspase-3, -8, and -9, poly(ADP-ribose)
polymerases (PARP), BH3-interacting domain death agonist (Bid),
Bcl-2-associated X protein (Bax), B-cell CLL/lymphoma 2 (Bcl-2),
phospho-Akt, Akt, β-actin and Z-VAD-FMK, were obtained from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell culture and cell viability
assay
The human A549 and NCI-H460 lung cancer cell lines
were cultured at 37°C in humidified 5% CO2 in RPMI-1640
medium supplemented with 10% fetal bovine serum (FBS), penicillin
(100 U/ml), and streptomycin (100 µg/ml) (all from GE
Healthcare Life Sciences, Logan, UT, USA). The non-transformed
human Hs27 foreskin fibroblast cell line was cultured in Dulbecco's
modified Eagle's medium (DMEM; GE Healthcare Life Sciences)
supplemented with 10% FBS. To determine cell viability, the MTT
assay was performed. The cells were seeded in 24-well culture
plates, cultured for 24 h, treated with or without various reagents
at the indicated concentrations, and then incubated in the dark
with MTT (0.5 mg/ml) at 37°C for 2 h. The formazan granules
generated by the live cells were dissolved in DMSO, and the
absorbance at 540 nm was monitored using a multi-well reader
(Thermo Fisher Scientific, Vantaa, Finland).
Annexin V/PI staining
To quantitatively determine the percentage of
apoptotic cells, we used the BD Pharmingen FITC Annexin V Apoptosis
Detection kit (BD Biosciences, San Diego, CA, USA) according to the
manufacturer's instructions. The cells were stained with propidium
iodide (PI) and Annexin V-fluorescein isothiocyanate (FITC)
solution at room temperature for 15 min in the dark. The stained
cells were then analyzed using flow cytometry within 1 h.
DNA fragmentation assay
The cells were lysed in a buffer containing 5 mM
Tris-HCl (pH 7.5), 5 mM ethylenedi-aminetetraacetic acid (EDTA),
and 0.5% Triton X-100 for 30 min on ice. After centrifugation at
27,000 × g for 15 min, the fragmented DNA in the supernatant was
treated with RNase, followed by proteinase K digestion, extraction
with a phenol/chloroform/isoamyl alcohol mixture (25:24:1, v/v/v),
and isopropanol precipitation. DNA was separated using a 1.6%
agarose gel, stained with ethidium bromide (0.1 µg/ml), and
visualized using an ultraviolet source.
Western blot analysis
The cells were harvested, lysed, and equal amounts
of protein were subjected to sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene
fluoride (PVDF) membranes for immunoblotting. Blots were probed
with the desired primary antibodies overnight, incubated with
horseradish peroxidase (HRP)-conjugated secondary antibodies (Santa
Cruz Biotechnology, Inc.), and then visualized using an enhanced
chemiluminescence (ECL) detection system (GE Healthcare,
Piscataway, NJ, USA).
Measurement of mitochondrial membrane
potential (MMP, ΔΨm)
MMP was determined using a flow cytometer and the
lipophilic cationic dye
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetra-ethyl-benzimidazolylcarbocyanine
iodide (JC-1; Calbiochem, San Diego, CA, USA). JC-1 is a dye that
stains the mitochondria of living cells in a membrane
potential-dependent manner. The cells were treated with various
concentrations of JC-1, harvested, and washed with cold PBS. The
cells were stained with 10 µM JC-1 for 20 min at 37°C in the
dark. The cells were subsequently washed with cold PBS and then
analyzed using an Accuri C6 flow cytometer (BD Biosciences, Ann
Arbor, MI, USA).
Flow cytometric analysis of sub-G1 phase
and cell cycle population
The DNA content was measured following the staining
of the cells with PI (Sigma-Aldrich Co., LLC, St. Louis, MO, USA).
After treatment with various concentrations of MHY-449, the cells
were collected, washed with cold PBS, and then fixed in 70% ethanol
at −20°C overnight. The fixed cells were washed with cold PBS and
then stained with cold PI solution (50 µg/ml in PBS) at 37°C
for 30 min in the dark. Analysis was performed using an Accuri C6
flow cytometer.
Statistical analysis
Results were presented as the mean ± standard
deviation (SD) of three separate experiments and analyzed using the
Student's t-test. The acceptable level of significance was
established at P<0.05.
Results
MHY-449 inhibits the proliferation of
lung cancer cells
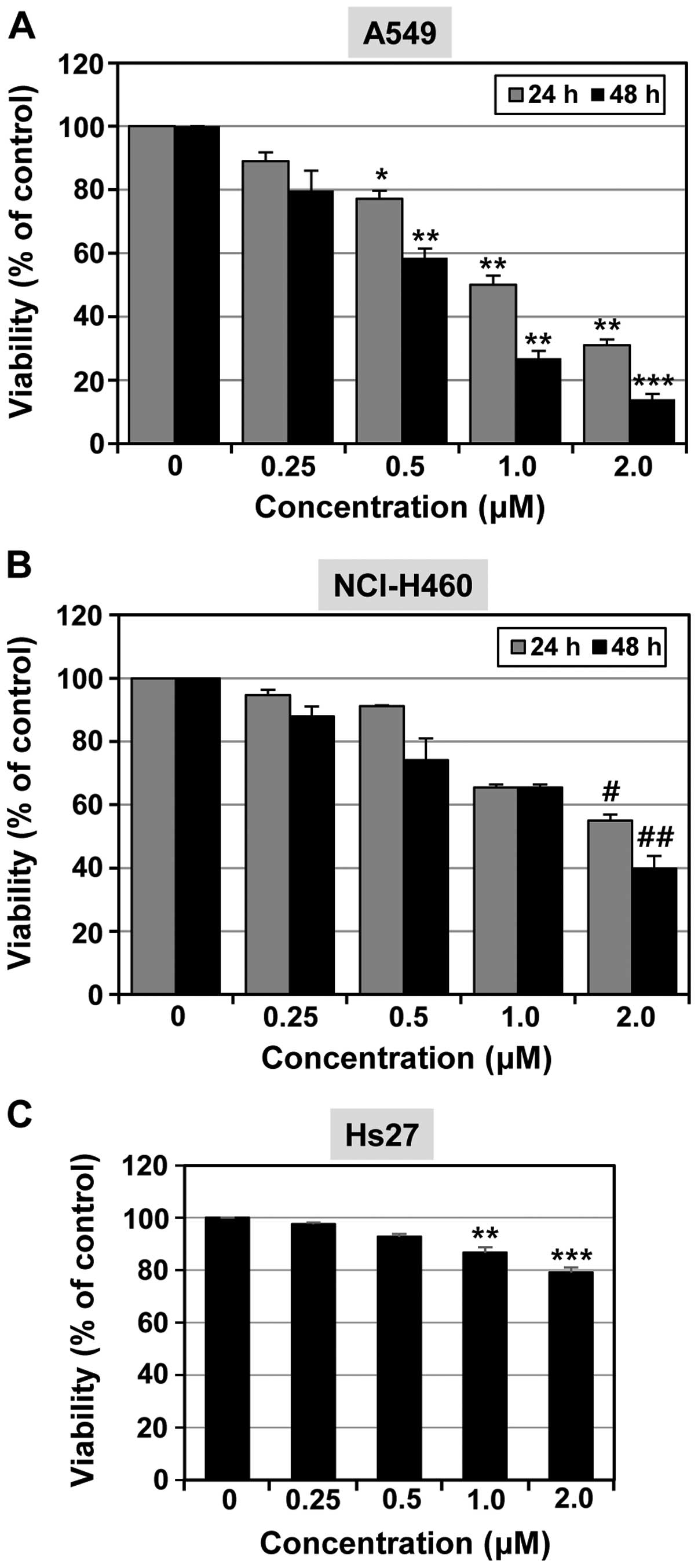
The anti-proliferative activities of MHY-449 on
NSCLC cell lines A549 and NCI-H460, were first evaluated using the
MTT cell viability assay. As shown in Fig. 2, MHY-449 reduced cell viability in a
concentration- and time-dependent manner in each of the cell lines.
The A549 cell line was more sensitive to the effects of MHY-449 in
comparison to the NCI-H460 cell line. The IC50 of
MHY-449 was found to be 0.66 µM for A549 cells (Fig. 2A) and 1.57 µM for NCI-H460
cells (Fig. 2B) at 48 h. In
subsequent experiments, we used MHY-449 at concentrations up to 1
and 2 µM for use with A549 and NCI-H460 cells,
respectively.
The effects of MHY-449 on the normal Hs27 cell line
were also analyzed (Fig. 2C).
MHY-449 (2 µM) inhibited >40% of the cell growth in A549
and NCI-H460 lung cancer cell lines, whereas little growth
inhibition was oberved in the non-transformed human Hs27 foreskin
fibroblast cell line.
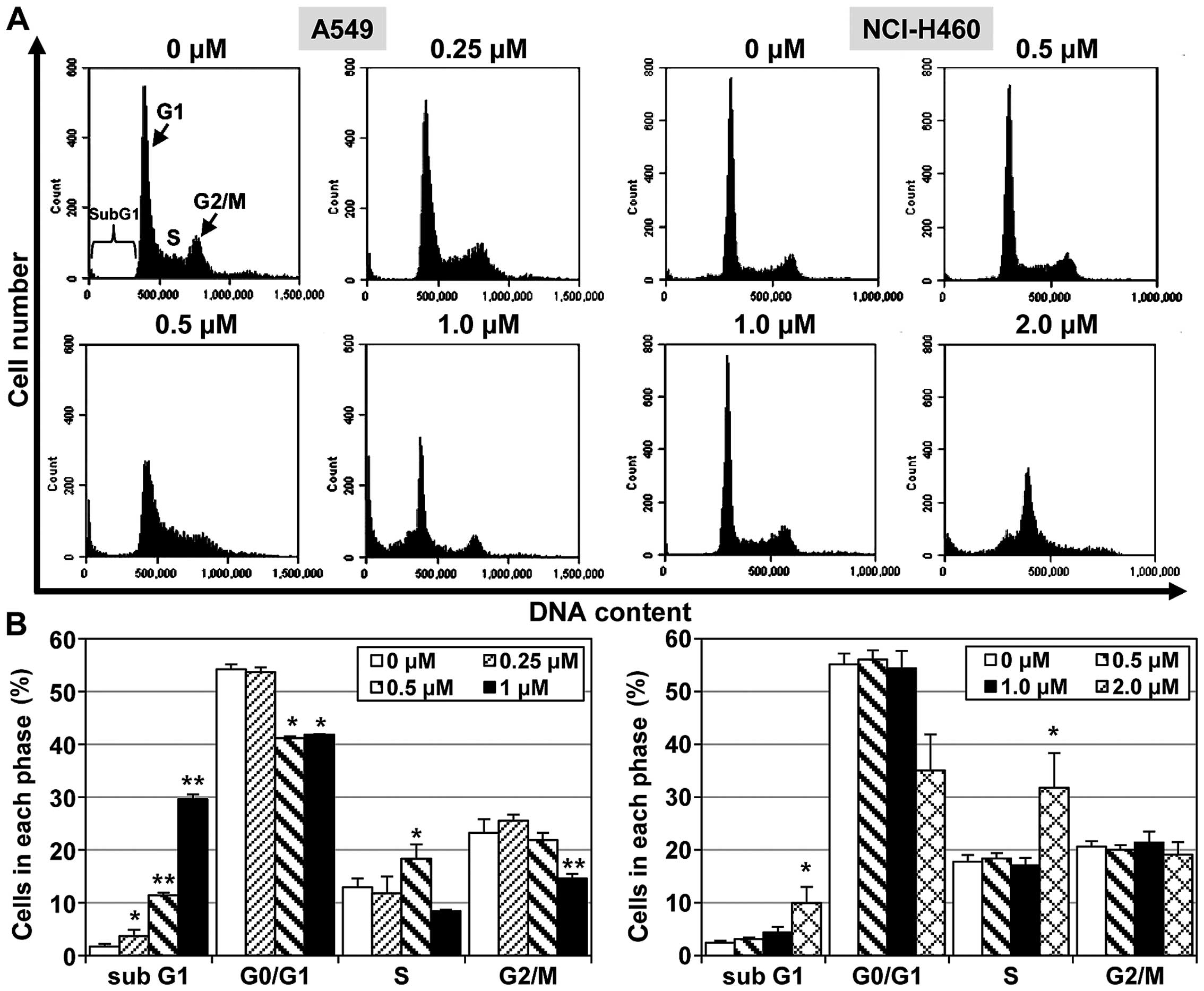
MHY-449 induces apoptotic cell population
in NSCLC cells
To examine the mechanisms responsible for
MHY-449-meditated cell growth inhibition, its effect on apoptosis
induction was examined in NSCLC cells. The A549 and NCI-H460 NSCLC
cell lines were treated with different concentrations of MHY-449
for 24 h. An increased proportion of A549 cells was found to be in
the sub-G1 phase (29.6%) 24 h after treatment with MHY-449 (1
µM) compared to the vehicle-treated control cells (1.7%).
However, the ability of MHY-449 to promote apoptosis in NCI-H460
cells was modest compared to this effect in A549 cells. As shown in
Fig. 3, MHY-449 only slightly
altered the percentage of NCI-H460 cells in the sub-G1 phase.
Specifically, MHY-449 (2 µM) treatment increased the sub-G1
phase cell population from 2.4% (vehicle-treated control) to 10%.
These results suggested that MHY-449 induces apoptosis in NSCLC
cells in a concentration-dependent manner.
MHY-449 treatment induces apoptosis in
NSCLC cells
To confirm that the effects of MHY-449 in NSCLC
cells are associated with the induction of apoptosis, we performed
flow cytometric analyses using cells stained with Annexin V/PI.
Exposure of A549 and NCI-H460 cells to increasing concentrations of
MHY-449 for 24 h resulted in a gradual increase in the apoptotic
cell population. Fig. 4A shows that
MHY-449 increased the proportions of early (lower-right quadrant)
and late (upper-right quadrant) apoptotic cells with increasing
concentration in the two cell lines. For example, 33.9% of cells
advanced to late apoptosis-induced death following treatment with
MHY-449 (1 µM) as compared to the number of apoptotic cells
in the vehicle-treated control (4.5%).
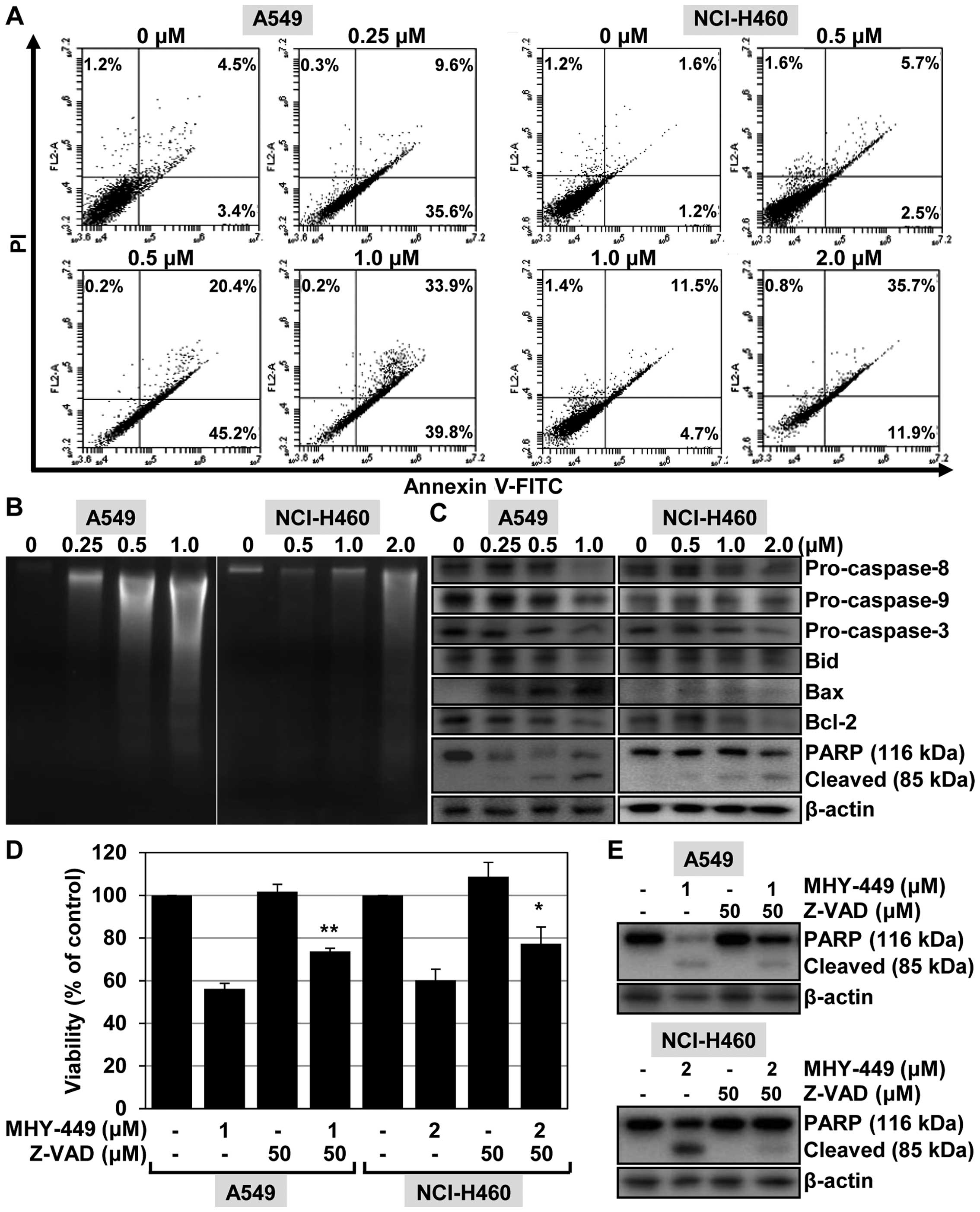 | Figure 4The effect of MHY-449 on the induction
of apoptosis in A549 and NCI-H460 cells. (A) To investigate the
effect of MHY-449 on cell death, the cells were treated for 24 h
with the indicated concentrations of MHY-449. The cells were
stained with Annexin V-FITC/PI and analyzed by flow cytometry. (B)
To analyze the fragmentation of genomic DNA, the DNA was extracted
from the cells and detected by 1.6% agarose gel electrophoresis in
the presence of EtBr. (C) The expression levels of proteins
involved in the extrinsic and intrinsic apoptotic pathways, such as
pro-caspase-8, -9 and -3, Bid, Bax, Bcl-2 and PARP (116 kDa), were
detected by western blot analysis. Proteins were visualized using
the ECL detection system. β-actin was used as an internal control.
(D) Cells were treated with the indicated concentrations of MHY-449
for 24 h after pretreatment with Z-VAD-FMK (50 µM) for 1 h.
The degree of cytotoxicity was determined using the MTT assay. Data
are shown as mean ± SD of three independent experiments
(**P<0.01 vs. MHY-449-treated A549 cells and
*P<0.05 vs. MHY-449-treated NCI-H460 cells). (E)
Cells were pretreated with Z-VAD-FMK (50 µM) for 1 h then
exposed to MHY-449 for 20 h. Total cell lysates were prepared and
analyzed by western blot analysis. β-actin was used as a protein
loading control. The representative results from three independent
experiments are shown. FITC, fluorescein isothiocyanate; PI,
propidium iodide; Bid, BH3-interacting domain death agonist; Bax,
Bcl-2-associated X protein; Bcl-2, B-cell CLL/lymphoma 2; PARP,
poly(ADP-ribose) polymerase. |
We determined the effect of MHY-449 on DNA
fragmentation in A549 and NCI-H460 cells, another hallmark event of
apoptosis. As shown in Fig. 4B, the
ladder pattern of DNA was observed in the NSCLC cells in a
concentration-dependent manner with respect to MHY-449. In A549
cells, this typical ladder pattern of internucleosomal
fragmentation resulted from exposure to 1 µM MHY-449,
whereas in NCI-H460 cells, 2 µM concentrations were required
(Fig. 4B).
These results indicated that MHY-449 induces
apoptosis in lung cancer cells. To determine the possible origin of
this effect, we investigated the influence of MHY-449 on
apoptosis-regulated gene expression. In particular, we assessed the
potential of MHY-449 to alter the expression levels of caspases in
NSCLC cells. We found that treating cells with MHY-449 resulted in
a concentration-dependent decrease of pro-caspase-8, -9, and -3
levels, and also cleavage of their substrate PARP (Fig. 4C).
As mitochondria play a critical role in apoptosis
triggered by a variety of stimuli (8), we investigated the effect of MHY-449
on signaling molecules known to be involved in these pathways.
Exposure of NSCLC cells to MHY-449 triggered a downregulation of
the whole form of pro-apoptotic protein Bid, resulting from Bid
cleavage and activation. MHY-449 was also found to increase the
levels of the pro-apoptotic protein Bax in A549 cells. No analogous
alterations of Bax expression were observed in NCI-H460 cells
because of MHY-449 treatment. A clear decrease in (anti-apoptotic
protein) Bcl-2 expression was observed in the two NSCLC cell lines
(Fig. 4C) following treatment with
MHY-449. These results suggested that, MHY-449 induces the
apoptosis of human lung cancer cells via activation of the caspase
cascade and through the mitochondrial pathway.
Caspases are involved in MHY-449-induced
apoptosis in NSCLC cells
To investigate the significance of caspase
activation in MHY-449-induced apoptosis, we determined the
viability of NSCLC cells pretreated with Z-VAD-FMK, a
broad-spectrum caspase inhibitor, for 1 h followed by treatment
with MHY-449 for 24 h. As shown in Fig.
4D, pretreatment of A549 cells with Z-VAD-FMK promoted their
viability as compared to cells treated with MHY-449 only. Similar
results were obtained using NCI-H460 cells (Fig. 4D). To verify the role of caspase
activation in MHY-449-induced apoptosis, we determined apoptotic
cell death by analyzing the progressive proteolytic cleavage
product of PARP, an activated caspase-3 substrate protein.
Pretreatment of the cells with Z-VAD-FMK was found to abolish
MHY-449-induced cleavage of PARP (Fig.
4E). These results suggested that activation of the caspase
cascade is involved in the induction of apoptosis by MHY-449.
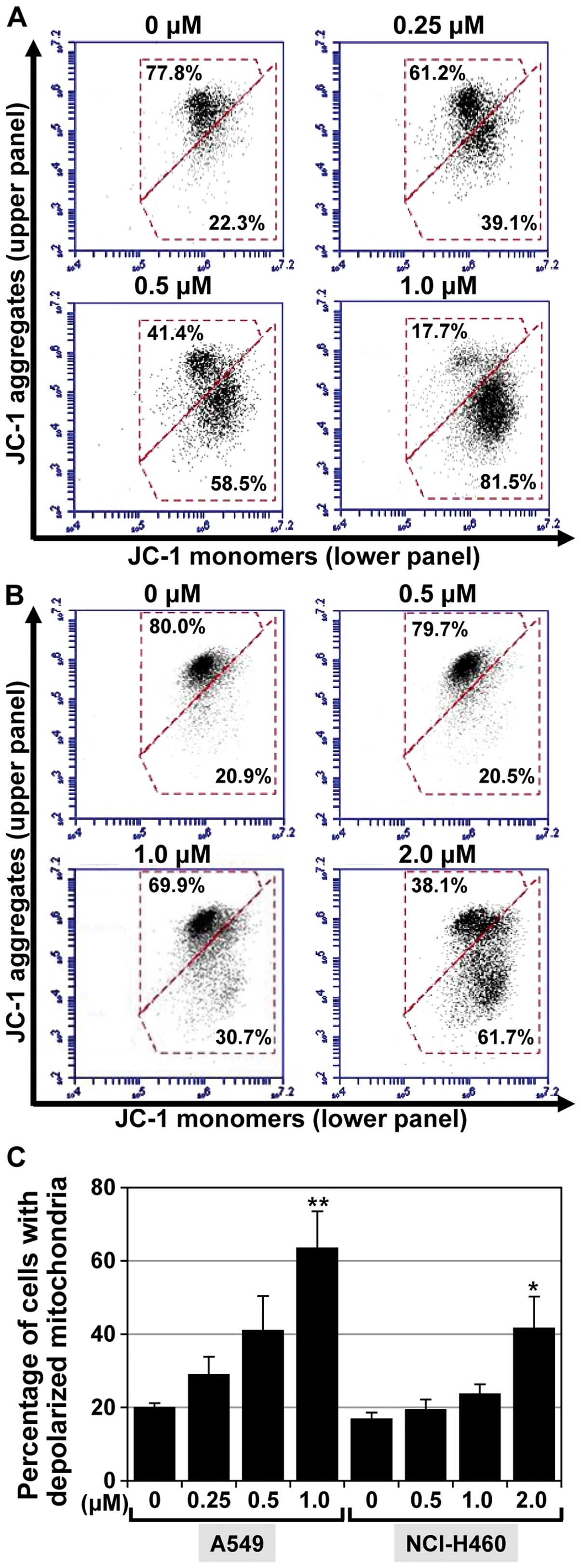
MHY-449 promotes loss of the
mitochondrial membrane potential
The alteration of Bax, Bid, and Bcl-2 proteins are
thought to contribute to apoptotic cell death by promoting the
release of apoptogenic molecules from the mitochondria into the
cytosol. As the expression levels of Bax, Bid, and Bcl-2 in NSCLC
cells were found to be influenced by MHY-449 (Fig. 4C), we determined whether the
resulting death of these cells was associated with disruption of
the mitochondrial membrane potential (MMP). The effect of MHY-449
on MMP was determined by flow cytometric analyses following
staining of the examined cells with JC-1, a lipophilic cationic dye
that selectively enters mitochondria. As shown in Fig. 5A and B, JC-1 in the control cells
exhibited red fluorescence due to the accumulation of J-aggregates
indicating intact MMP (upper panel). By contrast, treating the
cells with MHY-449 resulted in a concentration-dependent green
fluorescence (lower panel) resulting from the cytosolic
accumulation of monomeric JC-1. The populations of A549 cells with
a disrupted membrane potential were 20.2, 29.2, 41.2 and 63.7% at
0, 0.25, 0.5, and 1 µM MHY-449 concentrations, respectively
(Fig. 5C). MHY-449 (1 µM)
also increased the population of NCI-H460 cells with a disrupted
membrane potential to 41.6% compared to that observed in the
control (17%; Fig. 5C).
Collectively, these results indicated that MHY-449 induces a loss
of MMP in NSCLC cells.
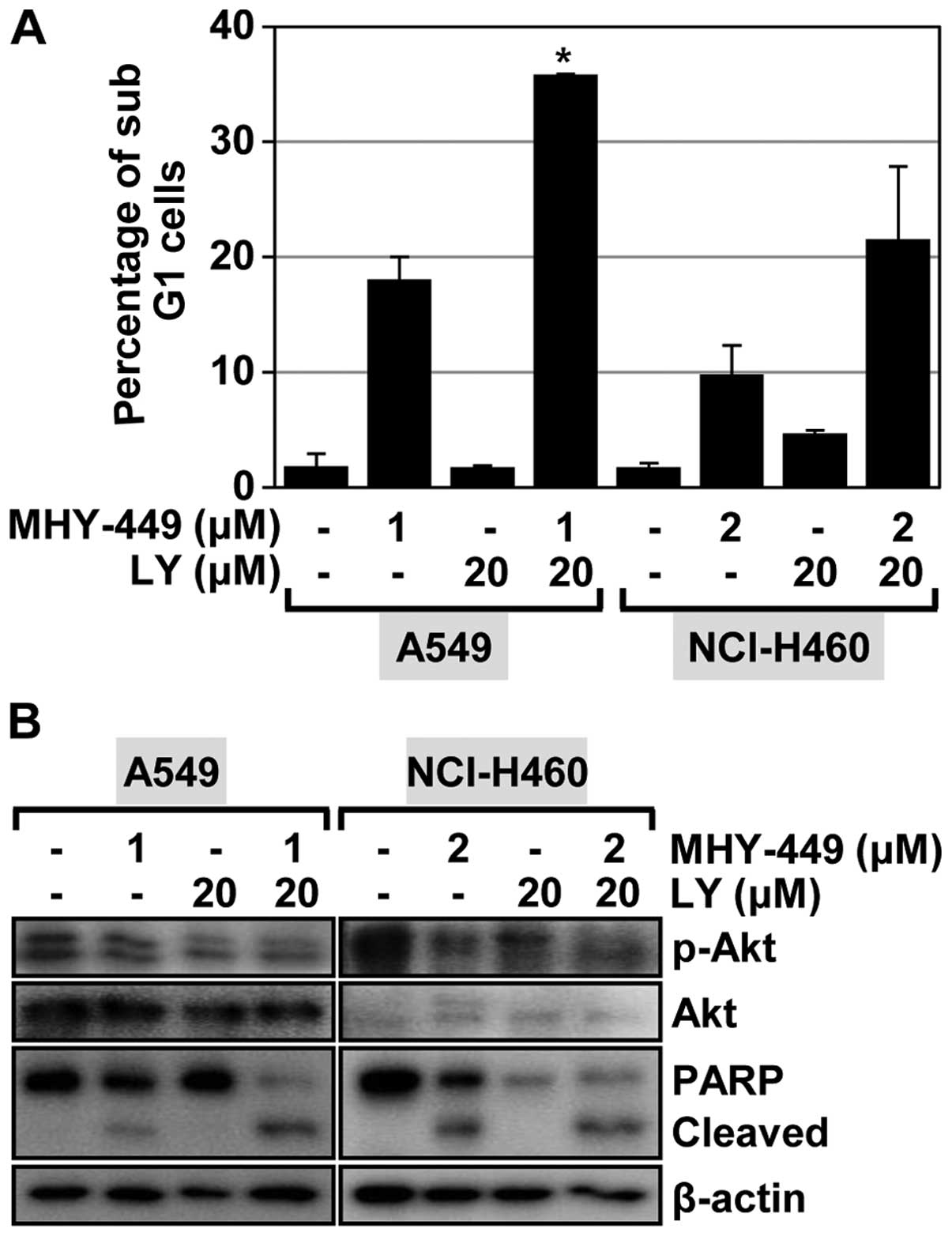
Akt inhibitor enhances apoptosis induced
by MHY-449
Recent evidence has determined that Akt signaling is
essential for NSCLC cell survival (9). Activation of the Akt pathway renders
cells resistant to apoptosis via the regulation of pro-and
anti-apoptotic proteins (10,11).
It was also reported that inhibition of the Akt pathway served to
arrest cancer cell proliferation and significantly delay tumor
growth (12,13). Thus, we examined the involvement of
Akt signaling in mediating apoptosis induced by MHY-449. Treatment
of A549 cells with the Akt inhibitor LY294002 alone had a minimal
effect on inducing apoptosis (Fig.
6A). We also observed no notable induction of apoptosis in
NCI-H460 cells treated with LY294002 (Fig. 6A). We also determined the influence
of Akt inhibition on MHY-449-induced apoptosis in NSCLC cells. As
shown in Fig. 6A, LY294002 enhanced
the proportion of cells that underwent MHY-449-induced apoptosis in
the NSCLC cell lines evaluated. Specifically, this proportion
increased from 18.1 to 35.9% in A549 cells and from 9.8 to 21.6% in
NCI-H460 cells. These results indicated that Akt inhibition
enhances the apoptotic effect of MHY-449 in NSCLC cells. To confirm
this result, we performed western blot analysis to investigate the
effect of LY294002, MHY-449, and their co-administration on Akt
activation and PARP cleavage. LY294002 was found to inhibit Akt
activation in the two lung cancer cell lines evaluated. Of note,
the concentrations of phosphorylated Akt (active form) were
markedly reduced after these cells were treated with MHY-449
(Fig. 6B). Treatment of the NSCLC
cells with LY294002 and MHY-449 was found to significantly suppress
Akt activation (Fig. 6B). As such,
the apoptogenic effect of MHY-449 on NSCLC cells was markedly
enhanced by LY294002 (Fig. 6B).
These results suggested that the Akt pathway is likely involved in
MHY-449-induced apoptosis of NSCLC cells.
Discussion
In the present study, we have determined that
synthetic MHY-449 exerts potent antitumor effects in NSCLC cells.
To the best of our knowledge, this is the first study describing
this effect of MHY-449 on NSCLC cells, despite its documented
cytotoxicity in numerous other cancer cell lines (5–7).
MHY-449 can effectively induce NSCLC cells to undergo apoptosis.
When the underlying mechanism for this effect was examined, we
found that MHY-449 triggered the caspase cascade, and also induced
apoptosis through the mitochondrial pathway. Therefore, a resulting
loss of mitochondrial membrane potential is likely a primary
mechanism by which MHY-449 exerts antitumor effects in NSCLC
cells.
We also demonstrated that concentrations of
phosphorylated Akt were markedly reduced in MHY-449-treated cells.
Furthermore, the pharmacologic inhibition of Akt was found to
markedly enhance MHY-449-induced NSCLC cell apoptosis. Constitutive
activation of the Akt pathway is frequently observed in NSCLC
cells, which acts to sustain their proliferation and survival.
MHY-449 may be considered in the treatment of lung cancer owing to
its apoptogenic properties in these cells, and ability to
effectively suppress the Akt pathway.
Apoptosis is an important event leading to
programmed cell death, which is often essential for the physiologic
development and maintenance of organisms (14). The present study demonstrates that
MHY-449 exerts a pronounced cytotoxic effect in two different NSCLC
cell lines. MHY-449 was shown to effectively decrease the
proliferation of A549 cells, which were more sensitive to this
agent than NCI-H460 cells. MHY-449 also produced clear increases in
sub-G1 populations and DNA fragmentation patterns in these cell
lines. Phosphatidylserine exposure on the external plasma membrane
leaflet in MHY-449-treated NSCLC cells was confirmed by Annexin V
staining. Collectively, these results suggest that MHY-449
effectively inhibits NSCLC cell proliferation and induces apoptosis
in a concentration-dependent manner.
The caspase family of cysteine proteases plays a
central role in regulating apoptosis. It is has been
well-established that certain caspases (e.g., caspase-8 and -9)
play upstream initiator roles in apoptosis by coupling cell death
to stimuli from the downstream effector caspases (e.g., caspase-3,
the most significant promotor of apoptosis) (15). Of note, the downregulation of
caspases-3, -9, and -8, as well as cleavage of PARP, was observed
in present study. The observed caspase-mediated properties of
MHY-449 are in agreement with previous studies using colon
(6) and prostate (7) cancer cells. We also demonstrated that
MHY-449, in the presence of Z-VAD-FMK, prevented the degradation of
PARP in these cells. However, Z-VAD-FMK did not effectively rescue
NSCLC cells from MHY-449-induced apoptosis. Thus, these results
suggest that MHY-449-triggered apoptosis is likely mediated, at
least in part, by the caspase cascade.
Since mitochondria play a crucial role in the
extrinsic and intrinsic pathways of apoptosis (16), we also examined the effect of
MHY-449 on mitochondrial function. Members of hte Bcl-2 family of
proteins are known to govern apoptotic cell death either as
activators (e.g., Bad, Bax, and Bid) or inhibitors (e.g., Bcl-2,
Bcl-xL, and Bcl-W) of mitochondrial outer membrane permeabilization
(16). For example, Bid is readily
cleaved by caspase-8 following stimulation of the extrinsic
apoptotic pathway (17). Our
results indicate that the expression of full-length Bid decreased
following exposure to MHY-449, which then led to its cleavage. The
results also demonstrated that MHY-449 served to simultaneously
decrease Bcl-2 expression and increase Bax expression. This
resulting decrease in the Bcl-2/Bax ratio is known to be a critical
factor in determining which cells undergo apoptosis (18). In addition, the level of MMP
disruption was enhanced by MHY-449 in NSCLC cells. Taken together,
these results suggest that MHY-449-induced apoptosis is associated
with alteration of the Bcl-2/Bax ratio and downregulation of the
full-length Bid, which collectively induce outer mitochondrial
membrane permeabilization and the loss of MMP.
We observed that MHY-449 represses phosphorylated
Akt expression in NSCLC cells. In agreement with this finding, Akt
activation has been shown to be negatively regulated by MHY-449 in
human prostate cancer cells (7). In
addition, we demonstrated that exposing NSCLC cells to an Akt
inhibitor (LY294002, 20 µM) alone only minimally induces
cell death. However, concomitant treatment of cells with LY294002
and MHY-449 resulted in enhanced apoptotic cell death. Given the
established role of the phosphatidylinositol-3 kinase/Akt/mammalian
target of rapamycin (PI3K/Akt/mTOR) signaling cascade as a pivotal
pathway in cancer cell growth and survival (19–21),
an agent that can perturb this signaling sequence may serve to
prevent and/or treat tumor progression. Previous studies have
indicated that NSCLC cells highly express phosphorylated and
activated Akt (22,23). Additionally, the tumor-suppressor
gene phosphatase and tensin homolog (PTEN) is involved in the
regulation of cell survival as well as apoptosis through the
PI3K/Akt pathway. Specifically, the downregulation of PTEN is
accompanied by a corresponding upregulation of phospho-Akt levels
(19). We found that A549 cells
were more sensitive to the combination of LY294002 and MHY-449 than
were NCI-H460 cells. The phosphorylation of Akt is known to occur
more readily in A549 cells than in NCI-H460 cells, since A549 cells
harbor more methylated promoter CpG sites in PTEN (11,24).
Although we have yet to determine the precise effect(s) of MHY-449
on PTEN, the known discrepancy in Akt phosphorylation between A549
and H460 cells may explain their difference in susceptibility to
concomitant LY294002/MHY-449 treatment.
In conclusion, the results of the present study have
provided evidence that MHY-449 induces human NSCLC cell death
stemming from activation of the caspase family and PARP cleavage.
In addition, MHY-449-induced apoptosis proceeds via
mitochondrial-mediated pathways involving activation and inhibition
of Akt. These documented anticancer properties make MHY-449 an
attractive candidate for further study in the prevention and
therapy of lung cancer.
Acknowledgments
This study was supported by the National Research
Foundation of Korea (NRF) grant, funded by the Korean Government
(MSIP, no. 2009-0083538). We would like to thank the Aging Tissue
Bank for providing research information.
References
|
1
|
Jung KW, Won YJ, Oh CM, Kong HJ, Cho H,
Lee DH and Lee KH: Prediction of cancer incidence and mortality in
Korea, 2015. Cancer Res Treat. 47:142–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung KW, Won YJ, Kong HJ, Oh CM, Cho H,
Lee DH and Lee KH: Cancer statistics in Korea: Incidence,
mortality, survival, and prevalence in 2012. Cancer Res Treat.
47:127–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Govindan R, Page N, Morgensztern D, Read
W, Tierney R, Vlahiotis A, Spitznagel EL and Piccirillo J: Changing
epidemiology of small-cell lung cancer in the United States over
the last 30 years: Analysis of the surveillance, epidemiologic, and
end results database. J Clin Oncol. 24:4539–4544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang JA, Yang Z, Lee JY, De U, Kim TH,
Park JY, Lee HJ, Park YJ, Chun P, Kim HS, et al: Design, synthesis
and anticancer activity of novel
dihydrobenzofuro[4,5-b][1,8]naphthyridin-6-one derivatives. Bioorg
Med Chem Lett. 21:5730–5734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hwang HJ, Kang YJ, Hossain MA, Kim DH,
Jang JY, Lee SH, Yoon JH, Moon HR, Kim HS, Chung HY, et al: Novel
dihydrobenzofuro[4,5-b][1,8]naphthyridin-6-one derivative, MHY-449,
induces apoptosis and cell cycle arrest in HCT116 human colon
cancer cells. Int J Oncol. 41:2057–2064. 2012.PubMed/NCBI
|
|
7
|
Lee SH, Kang YJ, Sung B, Kim DH, Lim HS,
Kim HR, Kim SJ, Yoon JH, Moon HR, Chung HY, et al: MHY-449, a novel
dihydrobenzofuro[4,5-b][1,8] naphthyridin-6-one derivative, induces
apoptotic cell death through modulation of Akt/FoxO1 and ERK
signaling in PC3 human prostate cancer cells. Int J Oncol.
44:905–911. 2014.PubMed/NCBI
|
|
8
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamada T, Takeuchi S, Fujita N, Nakamura
A, Wang W, Li Q, Oda M, Mitsudomi T, Yatabe Y, Sekido Y, et al: Akt
kinase-interacting protein1, a novel therapeutic target for lung
cancer with EGFR-activating and gatekeeper mutations. Oncogene.
32:4427–4435. 2013. View Article : Google Scholar
|
|
10
|
Zhou L, Luan H, Liu Q, Jiang T, Liang H,
Dong X and Shang H: Activation of PI3K/Akt and ERK signaling
pathways antagonized sinomenine-induced lung cancer cell apoptosis.
Mol Med Rep. 5:1256–1260. 2012.PubMed/NCBI
|
|
11
|
Lee MW, Kim DS, Lee JH, Lee BS, Lee SH,
Jung HL, Sung KW, Kim HT, Yoo KH and Koo HH: Roles of AKT1 and AKT2
in non-small cell lung cancer cell survival, growth, and migration.
Cancer Sci. 102:1822–1828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chandarlapaty S, Sawai A, Scaltriti M,
Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK,
Baselga J and Rosen N: AKT inhibition relieves feedback suppression
of receptor tyrosine kinase expression and activity. Cancer Cell.
19:58–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Puglisi M, Thavasu P, Stewart A, de Bono
JS, O'Brien ME, Popat S, Bhosle J and Banerji U: AKT inhibition
synergistically enhances growth-inhibitory effects of gefitinib and
increases apoptosis in non-small cell lung cancer cell lines. Lung
Cancer. 85:141–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taylor RC, Cullen SP and Martin SJ:
Apoptosis: Controlled demolition at the cellular level. Nat Rev Mol
Cell Biol. 9:231–241. 2008. View
Article : Google Scholar
|
|
15
|
Chang HY and Yang X: Proteases for cell
suicide: Functions and regulation of caspases. Microbiol Mol Biol
Rev. 64:821–846. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kantari C and Walczak H: Caspase-8 and
bid: Caught in the act between death receptors and mitochondria.
Biochim Biophys Acta. 1813:558–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stambolic V, Suzuki A, de la Pompa JL,
Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM,
Siderovski DP and Mak TW: Negative regulation of PKB/Akt-dependent
cell survival by the tumor suppressor PTEN. Cell. 95:29–39. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kennedy SG, Wagner AJ, Conzen SD, Jordán
J, Bellacosa A, Tsichlis PN and Hay N: The PI 3-kinase/Akt
signaling pathway delivers an anti-apoptotic signal. Genes Dev.
11:701–713. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim D, Cheng GZ, Lindsley CW, Yang H and
Cheng JQ: Targeting the phosphatidylinositol-3 kinase/Akt pathway
for the treatment of cancer. Curr Opin Investig Drugs. 6:1250–1258.
2005.PubMed/NCBI
|
|
22
|
Dinavahi SS, Prasanna R, Dharmarajan S,
Perumal Y and Viswanadha S: A novel, potent, small molecule AKT
inhibitor exhibits efficacy against lung cancer cells in vitro.
Cancer Res Treat. Jan 2–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vasudevan KM, Gurumurthy S and Rangnekar
VM: Suppression of PTEN expression by NF-kappa B prevents
apoptosis. Mol Cell Biol. 24:1007–1021. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung IL, Kang HJ, Kim KC and Kim IG:
PTEN/pAkt/p53 signaling pathway correlates with the radioresponse
of non-small cell lung cancer. Int J Mol Med. 25:517–523.
2010.PubMed/NCBI
|