Introduction
Thyroid cancer accounts for 96% of endocrine
malignancies and causes ~1,900 deaths annually in the USA (1). Except for medullary thyroid cancer,
thyroid cancers are derived from thyroid follicular cells. In the
known mutational spectrum of these follicular cell-derived thyroid
cancers, non-overlapping mutations activating the ERK pathway
predominate, including BRAF, RAS and RET-PTC gene re-arrangements
(2). Additional genetic alterations
specifically affect the PI3K pathway, including PIK3CA, PTEN and
AKT1 (2,3).
Systemic treatment of patients with advanced,
metastatic, radioiodine-refractory thyroid cancer has recently
evolved to include multikinase inhibitors (MKIs). These drugs
target VEGF receptors (principally VEGFR2), plus overlapping sets
of other receptor tyrosine kinases (RTKs). In addition to these
main targets of action, several of the kinase inhibitors used in
thyroid cancer, including sorafenib and pazopanib, have been shown
to more weakly target RAF kinases including BRAF (4,5).
Notably, RAF inhibition, particularly at sub-maximal
levels, has been shown to result in paradoxical ERK pathway
activation in certain settings. In one well-studied scenario, an
upstream RAS mutation was able to overdrive signaling through RAF
protein heterodimers, even when one RAF molecule is bound to a BRAF
inhibitor (6–9). This concern over paradoxical ERK
activation has led to the restriction of potent RAF inhibitor drugs
such as vemurafenib to BRAF mutant tumor settings, avoiding RAS
mutant tumors in particular. Weaker RAF inhibitors such as
sorafenib also have the potential to induce BRAF-CRAF heterodimers
and cause paradoxical ERK activation (10). In thyroid cancer cell lines treated
with vemurafenib, paradoxical ERK activation also occurs in BRAF
mutant settings, due to feedback activation of ERBB3 (11). There are currently insufficient data
in thyroid cancer to assess whether paradoxical RAF activation
occurs in patients treated with MKIs.
Pazopanib is a small molecule MKI that selectively
inhibits VEGFR1-3, PDGFR-α, PDGFR-β, KIT and FGFR1-3 in the low
nanomolar range (12). Similar to
sorafenib, pazopanib recently has been shown to be a RAF inhibitor
as well (5). In a cell-based assay,
the IC50 value was 59 nM for wild-type BRAF and 148 nM
for BRAFV600E. Pazopanib has been approved for use in
advanced renal cell carcinoma and advanced refractory soft tissue
sarcoma (13,14), and has been studied in phase II
trials for a variety of types of cancer, including breast, thyroid
and cervical cancer. Phase II data in advanced, progressive
differentiated thyroid cancer (DTC) from Bible et al,
indicate a 49% response rate, one of the highest response rates
reported for any agent in thyroid cancer (15). In this high-risk patient population
screened to have disease progression within 6 months, Bible et
al reported a median progression-free survival (PFS) of 11.7
months on pazopanib. Interestingly this study suggested a trend
toward a higher response rate in follicular than in papillary
thyroid cancer.
Trametinib (GSK1120212) is a potent, highly specific
allosteric inhibitor of MEK1 and MEK2 (IC50 value <5
nM). In patients with BRAF-mutant malignant melanoma in a phase III
clinical trial, single agent trametinib was associated with
enhanced progression-free survival (PFS) and overall survival (OS)
compared to chemotherapy (16).
Trametinib was approved by the FDA for unresectable or metastatic
BRAF-mutant melanoma in May 2013, and in combination with
dabrafenib for BRAF-mutant melanoma in January 2014.
Our goal in the present preclinical study was to
determine whether adding the potent MEK inhibitor trametanib to the
clinically useful MKI, pazopanib, could enhance the activity of
pazopanib in preclinical models of thyroid cancer. Based on the
reported ability of pazopanib to inhibit RAF in vitro
(5), we considered it likely that
paradoxical activation of ERK could be occurring in
pazopanib-treated tumor cells. Due to the high frequency of
mutations activating the ERK pathway in thyroid and the potential
for paradoxical ERK activation in some tumors, we hypothesized that
addition of trametinib could be a rational complement to pazopanib
in treating thyroid cancer.
Materials and methods
Cell lines
BCPAP, 8505C and CAL62 cells were obtained from the
German Collection of Microorganisms and Cell Culture (DSMZ,
Braunschweig, Germany). Cell lines were cultured in RPMI-1640 with
10% FBS. All media were supplemented with
penicillin-streptomycin.
Inhibitor treatments
Trametinib and pazopanib were obtained from
GlaxoSmithKline (King of Prussia, PA, USA). Stock solutions (10 mM)
were prepared in dimethyl sulfoxide (DMSO). For analysis of ERK
pathway inhibition, cultured cells were treated with indicated
doses of inhibitors for 4 h or for 5 days with medium change and
fresh drug at day 3. For growth inhibition assays, cells were
treated for 5 days with medium change and fresh drug at day 3.
Immunoblotting
Cells were treated for 4 h or 5 days as described
above, then washed with PBS and harvested by scraping with 1X
sodium dodecyl sulfate lysis buffer [2% sodium dodecyl sulfate and
62.5 mM Tris (pH 6.8)]. Lysates were electrophoresed on 4–20%
gradient polyacrylamide gels and transferred onto PVDF membranes.
Blots were probed at 4°C overnight with a primary antibody to pERK
(CST, Beverly, MA, USA; no. 9101) diluted 1:1,000 in 5% milk, total
ERK (CST; no. 9102) and GAPDH (Trevigen). Anti-rabbit secondary
antibodies (Santa Cruz) were diluted 1:2,000. Blots were visualized
using SuperSignal Pico Chemiluminescence (Pierce Chemical Co.,
Rockford, IL, USA).
Growth analyses
Growth assays were performed in triplicate using the
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazo-lium bromide (MTT)
assay (M2128; Sigma-Aldrich) following the manufacturer's
instructions. Cells were seeded in 24-well plates using phenol
red-free media. MTT absorbance was determined 5 days after exposure
to drugs or DMSO alone. Data are represented as the mean absorbance
± SEM, based on 3–6 independent experiments, normalized to control
cells. GI50 was determined as the x-intercept of
Log10(fa/fu) plotted vs.
Log10(concentration), determined by linear regression
(17). GI50 values were
reported as the means ± standard deviation of 3–5 independent
experiments.
Animal studies
Animal studies were approved by the Johns Hopkins
Animal Care and Use Committee and performed in accordance with NIH
guidelines. CAL62 or 8505C cells suspended in Matrigel
(5×106 cells/200 µl) were inoculated s.c. into
the right flank of 4- to 6-week-old female athymic nude
(nu/nu) mice (Harlan Laboratories, Indianapolis, IN, USA).
Once palpable, tumor volumes were calculated with calipers using
the formula: Length × width × height × 0.5236. After tumors reached
0.1–0.2 cm3 in size, animals were sorted into groups of
11 and randomized for drug assignment, to achieve equal
distribution of tumor size in all treatment groups. Animals were
treated with DMSO vehicle alone, trametinib (1 mg/kg daily),
pazopanib (100 mg/kg daily) or a combination of both drugs, by oral
gavage. Animals were euthanized by CO2 asphyxiation and
cervical dislocation when tumors reached ~1.5 cm3.
Kaplan-Meier analysis for tumor progression was performed with
GraphPad Prism version 5 software, using a 3-fold increase in tumor
volume from onset of treatment as a threshold for tumor
progression.
Immunohistochemistry
Four hours following treatment with drugs or DMSO
vehicle, mice were sacrificed, and tumors were harvested in 10%
paraformaldehyde overnight, followed by incubation in 70% ethanol.
Paraffin section slides were incubated with anti-pERK (CST; no.
4376; 1:100), pAKT (CST; no. 3787; 1:50) and developed using a
Vectastain ABC kit (Vector Labs, Burlingame, CA, USA) with DAB as
chromogen, and a hematoxylin counterstain. Anti-CD31 staining
(Abcam, Cambridge, MA, USA; ab28364; 1:50) followed a pressure
cooker antigen retrieval method according to the manufacturer's
protocol. Microvessel density was estimated as the mean of
CD31-reactive foci in ten 200 power fields in the tumor periphery,
using a two-tailed t-test with equal variance for significance.
Light microscopy was performed with an Olympus Vanox and Nikon
DMX1200 CCD camera, using Nikon ACT-1 image capture software.
Results
Growth inhibition of thyroid cancer cell
lines by trametinib and pazopanib
In preliminary dose-response studies, we found that
trametinib efficiently inhibited ERK activation and growth in
thyroid cancer cell lines at low nanomolar doses. Pazopanib
treatment caused in vitro growth inhibition at low
micromolar dose ranges. We therefore tested dose ranges from 0.1 to
10 nM of trametinib and from 0.5 to 20 µM of pazopanib to
determine GI50 values for these drugs. [Trough plasma
pazopanib concentrations achieved in clinical trials may exceed 30
µM at the typical 800 mg daily dose (18)]. Table
I shows calculated GI50 values for single agent
trametinib or pazopanib against the thyroid cancer cell lines
CAL62, BCPAP and 8505C. All three cell lines were highly sensitive
to trametinib with a GI50 ranging from 1.1 to 4.8 nM.
Pazopanib GI50 values ranged from 1.4 to 7.1 µM.
Clearly, trametinib alone was highly active in vitro for
thyroid cancer cell lines bearing either a RAS- or BRAF-mutant
genotype; pazopanib was relatively less active as a single agent in
a cell culture setting, as anticipated.
 | Table IInhibition of thyroid cancer growth
in vitro by trametinib and pazopanib. |
Table I
Inhibition of thyroid cancer growth
in vitro by trametinib and pazopanib.
| Cell line | Genotype | GI50
|
|---|
| Trametinib (nM) | Pazopanib
(µM) |
|---|
| CAL62 |
KRASG12R/G12R | 4.8±0.3 | 1.4±0.4 |
| BCPAP |
BRAFV600E/wt | 4.0±1.7 | 3.2±0.5 |
| 8505C |
BRAFV600E/V600E | 1.1±0.6 | 7.1±1.5 |
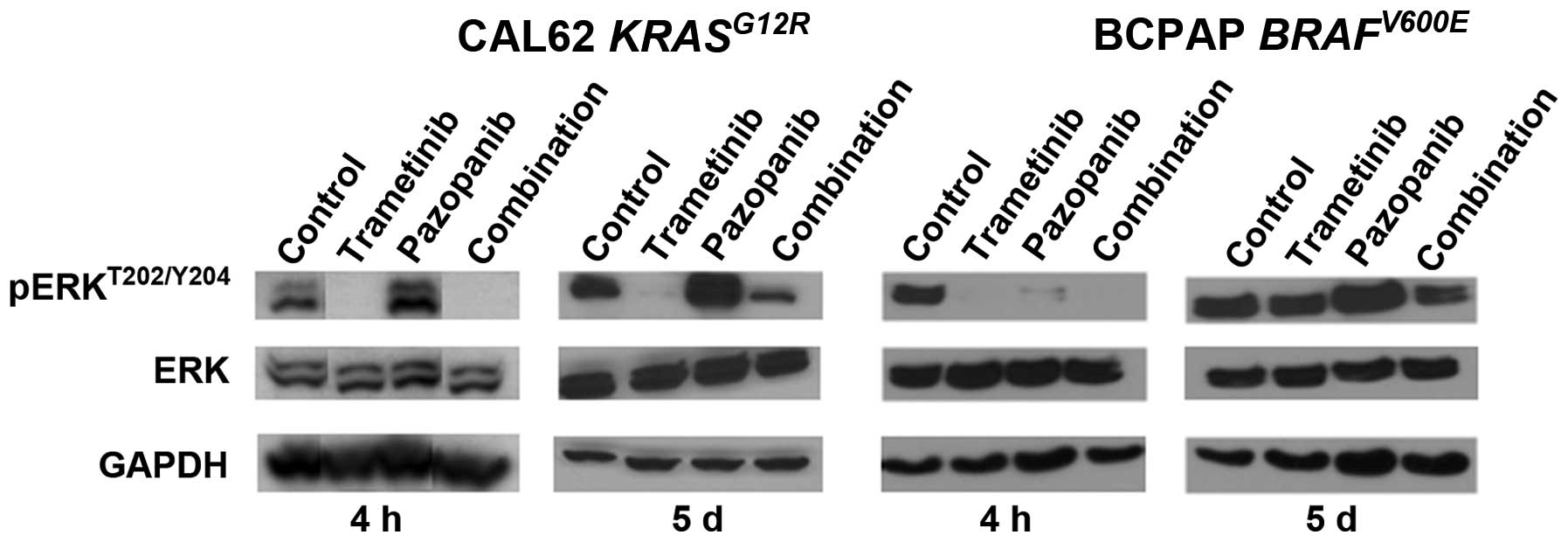
Trametinib abrogates ERK activation
caused by pazopanib
We hypothesized that pazopanib treatment could
potentially cause paradoxical ERK pathway activation in thyroid
cancer, based on studies performed in breast cancer and other types
of tumor (5). To explore the ERK
pathway signaling effects of pazopanib and trametinib in
vitro, we tested both acute and 5 day responses in CAL62 cells
(KRASG12R) and BCPAP cells (BRAFV600E).
Initial data indicated an IC50 value for trametinib of
1–2 nM for pERK inhibition in CAL62 cells and complete loss of pERK
signal at doses >4 nM (data not shown), confirming earlier data
derived from other types of tumor (19). Fig.
1 illustrates that pazopanib alone was associated with
upregulation of pERKT202/Y204 at 4 h and 5 days in the
RAS mutant line, CAL62. No corresponding changes were observed in
total ERK abundance. The pERK upregulation was fully abrogated by
combination with trametinib 10 nM at 4 h, and was significantly
reduced at 5 days. Pazopanib also was associated with ERK
activation in the BRAF-mutant cell line BCPAP at 5 days but not at
4 h. Combination treatment with trametinib again reduced this
activation. Since pazopanib has multiple effects in an in
vivo tumor setting relevant to ERK, beyond the in vitro
effects modeled here, we returned to this phenomenon in tumor
xenografts (see below, Fig. 3).
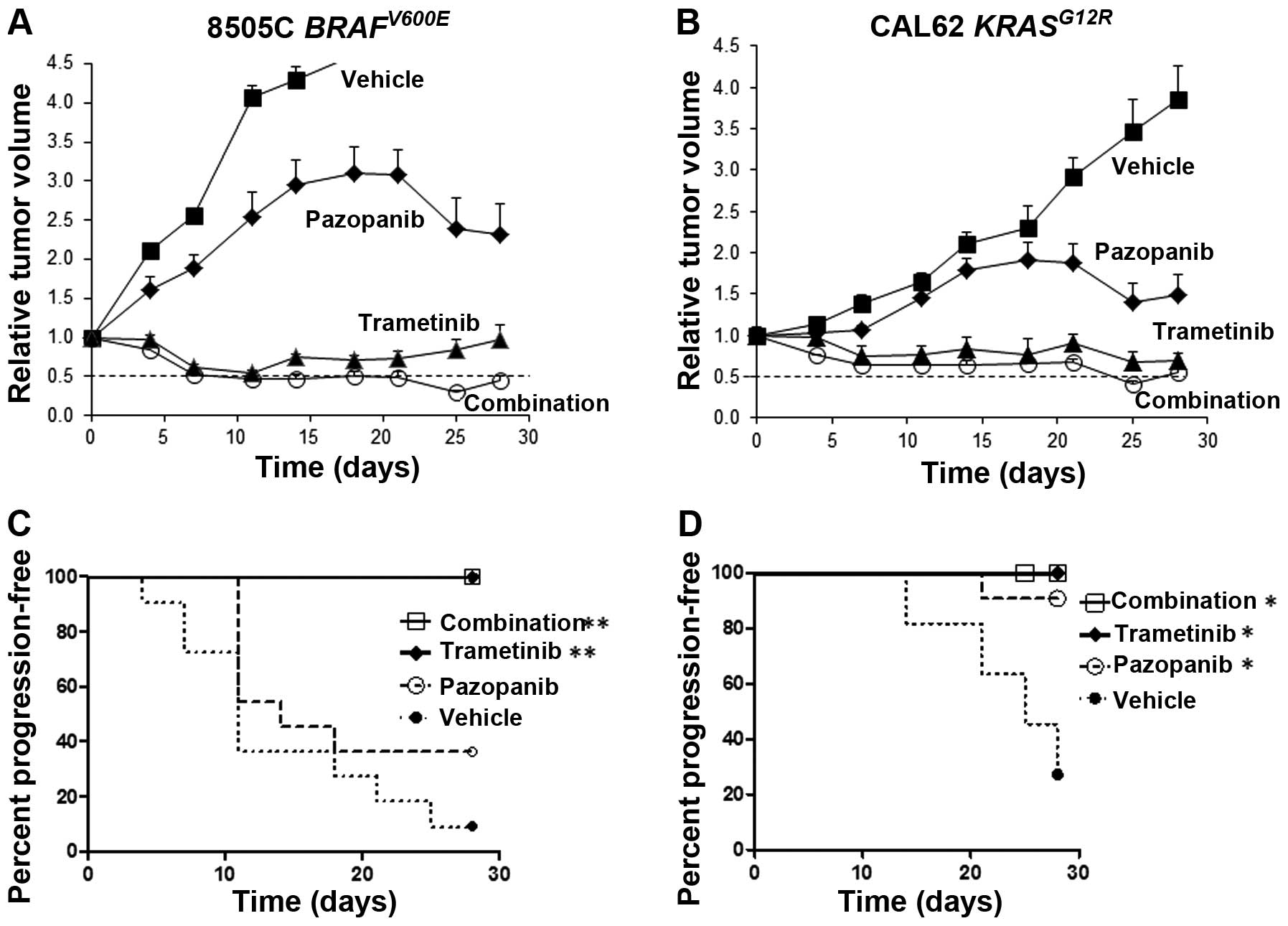
Tumor size reduction in thyroid cancer
xenografts treated with trametinib and pazopanib
In limited published studies to date, pazopanib has
been shown to inhibit the growth of xenografts derived from
anaplastic thyroid cancer cells (20). In order to test the in vivo
activity of pazopanib plus trametinib in thyroid cancer, athymic
nu/nu mice bearing palpable 8505C or CAL62 tumors were
randomized to receive daily trametinib, pazopanib, a combination of
both drugs or vehicle alone, by oral gavage. Dose levels were
selected optimizing for tolerability and to achieve plasma levels
comparable to achievable levels in human clinical trials. For 8505C
tumor xenografts bearing a BRAFV600E mutation, the drug
combination caused a rapid and sustained decrease in tumor size,
reaching a mean tumor volume reduction of 50% at 7 days and a peak
reduction of >65% at 25 days, compared to the starting tumor
volume (Fig. 2A). Trametinib alone
also was highly effective with sustained tumor size reductions.
Kaplan-Meier analysis showed highly significant retardation of
progression for trametinib or the combination (p<0.0001 vs.
vehicle), but not for single agent pazopanib (Fig. 2C).
The combination of trametinib and pazopanib also was
highly effective in CAL62 tumors bearing a KRASG12R
mutation, reaching a peak reduction in tumor volume by >50% at
25 days compared to the starting tumor volume (Fig. 2B). Trametinib also was effective as
a single agent in reducing the volume of CAL62 tumors. Trametinib,
pazopanib and the two-drug combination all were associated with
significant delays in tumor progression by Kaplan-Meier analysis
(p=0.03 vs. vehicle). The drugs were well tolerated and no
significant weight loss was noted with the drug combination or with
either drug separately.
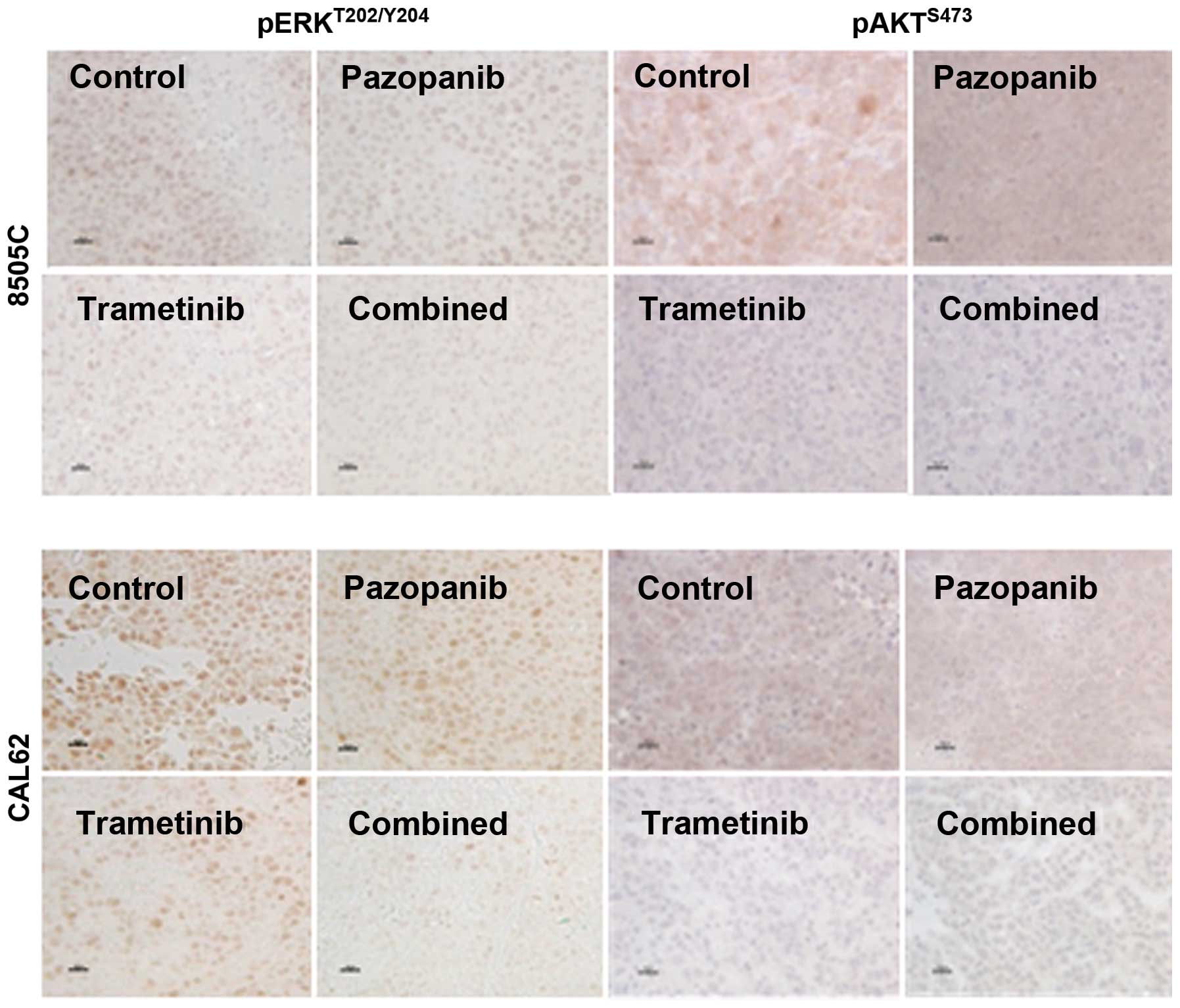
Pharmacodynamic analysis of treated
xenograft tumors
We performed IHC for pERKT202/Y204 and
pAKTS473 in order to qualitatively assess the ability of
trametinib and pazopanib to inhibit key signaling targets in
vivo. As shown in Fig. 3, 8505C
or CAL62 xenograft tumors from the vehicle-treated mice had dense,
predominantly nuclear reactivity for pERK. Four hours after
trametinib treatment, we observed a significant reduction in pERK
reactivity. The combination of the two drugs strongly reduced pERK
reactivity whereas pazopanib alone had little effect on nuclear
pERK compared to the vehicle-treated control. AKT phosphorylation
at serine 473 depends partially on PI3K-PDK1 activation, and also
on TORC2 activation, which may be independent of PI3K. We observed
downregulation of pAKTS473 by trametinib and by the
trametinib-pazopanib combination in both the CAL62 and 8505C
xenograft models, whereas pazopanib alone had a more limited
effect.
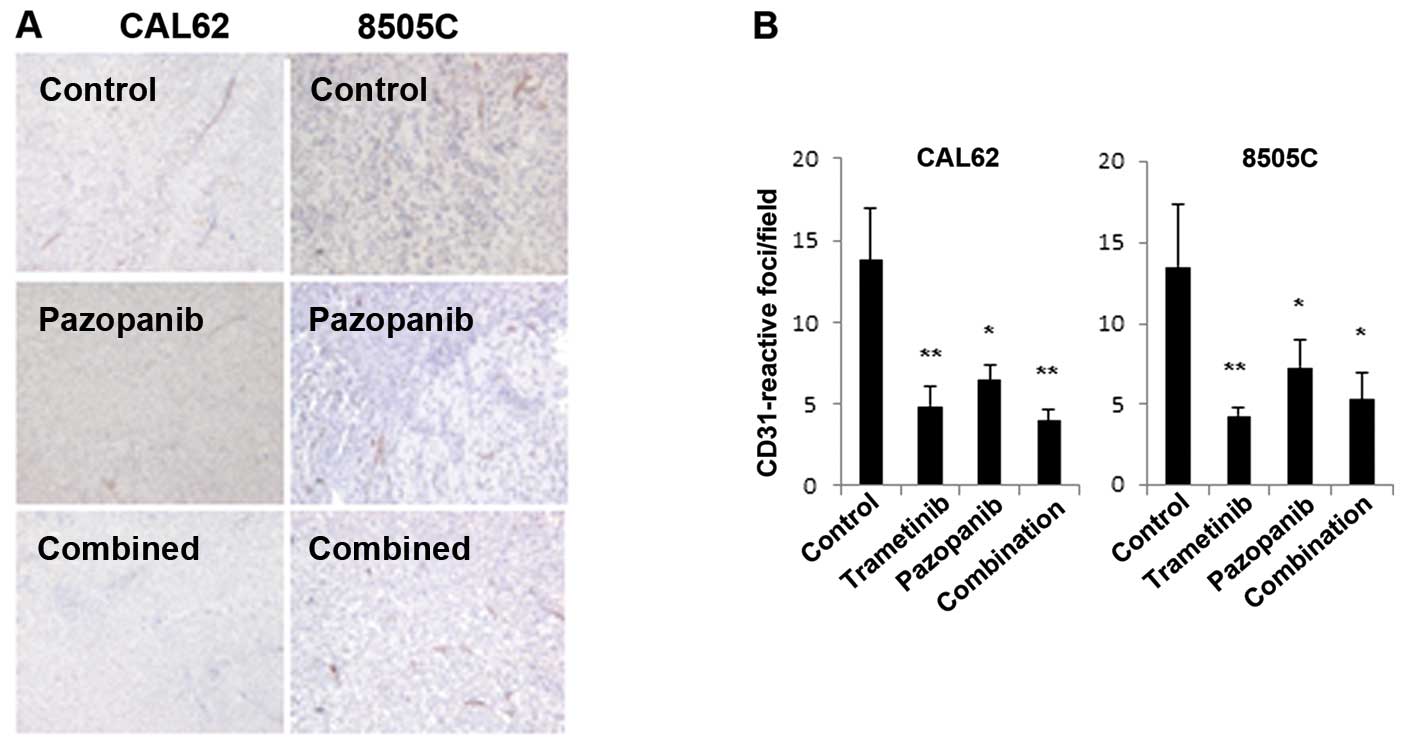
Based on the potential of pazopanib to inhibit
VEGFR2 signaling, we examined microvessel density (MVD), using
immunohistochemistry for CD31 (Fig.
4). In CAL62 and 8505C xenografts, pazopanib resulted in a
significant decline in MVD. Trametinib plus pazopanib together or
trametinib alone also caused significant reductions in MVD.
Based on these in vitro and xenograft data,
the MEK inhibitor trametinib appears highly effective in BRAF- and
RAS-mutant thyroid cancer preclinical models, both singly and in
combination with pazopanib. The combination of trametinib and
pazopanib appeared more effective than pazopanib alone in
downregulating pERK immunoreactivity; effects on microvessel
density were comparable.
Discussion
Highly selective MEK inhibitors have shown
significant clinical benefit in cancers with defined genotypes,
including both BRAF and RAS mutant malignant melanoma (21,22).
In published studies, RAF or MEK inhibitors alone or in combination
have shown significant activity against DTC cell lines and
xenografts (11,23,24).
Recently, the MEK inhibitor selumetinib (AZD6244) has shown utility
in a pilot study to increase radioiodine uptake in some
radioiodine-refractory DTC tumor lesions (25). In this preclinical study, we
explored the activity of trametinib, and whether trametinib could
augment the antitumor activity of pazopanib, an agent shown to have
promising activity in a phase II study of DTC (15). We found that trametinib induced
durable shrinkage of thyroid cancer xenografts, either singly or in
combination with pazopanib. Pazopanib alone was less effective in
the tested xenograft models. We found that pazopanib treatment led
to paradoxical ERK activation in vitro. While we did not
observe ERK activation by pazopanib in the xenograft models,
trametinib potently inhibited pERK immunoreactivity, whereas
pazopanib did not.
It is currently unknown whether ERK activation
occurs adaptively in the tumors of DTC patients treated with MKIs
such as sorafenib, and pazopanib, and whether this phenomenon could
contribute to drug resistance. Our preclinical data suggest that
ERK activation by pazopanib may occur in patients as well. Given
these preclinical data, as well as the high frequency of mutations
affecting the ERK pathway and the recent FDA approval of sorafenib
in DTC, the question of ERK activation by MKI's seems critical for
correlative studies in thyroid cancer clinical trials.
Most impressively in our present study, trametinib
showed marked single agent activity against both RAS- and
BRAF-mutant thyroid cancer models in vitro and in
vivo. This activity was retained or further augmented when
trametinib was combined with pazopanib. Notably, trametinib was
effective as a single agent against the RAS mutant CAL62 xenograft
model. We did not observe significant AKT activation with
trametinib in this model. Clinically, the MEK inhibitor MEK162
(Novartis) has shown significant single agent activity in
RAS-mutant melanoma patients, a subset not appropriate for BRAF
inhibitor treatment (26).
In summary, this preclinical study suggests that
single agent trametinib, and trametinib plus pazopanib in
combination, are highly active in thyroid cancer models, and may be
promising for clinical efficacy in thyroid cancer. Based on early
data from this study, we have initiated a phase I clinical trial
combining trametinib and pazopanib, including a DTC expansion
cohort (NCT01438554).
Acknowledgments
The authors thank Dr Tona Gilmer (GlaxoSmithKline,
King of Prussia, PA, USA) for the generous gift of trametinib and
pazopanib. The present research was supported by NIH Specialized
Programs of Research Excellence (SPORE) in Head and Neck Cancer
grant P50 DE19032.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ricarte-Filho JC, Ryder M, Chitale DA, et
al: Mutational profile of advanced primary and metastatic
radioactive iodine-refractory thyroid cancers reveals distinct
pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res.
69:4885–4893. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xing M: Genetic alterations in the
phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer.
Thyroid. 20:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gril B, Palmieri D, Qian Y, et al:
Pazopanib reveals a role for tumor cell B-Raf in the prevention of
HER2+ breast cancer brain metastasis. Clin Cancer Res.
17:142–153. 2010. View Article : Google Scholar
|
|
6
|
Poulikakos PI, Zhang C, Bollag G, Shokat
KM and Rosen N: RAF inhibitors transactivate RAF dimers and ERK
signalling in cells with wild-type BRAF. Nature. 464:427–430. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hatzivassiliou G, Song K, Yen I, et al:
RAF inhibitors prime wild-type RAF to activate the MAPK pathway and
enhance growth. Nature. 464:431–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heidorn SJ, Milagre C, Whittaker S, et al:
Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor
progression through CRAF. Cell. 140:209–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lito P, Pratilas CA, Joseph EW, et al:
Relief of profound feedback inhibition of mitogenic signaling by
RAF inhibitors attenuates their activity in BRAFV600E melanomas.
Cancer Cell. 22:668–682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arnault JP, Mateus C, Escudier B, et al:
Skin tumors induced by sorafenib; paradoxic RAS-RAF pathway
activation and oncogenic mutations of HRAS, TP53, and TGFBR1. Clin
Cancer Res. 18:263–272. 2012. View Article : Google Scholar
|
|
11
|
Montero-Conde C, Ruiz-Llorente S,
Dominguez JM, et al: Relief of feedback inhibition of HER3
transcription by RAF and MEK inhibitors attenuates their antitumor
effects in BRAF-mutant thyroid carcinomas. Cancer Discov.
3:520–533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumar R, Knick VB, Rudolph SK, et al:
Pharmacokinetic-pharmacodynamic correlation from mouse to human
with pazopanib, a multikinase angiogenesis inhibitor with potent
antitumor and antiangiogenic activity. Mol Cancer Ther.
6:2012–2021. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sternberg CN, Davis ID, Mardiak J, et al:
Pazopanib in locally advanced or metastatic renal cell carcinoma:
results of a randomized phase III trial. J Clin Oncol.
28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van der Graaf WT, Blay JY, Chawla SP, et
al EORTC Soft Tissue and Bone Sarcoma Group; PALETTE study group:
Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a
randomised, double-blind, placebo-controlled phase 3 trial. Lancet.
379:1879–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bible KC, Suman VJ, Molina JR, et al
Endocrine Malignancies Disease Oriented Group; Mayo Clinic Cancer
Center; Mayo Phase 2 Consortium: Efficacy of pazopanib in
progressive, radioiodine-refractory, metastatic differentiated
thyroid cancers: results of a phase 2 consortium study. Lancet
Oncol. 11:962–972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flaherty KT, Robert C, Hersey P, et al
METRIC Study Group: Improved survival with MEK inhibition in
BRAF-mutated melanoma. N Engl J Med. 367:107–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hurwitz HI, Dowlati A, Saini S, et al:
Phase I trial of pazopanib in patients with advanced cancer. Clin
Cancer Res. 15:4220–4227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gilmartin AG, Bleam MR, Groy A, et al:
GSK1120212 (JTP-74057) is an inhibitor of MEK activity and
activation with favorable pharmacokinetic properties for sustained
in vivo pathway inhibition. Clin Cancer Res. 17:989–1000. 2012.
View Article : Google Scholar
|
|
20
|
Bible KC1, Suman VJ, Menefee ME, et al
Mayo Phase 2 Consortium; Mayo Clinic Endocrine Malignances Disease
Oriented Group: A multiinstitutional phase 2 trial of pazopanib
monotherapy in advanced anaplastic thyroid cancer. J Clin
Endocrinol Metab. 97:3179–3184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Flaherty KT, Infante JR, Daud A, et al:
Combined BRAF and MEK inhibition in melanoma with BRAF V600
mutations. N Engl J Med. 367:1694–1703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim KB, Kefford R, Pavlick AC, et al:
Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients
with metastatic BRAF-mutant cutaneous melanoma previously treated
with or without a BRAF inhibitor. J Clin Oncol. 31:482–489. 2013.
View Article : Google Scholar
|
|
23
|
Ball DW, Jin N, Rosen DM, Dackiw A,
Sidransky D, Xing M and Nelkin BD: Selective growth inhibition in
BRAF mutant thyroid cancer by the mitogen-activated protein kinase
kinase 1/2 inhibitor AZD6244. J Clin Endocrinol Metab.
92:4712–4718. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leboeuf R, Baumgartner JE, Benezra M, et
al: BRAFV600E mutation is associated with preferential
sensitivity to mitogen-activated protein kinase kinase inhibition
in thyroid cancer cell lines. J Clin Endocrinol Metab.
93:2194–2201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ho AL, Grewal RK, Leboeuf R, et al:
Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer.
N Engl J Med. 368:623–632. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ascierto PA, Schadendorf D, Berking C, et
al: MEK162 for patients with advanced melanoma harbouring NRAS or
Val600 BRAF mutations: a non-randomised, open-label phase 2 study.
Lancet Oncol. 14:249–256. 2013. View Article : Google Scholar : PubMed/NCBI
|