Introduction
Hypoxia, which is detected in the central region of
solid tumors, is a fundamental determinant of malignant tumor
progression. It can also be a leading cause of angiogenesis by
activation of the expression of angiogenic factors, including
vascular endothelial growth factor (VEGF) (1,2).
Angiogenesis, the development of new blood vessels, is essential
for tumor progression since tumors require access to blood vessels
for a sufficient supply of oxygen and nutrients to maintain growth
and metastasis (3,4). Furthermore, an aggressive cancer
phenotype that is associated with resistance to radiation therapy,
chemotherapy and a poor treatment outcome can be generated as a
result of the hypoxic environment within the solid tumor (5,6). A key
factor in this process is hypoxia-inducible factor-1 (HIF-1), which
regulates transcription of hypoxia-activated genes and consists of
the HIF-1α and HIF-1β heterodimer (7). The α subunit of HIF-1α is rapidly
degraded under normoxic conditions and is stabilized under hypoxia,
while HIF-1β is constitutively expressed (8). Numerous studies have aimed to target
HIF-1α as an anticancer strategy, and the mechanisms of several
HIF-1α inhibitors have been well characterized (9,10).
Melatonin (MLT) is an indoleamine synthesized in the
pineal gland and other organs, and is a major regulator in the
coordination of circadian rhythms and seasonal reproduction with
antioxidant, oncostatic and antiproliferative activities (11–14).
It has been demonstrated that MLT exerts its complex actions by
binding and activating two distinct receptor types: membrane
receptors MT1 and MT2 and the nuclear receptors (15,16).
MLT membrane receptors mediate their functions through a
G-protein-coupled second messenger pathway and nuclear receptor
signaling appears to be mediated via the transcription factor
RZR/ROR, which is an orphan member of the nuclear receptor
superfamily (17,18). In mammals, the MLT membrane
receptors participate in the regulation of circadian and seasonal
rhythms (19). The nuclear orphan
receptors suggest that immunomodulatory and anti-tumor effects
through the intracellular action of MLT depend on nuclear signaling
(20,21).
Although MLT is known to inhibit the expression of
HIF-1α and VEGF, its underlying mechanisms still remain unclear
(22–25). Our previous studies found that MLT
inhibited growth activity in murine foregastric carcinoma cells
in vivo and in vitro (26,27).
In the present study, we report that pharmacological concentrations
of MLT have a direct anti-angiogenic effect, and we evaluated
whether the nuclear orphan receptor intracellular pathways are
involved in MLT-regulated hypoxia-induced HIF-1α stabilization and
angiogenesis in SGC-7901 human gastric cancer cells under
hypoxia.
Materials and methods
SGC-7901 cell culture
SGC-7901 cells were purchased from the Chinese
Academy of Sciences, Shanghai Institute for Biological Science.
SGC-7901 cells were cultured in RPMI-1640 medium supplemented with
10% fetal bovine serum (FBS). The cells were maintained at 37°C in
a humidified incubator with 5% CO2. CoCl2 was
added at a final concentration of 100 μM to mimic hypoxia.
Selecting cells in good condition for the study was performed, and
all experiments were repeated at least thrice. All cell culture
reagents were purchased from Gibco (Invitrogen, Carlsbad, CA,
USA).
Cellular proliferation and viability
assay
SGC-7901 cells were plated into 96-well plates
(5,000 cells/well). The cells were treated on the following day
(control, 0.01, 0.1, 1 and 3 mM MLT) and cultured for 0.2, 2, 16
and 24 h. Cell viability and proliferation were assayed using a
CCK-8 kit according to the manufacturer's protocol. Briefly, the
cells were incubated with CCK-8 solution (10 ml/well) for 1 h
before cell density was determined by measuring the absorbance at
450 nm using a Varioskan Flash (Thermo Scientific, USA).
Immunocytochemistry
SGC-7901 cells were fixed with 10% formaldehyde and
embedded in paraffin. Immunocytochemical staining for RZR/RORγ was
carried out. Images were captured with a Leica DM 4000B
photomicroscope (magnification, ×400).
VEGF ELISA assay
Cells were seeded into 60-mm diameter dishes and
continually incubated for 24 h; and then different concentrations
of MLT and 100 μM of CoCl2 were added to the
medium. After 24 h of culture in the dark, VEGF protein levels in
the supernatants secreted by the cultured cancer cells were
quantified by enzyme-linked immunosorbent assay (ELISA) methods.
The cultured supernatants were collected and centrifuged at 12,000
rpm at 4°C for 15 min, and then ELISA analysis was performed
according to the manufacturer's instructions (VEGF-ELISA kit;
R&D Systems, USA). The values of optical density (OD; A450
values) were measured at 450 nm. The total number of cells was
counted by the cell-counting plate repeated at least thrice. The
standard curve was determined by SPSS statistical software. The
supernatants were harvested with six replicates and the experiment
was performed thrice.
Western blot analysis
After treatment, the cells were harvested, washed
twice with phosphate-buffered solution (PBS), and lysed by adding
ice-cold lysis buffer containing 1 mM phenylmethylsulphone
fluoride, pH 7.4. The protein concentration was determined using
the BCA method. For western blot analysis, equal amounts of protein
were separated by 12% SDS-PAGE gel electrophoresis (110 V, 1.5 h)
and the membranes were blotted by wet transfer (110 V, 1.5 h, 4°C)
on polyvinyllidene fluoride (PVDF) membranes (Millipore, USA). The
membranes were blocked in 5% non-fat milk solution in PBS. The
membranes were then incubated with a primary antibody (dilution,
1:1,000) overnight at 4°C. The membranes were washed with
Tris-buffered saline Tween-20 (TBST) and then incubated for 1.5 h
at room temperature with a secondary antibody (dilution, 1:1,000).
After washing with TBST, the membranes were exposed to X-ray film
(1–15 min) for visualization of the immunoreactive bands.
Densitometric analysis of specific bands was performed by Quantity
One (Bio-Rad, USA). The quantity of target protein was calibrated
with respect to β-actin, and control value and relative intensities
were obtained.
Real-time reverse
transcriptase-polymerase chain reaction
Total RNA was isolated using TRIzol reagent
(Invitrogen) according to the manufacturer's instructions.
First-strand cDNA was generated from 2 mg of each RNA preparation
by reverse transcription using the First Strand cDNA Synthesis kit
(Promega, USA). Real-time quantitative polymerase chain reaction
(PCR) for the analysis of SGC-7901 expression of RZR/RORα,
RZR/RORβ, RZR/RORγ, SENP1, HIF-1α, VEGF and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes and cDNA was
amplified using the dye SYBR-Green (Stratagene, USA) on an
StepOnePlus Real-Time PCR System (Applied Biosystems). The PCR
cycling conditions (40 cycles) were as follows: 30 sec at 95°C; for
1 min at 60°C. The fold-change in expression of each gene was
calculated using the (2−ΔΔCt) method. Product quality of
PCR was monitored using post-PCR melting curve analysis at the end
of the amplification cycles. The primers were as follows: RZR/RORα,
5′-GCAGGTGAAGGAGCCAGAAGG-3′ and 5′-GGAACAACAGACGCCAGTAAGAAC-3′;
RZR/RORβ, 5′-CCTGTATGCTGAGGTGCAGA-3′ and 5′-GGTGCTAAC
TGCCCATTGTT-3′; RZR/RORγ, 5′-GAGGCCATTCAGTACGTGGT-3′ and
5′-GCAATCTCATCCTCGGAAAA-3′; SENP1, 5′-GAGGATGGATGCTGGAGAAG-3′ and
5′-TGTCTGAGGAAGGATTATCTGAG-3′; HIF1α,
5′-ACTCAGGACACAGATTTAGACTTG-3′ and 5′-ATCAGTGGTGGCAGTGGTAG-3′; VEGF
(A), 5′-CTTGCCTTGCTGCTCTAC-3′ and 5′-ACCACTTCGTGATGATTCTG-3′;
GAPDH, 5′-CCG AGAATGGGAAGCTTGTC-3′ and
5′-TTCTCGTGGTTCACACCCATC-3′.
RNA interference experiments
SGC-7901 cells were transfected with siRNA for
control or RZR/RORγ using PolyPlus siRNA transfection reagent
(Invitrogen) according to the manufacturer's instructions and then
treated with MLT for 4 h under hypoxia. In brief, siRNA (100 pmol)
was mixed with transfection reagent in Opti-MEM serum-free media
(Invitrogen) and incubated for 20 min at room temperature. The
siRNA/transfection reagent mixture was added to the cells for 24 h.
Medium was changed before the treatment with MLT under hypoxia. The
sramble and RZR/RORγ siRNA sequences were as follows: NC sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′; RZR/RORγ-368 (A) sense,
5′-CCCGAGAUGCUGUCAAGUUTT-3′ and antisense,
5′-AACUUGACAGCAUCUCGGGTT-3′; RZR/RORγ-629 (B) sense,
5′-CCUCAUAUUCCAACAACUUTT-3′ and antisense,
5′-AAGUUGUUGGAAUAUGAGGTT-3′; RZR/RORγ-713 (C) sense,
5′-GGCAGAGAGAGCUUCUAUATT-3′ and antisense,
5′-UAUAGAAGCUCUCUCUGCCTT-3′.
Statistical analysis
Results are presented as the mean values ± standard
error of the mean (SEM). Significance between experimental values
was determined by the Student's paired t-tests, and one-way ANOVA
was used to test differences in repeated measures across
experiments. Differences were considered to be statistically
significant at P<0.05. Values were analyzed using the
statistical package SPSS 16.0 (StatSoft, Tulsa, OK, USA).
Results
MLT nuclear receptor RZR/ROR expression
in SGC-7901 cells and the effects of MLT on the mRNA and the
protein expression of RZR/RORγ
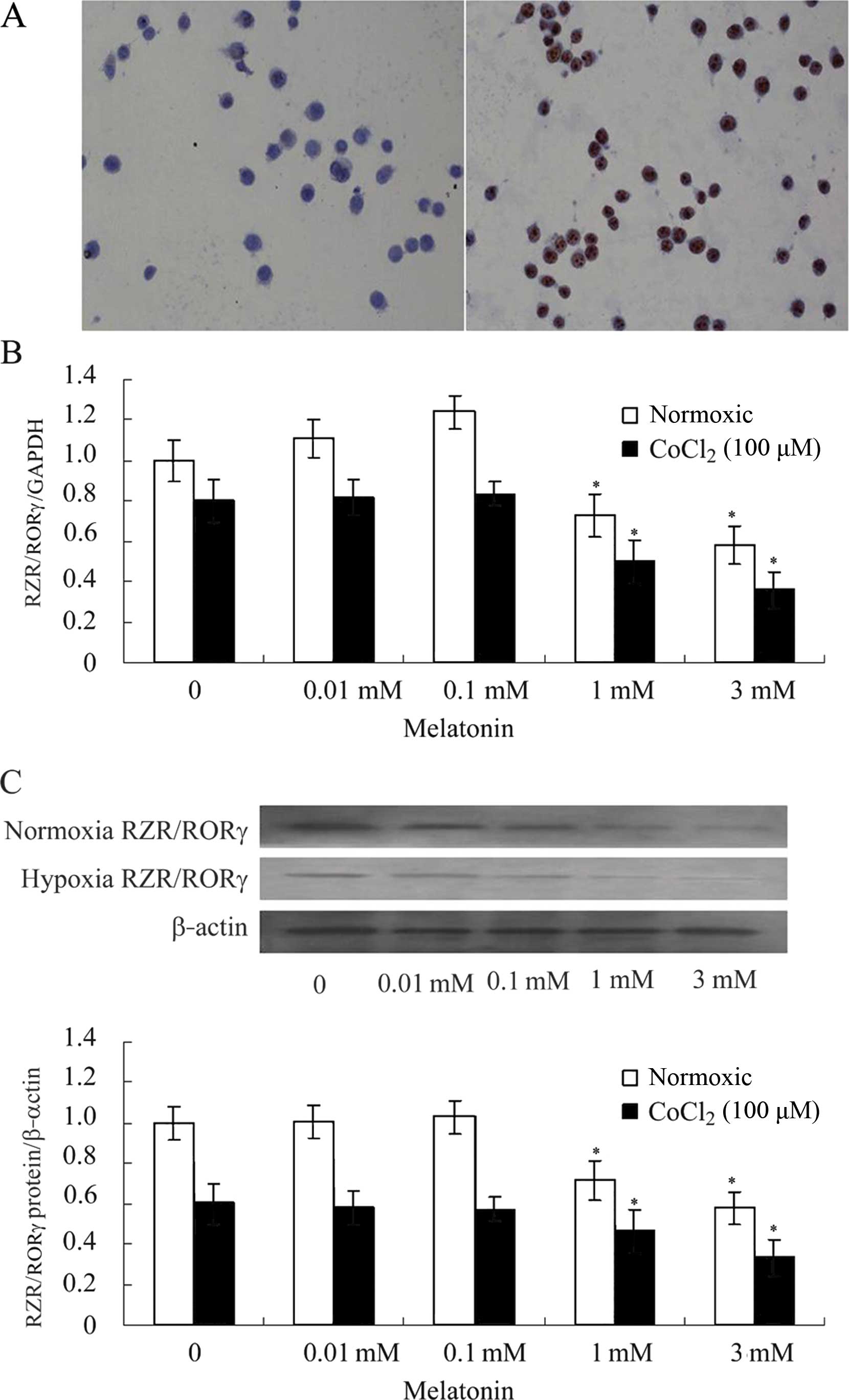
The results of immunocytochemistry showed that
RZR/RORγ was expressed in SGC-7901 human gastric cancer cells
(Fig. 1A), and RZR/RORα and
RZR/RORβ were not detected. Treatment of SGC-7901 cells with MLT
(0.01, 0.1, 1 and 3 mM) for 24 h resulted in decreased expression
of RZR/RORγ at the mRNA and protein levels compared with these
levels in the control group under hypoxic conditions (Fig. 1B and C).
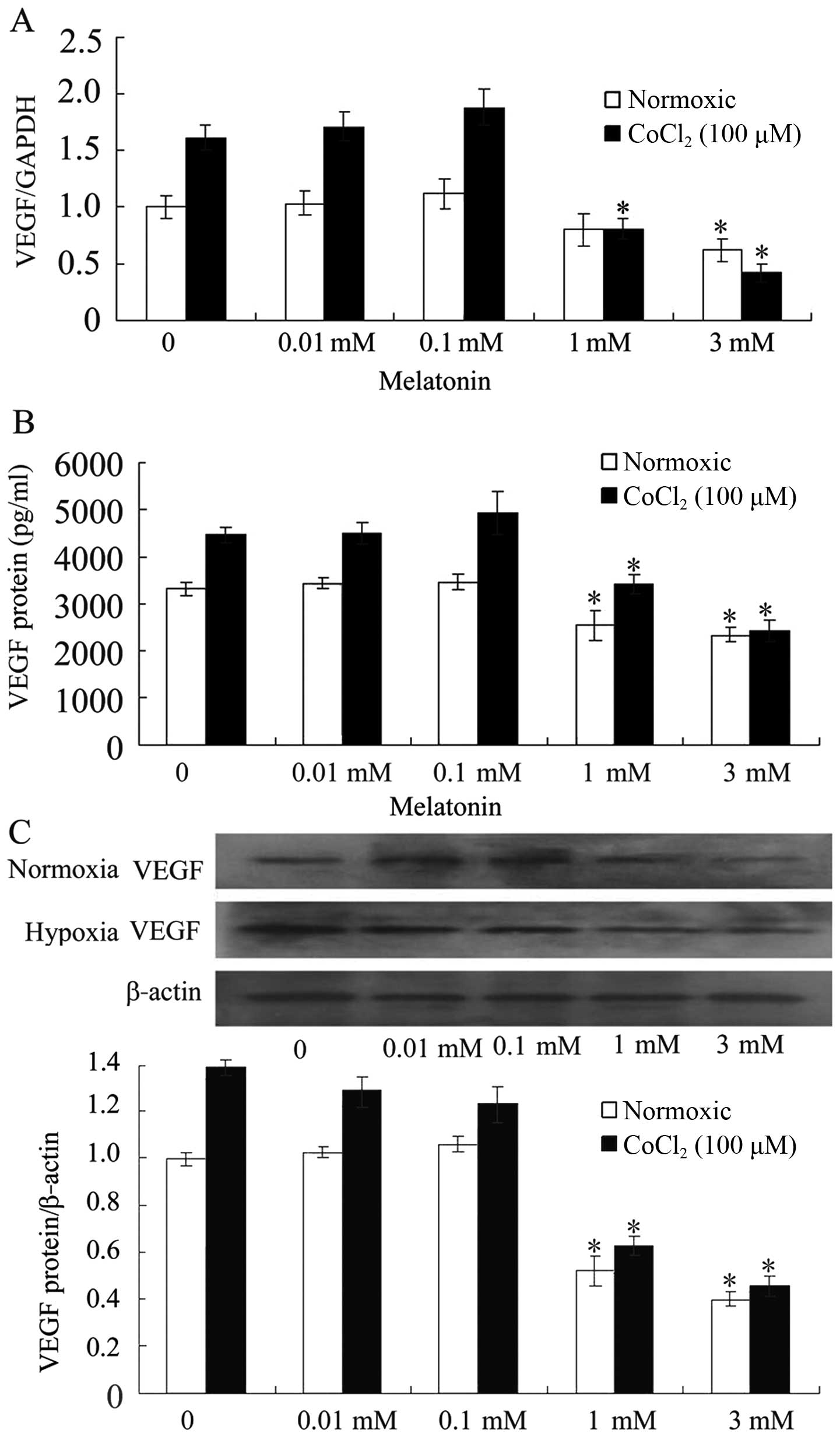
MLT decreases VEGF expression in SGC-7901
cells
To investigate the effect of pharmacological
concentrations of MLT on VEGF expression in cultured cells, the
levels of VEGF mRNA were detected by RT-PCR after incubation with
MLT for 24 h. The results showed that 0.01 mM MLT failed to
influence the basal levels and the CoCl2-induced levels
of VEGF mRNA. Exactly 1 mM of MLT downregulated the basal levels of
VEGF mRNA. The induced levels of VEGF mRNA were significantly
suppressed by 3 mM of MLT (Fig.
2A). We next assessed whether the influence on VEGF mRNA by MLT
resulted in a decreased production of VEGF protein. After 24 h of
incubation with different concentrations of MLT, VEGF protein
levels were notably decreased in the 1 and 3 mM MLT groups compared
with those of the control group and the 0.01 mM MLT group in the
CoCl2-treated cells (Fig. 2B
and C).
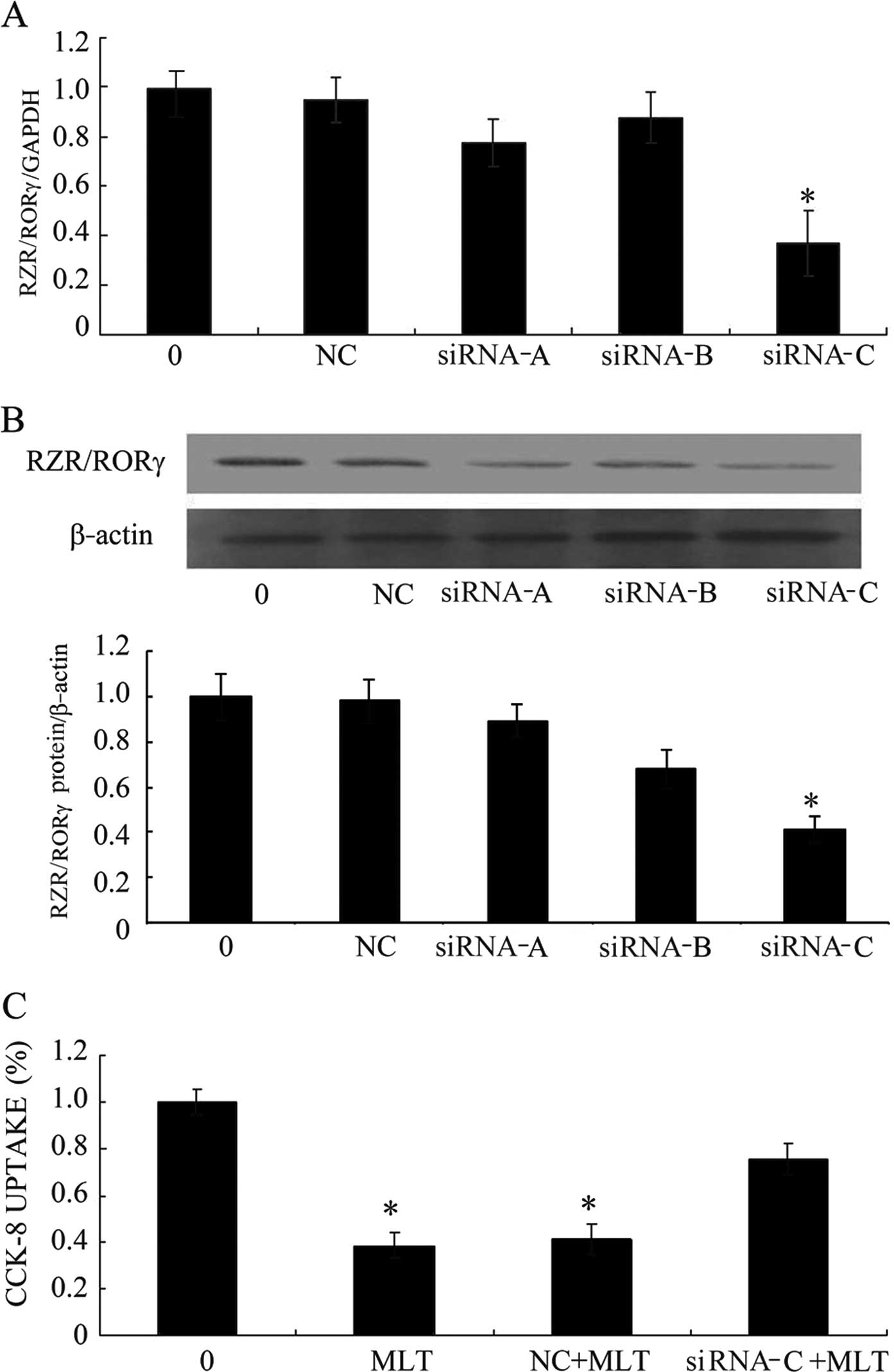
Decreased RZR/RORγ expression in SGC-7901
cells and the effects of MLT on cell viability following
application of siRNA RZR/RORγ
Application of siRNA technology to silence RZR/RORγ
decreased RZR/RORγ expression in the SGC-7901 cells (Fig. 3A and B) and obviously antagonized to
inhibit gastric cancer cell proliferation by MLT (Fig. 3C).
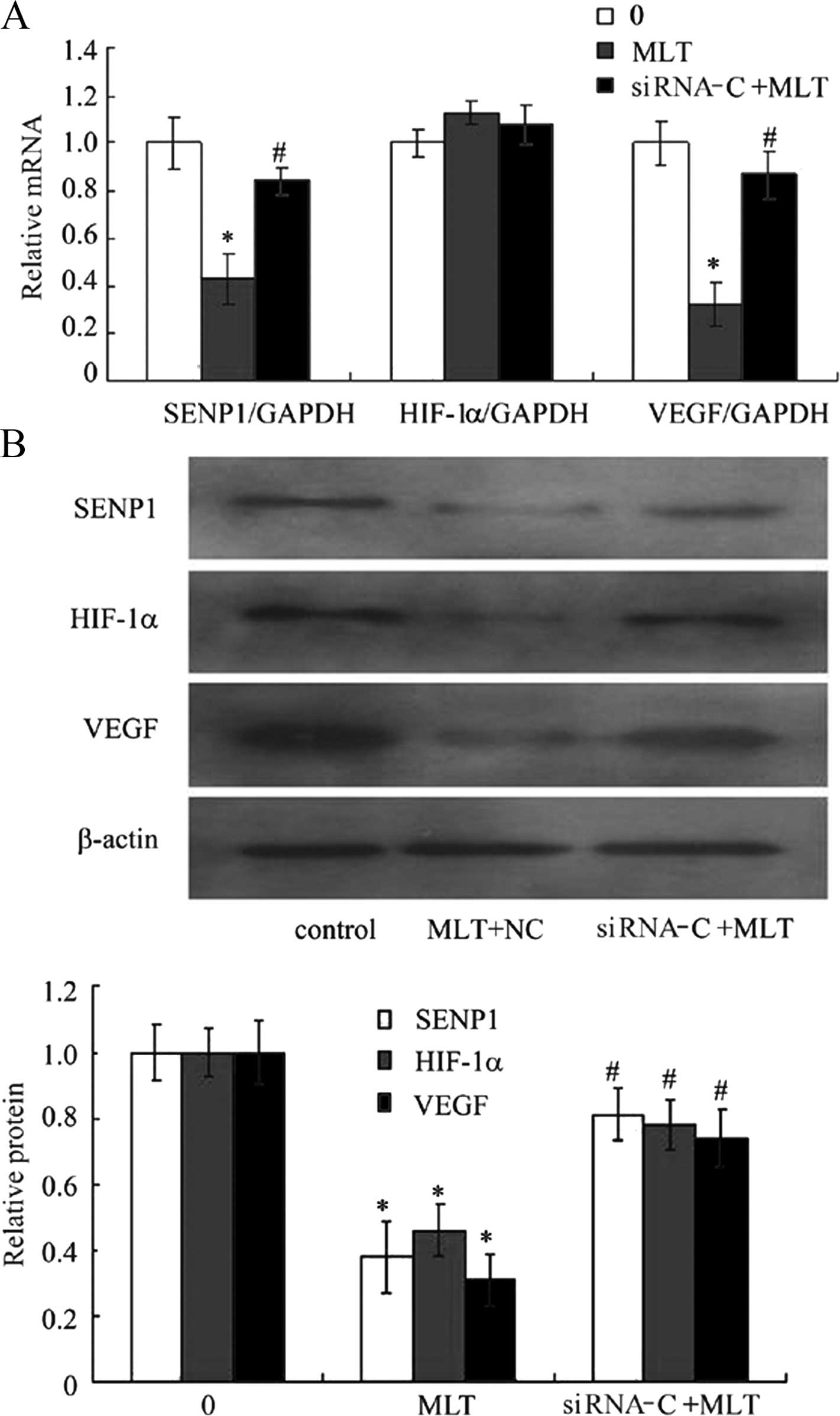
Effects of MLT on the mRNA and the
protein expression of SENP1, HIF-1α and VEGF after application of
siRNA RZR/RORγ
To investigate the effect of pharmacological
concentrations of MLT on RZR/RORγ, SENP1, HIF-1α and VEGF
expression in cultured cells, the levels of VEGF mRNA and protein
were detected by RT-PCR and western blotting after incubation with
3 mM MLT for 24 h. The results showed that 3 mM of MLT
downregulated basal levels of SENP1, HIF-1α and VEGF mRNA and
protein. After application of siRNA technology to silence RZR/RORγ,
3 mM MLT for 24 h obviously antagonized to inhibit the
downregulated basal levels of SENP1, HIF-1α and VEGF mRNA and
protein in the gastric cancer cells (Fig. 4A and B).
Discussion
Hypoxia inducible factors are transcription factors
that respond to hypoxia, a pathological condition in which the body
is deprived of an adequate oxygen supply (28). Notably, recent studies report that
melatonin (MLT) suppressed HIF-1α activation and angiogenesis in
cancer cells under hypoxia (22–24).
However, the underlying mechanisms responsible for MLT inhibition
of hypoxia-induced HIF-1α accumulation are not fully understood.
Thus, in the present study, we found that MLT inhibited HIF-1α
activation and VEGF secretion via the MLT nuclear receptor in
SGC-7901 human gastric cancer cells.
As a small lipophilic molecule, MLT easily crosses
cellular membranes and exerts its biological action through nuclear
signaling (29). It was proposed
that the putative nuclear MLT receptor is identical to and belongs
to a novel subclass of orphan nuclear receptors which suggests that
immunomodulatory and antitumor effects through the intracellular
action of MLT depend on nuclear signaling (17). The MLT nuclear receptors have been
cloned simultaneously by two different groups and received the
following names: retinoid Z receptor (RZR) and retinoid acid
receptor-related orphan receptor (ROR). The RZR/ROR family consists
of three subtypes (α, β and γ) and four splicing variants of the
α-subtype (30). In the present
study, we found that RZR/RORγ was highly expressed in SGC-7901
human gastric cancer cells while these cells did not express
RZR/RORα and RZR/RORβ. Treatment of SGC-7901 cells with MLT
resulted in decreased expression of RZR/RORγ in hypoxic conditions.
Notably, we found that MLT treatment significantly blocked RZR/RORγ
under hypoxia in SGC-7901 cells. Furthermore, RZR/RORγ siRNA
obviously antagonized to inhibit the action of the gastric cancer
cell SGC-7901 proliferation by MTL. Thus, MLT nuclear receptor
RZR/RORγ plays an important role to inhibit the action of gastric
cancer cell proliferation during hypoxia.
During normoxia, HIF1α is hydroxylated at two
critical proline residues by a family of oxygen-sensitive enzymes
prolyl 4-hydroxylases (PHD). Proline hydoxylated HIF1α then binds
to VHL, a component of the ubiquitin E3 ligase complex consisting
of Cul-2, VHL, elongin B and elongin C. Subsequently, HIF1α is
ubiquitinated and degraded by the proteasome (31). Hypoxia induces nuclear translocation
and SUMOylation of HIF1α, which binds to VHL in a hydroxyl
proline-independent manner, leading to ubiquitination and
proteasomal degradation. SUMO-specific protease 1 (SENP1), which is
predominately a nuclear protein, is well-positioned to regulate the
activity and stability of HIF1α in the nucleus by removing SUMO
(32–34). Thus, SENP1 plays a critical role to
control HIF1α stability during hypoxia. To further confirm the
involvement of SGC-7901 in MLT-mediated inhibition of HIF-1a during
hypoxia, we analyzed the effects of MLT on SENP1 since the
SENP1-dependent stabilization of HIF-1a is known to be mediated by
the SENP1 signaling pathway. In the present study, we confirmed
that MLT significantly downregulated basal levels of RZR/RORγ as
well as SENP1, HIF-1α and VEGF. RZR/RORγ siRNA obviously
antagonized to inhibit the downregulation of basal levels of SENP1,
HIF-1α and VEGF. Furthermore, the hypoxia-induced HIF-1α
accumulation was significantly suppressed in the presence of MLT,
consistent with previous studies.
Our data demonstrated that MLT significantly
prevented hypoxia-mediated RZR/RORγ and SENP1 in SGC-7901 cells.
Furthermore, RZR/RORγ siRNA transfection augmented the inhibitory
effect of MLT on SENP1 and HIF-1α accumulation in SGC-7901 cells
under hypoxia. Likewise, RZR/RORγ knockdown enhanced MLT-suppressed
RZR/RORγ activity under hypoxia, suggesting that MLT suppresses
hypoxia-induced HIF-1α inhibition via inactivation of RZR/RORγ in
gastric cancer cells. There is evidence that HIF-1α can mediate
VEGF secretion in cancer cells. In the present study, MLT reduced
the levels of secreted VEGF protein in SGC-7901 cells, suggesting
the strong inhibition of VEGF by MLT. Similarly, MLT revealed the
suppressive effects on tumor angiogenesis with VEGF inhibition by
targeting HIF-1α under hypoxia in prostate and colorectal cancer
cells (22). Importantly, blocking
RZR/RORγ activity prevented VEGF production in SGC-7901 cells,
strongly demonstrating that RZR/RORγ plays a critical role in
HIF-1α-mediated VEGF secretion under hypoxia.
Another group also reported that MLT suppressed
tumor angiogenesis by inhibiting HIF-1α stabilization under hypoxia
in HCT116 colon cancer cells (35).
In contrast, we focused on the important roles of the
RZR/RORγ-related pathways in MLT-induced HIF-1α inactivation and
antiangiogenic activity in gastric cancer cells under hypoxia.
The present study showed that MLT inhibits RZR/RORγ,
SENP1 and HIF-1α axis signaling and reduces VEGF production in
SGC-7901 cells under hypoxia. Consistently, siRNA-RZR/RORγ
effectively blocked the expression of SENP1, HIF-1α and VEGF
production in hypoxic SGC-7901 cells. These findings suggest that
MLT suppresses HIF-1α accumulation via inactivation of RZR/RORγ in
hypoxic SGC-7901 cells as a potent anticancer supplement for
gastric cancer therapy, which provides new ideas and approaches for
the treatment of gastric cancer.
Acknowledgments
This study was supported by the National Natural
Sciences Foundation Projects of China (nos. 30971541 and 81302601);
Grant sponsor, the Key Project of Science and Technology Commission
of Fujian Province of China (no. 2012Y0033), and the Major Projects
of Fujian Medical University (no. 09ZD018).
References
|
1
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Briançon-Marjollet A, Pépin JL, Weiss JW,
Lévy P and Tamisier R: Intermittent hypoxia upregulates serum VEGF.
Sleep Med. 15:1425–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ziche M and Gullino PM: Angiogenesis and
neoplastic progression in vitro. J Natl Cancer Inst. 69:483–487.
1982.PubMed/NCBI
|
|
4
|
Langsenlehner U, Hofmann G, Renner W,
Gerger A, Krenn-Pilko S, Thurner EM, Krippl P and Langsenlehner T:
Association of vascular endothelial growth factor - a gene
polymorphisms and haplotypes with breast cancer metastases. Acta
Oncol. 54:368–376. 2015. View Article : Google Scholar
|
|
5
|
Semenza GL: Intratumoral hypoxia,
radiation resistance, and HIF-1. Cancer Cell. 5:405–406. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lara PC, Lloret M, Clavo B, Apolinario RM,
Henríquez-Hernández LA, Bordón E, Fontes F and Rey A: Severe
hypoxia induces chemo-resistance in clinical cervical tumors
through MVP over-expression. Radiat Oncol. 4:292009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ratcliffe PJ, O'Rourke JF, Maxwell PH and
Pugh CW: Oxygen sensing, hypoxia-inducible factor-1 and the
regulation of mammalian gene expression. J Exp Biol. 201:1153–1162.
1998.PubMed/NCBI
|
|
8
|
Srinivas V, Zhang LP, Zhu XH and Caro J:
Characterization of an oxygen/redox-dependent degradation domain of
hypoxia-inducible factor alpha (HIF-alpha) proteins. Biochem
Biophys Res Commun. 260:557–561. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reddy KR, Guan Y, Qin G, Zhou Z and Jing
N: Combined treatment targeting HIF-1α and Stat3 is a potent
strategy for prostate cancer therapy. Prostate. 71:1796–1809. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Warfel NA and El-Deiry WS: HIF-1 signaling
in drug resistance to chemotherapy. Curr Med Chem. 21:3021–3028.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noche RR, Lu PN, Goldstein-Kral L, Glasgow
E and Liang JO: Circadian rhythms in the pineal organ persist in
zebrafish larvae that lack ventral brain. BMC Neurosci. 12:72011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
León J, Casado J, Jiménez Ruiz SM, Zurita
MS, González-Puga C, Rejón JD, Gila A, Muñoz de Rueda P, Pavón EJ,
Reiter RJ, et al: Melatonin reduces endothelin-1 expression and
secretion in colon cancer cells through the inactivation of FoxO-1
and NF-κβ. J Pineal Res. 56:415–426. 2014. View Article : Google Scholar
|
|
13
|
Yang Y, Sun Y, Yi W, Li Y, Fan C, Xin Z,
Jiang S, Di S, Qu Y, Reiter RJ, et al: A review of melatonin as a
suitable antioxidant against myocardial ischemia-reperfusion injury
and clinical heart diseases. J Pineal Res. 57:357–366. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lopes JR, Maschio LB, Jardim-Perassi BV,
Moschetta MG, Ferreira LC, Martins GR, Gelaleti GB and De Campos
Zuccari DA: Evaluation of melatonin treatment in primary culture of
canine mammary tumors. Oncol Rep. 33:311–319. 2015.
|
|
15
|
Cutando A, Aneiros-Fernández J,
López-Valverde A, Arias-Santiago S, Aneiros-Cachaza J and Reiter
RJ: A new perspective in oral health: Potential importance and
actions of melatonin receptors MT1, MT2, MT3, and RZR/ROR in the
oral cavity. Arch Oral Biol. 56:944–950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carocci A, Catalano A and Sinicropi MS:
Melatonergic drugs in development. Clin Pharmacol. 6:127–137.
2014.PubMed/NCBI
|
|
17
|
Wiesenberg I, Missbach M and Carlberg C:
The potential role of the transcription factor RZR/ROR as a
mediator of nuclear melatonin signaling. Restor Neurol Neurosci.
12:143–150. 1998.
|
|
18
|
Ekmekcioglu C: Melatonin receptors in
humans: Biological role and clinical relevance. Biomed
Pharmacother. 60:97–108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karamitri A, Vincens M, Chen M and Jockers
R: Involvement of melatonin MT2 receptor mutants in type
2 diabetes development. Med Sci. 29:778–784. 2013.In French.
|
|
20
|
Karasek M, Carrillo-Vico A, Guerrero JM,
Winczyk K and Pawlikowski M: Expression of melatonin MT(1) and
MT(2) receptors, and ROR alpha(1) receptor in transplantable murine
Colon 38 cancer. Neuro Endocrinol Lett. 23(Suppl 1): 55–60.
2002.PubMed/NCBI
|
|
21
|
Winczyk K, Pawlikowski M, Guerrero JM and
Karasek M: Possible involvement of the nuclear RZR/ROR-alpha
receptor in the antitumor action of melatonin on murine Colon 38
cancer. Tumour Biol. 23:298–302. 2002. View Article : Google Scholar
|
|
22
|
Cho SY, Lee HJ, Jeong SJ, Lee HJ, Kim HS,
Chen CY, Lee EO and Kim SH: Sphingosine kinase 1 pathway is
involved in melatonin-induced HIF-1α inactivation in hypoxic PC-3
prostate cancer cells. J Pineal Res. 51:87–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Liu Q, Wang F, Ling EA, Liu S,
Wang L, Yang Y, Yao L, Chen X, Wang F, et al: Melatonin antagonizes
hypoxia-mediated glioblastoma cell migration and invasion via
inhibition of HIF-1α. J Pineal Res. 55:121–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paroni R, Terraneo L, Bonomini F, Finati
E, Virgili E, Bianciardi P, Favero G, Fraschini F, Reiter RJ,
Rezzani R, et al: Antitumour activity of melatonin in a mouse model
of human prostate cancer: Relationship with hypoxia signalling. J
Pineal Res. 57:43–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang L, Zheng J, Xu R, Zhang Y, Gu L, Dong
J, Zhu Y, Zhou R, Zheng L, Zhang X, et al: Melatonin suppresses
hypoxia-induced migration of HUVECs via inhibition of ERK/Rac1
activation. Int J Mol Sci. 15:14102–14121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu H, Xu L, Wei JE, Xie MR, Wang SE and
Zhou RX: Role of CD4+ CD25+ regulatory T
cells in melatonin-mediated inhibition of murine gastric cancer
cell growth in vivo and in vitro. Anat Rec. 294:781–788. 2011.
View Article : Google Scholar
|
|
27
|
Xu L, Liu H, Zhang H, Wang RX, Song J and
Zhou RX: Growth-inhibitory activity of melatonin on murine
foregastric carcinoma cells in vitro and the underlying molecular
mechanism. Anat Rec. 296:914–920. 2013. View Article : Google Scholar
|
|
28
|
Ye LY, Zhang Q, Bai XL, Pankaj P, Hu QD
and Liang TB: Hypoxia-inducible factor 1α expression and its
clinical significance in pancreatic cancer: A meta-analysis.
Pancreatology. 14:391–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
García JJ, López-Pingarrón L,
Almeida-Souza P, Tres A, Escudero P, García-Gil FA, Tan DX, Reiter
RJ, Ramírez JM and Bernal-Pérez M: Protective effects of melatonin
in reducing oxidative stress and in preserving the fluidity of
biological membranes: A review. J Pineal Res. 56:225–237. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carlberg C and Wiesenberg I: The orphan
receptor family RZR/ROR, melatonin and 5-lipoxygenase: An
unexpected relationship. J Pineal Res. 18:171–178. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeong JW, Bae MK, Ahn MY, Kim SH, Sohn TK,
Bae MH, Yoo MA, Song EJ, Lee KJ and Kim KW: Regulation and
destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell.
111:709–720. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Geoffroy MC and Hay RT: An additional role
for SUMO in ubiquitin-mediated proteolysis. Nat Rev Mol Cell Biol.
10:564–568. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Q, Xia N, Li T, Xu Y, Zou Y, Zuo Y,
Fan Q, Bawa-Khalfe T, Yeh ET and Cheng J: SUMO-specific protease 1
promotes prostate cancer progression and metastasis. Oncogene.
32:2493–2498. 2013. View Article : Google Scholar
|
|
34
|
Gu J, Fan Y, Liu X, Zhou L, Cheng J, Cai R
and Xue S: SENP1 protects against myocardial ischaemia/reperfusion
injury via a HIF1α-dependent pathway. Cardiovasc Res. 104:83–92.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park SY, Jang WJ, Yi EY, Jang JY, Jung Y,
Jeong JW and Kim YJ: Melatonin suppresses tumor angiogenesis by
inhibiting HIF-1alpha stabilization under hypoxia. J Pineal Res.
48:178–184. 2010. View Article : Google Scholar : PubMed/NCBI
|