Introduction
Osteosarcoma is the most frequent primary malignant
bone tumor in children and young adults (1). Although combined neoadjuvant
chemotherapy has prolonged the survival rate greatly, the outcome
of osteosarcoma patients with a poor response to chemotherapy is
not encouraging. New strategies for treating recurrent and
metastatic osteosarcoma remains a critical aim in clinical
demands.
The focus of previous studies has been on the impact
of microRNAs (miRNA, miR) on tumorigenesis and cancer progression
(2–6). miRs are a class of small non-coding,
single-stranded endogenous RNA fragments of 19–25 nucleotides (nt)
in length that repress translation and cleaves mRNA by base-pairing
to the untranslated region of the target gene. In various types of
cancer, miRNA expression is significantly altered and this has
potential to be a prominent diagnostic and prognostic tool
(7). Elucidating the function of
miRNAs in tumor pathogenesis and progression is important as they
may play critical roles in the regulation of genes involved in
controlling the development, proliferation/differentiation,
apoptosis and drug resistance of tumor cells. Several studies
(2,8,9) have
found that miR-1908 is dysregulated in certain types of human
cancer. The expression pattern, clinical significance and
biological role of miR-1908 in osteosarcoma, however, remains
largely undefined.
In the present study, we showed that miR-1908 was
markedly upregulated in osteosarcoma cells and tissues compared
with normal bone tissues using RT-qPCR. miR-1908 upregulation in
osteosarcoma tissues was significantly associated with cell
proliferation, invasion, advanced TNM stage and tumor growth. Both
gain- and loss-of-function studies showed that miR-1908 markedly
increased the ability of osteosarcoma cells to proliferate and to
invade through Matrigel in vitro. miR-1908 significantly
increased the tumor volume and weight in vivo. Subsequent
investigations revealed that miR-1908 directly inhibited the
expression of phosphatase and tensin homolog deleted on chromosome
ten (PTEN). Collectively, miR-1908 promotes the proliferation and
invasion of osteosarcoma cells by repressing PTEN expression.
Materials and methods
Clinical specimens and cell culture
A total of 46 paraffin-embedded osteosarcoma
specimens and 9 normal muscle samples were obtained from the Fourth
Affiliated Hospital of China Medical University and were used in
accordance with the policies of the institutional review board of
the hospital. All the diagnoses were confirmed by light microscopy
and immunohistochemistry. No patient had received any anti-tumor
treatments before biopsy. The human osteosarcoma cell lines (143B,
U-2 OS, MG-63 and Saos-2) and hFOB 1.19 cell line were cultured in
RPMI-1640 medium (Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 10% FBS (Gibco Life Technologies, Carlsbad, CA,
USA) and 1% streptomycin/penicillin at 37°C with 5%
CO2.
RNA extraction, RT-PCR and RT-qPCR
Total RNA was extracted from paraffin-embedded
samples using acid phenol-chloroform method and from freshly-frozen
samples or cells with TRIzol reagent (Invitrogen Life
Technologies). Total RNA was reverse-transcribed using a First
Strand cDNA synthesis kit (Invitrogen Life Technologies). qPCR
reactions were conducted using Platinum SYBR-Green qPCR
SuperMix-UDG reagents (Invitrogen Life Technologies) on the Prism
7900HT system (Applied Biosystems, Foster City, CA, USA). Reactions
were performed in triplicate and reactions without reverse
transcriptase were used as negative controls. The U6 or GAPDH were
used as the endogenous controls and the 2−ΔΔCT equation
was used to calculate the relative expression levels.
Oligonucleotide transfection and
generation of stably transfected cell lines
Cells were seeded in 6-well plates, transfected with
miR-1908 mimics or miR controls (50 nM; GenePharma, Shanghai,
China) using Lipofactamine™ RNAiMAX (Invitrogen Life Technologies).
The cells were transfected with siMIF (100 nM; Invitrogen Life
Technologies) or siRNA controls using Lipofactamine 2000 reagent
(Invitrogen Life Technologies) and then harvested for assays 48 h
later. The lentiviral plasmid pEZX-MR03 (GeneCopoeia, Rockville,
MD, USA) expressing 1908 (Cat, HmiR0274-MR03) or scrambled miRNA
(Cat, CmiR0001-MR03) and Lenti-Pac HIV Expression Packaging mix
(GeneCopoeia) were co-transfected into glioma cells using the
EndoFectin Lenti transfection reagent (GeneCopoeia, Rockville, MD,
USA). After transfection for 48 h, lentiviral particles were
harvested and then transduced into the glioma cells and the stably
transfected cells were selected using puromycin and validated by
RT-qPCR and western blot analysis.
MTT and colony formation assays
Osteosarcoma cells were seeded at a density of
3×103 cells/well in 96-well plates after transfection.
An MTT assay was performed to test cell viability at 1, 2, 3 and 4
days, and the absorbance was measured at 490 nm with a
spectrophotometric plate reader. For colony formation assay, the
cells were plated at 500 cells/well in 6-well plates after
transfection and cultured for 14 days. Colonies were fixed with
methanol, stained with 0.5% crystal violet and counted under the
inverted microscope.
Western blot analysis
Equal amounts of protein were separated using SDS
polyacrylamide gels and were then electrotransferred to
polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA).
The membranes were immunoblotted overnight at 4°C with
primary antibodies, followed by their respective secondary
antibodies. β-actin was used as the loading control.
Cell migration and invasion assays
The effects of miR-1908 expression on cell migration
and invasion were assessed using the wound-healing and Transwell
assays as previously described (?).
In vivo tumor growth model
Male BALB/c athymic nude mice, aged 4–6 weeks, were
purchased from the Hunan Slac Jingda Laboratory Animal Co., Ltd.
(Changsha, China). For the tumor growth assay, cells stably
overexpressing miR-1908 or scrambled miRNA were resuspended in PBS
and 1×106 cells (200 µl) were subcutaneously
injected in the dorsal flank of nude mice. Tumor size was measured
every 3 days and tumor volumes were calculated using the formula:
volume = (L×W2)/2, in which L meant the longest diameter
and W meant the shortest diameter. After 22 days, the mice were
sacrificed and tumors were dissected and weighted. Animal handling
and research protocols were approved by the Animal Care and Use
Ethics Committee.
Immunohistochemical analysis
After 22 days, the mice were anesthetized and
sacrificed, and tumors were removed, photographed, weighed and
sectioned (5 mm in thickness), followed by immunostaining.
Following deparaffinization, the sections were
immunohistochemically analyzed using different antibodies,
respectively, and subsequently were pathologically confirmed for
the tumor phenotype and specific immunostaining. The positive cells
were counted and analyzed.
Luciferase reporter assay
The 3′-UTR (untranslated region) sequence of PTEN
was predicted to interact with miR-1908 or a mutated sequence
within the predicted target sites was produced and inserted into
the XbaI and FseI sites of the pGL3 control
luciferase reporter vector (Promega, Madison, WI, USA). The
luciferase reporter assay was performed as previously described
(?).
Statistical analysis
Experimental data are shown as mean ± standard
deviation (SD). The results from different treatment groups were
compared using a two-tailed Student's t-test. The Kaplan-Meier
method was used to estimate the probability of the patient survival
rate. A correlation analysis was performed to determine the
association between miR-1908 and the patients survival rate.
Differences were considered to be statistically significant at
P<0.05. statistical analysis was performed with SPSS/Win11.0
software (SPSS, Inc., Chicago, Illinois, USA).
Results
Aberrant expression of miR-1908 in human
osteosarcoma cells and tumors
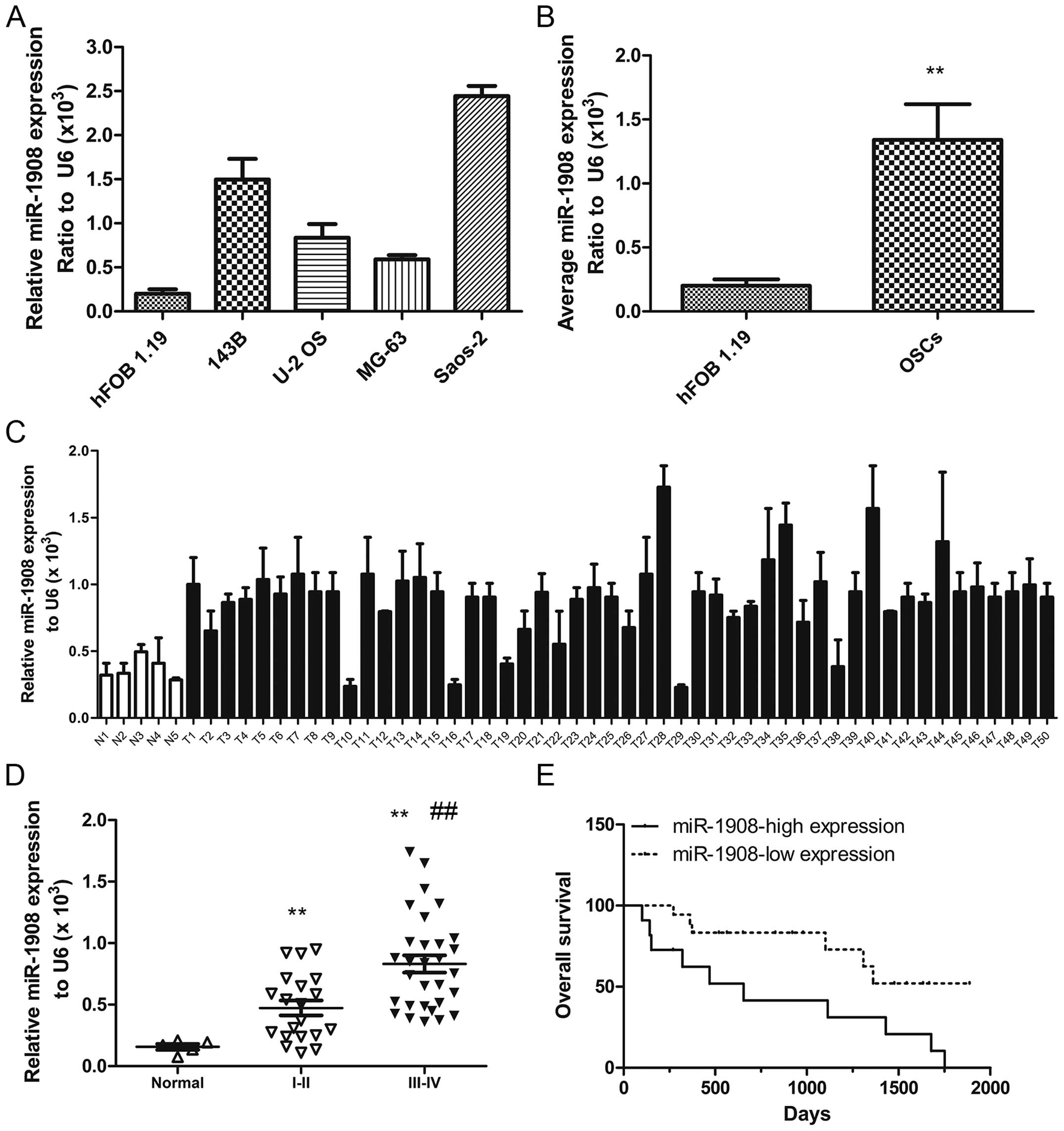
In order to confirm whether miR-1908 is associated
with osteosarcoma, miR-1908 levels were measured in osteosarcoma
cell lines (143B, U-2 OS, MG-63 and Saos-2) and a normal osteoblast
cell line (hFOB 1.19), as well as in human tumor specimens (n=46)
and normal muscle tissues (n=9). The results showed that, miR-1908
was significantly higher in osteosarcoma cells (Fig. 1A and B) than in the normal
osteoblast cell line and significantly higher in osteosarcoma
tumors than in the normal muscle tissue (Fig. 1C). miR-1908 was highest in stage
III-IV tumors and higher in stage I-II tumors as compared to normal
muscle tissue (Fig. 1D). miR-1908
was also associated with the overall patient survival rate.
Furthermore, more patients with lower miR-1908 survived following
treatment than those with higher miR-1908 (Fig. 1E). Collectively, these data
demonstrated that miR-1908 is upregulated in osteosarcoma and that
higher miR-1908 expression predicts a poor overall patient survival
rate.
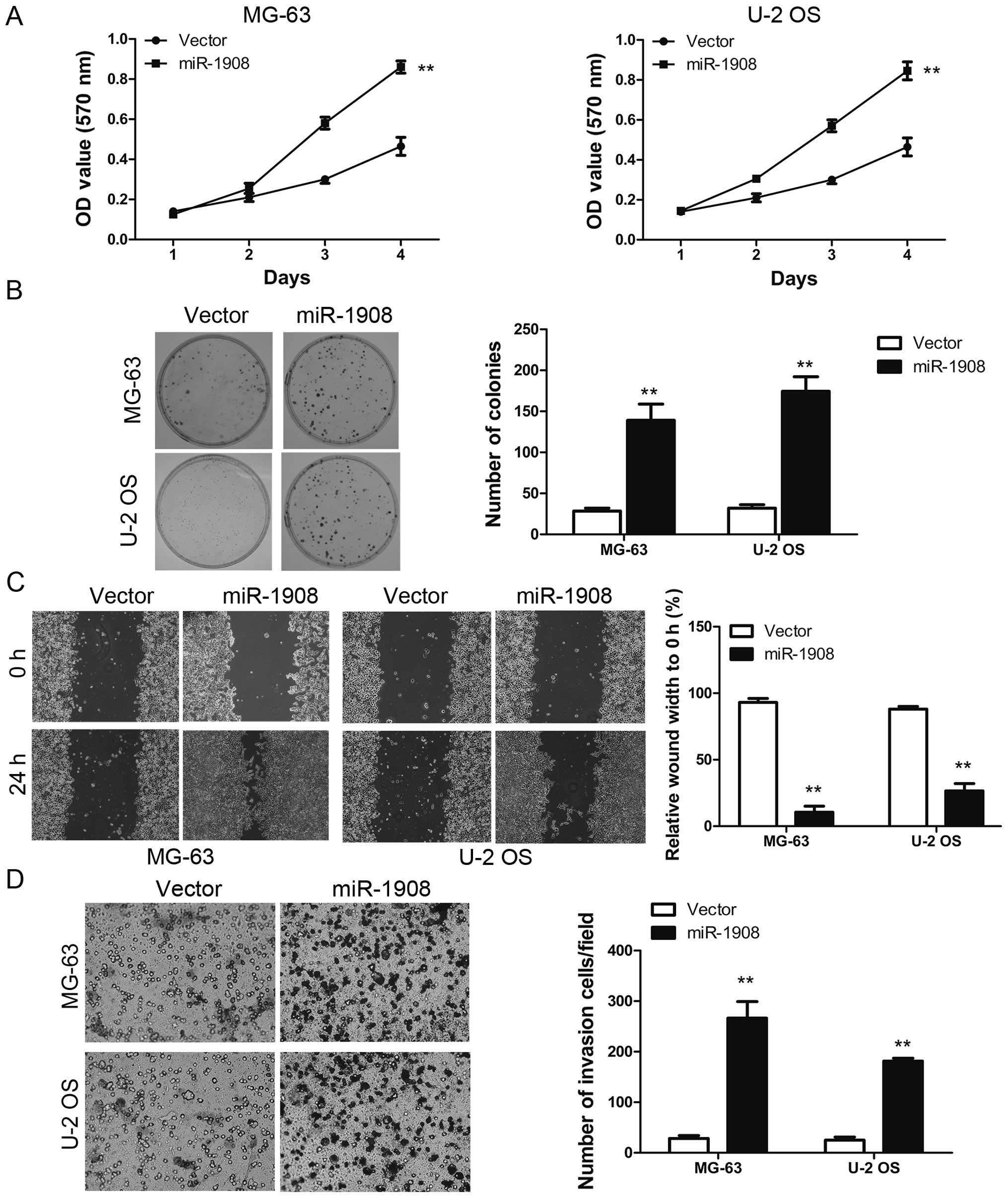
Overexpressed miR-1908 promotes
osteosarcoma cell proliferation, invasion and sphere formation
MTT and colony formation assays were carried out to
assess the functional role of miR-1908 in osteosarcoma cells by
determining the effects of miR-1908 overexpression in MG-63 and U-2
OS cell lines. Overexpression of miR-1908 in MG-63 and U-2 OS
resulted in a higher growth rate as compared with the controls
(Fig. 2A). In addition, the colony
formation assay revealed that miR-1908 promoted the viability of
osteosarcoma cells as compared with the controls (Fig. 2B). Collectively, these results
suggested that miR-1908 is an important regulator of proliferation
in osteosarcoma cells. The effect of miR-1908 on cell migration was
first assessed by the wound-healing assay. MG-63-miR-1908 and U-2
OS-miR-1908 cells had a significantly faster closure of the wound
area compared to their control cells (Fig. 2C). This result was confirmed by
Boyden's chamber assay (Fig. 2D).
Moreover, MG-63-miR-1908 and U-2 OS-miR-1908 cells showed a greater
degree of invasion through Matrigel (Fig. 2D). We then analyzed the effects of
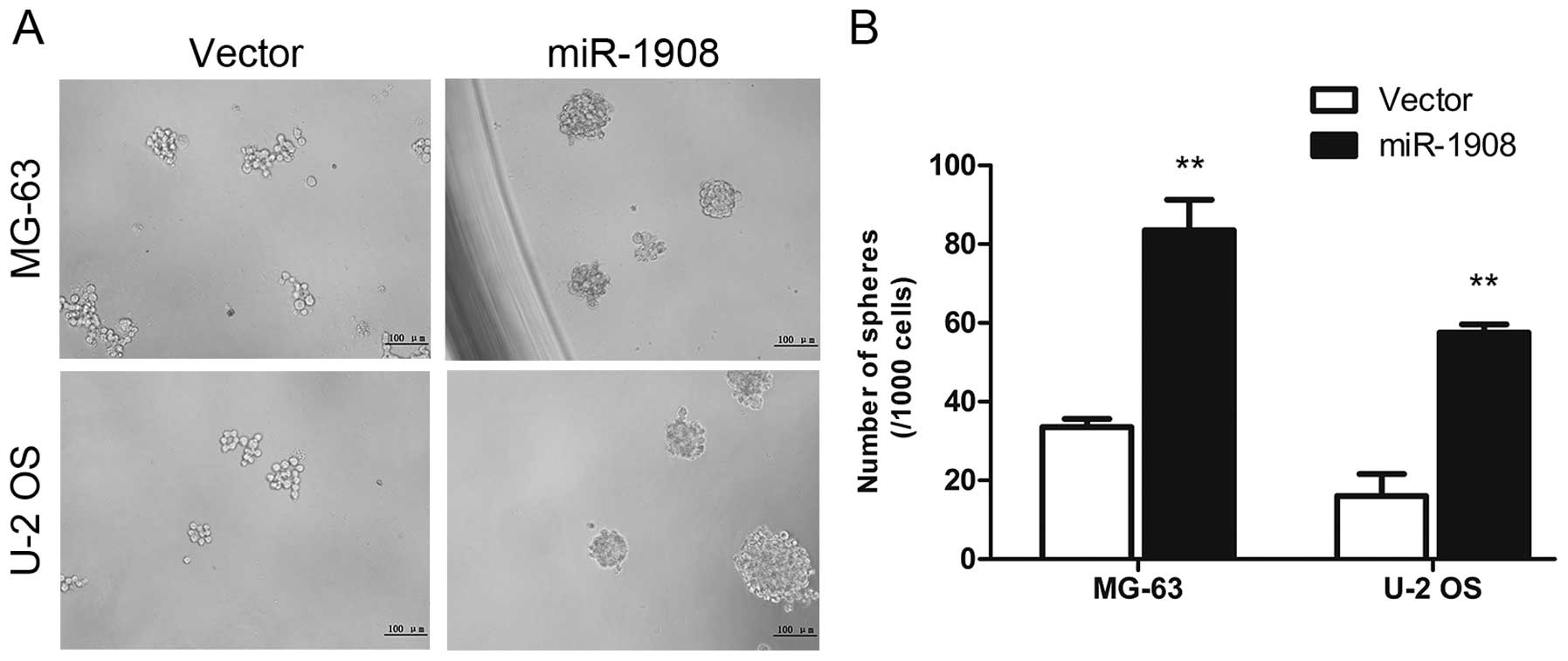
miR-1908 on osteosarcoma self-renewal using a sphere formation
assay. miR-1908 was transfected into osteosarcoma cells and sphere
formation was assessed for one week. miR-1908 overexpression
increased the sphere size and number (Fig. 3A and B). These data suggested that
miR-1908 promotes the self-renewal ability of osteosarcoma
cells.
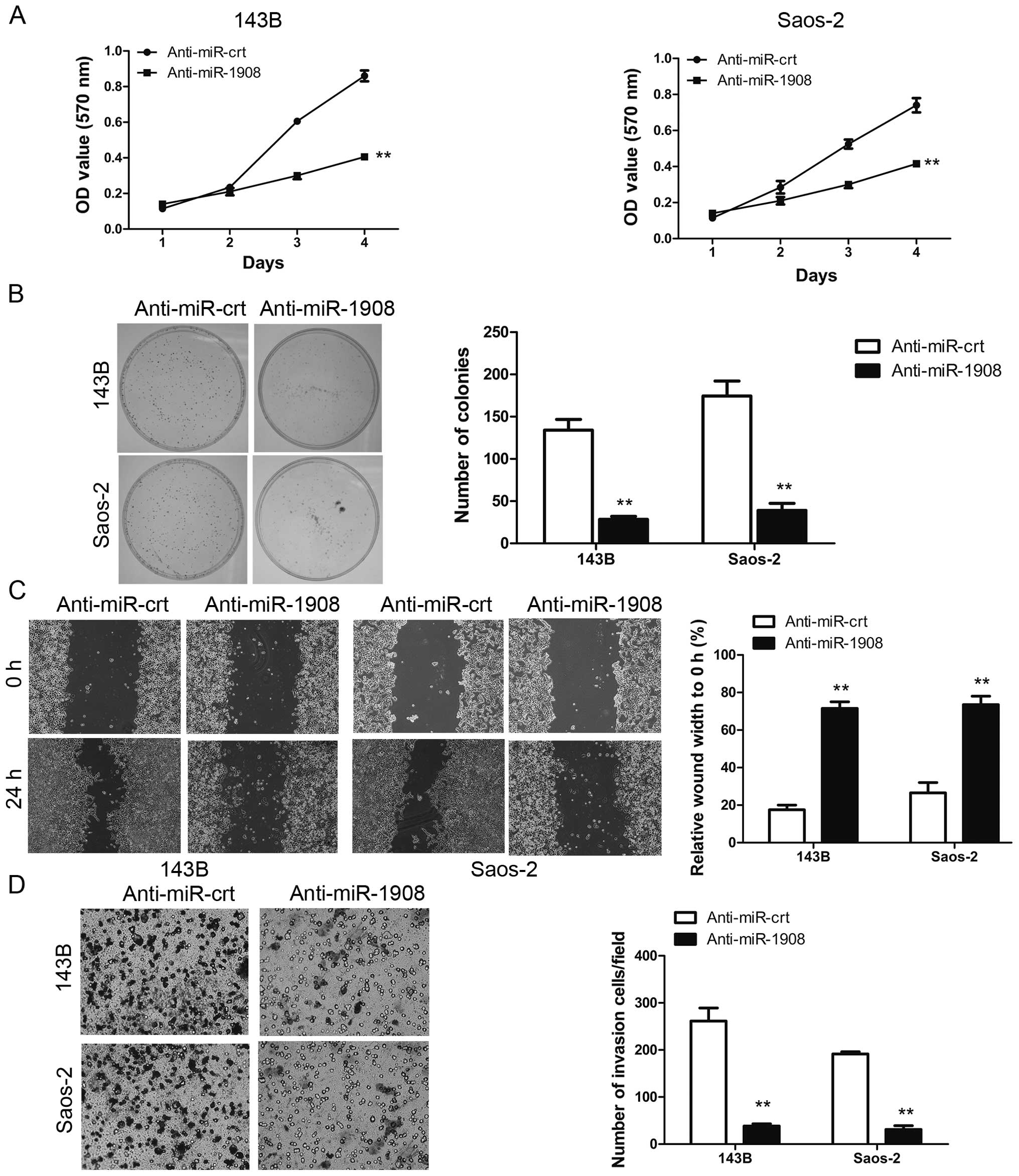
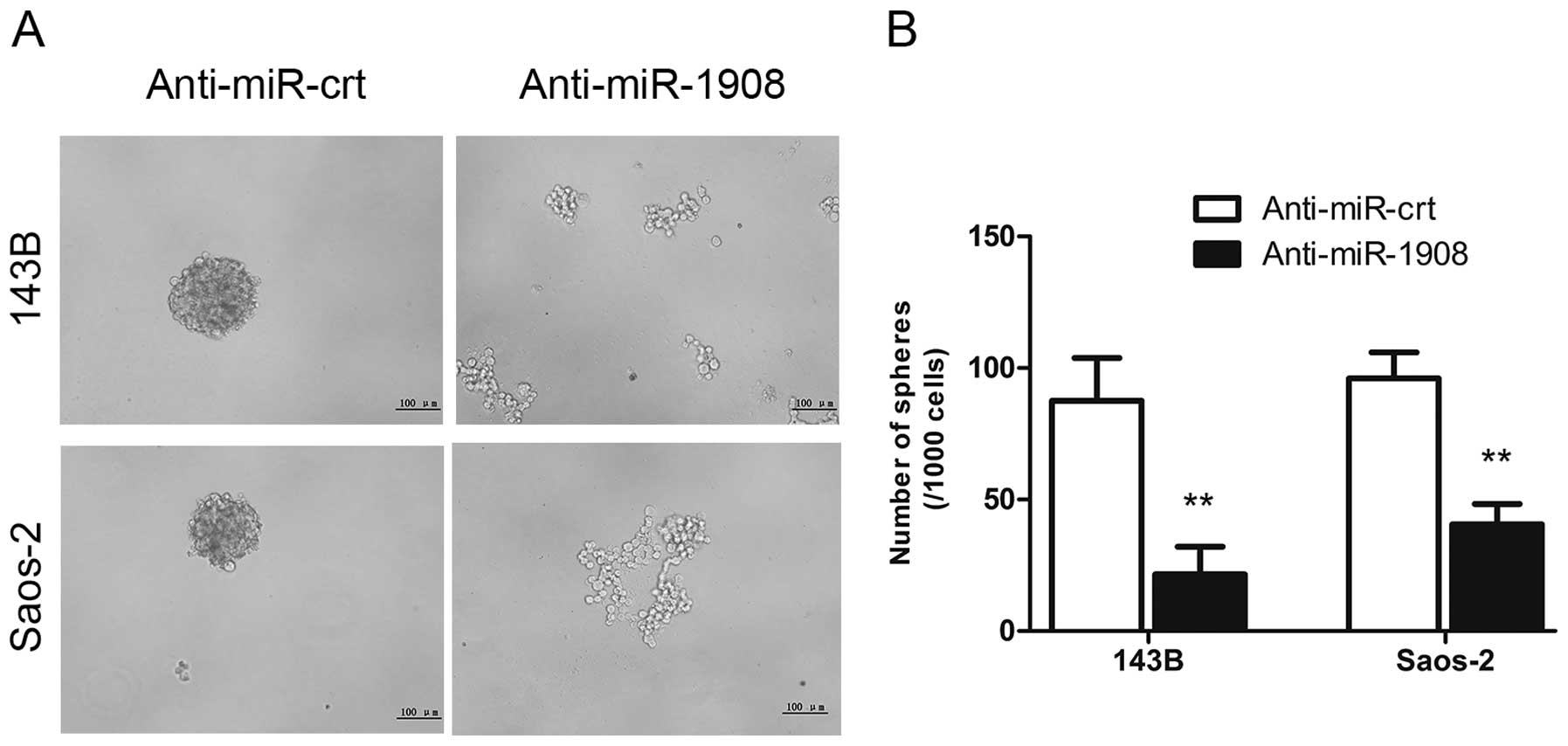
Silencing of miR-1908 inhibits
osteosarcoma cell proliferation, invasion and sphere formation
We determined whether the silencing of miR-1908
inhibits the malignant phenotype of osteosarcoma cells. Silencing
of miR-1908 in 143B and saos-2 cells significantly reduced cell
proliferation (Fig. 4A) and
clonogenicity (Fig. 4B). The effect
of silencing of miR-1908 on cell migration was first assessed by a
wound-healing assay.
The 143B-Anti-miR-1908 and Saos-2-Anti-miR-1908
cells reduced the migratory capacity compared to their control
cells (Fig. 4C). This result was
confirmed by the Boyden's chamber assay (Fig. 4D). In addition, 143b-Anti-miR-1908
and Saos-2-Anti-miR-1908 cells showed a lower degree of invasion
through Matrigel (Fig. 4D). The
sphere formation assay showed that silencing of miR-1908 decreased
sphere size and number (Fig. 5A and
B). This result indicated that miR-1908 promotes the
self-renewal ability of osteosarcoma cells. Collectively, the
results suggested that miR-1908 is an important regulator of
proliferation in osteosarcoma cells.
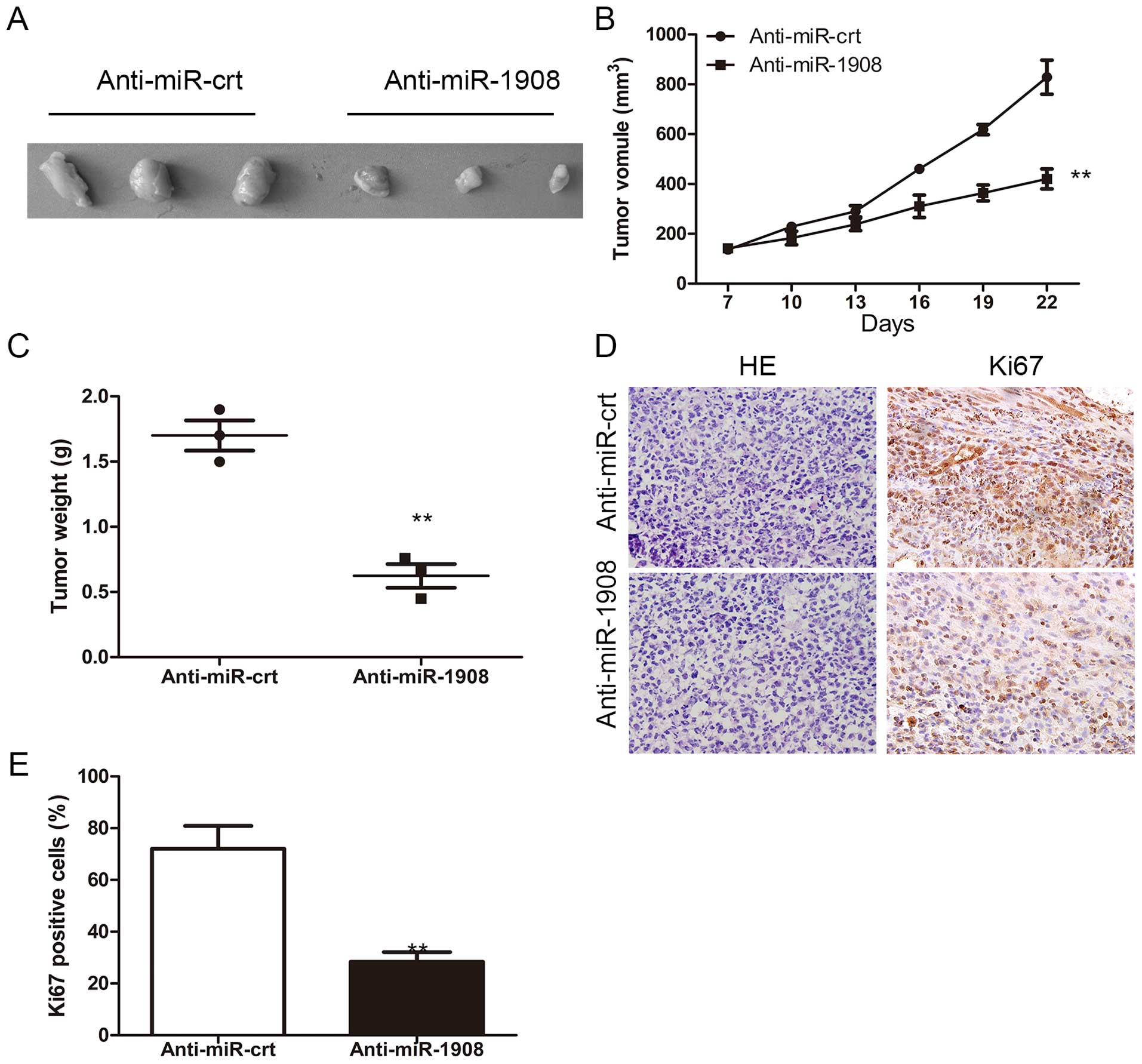
Silencing of miR-1908 inhibits
osteosarcoma tumor formation in vivo
To confirm whether the biologic effect of miR-1908
observed in cultured cells is relevant to osteosarcoma growth in
vivo, 143B-Anti-miR-1908 and control, respectively, were
subcutaneously inoculated into BALB/C athymic nude mice. As shown
in Fig. 6A, tumors formed by
miR-1908-silencing cells grew more slowly than those by the vector
control cells following inoculation and the difference in average
tumor volume between experimental and control animals continued to
increase 2 -fold at the experimental endpoint (22 days) (Fig. 6A and B). Concurrently, increases in
sizes and weights of tumors excised from animals of the miR-1908
overexpression group were also observed as compared with those of
the control group (Fig. 6B and C).
Consistent with the above observations, as shown in Fig. 6D and E, the proportion of
Ki67-positive cells increased in the control tumors compared to
that in miR-1908-silenced tumors.
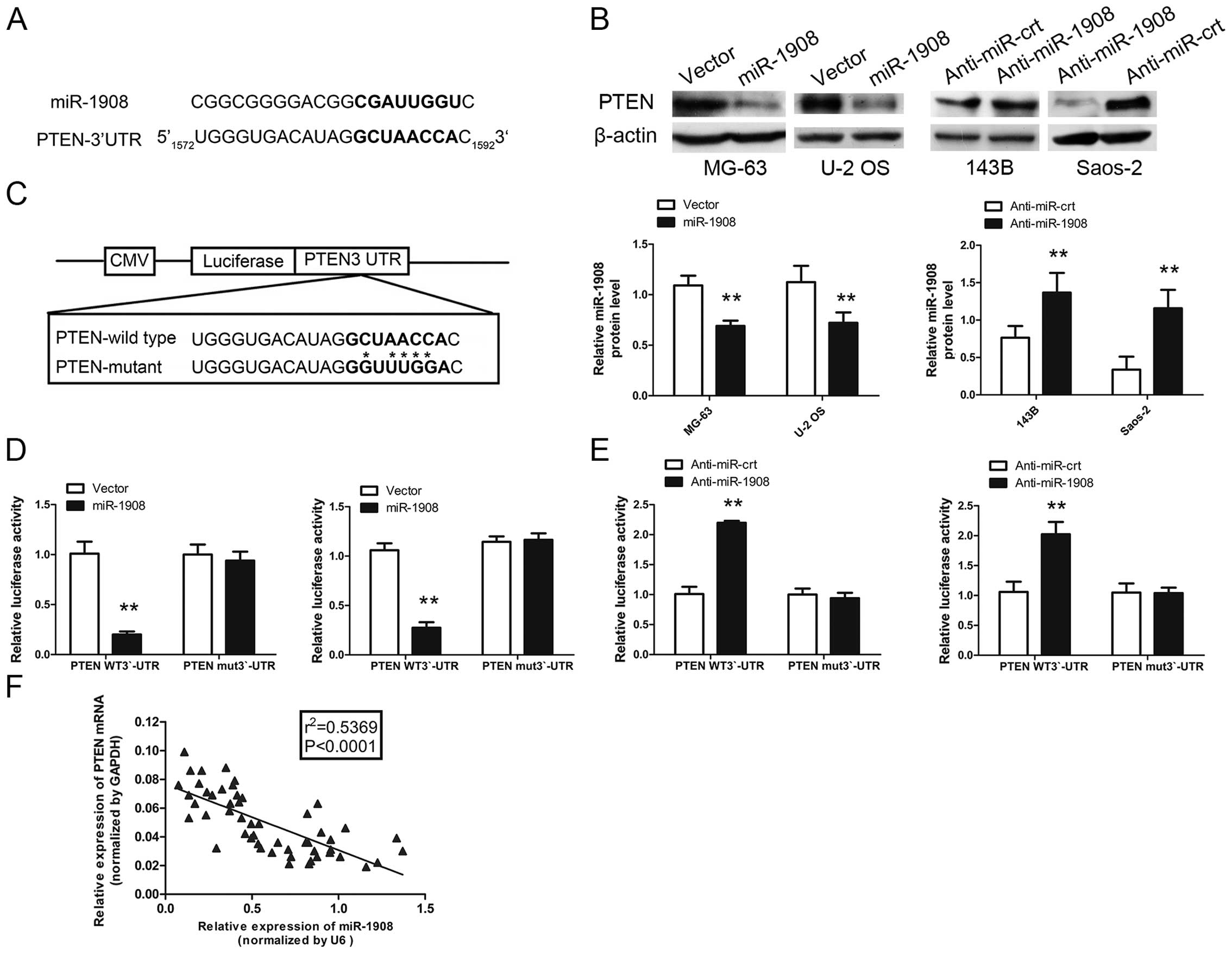
miR-1908 directly targets PTEN and PTEN
levels are inversely correlated with miR-1908 levels in
osteosarcoma tissues
To reveal mRNA targets of miR-1908 in osteosarcoma,
we used bioinformatics databases (TargetScan, Pictar, RNAhybrid) to
identify potential tumor-suppressor targets. The PTEN
(NM_000314) gene contained the predicted binding site for miR-1908
(Fig. 7A). To experimentally verify
this potential target, the cells were transfected with miR-1908 and
the protein and mRNA target levels were assessed by western blot
analysis. miR-1908 overexpression reduced and miR-1908 knockdown
increased the expression of PTEN in osteosarcoma cells (Fig. 7B). To determine whether PTEN 3′-UTR
is a direct target of miR-1908, PTEN 3′-UTR reporter constructs or
3′-UTR mutant controls were transfected into osteosarcoma cells
before transfection with miR-1908 and luciferase activity was
measured (Fig. 7C). As shown in
Fig. 7D and E, the luciferase
activity was significantly decreased in osteosarcoma cells
overexpressing miR-1908 co-transfected with 3′-UTR-PTEN-wt vector
and miR-1908 mimic compared with those co-transfected with
3′-UTR-PTEN-mut vector and miR-1908 mimic. By contrast, increased
activity was observed in osteosarcoma cells silencing miR-1908,
suggesting that the fragment at the 3′-UTR of the PTEN was the
complementary site for the miR-1908 seed region, and thus, that
PTEN was a direct target of miR-1908. In addition, PTEN expression
was inversely proportional to miR-1908 expression (Fig. 7F). Collectively, miR-1908 directly
affected PTEN expression.
Discussion
Osteosarcoma is one of the most common malignancies
and one of the leading causes of cancer-related mortality worldwide
(10,11). Despite recent advances in disease
management and treatment, osteosarcoma patients have a poor
long-term prognosis. The main challenges in the treatment of
osteosarcoma involve recurrence and metastasis, leading to the
prediction poor outcome for osteosarcoma patients.
The identification of critical molecules that
suppress these processes may lead to novel therapeutic targets for
improving the prognosis of these patients. Various studies have
identified some key signaling transduction cascades that are
involved in the progression, invasion and metastasis of
osteosarcoma, such as p53 (12,13),
the Ras/MAPK pathways (14,15) and the PTEN pathway (16–18).
However, the underlying molecular mechanisms of osteosarcoma
metastasis remain to be determined.
PTEN is located at 10q23.3 and encodes a
dual-specificity phosphatase with lipid and protein phosphatase
activities. PTEN suppressed the migration and genetic deletion of
the PTEN tumor suppressor gene, promoting cell motility and
overexpression or reconstitution of PTEN and inhibiting cell
motility in a variety of cell types (19–21).
Mechanistically, PTEN repressed cell motility through a variety of
pathways and PI3K/AKT is an important target of PTEN. miRNAs have
been shown to play an important role in the maintenance of normal
cell function and the dysregulation of miRNA expression may result
in cancer initiation and tumor progression. Hundreds of genes were
found to be the target of miRNA, which harbors the target sequence
in their 3′-UTR complementary to the seed region of the miRNA.
Previous studies have reported that certain miRNAs can directly
target PTEN (22–24).
miR-1908 is a new member of the microRNA family.
Although results of a previous study indicate that miR-1908
promoted invasion and angiogenesis in melanoma (25), angiogenesis has not been
investigated in this study. In this study, the expression,
function, and mechanisms of action of miR-1908 in osteosarcoma were
investigated. We found that miR-1908 was a risk factor in
osteosarcoma where it acts as an oncogene by regulating PTEN
expression. In the present study, it was demonstrated that
miRNA-1908 targets PTEN, resulting in the reduced expression of
PTEN and an increase in migration and proliferation of osteosarcoma
cells in vitro. In osteosarcoma cells, miR-1908
overexpression is associated with higher cell proliferation and
invasion in vitro. By contrast, silencing endogenous
miR-1908 markedly abrogated the proliferation and invasion of
osteosarcoma cells. miR-1908 overexpression and knockdown cell
lines were established separately. miR-1908 overexpression is
associated with higher cell proliferation and migration, while
silencing of miR-1908 is associated with lower cell proliferation
and migration. Of note, the close correlation between a high
miR-1908 expression and low expression of PTEN, as well as with the
malignant properties of osteosarcoma, was also observed in athymic
nude mice and in clinical osteosarcoma samples, suggesting a
possible role of miR-1908 in the development and progression of
osteosarcoma. miR-1908 directly targeted PTEN and PTEN levels are
inversely correlated with miR-1908 levels in osteosarcoma
tissues.
In summary, results of the present study have
demonstrated that miR-1908 is overexpressed in osteosarcoma cells
in vitro. Concurrently, miR-1908 overexpression is
associated with poor patient survival. The present study presents
evidence that indicates that miR-1908 has oncogenic, proliferation,
migration and invasion regulatory effects that are mediated by PTEN
in osteosarcoma cells. The present study therefore provides, to the
best of our knowledge, a first characterization of miR-1908 as an
oncogene and a potential therapeutic target in osteosarcoma.
Acknowledgments
The present study was supported by the Science and
Technology Research Foundation of Education Department of Liaoning
Province (no. L2010661) and the Science and Technology Projects of
Technology Department of Liaoning province (no. 2013021015).
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Long C, Jiang L, Wei F, Ma C, Zhou H, Yang
S, Liu X and Liu Z: Integrated miRNA-mRNA analysis revealing the
potential roles of miRNAs in chordomas. PLoS One. 8:e666762013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsu SH, Wang B, Kota J, Yu J, Costinean S,
Kutay H, Yu L, Bai S, La Perle K, Chivukula RR, et al: Essential
metabolic, anti-inflammatory, and anti-tumorigenic functions of
miR-122 in liver. J Clin Invest. 122:2871–2883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang Y, Xue JL, Shen Q, Chen J and Tian L:
MicroRNA-7 inhibits tumor growth and metastasis by targeting the
phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma.
Hepatology. 55:1852–1862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rawlings-Goss RA, Campbell MC and Tishkoff
SA: Global population-specific variation in miRNA associated with
cancer risk and clinical biomarkers. BMC Med Genomics. 7:532014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pencheva N, Tran H, Buss C, Huh D,
Drobnjak M, Busam K and Tavazoie SF: Convergent multi-miRNA
targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis
and angiogenesis. Cell. 151:1068–1082. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin JC, Jin XL, Zhang X, Piao YS and Liu
SP: Effect of OSW-1 on microRNA expression profiles of hepatoma
cells and functions of novel microRNAs. Mol Med Rep. 7:1831–1837.
2013.PubMed/NCBI
|
|
10
|
Geller DS and Gorlick R: Osteosarcoma: A
review of diagnosis, management, and treatment strategies. Clin Adv
Hematol Oncol. 8:705–718. 2010.
|
|
11
|
Zhang Y, Zhang L, Zhang G, Li S, Duan J,
Cheng J, Ding G, Zhou C, Zhang J, Luo P, et al: Osteosarcoma
metastasis: Prospective role of ezrin. Tumour Biol. 35:5055–5059.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He Y, de Castro LF, Shin MH, Dubois W,
Yang HH, Jiang S, Mishra PJ, Ren L, Gou H, Lal A, et al: p53 loss
increases the osteogenic differentiation of bone marrow stromal
cells. Stem Cells. 33:1304–1319. 2015. View Article : Google Scholar
|
|
13
|
Diller L, Kassel J, Nelson CE, Gryka MA,
Litwak G, Gebhardt M, Bressac B, Ozturk M, Baker SJ, Vogelstein B,
et al: p53 functions as a cell cycle control protein in
osteosarcomas. Mol Cell Biol. 10:5772–5781. 1990.PubMed/NCBI
|
|
14
|
Medema RH, Kops GJ, Bos JL and Burgering
BM: AFX-like Forkhead transcription factors mediate cell-cycle
regulation by Ras and PKB through p27kip1. Nature. 404:782–787.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng Z, Ding M, Ni J, Song D, Huang J and
Wang J: MiR-142 acts as a tumor suppressor in osteosarcoma cell
lines by targeting Rac1. Oncol Rep. 33:1291–1299. 2015.
|
|
16
|
Hu Y, Xu S, Jin W, Yi Q and Wei W: Effect
of the PTEN gene on adhesion, invasion and metastasis of
osteosarcoma cells. Oncol Rep. 32:1741–1747. 2014.PubMed/NCBI
|
|
17
|
Song D, Ni J, Xie H, Ding M and Wang J:
DNA demethylation in the PTEN gene promoter induced by
5-azacytidine activates PTEN expression in the MG-63 human
osteosarcoma cell line. Exp Ther Med. 7:1071–1076. 2014.PubMed/NCBI
|
|
18
|
Shen L, Chen XD and Zhang YH: MicroRNA-128
promotes proliferation in osteosarcoma cells by downregulating
PTEN. Tumour Biol. 35:2069–2074. 2014. View Article : Google Scholar
|
|
19
|
Yang YK, Xi WY, Xi RX, Li JY, Li Q and Gao
YE: MicroRNA-494 promotes cervical cancer proliferation through the
regulation of PTEN. Oncol Rep. 33:2393–2401. 2015.PubMed/NCBI
|
|
20
|
Zhang WL and Zhang JH: miR-181c promotes
proliferation via suppressing PTEN expression in inflammatory
breast cancer. Int J Oncol. 46:2011–2020. 2015.PubMed/NCBI
|
|
21
|
Hodakoski C, Fine B, Hopkins B and Parsons
R: Analysis of intracellular PTEN signaling and secretion. Methods.
77–78:164–171. 2015. View Article : Google Scholar
|
|
22
|
Zhang LY, Ho-Fun Lee V, Wong AM, Kwong DL,
Zhu YH, Dong SS, Kong KL, Chen J, Tsao SW, Guan XY, et al:
MicroRNA-144 promotes cell proliferation, migration and invasion in
nasopharyngeal carcinoma through repression of PTEN.
Carcinogenesis. 34:454–463. 2013. View Article : Google Scholar
|
|
23
|
Bar N and Dikstein R: miR-22 forms a
regulatory loop in PTEN/AKT pathway and modulates signaling
kinetics. PLoS One. 5:e108592010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garofalo M, Di Leva G, Romano G, Nuovo G,
Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P,
et al: miR-221&222 regulate TRAIL resistance and enhance
tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell.
16:498–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pencheva N, Tran H, Buss C, et al:
Convergent multi-miRNA targeting of ApoE drives LRP1/LRP8-dependent
melanoma metastasis and angiogenesis. Cell. 151:1068–1082. 2012.
View Article : Google Scholar : PubMed/NCBI
|