Introduction
A major obstacle to successful tumor chemotherapy is
multi-drug resistance (MDR), which has been widely investigated in
recent decades (1). Numerous
studies have been conducted into MDR mechanisms and strategies for
overcoming MDR. Unfortunately, most patients still die from their
disseminated cancer due to resistance to available anticancer drugs
(2). Therefore, the development of
an MDR model to screen novel therapy regimens for translation from
the laboratory to the clinic is desperately needed.
An important tool in MDR research, the tumor models
include three main types: the planar cell model, the
three-dimensional tumor model in vitro and the animal
xenograft model. The in vitro monolayer cell model is easy
to establish and is thus widely used in the early phase of tumor
research. Its value, however, is limited due to its lack of
relevance to clinical samples (3).
Moreover, apart from the MDR tumor cell itself, the tumor
microenvironment also plays an important, even dominant, role in
MDR (4). The interaction between
cells and extracellular matrices is extremely complex (5). To better mimic tumors in vivo,
the three-dimensional matrix model was developed, leading to more
physiologically relevant conditions for assessing drug responses
and studying biological mechanisms. In vitro 2-D cell and
3-D matrix models play an important role in understanding complex
tumor systems but both are hardly sufficient for evaluating the
real effects of products on tumors in vivo (6). Therefore, a whole animal xenograft
model needs to be developed.
When developing tumor models an issue with which
many groups are confronted is that it is difficult for certain
types of MDR tumor cell lines to grow with appropriate rate in
vivo after being inoculated subcutaneously, even though the
cells may grow quickly in a planar culture dish. In our laboratory,
we found an interesting scientific phenomenon that three resistant
tumor cell lines of KB-8-5, H460/Tax-R and NCI/ADR-RES with
gradually increasing expression of P-gp have similar proliferation
rate in vitro, but in the xenograft model the higher the
P-gp was expressed, the harder the model was to be establish.
Normally, the xenograft models of KB-8-5 and H460/Tax-R carcinoma
lines in nude mice can be established within 5 and 12 days or so,
while the tumor of NCI-ADR-RES cell lines in the nude mouse model
shows hardly any growth (data not shown). In addition, the
resistant KB-8-5 tumor models take on satisfactory reproduction and
stability. Some tumor cell lines with low tumorigenesis have been
reported (7), but few studies focus
on the tumorigenic ability of MDR cells that have high P-gp (also
known as MDR1; multidrug resistance protein 1) expression. Thus, we
undertook such a study and developed a fast growing whole animal
MDR tumor model with high level of P-gp expression.
Aside from the biological characteristics of tumor
cells themselves, the tumor microenvironment contributes greatly to
tumor growth and drug resistance (8). Fibroblasts, the most numerous and most
important cells found in the tumor microenvironment, could affect
tumor cell morphology, adhesion, proliferation and signaling by
both structural and chemical means (6,9).
Tumor-associated fibroblast (TAF) cells have been found to play a
critical role in regulating the growth of adjacent tumor cells and
vein endothelial cells via different growth factors such as VEGF,
HGF and BFGF. TAF cells promote tumorigenesis, and tumor cells that
fail to recruit fibroblasts develop slowly (9,10).
Some groups have employed fibroblasts to accelerate the growth rate
of tumors by co-inoculation with cancer cell lines MCF-RAS, human
PC-3 and MDA-436 (7,11). Solid tumor growth depends greatly on
the formation of the stroma. Without mesenchyme, a tumor <2 mm
in length cannot continue to grow (12).
Previous studies in our laboratory had already found
highly significant expression of TAF cells within the harvested
KB-8-5 tumor through the immunofluorescence of α-smooth muscle
actin (α-SMA) in the tumor parafin sections and paclitaxel (Taxol)
had no effect on the inhibition of tumor growth (unpublished data).
Consequently, we hypothesize the in vivo growth of resistant
tumor cells with high P-gp expression in nude mice depends on the
TAF cells to a certain extent. We investigated the correlations
among tumorgenesis, tumor cell growth profile in vitro, P-gp
expression levels and fibroblast distribution profiles in tumor
sections, and then develop a new in vivo MDR tumor mouse
model by co-inoculating with resistant tumor cells and NIH/3T3
fibroblasts, which may be useful for the screening of
antineoplastic agents and mechanistic studies of factors regulating
tumor growth and progression.
Materials and methods
Materials
Taxol injection was manufactured by Hospira, Inc.
(Lake Forest, IL, USA). gemcitabine was purchased from
Sigma-Aldrich (St. Louis, MO, USA). Antibodies against P-gp, α-SMA
and GAPDH, horseradish peroxidase-conjugated anti-mouse and
anti-rabbit whole IgG for western blot and fluorescence-conjugated
anti-mouse and anti-rabbit whole IgG for immunofluorescence assay,
were obtained from Santa Cruz Biotechnology (San Diego, CA, USA).
Matrigel™ Basement Membrane Matrix was purchased from BD
Biosciences (San Jose, CA, USA).
Sensitive KB-3-1 cell line (human mouth epidermal
carcinoma cells) and the P-gp-overexpressing NCI/ADR-RES cell line
(human ovarian carcinoma cells) were obtained from the National
Cancer Institute (Bethesda, MD, USA). Resistant KB-8-5 cells were
separated from KB-3-1 cells. H460/Tax-R cell line (non-small lung
carcinoma cells) was obtained from Dr Bingliang Fang at the MD
Anderson Cancer Center (Houston, TX, USA). NIH/3T3 fibroblast cells
(mouse embryonic fibroblast cell line) were originally obtained
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). Cells were maintained in RPMI-1640 or Dulbecco's modified
eagle's medium (DMEM) containing 10% fetal bovine serum (FBS), 100
U/ml penicillin and 100 µg/ml streptomycin (all from
Invitrogen, Carlsbad, CA, USA). Cells were cultivated at 37°C with
5% Co2 in a humidified incubator.
Nude mice were purchased from the National Cancer
Institute. All experiments performed on animals were in accordance
with and approved by the Institutional Animal Care and Use
Committee at the University of North Carolina at Chapel Hill.
Western blot analysis
For in vitro preparations adhered cells in
culture dishes were washed with ice-cold PBS, scraped off the dish
using a cold plastic cell scraper, and the suspension gently
transferred to a pre-cooled Eppendorf tube. For in vitro
studies the tumor-bearing mice were sacrificed and the tumors were
removed. Whole cell suspensions or tumor lysates were extracted
with RIPA buffer (~10 mg of tumor was mixed with 100 µl RIPA
buffer), and the concentration of protein was quantified using a
Pierce BCA Protein Assay kit (Thermo Scientific Inc., Rockford, IL,
USA). Approximately 50 µg of protein from each sample was
separated on a NuGAGE 12% SDS-polyacrylamine gel, and then
transferred to a polyvinylidene difluoride (PVDF) membrane
(Bio-Rad, Hercules, CA, USA). The membrane was blocked with 5%
skimmed milk in PBS for 1 h. After incubation with primary antibody
at 4°C overnight, the PVDF membrane was washed with PBST (0.2%
Tween-80 in PBS), and then incubated with secondary antibody for 1
h at room temperature. Antibodies against P-gp, α-SMA and GAPDH
were used at 1:2,000 dilutions. A HRP-conjugated anti-mouse
antibody at a dilution of 1:10,000 or an HRP-conjugated anti-rabbit
Igg at a dilution of 1:2,000 served as the secondary antibodies in
the experiment. The specific protein bands were visualized using a
chemiluminescence kit (Pierce, Rockford, IL, USA). Chemiluminiscent
signals were detected with the high-performance chemiluminescence
film (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA).
Targeted quantitative proteomic analysis
for P-gp determination
Samples were prepared by modification of previously
published targeted quantitative methods of ours (13,14).
Briefly, cells (~10 million), to which 0.5 ml 50 mM ammonium
bicarbonate had been added, were homogenized in a 2-ml glass
homogenizer (Wheaton, Millville, NJ, USA). Following centrifugation
at 16,000 × g for 10 min the supernatant was discarded and the
pellet solubilized, with further homogenization, in 0.5 ml of 1%
sodium deoxycholate. The homogenate was centrifuged again at 16,000
× g for 10 min and the pellet discarded. Total protein
concentration of the supernatant was measured using the Pierce BCA
Protein Assay kit (Thermo Scientific Inc.).
Membrane samples (25 µg/replicate) were
analyzed in duplicate. β-casein (0.5 µg, from bovine milk;
Sigma-Aldrich) was added as a general indicator of successful
digestion. Samples were denatured with heat, reduced with
dithiothreitol and carbamidomethylated with iodoacetamide. Trypsin
solution (0.01 µg/µl) was added to give a cell
protein to trypsin ratio of 20:1 and samples were digested at 37°C
for 4 h (pH ~8; 50 mM ammonium bicarbonate buffer), shaking at 300
rpm. The reaction was stopped by addition of 10% trifluoroacetic
acid, the amount added being 10% of the total reaction volume.
Following centrifugation at 13,400 × g for 5 min the supernatant
was treated with solid phase extraction (C18 cartridges, Strata-X,
33u, polymeric, 10 mg/ml; Phenomenex, Torrance, CA, USA). The
eluate was evaporated, reconstituted and analyzed by nanoUPLC-MS/MS
as previously described (13,14).
Two of the proteotypic P-gp tryptic peptides
targeted in the nano-UPLC-MS/MS analysis were
I368IDNKPSIDSYSK380 and
I896ATeAIeNFR905. Two MRMs were acquired per
peptide and peak areas were used to compare samples. The two MRM
areas were averaged and then the areas for the duplicates were
averaged. Results for peptides were compared between cell
samples.
Cell proliferation assay
Four types of tumor cell lines, namely KB-3-1,
KB-8-5, H460/Tax-R and NCI/ADR-RES cells, were seeded in 6-well
plates at a density of 40,000 cells/well and incubated at
atmospheric pressure at 37°C with 5 % Co2. over the
following week, cells in each well were harvested and counted using
a cell counting plate. The cell number was plotted against
time.
Tumor cell colony formation assay
To perform the tumor cell colony formation assay,
1.2 and 0.7% agarose solutions were first prepared. The solutions
were melted in a microwave oven and cooled to 40°C in a water bath
before use. An equal volume of 1.2% agarose was mixed with 2x
RPMI-1640 containing 20% FBS and antibiotics to give base agarose.
One milliliter of this mixture was added to each well of a 6-well
plate and allowed to solidify for 5 min. Following this, 1 ml of
0.7% agarose, mixed with 2x RPMI-1640 and 2,500 cells, was plated
gently onto the surface of the base agarose. 1x RPMI-1640 (0.5 ml)
of medium was then added to each well and the plate was incubated
at 37°C for 15 days. Cells were washed 1–2 times/week with cell
culture medium. At day 15, cell colonies were photographed after
staining with 0.5 ml of 0.005% crystal violet for 15 min.
Mouse xenograft model development and
tumor growth
Female nude mice (6–8 weeks) were used in all
studies. The mice were divided into 6 groups, each receiving the
following cells via subcutaneous injection into their right or left
flanks: i), KB-3-1 cells (5×106); ii), KB-8-5 cells
(5×106); iii), H460/Tax-R cells (5×106); iv),
NCI/ADR-RES cells (5×106); v), H460/Tax-R
(5×106) and NIH/3T3 cells (2.5×106); and vi),
NCI/ADR-RES (5×106) and NIH/3T3 cells
(2.5×106). Cells of group v and vi were mixed with
Matrigel™ Basement Membrane Matrix at a ratio of 2:1 (w/w) before
injection. Images of the mice were taken with a digital camera 16
days after tumor cell inoculation.
Sensitivity of the MDR tumor model to
chemotherapy
Tumor sensitivity to chemotherapy was evaluated in
the NCI/ADR-RES and H460/Tax-R cell-bearing nude mouse models. Once
the tumor mass in the xenograft was established, mice were randomly
divided into 3 groups (5 mice/group) and were injected, in the tail
vein, with normal saline (the control group), Taxol or gemcitabine.
Drug doses of 10 mg/kg PTX and 9 mg/kg gemcitabine were used for
all treatments. Therapy was continued at days 3, 5, 7 and 9 (5
doses in total). Mice were sacrificed when the tumor reached 2 cm
in length, and tumor volumes were calculated using the following
equation: (length × width2)/2.
Immunofluorescence assay
Harvested tumors were fixed in 4.0% paraformaldehyde
(PFA), paraffin-embedded, and sectioned at the UNC Lineberger
Comprehensive Cancer Center Animal Histopathology Facility. For
immunofluores-cence of P-gp and α-SMA, slides were subjected to
block by BSA after deparaffinization, dehydration and antigen
retrieval. Slides were then incubated with primary antibodies
(diluted by 1% BSA) at 4°C overnight and with secondary antibodies
at room temperature for 1 h. Nuclei were fluorescently stained with
4′,6-diamidino-2-phenylindole (DAPI) Vectashield (Vector
Laboratories, Inc., Burlingame, CA, USA). Finally, all slides were
photographed at 20-fold magnification. The primary antibodies for
P-gp and α-SMA were diluted 200-fold and the secondary antibodies
100-fold.
Results and Discussion
Tumorigenesis of various tumor cell lines
in vivo
KB-3-1, KB-8-5, H460/Tax-R and NCI/ADR-RES are the
four frequently used cell lines in tumor research. However, their
tumor growth characteristics in vivo vary greatly. As shown
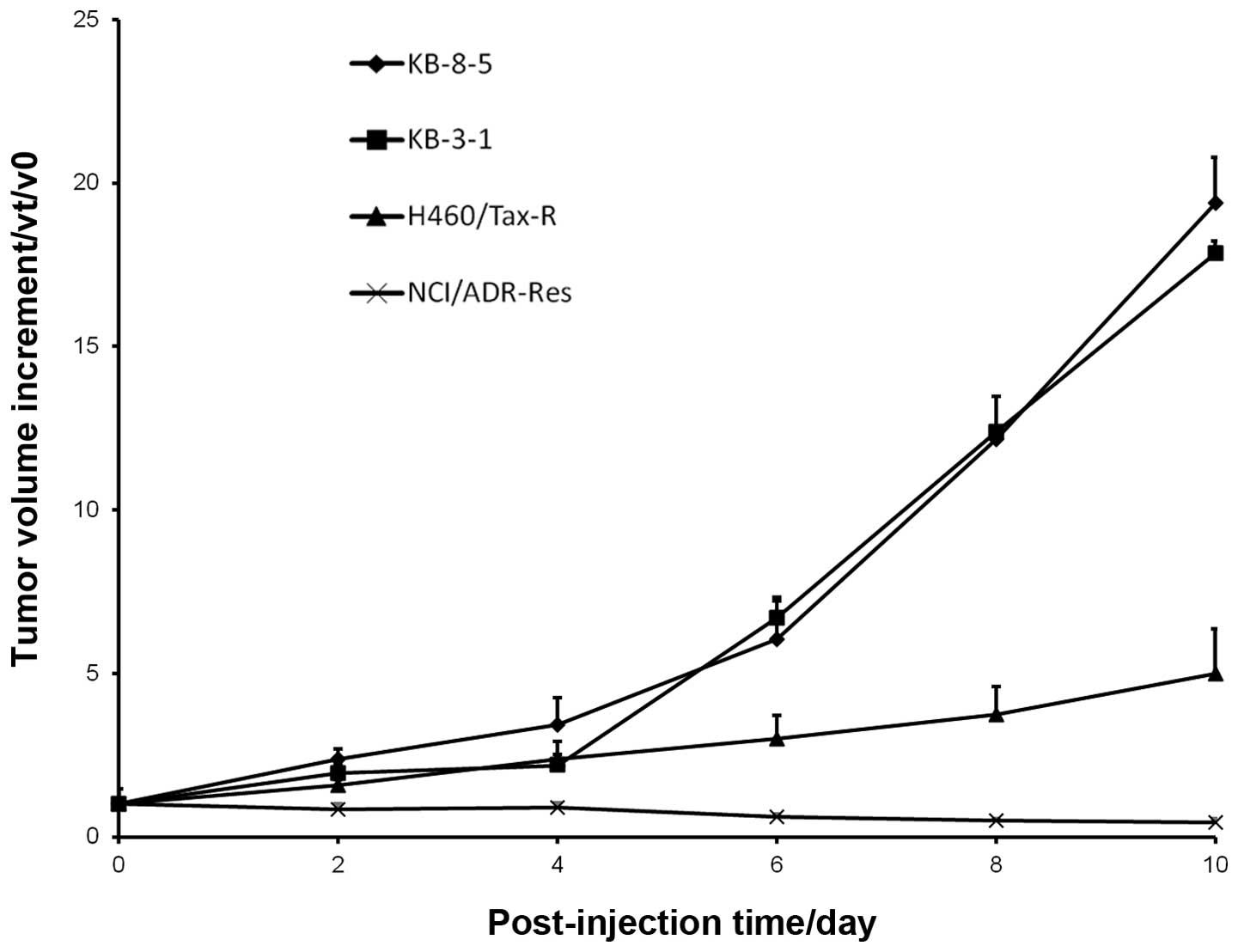
in Fig. 1, obvious differences in
tumorigenesis were observed in nude mice received subcutaneous
inoculation with one of the four types of cell line. The graph in
Fig. 1 shows that KB-3-1 and KB-8-5
grew much faster than H460/Tax-R and NCI/ADR-RES in vivo.
The tumor volume increment of KB-8-5 was about 5- and 56-fold
compared with that of H460/Tax-R and NCI/ADR-RES. It suggests that
KB-3-1 and KB-8-5 are the most tumorigenic of the cells. only a
'tumor dot' was observed in the mouse that was injected with
H460/Tax-R cells and no tumor was observed in the NCI/ADR-RES-
bearing mouse.
Correlations among cell proliferation,
colony formation, P-gp expression and tumorigenesis in vitro and in
vivo
To explore the reasons why tumorigenesis differs
among tumor cell types, we firstly compared the growth rate and
colony formation ability of the cell lines in vitro. As
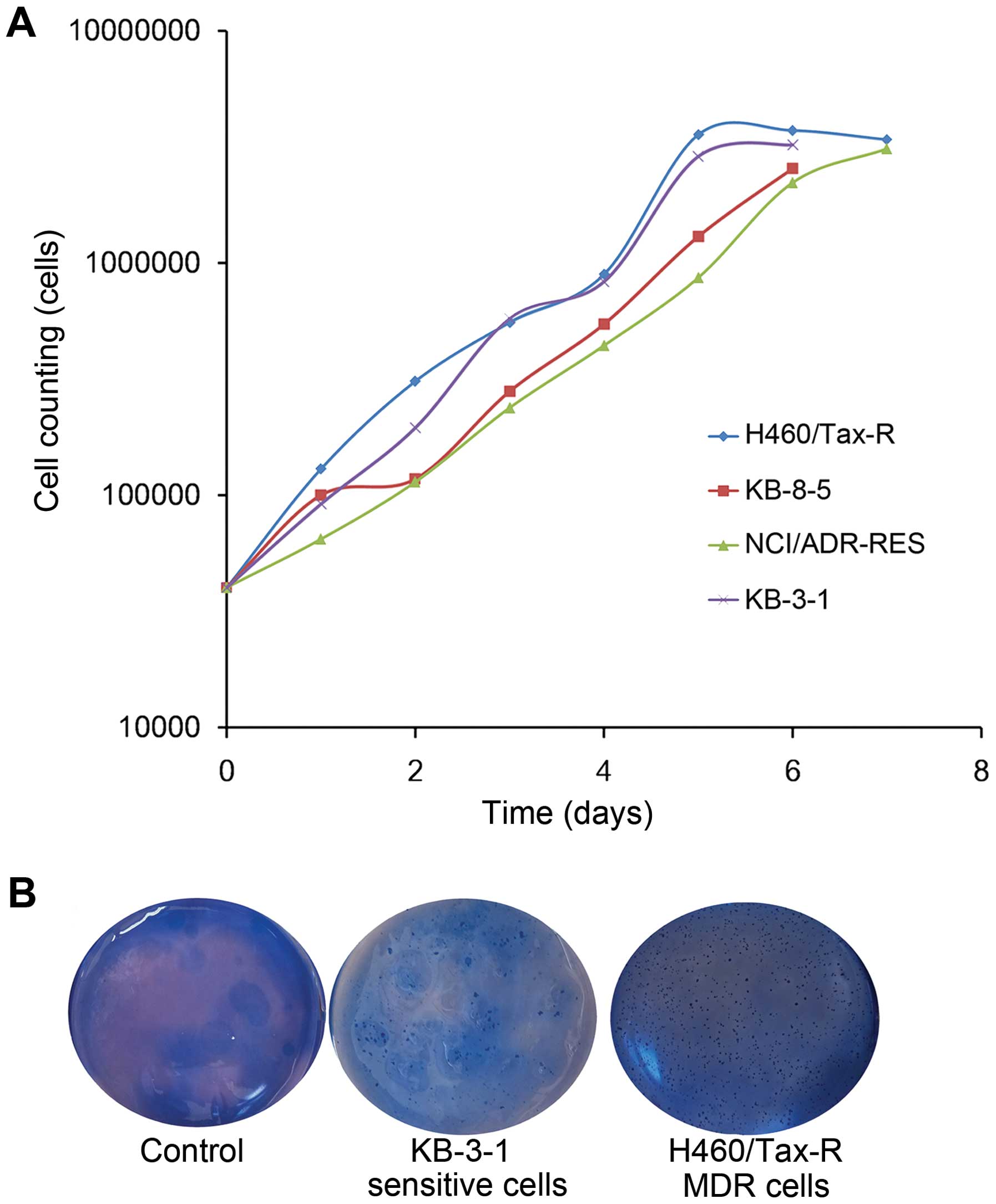
shown in Fig. 2A, comparable rates
of tumor cell proliferation was observed in plates regardless of
their cell type and sensitivity. All of the tumor cells grew
quickly in vitro and maintained proliferation activity.
The colony formation assay is an in vitro
cell survival assay based on the ability of a single cell to grow
into a colony, reflecting the possible malignant properties of
tumor cells. Correlations between tumor cell colony formation and
tumor growth in vivo have previously been reported (15,16).
In our comparisons, both of drug sensitive KB-3-1 cells and the
drug resistant H460/Tax-R cells preferred colony formation
(Fig. 2), but these two types of
cells presented completely different growth rate in the xenograft
mouse (Fig. 1). Hitherto, there
existed no correlation between colony formation and tumor growth
in vivo.
In the mouse xenograft models we found the growth of
drug resistant tumors (e.g., NCI/ADR-ReS and H460/Tax-R) to be slow
(Fig. 1). In order to characterize
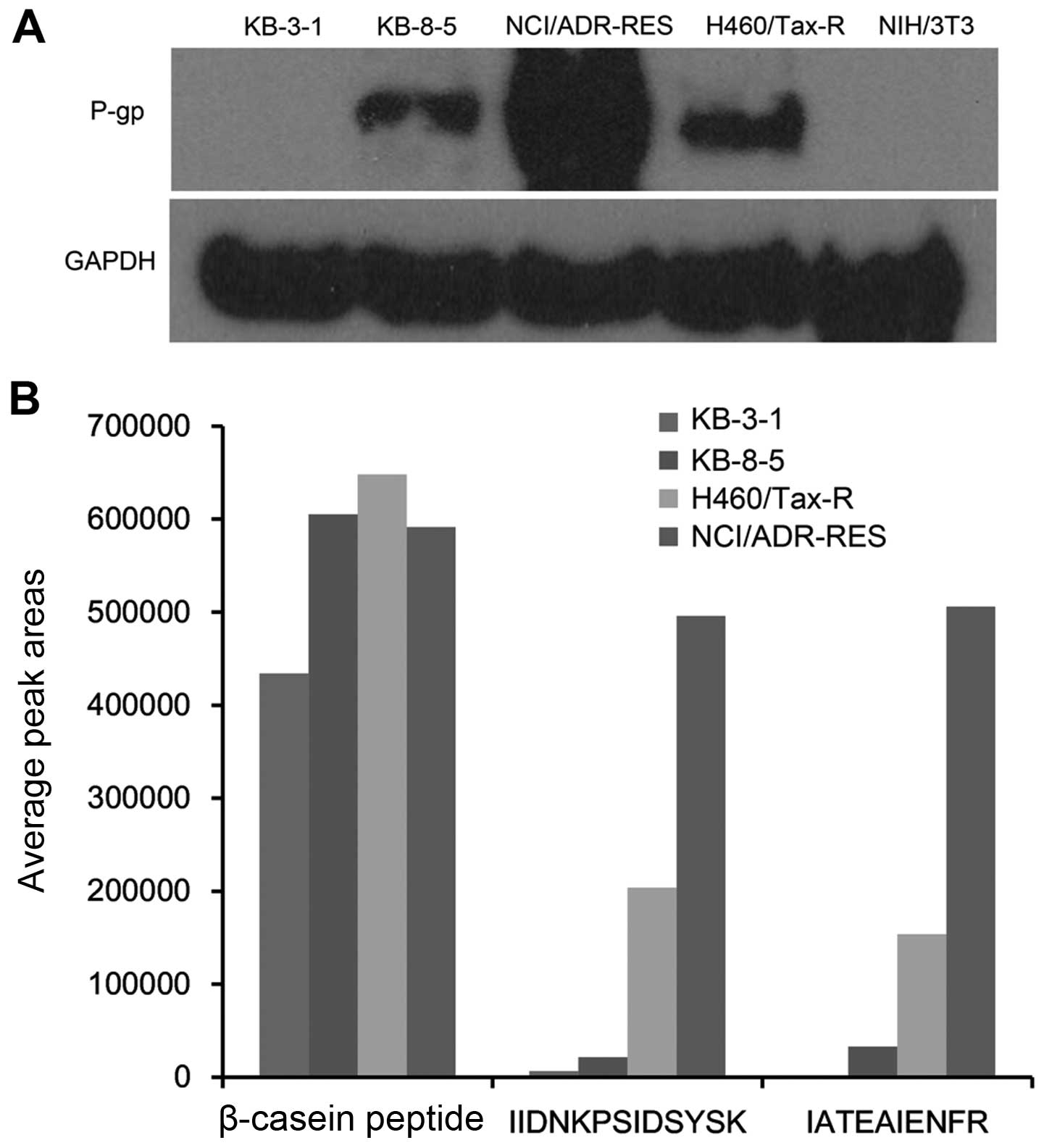
the drug resistance we measured P-gp expression in different cell
lines qualitatively using western blot analysis and nanoUPLC-MS/MS.
As shown in Fig. 3, both methods
showed the same trend of P-gp expression in the cell lines:
NCI/ADR-RES > H460/Tax-R > KB-8-5 > KB-3-1. Slight P-gp
expression was seen in KB-3-1 cells, which are sensitive to Taxol
(17). More P-gp expression was
observed in the other three resistant cell types (17). It followed that cells containing
high P-gp levels (Fig. 3) showed
low tumorigenesis in vivo (Fig.
1). It was therefore suggested that NIH/3T3 fibroblasts without
P-gp expression (Fig. 3) would be
tested as auxiliary cells for development of a resistant in
vivo tumor model.
Analysis of tissue section of the low
tumorigenesis tumor (H460/Tax-R)
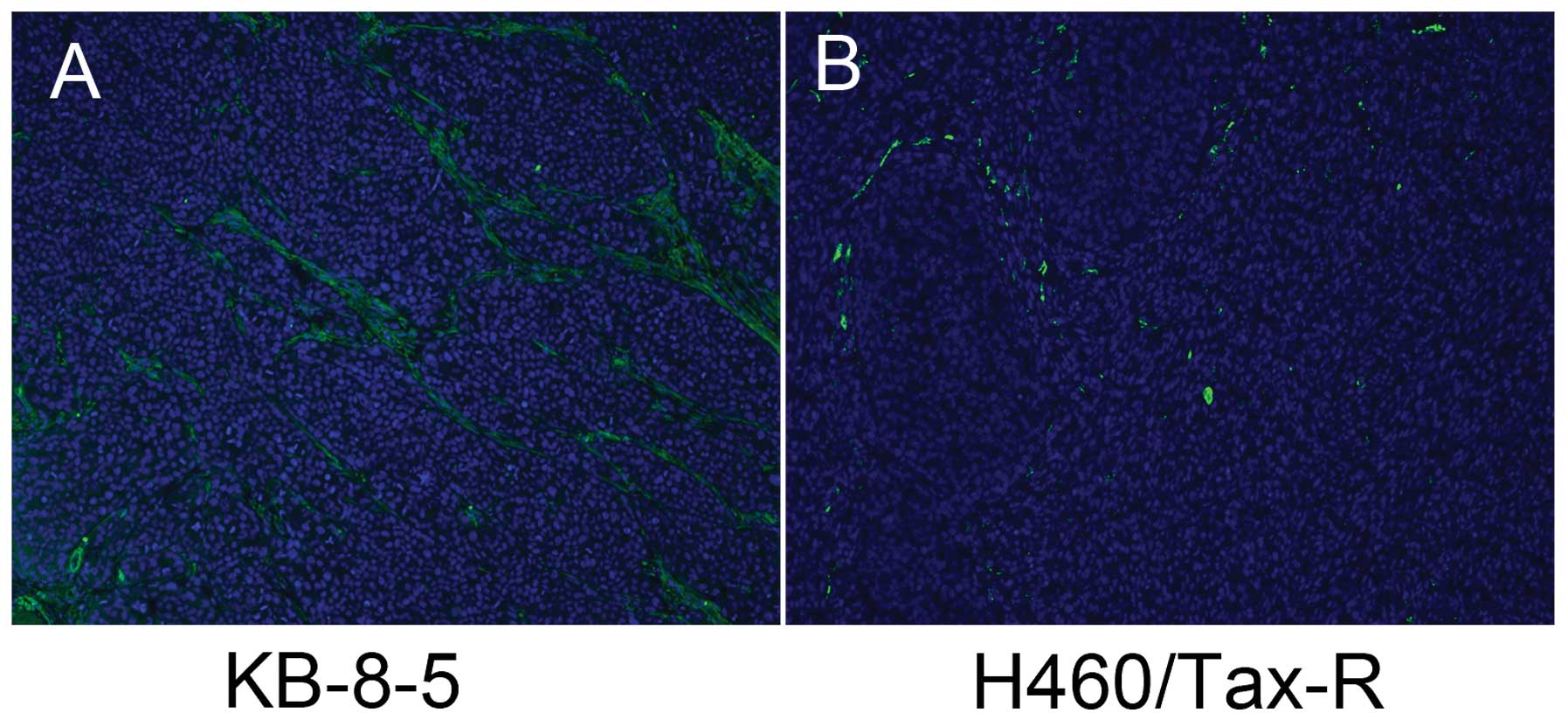
To further study the apparent negative correlation
between high P-gp levels and tumorigenesis, we assayed H460/Tax-R
tumor tissue sections by immunofluorescence staining. Staining for
α-SMA was performed because its expression is the major
morphological characteristic of myofibroblasts which contribute
greatly to tumor stroma and tumor growth in vivo (18). As shown in Fig. 4, abundant TAFs in the fast growing
KB-8-5 tumor, and few TAFs in the H460/Tax-R tumor were observed,
which had been a 'tumor dot' in the mouse xenograft model (Fig. 1). The strong effect of TAFs on tumor
growth has been widely reported (7,19). We
believe that tumor cells in vivo containing high levels of
P-gp have difficulty in recruiting TAFs, resulting in a lack of
structural and chemical support necessary for tumor progression
(20). We therefore decided to
develop and test an MDR xenograft mouse model produced by
co-inoculation with tumor cells and fibroblast cells.
Establishment and evaluation of a new
mouse xenograft model
As expected, the MDR tumor model was successfully
produced by co-inoculation with NIH/3T3 fibroblast (TAF) cells and
H460/Tax-R or NCI/ADR-RES cells (Fig.
5B and D). In our preliminary experiments, different ratios of
tumor cells to NIH/3T3 cells (2:1 or 1:1) were tested but no
obvious differences in tumor growth were observed. Moreover, the
NIH/3T3 cells did not form a tumor after being injected
subcutaneously. All the results suggested that improvement of
tumorigenesis is mainly due to modulation of the tumor by the
fibroblasts and not growth of the fibroblasts themselves.
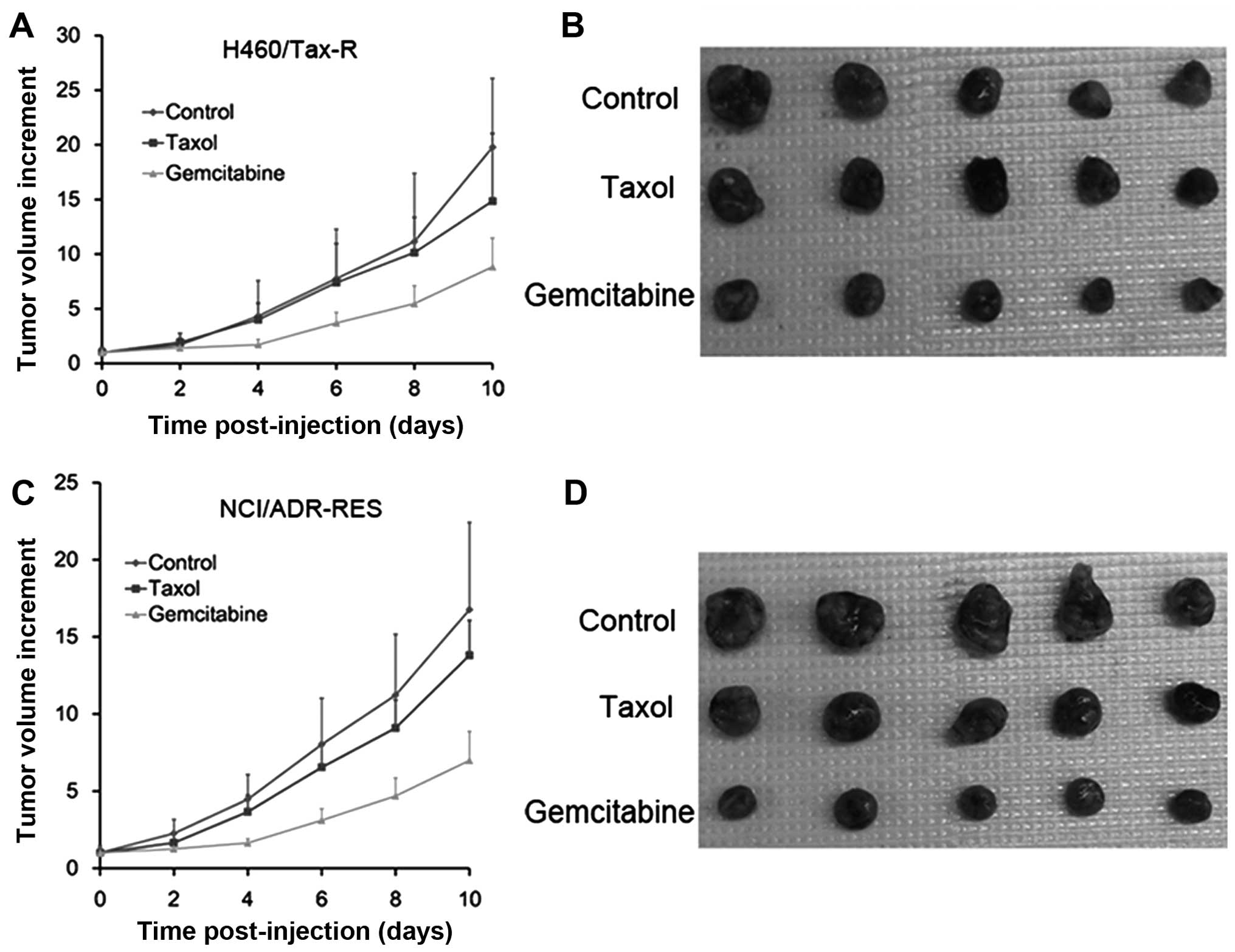
Tumor sensitivity to chemotherapy was investigated
in the developed H460/Tax-R and NCI/ADR-RES models. Taxol, a P-gp
substrate, was chosen as the tumor resistant test agent and
gemcitabine as the tumor sensitive agent (21–23).
As shown in Fig. 5, Taxol exerted
little effect on tumor growth for both cell types, indicating the
successful establishment of the mouse xenograft model modulated by
TAF cells. The observed anticancer activity of gemcitabine ruled
out the possibility that the poor response to Taxol was due to the
change in microenvironment of the tumors caused by the TAF cells.
It is well known that the weak response to PTx is caused by the
efflux of P-gp.
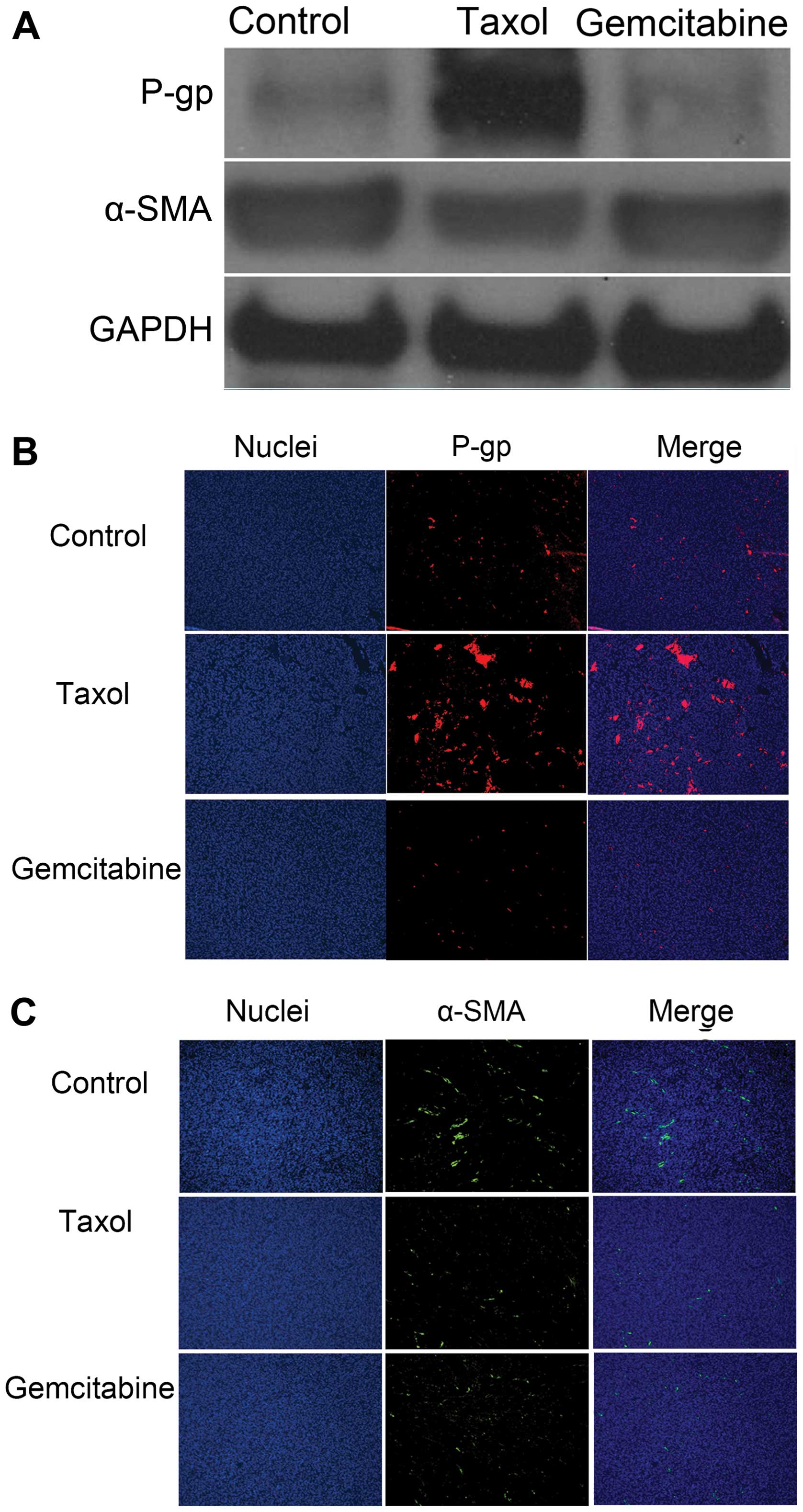
To further evaluate the applicability of the novel
MDR tumor model, P-gp and α-SMA levels in tumor tissues were
determined by western blot and immunofluorescence staining
analysis. As shown in Fig. 6B, P-gp
expression increased in the MDR tumor after Taxol treatment. This
is consistent with previous studies that P-gp expression can be
induced by Taxol (24). Higher P-gp
levels in the Taxol-treated group confirm that the novel MDR model
was successfully developed. In contrast, gemcitabine, to which MDR
tumors are sensitive, did not increase P-gp expression in the model
(Fig. 6B). The slightly higher
level of α-SMA expression were observed in the tumors treated with
Taxol compared to the control tumors. We supposed that the
resistance of Taxol may be related to the increased TAF besides the
efflux pump of P-gp. Further research is needed in our subsequent
study.
Overall, no antitumor effect was observed after
Taxol treatment, mainly because the drug could not kill the MDR
tumor cells or the fibroblasts, evident from an increase in P-gp
levels and no obvious change in α-SMA expression when compared to
the untreated group. In contrast, for gemcitabine, a significant
decrease in tumor size and little change in α-SMA expression
suggested that the gemcitabine acted mainly on the tumor cells.
Therefore, without being affected by the added fibroblasts, the new
human MDR mouse xenograft model should serve as a useful tool to
evaluate the antitumor activity of drugs.
In conclusion, in the present study we investigated
the common problems of developing a mouse xenograft model using
tumor cell lines that highly express P-gp and exhibit low
tumorigenesis in vivo.
Failure to recruit fibroblasts in vivo for
tumor cells containing high levels of P-gp lies in less structural
and chemical support during tumor progression. A new mouse
xenograft model applicable to MDR research has been successfully
developed by co-inoculating with tumor cells (NCI/ADR-RES or
H460/Tax-R) and fibroblast cells. The new MDR model is resistant to
Taxol treatment, which affects neither the tumor cells nor the
fibroblasts. In contrast, gemcitabine, to which MDR tumors are not
resistant, inhibits tumor growth by killing tumor cells but exerts
no obvious influence on fibroblasts. Fibroblasts in this tumor
model therefore would not disturb application of the model in the
research of MDR. The new mouse xenograft MDR model should serve as
a useful tool to evaluate the antitumor effect of drugs.
Acknowledgments
This study was supported by the National Cancer
Institute grant (5R01CA149387) and the National Nature Science
Foundation of China (no. 81403109) and the Youth elite Project of
guangzhou University of Chinese Medicine (no. QNYC20140107).
Notes
[1]
Dedication
This study was dedicated to the memory of Professor
Feng Liu, 1955–2014, University of North Carolina at Chapel
Hill.
References
|
1
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Borst P, Jonkers J and Rottenberg S: What
makes tumors multidrug resistant? Cell Cycle. 6:2782–2787. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gillet JP, Calcagno AM, Varma S, Marino M,
Green LJ, Vora MI, Patel C, Orina JN, Eliseeva TA, Singal V, et al:
Redefining the relevance of established cancer cell lines to the
study of mechanisms of clinical anti-cancer drug resistance. Proc
Natl Acad Sci USA. 108:18708–18713. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Correia AL and Bissell MJ: The tumor
microenvironment is a dominant force in multidrug resistance. Drug
Resist Updat. 15:39–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grinnell F, Rocha LB, Iucu C, Rhee S and
Jiang H: Nested collagen matrices: A new model to study migration
of human fibroblast populations in three dimensions. Exp Cell Res.
312:86–94. 2006.
|
|
6
|
Green JA and Yamada KM: Three-dimensional
microenvironments modulate fibroblast signaling responses. Adv Drug
Deliv Rev. 59:1293–1298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flach EH, Rebecca VW, Herlyn M, Smalley KS
and Anderson AR: Fibroblasts contribute to melanoma tumor growth
and drug resistance. Mol Pharm. 8:2039–2049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cirri P and Chiarugi P:
Cancer-associated-fibroblasts and tumour cells: A diabolic liaison
driving cancer progression. Cancer Metastasis Rev. 31:195–208.
2012. View Article : Google Scholar
|
|
10
|
Dong J, Grunstein J, Tejada M, Peale F,
Frantz G, Liang WC, Bai W, Yu L, Kowalski J, Liang X, et al:
VEGF-null cells require PDGFR alpha signaling-mediated stromal
fibroblast recruitment for tumorigenesis. EMBO J. 23:2800–2810.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Camps JL, Chang SM, Hsu TC, Freeman MR,
Hong SJ, Zhau He, von Eschenbach AC and Chung LW:
Fibroblast-mediated acceleration of human epithelial tumor growth
in vivo. Proc Natl Acad Sci USA. 87:75–79. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Folkman J: Tumor angiogenesis and tissue
factor. Nat Med. 2:167–168. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fallon JK, Neubert H, Hyland R, Goosen TC
and Smith PC: Targeted quantitative proteomics for the analysis of
14 UGT1As and -2Bs in human liver using NanoUPLC-MS/MS with
selected reaction monitoring. J Proteome Res. 12:4402–4413. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Higgins JW, Bao JQ, Ke AB, Manro JR,
Fallon JK, Smith PC and Zamek-Gliszczynski MJ: Utility of
Oatp1a/1b-knockout and OATP1B1/3-humanized mice in the study of
OATP-mediated pharmacokinetics and tissue distribution: Case
studies with pravastatin, atorvastatin, simvastatin, and
carboxydichlorofluorescein. Drug Metab Dispos. 42:182–192. 2014.
View Article : Google Scholar
|
|
15
|
Shin SI, Freedman VH, Risser R and Pollack
R: Tumorigenicity of virus-transformed cells in nude mice is
correlated specifically with anchorage independent growth in vitro.
Proc Natl Acad Sci USA. 72:4435–4439. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Colburn NH, Bruegge WF, Bates JR, gray RH,
Rossen JD, Kelsey WH and Shimada T: Correlation of
anchorage-independent growth with tumorigenicity of chemically
transformed mouse epidermal cells. Cancer Res. 38:624–634.
1978.PubMed/NCBI
|
|
17
|
Chung HC, Rha SY, Kim JH, Roh JK, Min JS,
Lee KS, Kim BS and Lee KB: P-glycoprotein: The intermediate end
point of drug response to induction chemotherapy in locally
advanced breast cancer. Breast Cancer Res Treat. 42:65–72. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hinz B, Celetta G, Tomasek JJ, Gabbiani G
and Chaponnier C: Alpha-smooth muscle actin expression upregulates
fibroblast contractile activity. Mol Biol Cell. 12:2730–2741. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ostman A and Augsten M: Cancer-associated
fibroblasts and tumor growth - bystanders turning into key players.
Curr Opin Genet Dev. 19:67–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishii G, Sangai T, Ito T, Hasebe T, Endoh
Y, Sasaki H, Harigaya K and Ochiai A: In vivo and in vitro
characterization of human fibroblasts recruited selectively into
human cancer stroma. Int J Cancer. 117:212–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Casazza AM and Fairchild CR: Paclitaxel
(Taxol): Mechanisms of resistance. Cancer Treat Res. 87:149–171.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Davidson JD, Ma L, Flagella M, Geeganage
S, Gelbert LM and Slapak CA: An increase in the expression of
ribonucleotide reductase large subunit 1 is associated with
gemcitabine resistance in non-small cell lung cancer cell lines.
Cancer Res. 64:3761–3766. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bergman AM, Pinedo HM, Talianidis I,
Veerman G, Loves WJ, van der Wilt CL and Peters GJ: Increased
sensitivity to gemcitabine of P-glycoprotein and multidrug
resistance-associated protein-overexpressing human cancer cell
lines. Br J Cancer. 88:1963–1970. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yusuf RZ, Duan Z, Lamendola DE, Penson RT
and Seiden MV: Paclitaxel resistance: Molecular mechanisms and
pharmacologic manipulation. Curr Cancer Drug Targets. 3:1–19. 2003.
View Article : Google Scholar : PubMed/NCBI
|