Introduction
Lung cancer is the most deadly malignant tumor
(1). There are two major types,
non-small cell lung cancer (NSCLC) and small-cell lung cancer
(SCLC) which comprise 85 and 15% of all cases, respectively.
Tumor-initiating cells, also termed cancer stem cells (CSCs), are a
rare population of cancer cells which possess the ability to
self-renew and differentiate (2).
CSCs were first isolated in 1997 in leukemia (3). Subsequently, CSCs have been identified
in solid tumors, including breast (4), brain (5), prostate (6), melanoma (7), colon (8,9) and
lung cancer (10,11). Since the isolation of CSCs in 1997,
a growing body of literature has demonstrated that tumor
initiation, recurrence and drug resistance are highly related to
CSCs (12–14). Thus, CSCs is likely a plausible
target for cancer therapy (15).
CSCs were identified using flow cytometry-based cell sorting of
specific surface markers or tumor sphere forming assay. Tumor
spheres, which could reflect tumor cell stem-like property, are
multicellular three-dimensional clones enriching CSCs (2,3).
Suppressing tumor spheres may impair cancer cell stem-like
property.
The JAK2/STAT3 pathway mediates the effects of a
spectrum of cytokines and growth factors. It can be transiently
activated in normal cells upon stimulation while in cancer cells,
it is constitutively active (16,17).
JAK2/STAT3 signaling pathway plays a crucial role in various cancer
initiation and development stages, and active STAT3 is commonly
associated with a worse prognosis (18–21).
It is also reported that JAK2/STAT3 signaling pathway is involved
in CSCs in many malignant, including lung CSCs and aberrant
expression of JAK2/STAT3 signaling pathway in CSCs can promote
cancer initiation (22–24). Thus, JAK2/STAT3 pathway could be a
targetable pathway for CSC elimination.
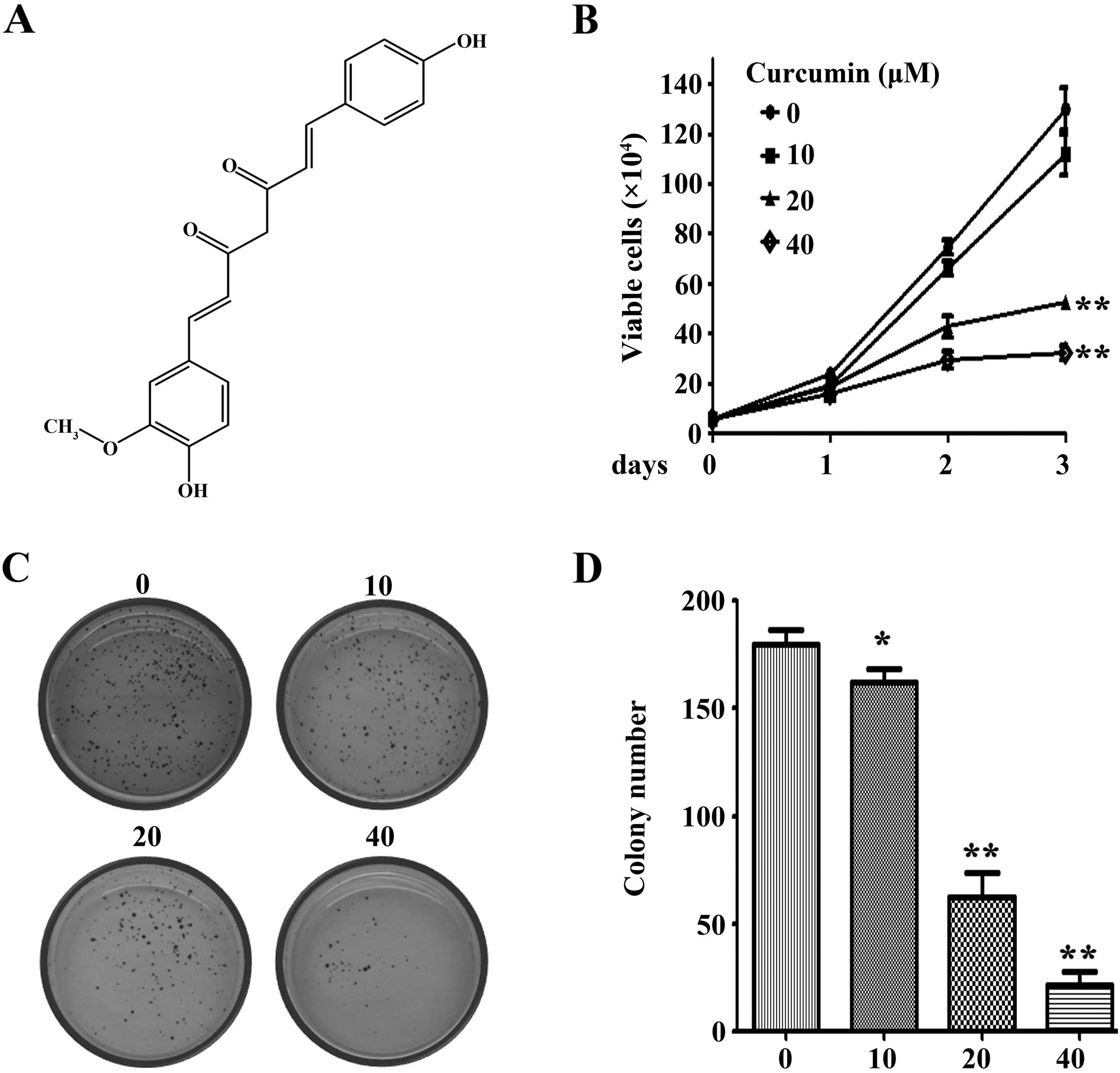
Curcumin (diferuloylmethane, Fig. 1A), a phenolic compound isolated from
the plant Curcuma longa, has been used in traditional
medicines in China for thousands of years. It exhibits anticancer
effects by induction of growth inhibition, cell cycle arrest and
apoptosis in various types of cancer (25–28).
It is also reported that curcumin effectively prevented emergence
of chemoresistance and eliminate CSCs in breast, glioblastoma,
pancreatic and colon cancer (29–33).
The antiproliferation effects of curcumin on lung cancer have been
reported to induce apoptosis in A549 and NCI-H460 cells through ER
stress and caspase cascade- and mitochondria-dependent pathways
(34–36), however, the effects of curcumin on
lung cancer stem-like cells remain obscure. In the present study,
we investigated the underlying mechanisms of curcumin on lung tumor
spheres.
Materials and methods
Cell culture and reagents
The lung cancer cell line NCI-H460 was purchased
from American Type Culture Collection (ATCC) and cultured in
RPMI-1640 supplemented with 10% fetal bovine serum (FBS). Curcumin
and Taxol were purchased from Sigma. MTT was purchased from
Amresco. The antibodies used in the present study were as follows:
anti-β-actin (A5441; Sigma); p-JAK2 (#3776), JAK2 (#3230), p-STAT3
(#9138), STAT3 (#4904) (all from CST); C-myc (sc-40; Santa Cruz),
anti-myc (#MA1-21316-D680; Invitrogen), cyclin D1 (ab16663; Abcam).
The growth factors used in the present study were: N2 supplement
(Gibco), FGF, EGF (R&D Systems), and STAT3 siRNA (#6582;
CST).
Cell viability and clonogenic assay
For cell viability assay, 8×104 cells
were seeded into 12-well plates. Then, different concentrations of
curcumin and dimethyl sulfoxide (DMSO) were added into plates for
indicated times. Viable cells were counted using trypan blue dye
exclusion analysis. For the clonogenic assay, NCI-H460 cells were
suspended in 1 ml RPMI-1640 containing 0.3% low melting point
agarose (Amresco) and 10% FBS, and plated on a bottom layer
containing 0.6% agarose and different doses of curcumin in 35 mm
plates (1,000 cells/plate). Ten days later, cells were stained with
0.005% crystal violet and clones >50 cells were counted.
Tumor sphere assay
NCI-H460 cells were cultured in tumor sphere medium
consisting of serum-free DMEM/F12 medium, 10 ng/ml human
recombinant fibroblast growth factor-basic (FGF), 10 ng/ml
epidermal growth factor (EGF), and N2 supplement. Then cells
suspended in sphere medium containing different doses of curcumin
and Taxol were plated into ultra low-attachment 24 well-plate at a
density of 1,000/well. Five days later, tumor sphere formation was
observed and photographed by a microscope.
Western blotting assay
NCI-H460 cells were suspended in tumor sphere medium
to form tumor spheres. Then medium was replaced with fresh tumor
sphere medium containing different doses of curcumin and DMSO and
tumor spheres were cultured for indicated time points. Cell lysates
of tumor spheres treated with curcumin or DMSO at indicated times
or different doses were subjected to SDS-PAGE to detect related
protein expression.
STAT3 overexpression and RNAi
knockdown
STAT3 was overexpressed by transient transfection
with pcDNA3.0-myc-STAT3 plasmids in H460 cells (vector pcDNA3.0-myc
as control). To downregulate STAT3 expression in NCI-H460 cells,
specific stat3 RNAi (CST) was used. After transfection, cells were
suspended in tumor sphere medium in ultra low attachment 24-well
plates. One week later, the formed tumor spheres were photographed
under a microscope.
Murine models
All animal studies were conducted according to the
protocols approved by the Animal Ethics Committee of Guangxi
Medical University and maintained under conventional conditions.
NCI-H460 derived tumor spheres were digested into single cells and
injected into each mouse (100 cells for each) by subcutaneously
inoculation in the right flank of nude mice. When tumor volume
reached 50 mm3, mice were randomized into 3 groups (n=5
for each group) and treated with curcumin (40 mg/kg), Taxol (5
mg/kg) or vehicle control (polyoxyethylenated castor oil:
ethanol=1:1) for 2 weeks every 2 days with intraperitoneal
injection (i.p). Tumor growth and mouse body weight were monitored
every other day for 15 days. Tumor volume was calculated using the
formula: V = 0.5 × L × W2, where L and W represented the
long and short diameter of the tumor, respectively. At the time of
the animal sacrifice, tumors were excised; cells were separated and
lyzed for western blotting.
Molecular docking
Computational docking test was performed using
MOE2008.10 (Molecular Operating Environment) to investigate the
interaction between JAK2 and curcumin at a molecular level. X-ray
crystal structures of JAK2 (PDB ID: 5AEP) and its ligand was
obtained from Protein Data Bank (http://www.rcsb.org). Water molecules were manually
removed from the protein structures. Docking process was as
described previously (39).
Statistical analysis
Differences between data groups were evaluated using
Student's t-test. P-values <0.05 were considered to indicate a
statistically significant result. Data are presented as the mean ±
SD unless otherwise noted.
Results
Curcumin inhibits proliferation and
colony formation of NCI-H460 lung cancer cells
The effects of curcumin on lung cancer cell
proliferation was determined using trypan blue dye exclusion
analysis. The results demonstrated that curcumin significantly
inhibited the growth of NCI-H460 cells at 20 and 40 µM
(Fig. 1B). The soft agar assay
showed that curcumin markedly reduced colony number of NCI-H460
cells in a dose-dependent manner (Fig.
1C and D). Curcumin significantly inhibited the colony forming
activity of NCI-H460 cells at a concentration of 10 µM.
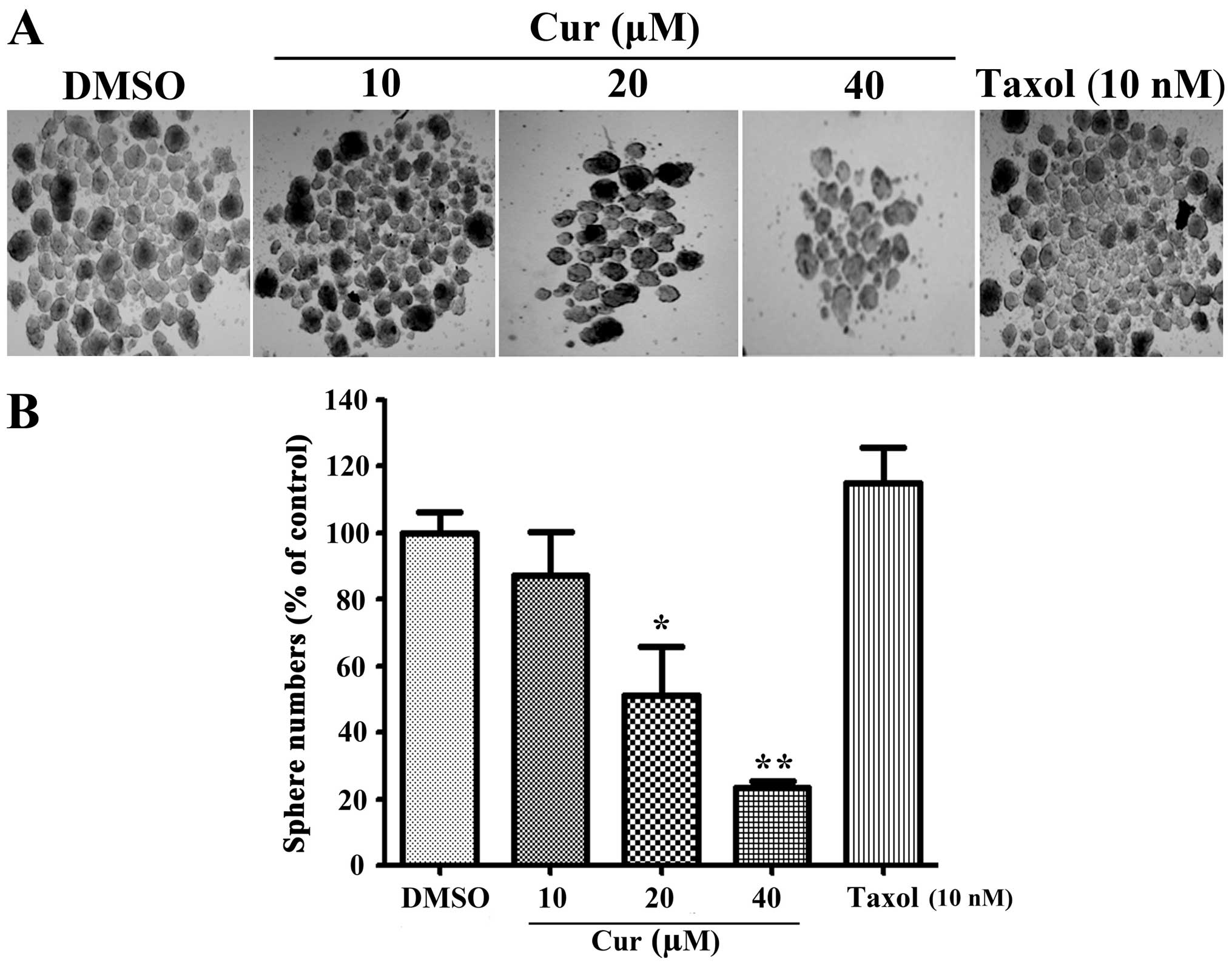
Curcumin impairs tumor sphere formation
of NCI-H460 lung cancer cells in vitro
To determine the effects of curcumin on lung tumor
spheres, we used NCI-H460 lung cancer cells as a in vitro
model and carried out tumor sphere assays. The results suggested
that curcumin reduced NCI-H460 tumor spheres in a dose-dependent
manner (Fig. 2A). Compared with the
traditional chemotherapy drug Taxol, which could not decrease tumor
spheres (37), curcumin
significantly reduced tumor spheres at concentrations of 20 and 40
µM (Fig. 2B). These data
implied curcumin suppressed tumor spheres growth in
vitro.
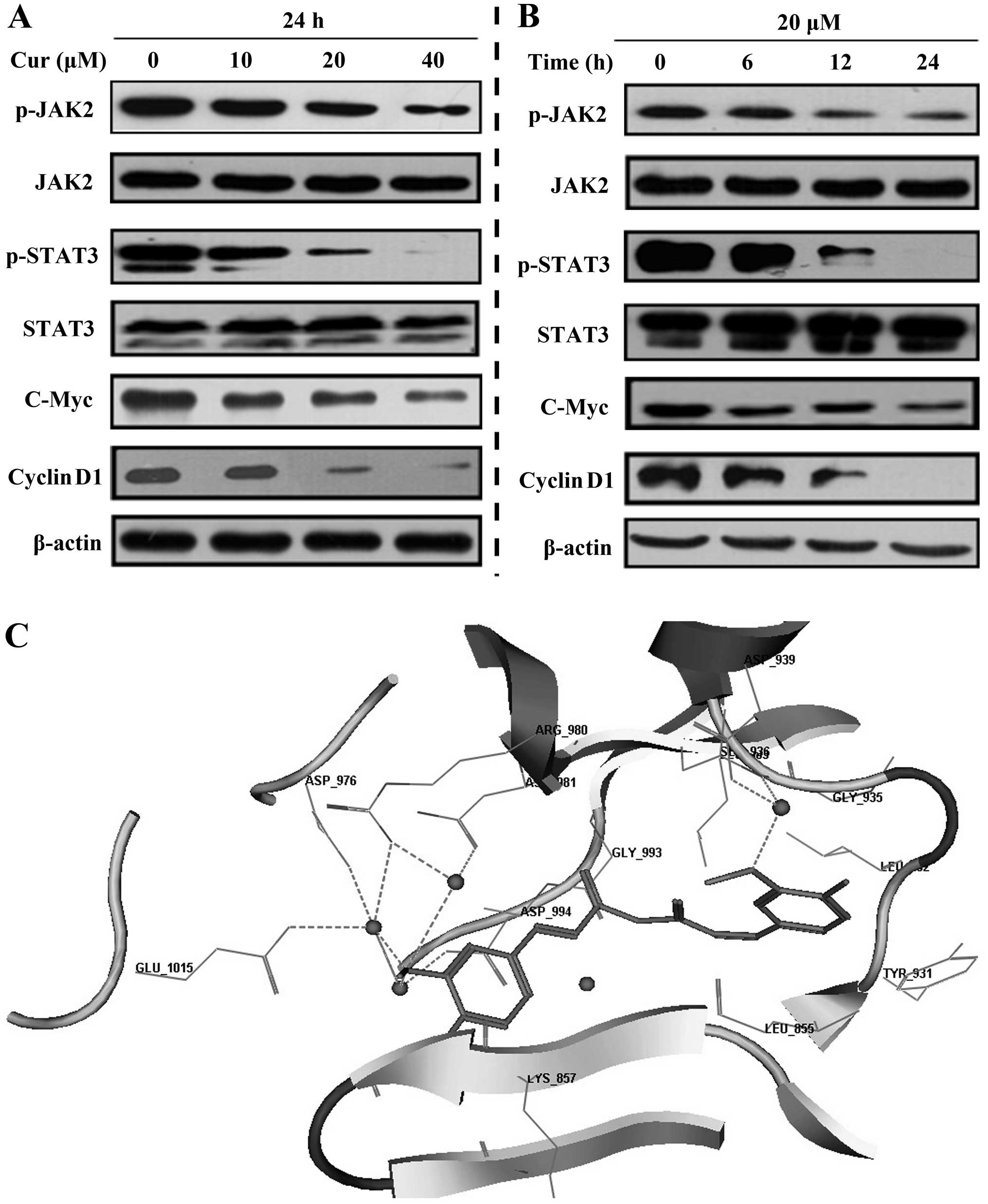
Curcumin inhibits JAK2/STAT3 signaling
pathway activity
Considering the significant role of JAK2/STAT3
signaling pathway in lung CSCs development (23,38),
we carried out western blotting to detect whether curcumin
disturbed the JAK2/STAT3 signaling pathway. The results show that
under curcumin treatment, p-JAK2 and p-STAT3 were downregulated in
a time- and dose-dependent manner. The downstream molecules of the
JAK2/STAT3 pathway, cyclin D1 and C-myc were also downregulated
(Fig. 3A and B). Moreover, by
molecular docking analysis (39),
we discovered that curcumin could potently bind to JAK2. The
three-dimensional structure revealed that curcumin can be perfectly
embedded into the long and narrow hydrophobic pocket formed by
Leu_855, Leu_983, Gly_858, Asp_994 and Gly_935 of JAK2 (Fig. 3C). The methoxyl group of curcumin
formed hydrogen bonds with the side chains of Asp_976, Asp_994,
Glu_1015, Arg_980 and Asn_981 of JAK2 through water molecules
(Fig. 3A). These results inferred
that curcumin attenuated the JAK2/STAT3 pathway.
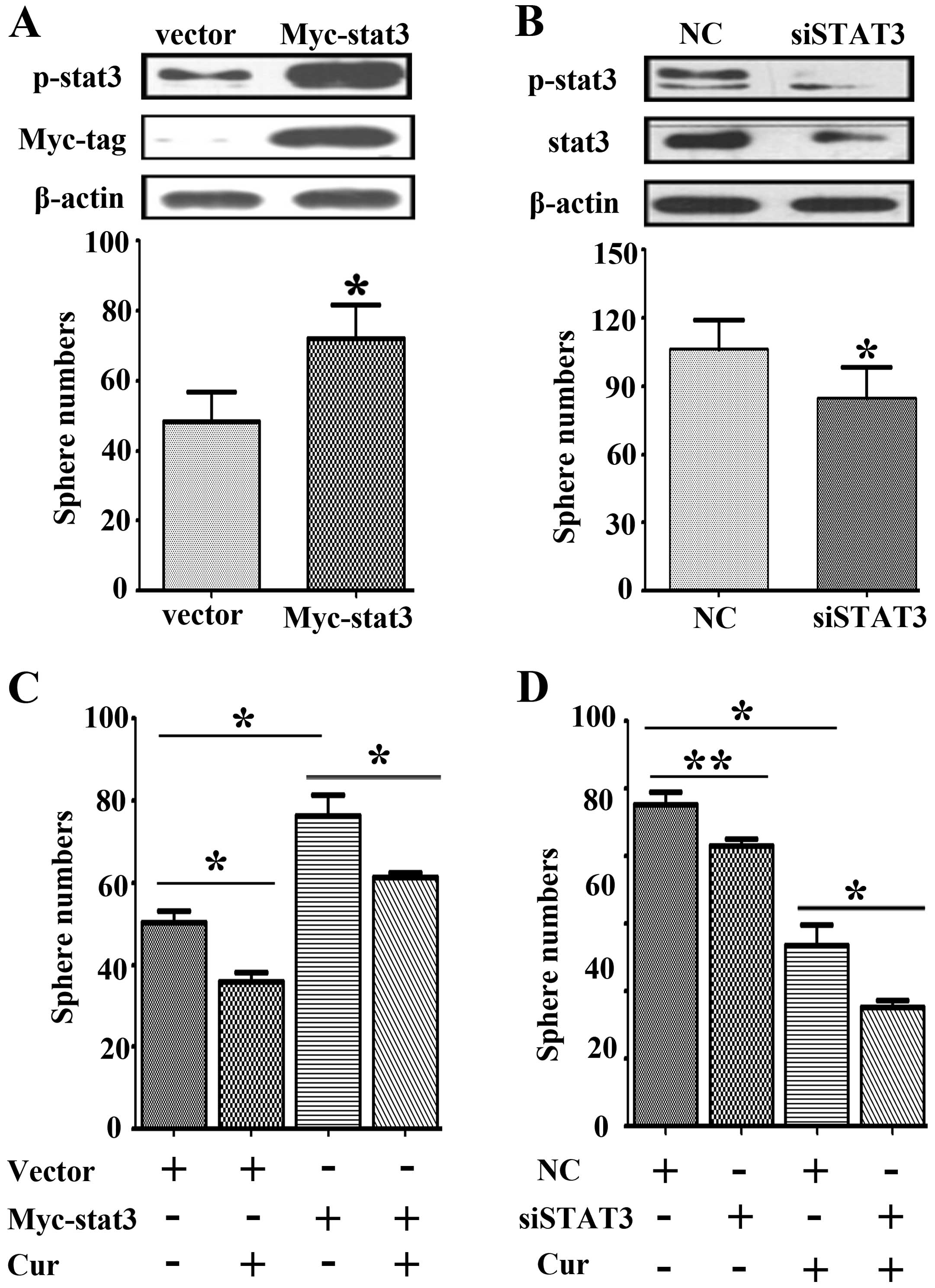
The role of STAT3 in curcumin-induced
tumor sphere suppression
To further clarify JAK2/STAT3 activity in
curcumin-induced tumor sphere suppression, we first evaluated STAT3
activity in NCI-H460 tumor sphere formation. STAT3 overexpressing
and knockdown assays were carried out using plasmids and siRNA. We
found when overexpressing STAT3, not only the number but the size
of tumor spheres were increased (Fig.
4A). Conversely, when STAT3 was knoced down, both the number
and size of tumor spheres were decreased (Fig. 4B). These results indicate that
active stat3 promotes tumor sphere formation of NCI-H460 lung
cancer cells. Next, by stat3 overexpression and curcumin
co-treatment, stat3 overexpression can restore curcumin-induced
tumor sphere inhibition (Fig. 4C).
Further, stat3 knockdown and curcumin treatment can synergistically
inhibit tumor spheres of NCI-H460 lung cancer cells (Fig. 4D). Taken together, these results
inferred curcumin could reduce tumor spheres via inhibiting the
JAK2/STAT3 signaling pathway.
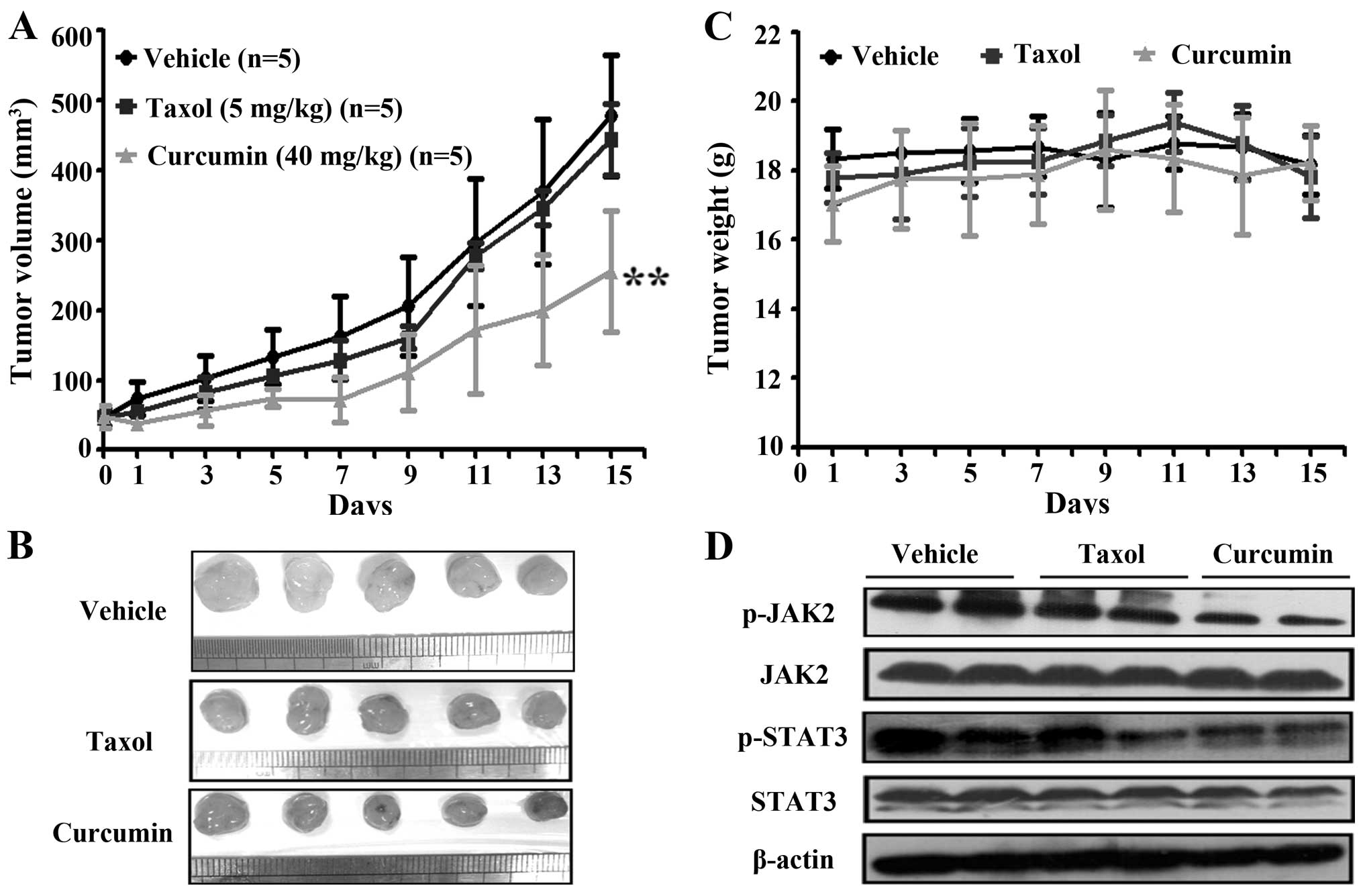
Curcumin inhibits tumor spheres growth of
NCI-H460 lung cancer cells in vivo
We next assessed whether curcumin could inhibit
stem-like tumor growth in vivo. NCI-H460 derived tumor
spheres were digested into single cells and subcutaneously
inoculated into nude mice in the right flank area (100 cells for
each mouse). When the tumors reached 50 mm3, the mice
were randomized into 3 groups (n=5 for each group). The mice were
administered curumin (40 mg/kg) or Taxol (5 mg/kg) every 2 days
with intraperitoneal injection (i.p). Tumor growth and mice body
weight were monitored every other day for 15 days. Intriguingly, we
found that curcumin significantly inhibited tumor growth compared
with vehicle control or Taxol (P<0.01) (Fig. 5A and B). Moreover, body weight loss
was not observed in curcumin-treated groups compared to vehicle and
Taxol groups (Fig. 5C). Proteins
were extracted from tumor samples and western blot assay was
performed. We found that in curcumin treatment group, the
expression of p-JAK2 and p-STAT3 were downregulated as compared
with the samples separated from vehicle or Taxol control mice
(Fig. 5D). These results
demonstrate that curcumin suppressed tumor sphere growth in
vivo.
Discussion
Tumor recurrence and drug resistance are the primary
causes of poor survival rates in patients with advanced cancer.
Since the isolation of CSCs in 1997, substantial research has
demonstrated CSCs are highly related to tumor recurrence and drug
resistance (12–14). Thus, eliminating CSCs may be a
feasible approach for cancer therapy (40). Previous research showed that tumor
spheres enrich cells with CSC-like property and are capable of
forming tumors in vivo (41,42).
Unlike the main population of cancer cells, CSC-like cells can form
tumor spheres in non-adherent conditions and serum-free medium
(43). Tumor sphere-forming assay
has its own advantage, as it does not rely on specific markers to
identify CSCs, which could vary greatly from one cell line to
another (44). Curcumin has been
reported to be an effective drug by preventing emergence of
chemoresistance and eliminating CSCs in breast, glioblastoma,
pancreatic and colon cancer (29–33).
To date, the effects and molecular mechanisms of curcumin on lung
CSCs still remain unclear. In the present study, we performed tumor
sphere assays to determine the effects of curcumin on lung CSCs and
found curcumin impaired the ability of tumor spheres in NCI-H460
lung cancer cells (Fig. 2A and B).
Moreover, in the in vivo nude mouse model, 100 cancer
stem-like cells derived from NCI-H460 tumor spheres were injected
into each mouse and tumors formed. Curcumin strongly repressed
tumor growth compared to vehicle or Taxol treatment groups
(Fig. 5A and B). These results
indicated that curcumin suppressed tumor sphere growth of NCI-H460
lung cancer cells in vitro and in vivo.
Attempts to eliminate CSCs by targeting relevant
signaling pathways are being carried out in several preclinical
studies. In lung CSCs, interference with Wnt/β-catenin signaling by
RNAi markedly decreased cancer cell proliferation, clone formation
and drug resistance (45). PTEN,
Hedgehog, JAK-STAT, Notch and PI3K/AKT pathways also offer latent
intervention targets against CSCs (46,47).
In the present study, by molecule docking we found curcumin
interacted with the residues of JAK2 by forming hydrogen bonds
though water molecules (Fig. 3C).
We assumed that curcumin could inhibit JAK2 activity and repress
the JAK2/STAT3 signaling pathway. Western blotting results
confirmed our hypothesis. Under curcumin treatment, the activity of
JAK2/STAT3 pathway was inhibited in vitro and in vivo
(Figs. 3A and B, and 5D). Further, to elucidate the role of
STAT3 in tumor sphere formation of NCI-H460 lung cancer cells, we
conducted an overexpressing and RNAi knockdown assay. By
transfection with STAT3 overexpression plasmid or STAT3 siRNA, we
found STAT3 activation promotes tumor sphere formation, while STAT3
inhibition suppresses this effect (Fig.
4A and B). Furthermore, by stat3 overexpression and curcumin
co-treatment, stat3 overexpression restored curcumin-induced tumor
sphere inhibition, and stat3 knockdown with curcumin could
synergistically inhibit tumor sphere formatioin (Fig. 4C and D). Taken together, our results
infer that curcumin repressed tumor spheres of NCI-H460 lung cancer
cells by inhibiting the JAK2/STAT3 signaling pathway.
In conclusion, we have documented the antitumor
sphere formation effects of curcumin in vitro and in
vivo. Our findings highlighted the fact that curcumin could
inhibit lung cancer cell proliferation, colony formation and tumor
spheres. The underlying mechanisms of curcumin-induced tumor
spheres suppression are mainly due to the inhibition of the
JAK2/STAT3 signaling pathway. The present study implies that
curcumin may be a potential drug in lung CSC elimination and cancer
therapy.
Acknowledgments
The present study was financially supported by the
Natural Science Foundation of Zhejiang Province of China (no.
Y12H300003), the Postdoctoral Science Foundation of China and the
Postdoctoral Science Foundation of Guangxi Province of China. We
thank Professor Hu Tingjun of School of Zoology Science and
Technology of Guangxi University for the experimental support.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
6
|
Miki J, Furusato B, Li H, Gu Y, Takahashi
H, Egawa S, Sesterhenn IA, McLeod DG, Srivastava S and Rhim JS:
Identification of putative stem cell markers, CD133 and CXCR4, in
hTERT-immortalized primary nonmalignant and malignant tumor-derived
human prostate epithelial cell lines and in prostate cancer
specimens. Cancer Res. 67:3153–3161. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang D, Nguyen TK, Leishear K, Finko R,
Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE and Herlyn M: A
tumorigenic subpopulation with stem cell properties in melanomas.
Cancer Res. 65:9328–9337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar
|
|
9
|
O'Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar
|
|
10
|
Kim CF, Jackson EL, Woolfenden AE,
Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT and Jacks T:
Identification of bronchioalveolar stem cells in normal lung and
lung cancer. Cell. 121:823–835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bertolini G, Roz L, Perego P, Tortoreto M,
Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S,
et al: Highly tumorigenic lung cancer CD133+ cells
display stem-like features and are spared by cisplatin treatment.
Proc Natl Acad Sci USA. 106:16281–16286. 2009. View Article : Google Scholar
|
|
12
|
Vinogradov S and Wei X: Cancer stem cells
and drug resistance: The potential of nanomedicine. Nanomedicine.
7:597–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen K, Huang YH and Chen JL:
Understanding and targeting cancer stem cells: Therapeutic
implications and challenges. Acta Pharmacol Sin. 34:732–740. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eyler CE and Rich JN: Survival of the
fittest: Cancer stem cells in therapeutic resistance and
angiogenesis. J Clin Oncol. 26:2839–2845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shigdar S, Lin J, Li Y, Yang CJ, Wei M,
Zhus Y, Liu H and Duan W: Cancer stem cell targeting: The next
generation of cancer therapy and molecular imaging. Ther Deliv.
3:227–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sansone P and Bromberg J: Targeting the
interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol.
30:1005–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Moretti L, Giacalone NJ, Schleicher
S, Speirs CK, Carbone DP and Lu B: Inhibition of JAK2 signaling by
TG101209 enhances radiotherapy in lung cancer models. J Thorac
Oncol. 6:699–706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colomiere M, Ward AC, Riley C, Trenerry
MK, Cameron-Smith D, Findlay J, Ackland L and Ahmed N: Cross talk
of signals between EGFR and IL-6R through JAK2/STAT3 mediate
epithelial-mesenchymal transition in ovarian carcinomas. Br J
Cancer. 100:134–144. 2009. View Article : Google Scholar :
|
|
19
|
Behera R, Kumar V, Lohite K, Karnik S and
Kundu GC: Activation of JAK2/STAT3 signaling by osteopontin
promotes tumor growth in human breast cancer cells. Carcinogenesis.
31:192–200. 2010. View Article : Google Scholar
|
|
20
|
Zhao M, Gao FH, Wang JY, Liu F, Yuan HH,
Zhang WY and Jiang B: JAK2/STAT3 signaling pathway activation
mediates tumor angiogenesis by upregulation of VEGF and bFGF in
non-small-cell lung cancer. Lung Cancer. 73:366–374. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan DS, Agarwal R and Kaye SB: Mechanisms
of transcoelomic metastasis in ovarian cancer. Lancet Oncol.
7:925–934. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abubaker K, Luwor RB, Zhu H, McNally O,
Quinn MA, Burns CJ, Thompson EW, Findlay JK and Ahmed N: Inhibition
of the JAK2/STAT3 pathway in ovarian cancer results in the loss of
cancer stem cell-like characteristics and a reduced tumor burden.
BMC Cancer. 14:3172014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsu HS, Lin JH, Hsu TW, Su K, Wang CW,
Yang KY, Chiou SH and Hung SC: Mesenchymal stem cells enhance lung
cancer initiation through activation of IL-6/JAK2/STAT3 pathway.
Lung Cancer. 75:167–177. 2012. View Article : Google Scholar
|
|
24
|
Marotta LL, Almendro V, Marusyk A,
Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ,
Choudhury SA, Maruyama R, et al: The JAK2/STAT3 signaling pathway
is required for growth of CD44+CD24− stem
cell-like breast cancer cells in human tumors. J Clin Invest.
121:2723–2735. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chauhan DP: Chemotherapeutic potential of
curcumin for colorectal cancer. Curr Pharm Des. 8:1695–1706. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leu TH and Maa MC: The molecular
mechanisms for the anti-tumorigenic effect of curcumin. Curr Med
Chem Anticancer Agents. 2:357–370. 2002. View Article : Google Scholar
|
|
27
|
Karunagaran D, Rashmi R and Kumar TR:
Induction of apoptosis by curcumin and its implications for cancer
therapy. Curr Cancer Drug Targets. 5:117–129. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duvoix A, Blasius R, Delhalle S,
Schnekenburger M, Morceau F, Henry E, Dicato M and Diederich M:
Chemopreventive and therapeutic effects of curcumin. Cancer Lett.
223:181–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fong D, Yeh A, Naftalovich R, Choi TH and
Chan MM: Curcumin inhibits the side population (SP) phenotype of
the rat C6 glioma cell line: Towards targeting of cancer stem cells
with phytochemicals. Cancer Lett. 293:65–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kakarala M, Brenner DE, Korkaya H, Cheng
C, Tazi K, Ginestier C, Liu S, Dontu G and Wicha MS: Targeting
breast stem cells with the cancer preventive compounds curcumin and
piperine. Breast Cancer Res Treat. 122:777–785. 2010. View Article : Google Scholar
|
|
31
|
Lim KJ, Bisht S, Bar EE, Maitra A and
Eberhart CG: A polymeric nanoparticle formulation of curcumin
inhibits growth, clonogenicity and stem-like fraction in malignant
brain tumors. Cancer Biol Ther. 11:464–473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin L, Liu Y, Li H, Li PK, Fuchs J,
Shibata H, Iwabuchi Y and Lin J: Targeting colon cancer stem cells
using a new curcumin analogue, GO-Y030. Br J Cancer. 105:212–220.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bao B, Ali S, Banerjee S, Wang Z, Logna F,
Azmi AS, Kong D, Ahmad A, Li Y, Padhye S, et al: Curcumin analogue
CDF inhibits pancreatic tumor growth by switching on suppressor
microRNAs and attenuating EZH2 expression. Cancer Res. 72:335–345.
2012. View Article : Google Scholar
|
|
34
|
Radhakrishna Pillai G, Srivastava AS,
Hassanein TI, Chauhan DP and Carrier E: Induction of apoptosis in
human lung cancer cells by curcumin. Cancer Lett. 208:163–170.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin SS, Huang HP, Yang JS, Wu JY, Hsia TC,
Lin CC, Lin CW, Kuo CL, Gibson Wood W and Chung JG: DNA damage and
endoplasmic reticulum stress mediated curcumin-induced cell cycle
arrest and apoptosis in human lung carcinoma A-549 cells through
the activation caspases cascade- and mitochondrial-dependent
pathway. Cancer Lett. 272:77–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu SH, Hang LW, Yang JS, Chen HY, Lin HY,
Chiang JH, Lu CC, Yang JL, Lai TY, Ko YC, et al: Curcumin induces
apoptosis in human non-small cell lung cancer NCI-H460 cells
through ER stress and caspase cascade- and mitochondria-dependent
pathways. Anticancer Res. 30:2125–2133. 2010.PubMed/NCBI
|
|
37
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shao C, Sullivan JP, Girard L, Augustyn A,
Yenerall P, Rodriguez-Canales J, Liu H, Behrens C, Shay JW, Wistuba
II, et al: Essential role of aldehyde dehydrogenase 1A3 for the
maintenance of non-small cell lung cancer stem cells is associated
with the STAT3 pathway. Clin Cancer Res. 20:4154–4166. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Padilla F, Bhagirath N, Chen S, Chiao E,
Goldstein DM, Hermann JC, Hsu J, Kennedy-Smith JJ, Kuglstatter A,
Liao C, et al: Pyrrolopyrazines as selective spleen tyrosine kinase
inhibitors. J Med Chem. 56:1677–1692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li RJ, Ying X, Zhang Y, Ju RJ, Wang XX,
Yao HJ, Men Y, Tian W, Yu Y, Zhang L, et al: All-trans retinoic
acid stealth liposomes prevent the relapse of breast cancer arising
from the cancer stem cells. J Control Release. 149:281–291. 2011.
View Article : Google Scholar
|
|
41
|
Grimshaw MJ, Cooper L, Papazisis K,
Coleman JA, Bohnenkamp HR, Chiapero-Stanke L, Taylor-Papadimitriou
J and Burchell JM: Mammosphere culture of metastatic breast cancer
cells enriches for tumorigenic breast cancer cells. Breast Cancer
Res. 10:R522008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cioce M, Gherardi S, Viglietto G, Strano
S, Blandino G, Muti P and Ciliberto G: Mammosphere-forming cells
from breast cancer cell lines as a tool for the identification of
CSC-like- and early progenitor-targeting drugs. Cell Cycle.
9:2878–2887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park SY, Lee HE, Li H, Shipitsin M, Gelman
R and Polyak K: Heterogeneity for stem cell-related markers
according to tumor subtype and histologic stage in breast cancer.
Clin Cancer Res. 16:876–887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Teng Y, Wang X, Wang Y and Ma D:
Wnt/beta-catenin signaling regulates cancer stem cells in lung
cancer A549 cells. Biochem Biophys Res Commun. 392:373–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yilmaz OH, Valdez R, Theisen BK, Guo W,
Ferguson DO, Wu H and Morrison SJ: Pten dependence distinguishes
haematopoietic stem cells from leukaemia-initiating cells. Nature.
441:475–482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin L, Fuchs J, Li C, Olson V, Bekaii-Saab
T and Lin J: STAT3 signaling pathway is necessary for cell survival
and tumorsphere forming capacity in
ALDH+/CD133+ stem cell-like human colon
cancer cells. Biochem Biophys Res Commun. 416:246–251. 2011.
View Article : Google Scholar : PubMed/NCBI
|