Introduction
Cancer is the second cause of mortality, with colon
cancer being the second most lethal cause of cancer in women and
the third in men, worldwide (1).
Approximately 102,900 cases were diagnosed with and 51,370 patients
succumbed to causes associated with colon and rectal cancer
combined in the United States in 2010 (2). Previous findings showed that colon
cancer is a multifactorial and multistep disease and is associated
with the mutations of many specific genes and the alterations of
epigenetic and non-genotoxic factors (3). Thus, gaining know ledge on the
specific molecular markers is crucial for the diagnosis and
treatment of this disease. One of the factors involved in
understanding the mechanism of tumor is the activity of oncogenes
or tumor suppressors (4).
Alteration of oncogenes or tumor-suppressor activity often occurs
at the critical points of the development of colon cancer (4). For example, cytokines from the TNF
family have potent inflammatory activities and play an important
role in cancer development by regulating apoptosis in colon cancer
(5). Chen et al reported
that the tumor suppressor p53 promotes mitochondrial DNA base
excision to regulate the development of colon cancer (6). However, the molecular mechanism of
colon cancer development and progression remains unknown.
For patients, most early lesions are treated using
surgery, radiation and chemotherapy. However, resistance to
chemotherapy is the leading cause of treatment failure in colon
cancer (7). The main reason for
this failure is that cancer cells develop resistance to
chemotherapy drugs. Thus, clarifying the potential mechanisms
underlying drug resistance in cancer can improve the survival rate
in patients. Depending on the type of drug resistance, there are
different structures and mechanisms of action (8). The well-known ABC transporter family
can partly explain the mechanisms of drug resistance (9). The ABC transporter family includes
P-glycoprotein (P-gp, encoded by the ABCB1 gene),
MDR-associated protein 1 (MRP1, encoded by the ABCC1 gene)
and ABC subfamily G member 2 (ABCG2) (10). It has been shown that oncogenes or
tumor suppressors, such as octamer-binding protein 4 (OCT4), which
contribute to the tumorigenesis and progression of cancer, also
regulate the chemosensitivity of tumor cells.
OCT4 is a POU homeodomain transcription factor and
encodes three transcripts including a long main variant (OCT4A) and
two alternatively spliced variants (OCT4B and OCT4B1) (11). The variants are associated with the
pluripotency and self-renewal properties of embryonic stem (ES)
cells (7,12). Based on the pluripotency and
self-renewal role in ES cells, OCT4 plays important roles in the
tumorigenesis and progression of cancer. Li et al reported
that OCT4 is capable of repressing Tgf β 3 and Tgf β
R3 and downregulating the epithelial-to-mesenchymal transition
(EMT) regulator Snail (13). In
esophageal carcinoma, OCT4 regulates CCND1 expression to promote
cell progression and accelerate cell proliferation and invasion
(14). Atlasi et al reported
that the long variant OCT4A was found to be required for the
pluripotency properties of ES cells (11). OCT4B1, which lacks DNA-binding
activities and is located in the cytoplasm, has also been suggested
to have a vital catalytic role in pluripotency. Accumulating
evidence suggests that OCT4B1 contributes to the initiation and
progression of cancer (15). In
bladder cancer, OCT4B1 was upregulated and promoted the tumorigenic
process (15). Asadi et al
and Mirzaei et al also found that OCT4B1 was highly
expressed in gastric cancer and acts as an anti-apoptotic factor
(16,17). However, the function of OCT4B1 in
colon cancer and drug-resistant cells remain to be determined.
In the present study, we investigated the role of
OCT4B1 in the malignant phenotype of colon cancer and
drug-resistant cancer cells. We found that OCT4B1 promoted the
growth of colon cancer and drug-resistant cancer cells, by
maintaining the activity of ES cells, and by facilitating
transition of the cell cycle and reducing apoptosis. OCT4B1 reduced
sensitivity to oxaliplatin by altering the expression of P-gp and
ABCG2. In addition, the results revealed that, OCT4B1 contributes
to the migration and invasion by altering EMT in colon cancer and
drug-resistant cells. Taken together, the results indicate that
OCT4B1 functions as an oncogene and a mediator of the drug
resistance process in colon cancer.
Materials and methods
Cell culture, transfection and stable
cell line selection
The human SW480 and SW620 colon cancer cell lines
were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco,
Gaithersburg, MD, USA), supplemented with 10% dialyzed fetal bovine
serum (FBS) and 1% PS (100 U/ml penicillin and 100 g/ml
streptomycin). The cell lines were incubated at 37°C in a
humidified chamber supplemented with 5% CO2.
Transfection was performed with Lipofectamine 3000 reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions.
Stably transfected cells were selected by adding
G418 to the culture medium after transfection 48 h later.
Individual colonies were selected 2 weeks after transfection, and
OCT4B1 levels were quantified using western blot analysis.
Construction of vectors
The CDS of human OCT4B1 was amplified from human
SW480 cDNA by PCR using the oligonucleotide primers: sense,
5′-CGCGGATCCCGCCACCATGCACTTCTAC-3′ and antisense,
5′-CCGGAATTCCTACTCCTCTTCATGGGTGAG-3′. PCR was performed by
denaturing the DNA at 94°C for 4 min, followed by 30 cycles of
amplification: 94°C for 45 sec, 56°C for 40 sec, 72°C for 1 min,
and a final extension step at 72°C for 10 min. The product was 572
bp, and it was cloned into the pcDNA3.1 vector sites (EcoRI
and BamHI). The resulting construct pcDNA3.1/OCT4B1 (OCT4B1)
was confirmed by DNA sequencing.
The shRNA of OCT4B1 (shR-OCT4B1) was annealed and
cloned into the pSilencer2.1 vector sites. A 70-bp double-strand
fragment was obtained via an annealing reaction using the two
single strands: ShR-OCT4B1-Top,
5′-GATCCCAGACTACCCTCACCCATGTTCAAGAGACATGGGTGAGGGTAGTCTGTTTTTTGGAAA-3′;
ShR-OCT4B1-Bot,
5′-AGCTTTTCCAAAAAACAGACTACCCTCACCCATGTCTCTTGAACATGGGTGAGGGTAGTCTGCG-3′.
The negative control pSilencer2.1/negative control (shR-Ctrl)
expressed a hairpin shRNA with limited homology to all known
sequences in the human genome.
RNA extraction and RT-qPCR
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen Life Technologies) according to the
manufacturer's instructions. For RNA integrity assessment, part of
an RNA sample was used for concentration and purity measurement (by
A260 and A280 spectrophotometry) and another part of the sample was
run on a 1.5% denaturing agarose gel stained with ethidium bromide.
A ratio of the absorbance at 260 and 280 nm (A260/280) of 1.8–2 was
accepted. Sharp, clear 28S and 18S rRNA bands at a 2:1 ratio
(28S:18S) were good indicator that the RNA was completely
intact.
Reverse transcriptase-quantitative PCR (RT-qPCR) was
performed to detect the relative transcript levels of OCT4B1
according to the manufacturer's instructions and analyzed using the
ABI 7300 RT-PCR system (Life Technologies, Carlsbad, CA, USA).
Briefly, a cDNA library was generated using M-MLV reverse
transcriptase (Promega, Madison, WI, USA) and oligo (dT) with 5 lg
of extracted RNA, and this cDNA was used for the amplification of
OCT4B1 and β-actin. PCR was performed under the following
conditions: 94°C for 4 min followed by 40 cycles of 94°C for 1 min,
56°C for 1 min and 72°C for 1 min. The primers used were: OCT4B1
forward, 5′-TGAATCCCGAATGGAAAGG-3′ and reverse,
5′-GGAACCCACCAAATAGAAC-3′; and GAPDH forward,
5′-AACGGATTTGGTCGTATTG-3′ and reverse, 5′-GGAAGATGGTGATGGGATT-3′.
The primers were produced by AuGAT Inc. (Beijing, China). The
relative expression levels of the gene of interest were calculated
using the 2−ΔΔCt method.
Cell growth and cytotoxicity assay
The SW480 and SW620 cells were seeded in 96-well
plates at 1×103–1×104 cells/well. For cell
cytotoxicity assays, 24, 48 and 72 h after being seeded, the cells
were incubated with 15 µl of MTT (at a final concentration
of 0.5 mg/ml) at 37°C for another 4 h. After incubation the medium
was removed and the precipitated formazan was dissolved in 100 ml
of DMSO. Following agitation for 10 min, the absorbance at 570 nm
was detected using a Quant Universal microplate spectrophotometer
(Bio-Tek Instruments, Winooski, VT, USA). Each experiment was
repeated in triplicate.
In cytotoxicity assays, cells were seeded in a
96-well plate (5×103 cells/well). After 24 h, the cell
medium was replaced with fresh medium containing six different
concentrations of oxaliplatin (0, 0.3125, 0.625, 1.25, 2.5 and 5
mg/ml). The cells were then incubated at 37°C for 24 h and cell
viability was determined by MTT assays. The absorbance at 570 nm
(A570) of each well was read on a spectrophotometer. The inhibition
rate (IR) was calculated according to the formula: IR = [(XY)/X] ×
100% from each group. The concentration at which each drug produced
50% inhibition of growth (IC50) was estimated using the
IR. Three independent experiments were performed in triplicate.
Cell cycle analysis and apoptosis by flow
cytometry
Stably transfected cells were plated in duplicate in
6-well plates and incubated for 24 h in complete culture medium.
One group of cells was deprived of serum for 24 h prior to being
harvested, whereas another group of cells was returned to complete
medium for another 24 h prior to being harvested. The cells were
collected by centrifugation, fixed in 95% (v/v) ethanol and stored
at −20°C overnight. After washing with phosphate-buffered saline
(PBS), the cells were resuspended in propidium iodide (PI) staining
buffer (PBS, 0.1% Triton X-100, 60 g/ml PI, 0.1 mg/ml DNase-free
RNase and 0.1% trisodium citrate) for 30 min on ice. The DNA
content was analyzed using a FACSCalibur flow cytometer (BD
Biosciences, San Jose, CA, USA) and Cell Quest software (BD
Biosciences). For cell apoptosis, stably transfected cells were
determined using a FITC-Annexin V/PI Apoptosis Detection kit
(KeyGEN, Nanjing, China) by FCM (BD Biosciences). The percentage of
apoptotic cells was analyzed by Cell Quest software.
Western blot analysis
Total cell extracts were extracted using
radioimmunoprecipitation assay (RIPA) lysis buffer lysed at 4°C for
30 min and the proteins were harvested. The cell extracts were
cleared by centrifugation at 12,000 × g for 10 min at 4°C, and the
supernatant was used for western blot analysis. The protein
concentration of the cell lysates was determined using BCA reagents
from Promega. Approximately 25 µg/lane of proteins was
loaded onto a 10% SDS denaturing polyacrylamide gel, separated by
electrophoresis and transferred to nitrocellulose membranes. The
membranes were incubated overnight at 4°C with anti-OCT4B1,
anti-E-cadherin, anti-N-cadherin, anti-vimentin, anti-CD44,
anti-CD133, anti-P-gp, anti-ABCG2 and anti-GAPDH (Tianjin Saier
Biotech, Tianjin, China). The membranes were washed and incubated
with a horseradish peroxidase (HRP)-conjugated secondary antibody.
Protein was visualized using enhanced chemiluminescence and
exposing the membranes to autoradiographic film. LabWorks™ Image
Acquisition and Analysis software (UVP) were used to quantify the
band intensities.
In vitro invasion assays
In vitro cell invasion assays were performed
using Transwell chambers (pore size of 8 µM; Costar,
Corning, NY, USA) with 2 mg/ml Matrigel (Clontech, Mountain View,
CA, USA) according to the manufacturer's instructions. Transfected
cells were seeded in the upper chamber of the insert at a density
of 1×105 cells (SW480 or SW620) and covered with 250
µl of serum-free medium. For screening, any cells that had
not migrated after 20 h were removed and the membranes were stained
with a 2% crystal violet solution for 10 min and placed on a glass
slide. The cells adhering to the lower membrane of the inserts were
counted. Three different fields of images (magnification, ×5) were
taken for each membrane, and the number of migratory cells was
counted. The mean of triplicate assays for each experimental
condition was used.
In vitro wound-healing assay
A cell wound-healing assay was performed as
described previously (19).
Transfected cells were seeded into 24-well plates coated with
gelatin prior to wounding. Cell wound-healing assay was induced by
adding medium supplemented with 10% FBS and 1% PS (100 U/ml
penicillin and 100 g/ml streptomycin). The wound was created by
scraping a conventional pipette tip across the monolayer. It
typically took 12 and 24 h for wound closure to occur in SW480 and
SW620 cells. Images of three different fields were captured using
NIS Elements F 2.20 imaging software (Nikon, Tokyo, Japan).
Statistical analysis
Data were expressed as means±SD. Statistical
analyses were performed using a paired t-test to compare data.
Statistical significance (P<0.05) was determined using a
Student's t-test.
Results
Screening of OCT4B1 stably expressed
cells
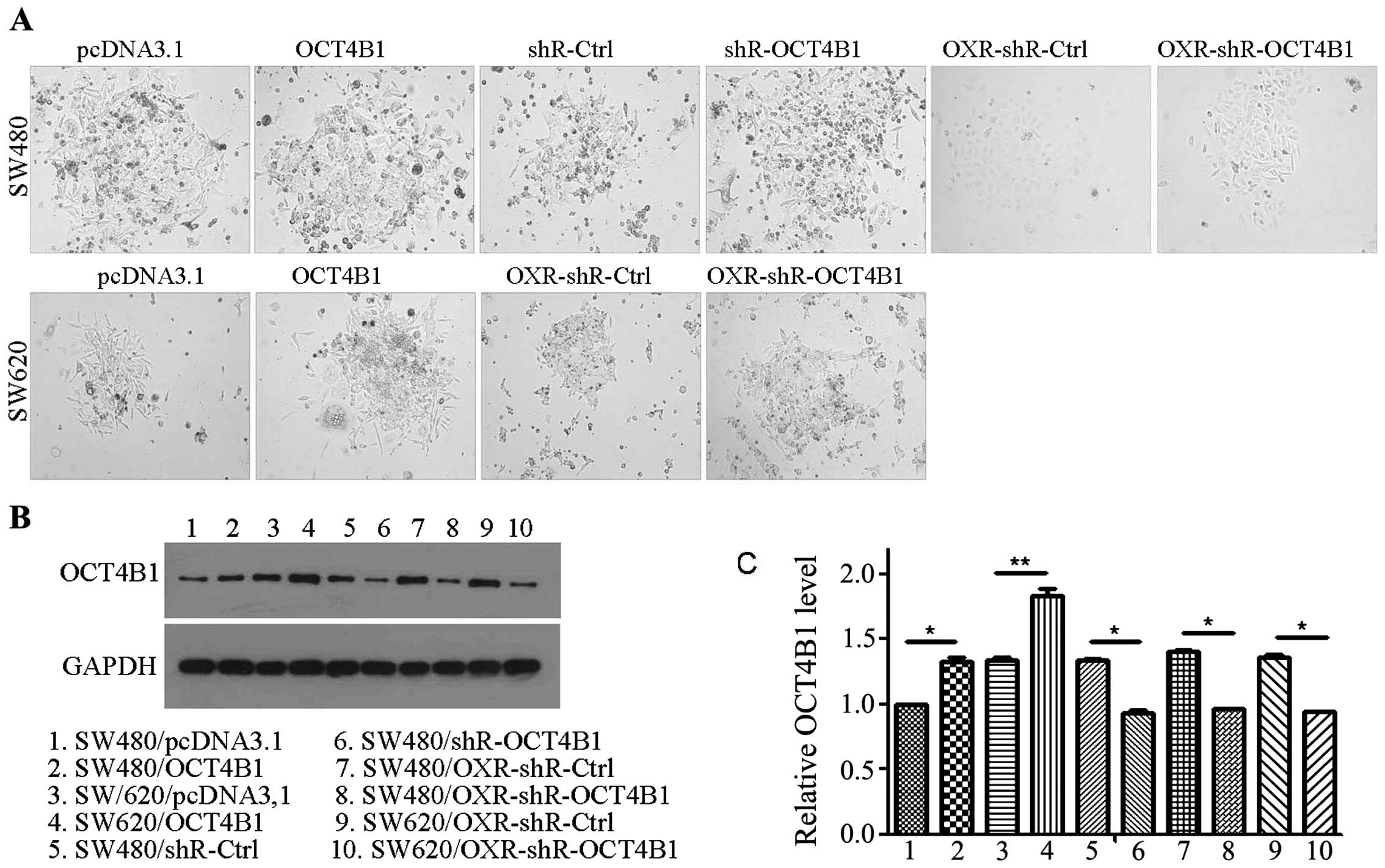
To examine the role of OCT4B1 on cell phenotypes in
colon cancer, we first constructed an OCT4B1 overexpression
construct (pcDNA3.1/OCT4B1, OCT4B1) and an OCT4B1 interference
vector (pSilencer 2.1-U6/OCT4B1, shR-OCT4B1) to overexpress or
knockdown OCT4B1. We then screened single-cell cloning cells with
G418 to gain a stable expression of OCT4B1 in colon cancer cells.
We obtained four stably expressed cells that were overexpressed by
OCT4B1 (SW480/OCT4B1 and SW620/OCT4B1) and the negative control
cells (SW480/pcDNA3.1 and SW620/pcDNA3.1) (Fig. 1A). The expression of OCT4B1 was
assessed by western blot analysis in the stably expressed cells
(Fig. 1B and C). As expected, in
the OCT4B1 overexpression group the protein level of OCT4B1 was
increased ~21.05% (SW480/OCT4B1) and 40.04% (SW620/OCT4B1) compared
with the control group (Fig. 1B and
C). Concurrently, we obtained the OCT4B1 knockdown stably
expressed cells in colon cancer (SW4801shR-OCT4B1) and their
negative control cells (SW480/shR-Ctrl) (Fig. 1A). OCT4B1 expression decreased
~30.41% compared with the negative control (Fig. 1B and C). To examine the role of
OCT4B1 in the formation and development of drug resistance the
oxaliplatin-resistant stably expressed cells (SW480/OXR-shR-OCT4B1
and SW620/OXR-shR-OCT4B1) and their negative control cells
(SW480/OXR-shR-Ctrl and SW620/OXR-shR-Ctrl) were obtained (Fig. 1A). OCT4B1 expression decreased
~31.44% (SW480/OXR-shR-OCT4B1) and 31.09% (SW620/OXR-shR-OCT4B1)
compared with the negative control (Fig. 1B and C). Thus, we screened and
identified the stably expressed cells that altered the OCT4B1
expression for further study.
OCT4B1 enhances cell growth by
accelerating cell cycle progression and reducing apoptosis and
maintaining EC cell property
Given that OCT4B1 acts as an oncogene in many types
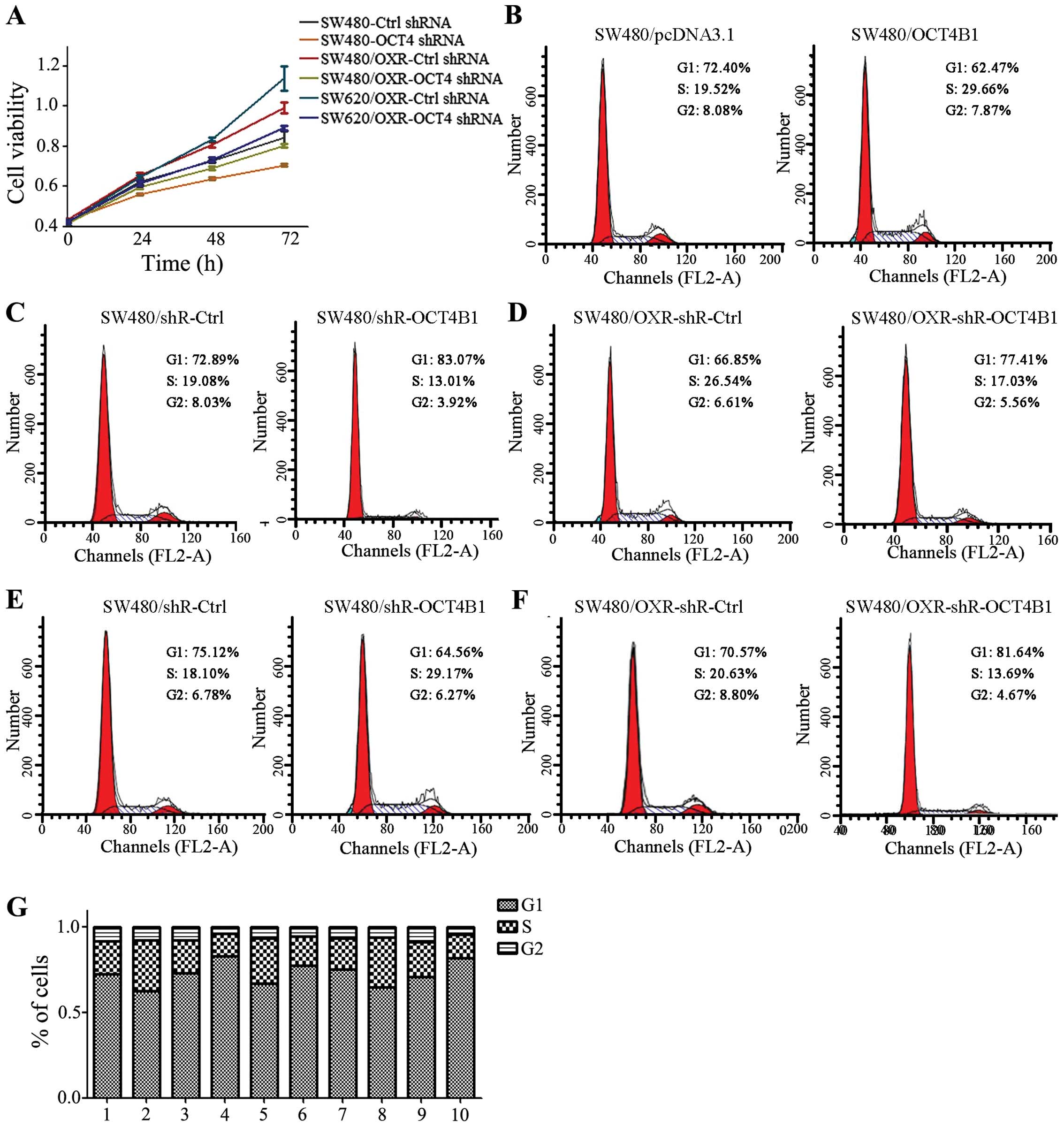
of cancer, we performed an MTT assay to examine the impact of
OCT4B1 on the cell growth of colon cancer. OCT4B1 knockdown stably
expressed cells were used. Knockdown of OCT4B1 caused a reduction
of cell viability in SW480/shR-OCT4B1 compared with the negative
control (Fig. 2A). In the
drug-resistant cells, the knockdown of OCT4B1 (SW480/OXR-shR-OCT4B1
and SW620/OXR-shR-OCT4B1) also reduced cell viability.
Mounting evidence indicates that cell cycle and
apoptosis can partially explain the change of cell viability
(18). To investigate the
mechanisms underlying the regulation of cell viability, we examined
the alteration in cell cycle progression caused by OCT4B1 in colon
cancer stably expressed cells. Results of the flow cytometric
analysis (FCM) showed that the overexpression of OCT4B1 in SW480
cells decreased the percentage of G1 phase from 72.40 to 62.47% and
increased the percentage of cells in the S phase from 19.52 to
29.66% (Fig. 2B). Similarly, OCT4B1
overexpression led to an increment of G1 phase from 72.89 to
83.07%, and a reduction of S phase from 19.08 to 13.01% in SW620
stably expressed cells (Fig. 2C).
By contrast, the inhibition of OCT4B1 in SW480/shR-OCT4B1 cells
increased the percentage of G1 phase and decreased the percentage
of S phase (Fig. 2D). These results
indicated that OCT4B1 contributes to cell growth by promoting the
transition from G1 and S phases to G2 phase. Furthermore, we
detected the effect of OCT4B1 on the drug-resistant cells. The
percentage of G1-phase cells was increased from 66.85 to 77.41%,
whereas the percentage of S and G2 phase cells was decreased from
26.54 and 6.61% to 17.03 and 5.56% in SW480/shR-OCT4B1-OXR cells
compared with the negative control (Fig. 2E). Similar results were obtained in
the SW620/shR-OCT4B1-OXR stably expressed cells (Fig. 2F). Thus, these results suggesetd
that OCT4B1 is capable of altering cell cycle progression in colon
cancer, especially in drug-resistant cancer.
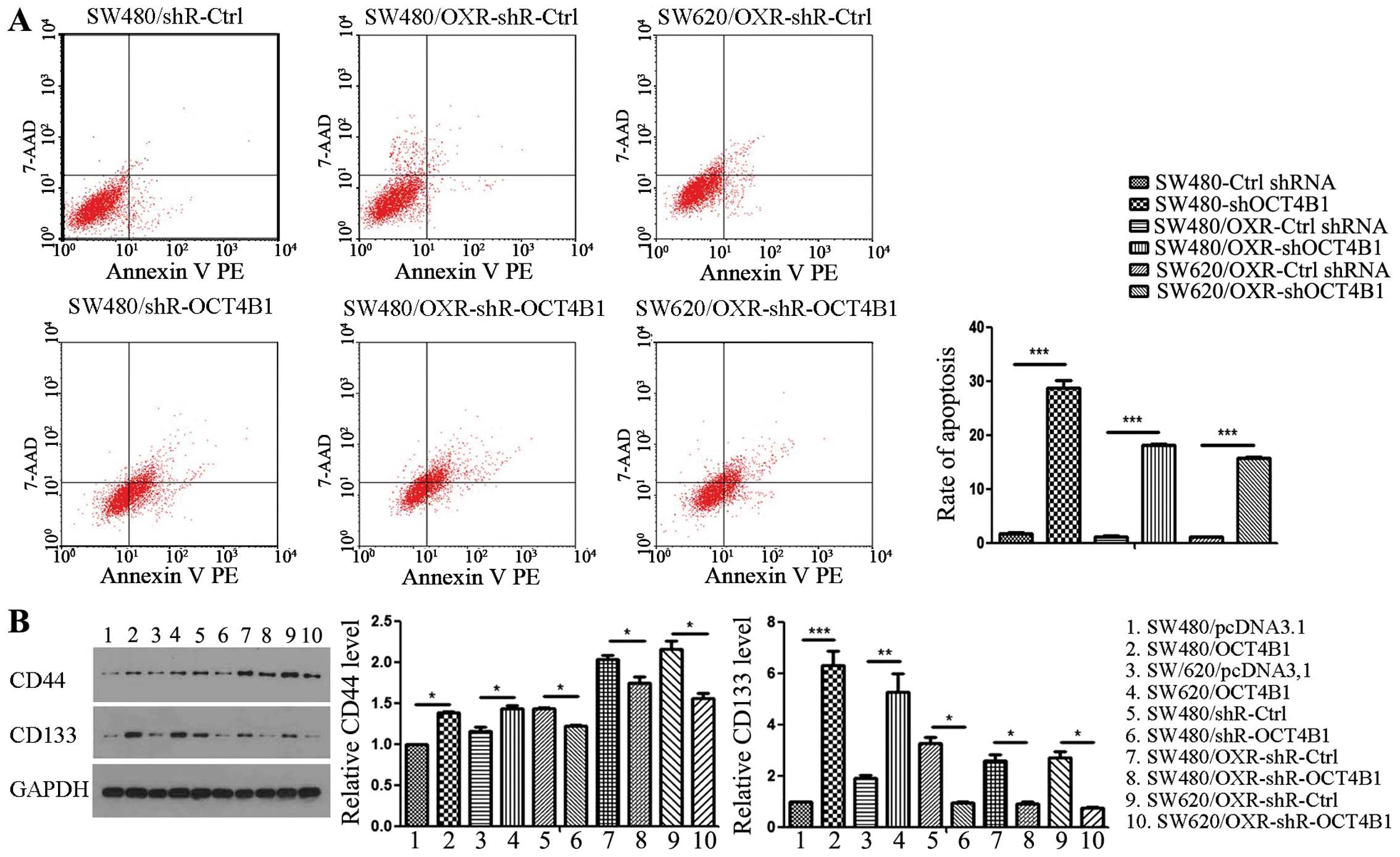
In parallel, the apoptosis index of OCT4B1 knockdown
stably expressed cells was detected by FCM. The apoptosis index of
SW480/shR-OCT4B1 cells was increased by ~29-fold compared with the
control group (Fig. 3A). Similarly,
the effect of OCT4B1 on the drug-resistant cells was identified.
The apoptosis index of SW480/OXR-shR-OCT4B1 and SW620/OXR-shR-Ctrl
stably expressed cells was increased by ~18- and ~16-fold,
respectively, compared with the negative control (Fig. 3A).
We also detected the effect of EC cell property. Two
EC cell biomarkers, CD44 and CD133, were examined using western
blot analysis. The expression of CD44 and CD133 was 1.40- and
6.29-fold in the SW480/OCT4B1 cells compared with the control
group. Knockdown of OCT4B1 led to a 15.17 and 249.13% reduction of
CD44 and CD133 in SW480/shR-OCT4B1. In addition, the protein level
of CD44 and CD133 was reduced in the drug-resistant cells of
SW480/shR-OCT4B1-OXR (Fig. 3B).
These results suggested that OCT4B1 enhances cell survival by
accelerating cell cycle progression, reducing apoptosis and
maintaining EC cell property in colon cancer.
Overexpression of OCT4B1 reduces
sensitivity to oxaliplatin in vitro
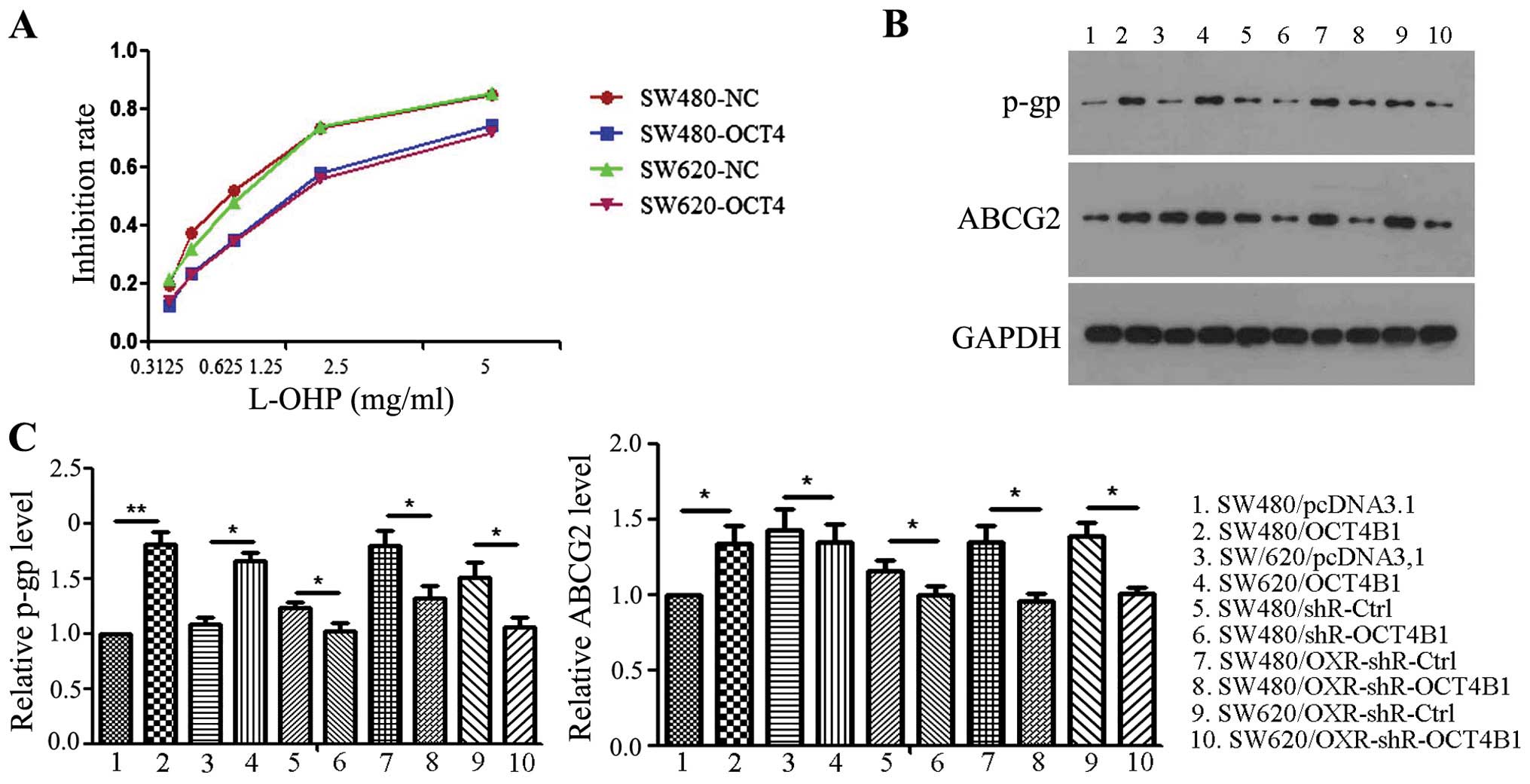
To investigate the role of OCT4B1 with regard to
sensitivity to oxaliplatin, we confirmed cytotoxicity assays using
OCT4B1-overexpressed stably expressed cells (SW480/OCT4B1 and
SW620/OCT4B1). As shown in Fig. 4A,
the viability of SW480/OCT4B1 was significantly higher than that of
the negative control. The amount of oxaliplatin requirement was
much higher in SW480/OCT4B1 to achieve the same level of cell death
as the SW480/pcDNA3.1. The respective IC50 doses for
oxaliplatin were 2.5 g/ml (SW480/OCT4B1) and 1.25 g/ml
(SW480/pcDNA3.1). Similar results were obtained in the SW620/OCT4B1
and SW620/pcDNA3.1 groups (Fig.
4A).
As P-gp and ABCG2 play important roles on the drug
resistance of cancer cells, we examined the mechanism of reduced
sensitivity to oxaliplatin by OCT4B1. Overexpression of OCT4B1 led
to an increase while the knockdown of OCT4B1 led to a decrease in
the level of P-gp and ABCG2 (Fig. 4B
and C). In the drug-resistant cells, knockdown of OCT4B1
suppressed the expression of P-gp and ABCG2 in SW480/OXR-shR-OCT4B1
and SW620/OXR-shR-OCT4B1 compared to the control group (Fig. 4B and C). These results suggested
that OCT4B1 reduces sensitivity to oxaliplatin by increasing the
two important mediators of the drug resistance process in colon
cancer.
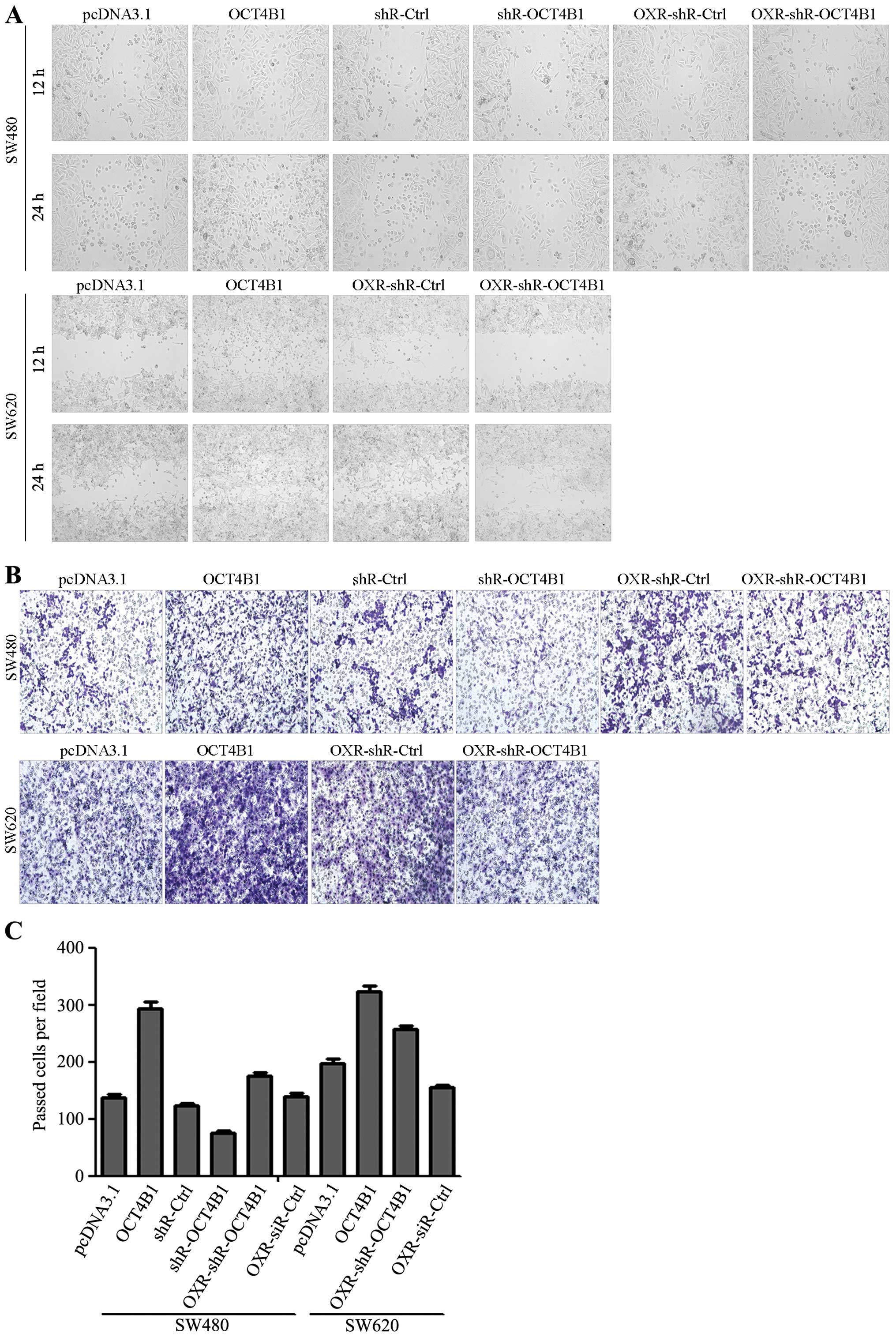
OCT4B1 promotes migration and invasion
via regulation of the EMT process in vitro
Migration and invasion are essential for tumor
metastasis. To determine whether OCT4B1 can affect the migration
and invasion properties of colon cancer cells, wound-scratch and
Transwell assays with Matrigel were carried out in the stably
expressed cells. The wound-scratch assay showed that overexpression
of OCT4B1 promoted wound recovery in SW480/OCT4B1 and SW620/OCT4B1
cells. By contrast, the inhibition of OCT4B1 delayed wound recovery
in SW480/shR-OCT4B1 compared with the control group (Fig. 5A). We also examined the effects of
OCT4B1 on drug-resistant cells. As expected, knockdown of OCT4B1
delayed wound recovery in drug resistance cells (Fig. 5A). The drug-resistant cells,
SW480/OXR-shR-OCT4B1 and SW620/OXR-shR-OCT4B1, obtained the same
results (Fig. 5A). Transwell assay
with Matrigel showed that overexpression of OCT4B1 increased cell
invasion ~2.12- and 1.64-fold in SW480 and SW620 cells
overexpressed with OCT4B1, respectively, compared with the control
group (Fig. 5B and C). The
inhibition of OCT4B1 resulted in a 39.40, 20.11 and 40.05%
reduction in SW480/shR-OCT4B1, SW480/OXR-shR-OCT4B1 and
SW620/OXR-shR-OCT4B1, respectively (Fig. 5B and These results indicated that
OCT4B1 promoted the migration and invasion of colon cancer in
vitro.
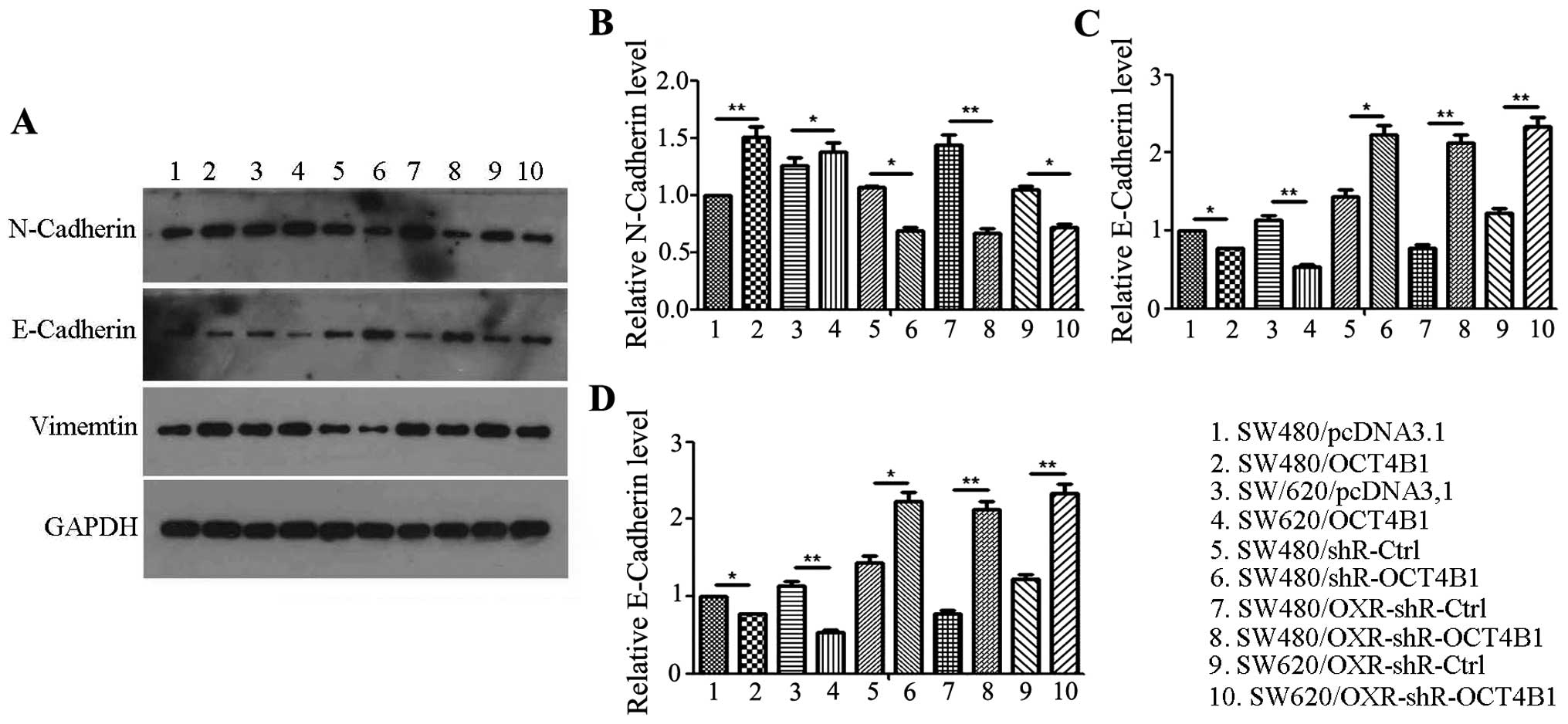
As the results showed that OCT4B1 promotes the
migration and invasion of colon cancer, we investigated the
mechanism underlying the function of OCT4B1 in colon cancer. First,
we examined the typical molecular alterations of EMT. The
expression of the adherent marker E-cadherin and tight junction
marker N-cadherin and vimentin was examined. The protein level of
E-cadherin was reduced 23.01% in SW480/OCT4B1 single-cell cloning
cells compared with the negative control (Fig. 5A and C). The level of N-Cadherin and
vimentin was increased 50.21 and 25.52%, respectively, in
SW480/OCT4B1 stably expressed cells (Fig. 6A, B and In the SW480/shR-OCT4B1
group, the protein level of E-cadherin was increased 55.23%,
whereas N-cadherin and vimentin was reduced 53.96 and 8.21%,
respectively, compared with the control group (Fig. 6). The alteration of EMT was also
examined in the drug-resistant cells. The expression of E-cadherin
increased whereas that of N-cadherin and vimentin was reduced in
SW480/OXR-shR-OCT4B1 and SW620/OXR-shR-OCT4B1 cells compared with
the control group, suggesting that OCT4B1 participated in the
malignant phenotype of drug resistance in colon cancer (Fig. 6). Similar results were obtained in
SW620 cells (Fig. 6). These results
indicated that OCT4B1 promotes the migration and invasion of colon
cancer through regulation of the EMT process.
Discussion
OCT4B1, also known as POU5F1, is a novel transcript
in the human OCT4 gene, which appears to be a master
regulator in maintaining the pluripotency and self-renewal of ES
cells (7). Since OCT4A and OCT4B
were identified in 1992, studies have focused on the activity of
the transcription factor responsible for the stemness properties of
OCT4A and the cell stress-related funtion of OCT4B (20,21).
The function of OCT4B1 in pluripotency and self-renewal has been
identified. Asadzadeh et al have reported that OCT4B1 is
expressed in pleuripotent cells (15). Based on the characteristic of stem
cells, the cancer stem cell (CSC) renders the self-renewal
potential, tumorigenesis, and resistance to therapy characteristic
of cancer (22). The high
tumorigenicity of cancer cells occurred as a result of the
dysregulation of the self-renewal process in stem cells (23,24).
It was previously reported that OCT4 variants may function as
oncogenes or tumor-suppressing genes in cancer. In gastric
adenocarcinoma, OCT4B1 acts as an anti-apoptotic factor (25). Addtionally, OCT4B1 has been
expressed in gastric and colorectal cancers (25,26).
However, the role of OCT4B1 in the development of colon cancer
remains to be determined. The present study focused on the
contribution of OCT4B1 in the pathogenesis of colon cancer as well
as cancer drug-resistant cells.
Identification of the role of OCT4B1 is critical for
understanding the molecular mechanisms in tumorigenesis. We first
screened the stably expressed cells overexpressing or knocking down
OCT4B1 in SW480 and SW620 cells and their drug-resistant cells,
respectively. Subsequently, we examined the effect of OCT4B1 on
cell growth. In the present study, we found that OCT4B1 promotes
cell growth in colon cancer and drug-resistant cells, which
indicates that OCT4B1 participates in the growth of colon cancer as
well as drug-resistant cells. Furthermore, we examined the
mechanisms of the growth supporting role of OCT4B1 in colon cancer.
First, we found that OCT4B1 promotes the ES cell property. Cell
cycle and apoptosis were then detected in the OCT4B1-altered cells.
The cell cycle analysis indicated that OCT4B1 preferentially
promotes cell proliferation by facilitating the G1/S and S/G2 phase
transitions, while OCT4B1 reduced cell apoptosis in colon cancer
and drug-resistant cells. Thus, OCT4B1 promotes the growth of
cancer cells via the ES cell property as well as alterations in the
cell cycle and apoptosis.
In addition, we found that OCT4B1 reduced
sensitivity to oxaliplatin, which further supports its oncogenic
role in colon cancer. It is well known that the ABC transporter
family participates in the formation of cancer drug resistance
(27). P-gp and ABCG2 are two major
mediators of drug resistance in cancer (28). Therefore, we hypothesized that
OCT4B1 reduced the sensitivity to oxaliplatin due to the alteration
of ABC transporter family expression. As expected, OCT4B1 was able
to increase the expression of P-gp and ABCG2, which explains that
OCT4B1 reduced the sensitivity to oxaliplatin in colon cancer.
These results suggest that OCT4B1 reduces the sensitivity to
oxaliplatin and potentiates P-gp and ABCG2-mediated drug
resistance.
Another important characteristic of cancer is
metastasis. This is a complicated event whereby cells detach from
the primary tumors, increase motility and invade the tissue stroma.
The invasive tumor cells enter the circulation through blood
vessels or lymphatic channels (29). Subsequently, the circulating tumor
cells invade another organ and undergo metastatic growth (30). During this process, the cancer cells
undergo an epithelial-mesenchymal transition (EMT) transition.
Thus, we determined the effect of OCT4B1 on migration and invasion
in colon cancer. The wound-healing assay revealed that OCT4B1
promotes the migarion of colon cancer and drug-resistant cells.
Similarly, OCT4B1 contributes to the invasion in colon cancer
performed by invasion assays. Of note, the migratiory and invasive
abilities of OCT4B1 are resulted from EMT (31). OCT4B1 promotes the switch from the
biomarker of the epithelial cells, E-cadherin, to vimentin and
N-cadherin, which are the mesenchymal biomarkers. These results
suggest that OCT4B1 enhances the EMT by a reduction in the levels
E-cadherin and an increase in the level of vimentin and N-cadherin,
further stimulating the ability of migration and invasion in the
colon cancer and drug-resistant cells.
In summary, results of the present study have
demonstrated a novel function by which OCT4B1 stimulated colon
cancer cell growth, migration, invasion and suppression of the
sensitivity to oxaliplatin. In addition, OCT4B1 contributes the
malignant phenotype of drug resistance in colon cancer cells.
Therefore, inhibition of OCT4B1 may be considered to be a novel
molecular treatment strategy for colon cancer and the drug
resistance patients in the future.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81260369) and the Science and
Technology Fund Foundation of Guizhou [no. (2012)2365].
References
|
1
|
Slattery ML and Fitzpatrick FA:
Convergence of hormones, inflammation, and energy-related factors:
A novel pathway of cancer etiology. Cancer Prev Res (Phila).
2:922–930. 2009. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grady WM and Carethers JM: Genomic and
epigenetic instability in colorectal cancer pathogenesis.
Gastroenterology. 135:1079–1099. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vogelstein B and Kinzler KW: Cancer genes
and the pathways they control. Nat Med. 10:789–799. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hofmanová J, Straková N, Vaculová AH,
Tylichová Z, Safaříková B, Skender B and Kozubík A: Interaction of
dietary fatty acids with tumour necrosis factor family cytokines
during colon inflammation and cancer. Mediators Inflamm.
2014:8486322014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen D, Yu Z, Zhu Z and Lopez CD: The p53
pathway promotes efficient mitochondrial DNA base excision repair
in colorectal cancer cells. Cancer Res. 66:3485–3494. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nichols J, Zevnik B, Anastassiadis K, Niwa
H, Klewe-Nebenius D, Chambers I, Schöler H and Smith A: Formation
of pluripotent stem cells in the mammalian embryo depends on the
POU transcription factor Oct4. Cell. 95:379–391. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fankam AG, Kuiate JR and Kuete V:
Antibacterial activities of Beilschmiedia obscura and six other
Cameroonian medicinal plants against multi-drug resistant
Gram-negative phenotypes. BMC Complement Altern Med. 14:2412014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sui H, Liu X, Jin BH, Pan SF, Zhou LH, Yu
NA, Wu J, Cai JF, Fan ZZ, Zhu HR, et al: Zuo Jin Wan, a traditional
Chinese herbal formula, reverses P-gp-mediated MDR in vitro and in
vivo. Evid Based Complement Alternat Med. 2013:9570782013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sui H, Fan ZZ and Li Q: Signal
transduction pathways and transcriptional mechanisms of
ABCB1/Pgp-mediated multiple drug resistance in human cancer cells.
J Int Med Res. 40:426–435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Atlasi Y, Mowla SJ, Ziaee SA, Gokhale PJ
and Andrews PW: OCT4 spliced variants are differentially expressed
in human pluripotent and nonpluripotent cells. Stem Cells.
26:3068–3074. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X and Dai J: Concise review: Isoforms
of OCT4 contribute to the confusing diversity in stem cell biology.
Stem Cells. 28:885–893. 2010.PubMed/NCBI
|
|
13
|
Li R, Liang J, Ni S, Zhou T, Qing X, Li H,
He W, Chen J, Li F, Zhuang Q, et al: A mesenchymal-to-epithelial
transition initiates and is required for the nuclear reprogramming
of mouse fibroblasts. Cell Stem Cell. 7:51–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Li X, Li C, Su Y, Fang W, Zhong C,
Ji W, Zhang Q and Su C: Transcription factor OCT4 promotes cell
cycle progression by regulating CCND1 expression in esophageal
carcinoma. Cancer Lett. 354:77–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asadzadeh J, Asadi MH, Shakhssalim N,
Rafiee MR, Kalhor HR, Tavallaei M and Mowla SJ: A plausible
anti-apoptotic role of up-regulated OCT4B1 in bladder tumors. Urol
J. 9:574–580. 2012.PubMed/NCBI
|
|
16
|
Asadi MH, Mowla SJ, Fathi F, Aleyasin A,
Asadzadeh J and Atlasi Y: OCT4B1, a novel spliced variant of OCT4,
is highly expressed in gastric cancer and acts as an antiapoptotic
factor. Int J Cancer. 128:2645–2652. 2011. View Article : Google Scholar
|
|
17
|
Mirzaei MR, Najafi A, Arababadi MK, Asadi
MH and Mowla SJ: Altered expression of apoptotic genes in response
to OCT4B1 suppression in human tumor cell lines. Tumour Biol.
35:9999–10009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valianou M, Cox AM, Pichette B, Hartley S,
Paladhi UR and Astrinidis A: Pharmacological inhibition of
polo-like kinase 1 (PLK1) by BI-2536 decreases the viability and
survival of hamartin and tuberin deficient cells via induction of
apoptosis and attenuation of autophagy. Cell Cycle. 14:399–407.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen L, Zhang JJ and Huang XY: cAMP
inhibits cell migration by interfering with Rac-induced
lamellipodium formation. J Biol Chem. 283:13799–13805. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee J, Kim HK, Rho JY, Han YM and Kim J:
The human OCT-4 isoforms differ in their ability to confer
self-renewal. J Biol Chem. 281:33554–33565. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Zhao Y, Xiao Z, Chen B, Wei Z,
Wang B, Zhang J, Han J, Gao Y, Li L, et al: Alternative translation
of OCT4 by an internal ribosome entry site and its novel function
in stress response. Stem Cells. 27:1265–1275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pardal R, Clarke MF and Morrison SJ:
Applying the principles of stem-cell biology to cancer. Nat Rev
Cancer. 3:895–902. 2003. View
Article : Google Scholar
|
|
23
|
Al-Hajj M and Clarke MF: Self-renewal and
solid tumor stem cells. Oncogene. 23:7274–7282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lobo NA, Shimono Y, Qian D and Clarke MF:
The biology of cancer stem cells. Annu Rev Cell Dev Biol.
23:675–699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Junker K, Wolf M and Schubert J: Molecular
clonal analysis of recurrent bladder cancer. Oncol Rep. 14:319–323.
2005.PubMed/NCBI
|
|
26
|
Engers R: Reproducibility and reliability
of tumor grading in urological neoplasms. World J Urol. 25:595–605.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang XK, To KK, Huang LY, Xu JH, Yang K,
Wang F, Huang ZC, Ye S and Fu LW: Afatinib circumvents multidrug
resistance via dually inhibiting ATP binding cassette subfamily G
member 2 in vitro and in vivo. Oncotarget. 5:11971–11985. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Greenberg RM: Schistosome ABC multidrug
transporters: From pharmacology to physiology. Int J Parasitol
Drugs Drug Resist. 4:301–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Servant G, Weiner OD, Herzmark P, Balla T,
Sedat JW and Bourne HR: Polarization of chemoattractant receptor
signaling during neutrophil chemotaxis. Science. 287:1037–1040.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|