Introduction
Medullary thyroid carcinoma (MTC) is a rare, but
aggressive neuroendocrine tumor that arises from calcitonin
(CT)-producing parafollicular cells (C cells) of the thyroid, and
accounts for 5–8% of all thyroid cancers (1,2). Based
on the germline RET gene mutation status and clinical phenotype,
MTC can be classified as 'sporadic' (75%) or 'hereditary' (25%)
(3). It has a slow but progressive
clinical course with an early involvement of lymph nodes. It is
challenging to diagnose MTC in clinical practice. Use of
fine-needle aspiration cytology is helpful in diagnosing cancer
including MTC, but some results of this technique are
indeterminate, benign or have inadequate cytological studies
(4–9); a major difficulty is in obtaining
sufficient tumor tissue. Also, ultrasonography is frequently 'not
suspicious' in diagnosing MTC (10–16).
Thyroid C cells produce and secrete CT, which is a more specific
circulating marker and is widely used for diagnosis and monitoring.
Nevertheless, the issue of screening serum CT in patients with
thyroid nodules is partially unsettled due to analytical problems,
low prevalence of MTC, increased cost of routine determination and
risk of inappropriate surgery after misleading diagnosis (17). MTC cannot be treated using
radionuclide therapy as it does not fulfill the necessary
conditions. Also, it is not sensitive to radiotherapy. Surgery is
the main and only effective treatment, with total thyroidectomy
plus cervical lymph node dissection and should be performed before
the occurrence of distant metastasis (18). Hence, there is an urgent need to
develop accurate and non-invasive methods for early diagnosis of
MTC.
Monoclonal antibodies that recognize specific
markers expressed on tumor cells have been widely used in the
research and diagnosis of cancer. Anti-carcinoembryonic antigen
monoclonal antibody was successfully used in tumor imaging in 1978
(19). However, the use of murine
antibodies, which are produced using hybridoma technique, in large
intact antibodies in solid tumors is limited by the response of
human anti-murine antibody (HAMA) (20), thereby weakening the effectiveness
of the treatment. In 1988, a single-chain fragment of variable
(scFv) antibody was first constructed using genetic fusion of
variable regions of the heavy (VH) and light chains (VL) (21). The following year, surface display
phage antibody library was established. The phage antibody not only
can identify and bind the corresponding antigen, but also can
infect the host bacteria for amplification. Sufficient amounts of
heterologous proteins are produced by efficient microbial
production systems (22,23). Also, scFv has shown distinct
advantages as it can be prepared through chemical synthesis at a
relatively low cost, is less responsive to HAMA and has rapid blood
clearance. Thus, this technique was used to construct single-chain
antibody phage libraries for anti-MTC.
Recently, molecular imaging has been widely used for
diagnosing solid tumors using positron emission tomography (PET)
and single photon emission computed tomography (SPECT) (24,25).
Due to their non-invasive character in patients and high
specificity to tumor lesions, antibodies against tumor
cell-specific surface markers have become an ideal method for tumor
imaging. Hence technetium-99m (99mTc), with a lower
energy (140 keV) and shorter half-life, has been widely used in the
departments of nuclear medicine worldwide. However,
99mTc is not the best choice for SPECT imaging due to
its limitation in treatment. In contrast, iodine-131
(131I) is easy to obtain, has higher energy (364 keV)
and relatively long half-life (8 days). Moreover, 131I
not only can emit γ-ray for imaging but also can emit β-rays for
treatment. Pavlinkova et al reported the use of
131I-labeled scFv in nude mice bearing colon cancer
cells and found that the tumors completely subsided with a
probability of 60% in the treatment group (25). This may be a new treatment targeting
tumors.
In the present study, the phage display technique
was used to construct a human single-chain antibody library of MTC.
Later, this library was panned with thyroid epithelial cell lines
(TECs) and MTC cell lines (TTs). Panned scFv was identified using
western blotting and enzyme-linked immunosorbent assay (ELISA).
After purification, the scFv was labeled with 131I and
SPECT-CT tomography imaging was performed in nude mice bearing TT
cells. These results may provide a basis for the future development
of diagnosis and therapeutics of MTC.
Materials and methods
Construction and stitching of gene
library
Phagemid vector pCANTAB-5E, helper phage M13K07 and
Escherichia coli TG1 (E. coli TG1) were obtained from
New England BioLabs (New England). Based on the literature
(26,27), polymerase chain reaction (PCR)
primers were designed and commissioned by Chongqing MacKay Ltd.
(Chongqing, China). Reverse transcription (RT)-PCR reaction
synthesis of first-strand complementary DNA (cDNA) was performed
based on the instructions in the kit. Later the heavy chain linker
(VH-linker) and the light chain linker (VL-linker) were amplified
from the cDNA. A sequence encoding E-tag was included in the
forward primer. The PCR protocol was as follows: initial
denaturation at 94°C for 5 min, 35 cycles of melting at 94°C for 30
sec, annealing at 53°C for 30 sec and extension at 72°C for 30 sec,
1 cycle at 72°C for 10 min. The purified PCR products were digested
and inserted into the pCANTAB-5E and then transformed into E.
coli TG1 cells (E. coli TG1 was used as the main host
for gene cloning and library screening). Transformed E. coli
TG1 cells were selected from lysogeny broth medium containing
ampicillin. PCR amplification was performed according to the
instructions of the plasmid extraction kit (purchased from Beijing
Hundred Tektronix Biotechnology Co., Beijing, China). The product
was subjected to 1.0% agarose gel electrophoresis to identify the
rate of insertion of the antibody gene. Positive clone was
identified using SfiI and NotI double digestion and
analyzed using 1.0% agarose gel electrophoresis to identify whether
there was visible release of a fragment. The positive plasmid was
sequenced by Shanghai Handsome Positive Biotechnology (Shanghai,
China).
Preparation and screening of phage
antibody library
The transformed E. coli TG1 cells were
inoculated into 2X YT medium containing 2% (w/v) glucose at 37°C
for 1 h, then M13K07 helper phages were added and oscillated at 250
rpm for 1 h and then centrifuged, and the cells were transferred to
fresh 2X YT-AK medium (2XYT containing ampicillin and kanamycin)
and were incubated overnight at 37°C and 250 rpm. The recombinant
phages were recovered from the overnight culture and precipitated
using polyethylene glycol-NaCl. The phage antibody was panned with
TEC for blocking non-specific binding sites of the antibody, then
unbounded phage was added to the immune-coated tube, which has been
coated with TT cells, rocked gently in a warm bath for 1.5 h, then
allowed to rest for 30 min and the supernatant was removed. Cells
were gently washed thrice using phosphate-buffered saline (PBS)
with Tween-20 (PBST) and PBS followed by elution using 0.2 M
glycine-HCl (pH 2.5) and neutralized using Tris-HCl (pH 7.5).
Recombinant phages were transformed into E. coli TG1 cells
[optical density (OD), 0.6] and plated on 2X YT-A medium at 37°C
for 1 h. The bacteria were next cultured in 2X YT-AK medium
supplemented with 4×1010 pfu/mol M13K07 helper phages at
37°C overnight. Overnight bacteria solution was centrifuged at
10,000 rpm for 20 min and the supernatant was removed. Thus, the
first round of panning was completed. The next four rounds of
panning were identical to the first except that washing was more
stringent (5X PBST and 5X PBS). A small amount of antibody library
before and after the screening was taken to infect E. coli
TG1 cells in SOBAG agar plates to calculate the titer of antibody
library and input-output ratio, as an enriched index of specific
phage antibodies.
Phage ELISA and scFv ELISA detect
recombinant antibodies
Phage ELISA and scFv ELISA were used to detect the
presence of M13K07 helper phages and E-tagged scFv (the transformed
E. coli TG1 cells injected with M13K07 helper phages and
pCANTAB-5E contain a sequence encoding for a peptide E-tag and thus
yield E-tagged scFv). In phage ELISA, the supernatant of the fifth
screening in the previous step was added to the TT cells in
well-packaged microtiter plates as the first antibody. In scFv
ELISA, the transformed E. coli TG1 cells were induced using
1 mM isopropyl-β-D-thiogalactopyranoside (IPTG). Under these
conditions, soluble scFv was released into the growth medium where
it can be used for detection. Soluble scFv was added to the TT
cells in well-packaged microtiter plates as the first antibody. The
control group was established by the addition of PBS. Horseradish
peroxidase (HRP)/anti-M13 monoclonal antibody and HRP/anti-E-tag
monoclonal antibody (both from Abcam Company, Shanghai, China) were
added to each well for secondary antibody. 3,5,5-Tetramethyl
benzidine dihydrochloride (TMB) was added to each well and kept in
dark for 45 min. A stop solution was added and the reading was
obtained in the ELISA reader at 450 nm.
Expression and purification of scFv
A few phage clones that underwent reaction with the
TT cells using ELISA were selected and transferred into E.
coli HB2151 (New England BioLabs). Transformed cells were
inoculated into the SOBAG-N medium containing nalidixic acid at
30°C overnight. The cells were transferred into a fresh 2X YT-AI
medium containing IPTG and collected using centrifugation when the
shock time was 4 and 6 h, respectively. The cells were resuspended
in PBS, frozen in liquid nitrogen for 30 min and thawed at 37°C.
Ultrasound was used to break down bacteria after freezing and
thawing thrice. The resulting supernatant was collected using
centrifugation and contained soluble scFv from whole cells. Soluble
scFv was purified using HiTrap™ anti-E-tag column. Each tube was
monitored at A280nm and a few tubes were collected at
the highest OD value of A280nm. This results in the
purified production of soluble antibodies. Purified soluble scFv
was stored at 4°C for further use.
Sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and western blotting
To examine the expression of scFv, the supernatant
from the uninduced and induced culture at 4 and 6 h of the
recombinant clone in E. coli HB2151 was obtained. Purified
soluble scFv was run on sodium dodecyl sulfate-poly-acrylamide gel
electrophoresis (SDS-PAGE), followed by Coomassie brilliant blue.
E. coli HB2151 induced at 4 and 6 h was run on SDS-PAGE with
uninduced E. coli HB2151 as the control group, then
transferred onto nitrocellulose membrane followed by blocking with
MPBS (2% skim milk in PBS) for 1 h at room temperature with gentle
shaking. After washing, the membrane was incubated with the
HRP-mouse anti-E-tag monoclonal antibody as the first antibody and
HRP-anti-mouse antibody as the secondary antibody. It underwent
chemiluminescence, then developed and fixed.
Cell ELISA detection of soluble
antibodies immune activity
TT cells, TEC, and SW480 cells were cultured into
96-well plates at 37°C for 48 h, then cells were washed thrice with
PBS, dried by placing the cells in the incubator, and fixed with
0.25% glutaraldehyde for 10 min. After blocking and washing, cells
were washed thrice with PBST and PBS, respectively. To each 96-well
plate, purified soluble recombinant antibodies were added.
HRP/anti-E-tag monoclonal antibody was used as the secondary
antibody (1/10,000 dilution, 37°C, 1 h). PBS was used instead of
purified scFv TT cells as the blank control group. TMB was added to
each plate and kept in the dark for 45 min. Reading was obtained in
the ELISA reader at 450 nm, and photographed using light
microscopy.
Methyl thiazolyl tetrazolium assay
exploring the inhibition of the proliferation rate
TT and SW480 cells were grown in 96-well plates.
Serial dilutions of scFv solution at concentrations of
10−4, 10−3, 10−2, 10−1,
1 and 10 µmol/l (50 µl/well) were added to each
96-well plate the next day, PBS was added to the control group and
five replicate wells were set in each group. After these cells were
incubated in a cell incubator for 48 h, MTT solution was added (20
µl/well) for 4 h, then the reaction was terminated and later
dimethyl sulfoxide (150 µl/well) was added to each well.
After low temperature shock for 10 min, reading was obtained in the
absorbance microplate reader at A490nm and then the inhibition rate
was calculated. Inhibition rate (%) = (control group A -
experimental group A)/(control group A) × %.
Radiolabeling, purification and
radiochemical purity test
131I was labeled using a modification of
the chloramine-T method. Briefly, soluble scFv was added to freshly
prepared 131I, chloramine-T solution was added 3 min
later and 200 µl potassium iodide was then added 1 min later
to stop the reaction. This solution was purified using Sephadex
G-200 and filter sterilized. Labeling efficiency was measured using
trichloroacetic acid precipitation. Paper chromatography was used
to determine the radiochemical purity of 131I-scFv and
to calculate the specific activity of radioactivity. Purified
131I-scFv was added to the fresh human serum (obtained
from the blood bank of the First Affiliated Hospital of Chongqing
Medical University, Chongqing, China) at 37°C for 24 h to test the
stability of the serum. Later these were analyzed at 1, 6, 12 and
24 h, using paper chromatography.
Animal models and biodistribution
studies
Animal biodistribution experiments and SPECT-CT
imaging were performed in 4- to 6-week-old male nude mice
(Department of Laboratory Animal Center at Chongqing Medical
University), which were xenografted with TT cells. Cells were
injected subcutaneously into the right forelimb of the nude mice.
After 6 weeks, the diameter of tumors was ~1.0 cm. Twelve
tumor-burdened nude mice were divided into four groups, with three
in each group. 131I-scFv was injected into the tail
veins of nude mice. The animals were sacrificed and dissected at 12
h, 1, 2 and 3 days after the injection of 131I-scFv.
Tumor tissues, heart, liver, spleen, lung, kidney, stomach,
intestines, brain and muscle were removed and weighed. The
radioactivity of the tissues was measured using a γ-counter.
Results were expressed as the percentage injected dose/gram of
tissue (% ID/g). Ethics approval for the animal studies was given
by the First Affiliated Hospital of Chongqing Medical University
Biomedical Ethics Committee.
SPECT-CT imaging
Thyroid of nude mice bearing TT cells was sealed
using potassium iodide and injected with 131I-scFv.
SPECT-CT was used for anteroposterior static imaging (SPECT-CT
Symbia T2; Siemens, Germany), using a single head rotating
scintillation camera at 12 h, 1, 2 and 3 days after the injection
of 131I-scFv to observe the radioactivity in tumor
(high-energy collimator, matrix 256×256, peak energy of 364 keV,
acquisition time for each frame 15 min). Image fusion was performed
using the SPECT-CT when the tumor tissues were clearly visible.
Results
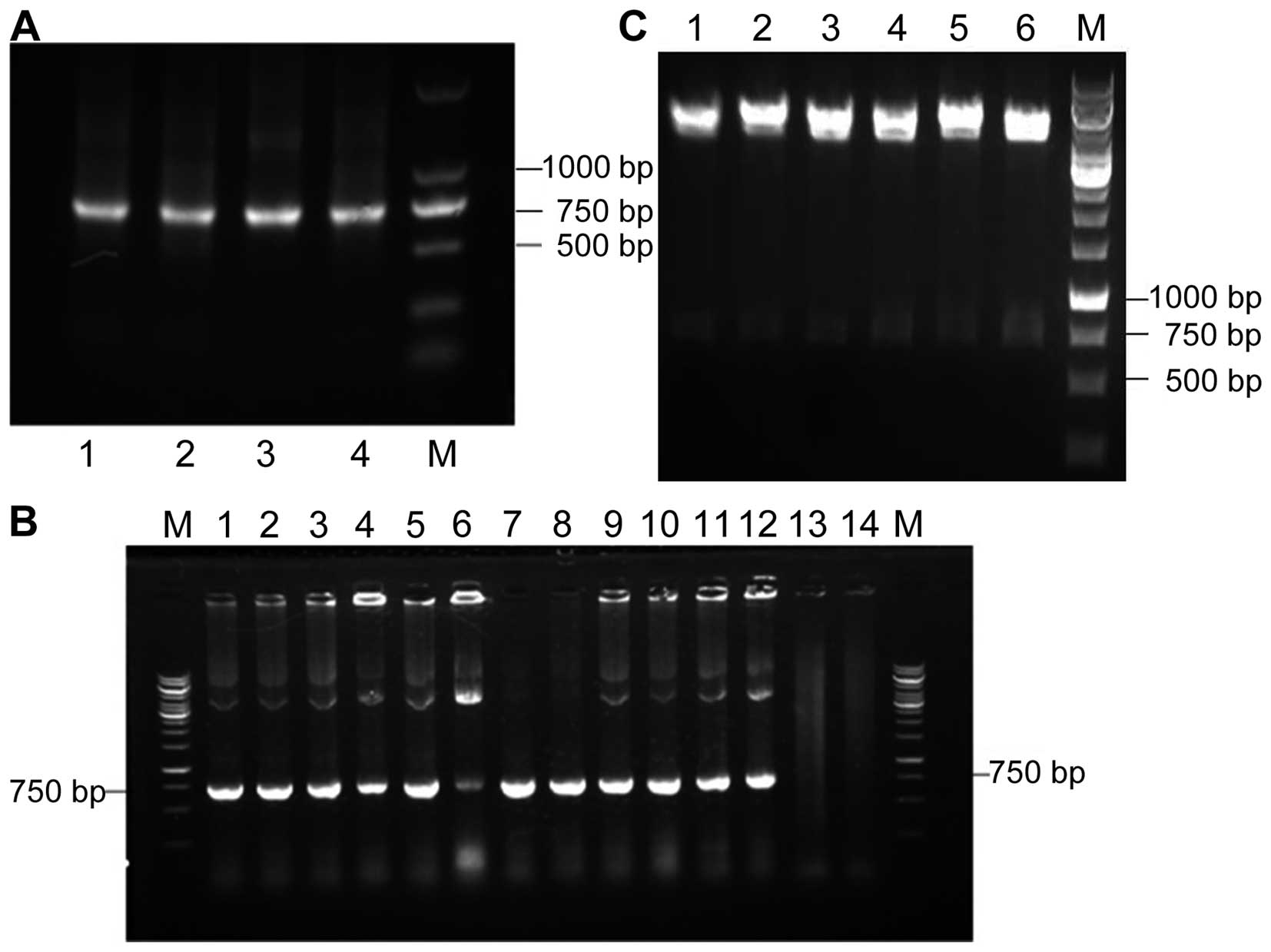
Construction of gene library
The VH and VL genes were amplified from the cDNA
derived from the mRNA, which was extracted from the lymph nodes
near the MTC tumor tissue, and were visualized on 1% agarose gel as
370- and 350-bp bands, respectively. A 750-bp scFv DNA was produced
by assembling VH and VL DNA fragments and was subsequently cloned
to express recombinant E-tag scFv (Fig.
1A). As shown in Fig. 1B, the
gene encoding the Dmab (scFv)-Fc antibody was amplified from 12 of
the 16 colonies. Six monoclonal plasmids were randomly chosen and
double digested using SfiI and NotI. The release of
the fragment can be observed (Fig.
1C). These results showed that MTC-specific scFv was
successfully obtained.
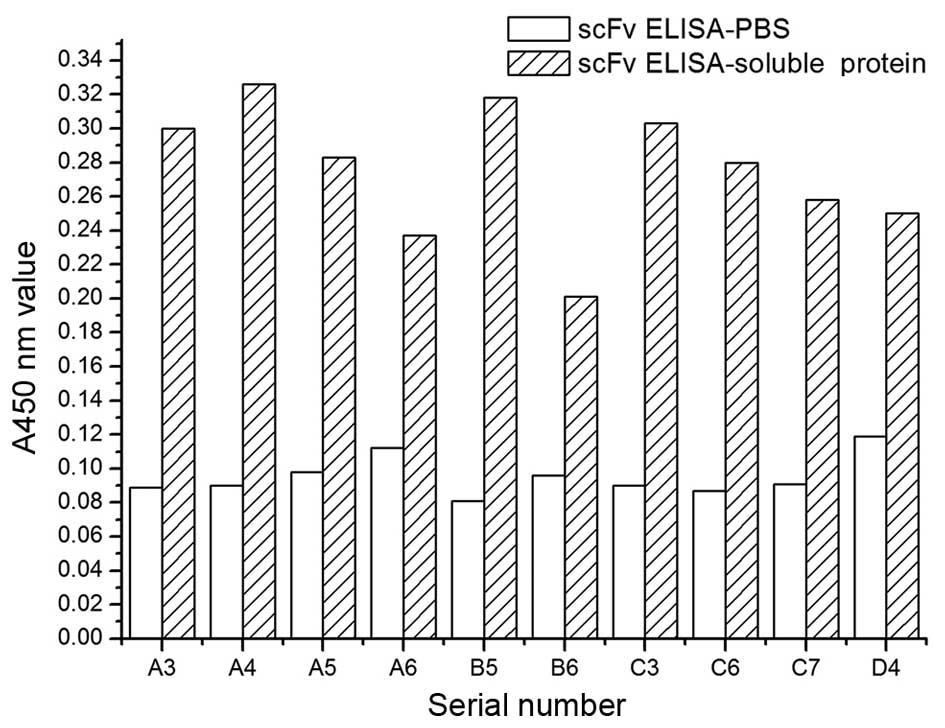
Detection of soluble antigen-positive
recombinant antibodies using phage ELISA and scFv ELISA
After five rounds of
'adsorption-elution-amplification', the rates of harvest of the
first and the fifth rounds were 1.96×10−6 and was
2.84×10−4 %, respectively, showing an increase of
145-fold. Anti-MTC antibodies have been significantly enriched. The
enriched scFv library was first detected using phage ELISA and then
using scFv ELISA; 13 and 10 bacterial clones in 20 showed a
positive reaction to TT cells, which were detected using phage
ELISA and scFv ELISA with a positive rate of 65 and 50%,
respectively. The data also indicated that positive wells
corresponding to coding have a high consistency of detection
between the two methods, which suggests that phage-infected E.
coli TG1-induced protein initially showed soluble expression
(Fig. 2).
SDS-PAGE and western blotting
HiTrap™ anti-E-tag was used to purify soluble
proteins. Five tubes have higher readings at A280nm, and their
readings were as follows: (no. 12) 0.1; (no. 13) 0.15; (no. 14)
0.1; (no. 15) 0.09; and (no. 16) 0.05, merging the five tubes that
have highest A280nm reading as purified expression of soluble scFv.
Fig. 3A and B shows the results of
SDS-PAGE followed by Coomassie brilliant blue staining and western
blot analyses for the purified scFv proteins. Supernatant of the
uninduced E. coli HB2151, the induced supernatant 4 and 6 h
of the recombinant clone in E. coli HB2151, and purified
soluble scFv were obtained using SDS-PAGE. Data showed the soluble
expression of antibody molecules after the induction of IPTG
(Fig. 3A, lanes 2, 3 and 4);
soluble proteins were not expressed in lane 1. Western blot
analysis showed that for developing clear bands, the relative
molecular weight of soluble proteins should be ~28 kDa, indicating
the soluble expression of the antibody. The expression levels of
the soluble scFv were not significantly increased after being
induced for 4 and 6 h.
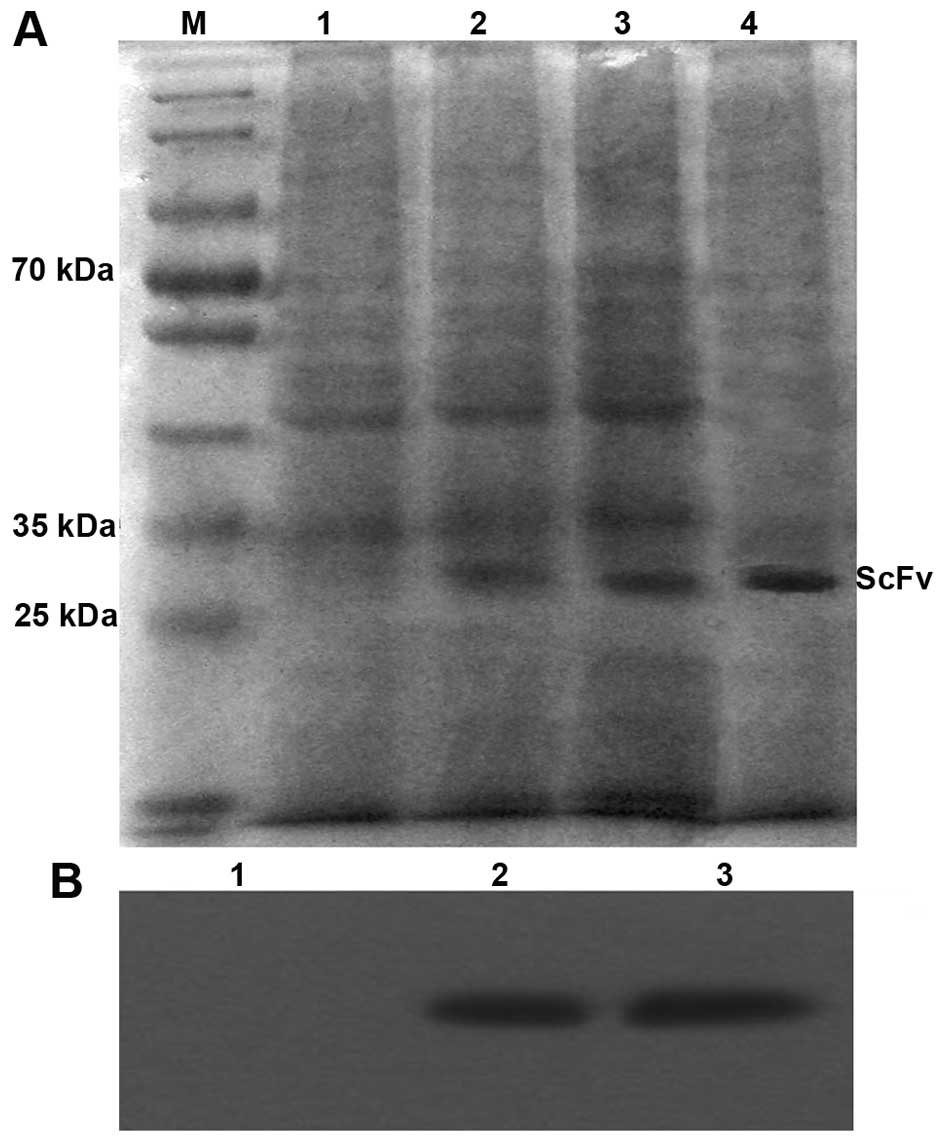 | Figure 3SDS-PAGE and western blot analysis of
the scFv expressed in E. coli HB2151. (A) M, protein
molecular weight marker; lane 1, uninduced E. coli HB2151;
lane 2, expression of scFv supernatants induced for 4 h; lane 3,
expression of scFv supernatants induced for 6 h; lane 4, purified
scFv. (B) Detection of purified scFv using western blotting. Lane
1, uninduced E. coli HB2151 with a negative result; lane 2,
purified scFv induced for 4 h; lane 3, purified scFv induced for 6
h. scFv, single-chain fragment of variable; SDS-PAGE, sodium
dodecylsulfate-polyacrylamide gel electrophoresis. |
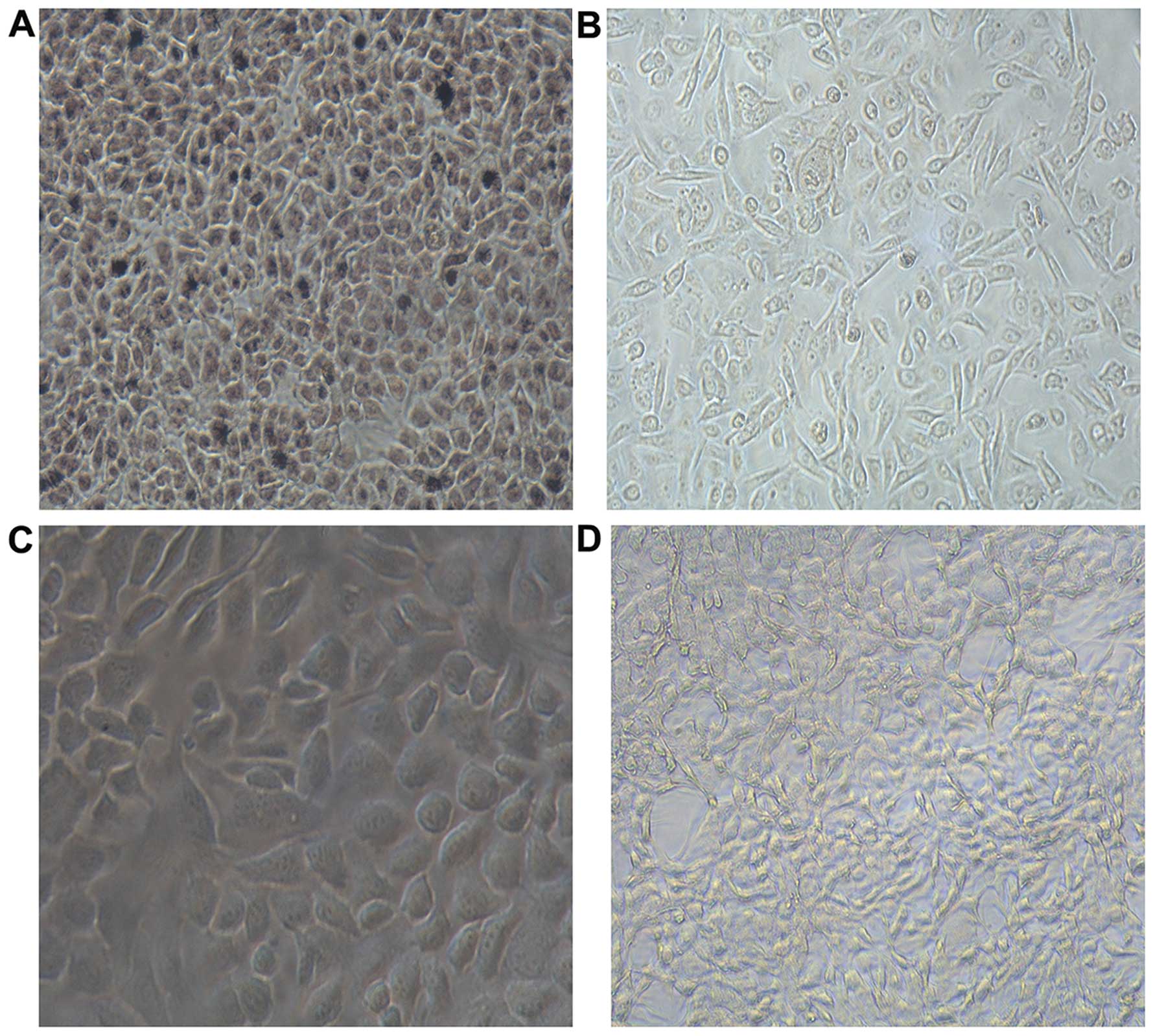
Detection of soluble scFv immune activity
through cell ELISA
Absorbance at A450nm was detected using ELISA.
Results of variance analysis showed that the TT cell group
(0.41±0.12), TEC group (0.13±0.01), SW480 cell group (0.20±0.03)
and PBS group (0.07±0.01) have statistically significant
differences (F=103.626, P=0.000). Multiple comparisons showed that
the difference between the TT cell and the other three groups were
statistically significant (P=0.000). The results showed that the
binding ability of scFv to TT cells was significantly higher than
that of the other groups, which means scFv has higher specificity
in TT cells. Light microscope images showed that scFv has high
immune activity (Fig. 4).
Inhibition of cell proliferation
test
Difference in concentrations of scFv on the
inhibition rate of TT and SW480 cells is shown in Table I. It indicated that inhibition rates
tend to increase with the increase in the concentration of drug in
the TT cell group. When the concentration of scFv was 0.1, 1 and 10
µmol/l, inhibition rate between the TT and SW480 cell groups
at the same concentration was statistically significant, and were
t=2.881, P=0.02; t=5.073, P=0.006; and t=7.324, P=0.000. These data
indicated that inhibition rate between two groups was different
when the concentration was 0.1, 1 and 10 µmol/l, while the
inhibition rate was not significantly different in other
concentrations. The inhibition rate of TT cells reached the peak
with a value of 0.59±0.15% when the concentration of scFv was 10
µmol/l. The comparison between TT cell groups at different
concentrations of scFv was statistically significant (F=4.767,
P=0.004); pairwise comparison showed no significant differences
between 10 and 1 µmol/l groups (P=0.253), but when 10
µmol/l group was compared with other concentrations they
were statistically significant (P<0.05). The comparison between
SW480 cell groups at different concentrations of scFv did not show
statistically significant difference (F=1.666, P=0.181).
 | Table IDifference in concentrations of scFv
on the inhibition rate of TT and SW480 cells (mean ± standard
deviation, %, n=5). |
Table I
Difference in concentrations of scFv
on the inhibition rate of TT and SW480 cells (mean ± standard
deviation, %, n=5).
| Concentration
(µmol/l) | TT cells | SW480 cells | t | P-value |
|---|
| 0.0001 | 0.03±0.32a | −0.06±0.17 | 0.562 | 0.589 |
| 0.001 | 0.11±0.20a | 0.02±0.07 | 0.964 | 0.362 |
| 0.01 | 0.28±0.23a | 0.06±0.13 | 1.992 | 0.08 |
| 0.1 | 0.31±0.16ab | 0.10±0.06b | 2.881 | 0.02 |
| 1 | 0.43±0.17ab | 0.04±0.03b | 5.073 | 0.006 |
| 10 | 0.59±0.15ab | 0.08±0.05b | 7.324 | 0.000 |
Radiolabeling, purification and
radiochemical purity test
Results showed that there were two radioactive peaks
after chromatography on the Sephadex G-200 column. The first
radioactive peak was at fraction 35–45 and the second at fraction
60–68. The first radioactive peak was taken and purified, labeled
131I-scFv. Labeling rate of 131I-scFv was
78.6±0.083%, when measured using trichloroacetic acid. The
radiochemical purity of purified 131I-scFv was
87.1±0.78% and specific activity was 2.9±0.32 MBq/µg. After
storage at 37°C in the human blood serum, the radiochemical
purities of 131I-scFv at 1, 6, 12 and 24 h were 95.1,
94.2, 93.1 and 92.6%, respectively, showing >90% purity.
Biodistribution studies
Biodistribution data in nude mice with TT cell
xenografts are shown in Table II.
131I-scFv was mainly distributed in the blood, liver,
kidney and intestines 12 h after the injection. High uptake (%
ID/g) was still noted 1 day after the injection. The kidney showed
high radioactivity uptake in all the tissues, which indicated that
kidney is the primary route of excretion of the label. However,
brain tissue showed less distribution, which indicates the
difficulty of the drug in crossing the blood-brain barrier. At 12 h
after injection, the tumors accumulated 4.32±0.12% ID/g, which
decreased to 2.33±0.11% ID/g at 3 days after the injection, but it
still showed a higher uptake (% ID/g) compared with other tissues.
131I-scFv in tumor tissue shows long residence time and
slow rate of clearance. The ratio of radioactivity of tumor:blood
and tumor:muscle increased with time gradually, reached a peak at
48 h; the tumor:blood ratio was 4.31, whereas, tumor:muscle ratio
was 5.19 at the peak of 48 h and, then both decreased.
 | Table IIBiodistribution of scFv in the nude
mice bearing medullary thyroid carcinoma cells (% ID/g, mean ±
standard deviation, n=3). |
Table II
Biodistribution of scFv in the nude
mice bearing medullary thyroid carcinoma cells (% ID/g, mean ±
standard deviation, n=3).
| 12 h | 1 day | 2 days | 3 days |
|---|
| Tumor | 4.32±0.12 | 4.05±1.25 | 3.58±0.65 | 2.33±0.11 |
| Blood | 5.35±1.21 | 4.11±1.19 | 0.83±0.12 | 0.58±0.07 |
| Liver | 10.22±0.57 | 7.02±1.33 | 2.41±0.33 | 1.08±0.11 |
| Kidney | 9.01±0.66 | 7.01±1.52 | 6.59±0.29 | 4.00±0.06 |
| Spleen | 5.32±0.86 | 2.33±0.81 | 1.66±0.72 | 0.51±0.23 |
| Heart | 4.21±1.12 | 1.65±0.38 | 0.60±0.72 | 0.43±0.11 |
| Lung | 2.52±1.04 | 1.32±0.52 | 0.52±0.66 | 0.32±0.20 |
| Stomach | 3.42±1.25 | 1.63±1.21 | 1.18±0.58 | 0.64±0.02 |
| Intestines | 6.66±1.05 | 4.08±0.87 | 3.26±0.53 | 0.94±0.32 |
| Brain | 1.22±0.11 | 0.85±0.12 | 0.66±0.26 | 0.32±0.08 |
| Muscle | 2.98±0.28 | 1.45±0.39 | 0.69±0.32 | 0.52±0.65 |
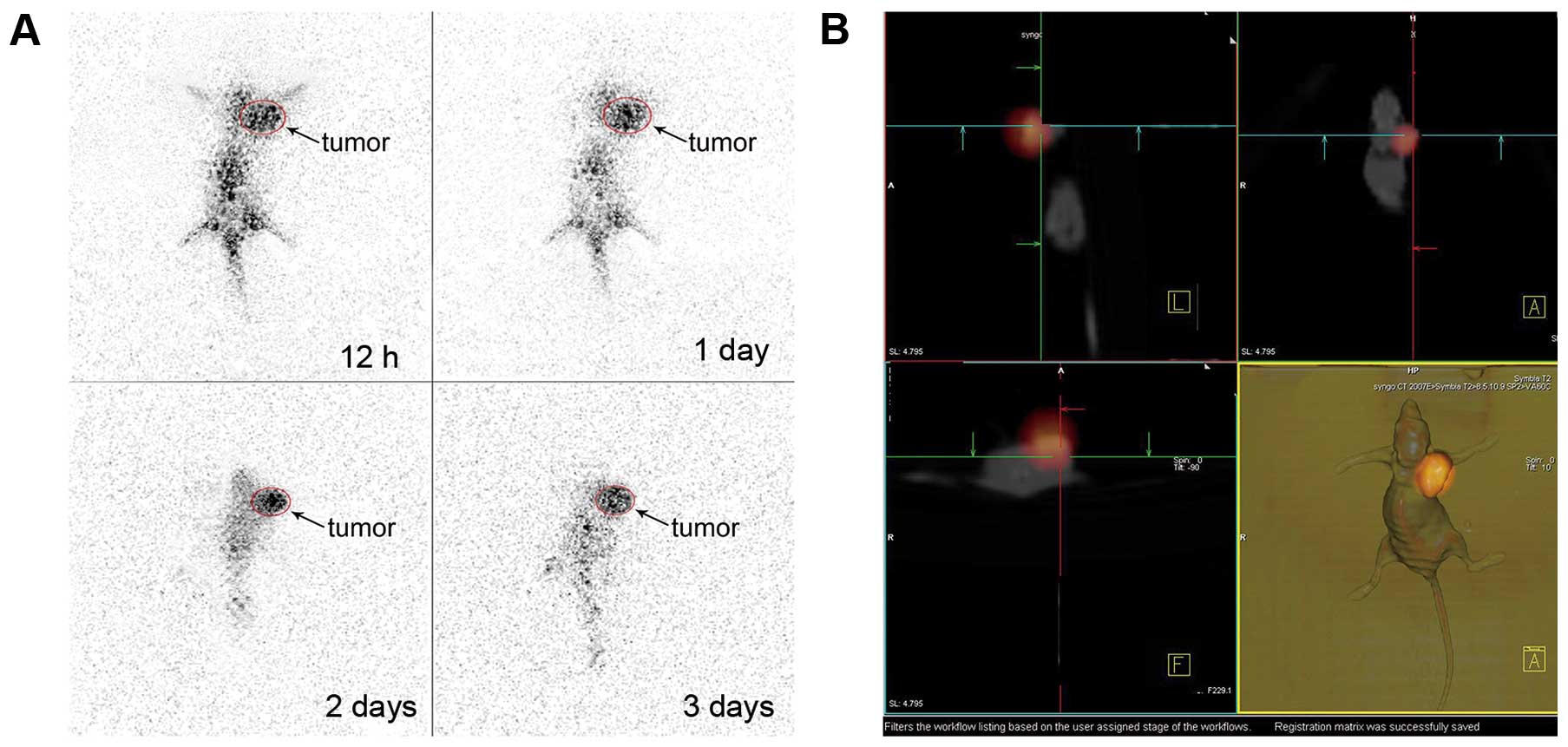
SPECT imaging
The static SPECT imaging of nude mice bearing human
MTC at 12 h, 1, 2 and 3 days after injection of
131I-scFv is shown in Fig.
5A. A high concentration of radioactivity in the tumor tissues
is shown, the background at 12 h after the injection of
131I-scFv was relatively high, and radioactivity
accumulated mainly in the liver and kidney. At 1 day after
administration, a high concentration of radioactivity accumulated
in the tumor of the mice, and the background was <12 h. At 2
days, concentration of radioactivity of other body tissues was very
low compared with tumor tissues, which showed the outline of tumor
tissue very clearly; at this point, rows of SPECT-CT fusion imaging
and area of fusion tumor are developing well. Fig. 5B shows the results.
Discussion
Monoclonal antibodies have increasingly become
important in the diagnosis and treatment of tumors. Though a
variety of anticancer drugs are available, their clinical
application is limited due to their failure to distinguish cancer
cells from normal cells, which cause toxic adverse effects
(28,29). Monoclonal antibodies directly
deliver the drug to the tumor, resulting in a high drug
concentration in the tumor. Monoclonal antibodies labeled with a
radionuclide are ideal vehicles for imaging since they easily reach
the tumor due to their small molecular weight and strong
penetrating force. In the present study, a human single-chain
antibody library of MTC was constructed and special scFv was
labeled with 131I. It was hypothesized that
131I-scFv can be a candidate for molecular probes in the
non-invasive imaging of tumor angiogenesis. The main finding of the
present study was that the new molecular probe preferentially
adhered to tumor angiogenesis. The data support the hypothesis that
131I-scFv can selectively accumulate in tumor tissues of
nude mice-bearing TT cells, indicating that the
131I-scFv is specific to MTC.
However, the ability to obtain the desired antibody
from the antibody library is constrained by many factors including
the capacity, diversity amplification, conditions of amplification
and screening of antibody library. Among these factors, the most
important being the amplification of all the antibody genes, making
design and application of a good primer particularly important in
increasing the storage capacity and maintaining the diversity of
the antibody libraries. Primer design should contain as much of the
variable region gene as possible (30). Variable region gene family is mostly
common in Vγ3 and Vκ3 types. Linker peptide sequence was also
designed in the process assembly of PCR primers, which makes
connecting peptide synthesis easier and increases the diversity of
the scFv gene. It is important to construct a large antibody
library to ensure high-affinity antigen-antibody screening
(31,32). Data showed that the capacity of our
library is 3×105, which is relatively small compared
with the diversity of natural human antibody. Although the size of
our library is not large, the quality was enough for use in
isolating the anti-MTC antibody. Our libraries were subjected to
inframe selection by fusion with kanamycin and ampicillin
resistance selection. The quality was validated using PCR. Gene
insertion rate of scFv was 87.5%, which further validates the
reliability of the antibody library.
Although classical screening technique could provide
successful screening results, it does not mean that the use of
screening strategy guarantees successful screening of antibody
against any antigens. It can only be used when the nature of the
antigen is clear and the antigen can be purified. For antigens such
as tissues or cell surface receptors, the cell surface, as well as
novel cell surface markers at specific differentiation or
disease-induced state. For those screening methods that cannot be
purified or are indeterminate, conventional screening method is no
longer applicable (33). A number
of studies have shown that complex antigen screening can lead to
better results in cell, organization and body panning method
(34). In the present study, TECs
were used as negative selection, thus removing some non-specific
phage antibody. Later TT cells acted as complex antigen conditions.
After five positive screening, MTC-associated antibody phage was
significantly enriched.
Use of 131I-labeled polypeptide is
feasible in the present study. Labeling rate of
131I-scFv was 78.6±0.083%, the radio-chemical purity of
purified 131I-scFv was 87.1±0.78% and the radiochemical
purity of 131I-scFv which was stored at 37°C in human
blood serum at 48 h was 92.6%. These results showed that
131I-scFv has good stability in vitro, which
meets the requirements of in vivo experimental studies on
peptide. SPECT imaging can directly observe the dynamic changes in
the imaging agents in the in vivo distribution. Results of
131I-scFv polypeptide imaging showed that in nude mice
xenografted with TT cells, dynamic changes in distribution in the
imaging agent in vivo can be directly observed using the
SPECT imaging, which was closer to clinical practice. The selection
of radionuclide is a critical factor to consider for
131I-scFv. Results of 131I-scFv in
vivo imaging of nude mice showed more accumulation of
radiotracer in the liver, which was also observed in the
biodistribution analysis, the liver fades with time. However,
imaging showed a low level of radioactivity in the intestine.
Concentrations of radioactivity were not found in the thyroid at
any time after injection mainly since the labeled compound can
target tumor vasculature with high affinity and specificity and it
is stable without iodothyronine. This is consistent with the
results of measurement of in vitro stability of
131I-scFv. SPECT imaging using 131I-scFv
revealed a higher tumor uptake in the mice-bearing TT cell
xenograft at 12 h as well as the whole body. With passage of time,
no obvious contrast was observed between the tumor and other
tissues, concentration of radioactivity in the tumor decreased, but
the rate of decline was slower than the body tissues. At 48 h,
tumor imaging was mostly clear mainly due to the contrast as
compared with the whole body. Hence, SPECT-CT imaging fusion which
shows the tumor site clearly, was performed. The results of
biodistribution suggested that the radio-labeled probe can
particularly accumulate in tumor tissues.
In conclusion, the biological characteristics, in
vitro stability, biodistribution and imaging properties of
131I-scFv were evaluated. High tumor uptake and
retention suggested that this radio-labeled peptide has the
potential to be used as a molecular probe for imaging tumor
angiogenesis in MTC. The use of 131I-scFv in diagnosing
different types of malignant tumors is expected to be explored.
Acknowledgments
The present study was funded by the National Natural
Science Foundation of China (no. 81071171).
References
|
1
|
Cerrato A, De Falco V and Santoro M:
Molecular genetics of medullary thyroid carcinoma: The quest for
novel therapeutic targets. J Mol Endocrinol. 43:143–155. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilliland FD, Hunt WC, Morris DM and Key
CR: Prognostic factors for thyroid carcinoma. A population-based
study of 15,698 cases from the Surveillance, Epidemiology and End
Results (SEER) program 1973–1991. Cancer. 79:564–573. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Monson JP: The epidemiology of endocrine
tumours. Endocr Relat Cancer. 7:29–36. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo T, Miyauchi A, Ito Y, Takamura Y,
Amino N and Hirokawa M: Diagnosis of medullary thyroid carcinoma by
calcitonin measurement in fine-needle aspiration biopsy specimens.
Thyroid. 17:635–638. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jo VY, Renshaw AA and Krane JF: Relative
sensitivity of thyroid fine-needle aspiration by tumor type and
size. Diagn Cytopathol. 41:871–875. 2013.PubMed/NCBI
|
|
6
|
Trimboli P, Cremonini N, Ceriani L,
Saggiorato E, Guidobaldi L, Romanelli F, Ventura C, Laurenti O,
Messuti I, Solaroli E, et al: Calcitonin measurement in aspiration
needle washout fluids has higher sensitivity than cytology in
detecting medullary thyroid cancer: A retrospective multicentre
study. Clin Endocrinol. 80:135–140. 2014. View Article : Google Scholar
|
|
7
|
Pusztaszeri MP, Bongiovanni M and Faquin
WC: Update on the cytologic and molecular features of medullary
thyroid carcinoma. Adv Anat Pathol. 21:26–35. 2014. View Article : Google Scholar
|
|
8
|
Trimboli P, Treglia G, Guidobaldi L,
Romanelli F, Nigri G, Valabrega S, Sadeghi R, Crescenzi A, Faquin
WC, Bongiovanni M, et al: Detection rate of FNA cytology in
medullary thyroid carcinoma: A meta-analysis. Clin Endocrinol.
82:280–285. 2015. View Article : Google Scholar
|
|
9
|
Essig GF Jr, Porter K, Schneider D, Debora
A, Lindsey SC, Busonero G, Fineberg D, Fruci B, Boelaert K, Smit
JW, et al: Fine needle aspiration and medullary thyroid carcinoma:
The risk of inadequate preoperative evaluation and initial surgery
when relying upon FNAB cytology alone. Endocr Pract. 19:920–927.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee S, Shin JH, Han BK and Ko EY:
Medullary thyroid carcinoma: Comparison with papillary thyroid
carcinoma and application of current sonographic criteria. AJR Am J
Roentgenol. 194:1090–1094. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi N, Moon WJ, Lee JH, Baek JH, Kim DW
and Park SW: Ultrasonographic findings of medullary thyroid cancer:
Differences according to tumor size and correlation with fine
needle aspiration results. Acta Radiol. 52:312–316. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SH, Kim BS, Jung SL, Lee JW, Yang PS,
Kang BJ, Lim HW, Kim JY, Whang IY, Kwon HS, et al: Ultrasonographic
findings of medullary thyroid carcinoma: A comparison with
papillary thyroid carcinoma. Korean J Radiol. 10:101–105. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trimboli P, Nasrollah N, Amendola S, Rossi
F, Ramacciato G, Romanelli F, Aurello P, Crescenzi A, Laurenti O,
Condorelli E, et al: Should we use ultrasound features associated
with papillary thyroid cancer in diagnosing medullary thyroid
cancer? Endocr J. 59:503–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Andrioli M, Trimboli P, Amendola S,
Valabrega S, Fukunari N, Mirella M and Persani L: Elastographic
presentation of medullary thyroid carcinoma. Endocrine. 45:153–155.
2014. View Article : Google Scholar
|
|
15
|
Fukushima M, Ito Y, Hirokawa M, Miya A,
Kobayashi K, Akasu H, Shimizu K and Miyauchi A: Excellent prognosis
of patients with nonhereditary medullary thyroid carcinoma with
ultrasonographic findings of follicular tumor or benign nodule.
World J Surg. 33:963–968. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trimboli P, Giovanella L, Valabrega S,
Andrioli M, Baldelli R, Cremonini N, Rossi F, Guidobaldi L,
Barnabei A, Rota F, et al: Ultrasound features of medullary thyroid
carcinoma correlate with cancer aggressiveness: A retrospective
multicenter study. J Exp Clin Cancer Res. 33:872014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trimboli P and Giovanella L: Serum
calcitonin negative medullary thyroid carcinoma: A systematic
review of the literature. Clin Chem Lab Med. 53:1507–1514. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rubello D, Rampin L, Nanni C, Banti E,
Ferdeghini M, Fanti S, Al-Nahhas A and Gross MD: The role of
18F-FDG PET/CT in detecting metastatic deposits of
recurrent medullary thyroid carcinoma: A prospective study. Eur J
Surg Oncol. 34:581–586. 2008. View Article : Google Scholar
|
|
19
|
Goldenberg DM, DeLand F, Kim E, Bennett S,
Primus FJ, van Nagell JR Jr, Estes N, DeSimone P and Rayburn P: Use
of radiolabeled antibodies to carcinoembryonic antigen for the
detection and localization of diverse cancers by external
photo-scanning. N Engl J Med. 298:1384–1386. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blottière HM, Steplewski Z, Herlyn D and
Douillard JY: Human anti-murine immunoglobulin responses and immune
functions in cancer patients receiving murine monoclonal antibody
therapy. Hum Antibodies Hybridomas. 2:16–25. 1991.PubMed/NCBI
|
|
21
|
Bird RE, Hardman KD, Jacobson JW, Johnson
S, Kaufman BM, Lee SM, Lee T, Pope SH, Riordan GS and Whitlow M:
Single-chain antigen-binding proteins. Science. 242:423–426. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Subedi GP, Satoh T, Hanashima S, Ikeda A,
Nakada H, Sato R, Mizuno M, Yuasa N, Fujita-Yamaguchi Y and
Yamaguchi Y: Overproduction of anti-Tn antibody MLS128 single-chain
Fv fragment in Escherichia coli cytoplasm using a novel pCold-PDI
vector. Protein Expr Purif. 82:197–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marasco WA and Dana Jones S: Antibodies
for targeted gene therapy: Extracellular gene targeting and
intracellular expression. Adv Drug Deliv Rev. 31:153–170. 1998.
View Article : Google Scholar
|
|
24
|
Leyton JV, Olafsen T, Sherman MA, Bauer
KB, Aghajanian P, Reiter RE and Wu AM: Engineered humanized
diabodies for microPET imaging of prostate stem cell
antigen-expressing tumors. Protein Eng Des Sel. 22:209–216. 2009.
View Article : Google Scholar :
|
|
25
|
Pavlinkova G, Booth BJ, Batra SK and
Colcher D: Radio-immunotherapy of human colon cancer xenografts
using a dimeric single-chain Fv antibody construct. Clin Cancer
Res. 5:2613–2619. 1999.PubMed/NCBI
|
|
26
|
Marks JD, Hoogenboom HR, Bonnert TP,
McCafferty J, Griffiths AD and Winter G: By-passing immunization.
Human antibodies from V-gene libraries displayed on phage. J Mol
Biol. 222:581–597. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Osbourn J, Jermutus L and Duncan A:
Current methods for the generation of human antibodies for the
treatment of autoimmune diseases. Drug Discov Today. 8:845–851.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Puleo S, Mauro L, Gagliano G, Lombardo R,
Li Destri G, Petrillo G and Di Carlo I: Liver damage after
transarterial chemoembolization without embolizing agent in
unresectable hepatocellular carcinoma. Tumori. 89:285–287.
2003.PubMed/NCBI
|
|
29
|
Chung KY and Saltz LB: Antibody-based
therapies for colorectal cancer. Oncologist. 10:701–709. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Staelens S, Desmet J, Ngo TH, Vauterin S,
Pareyn I, Barbeaux P, Van Rompaey I, Stassen JM, Deckmyn H and
Vanhoorelbeke K: Humanization by variable domain resurfacing and
grafting on a human IgG4, using a new approach for
determination of non-human like surface accessible framework
residues based on homology modelling of variable domains. Mol
Immunol. 43:1243–1257. 2006. View Article : Google Scholar
|
|
31
|
Beckmann C, Brittnacher M, Ernst R,
Mayer-Hamblett N, Miller SI and Burns JL: Use of phage display to
identify potential Pseudomonas aeruginosa gene products relevant to
early cystic fibrosis airway infections. Infect Immun. 73:444–452.
2005. View Article : Google Scholar :
|
|
32
|
Sidhu SS, Lowman HB, Cunningham BC and
Wells JA: Phage display for selection of novel binding peptides.
Methods Enzymol. 328:333–363. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hegmans JP, Radosevic K, Voerman JS,
Burgers JA, Hoogsteden HC and Prins JB: A model system for
optimising the selection of membrane antigen-specific human
antibodies on intact cells using phage antibody display technology.
J Immunol Methods. 262:191–204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Foy BD, Killeen GF, Frohn RH, Impoinvil D,
Williams A and Beier JC: Characterization of a unique human
single-chain antibody isolated by phage-display selection on
membrane-bound mosquito midgut antigens. J Immunol Methods.
261:73–83. 2002. View Article : Google Scholar : PubMed/NCBI
|