Introduction
In the past decades, lung cancer has become the
leading cause of cancer-related deaths globally, of which non-small
cell lung cancer (NSCLC) accounts for over 80% (1–3). Most
NSCLC patients are diagnosed at an advanced stage, with a 5-year
overall survival rate of 15% because of recurrence and metastasis
(4–6). Therefore, exploring the molecular
mechanisms underlying the development and progression of NSCLC is
urgently required for developing new preventable and therapeutic
strategies in clinic.
The accumulation of genetic and epigenetic
alterations play important roles in the progression of cancers
(7,8). Jumonji domain containing 2A (JMJD2A),
one of the histone demethylases, targets histone H3 on lysines 9
and lysines 36 as well as histone H1.4 on lysine 26. Several
studies have shown that JMJD2A was aberrantly expressed in lung,
gastric, bladder, breast, and other tumors and involved in the
regulation of tumor progression (9–16). For
instance, JMJD2A is significantly upregulated in gastric cancer,
indicating a poor prognosis (11).
Additionally, deregulation of JMJD2A is involved in the human
carcinogenesis through regulation of the G1/S transition (13). Our previous study uncovered that
SIRT2 suppressed NSCLC growth in a JMJD2A-dependent manner and
JMJD2A was negatively correlated with SIRT2 in NSCLC (15). However, the role of JMJD2A in NSCLC
and the underlying mechanisms are still poorly understood.
MicroRNAs (miRNAs), identified as the important
post-transcriptional regulatory factors, participate in several
important biological processes, such as proliferation,
differentiation, apoptosis and development (17–19).
Previous research has reported that miR-150 dysregulation was
involved in important processes of cancer, including lung, gastric
and prostate cancer (20–23). miR-150 has been confirmed to promote
the proliferation and migration of lung cancer cells by targeting
SRC kinase signaling inhibitor 1 or p53 (22,23),
but its interaction with JMJD2A remains unclear.
We investigated the role of JMJD2A in NSCLC and
sought to explore the underlying mechanisms. We identified
significant overexpression of JMJD2A in NSCLC, which was associated
with the proliferation and apoptosis of NSCLC cells. Moreover,
JMJD2A was also found to function in the progression of NSCLC via
regulating miR-150. JMJD2A shows promise as a potential therapeutic
target for NSCLC.
Materials and methods
Patients
One hundred and fifty samples of NSCLC tissues and
sixteen normal lung tissues were obtained from the Second
Affiliated Hospital of Soochow University. The diagnosis of NSCLC
was established using World Health Organization morphological
criteria. A written form of informed consent was obtained from all
patients and donors. The study was approved by the Clinical
Research Ethics Committee of the Second Affiliated Hospital of
Soochow University (Jiangsu, China).
Cell culture
Two normal lung cell lines (HBE and MRC-5) and four
NSCLC cell lines (H460, H1299, A549 and H520) were purchased from
the American Type Culture Collection (ATCC). The culture conduction
was according to our previous study (15). All cells were maintained at 37°C
with 5% CO2.
Cell proliferation and apoptosis
assay
Cell proliferation was monitored by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltet-razolium bromide (MTT)
Cell Proliferation/Viability Assay kit (Sigma, Germany) according
to the guidelines.
Apoptosis was evaluated using an Annexin V-Fluos and
Propidium Iodide (PI) Apoptosis Detection kit (Sigma) according to
the manufacturer's protocol.
Soft sugar colony formation assay
Colony formation assay was based on our previous
study (15). A549 cells were
suspended in 1.5 ml complete medium supplemented with 0.45% low
melting point agarose (Invitrogen, USA). The cells were placed in
35-mm tissue culture plates containing 1.5 ml complete medium and
agarose (0.75%) on the bottom layer. The plates were incubated at
37°C with 5% CO2 for 2 weeks. Cell colonies were stained
with 0.005% crystal violet and analyzed using a microscope.
Tumor xenograft experiments
Xenograft mouse experiments were performed as
described previously (15). The
tumors were harvested and weighed at the end of the experiments.
n=15 in each group.
Luciferase assay
The promoter activity of miR-150 was analyzed using
luciferase assay according to a previous study (11). In brief, the human miR-150 promoter
was cloned into the pGL4 reporter vector (Promega, USA) to generate
a miR-150-luc reporter vector. The cells were co-transfected with
miR-150-Luc/p-RL-luc and infected with control and JMJD2A virus.
Relative luciferase assays (Promega) were performed as described by
the manufacturer.
Quantitative RT-PCR (qRT-PCR) and western
blotting
Quantitative RT-PCR (qRT-PCR) and western blotting
analysis were performed as described previously (15). The relative fold change of mRNAs was
calculated using the 2−ΔΔCt method. GAPDH was used as
protein loading control. Primary antibodies against JMJD2A, Bcl-2,
Bax, pro-caspase-3, active-caspase-3, GAPDH and the corresponding
secondary antibodies were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA).
Cell infection and transfection
For JMJD2A overexpression or knockdown, A549 cell
lines were infected with adenovirus expressing ad-JMJD2A or
retrovirus expressing sh-JMJD2A, respectively, according to our
previous study (15).
For the miR-150 knockdown, miR-150 inhibitor
(LNA-anti-miR-150; Exiqon, Denmark) was added to the culture medium
according to a previous study (24). The transfection medium was replaced
4 h post-transfection by regular culture medium.
Statistical analysis
All experiments were performed at least three times.
Data are shown as mean ± standard deviation (SD). Statistical
differences among groups were determined using either Student's
t-test or two-way ANOVA. Predictors of differences in overall and
disease-free survival were analyzed using Kaplan-Meier analyses.
The correlation between JMJD2A and SIRT2 were analyzed using linear
regression. Statistical analysis was performed using SPSS software
version 16.0. p<0.05 was considered statistically
significant.
Results
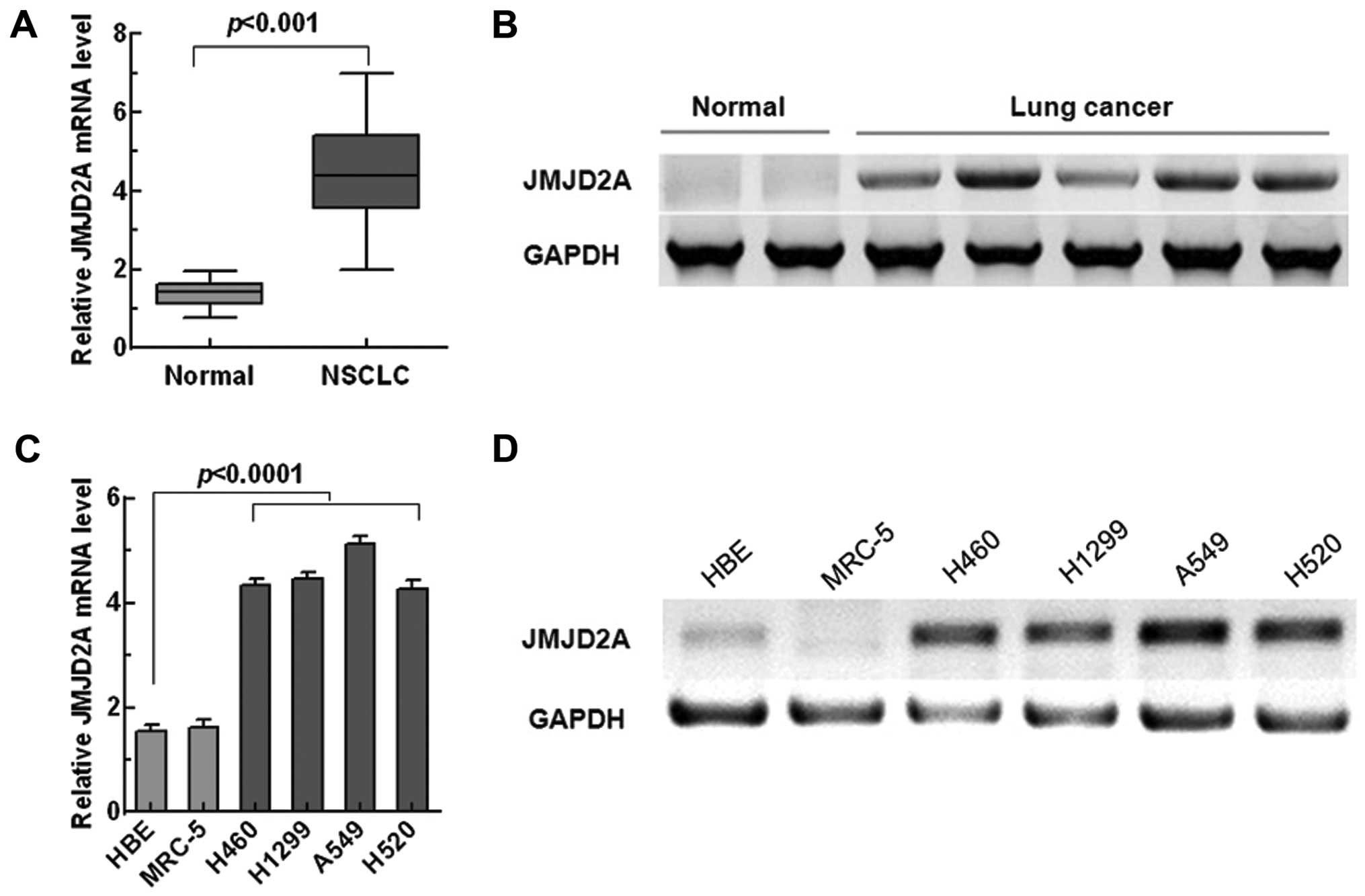
JMJD2A is overexpressed in NSCLC tissues
and cell lines
Several studies have reported that JMJD2A was
overexpressed in lung cancer (13,14).
To confirm whether JMJD2A was aberrantly expressed in NSCLC, we
tested the expression of JMJD2A in NSCLC tissues and cell lines
through qRT-PCR and western blotting. As shown in Fig. 1A and B, compared with normal lung
tissues, JMJD2A was significantly overexpressed in NSCLC tissues.
Similarly, significant overexpression of JMJD2A was observed in
NSCLC cell lines (p<0.0001, Fig. 1C
and D).
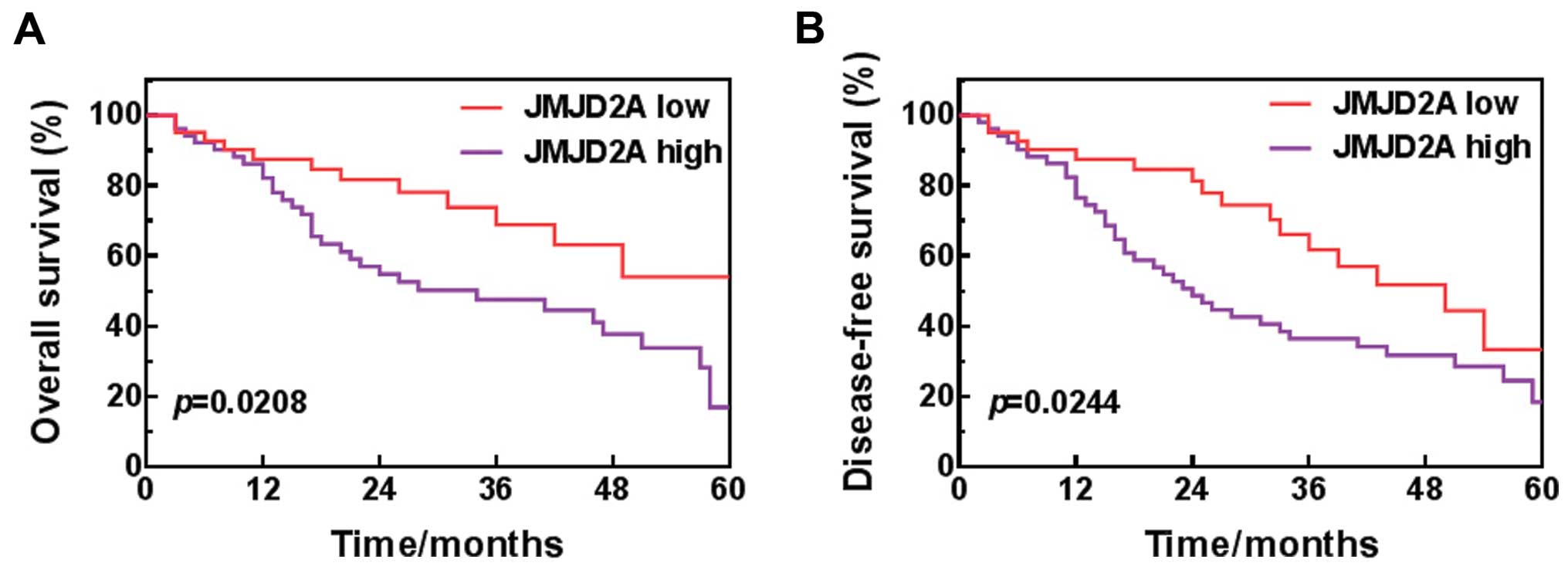
High level of JMJD2A is correlated with
poor prognosis
To explore the relationship between JMJD2A and NSCLC
patient prognosis, Kaplan-Meier analysis was performed to assess
the rate of overall survival and disease-free survival of patients.
We differentiated low-level JMJD2A (n=42) from high-level JMJD2A
(n=51) by choosing the mean JMJD2A level of the NSCLC patients as
the cut-off point. The results showed that the patients with high
level of JMJD2A had poorer overall survival and disease-free
survival than those with low level of JMJD2A (Fig. 2), indicating that JMJD2A could serve
as a prognostic predictor for NSCLC.
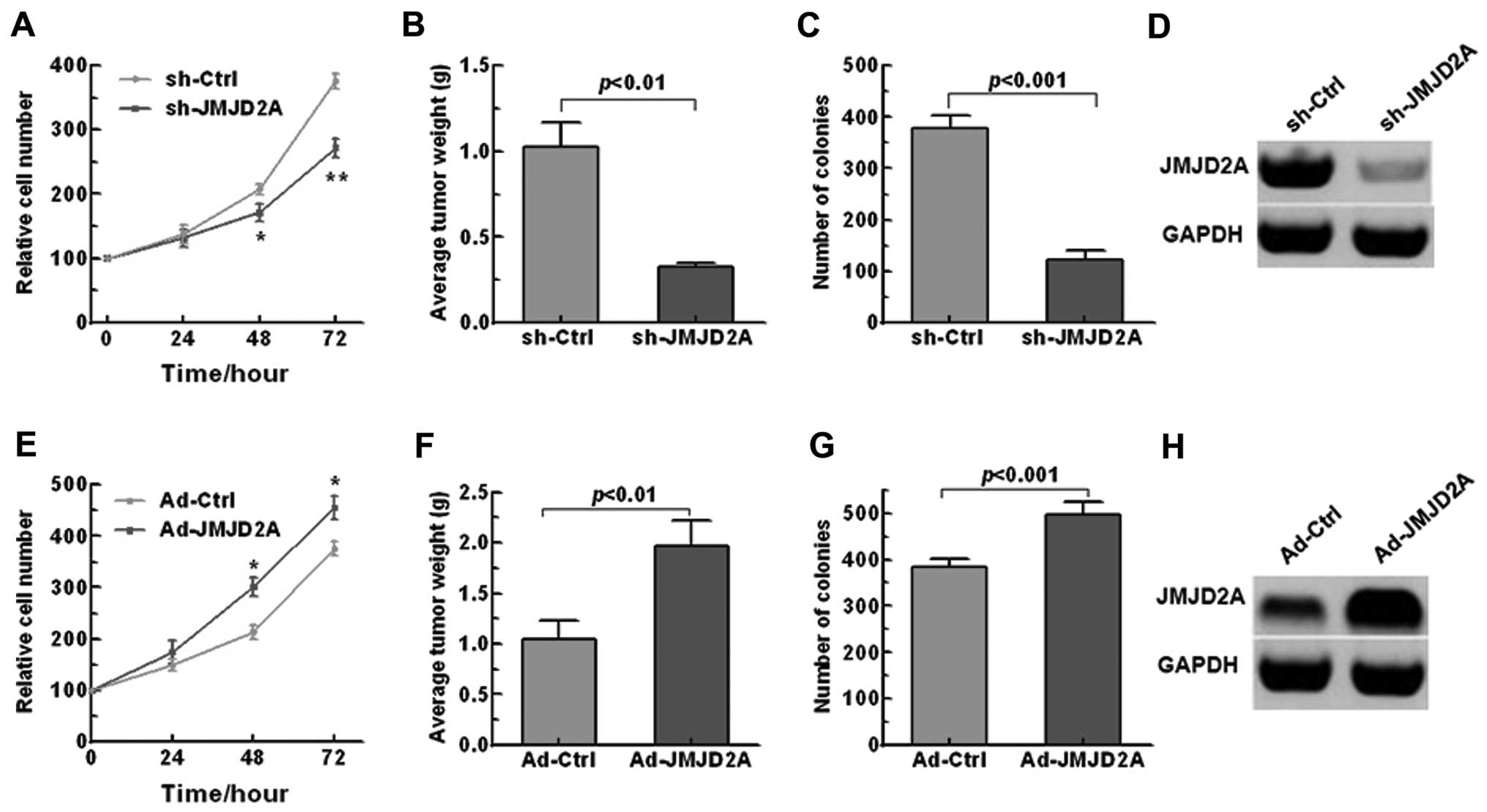
JMJD2A facilitates NSCLC growth and
transformation, inhibiting cell apoptosis
The significant overexpression of JMJD2A in NSCLC
tissues suggested possible biological significance in
tumorigenesis. To investigate the effects of JMJD2A on the tumor
progression in NSCLC, we knocked down or overexpressed JMJD2A in
A549 cells (Fig. 3D and H). We
found that JMJD2A knockdown significantly inhibited cell
proliferation and colony formation (Fig. 3A and C) while JMJD2A overexpression
promoted the NSCLC growth and transformation (Fig. 3E and G). To confirm the above
findings in vivo, a xenograft mouse model was adopted. As
shown in Fig. 3B and F, JMJD2A
knockdown significantly decreased tumor weight while AD-JMJD2A had
the opposite effect.
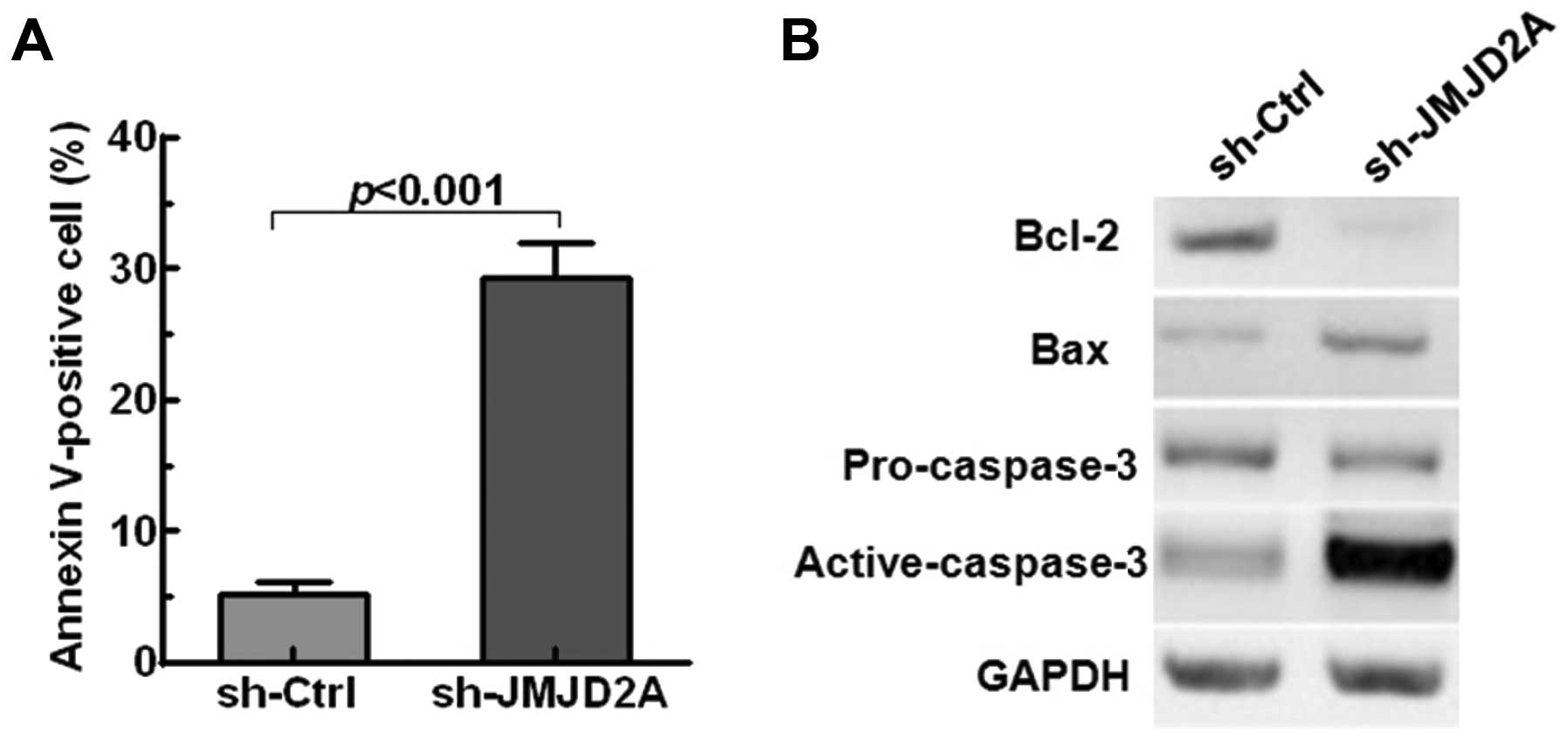
Furthermore, quantitative analysis of apoptotic
cells was performed. The results suggested that JMJD2A knockdown
significantly accelerated NSCLC cells apoptosis (p<0.001,
Fig. 4A). Moreover, JMJD2A
knockdown suppressed the expression of the anti-apoptotic proteins
Bcl-2 and promoted the levels of the pro-apoptotic proteins (Bax
and active-caspase-3) in A549 cells (Fig. 4B). All these results implied that
JMJD2A may facilitate tumor growth and regulate cell apoptosis in
NSCLC.
JMJD2A positively regulates the
expression of miR-150
We have revealed that JMJD2A was correlated with
poor prognosis and regulated NSCLC growth, the potential mechanisms
underlying the role of JMJD2A in NSCLC were investigated. miR-150
has been reported to promote the proliferation and migration of
lung cancer cells (23). Therefore,
we investigated whether JMJD2A regulated miR-150 in NSCLC. We first
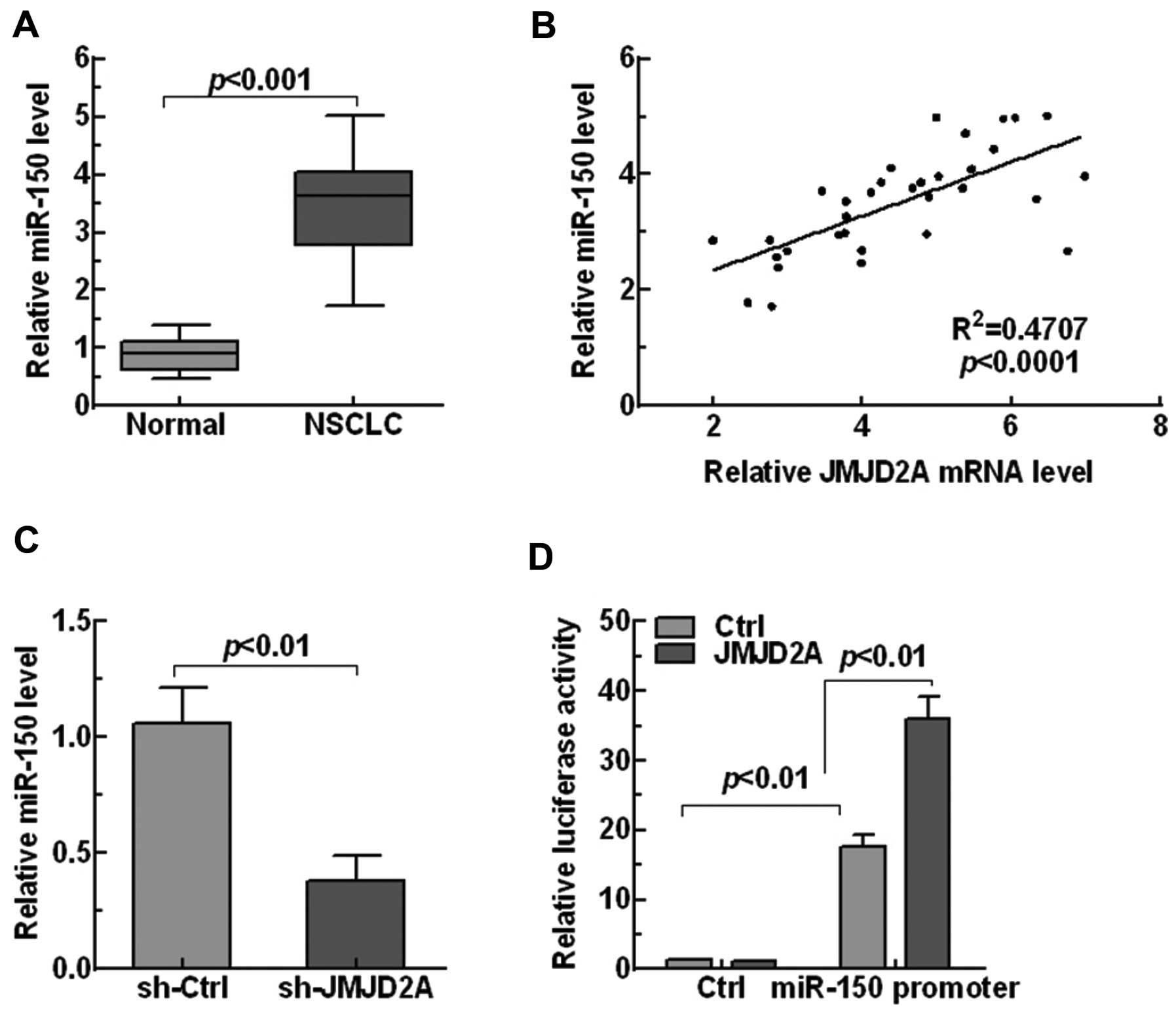
explored the expression of miR-150 in NSCLC tissues. The results
showed that miR-150 was markedly upregulated in NSCLC tissues
compared with the normal tissues (p<0.001, Fig. 5A). Moreover, linear regression
analysis showed that miR-150 level was significantly positively
related with JMJD2A level (p<0.0001, Fig. 5B), indicating JMJD2A may regulate
miR-150 level in NSCLC. Next, the relationship between JMJD2A and
miR-150 was explored. Knockdown of JMJD2A in human A549 lung cell
lines significantly reduced the miR-150 expression (p<0.001,
Fig. 5C). In addition, luciferase
analysis also showed that JMJD2A enhanced the promoter activity of
miR-150 (Fig. 5D). Our findings
revealed that JMJD2A positively regulated the expression of miR-150
in NSCLC.
JMJD2A regulates NSCLC cell growth and
apoptosis in a miR-150-dependent manner
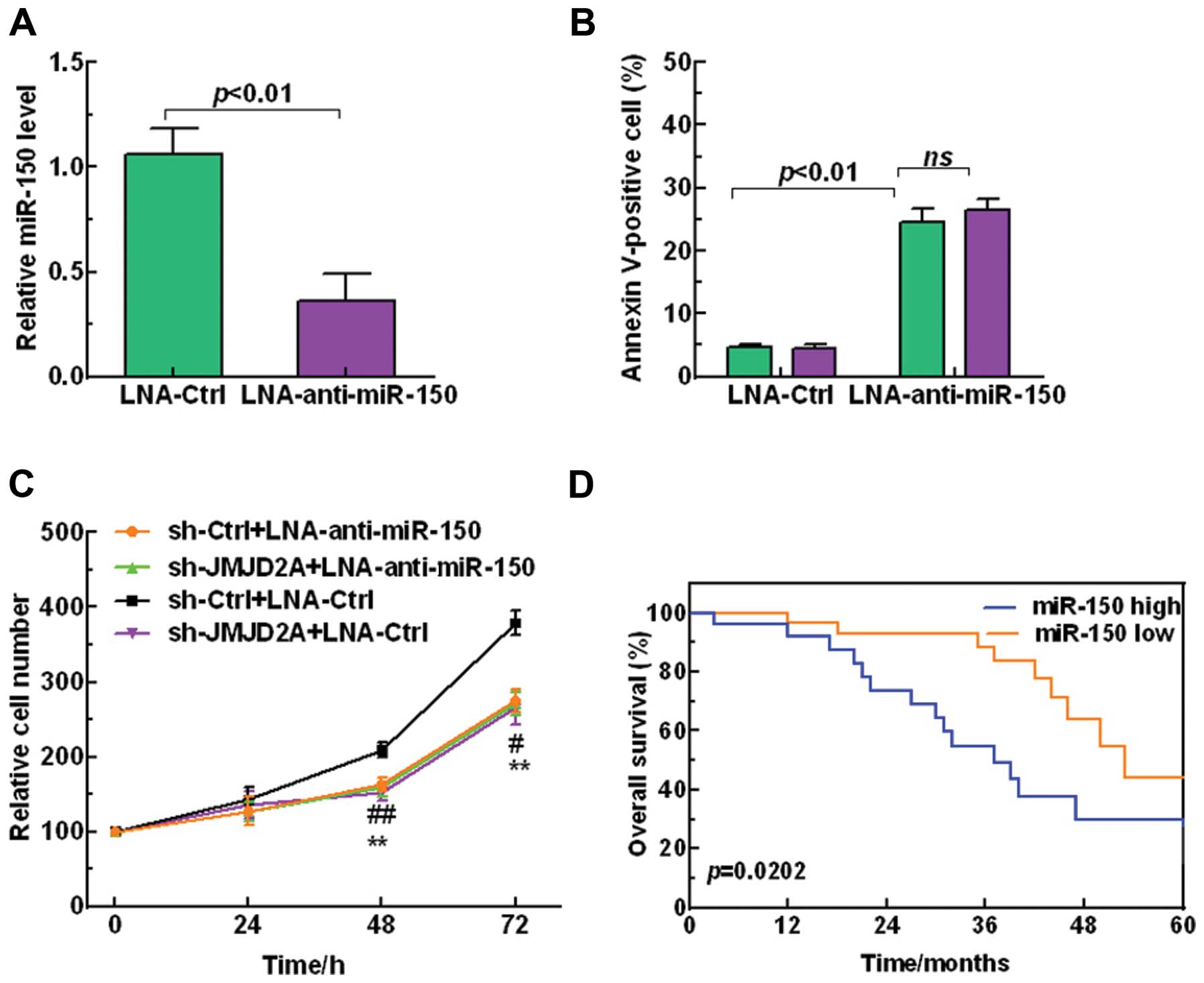
We further investigated the regulatory mechanism
between JMJD2A and miR-150 in the regulation of NSCLC
tumorigenesis. Firstly, LNA-anti-miR-150 was employed to silence
miR-150 (p<0.01, Fig. 6A).
Furthermore, Fig. 6B shows that
miR-150 reduction significantly promoted A549 cell apoptosis.
Moreover, we demonstrated that JMJD2A knockdown inhibited NSCLC
cell proliferation while silencing the miR-150 attenuated the
inhibition effect on cell proliferation (Fig. 6C), suggesting that the effect of
JMJD2A on NSCLC cells growth was dependent on miR-150. In addition,
Kaplan-Meier survival analysis showed that high miR-150 level
predicted a poor overall survival (Fig.
6D). Taken together, these results revealed that JMJD2A
regulated the tumor progression in NSCLC in a miR-150-dependent
manner.
Discussion
Notwithstanding that JMJD2A was aberrantly expressed
in various tumors and involved in the regulation of tumor
progression (9–16), its role in NSCLC growth was still
unknown. Our previous study uncovered that SIRT2 suppressed NSCLC
growth in a JMJD2A-dependent manner, which was negatively
correlated with JMJD2A in NSCLC (15). Thus, we conjectured that JMJD2A may
participate in the regulation of the NSCLC growth. To determine
this hypothesis, we first analyzed the expression of JMJD2A in
NSCLC tissues and cell lines. Consistent with the findings of
previous research (13–15), JMJD2A was overexpressed in NSCLC
tissues and cell lines. We also found that high level of JMJD2A was
associated with a poor prognosis in NSCLC. Additionally, JMJD2A was
knocked down or overexpressed to explore its functional role in
NSCLC progression. Our results showed that JMJD2A overexpression
promoted cell proliferation, colony formation while inhibited cell
apoptosis in NSCLC. Taken together, JMJD2A showed porential to play
a pivotal role in the tumorigenesis of NSCLC.
Furthermore, we explored the potential mechanisms
underlying the role of JMJD2A in NSCLC. Several miRNAs have been
reported to function as oncogenes or tumor suppressors in various
tumors (25–29). Recent studies have revealed that
miR-150 was aberrantly expressed in various types of diseases,
including pediatric intestinal Burkitt's lymphoma, irritable bowel
syndrome, dengue haemorrhagic fever, acute myeloid leukemia,
systemic sclerosis, and gastric cancer (20,30–34).
Importantly, miR-150 was significantly upregulated in lung cancer
tissues and promoted the proliferation and migration of lung cancer
cells (22,23). Thus, we speculated that JMJD2A may
have correlations with miR-150 in NSCLC. Our results showed that
miR-150 was markedly upregulated in NSCLC tissues and positively
related with JMJD2A. In addition, miR-150 was significantly
downregulated in NSCLC cells with JMJD2A knockdown, implying that
miR-150 may be regulated by JMJD2A in NSCLC. Luciferase analysis
also suggested that JMJD2A enhanced the promoter activity of
miR-150. All these findings indicated that miR-150 was a target of
JMJD2A in NSCLC. Besides, silencing the miR-150 significantly
promoted cell apoptosis in A549 cells, which agreed with the
results of previous studies (22,23).
To further confirm whether JMJD2A regulated NSCLC growth through
miR-150, we knocked down JMJD2A and miR-150, respectively, or
simultaneously in NSCLC cells. Obviously, JMJD2A knockdown
inhibited NSCLC cell proliferation while silencing miR-150
attenuated the inhibition effect on cell proliferation, indicating
that miR-150 was critically essential for the function of JMJD2A in
NSCLC. The results suggested that JMJD2A regulated NSCLC growth by
regulating miR-150.
Our previous study reported that SIRT2 suppressed
NSCLC growth in a JMJD2A-dependent manner (15). In the present study, we also
identified that JMJD2A promoted NSCLC growth by regulating miR-150.
Unfortunately, the deficiency is, the relationship between SIRT2
and miR-150 remaining largely unknown, which needs our further
attention.
In summary, this study demonstrates that JMJD2A
contributes to tumorigenesis in NSCLC by regulating miR-150.
Additionally, JMJD2A overexpression is associated with a poor
prognosis for NSCLC patients. JMJD2A may serve as a potential
therapeutic target for NSCLC.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
World Health Organization (WHO): The top
10 causes of death. July. 2013, Available at: http://who.int/mediacentre/factsheets/fs310/en/urisimplewho.int/mediacentre/factsheets/fs310/en/.
(Last accessed: October 2013).
|
|
4
|
Mostafa AA and Morris DG: Immunotherapy
for lung cancer: Has it finally arrived? Front Oncol. 4:2882014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manser R, Lethaby A, Irving LB, Stone C,
Byrnes G, Abramson MJ and Campbell D: Screening for lung cancer.
Cochrane Database Syst Rev. 6:CD0019912013.PubMed/NCBI
|
|
6
|
Patz EF Jr, Goodman PC and Bepler G:
Screening for lung cancer. N Engl J Med. 343:1627–1633. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Risch A and Plass C: Lung cancer
epigenetics and genetics. Int J Cancer. 123:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Langevin SM, Kratzke RA and Kelsey KT:
Epigenetics of lung cancer. Transl Res. 165:74–90. 2015. View Article : Google Scholar
|
|
9
|
Berry WL, Shin S, Lightfoot SA and
Janknecht R: Oncogenic features of the JMJD2A histone demethylase
in breast cancer. Int J Oncol. 41:1701–1706. 2012.PubMed/NCBI
|
|
10
|
Berry WL and Janknecht R: KDM4/JMJD2
histone demethylases: Epigenetic regulators in cancer cells. Cancer
Res. 73:2936–2942. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu CE, Liu YC, Zhang HD and Huang GJ:
JMJD2A predicts prognosis and regulates cell growth in human
gastric cancer. Biochem Biophys Res Commun. 449:1–7. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kauffman EC, Robinson BD, Downes MJ,
Powell LG, Lee MM, Scherr DS, Gudas LJ and Mongan NP: Role of
androgen receptor and associated lysine-demethylase coregulators,
LSD1 and JMJD2A, in localized and advanced human bladder cancer.
Mol Carcinog. 50:931–944. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kogure M, Takawa M, Cho HS, Toyokawa G,
Hayashi K, Tsunoda T, Kobayashi T, Daigo Y, Sugiyama M, Atomi Y, et
al: Deregulation of the histone demethylase JMJD2A is involved in
human carcinogenesis through regulation of the G(1)/S transition.
Cancer Lett. 336:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mallette FA and Richard S: JMJD2A promotes
cellular transformation by blocking cellular senescence through
transcriptional repression of the tumor suppressor CHD5. Cell Rep.
2:1233–1243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu W, Jiang K, Shen M, Qian Y and Peng Y:
SIRT2 suppresses non-small cell lung cancer growth by targeting
JMJD2A. Biol Chem. 396:929–936. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Das A, Chai JC, Jung KH, Das ND, Kang SC,
Lee YS, Seo H and Chai YG: JMJD2A attenuation affects cell cycle
and tumourigenic inflammatory gene regulation in lipopolysaccharide
stimulated neuroectodermal stem cells. Exp Cell Res. 328:361–378.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bueno MJ, Pérez de Castro I and Malumbres
M: Control of cell proliferation pathways by microRNAs. Cell Cycle.
7:3143–3148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Conrad R, Barrier M and Ford LP: Role of
miRNA and miRNA processing factors in development and disease.
Birth Defects Res C Embryo Today. 78:107–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schoolmeesters A, Eklund T, Leake D,
Vermeulen A, Smith Q, Force Aldred S and Fedorov Y: Functional
profiling reveals critical role for miRNA in differentiation of
human mesenchymal stem cells. PLoS One. 4:e56052009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Q, Jin H, Yang Z, Luo G, Lu Y, Li K,
Ren G, Su T, Pan Y, Feng B, et al: miR-150 promotes gastric cancer
proliferation by negatively regulating the pro-apoptotic gene EGR2.
Biochem Biophys Res Commun. 392:340–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Waltering KK, Porkka KP, Jalava SE,
Urbanucci A, Kohonen PJ, Latonen LM, Kallioniemi OP, Jenster G and
Visakorpi T: Androgen regulation of micro-RNAs in prostate cancer.
Prostate. 71:604–614. 2011. View Article : Google Scholar
|
|
22
|
Zhang N, Wei X and Xu L: miR-150 promotes
the proliferation of lung cancer cells by targeting P53. FEBS Lett.
587:2346–2351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao M, Hou D, Liang H, Gong F, Wang Y, Yan
X, Jiang X, Wang C, Zhang J, Zen K, et al: miR-150 promotes the
proliferation and migration of lung cancer cells by targeting SRC
kinase signalling inhibitor 1. Eur J Cancer. 50:1013–1024. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong S, Cheng Y, Yang J, Li J, Liu X, Wang
X, Wang D, Krall TJ, Delphin ES and Zhang C: MicroRNA expression
signature and the role of microRNA-21 in the early phase of acute
myocardial infarction. J Biol Chem. 284:29514–29525. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang LQ, Zhang Y, Yan H, Liu KJ and Zhang
S: MicroRNA-373 functions as an oncogene and targets YOD1 gene in
cervical cancer. Biochem Biophys Res Commun. 459:515–520. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
27
|
Pham H, Rodriguez CE, Donald GW, Hertzer
KM, Jung XS, Chang HH, Moro A, Reber HA, Hines OJ and Eibl G:
miR-143 decreases COX-2 mRNA stability and expression in pancreatic
cancer cells. Biochem Biophys Res Commun. 439:6–11. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Dong Y, Ti H, Zhao J, Wang Y, Li
T and Zhang B: Down-regulation of miR-145 and miR-143 might be
associated with DNA methyltransferase 3B overexpression and worse
prognosis in endometrioid carcinomas. Hum Pathol. 44:2571–2580.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Puerta-Gil P, García-Baquero R, Jia AY,
Ocaña S, Alvarez-Múgica M, Alvarez-Ossorio JL, Cordon-Cardo C, Cava
F and Sánchez-Carbayo M: miR-143, miR-222, and miR-452 are useful
as tumor stratification and noninvasive diagnostic biomarkers for
bladder cancer. Am J Pathol. 180:1808–1815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang M, Yang W, Li M and Li Y: Low
expression of miR-150 in pediatric intestinal Burkitt lymphoma. Exp
Mol Pathol. 96:261–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fourie NH, Peace RM, Abey SK, Sherwin LB,
Rahim-Williams B, Smyser PA, Wiley JW and Henderson WA: Elevated
circulating miR-150 and miR-342-3p in patients with irritable bowel
syndrome. Exp Mol Pathol. 96:422–425. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen RF, Yang KD, Lee IK, Liu JW, Huang
CH, Lin CY, Chen YH, Chen CL and Wang L: Augmented miR-150
expression associated with depressed SOCS1 expression involved in
dengue haemorrhagic fever. J Infect. 69:366–374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang X, Huang H, Li Z, Li Y, Wang X,
Gurbuxani S, Chen P, He C, You D, Zhang S, et al: Blockade of
miR-150 maturation by MLL-fusion/MYC/LIN-28 is required for
MLL-associated leukemia. Cancer Cell. 22:524–535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Honda N, Jinnin M, Kira-Etoh T, Makino K,
Kajihara I, Makino T, Fukushima S, Inoue Y, Okamoto Y, Hasegawa M,
et al: miR-150 down-regulation contributes to the constitutive type
I collagen overexpression in scleroderma dermal fibroblasts via the
induction of integrin β3. Am J Pathol. 182:206–216. 2013.
View Article : Google Scholar
|