Introduction
Colorectal cancer (CRC) is one of the most common
cancers worldwide and is the second leading cause of cancer-related
mortality in the developed countries (1). Hepatic metastasis (e.g., liver
metastasis) as an ominous event in the natural history and
progression of CRC contributing to the major cause of mortality in
CRC patients (2,3).
Cancer metastasis is a multistep process by which
cancer cells disseminate from primary tumors and establish
secondary lesions in distant organs (4). Invasive migration and proteolytic
extracellular matrix (ECM) remodelling are believed to be
independent processes that control cancer cell invasion (5). Matrix metalloproteinases (MMPs) are
members of pericellular proteases that are able to degrade ECM
components (6). Upregulation of
MMP-2 and MMP-9 was reported to accelerate cell migration and
invasion in CRC (7). In particular,
results from two recent high throughput-based investigations also
pointed to the vital roles of MMPs in CRC invasion and metastasis
(8,9).
Nicotine is one of the active ingredients and the
major addictive component of cigarette smoking. By binding and
activating of nicotinic acetylcholine receptors (nAChRs), nicotine
can activate several signalling pathways. The link between nicotine
to colon cancer tumorigenesis, i.e. cell proliferation and
angiogenesis have been extensively studied (10). Besides, there is also evidence
showing that nicotine can enhance colon cancer cell migration by
induction of fibronectin (11,12).
Nevertheless, the effects and novel mechanisms for nicotine on the
capacities for colon cancer cell invasion and metastasis are not
fully illustrated yet. In the present study, using LOVO and SW620
colon cancer cells grown in vitro, we revealed that nicotine
increased the cancer cells invasion along with enhanced activities
and expressions of metastasis-related MMP-1, -2 and -9. We also
proved that the bioactivities of nicotine we observed were depended
on activations of the nAchRs and downstream p38 MAPK signaling
pathway. Taken together, we reported that nicotine enhances
invasion and metastasis of human CRC cells through nAChRs
downstream p38 MAPK signaling pathway. Therefore, p38 MAPK may
serve as a novel therapeutic target for smoking-related human CRC
metastasis.
Materials and methods
Materials
Cell culture RPMI-1640 medium and fetal bovine serum
(FBS) were purchased from Invitrogen (Shanghai, China); antibodies
for MMP-1, MMP-2, MMP-9 and MMP-10 were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA); antibodies for
phospho-ERK1/2, phospho-Akt (Ser473), phospho-JNK, phospho-p38,
phospho-p65 (NF-κB), phospho-PI3K (55/85), ERK1/2, Akt, JNK, p38
and p65NF-κB were purchased from Cell Signaling Technology
(Beverly, MA, USA); antibody for β-actin was purchased from
Sigma-Aldrich (St. Louis, MO, USA); inhibitors of U0126, LY294002
and SB239063 were purchased from Beyotime Institute of
Biotechnology (Shanghai, China); Hexamethonium (Hex) bromide was
from Sigma-Aldrich (Shanghai, China). Goat anti-rabbit IgG antibody
conjugated to horseradish peroxidase and goat anti-rat IgG antibody
conjugated to horseradish peroxidase were purchased from Santa Cruz
Biotechnology, Inc.
Cell culture
The LOVO and SW620 human CRC cells were obtained
from the American Type Culture Collection (ATCC; USA). The cells
were grown in RPMI-1640 medium containing 10% of FBS, glutamine and
antibiotics (penicillin/streptomycin), and were maintained in a
humidified incubator at 37°C with a supply of 5% CO2/95%
air atmosphere.
Invasion assay
Cell invasion assays were carried out using modified
Boyden chambers consisting of Transwell (8-µm pore size;
Corning Costar Corp., Cambridge, MA, USA) membrane filter inserts
into 24-well tissue culture plates. In brief, the upper surfaces of
the membranes were coated with 50 µl Matrigel (BD
Biosciences, San Jose, CA, USA) 37°C for 6 h. SW620 or LOVO cells
(1.0×105) in 200 µl serum-free RPMI-1640 were
added to each Transwell chamber supplied with or without nicotine
or/and inhibitors as indicated. Cell culture media with 20% of FBS
were added in the lower chamber. Cells were allowed to invade
toward the underside of the membrane under cell culture condition
for 48 h before the non-invading cells were removed by wiping the
upper side of the membrane with a cotton swab and the invaded cells
were fixed with ice-cold methanol, then the inserts were stained
with 0.1% crystal violet in 20% ethanol for 30 min. The cells that
invaded to the underside of the membrane were quantitated by cell
counting under the light microscopy in five predetermined fields at
a magnification of ×100.
Gelatin zymography assay for MMP-2 and -9
activities
The gelatin zymography assay was used to determine
the activities of MMP-2 and MMP-9. Cell culture supernatants were
collected and concentrated in Amicon Ultra-4 Centrifugal Filter
Devices before loading with SDS sample buffer and electrophorese on
a 10% SDS polyacrylamide gel polymerized with 5 mg/ml gelatin.
After electrophoresis, the gels were renatured by soaking for 30
min at room temperature in 2.5% Triton X-100. To visualize the
bands, the gels were incubated in a developing buffer [50 mM
Tris-HCl buffer (pH 7.4), 10 mM CaCl2] overnight at 37°C
before they were stained with 0.5% Coomassie brilliant blue R-250
dissolved in 10% acetic acid and 30% carbinol, and were destained
in the washing solution without dye. Gelatinolytic bands were
observed as clear zones against the blue background.
Western blotting
Total cellular protein was prepared by lysing the
cells with 1xRIPA buffer (CST, Danvers, MA, USA) containing 1 mM
phenylmethylsulfonyl fluoride (PMSF). Protein concentration was
determined by the Bradford protein assay. A total of 40 µg
protein lysates from each sample were separated in 10% SDS-PAGE,
and were then transferred to polyvinylidene fluoride (PVDF)
membranes (Millipore, Billerica, MA, USA). After blocking with 5%
non-fat milk, the membranes were incubated overnight on ice with
primary antibodies against MMP-1, MMP-2, MMP-9, MMP-10,
phospho-ERK1/2, phospho-Akt, phospho-JNK, phospho-p38, phospho-p65
(NF-κB), phosph-PI3K (55/85), ERK1/2, Akt, JNK, p38, p65 (NF-κB)
and β-actin (all with 1:1,000 dilution). After washing, the
membranes were probed with a horseradish peroxidase-conjugated goat
anti-rabbit or anti-rat secondary antibody followed detection of
signals with the FluorChem E system (Protein Simple, Santa Clara,
CA, USA).
Statistical analysis
All assays were performed in triplicate, and
experiments were repeated at least three times. Data are presented
as the means ± SEM. Significant differences between two groups were
determined by Student's t-test with significance set at
p<0.05.
Results
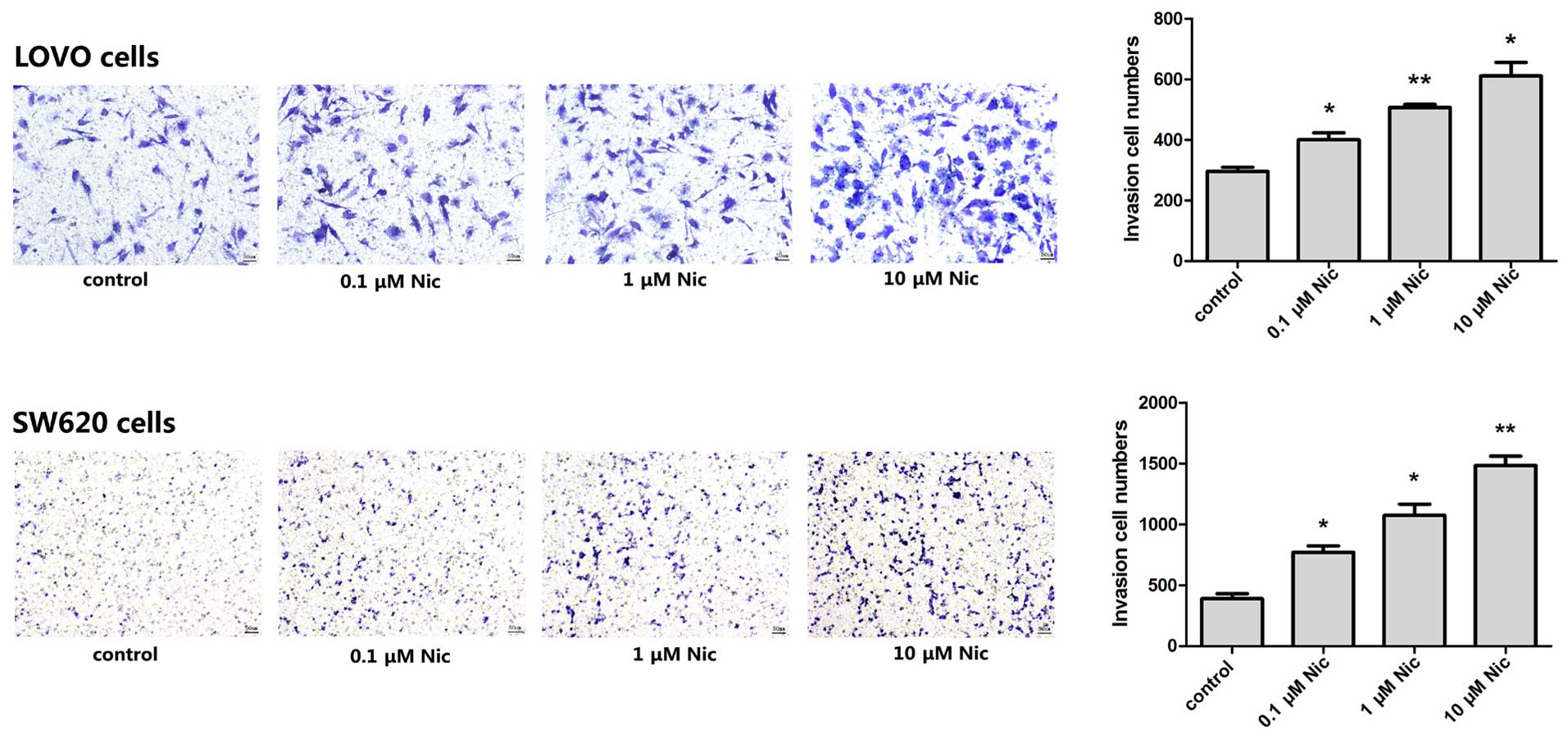
Nicotine promotes LOVO and SW620 human
CRC cells invasion in vitro
Firstly, we investigated whether nicotine affects
the invasion of CRC cells in a Transwell assay and BD Matrigel™ was
used to imitate the ECM. LOVO and SW620 colon cancer cells were
grown in the upper chamber in serum-free media without or with the
supplement of nicotine in a final concentration of 0.1, 1 and 10
µM, respectively (11–14).
Results showed that both starved LOVO and SW620 cells were able to
invade through the Matrigel with the attracting of serum rich media
in the lower chamber. Certain numbers of invaded cells stained in
blue were counted after 48 h (Fig.
1, controls). The presence of nicotine increased the number of
the invaded cells in a dose-dependent manner, and for both of the
LOVO and SW620 cells (Fig. 1).
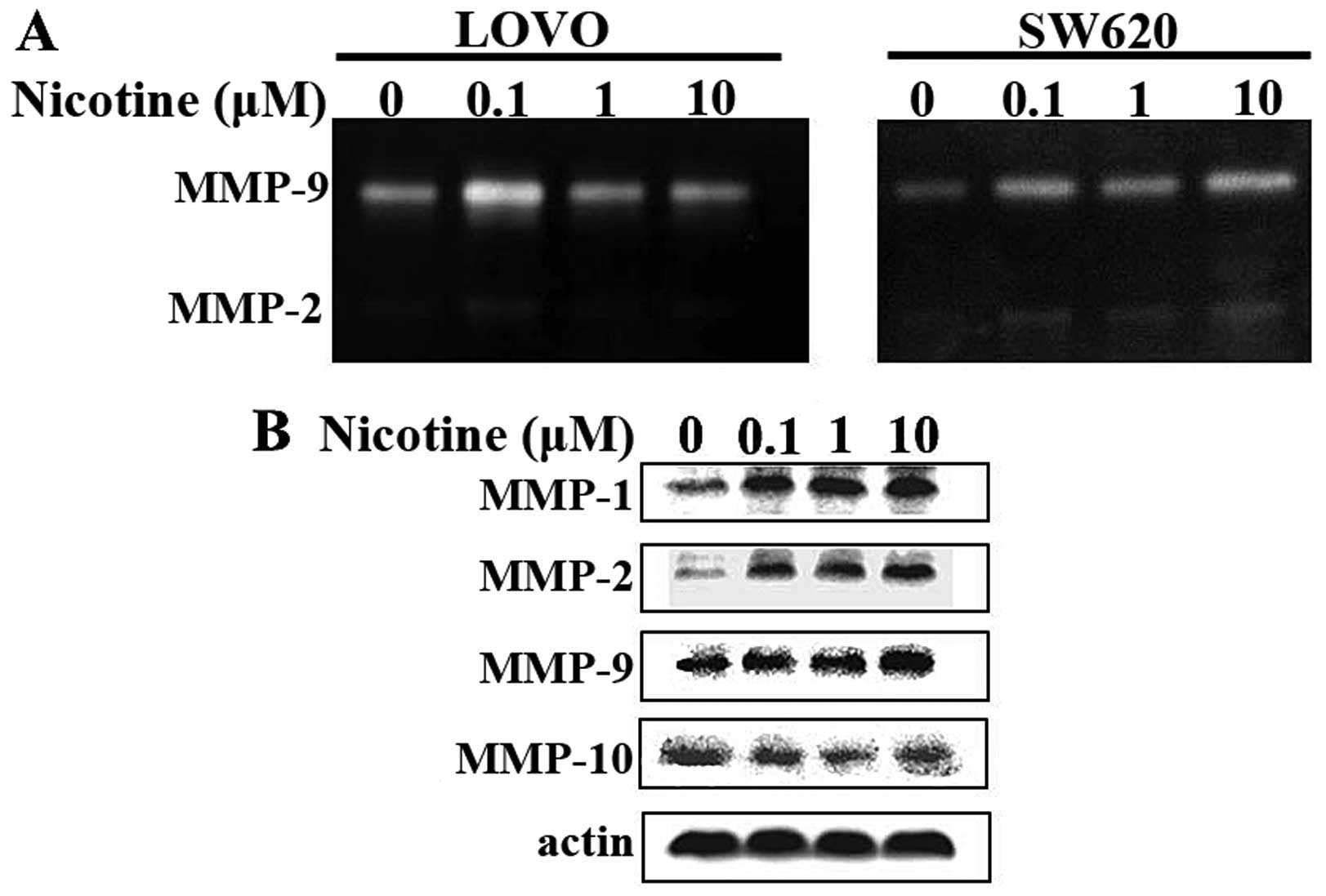
Nicotine promotes the activities and
expressions of MMPs in LOVO and SW620 human CRC cells
Next, we detected the activities and expressions of
metastasis-related MMPs for LOVO and SW620 human CRC cells without
or with the presence of nicotine. A gelatin zymography assay was
applied to detect the activity for secreted MMP-9 and -2. In brief,
LOVO and SW620 human CRC cells were grown in serum-free medium
containing various concentrations of nicotine as indicated above,
24 h before the cell culture supernatants were collected and
concentrated for gelatin zymography assay. As indicated in Fig. 2A, there were gelatinolytic bands for
MMP-9 and MMP-2, respectively. The nicotine treatments deepen the
bands for both MMPs, and in both cell lines.
In a similar experimental setting, western blot
assays were applied to detect the expression of a panel of MMPs
including MMP-1, -2, -9 and -10 in LOVO cells. As shown in Fig. 2B, the nicotine treatments increased
the expression of MMP-1, -2 and -9, but had no effect on
MMP-10.
Nicotine activates number of signal
transduction pathways in LOVO cells
We investigated the activation of relevant
intracellular MAPK (JNK, p38, ERK), NF-κB and PI3K/Akt signaling
pathways caused by nicotine stimulation. To this end, serum-starved
LOVO cells were stimulated without or with 10 µM nicotine
for 15, 30, 60 and 120 min, respectively, and then were subjected
to western blot assays examining the phosphorylation of JNK, p38,
ERK, NF-κB (p65), Akt and PI3K (p55/p85). As shown in Fig. 3, with total amount of protein was
the same, the presence of nicotine brought up the phosphorylation
levels of p38, ERK, Akt and PI3Kp55, but did not change that of JNK
and NF-κB (p65).
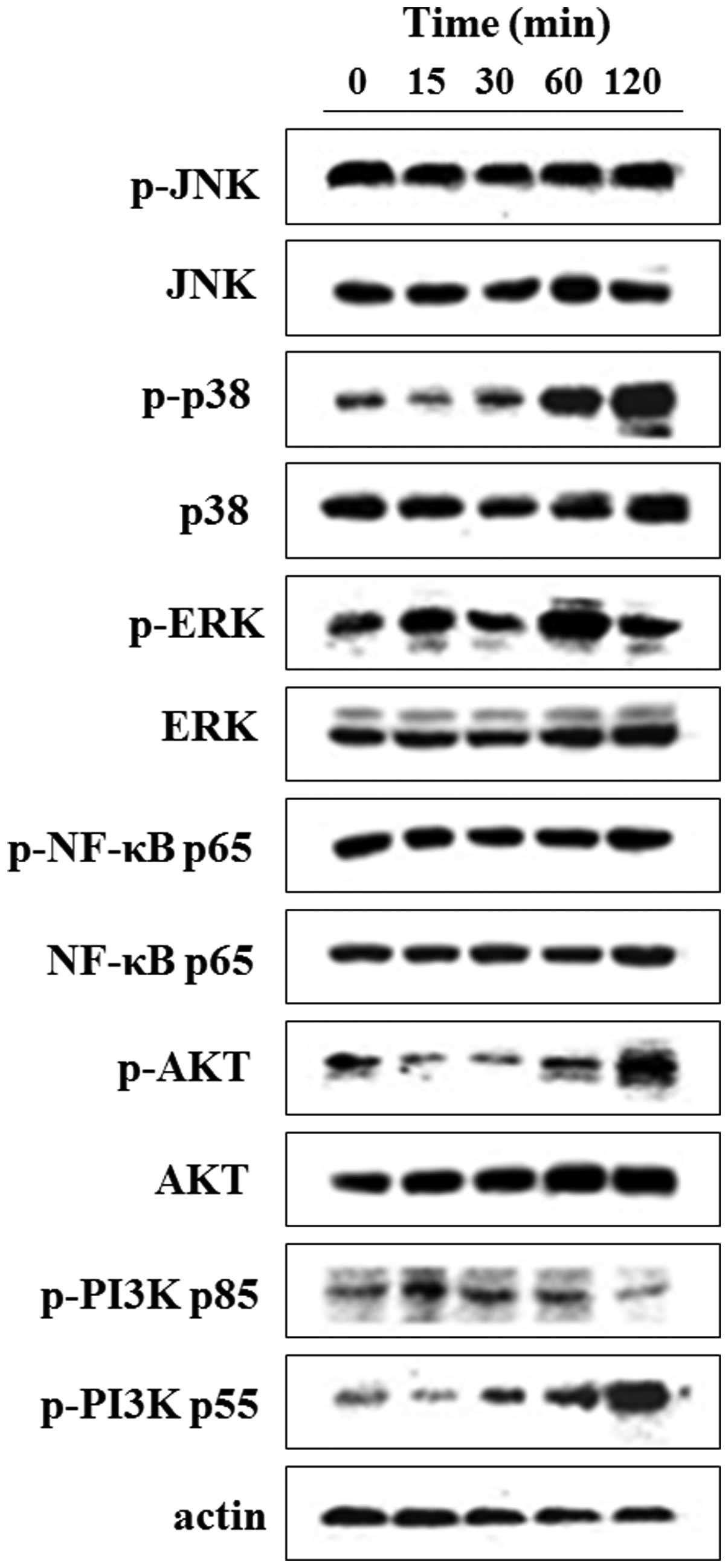 | Figure 3Nicotine activates number of signal
transduction pathways in LOVO cells. Serum-starved LOVO cells
(1.0×105) were incubated with or without 10 µM
nicotine for 15, 30, 60 and 120 min, and then cells were harvested
and cell lysates (40 µg of protein) were subjected to
western blotting with antibodies against p-JNK, JNK, p-p38, p38,
p-ERK, ERK, p-NF-κBp65, NF-κBp65, p-AKT, AKT, p-PI3Kp85, p-PI3Kp55
and β-actin, respectively. Results are representative of three
independent experiments. |
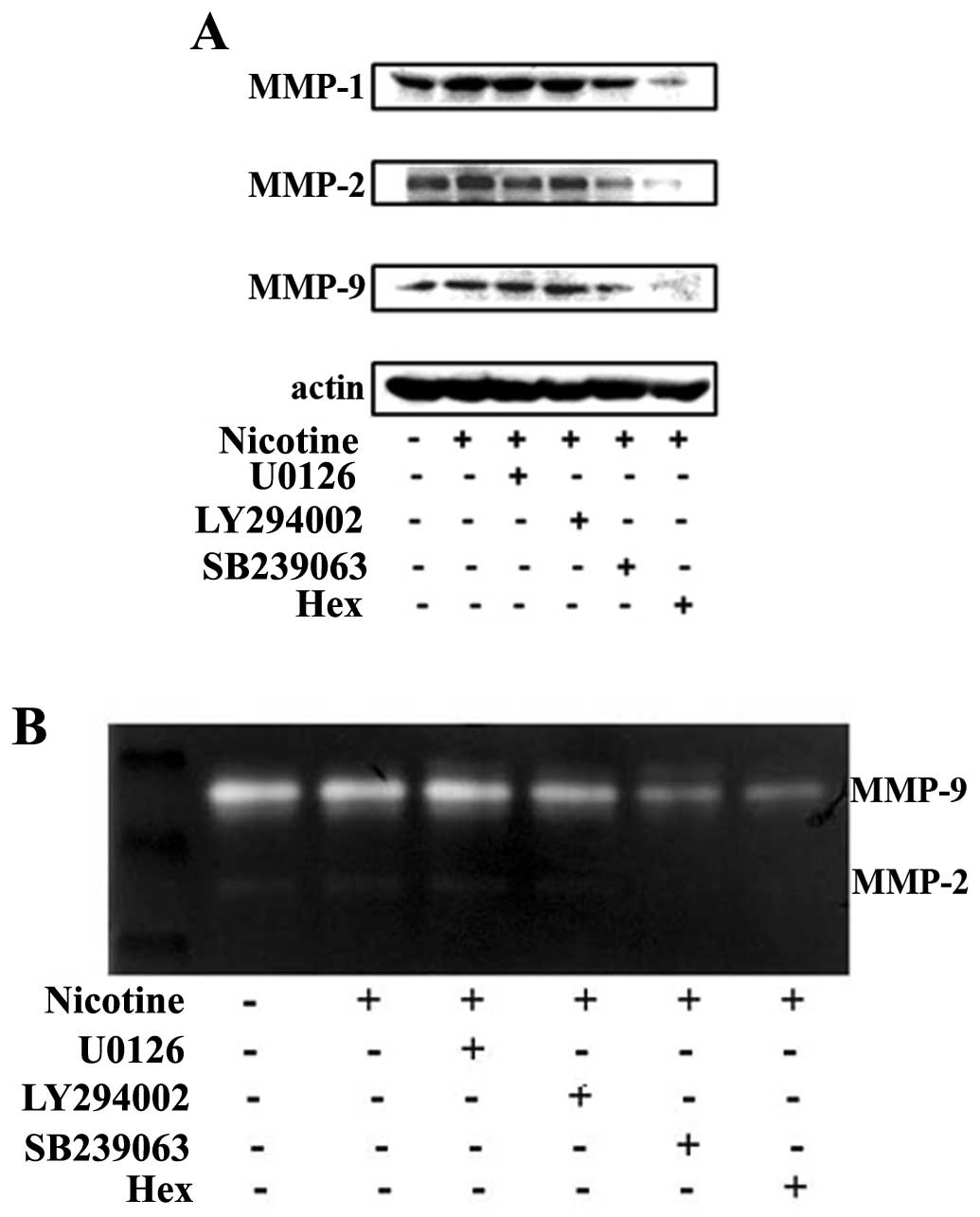
Stimulative effect of nicotine on MMPs
involves nAchRs and p38 MAPK in LOVO cells
Having shown that nicotine stimulates the activation
of MAPK/ERK, MAPK/p38 and PI3K (p55)/Akt signaling pathways, we
then tried to dissect their relationship to the observed
bioactivities for nicotine in regards of stimulating metastasis
related MMPs expression in colon cancer cells. To this end, a panel
of pharmaceutical inhibitors was applied before the cells were
exposed to nicotine stimulation. The activities and expressions of
MMPs were assayed by both gelatin zymography and western blotting.
In addition, we also included Hex, a pharmaceutical inhibitor for
nAChR in the experimental setting to illustrate the role for
receptor binding of nicotine.
As detailed in Fig.
4A, western blot results revealed that the pre-incubation of
cells with the p38 MAPK inhibitor SB239063 and nAChR antagonist
Hex, but not selective MAPK/ERK inhibitor U0126 and the PI3Ks
inhibitor LY294002 attenuated the stimulative effect of nicotine
for MMP-1, -2 and -9 expression levels. Similar results are also
shown in Fig. 4B, as the gelatin
zymography assay result revealed that the SB239063 and Hex
attenuated the stimulative effect of nicotine on the activation of
MMP-2 and -9.
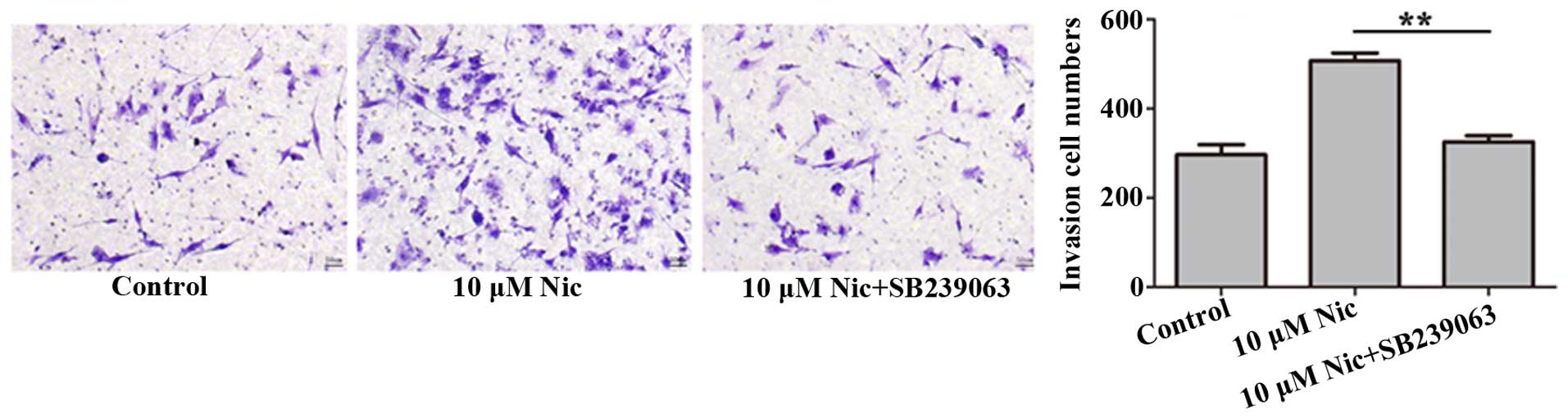
p38 MAPK are required for the stimulative
effect of nicotine on LOVO cell invasion in vitro
We further examined whether p38 MAPK also was
affected by stimulative effect of nicotine on LOVO cell invasion
in vitro. In this regards, we performed the Transwell
chamber invasion assay as described above with an additional step
of pre-incubation of the cells with SB239063. Results showed that
compared to nicotine-treated cells, the application of SB239063
indeed decreased the number of invaded cells (p<0.05), almost
down to control levels, i.e. without nicotine treatment (Fig. 5).
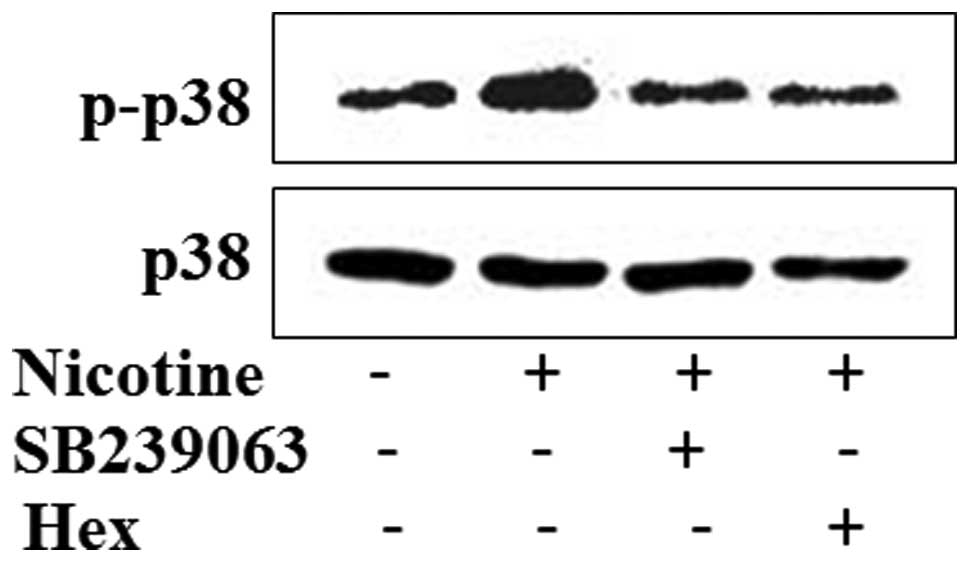
Nicotine activation is through nAchRs and
downstream of the p38 MAPK signaling pathway in LOVO cells
Finally, we addressed the question whether
activation of p38 MAPK in LOVO cells is indeed dependent on the
activation of nAChRs complex. To this end, LOVO cells were
pre-treated with SB239063 or Hex for 1 h, respectively, before
cells were exposed to nicotine stimulation for 24 h and
subsequently subjected to western blotting. As shown in Fig. 6, the application of Hex abolished
the enhanced p38 phosphorylation caused by nicotine stimulation, to
an extent similar to SB239063, the known p38 MAPK inhibitor
treatment.
Discussion
Sufficient evidence has revealed the association of
pharmaceutical stimulation of nicotine with tumor cell metastatic
dissemination. In mouse tumor transplantation models, nicotine
increases the growth and metastasis of various tumor cells
including the liver metastasis of orthotopically implanted
pancreatic adenocarcinoma cells, subcutaneously injected head and
neck squamous cell carcinoma cell lines and NNK-induced lung tumors
(15–17). In particular, Wei et al
showed that nicotine enhanced colon cancer cell migration through
activation of α7 nicotinic acetylcholine receptor (nAChR) and
induction of E-cadherin (12). In
the present study, the stimulatory effect on human colon cancer
cell invasion could also be observed after nicotine treatment in a
dose-dependent manner ranging from 0.1 to 10 µM after 48 h.
These concentrations of nicotine were similar to the amount of
nicotine intake in cigarette smokers (18). Therefore, nicotine does affect
colorectal cancer (CRC) tumor cell invasion and migration.
Matrix metalloproteinases (MMPs) are members of
pericellular proteases that are able to degrade proteolytic
extracellular matrix (ECM) components (6). Despite abundant evidence showing that
overexpression of MMPs correlates with tumor aggressiveness and
metastatic potential in various cancers such as ovarian, lung,
prostate, breast and pancreatic, the role of nicotine in this
scenario is not fully understood. There is only one previous study
revealing that nicotine contributes to pancreatic ductal
adenocarcinoma (PDA) metastasis by inducing MMP9 among others
(19). In the present study, we
showed that nicotine indeed increased LOVO and SW620 CRC cell
invasion along with enhanced activity and expression of MMP-1, -2
and -9. We believe that nicotine directly induced this effect via
the activation of nAchRs since the application of Hex, the
pharmaceutical nAChRs inhibitor abolished the increased expression
of the MMPs.
Little is known concerning the molecular mechanisms
by which nicotine promoted tumor development, particularly on the
metastatic process of CRC. In other cancer cells, nicotine has been
shown to affect various signaling cascades that initiated by the
binding of its receptor. In breast cancer cells, nicotine was shown
to promote cell migration through a signaling cascade involving
protein kinase C (PKC) activation and its downstream effector cdc42
(20). In PDA cells, nicotine was
shown to induce the expression of pro-metastasis and
pro-angiogenesis osteopontin (OPN) through nAChR and downstream
ERK1/2-dependent pathway (21,22).
Additionally, activation of the EGFR and downstream AKT and ERK
pathways was shown related to the enhancement of cell migration of
human malignant glioma cells as response to low concentrations of
nicotine treatment (23). In the
present study, we revealed that stimulation of CRC cells with
nicotine resulted in the activation MAPK/ERK, MAPK p38 and PI3K
(p55)/Akt signaling cascades but notably only MAPK p38 was
responsible for the MMPs related CRC cell invasion and migration.
Amongst the panel of pharmaceutical inhibitors, only p38 MAPK
inhibitor SB239063, but not selective MAPK/ERK inhibitor U0126 or
the PI3K inhibitor LY294002 attenuated the stimulative effect of
nicotine on CRC cell invasion and MMP-1, -2 and -9 expression.
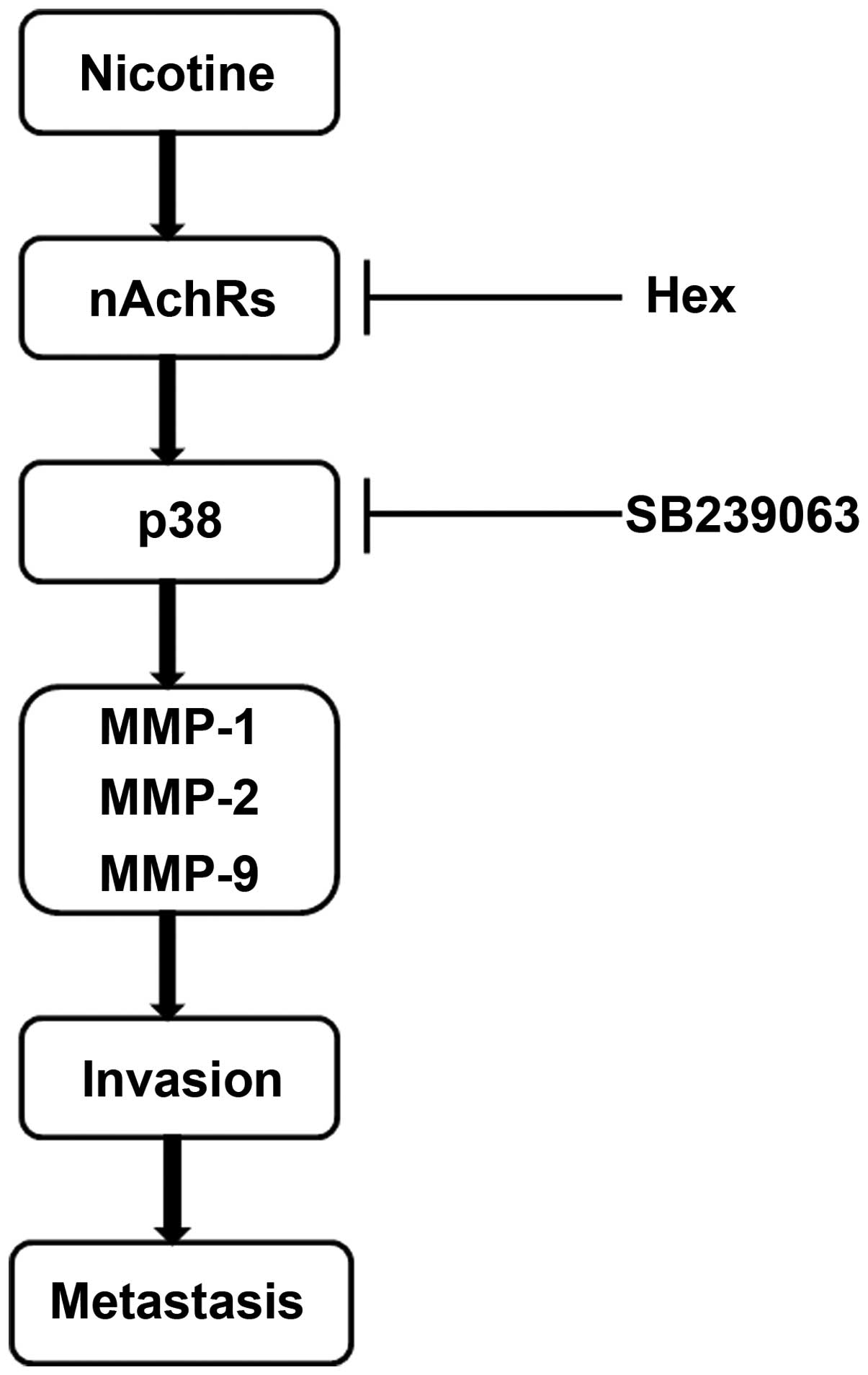
In summary, we demonstrated for the first time that
nicotine promotes CRC cell invasion by simultaneously upregulating
the expression and activity of MMP-1, -2 and -9 through
nAChR-mediated p38 MAPK signaling pathway (illustrated in Fig. 7). The p38 MAPK signaling pathway may
exert a central effect in the nicotine-mediated promotion of cell
invasion, suggesting that p38 MAPK may be a promising target for
the treatment of smoking-related human CRC metastasis.
Acknowledgments
This study was supported by grants from the Key
Project of Natural Science Foundation of Zhejiang Province
(LZ16H160003 to W.-B.C.), Qianjiang Talent Project of Zhejiang
Province (2013R10034 to J.Q.), and National Natural Science
Foundation of China (30600596 to W.-B.C.).
Abbreviations:
|
nAChR
|
nicotinic acetylcholine receptor
|
|
CRC
|
colorectal cancer
|
|
MMPs
|
matrix metalloproteinases
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NF-κB
|
nuclear factor-κB
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
References
|
1
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rudmik LR and Magliocco AM: Molecular
mechanisms of hepatic metastasis in colorectal cancer. J Surg
Oncol. 92:347–359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin K, Gao W, Lu Y, Lan H, Teng L and Cao
F: Mechanisms regulating colorectal cancer cell metastasis into
liver (Review). Oncol Lett. 3:11–15. 2012.PubMed/NCBI
|
|
5
|
Wolf K, Wu YI, Liu Y, Geiger J, Tam E,
Overall C, Stack MS and Friedl P: Multi-step pericellular
proteolysis controls the transition from individual to collective
cancer cell invasion. Nat Cell Biol. 9:893–904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sevenich L and Joyce JA: Pericellular
proteolysis in cancer. Genes Dev. 28:2331–2347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mook OR, Frederiks WM and Van Noorden CJ:
The role of gelatinases in colorectal cancer progression and
metastasis. Biochim Biophys Acta. 1705:69–89. 2004.PubMed/NCBI
|
|
8
|
Del Rio M, Mollevi C, Vezzio-Vie N, Bibeau
F, Ychou M and Martineau P: Specific extracellular matrix
remodeling signature of colon hepatic metastases. PLoS One.
8:e745992013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naba A, Clauser KR, Whittaker CA, Carr SA,
Tanabe KK and Hynes RO: Extracellular matrix signatures of human
primary metastatic colon cancers and their metastases to liver. BMC
Cancer. 14:5182014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grando SA: Connections of nicotine to
cancer. Nat Rev Cancer. 14:419–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dasgupta P, Rizwani W, Pillai S, Kinkade
R, Kovacs M, Rastogi S, Banerjee S, Carless M, Kim E, Coppola D, et
al: Nicotine induces cell proliferation, invasion and
epithelial-mesenchymal transition in a variety of human cancer cell
lines. Int J Cancer. 124:36–45. 2009. View Article : Google Scholar
|
|
12
|
Wei PL, Kuo LJ, Huang MT, Ting WC, Ho YS,
Wang W, An J and Chang YJ: Nicotine enhances colon cancer cell
migration by induction of fibronectin. Ann Surg Oncol.
18:1782–1790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi D, Guo W, Chen W, Fu L, Wang J, Tian
Y, Xiao X, Kang T, Huang W and Deng W: Nicotine promotes
proliferation of human nasopharyngeal carcinoma cells by regulating
α7AChR, ERK, HIF-1α and VEGF/PEDF signaling. PLoS One.
7:e438982012. View Article : Google Scholar
|
|
14
|
Wei PL, Chang YJ, Ho YS, Lee CH, Yang YY,
An J and Lin SY: Tobacco-specific carcinogen enhances colon cancer
cell migration through alpha7-nicotinic acetylcholine receptor. Ann
Surg. 249:978–985. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Treviño JG, Pillai S, Kunigal S, Singh S,
Fulp WJ, Centeno BA and Chellappan SP: Nicotine induces inhibitor
of differentiation-1 in a Src-dependent pathway promoting
metastasis and chemoresistance in pancreatic adenocarcinoma.
Neoplasia. 14:1102–1114. 2012. View Article : Google Scholar
|
|
16
|
Yu MA, Kiang A, Wang-Rodriguez J, Rahimy
E, Haas M, Yu V, Ellies LG, Chen J, Fan JB, Brumund KT, et al:
Nicotine promotes acquisition of stem cell and
epithelial-to-mesenchymal properties in head and neck squamous cell
carcinoma. PLoS One. 7:e519672012. View Article : Google Scholar
|
|
17
|
Davis R, Rizwani W, Banerjee S, Kovacs M,
Haura E, Coppola D and Chellappan S: Nicotine promotes tumor growth
and metastasis in mouse models of lung cancer. PLoS One.
4:e75242009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benowitz NL: Drug therapy Pharmacologic
aspects of cigarette smoking and nicotine addition. N Engl J Med.
319:1318–1330. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lazar M, Sullivan J, Chipitsyna G, Gong Q,
Ng CY, Salem AF, Aziz T, Witkiewicz A, Denhardt DT, Yeo CJ, et al:
Involvement of osteopontin in the matrix-degrading and
proangiogenic changes mediated by nicotine in pancreatic cancer
cells. J Gastrointest Surg. 14:1566–1577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo J, Ibaragi S, Zhu T, Luo LY, Hu GF,
Huppi PS and Chen CY: Nicotine promotes mammary tumor migration via
a signaling cascade involving protein kinase C and CDC42. Cancer
Res. 68:8473–8481. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chipitsyna G, Gong Q, Anandanadesan R,
Alnajar A, Batra SK, Wittel UA, Cullen DM, Akhter MP, Denhardt DT,
Yeo CJ, et al: Induction of osteopontin expression by nicotine and
cigarette smoke in the pancreas and pancreatic ductal
adenocarcinoma cells. Int J Cancer. 125:276–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sullivan J, Blair L, Alnajar A, Aziz T, Ng
CY, Chipitsyna G, Gong Q, Witkiewicz A, Weber GF, Denhardt DT, et
al: Expression of a prometastatic splice variant of osteopontin,
OPNC, in human pancreatic ductal adenocarcinoma. Surgery.
146:232–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Khalil AA, Jameson MJ, Broaddus WC, Lin PS
and Chung TD: Nicotine enhances proliferation, migration, and
radioresistance of human malignant glioma cells through EGFR
activation. Brain Tumor Pathol. 30:73–83. 2013. View Article : Google Scholar
|