Introduction
Human chorionic gonadotropin (hCG), which together
with lutropin (LH), thyrotropin (TSH) and follitropin (FSH) belong
to one glycoprotein hormone family, is produced during pregnancy by
trophoblast cells (1). The hormone
controls a number of processes, including embryo implantation,
angiogenesis and development of the chorion (2).
The hormone is a glycoprotein composed of subunits α
(hCGα) and β (hCGβ). Expression of human chorionic gonadotropin and
in particular its β subunit (hCGβ) has been also documented for
30–60% of tumors of different origin (2,3). What
is more, it has been shown that transcriptional activity of genes
encoding hCGβ is higher in advanced cancers. Its presence in serum
and urine of cancer patients and in tumor tissues in many cases is
of prognostic significance as it correlates with a poor response to
therapy and thus poor prognosis (4).
It is postulated that, similarly to the way
chorionic gonadotropin promotes angiogenesis during placenta
formation and modulates the mother's immune system towards
immunotolerance of the fetus, the hormone can play analogous
functions in oncogenesis. This action of CG is said to contribute
to neovascularization as well as desensitization of the
immunological system towards cancer cells (5,6).
Several publications also demonstrate that CG promotes tumor growth
by exhibiting anti-apoptotic and/or proliferative effects (7,8).
Despite numerous studies on the role of CG and its
free subunits in carcinogenesis, the biological functions and
mechanisms behind their action remain unknown.
One hypothesis, which explains hCGβ action in
cancers, is based on the hormone's structural similarity to growth
factors such as TGFβ (transforming growth factor β), PDGFβ
(platelet-derived growth factor β) and NGF (nerve growth factor)
characterized by the presence of a cysteine knot motif. It is
suggested that due to this structural similarity, CG, like the
aforementioned growth factors, may affect cells by regulation of
their proliferation (9).
Recent studies have shown that the interaction of
the β subunit of hCG with its receptor (LHCGR, luteinizing
hormone/choriogonadotropin receptor) may lead to initiation of
signaling cascades associated with extracellular signal-regulated
kinases (ERK) and protein kinase B (PKB/Akt). The kinases are
involved in cell cycle regulation, apoptosis and cancer
pathogenesis (6,10). Thus, CG might also be considered a
tumor-growth promoter.
Results of in vitro studies obtained
independently by Hamada et al and our group support this
hypothesis showing that silencing of CGB genes induces
apoptosis (11,12). We demonstrated that the inhibition
of CGB expression caused apoptosis both in cells expressing the
anti-CGB construct as well as in neighboring cells, lacking
the construct. This neighboring cell effect could be explained by
the overall decreased level of hCGβ in culture medium, a state
which presumably allows more interactions between receptors and
ligands essential for induction of apoptosis (12).
The in vitro results were verified on animal
models. These experiments proved in turn that non-cancerous ovarian
epithelial cells stably transfected with CGB overexpressed
BCL-X(L) (B-cell lymphoma-extra large) and were characterized by a
lower rate of apoptosis as well as more intense proliferation with
increased levels of cyclin E/D1 and Cdk2/4/6. In consequence, cell
phenotype changes led to increased tumorigenesis of xenografts in
athymic nude mice. Histopathological analysis of tumors arising
from implanted xenografts overexpressing hCGβ demonstrated that the
cells were poorly differentiated (13).
What is more it has been shown that expression of
hCGβ correlates with a decreased apoptosis rate in human cervical
carcinomas (14). This suggests
that the presence of hCG, produced by tumors, protects cells from
initiation of apoptosis, allowing tumor development and growth.
Thus, in the present study we attempted to verify
the association between expression of CGB and factors
regulating apoptosis such as BCL2 (B-cell lymphoma 2),
BAX (Bcl-2-like protein 4) and BIRC5 (survivin).
Materials and methods
Specimens of ovarian cancer tissue were obtained
from 45 patients with ovarian cancer treated by surgery at the
Department of Gynecologic Oncology, Poznan University of Medical
Sciences. Histological subtypes and the grade of the carcinomas are
presented in Table I.
 | Table IThe histological subtype and grade of
the studied ovarian carcinomas. |
Table I
The histological subtype and grade of
the studied ovarian carcinomas.
| Tumor grade | Ovarian carcinoma
subtype
|
|---|
| Serous | Endometrial | Mucinous | Clear cell |
|---|
| G1 | 0 | 0 | 0 | 0 |
| G2 | 10 | 5 | 2 | 2 |
| G3 | 22 | 3 | 0 | 0 |
| Not determined | 2 | 0 | 0 | 0 |
| Total | 34 | 8 | 2 | 2 |
The control group included samples of ovaries (n=8)
and fallopian tubes (n=6) that lacked cancerous transformations as
assessed by macroscopic and microscopic examination by a
pathologist. Control samples were obtained from postmenopausal
patients who underwent total hysterectomy with additional
oophorectomy due to myomas.
Both ovarian cancer tissues and control tissues were
maintained in RNAlater buffer (Sigma Life Sciences, St. Louis, Mo,
USA) at −80°C prior to further processing.
The study was approved by the Ethics Review Board of
Poznan University of Medical Sciences (resolution no. 748/08) and
all patients participated after informed consent.
SKOV-3 and OVCAR-3 cell lines, established from
ovarian carcinomas and used as an in vitro model of cancer,
were obtained from the Global Bioresource Center, American Type
Culture Collection (ATCC, Manassa, VA, USA; SKOV-3 ATCC®
HTB-77™, OVCAR-3 ATCC® TB-161™). The choice of cell
lines was determined by the fact that SKOV-3 is characterized by
the lack of LHCGR expression, while OVCAR-3 cells express
the receptor (15). The presence
and lack of LHCGR expression in the analyzed cells was also
validated experimentally by quantitative polymerase chain reaction
(qPCR) (data not shown). Cells were cultured and passaged under
standard conditions.
Cell culture and transfection
Cells were seeded so as to at the time of
transfection obtain the optimal 70–80% confluence. Transfection
with the construct carrying the CGB5 gene, chosen as one of
the most transcriptionally active CGB genes (17), utilizing TurboFect (Thermo
Scientific, Rockford, IL, USA) was performed according to the
manufacturer's protocol. The CGB5 construct was prepared as
described previously (16).
Non-transfected cells were used as control.
Additionally in order to estimate the transfection efficiency, an
equal amount of reporter eGFP plasmid (invitrogen, San Diego, CA,
USA) was used to transfect the study cell lines.
Analyses were conducted 72 h after transfection. All
experiments were performed in triplicates.
RNA isolation
Total RNA from the SKOV-3 and OVCAR-3 cells as well
as from the analyzed tissues (100–300 mg) was isolated using
TriPure Isolation reagent (Roche Diagnostics, Mannheim, Germany)
according to the manufacturer's protocol. An air-dried pellet of
RNA was suspended in UltraPure DNase/RNase-Free Distilled Water
(Invitrogen, Carlsbad, CA USA). RNA was stored at −80°C prior to
subsequent steps.
cDNA synthesis
One microgram of RNA was used for each reverse
transcription reaction with universal primer p(dT)10 and
Transcriptor Reverse Transcriptase (Roche Diagnostics), according
to the delivered protocol.
qPCR
To assess the expression level of the analyzed
genes, qPCR with sequence-specific primers, hydrolysis probes and
the LightCycler® TaqMan® Master kit (Roche
Diagnostics) was performed. Probes and primers used in the
reactions are presented in Table
II.
 | Table IIPrimers and hydrolysis probes used in
qPCR assays. |
Table II
Primers and hydrolysis probes used in
qPCR assays.
| Gene(s) | Starters and
probes |
|---|
| CGB1-9 |
5′-TACTGCCCCACCATGACC-3′ |
|
5′-CACGGCGTAGGAGACCAC-3′ |
| #71 (Roche
Diagnostics UPL) |
| LHCGR |
5′-CACTCGACTATCATCTGCCTACCTCC-3′ |
|
5′-GAAAAGCATTTCCTGGTATGGTGG-3′ |
|
6FAM-CAGGCATCAGAAAGTTTCCAGATGTTACGA-
BBQ |
| (TIB MOLBIOL) |
| BAX |
5′-ATGTTTTCTGACGGCAACTTC-3′ |
|
5′-ATCAGTTCCGGCACCTTG-3′ |
| #57 (Roche
Diagnostics UPL) |
| BCL2 |
5′-TACCTGAACCGGCACCTG-3′ |
|
5′-GCCGTACAGTTCCACAAAGG-3′ |
| #75 (Roche
Diagnostics UPL) |
| BIRC5 |
5′-TCTGCTTCAAGGAGCTGGA-3′ |
|
5′-AAAGTGCTGGTATTACAGGCGTA-3′ |
| #36 (Roche
Diagnostics UPL) |
| HPRT | Human HPRT Gene
Assay cat no. 05046157001 |
| (Roche Diagnostics
UPL) |
The reaction mix for the TaqMan reactions contained:
5 µl of cDNA, 1X TaqMan Master Mix (Roche Diagnostics), 0.1
µM hydrolysis probe (TaqMan) and 0.4 µM of each
primer. qPCR program consisted of initial denaturation at 95°C for
10 min followed by 45 3-step cycles: 95°C/10 sec hold for
denaturation, 60°C/30 sec hold for primers and probe hybridization
and product extension, and 72°C/1 sec hold for data
acquisition.
Relative expression of genes analyzed by TaqMan
assays was normalized against HPRT expression (Human HPRT
Gene Assay; Roche Diagnostics). All experiments were performed in
triplicates using independently synthesized cDNA.
Statistical analysis
Data were analyzed using the Statistica 10 software
package (StatSoft, Krakow, Poland) with Mann-Whitney U test in case
of cell lines and Kruskal-Wallis test with Dunn's post hoc test in
case of tissues. Differences were considered statistically
significant if p-value was <0.05. Associations between
particular gene expression were tested using Spearman's rank
correlation.
For graphical representation, qPCR raw data were
transformed and presented as the logarithm to base ten.
Results
Overexpression of CGB affects the
expression of BCL2, BAX and BIRC5 in the ovarian cancer cell
lines
In order to test the hypothesis that hCGβ
synthesized by cancer cells protects them against the induction of
apoptosis, ovarian cancer cell lines, OVCAR-3 and SKOV-3, were
transfected with a construct carrying the CGB5 gene, which
is one of the most transcriptionally active CGB genes
(17).
Introduction of the construct into OVCAR-3 and
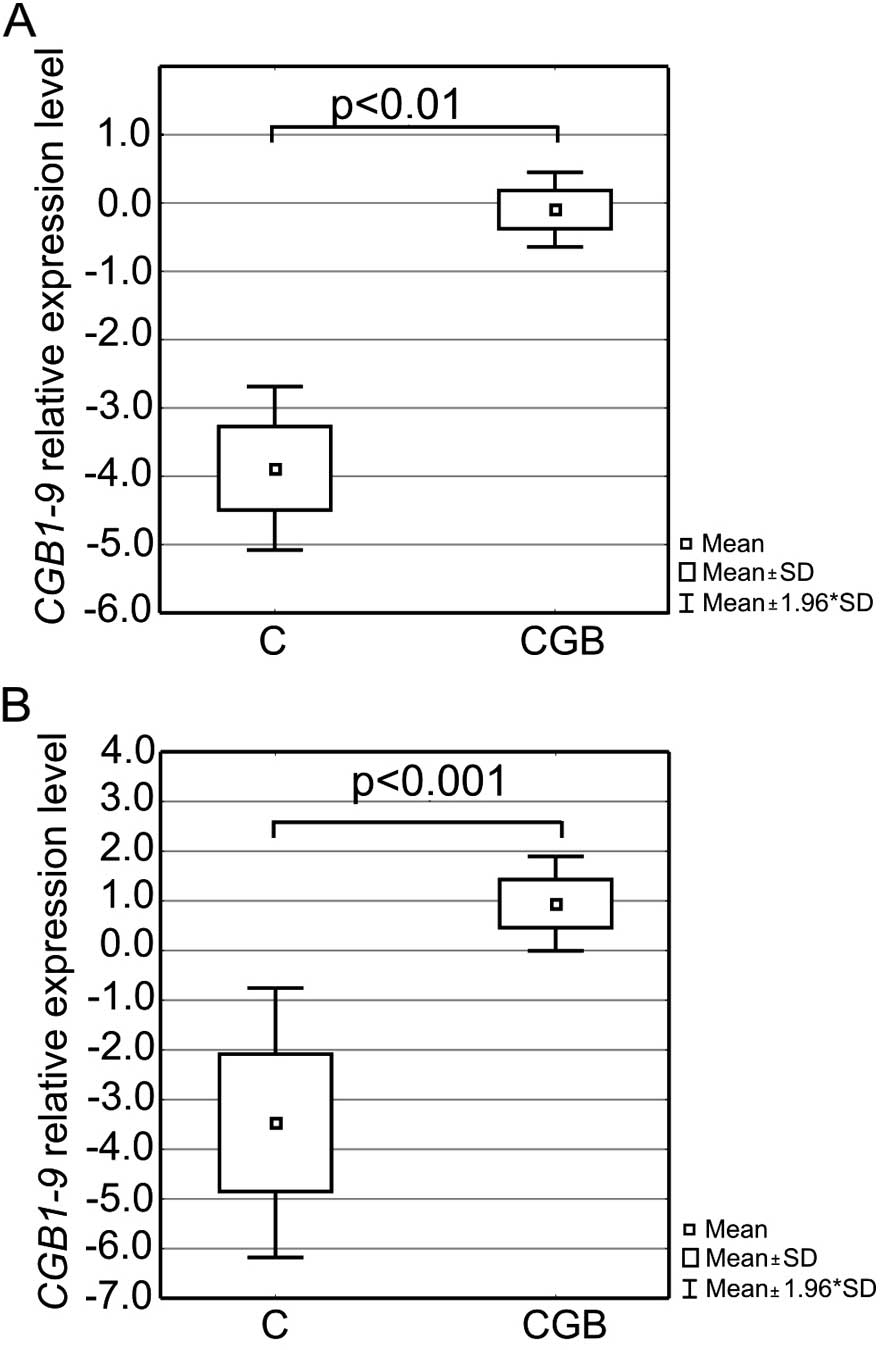
SKOV-3 cells resulted on average in 80% efficiency of transfection
and thus a profound overexpression of CG β subunit mRNA in
both cell lines. The relative level of CGB1-9 transcripts in
the transfected OVCAR-3 and SKOV-3 cells was on average >3,000-
and 15,000-fold higher than that in the control (non-transfected)
cells, respectively. Differences for both cell lines were
statistically significant (p<0.001 and p<0.001, respectively,
Fig. 1).
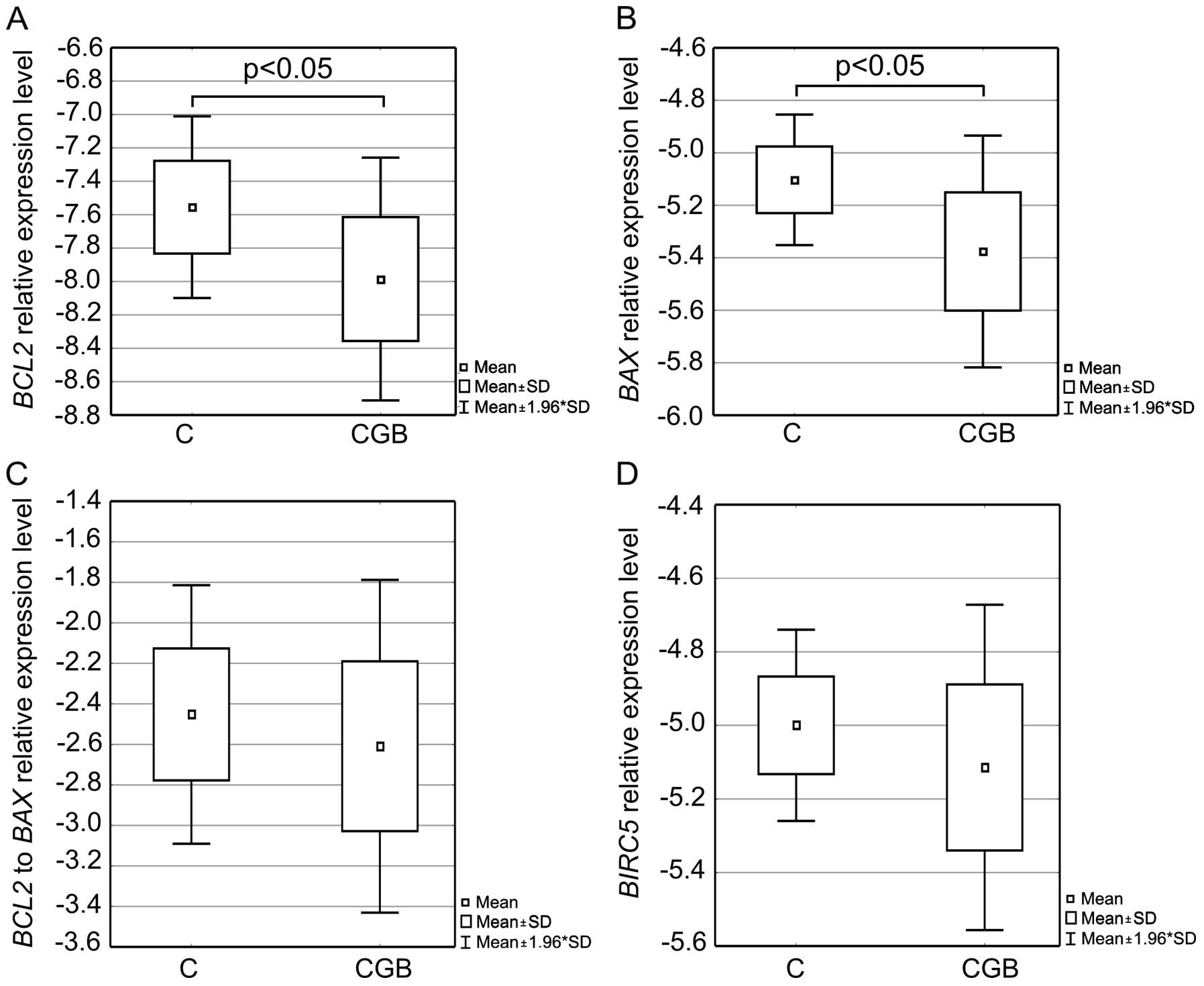
Analysis of BCL2 and BAX expression in
the cell lines overexpressing CGB5 showed that the mRNA
level of these agents declined in the transfected cells (Fig. 2A and B and Fig. 3A and B). In the case of the
transfected ovCAR-3 cells, expression of BCL2 and BAX
was on average 2-fold lower than that in the control cells;
differences were statistically significant (p=0.05 and 0.05,
respectively). In the SKov-3 cells, significant differences were
noted for BAX expression only (p<0.01), where in the
control cells the gene expression was on average >4-fold
lower.
No significant differences in the BCL2 to
BAX expression ratio were observed between the control and
transfected cell (Fig. 2C and
Fig. 3C).
Similarly to BCL2 and BAX, expression
level of the BIRC5 gene encoding survivin was noted to
decline in the transfected cells (Fig.
2D and Fig. 3D). Statistically
significant differences were noted between the control and
transfected SKOV-3 cells (p<0.01); transfected cells were
characterized by an average >3-fold lower BIRC5
expression than the control cells.
Since the physiological effect of CG is mediated via
the receptor for luteinizing hormone and hCG, transfected cells
were analyzed in terms of the receptor expression. In the case of
OVCAR-3 cells, CGB5 overexpression was associated with an
almost 2-fold decrease in the relative level of LHCGR mRNA,
but the difference was not statistically significant (Fig. 4). Increased expression of
CGB5 in the SKOV-3 cells, lacking LHCGR, did not influence
the expression of the receptor; no LHCGR expression was
noted (data not shown).
All analyses were made on the basis of three
independent experiments and from each three independent
experiments, cDNA was used for qPCR.
Ovarian cancer is characterized by
altered expression of the BCL2, BAX and BIRC5 genes
The experiments conducted using RNA isolated from
ovarian cancers, fallopian tubes and healthy ovaries showed that
all analyzed tissues expressed CGB1-9 and the level of gene
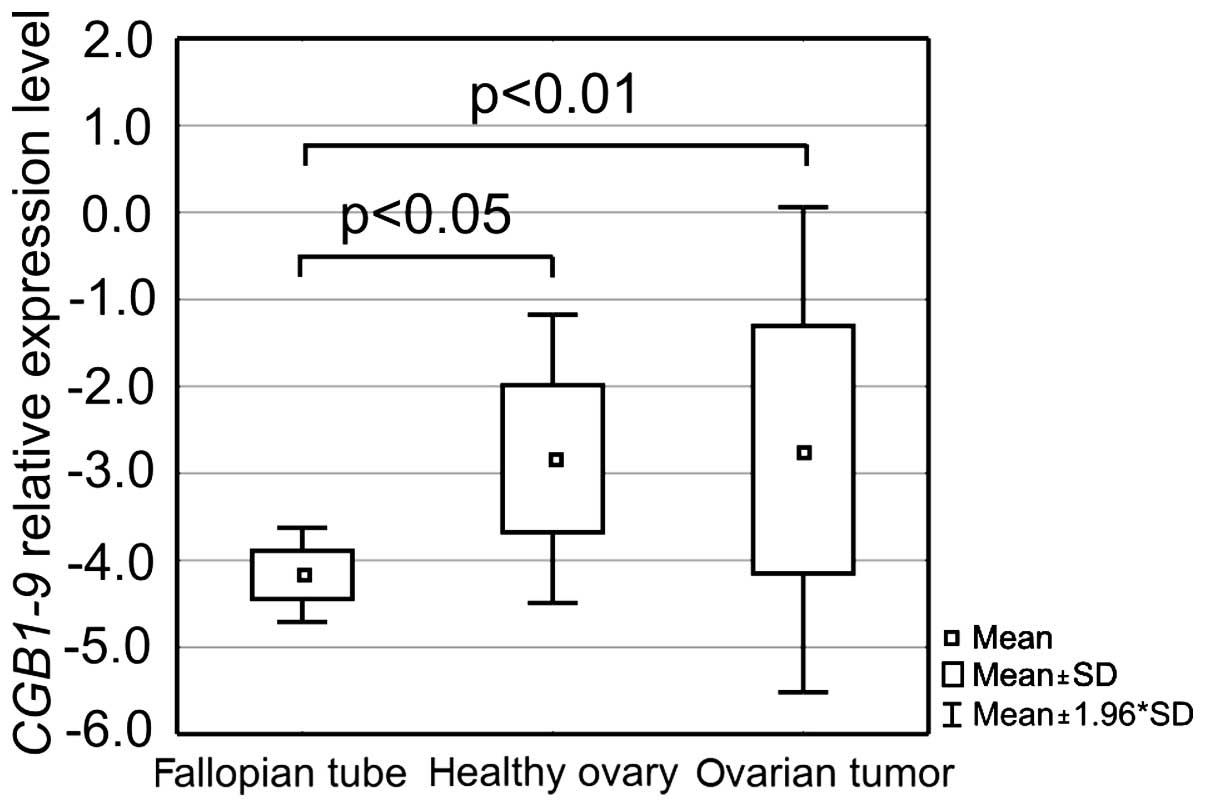
expression differed between the groups (Fig. 5).
Fallopian tube tissues were characterized by lower
CGB1-9 expression than the other analyzed groups. On average
it was ~60,000- and 40,000-fold lower than the level in the healthy
ovaries and ovarian cancer, respectively. Thus, statistically
significant differences were observed between the group of
fallopian tubes and healthy ovaries (p<0.05) as well as between
fallopian tubes and ovarian cancers (p<0.01).
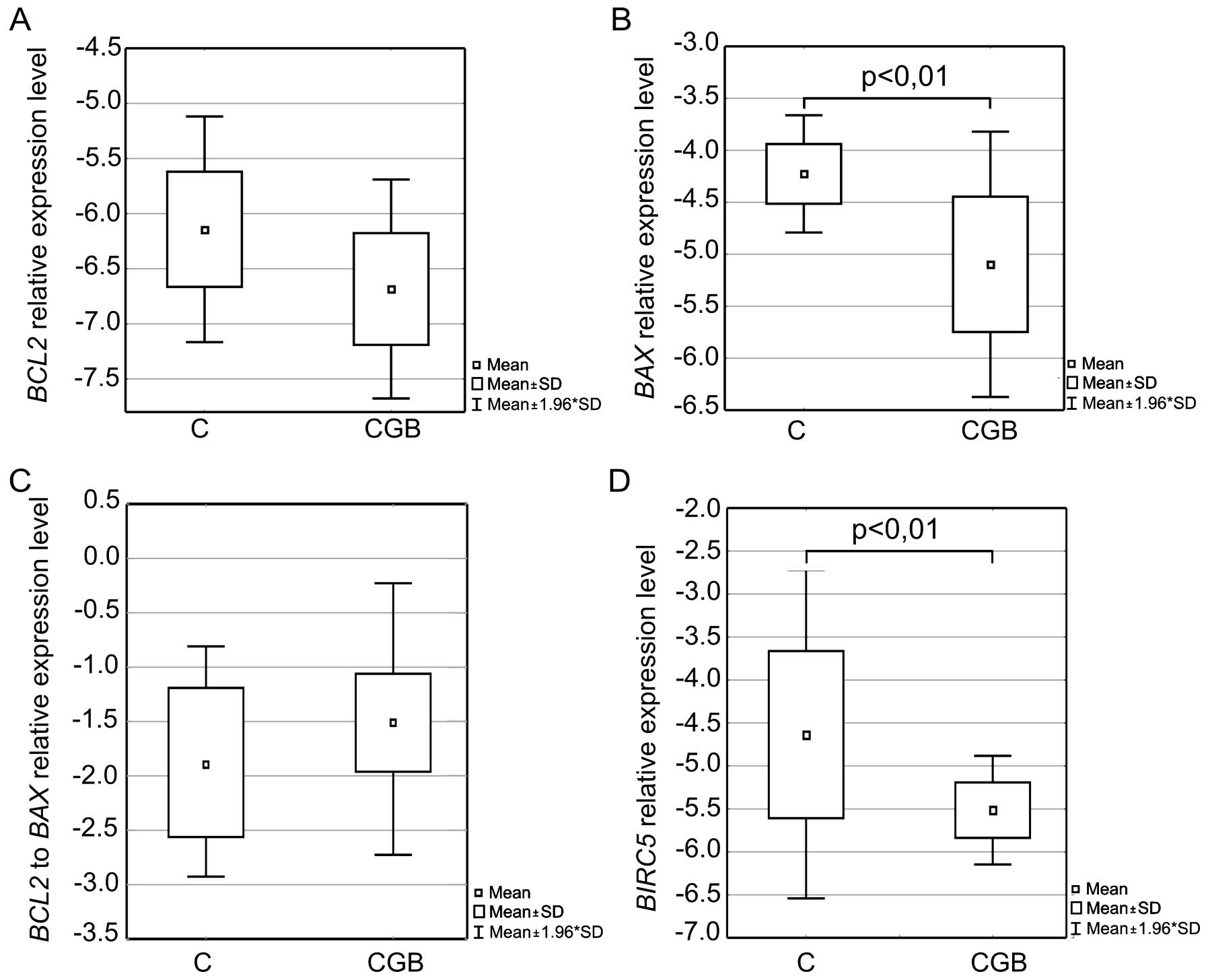
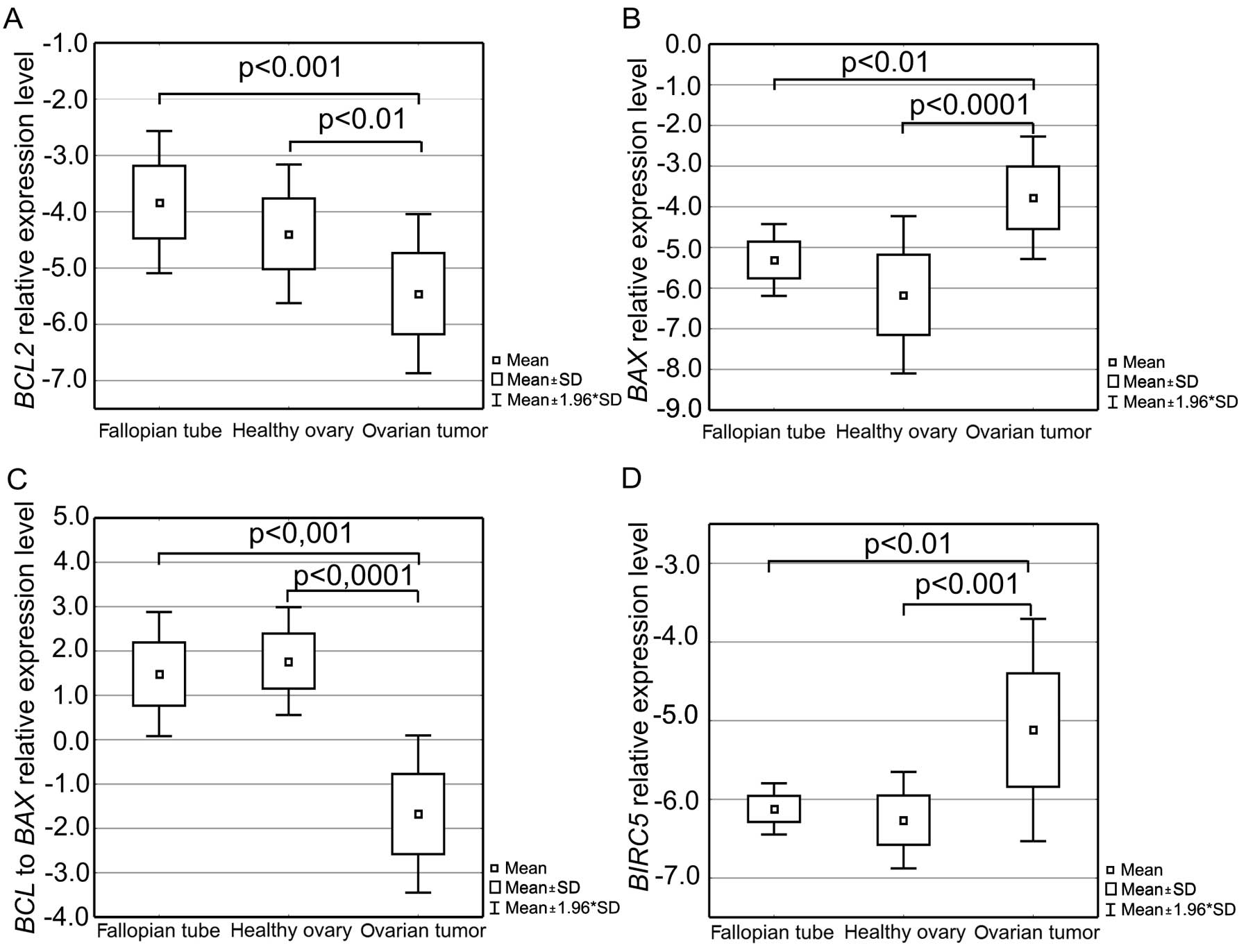
Assessment of the BCL2 gene, encoding an
anti-apoptotic factor, showed that ovarian cancers had a
statistically significantly lower BCL2 expression level than
the fallopian tube (p<0.001) and healthy ovary tissues
(p<0.01, Fig. 6A) and that it
was on average almost 30- and 10-fold lower, respectively. An
opposite phenomenon was observed in case of the pro-apoptotic
BAX gene analysis. Evaluation of BAX expression
showed that the group of ovarian cancer tissues was characterized
by distinctly higher amount of the gene transcripts than the
fallopian tubes and healthy ovaries (Fig. 6B). On average it was almost 50- and
>100-fold higher, respectively, and the differences proved to be
statistically significant (p<0.01 and p<0.001,
respectively).
Consequently a decrease in the BCL2 to
BAX expression ratio was reported (Fig. 6C). Differences in the
BCL2/BAX between the ovarian cancer and fallopian tube
tissues as well as the ovarian cancers and healthy ovaries were
statistically significant (p<0.001 and p<0.0001,
respectively).
The BIRC5 gene, coding for yet another factor
regulating apoptosis, was shown to have on average a >30-fold
higher expression level in case of the ovarian cancer than the
level in the fallopian tube and healthy ovarian tissues.
Differences in BIRC5 expression between the groups were also
found to be statistically significant (p<0.01 and p<0.001,
respectively, Fig. 6D).
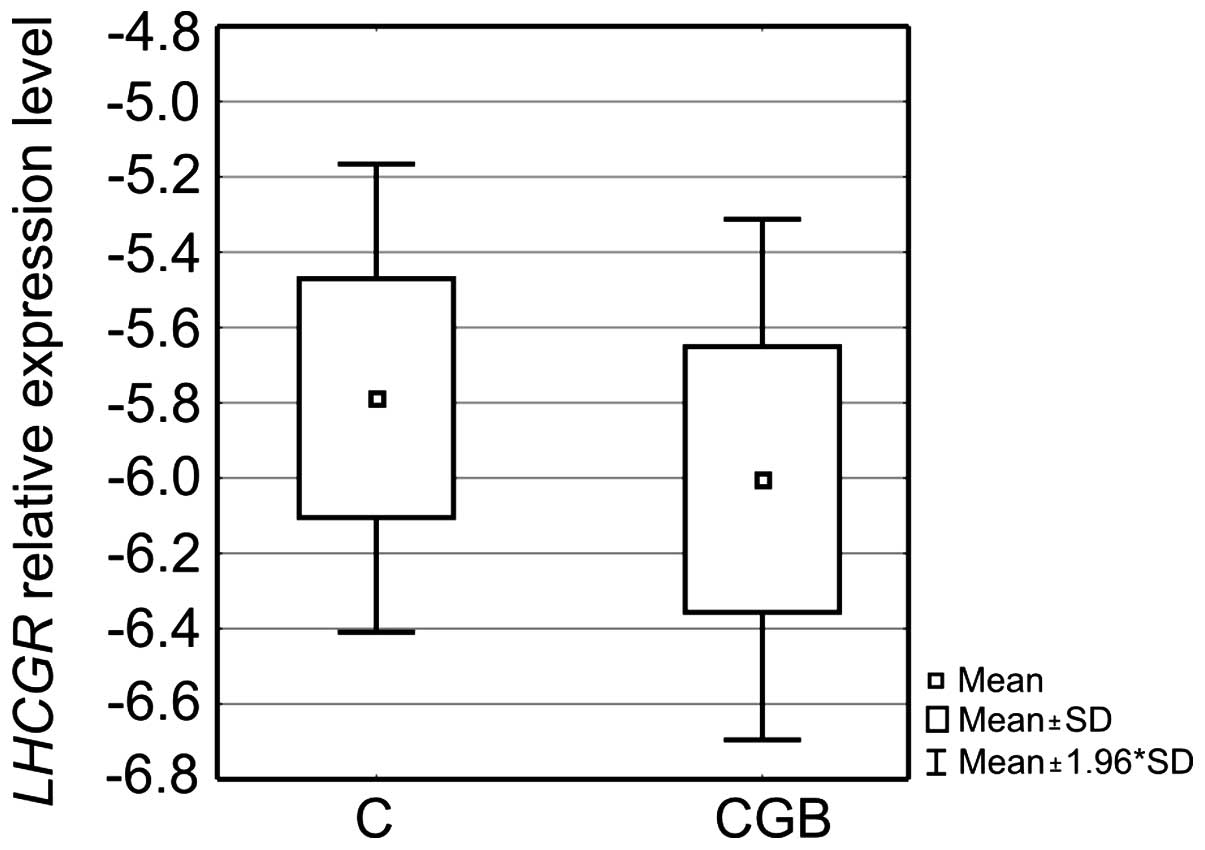
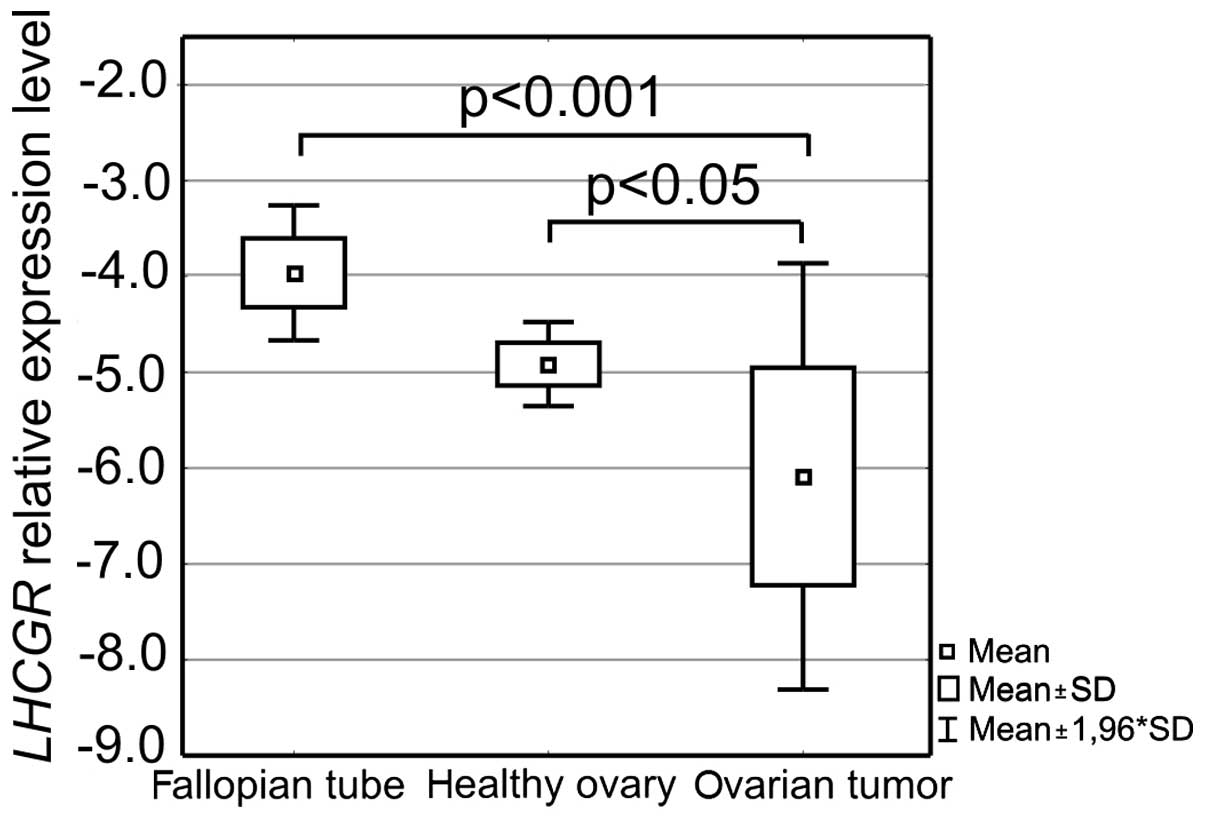
In all studied tissues, the expression level of the
receptor for luteinizing hormone and human chorionic gonadotropin
was also evaluated. The results confirmed that not all analyzed
samples expressed LHCGR at the RNA level. In 13 out of 45
ovarian cancer tissue samples, LHCGR expression was found to
be below the adopted qPCR assay's detection range. In these cases
zero was replaced with a value 10% lower than the lowest
LHCGR expression level observed in the assay. This allowed
performing full statistical analysis, which proved that again the
ovarian cancer tissue expression profile was different from the
profiles of the fallopian tube and healthy ovarian tissues. The
ovarian cancer tissue group was discriminated by a lower
LHCGR expression level than the level in the other two
analyzed groups. It was especially distinct between cancers and
fallopian tubes as it was on average >10-fold lower. Differences
in the gene expression between ovarian cancer and fallopian tube
tissues as well as ovarian cancer and healthy ovarian tissues were
shown to be statistically significant (p<0.001 and p<0.05,
respectively, Fig. 7).
Spearman's rank order correlation analysis pointed
to statistically significant relations within the three studied
tissue groups. Among the cancer tissue cases, the relative level of
LHCGR transcripts was negatively correlated with the
expression level of BIRC5 encoding survivin (p<0.05;
R=−0.39).
On the other hand, in the group of fallopian tube
tissues, LHCGR expression level showed a very strong
positive correlation with the number of BCL2 transcripts
(p<0.05; R=0.94).
The highest number of statistically significant
correlations was however noted for the group of healthy ovaries. In
these tissues, CGB expression showed a very strong positive
correlation with survivin expression (p<0.05; R=0.80) as well as
a strong negative correlation with transcriptional activity of
LHCGR (p<0.05; R=−0.74). In this group, BAX
expression was also correlated positively with the BCL2
expression level (p<0.05; R=0.74), while LHCGR was
correlated negatively with the survivin mRNA level (p<0.05;
R=−0.79).
All analyses were carried out based on the results
obtained in qPCR with the use of three independent cDNAs
synthesized for each RNA sample.
Discussion
Production of CG and its free β subunit by tumors of
different origins is a well-known phenomenon (4). However, the mechanism by which these
interchangeable cancer promoters achieve their biological effects
is not fully understood. The two most often pursued hypotheses
concern direct influence on cancer cell proliferation or/and
indirect cancer cell survival promotion by inhibiting apoptosis
signals (7,11,12,18).
The results of our previous studies as well as data
published by Hamada's group demonstrated that silencing of the
chorionic gonadotropin β subunit leads to induction of programmed
cell death in cancer cells cultured in vitro (11,12).
What is more it was shown that the expression of hCGβ was
correlated with a decreased apoptosis rate in human cervical
carcinoma samples analyzed by IHC (14). This suggests that hCG produced by
tumors may protect cells from initiation of apoptosis, allowing
tumor development and growth.
In the present study, the expression level of
BCL2, BAX, and BIRC5 genes involved in the
apoptosis of ovarian cancers expressing CGB was analyzed. Selection
of these factors was based on the fact that they are key players in
the activation of caspases in the final stages of apoptosis.
Moreover, their aberrant expression marks a variety of tumors
(19).
In the first step of the study, the influence of
hCGβ on the expression of BCL2, BAX and BIRC5
in an in vitro model was examined. Introduction of the
construct carrying the CGB5 gene into OVCAR-3 and SKOV-3
cells resulted in a profound, statistically significant increase in
CGB expression in both cell lines.
Overexpression of the hormone's β subunit caused a
decrease in BCL2 and BAX transcript level in both
transfected cell lines. However no significant differences were
observed in the BCL2 to BAX expression ratio between
the transfected and control cells.
Similarly to BCL2 and BAX, the
expression of the BIRC5 gene encoding survivin was also
noted to decline in the transfected cells compared to the control
(non-transfected) cells.
The same analyses were conducted on RNA isolated
from the ovarian cancer tissues.
Ovarian cancer can be very heterogeneous at both the
histological and genetic level. It was suggested recently that the
source of both low- and high-grade serous carcinoma is the
fallopian tube epithelium (benign or malignant) implanted on the
ovary, rather than the ovarian surface epithelium itself, as
previously believed (20). In the
view of this new model of ovarian tumorigenesis, the control groups
of our study comprised healthy ovaries and fallopian tubes.
All studied tissues, both cancer and healthy, were
characterized by the presence of CGB transcripts. However,
the relative expression level of the hormone's subunit varied.
Fallopian tube tissues were distinguished by a lower CGB1-9
expression level than that in the other analyzed tissues.
Statistically significant differences were observed between the
group of fallopian tubes and healthy ovaries as well as the
fallopian tubes and ovarian cancers. Such diversity in CGB
expression in ovarian cancer was previously shown (21).
Analysis of apoptotic agents in ovarian cancers
expressing CGB demonstrated a significantly lower
BCL2 mRNA level in cancer than in the fallopian tubes and
healthy ovaries. In contrast, a higher level of BAX gene
transcripts was found in the ovarian cancer tissues compared to the
control groups.
Changes in the expression of BCL2 and BAX are
essential for the regulation of cell cycle and apoptosis.
Overexpression of BCL2 protects normal cells from apoptosis
(22), whereas excess of BAX can
lead to cell death induction (23).
Thus, the level of both factors in ovarian cancer, regulated by
hCG, may affect tumor cell growth and survival. This scenario seems
possible since CG controlling the apoptosis of the corpus luteum
through BCL2 and BAX was previously documented (24). On the other hand, these factors
expressed in tumors may act differently than in normal tissues. In
cancer, a high level of BCL2 protein is known to correlate with
tumor differentiation and better prognosis, while overexpression of
BAX with a worse prognosis. In ovarian cancer patients, BAX, but
not BCL2 expression, correlates with significantly reduced survival
compared to individuals characterized by the expression of both
factors (25). Thus, the results of
the present study, demonstrating increased transcriptional activity
of the BAX gene in ovarian cancer tissues, are in agreement
with previously published data. What is more, they suggest that the
difference in both BCL2 and BAX gene expression may
be responsible for more malignant phenotype of cancer cells
overexpressing CGB.
Numerous studies have shown that the quantitative
ratio of BCL2 and BAX expression (BCL2/BAX) and their interactions
are a key element of the cell fate (survival or death) (26,27).
BCL2/BAX is a characteristic feature of a given cell type. A
decline in BCL2/BAX observed in advanced stages of ovarian cancer
is associated with the degree of tumor differentiation but appears
to have no correlation with its proliferation rate (26).
Taking this into consideration, we estimated the
BCL2/BAX ratio for all studied tissue groups. The highest value was
noted for healthy tissues, fallopian tubes and ovaries, and the
lowest characterized ovarian cancer tissue. Thus, these results and
the fact that tumors expressing hCGβ are often metastatic and have
worse prognosis may suggest that the β subunit of hCG protects
tumor cells from apoptosis and allows development of a malignant
phenotype through modulation of BCL2 and BAX
expression.
Aberrant apoptosis of ovarian cancer cells was
additionally confirmed by the assessment of BIRC5
expression. The BIRC5 gene encodes survivin, a member of the
IAP family proteins which play a role in oncogenesis via their
effective suppression of apoptosis (27). Even though a direct link between
CGB and BIRC5 expression has not been shown,
stimulation of survivin synthesis by CG in granulosa cells of the
ovary was previously documented (28). This implies that survivin-mediated
suppression of apoptosis in ovarian cancer is regulated by
endogenously synthesized hCGβ. The obtained results showed that the
highest expression level of BIRC5 characterized cancer
tissues. They are also in agreement with current research on the
role of survivin in tumorigenesis, showing that a high level of
survivin in tumors, similarly to CGB, is often associated with an
invasive phenotype and resistance to chemotherapy and radiotherapy
and consequently with poor prognosis (27).
In both cell lines CGB overexpression led to
a decrease in all analyzed apoptosis-related factors, while in the
ovarian cancer tissues CGB expression was associated with an
increase in BAX and BIRC5 expression. The reason why
the results of our in vivo studies did not completely match
the results of the in vitro study may be related to the fact
that the in vitro model did not completely reflect the
complexity of the tissue. Therefore, it must be taken into
consideration that the final effect of hCGβ action in tissues
depends on the interaction between different cell types and
responses induced by the hormone.
CG acts via the receptor for luteinizing hormone and
human chorionic gonadotropin, thus the expression level of
LHCGR in the studied cell lines and tissues was evaluated.
SKOV-3 cells lack LHCGR, and overexpression of CGB did not
influence LHCGR transcriptional activity. On the other hand,
in the transfected OVCAR-3 cells, a decrease in the amount of
LHCGR mRNA was observed, however differences were not
statistically significant.
These results were confirmed also by the analysis
performed on RNA isolated from cancer tissues. In accordance with
other studies showing that not all tumors with confirmed hCGβ
expression were characterized by the presence of LHCGR (12), only part of the analyzed samples
expressed LHCGR. In fact in 13 out of 45 ovarian cancer
cases, LHCGR was not detected. What is more, ovarian cancers were
discriminated by a significantly lower LHCGR expression
level than the one noted for healthy ovarian and fallopian tube
tissues.
Thus, both in vitro and in vivo
studies suggested that CGB expression in cancer cells
regulates LHCGR expression. Negative LHCGR regulation under
CG and hCGβ influence has been previously reported at both the mRNA
and protein levels (17,29). Our data together with the fact that
hCGβ affects SKOV-3 cells lacking the receptor, suggests that the
effect of the hormone on cancer cells is LHCGR-independent.
In the present study, the analyzed tumor tissues
consisted of ovarian cancers, most of which, according to the
newest classification, belonged to type ii tumors (present in
advanced stage and comprising high-grade serous, high-grade
endometrioid, malignant mixed mesodermal tumors, carcinosarcomas,
and undifferentiated carcinomas) (19). Since the analyzed cancer tissues
were a rather homogenous group, no attempt was made to correlate
the obtained results with clinical data.
The correlations we found linked CGB
positively with BIRC5 and negatively with LHCGR
expression in healthy ovaries. Within this tissue group, strong
positive correlation between BAX and BCL2 expression
was also found. Surprisingly a very strong positive correlation
between LHCGR and BCL2 in the fallopian tube group
was noted while in cancer LHCGR expression was negatively
related with BIRC5. The meaning of these correlations is not
clear.
In conclusion, even though the exact molecular
mechanism of hCGβ action in cancer cells still needs to be
established, the results of the present study together with the
fact that tumors expressing the free β subunit of hCG are
characterized by a more malignant phenotype and worse prognosis,
suggest that the biological effect of hCGβ is related to
suppression of apoptosis. Protection of tumor cells from programmed
cell death induction may be achieved by expression modulation of
genes regulating apoptosis such as BCL2, and BAX and
BIRC5.
Acknowledgments
The present study was supported by the Polish
National Science Centre Awards (NN 407459138 to A.S. and NN
407275439 to A.J.).
References
|
1
|
Pierce JG and Parsons TF: Glycoprotein
hormones: Structure and function. Annu Rev Biochem. 50:465–495.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cole LA: hCG, five independent molecules.
Clin Chim Acta. 413:48–65. 2012. View Article : Google Scholar
|
|
3
|
Iles RK and Butler SA: Gonadotropins and
gonadotropin receptors - evolutional genetics, signalling
mechanisms, extra gonadal function and roles in oncogenesis. Mol
Cell Endocrinol. 329:1–2. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iles RK, Delves PJ and Butler SA: Does hCG
or hCGβ play a role in cancer cell biology? Mol Cell Endocrinol.
329:62–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsampalas M, Gridelet V, Berndt S, Foidart
JM, Geenen V and Perrier d'Hauterive S: Human chorionic
gonadotropin: A hormone with immunological and angiogenic
properties. J Reprod Immunol. 85:93–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zygmunt M, Herr F, Keller-Schoenwetter S,
Kunzi-Rapp K, Münstedt K, Rao CV, Lang U and Preissner KT:
Characterization of human chorionic gonadotropin as a novel
angiogenic factor. J Clin Endocrinol Metab. 87:5290–5296. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burczynska BB, Kobrouly L, Butler SA,
Naase M and Iles RK: Novel insights into the expression of CGB1 and
2 genes by epithelial cancer cell lines secreting ectopic free
hCGβ. Anticancer Res. 34:2239–2248. 2014.PubMed/NCBI
|
|
8
|
Lee CL, Chiu PC, Hautala L, Salo T, Yeung
WS, Stenman UH and Koistinen H: Human chorionic gonadotropin and
its free β-subunit stimulate trophoblast invasion independent of
LH/hCG receptor. Mol Cell Endocrinol. 375:43–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Butler SA and Iles RK: The free monomeric
beta subunit of human chorionic gonadotrophin (hCG beta) and the
recently identified homodimeric beta-beta subunit (hCG beta beta)
both have autocrine growth effects. Tumour Biol. 25:18–23. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prast J, Saleh L, Husslein H, Sonderegger
S, Helmer H and Knöfler M: Human chorionic gonadotropin stimulates
trophoblast invasion through extracellularly regulated kinase and
AKT signaling. Endocrinology. 149:979–987. 2008. View Article : Google Scholar
|
|
11
|
Hamada AL, Nakabayashi K, Sato A, Kiyoshi
K, Takamatsu Y, Laoag-Fernandez JB, Ohara N and Maruo T:
Transfection of antisense chorionic gonadotropin β gene into
choriocarcinoma cells suppresses the cell proliferation and induces
apoptosis. J Clin Endocrinol Metab. 90:4873–4879. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jankowska A, Gunderson SI, Andrusiewicz M,
Burczynska B, Szczerba A, Jarmolowski A, Nowak-Markwitz E and
Warchol JB: Reduction of human chorionic gonadotropin beta subunit
expression by modified U1 snRNA caused apoptosis in cervical cancer
cells. Mol Cancer. 7:26–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo X, Liu G, Schauer IG, Yang G,
Mercado-Uribe I, Yang F, Zhang S, He Y and Liu J: Overexpression of
the β subunit of human chorionic gonadotropin promotes the
transformation of human ovarian epithelial cells and ovarian
tumorigenesis. Am J Pathol. 179:1385–1393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li D, Wen X, Ghali L, Al-Shalabi FM,
Docherty SM, Purkis P and Iles RK: hCG beta expression by cervical
squamous carcinoma - in vivo histological association with tumour
invasion and apoptosis. Histopathology. 53:147–155. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mandai M, Konishi I, Kuroda H and Fujii S:
LH/hCG action and development of ovarian cancer - a short review on
biological and clinical/epidemiological aspects. Mol Cell
Endocrinol. 269:61–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Głodek A and Jankowska A: CGB activates
ERK and AKT kinases in cancer cells via LHCGR-independent
mechanism. Tumour Biol. 35:5467–5479. 2014. View Article : Google Scholar
|
|
17
|
Hotakainen K, Lintula S, Jarvinen R, Paju
A, Stenman J, Rintala E and Stenman UH: Overexpression of human
chorionic gonadotropin beta genes 3, 5 and 8 in tumor tissue and
urinary cells of bladder cancer patients. Tumour Biol. 28:52–56.
2007. View Article : Google Scholar
|
|
18
|
Iles RK: Ectopic hCGbeta expression by
epithelial cancer: Malignant behaviour, metastasis and inhibition
of tumor cell apoptosis. Mol Cell Endocrinol. 260–262:264–270.
2007. View Article : Google Scholar
|
|
19
|
Philchenkov A: Caspases: Potential targets
for regulating cell death. J Cell Mol Med. 8:432–444. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurman RJ: Origin and molecular
pathogenesis of ovarian high-grade serous carcinoma. Ann oncol.
24(Suppl 10): x16–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kubiczak M, Walkowiak GP, Nowak-Markwitz E
and Jankowska A: Human chorionic gonadotropin beta subunit genes
CGB1 and CGB2 are transcriptionally active in ovarian cancer. Int J
Mol Sci. 14:12650–12660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin C, Knudson CM, Korsmeyer SJ and van
Dyke T: Bax suppresses tumorigenesis and stimulates apoptosis in
vivo. Nature. 385:637–640. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sugino N, Suzuki T, Kashida S, Karube A,
Takiguchi S and Kato H: Expression of Bcl-2 and Bax in the human
corpus luteum during the menstrual cycle and in early pregnancy:
Regulation by human chorionic gonadotropin. J Clin Endocrinol
Metab. 85:4379–4386. 2000.PubMed/NCBI
|
|
25
|
Basu A and Haldar S: The relationship
between Bci2, Bax and p53: Consequences for cell cycle progression
and cell death. Mol Hum Reprod. 4:1099–1109. 1998. View Article : Google Scholar
|
|
26
|
Yoon O and Roh J: Downregulation of KLF4
and the Bcl-2/Bax ratio in advanced epithelial ovarian cancer.
Oncol Lett. 4:1033–1036. 2012.PubMed/NCBI
|
|
27
|
Waligórska-Stachura J, Jankowska A, Waśko
R, Liebert W, Biczysko M, Czarnywojtek A, Baszko-Błaszyk D, Shimek
V and Ruchała M: Survivin - prognostic tumor biomarker in human
neoplasms - review. Ginekol Pol. 83:537–540. 2012.
|
|
28
|
Kumazawa Y, Kawamura K, Sato T, Sato N,
Konishi Y, Shimizu Y, Fukuda J, Kodama H and Tanaka T: HCG
up-regulates survivin mRNA in human granulosa cells. Mol Hum
Reprod. 11:161–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hoffman YM, Peegel H, Sprock MJ, Zhang QY
and Menon KM: Evidence that human chorionic
gonadotropin/luteinizing hormone receptor down-regulation involves
decreased levels of receptor messenger ribonucleic acid.
Endocrinology. 128:388–393. 1991. View Article : Google Scholar : PubMed/NCBI
|