Introduction
Toll-like receptors (TLRs) are fundamental elements
of the immune system, which facilitate our understanding of the
innate and adaptive immune pathways (1). TLRs are expressed on a variety of
cells, including B cells, specific types of T cells, DCs and
macrophages. All the TLRs can recognize distinct molecular
components of bacteria, viruses, fungi or other pathogens (1,2). Also
some synthetic small molecules can activate certain TLR pathways
(3). Particularly, TLR ligands can
control the activation of dendritic cells (DCs), trigger the
maturation program of DCs and lead to the secretion of
proinflammatory cytokines (4). Most
TLR ligands possess important properties that can initiate the host
innate and adaptive immune response for the immunotherapy against
cancer (5). They have the ability
to promote tumor-specific Th1 immune response and cytotoxic T
lymphocyte responses (6,7).
Most conventional cancer therapies (surgery,
chemotherapy and radiation) are not tumor-specific, so
immunotherapy is emerging as a powerful tumor-specific approach to
cancer treatment. However, some immunotherapies are likely to have
a negative influence of the tumor immune escape. Tumor cells
generated occasionally can be recognized and removed as non-self by
immune cells. However, cancer progression occurs as a result of a
failure in immune surveillance. Increasing research effort is
focused on dysfunctional immune cells and immune cells in the tumor
microenvironment (8-10). The goal of cancer immunotherapy is
directed not only at stimulation of innate and adaptive immune but
reversal of immune dysfunction. Recent reports based on the
antitumor effect of TLR agonists provide new evidence that TLR
agonists can modulate the tumor microenvironment, especially the
type, location, and density of immune cells. FDA has approved seven
TLR agonists on cancer immunotherapy, including imiquimod (TLR7
agonists), BCG (mixed TLR2/TLR4 agonists) and glucopyranosyl lipid
adjuvant (TLR4 agonists) (11). As
is known that only TLR 7 ligand can be a small-molecule synthetic
agonist, several synthesized low molecular weight TLR7 agonists
were found, including imidazoquinolines and purine-like molecules,
to activate immune cells via the TLR7-MyD88-dependent signaling
pathway (12,13). Imiquimod is being successfully used
for the treatment of many primary skin tumors and cutaneous
metastases as the single antitumor agent with immunostimulatory
capacity (11,14).
In the present study, we examined the effects of a
novel adenine type of TLR7 agonists (SZU101) on the magnitude of
both innate and adapt immune activation in vitro and in
vivo. We established local and distant tumor-bearing mice
derived from murine mammary carcinoma cell line (4T1) to model
metastatic disease. We report on the elicited antitumor effect on
tumors by multiple mechanisms, inducing tumor-specific immune
response, activating innate immune cells and modulation of the
tumor microenvironment. We also found that intratumoral
administration of SZU101 can change the tumor microenvironment and
delay distant tumor growth.
Materials and methods
Cell line and mice
The 4T1 were maintained in our lab and cultured in
RPMI-1640, supplemented with 10% fetal bovine serum (FBS), 100
µg/ml penicillin and 100 µg/ml streptomycin (all from
Hyclone, Logan, UT, USA). Cells were cultured at 37°C in a
humidified atmosphere with 5% CO2. Female BALB/c mice at
6 weeks of age were purchased from the Medical Laboratory Animal
Centre, Guangdong, China. All animals were housed in laminar flow
cages and were permitted ad lib access to sterile food and water.
The experiments were carried out in accordance with recommendations
cited in the Guide for the Care and Use of Laboratory Animals of
the Medical Laboratory Animal Center of Guangdong, China.
Synthesis of TLR7 agonist
TLR7 agonist (SZU101) was synthesized in our
laboratory according the following scheme (15).
Mouse model and treatments
The animals were injected subcutaneously with 100
µl of 1.5×105 4T1 cells in PBS. The first
injection was on day 0 in the right flank, and the second injection
was on day 3 in the left flank. Mice were randomized at day 7 after
inoculation to treatment by intratumoral injection with 0.1 ml of
either 1 mg/ml (~5 mg/kg) TLR7 agonist or placebo control (PBS, pH
7.2) in the right flank tumor every three days. Mice were
sacrificed on day 30.
Western blot analysis
Cells were lysed by Cell Lysis Buffer (Beyotime
Institute of Biotechnology, China). Total protein was measured with
a BCA assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Samples
were loaded onto SDS-polyacrylamide gels, transferred onto
microporous polyvinylidene difluoride membranes and incubated with
appropriate antibodies. Blotting was performed with the following
primary antibodies: IκBα, NF-κBp65, mTLR7, β-actin and GAPDH,
horseradish peroxidase-conjugated anti-mouse or anti-rabbit
secondary antibody (1:1,500 dilution; Cell Signaling Technology,
Inc., Danvers, MA, USA). Protein bands were visualized using ECL
substrate (Pierce, Rockford, IL, USA).
Determination of cytokine production in
vitro
Mouse spleen lymphocytes, BMDC and NK cells were
isolated from BALB/c mice and grown in RPMI-1640 medium with 10%
FBS. Cells were seeded in 24-well plates at a density of
5×104 cells per well. For spleen lymphocytes and NK
cells, compounds were added to 24-h cultures at a final
concentration ranging from 5 to 100 µM or as otherwise
indicated. For BMDC, compounds were added to 6-day cultures, and
they were incubated for 24 h, culture supernatants were collected
and assayed for cytokine inductions by enzyme-linked immunosorbent
assay (ELISA) (eBioscience, Inc., San Diego, CA, USA), according to
the manufacturer's instructions. Murine macrophage-like cell line,
RAW264.7 cells, was used as murine macrophage to determin the
cytokines. The data were calculated from triplicate wells and are
presented as a mean ± SD.
Determinations of antibody titers
On day 30, mice were sacrificed, blood samples were
collected from the mice and centrifuged at 3,000 × g for 15 min to
obtain serum samples. Antibody titers in serum were determined by
ELISA method using an alkaline phosphate-conjugated detection
antibody (Millipore, Billerica, MA, USA) for 4T1 antibody, IgG1 and
IgG2a.
Determinations of CTL
At the time of sacrifice, lymphocytes, separated
from the spleen of each mouse by Mouse Lymphocyte Separation medium
(Dakewe, Beijing, China), were used as effectors. 4T1 tumor cells
were used as target cells and incubated with lymphocytes for 4 h at
an effector-to-target cell ratio of 100:1. Cytotoxicity was also
measured by LDH method using Non-Radioactive Cytotoxicity assay
(Promega, Madison, WI, USA), according to the supplier's
manual.
Determinations of ADCC
At the time of sacrifice, serum samples from the
mice were 1:25 diluted, and incubated with 4T1 tumor cells for 30
min at 37°C. NK cells, isolated from normal BALB/c mouse by Mouse
NK cells Separation kit (Hao Yang, Tianjin, China), were used as
effectors and seeded with the antibody-labeled 4T1 cells for 4 h at
an effector-to-target cell ratio of 30:1. Cytotoxicity was measured
by LDH method using Non-Radioactive Cytotoxicity assay (Promega),
according to the supplier's manual. Briefly, after incubation,
culture supernatants were transferred to an ELISA plate, followed
by the addition of substrate solution for 30 min at room
temperature. Finally, stop solution was filled in, and the optical
density was measured at 490 nm with a spectrophotometer (BioTek,
Winooski, VT, USA).
Flow cytometric analysis (16)
On day 30, mice were sacrificed. Spleens were
homogenized by repeated pipetting and filtered through a
70-µm nylon filter. Tumors from each group were minced with
scissors prior to incubation with 1 mg/ml final concentration
clostridiopeptidase A (Sigma-Aldrich, St. Louis, MO, USA), 0.25%
trypsin (Hyclone) and 0.2 mg/ml DNase (Sigma) for 30 min at 37°C.
Single cell suspensions of splenocytes and tumors were collected
and washed with FACS (5% calf serum in PBS) 3 times and incubated
with FACS for 1 h, stained with appropriate antibodies at 1
µg/ml final concentration, on ice overnight and then washed
with FACS 3 times and immediately analyzed by flow cytometry
(Becton-Dickinson, San Jose, CA, USA). For intracellular staining,
samples were fixed and permeabilized before incubation with
antibody. Antibodies used for flow cytometry were purchased from
eBioscience (PE-anti-mouse CD4, APC-anti-mouse CD25,
FITC-anti-mouse Foxp3, APC-anti-mouse CD8 and FITC-anti-mouse CD3).
Data were analyzed with FlowJo software (Tree Star, Inc., Ashland,
OR, USA).
Statistical analysis
Statistical comparisons of mean values were
performed using the Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
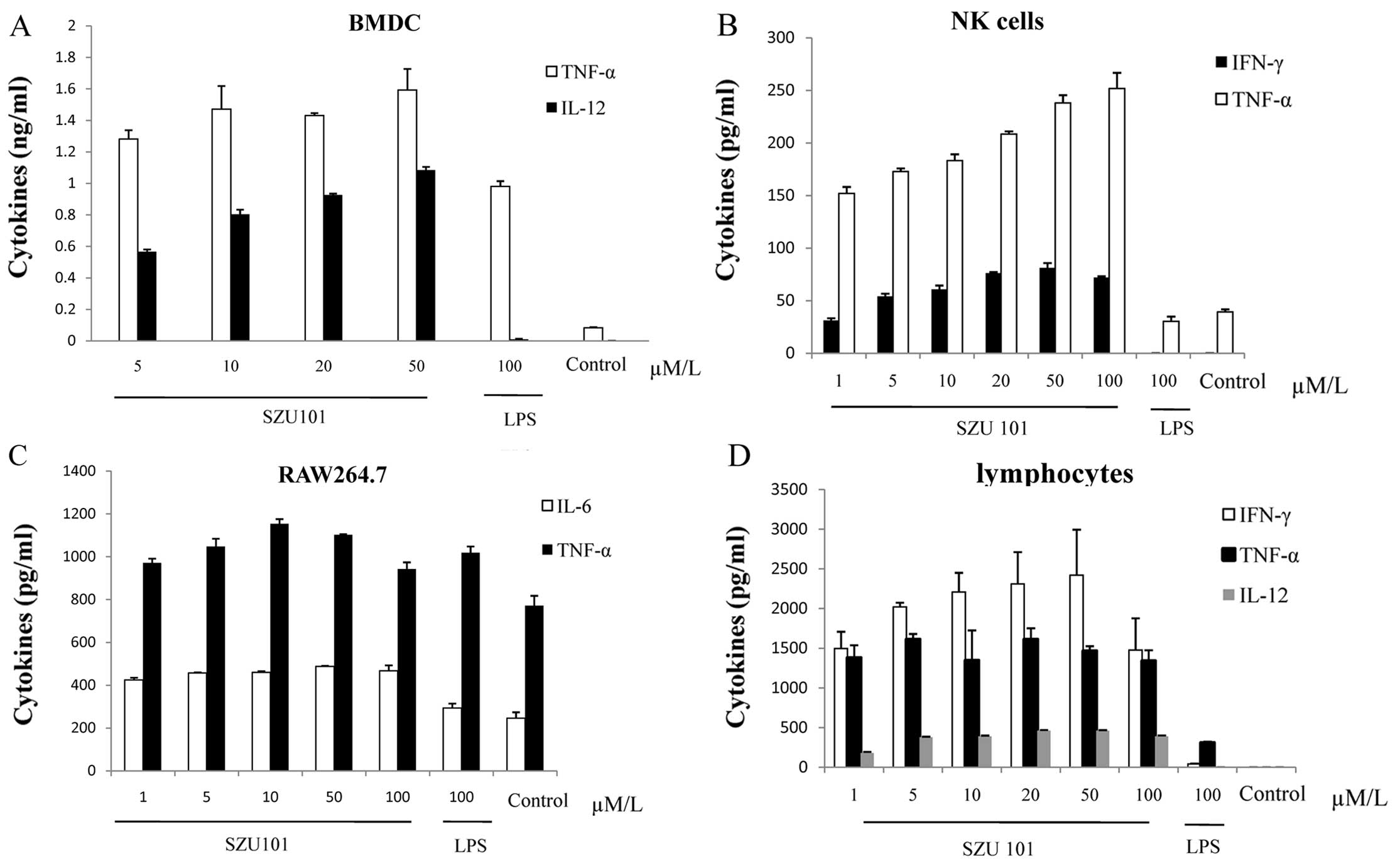
Potent in vitro cytokine release in
response to SZU101
To investigate the potency on murine TLR7 and the
immunological activity of SZU101, bone marrow derived dendritic
cells (BMDC), mouse nature killer cells (NK) and spleen lymphocytes
isolated from BALB/c mice were stimulated with SZU101 at 5, 10, 20,
50 and 100 µM concentrations and LPS at 100 µM as
positive control in vitro (Fig.
1). RAW264.7 cells were treated as mentioned above. The values
are shown in Fig. 1A–D for IL6,
IL12, TNF-α and IFN-γ of each cell type. Incubation of BMDC with
SZU101 alone stimulated cytokine release (Fig. 1A). IL12 and TNF-α production was
concentration-dependent from 5 to 50 µM, comparing with the
concentration of LPS at 100 µM, both IL12 and TNF-α
production of SZU101-treated group was higher than LPS positive
control. For NK cell, we detected the release of TNF-α and INF-γ
(Fig. 1B). We also used the mouse
macrophage cell line RAW264.7 to evaluate the production of IL6 and
TNF-α, with similar results (Fig.
1C). SZU101 was able to stimulate lymphocytes to release
cytokines, which were related with adopted immunity, at the very
high level compared with other innate immunity cells at the same
concentration (Fig. 1D).
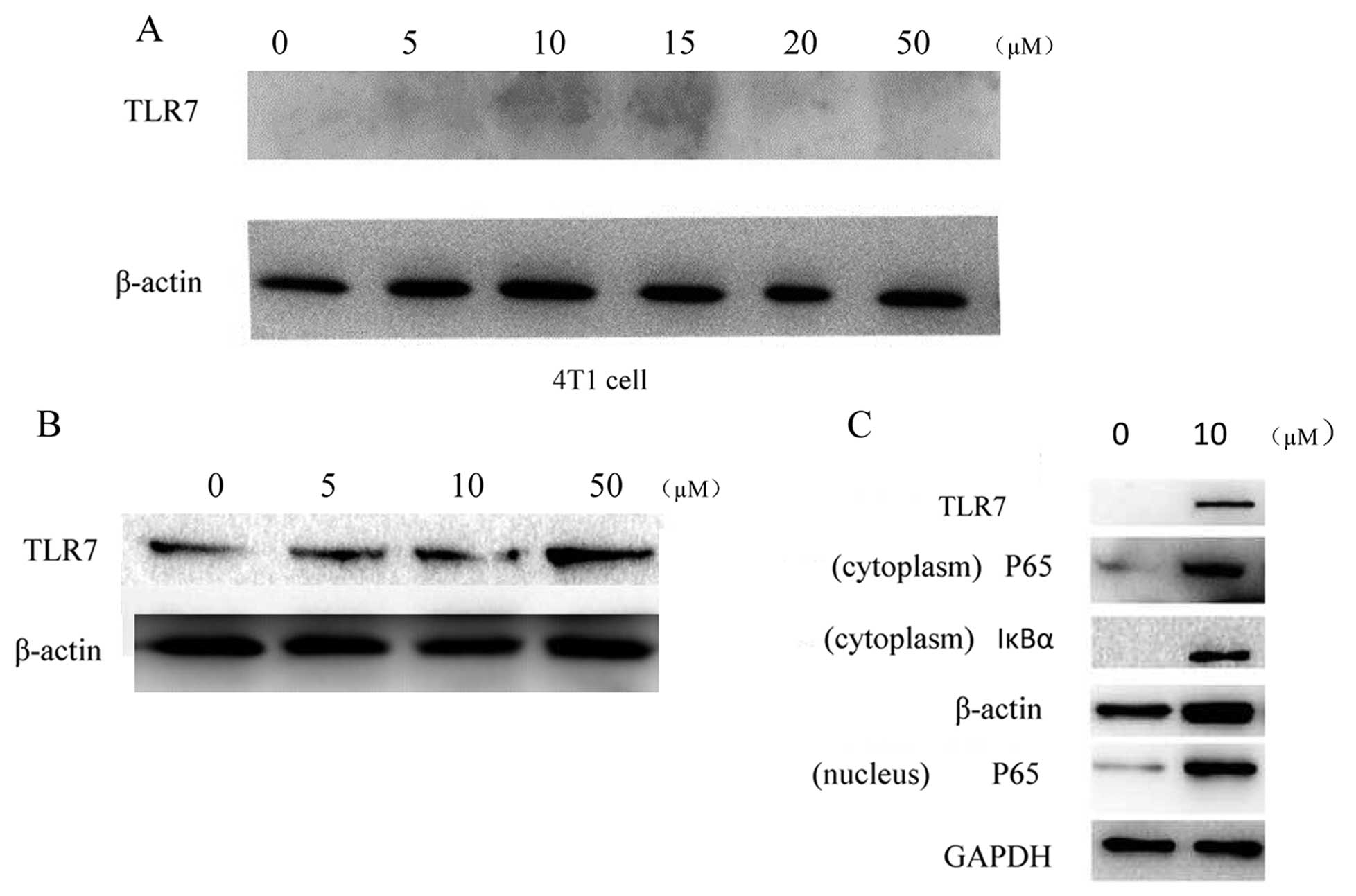
Expression of TLR7 on immune cells and
activation of NF-κB pathway
To confirm that SZU101 was indeed the TLR7 ligand,
we detected the expression of TLR7 on immune cells by western blot
analysis. BMDC were treated with SZU101 to identify endogenous TLR7
expression for innate immune and spleen lymphocytes for adoptive
immune. The western blot results showed that the expression of TLR7
increased after the SZU101 treatment in a concentration-dependent
manner (Fig. 2B). We also detected
the expression of TLR7 on the 4T1 cells. There was no expression of
TLR7 on the 4T1 cells (Fig. 2A).
For the activation of NF-κB pathway, NF-κBp65 was the key protein.
NF-κB activation was observed by detected p65 expression on both
cytoplasm and the nucleus (Fig.
2C).
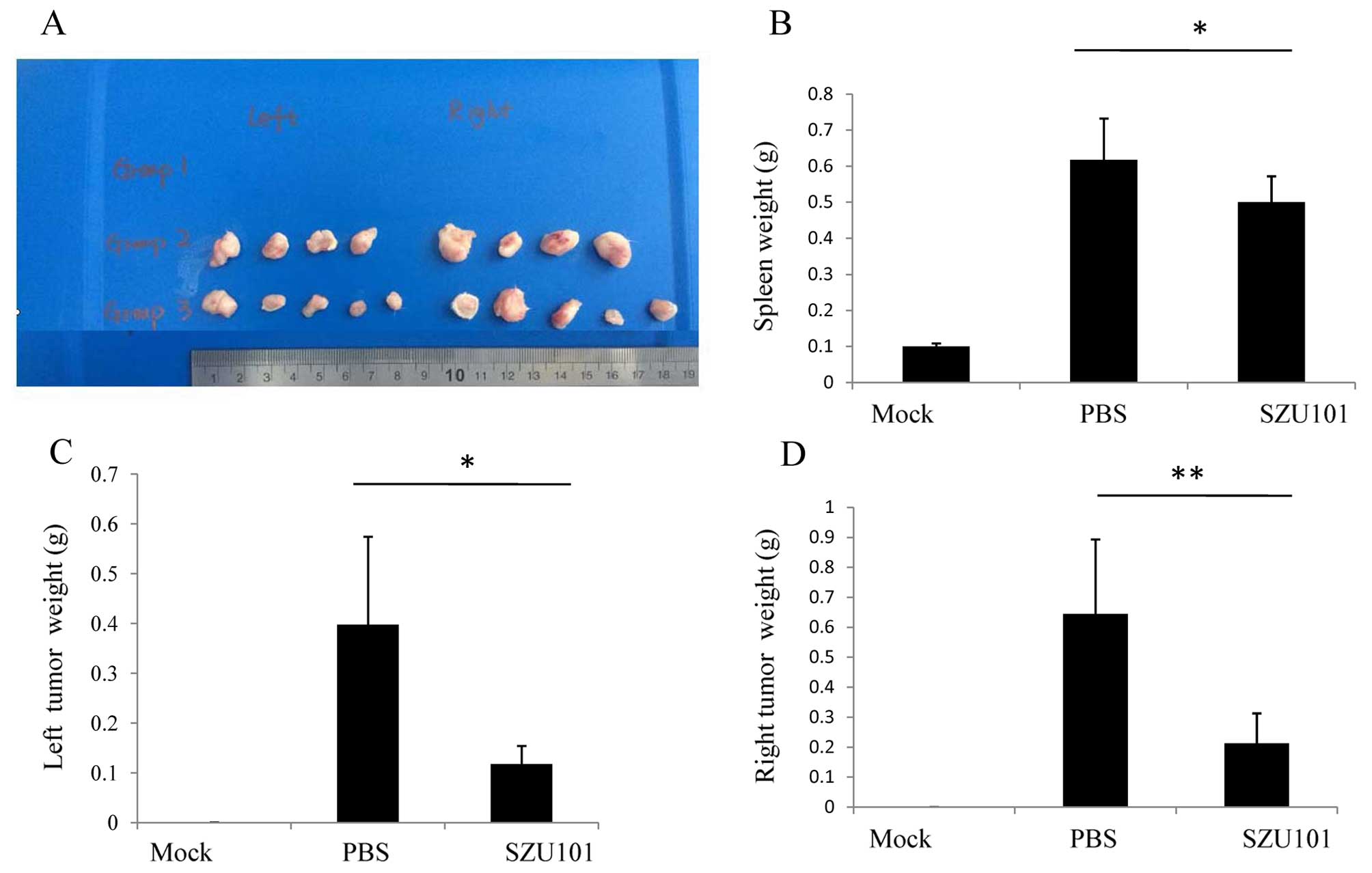
Intratumoral injection of SZU101 to 4T1
tumor-bearing BALB/c mice can reduce tumor growth
4T1 tumor-bearing BALB/c mice were treated every
three days from day 7 to day 30 with SZU101 (n=5) or PBS (n=5). On
day 30, the mice were sacrified, the weight of spleen, and both
sides of the tumor was measured. As shown in Fig. 3, the mean spleen weight of
tumor-bearing BALB/c mice was higher than the healthy mice. The 4T1
tumor cells increased the spleen size (Fig. 3B). In our experiment, there was a
positive correlation between the weight of the spleen and the
tumor. When the tumor was large, the spleen was also large.
Although SZU101 could reduce both sides of the tumors, it could not
reduce the size of the spleen efficiently. We injected SZU101 into
the right tumor (we called it local tumor), and the result showned
that SZU101 inhibited the local tumor (Fig. 3D). We also found that the growth of
the left tumor was inhibited (Fig.
3C). The results showed that intratumoral SZU101 injection in
local tumor was inhibited on the other side of the tumor.
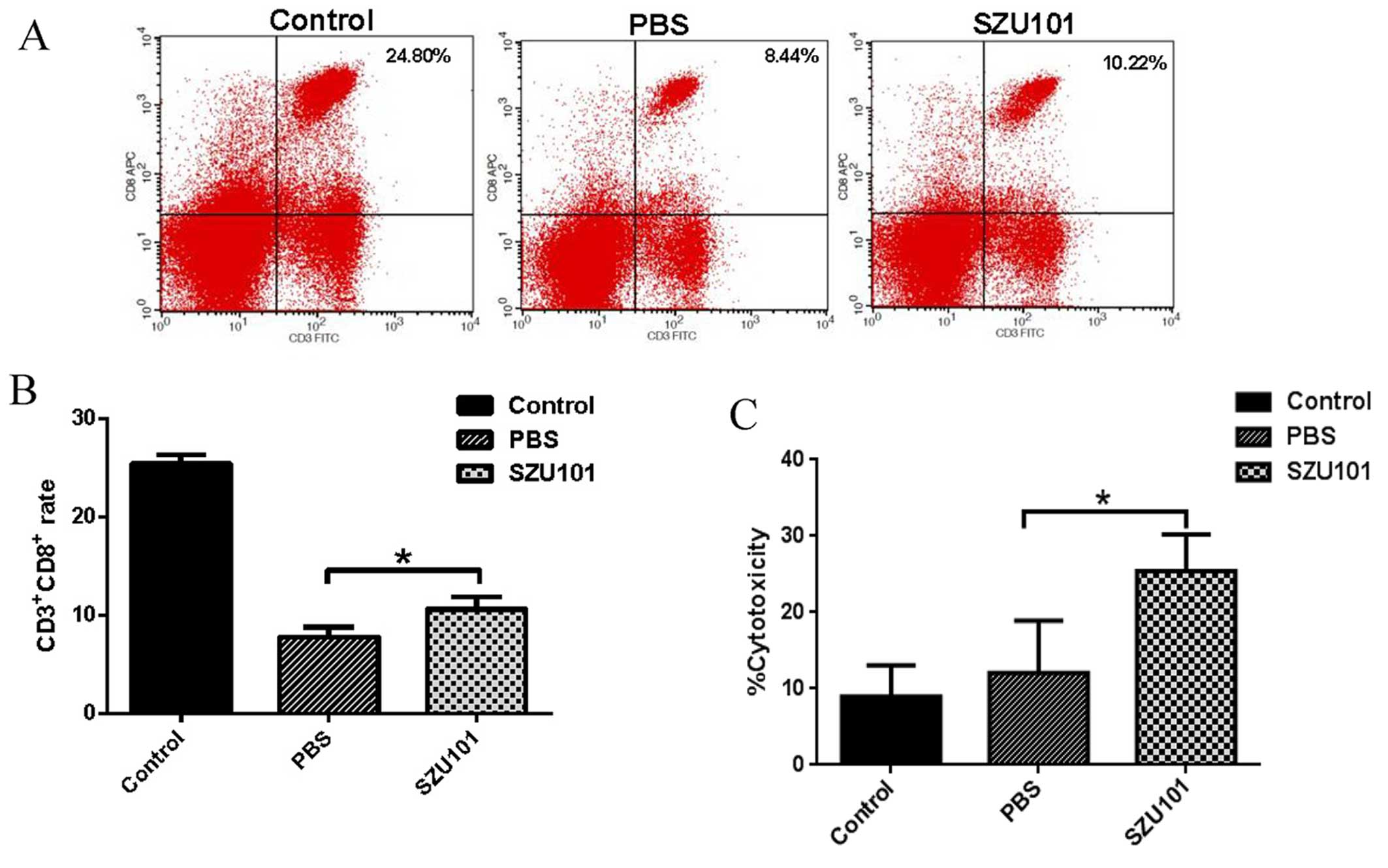
CTL assay
It has been demonstrated that the proportion of CD8
T cell not only in the TILs but also in the spleen was significant.
To assess the ability of the SZU101 to activate CTLs, we determined
the percentage of CD3+/CD8+ T cells, and the
cytotoxicity rates of splenic lymphocytes on 4T1 tumor cells. As
shown in Fig. 4A, the percentages
of CD3+/CD8+ T cells in group SZU101 are
remarkably higher than that in the control (Fig. 4B). The cytotoxicity rates of 4T1
cells induced by splenic lymphocytes in group SZU101 is higher than
that in the control (Fig. 4C).
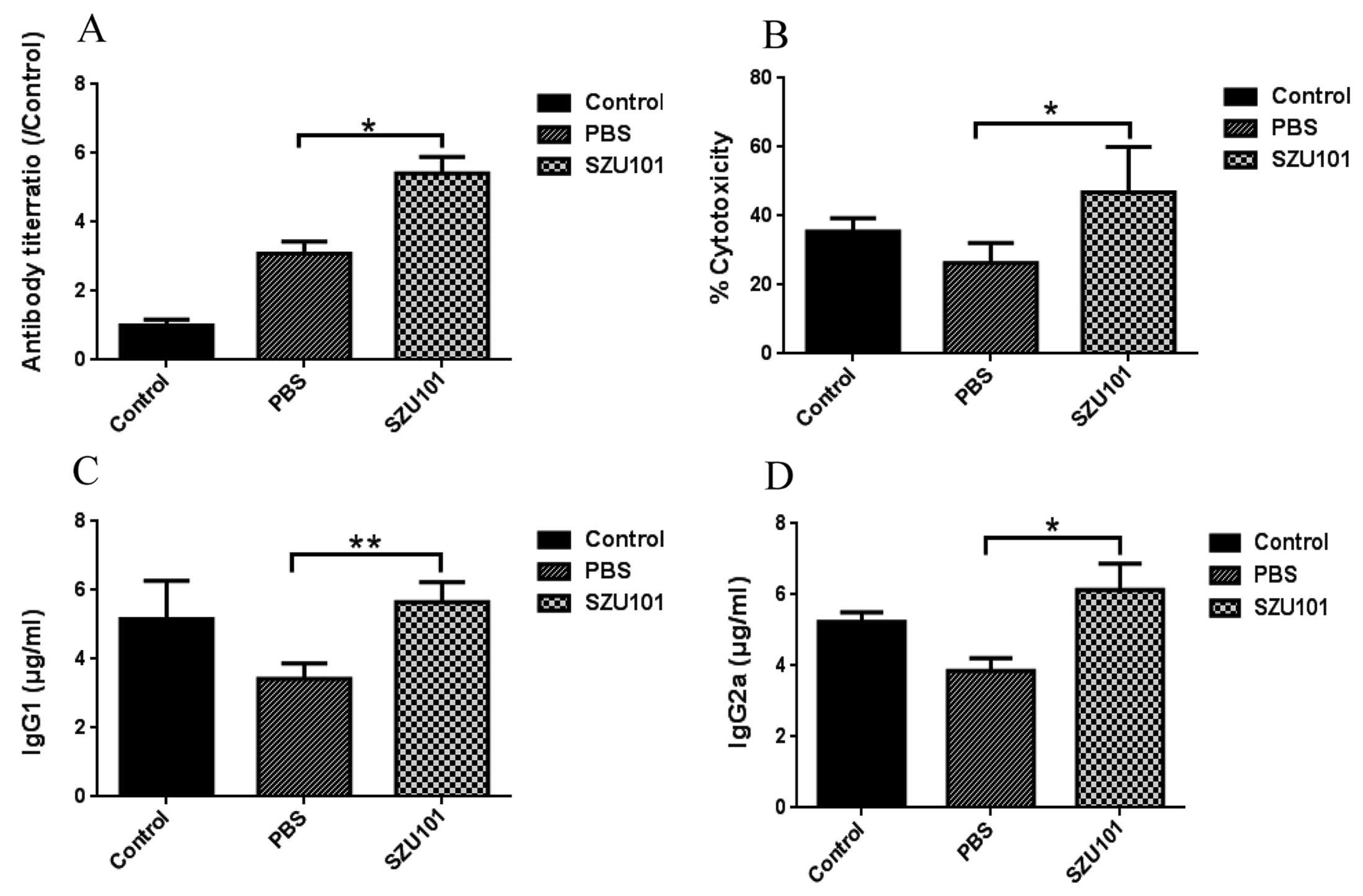
The humoral immune assay
Serum antibody titers against 4T1 tumor cells were
determined by ELISA assay coating microtiter plates with whole
protein of 4T1 tumor cells. As shown in Fig. 5, 4T1 tumor cell antibody increased
significantly in SZU101 group compared to control, but the control
group also had high antibody titers (Fig. 5A). SZU101 was effective in eliciting
IgG responses in serum, and these responses were generally
dominated by IgG2a, which is thought to reflect Th1-driven
response. IgG2a titers were significantly different between
SZU101-treated group and control group (Fig. 5D). Antibody-dependent cell-mediated
cytotoxicity (ADCC) was determined by addition of serum samples and
NK cells (cytotoxic effector cells) to 4T1 tumor cells (target
cells), and measurement of released LDH activity (Fig. 5B). Compared with PBS control,
control group was not significantly different tumor from cell
lysis. However, SZU101 treated group induced significant cancer
cell lysis compared with the other two groups. ADCC is primarily
mediated by IgG1. IgG1 titers were increased in Fig. 5C.
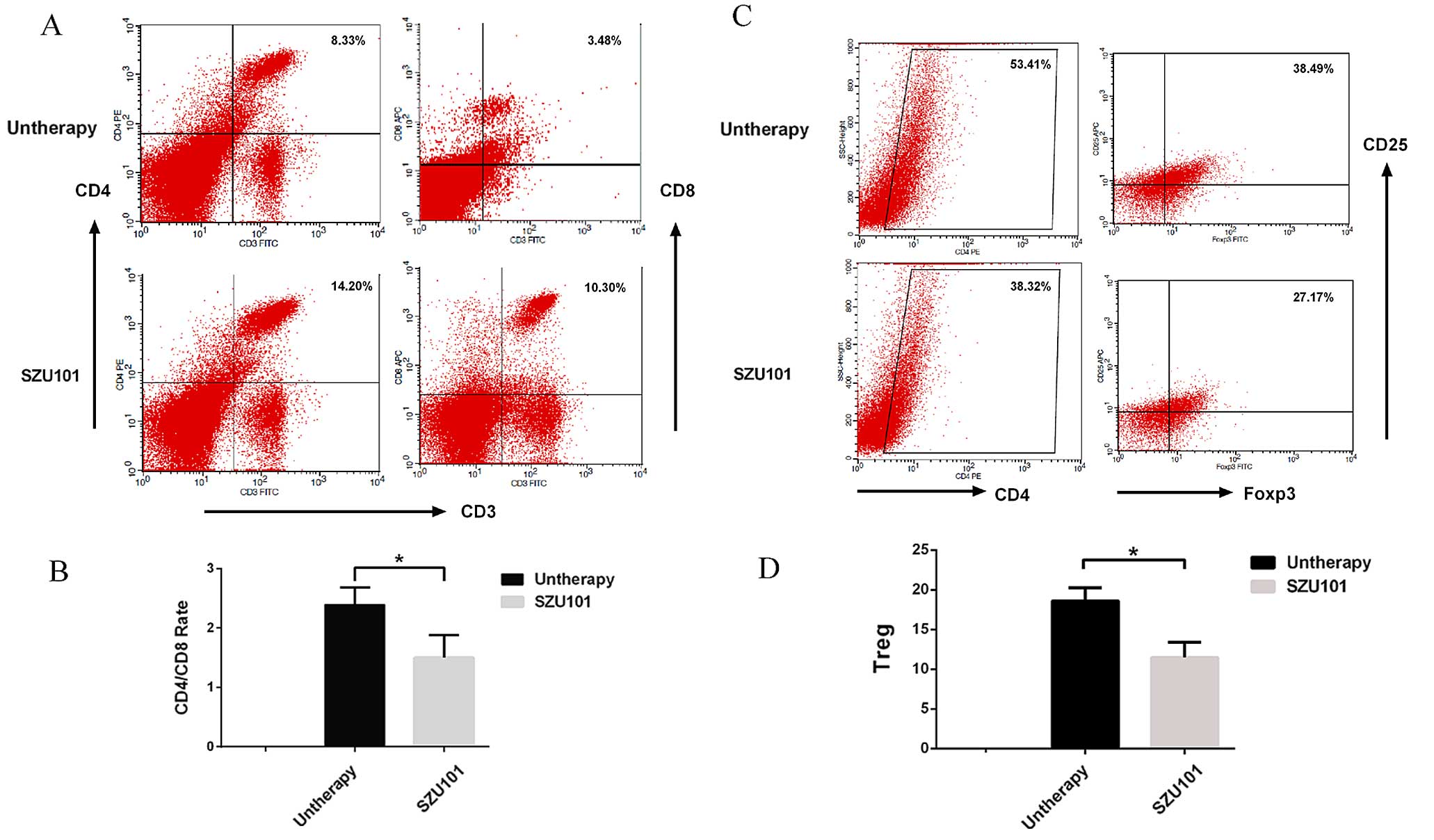
SZU101 effect on the frequency of
intratumoral immune cell infiltration
To examine the 4T1 tumor microenvironment in the
treated mouse and to study if the treatment of tumor-bearing mice
with SZU101 would lead to memory responses in the mice, we
collected and processed distant tumors for analysis of infiltrating
cells (Fig. 6). The percentage of T
helper cells (CD4+/CD3+) in SZU101-treated
mice significantly increased compared with untreated mice (Fig. 6A). We also found a strong increase
of CD8+ T cells in the distant tumor (Fig. 6A). The
CD4+/CD8+ ratio was reduced in SZU101 treated
group (Fig. 6B). To determine the
effect of SZU101 treatment on Tregs at the distant tumor site, CD25
and Foxp3 expression on CD4+ cells was assessed by flow
cytometry. The distribution of CD25+, Foxp3+,
or CD25+Foxp3+ (Treg) cells in the distant
tumor are shown in Fig. 6C and D,
respectively. In the distant tumor, the frequency of
CD25+Foxp3+ cells was significantly reduced
in SZU101-treated mice compared with (Fig. 6D). The untreated mice had 38.49±2.1%
Tregs (Fig. 6C), whereas mice
treated with SZU101 had fewer Tregs at the distant tumor site
(27.17±3.6%, P<0.05) (Fig.
6C).
Discussion
For most solid tumors, surgery is still the main
method, but small metastatic tumors cannot always be found and
removed totally. Normally our immune systems are able to recognize
tumor cells and remove them, but the tumor-bearing host loses the
ability to recognize tumor cells as exogenous and fails to defend
transformed cells. Immunotherapy attempts to stimulate the
patient's own natural ability of the immune system to fight cancer,
it is becoming increasingly widely used as more successful
approaches are discovered (17).
TLR agonists are of great interest in cancer immunotherapy
(5). TLR agonists used as single
agents especially when applied locally can effectively eradicate
tumors due to their potent stimulation of innate and adaptive
immunity as well as their effects on the tumor microenvironment
(6,18). In the present study, we set up a
local administration strategy to trigger systemic antitumor immune
response on a mouse breast cancer model. The antitumor effect of
SZU101 was associated with TLR7 activation of both innate immune
cells and adaptive immune cells, especially reversal of tolerance
in tumor microenvironment.
Non-specific innate immune cells play an important
role in cancer therapy, particularly in the elimination of tumor
metastases and small tumors. Activited NK cells have not only the
capacity to detect changes in transformed cells, but also the
responsiblity for tumor rejection in a direct manner (19). Low natural killer (NK) activity has
been reported as a high risk for developing malignancy (20). NK cells produce perforin to lyse
tumor cells directly as shown in vivo and in vitro
(21,22). TLR agonists can increase NK cell
activity to enhance antibody-dependent cell-mediated cytotoxicity
(ADCC) (23,24). NK cells produce type 1 cytokines
such as IFN-γ and TNF-α during tumor occurrence to further enhance
their cytotoxicity and modulate the adaptive immune cells, such as
dendritic cells and T cells (25).
SZU101 stimulated NK cells to produce more TNF-α and INF-γ than LPS
control (Fig. 2B). In ADCC, the
SZU101 group had higher ADCC activity than the other groups. There
are two major kinds of macrophages: classical M1 and alternative M2
macrophages. The M1 macrophage can be activated by TLR agonists and
INF-γ, and secrete high levels of TNF-α, it is related to the
inflammatory response, pathogen clearance, and antitumor immunity.
In contrast, M2 macrophage influences an anti-inflammatory
response, wound healing, and pro-tumorigenic properties (26,27).
Tumor-associated macrophages (TAMs) are considered mainly M2
macrophages with poor response to therapy (28). Activation of macrophages to the M1
phenotype leads to upregulation of several pro-inflammatory
cytokines and chemokines (29). We
utilized RAW264.7 as the model in vitro. The results showed
that SZU101 stimulated RAW264.7 to produce high level of TNF-α and
IL-6, which were markers of the M1 macrophage (Fig. 1C). In our study, SZU101 could not
only elicit the non-specific antitumor responses but also
strengthen the specific humoral and cellular immune responses. DCs
play a crucial role in linking innate and adaptive immunity, and in
the generation of a protective immune response against both
infectious diseases and tumors. DCs are always immature in tumor
microenvironment (30). Here, DCs
were activated and matured by SZU101 in vitro, and we
detected the secretion of IL12, TNF-α at a high secretive level
(Fig. 1A). The major function of
DCs is as professional antigen presenting cells (APCs) to induce
naïve T cell into protective CD8 T cells and CD4 T helper cells.
Antigen delivery to DCs and maturation of DCs is relevant to
antitumor success. Our CTL assay data showed that SZU101 stimulated
systemic antitumor CD8 T cell response. SZU101 was injected into
local tumor site (right frank), but the distant tumor was inhibited
at the same time (Fig. 2). It
implied that SZU101 may induce the CD8 T cells into T memory cells
and CTL to aim to distant tumor. Intratumoral administration of
TLR7 agonist generated systemic antitumor immunity and suppressed
both injected and distant, uninjected wild-type B16F10 melanomas in
a recent study. It showed that CD8 T cells, B cells, type I IFN,
IFN-γ and plasmacytoid dendritic cells contributed to efficient
tumor suppression (31). The
microenvironment of solid tumor typically contains various cell
subsets of the innate immune system (32). The immune cells have been shown to
influence the immune system to promote either antitumor immunity,
or tumor progression in the tumor microenvironment. Diederichsen
et al showed a significantly higher 5-year survival in
patients with a low CD4+/CD8+ ratio in the
tumor infiltrating lymphocytes (33). Bloomfield et al investigated
the potential of locally delivered imiquimod in a murine model of
malignant mesothelioma (AB1-HA) with primary and distal tumors
(dual tumor). They found imiquimod injection locally could
stimulate an effective systemic antitumor response required both
CD8 T cells and NK cells, but not CD4 T cells (34).
CD8+ T cells within cancer nests were
shown to be better predictors of outcome than the same cells found
in other areas of the tumor in NSCLC (35). Similarly, tumor specific
CD8+ T cell activity determines colorectal cancer
patient prognosis (36). In
accordance with these results, our results showed that infiltration
of CD8+ T cells increased, and
CD4+/CD8+ T cells decreased in distant tumor
by SZU101 injection (Fig. 6). As
shown previously, SZU101 injection group delayed distant tumor
growth. Clinical antitumor resistance has been correlated with
increased intratumoral levels of immunosuppressive regulatory T
cells (Tregs), and the phenotype of Tregs is
CD4+/FOXP3+/CD25+ (37). We detected Tregs in the distant
tumor, and representative dot plots are displayed in Fig. 6C. The results suggested that SZU101
reduced the number of Tregs in the distant tumor microenvironment.
Some studies showed that Treg cell-mediated suppression could be
overcome by the stimulation of TLRs on DCs (4). In a recent study, loxoribin, one of
the TLR7 ligands, inhibited tumor growth in xenograft models of
colon cancer and lung cancer by reversing Treg-mediated suppression
via dendritic cells (DCs) (38).
In conclusion, the immunostimulatory properties of
TLR ligands have been exploited to increase the efficacy of cancer
immunotherapy. Our results confirmed that SZU101 could induce both
innate and adaptive immune cells to strong Th-1-bias immune
responses and the release of proinflammatory cytokines, such as
TNF-α, IFN-γ, IL-6 and IL-12 in vitro. In a previous study,
our group used SZU-101 to treat tumors in a murine model of T cell
lymphoma. SZU-101 could activated TLR7 NF-κB signaling in a
TLR7-specific system at a low concentration of 1 µM after 6
h of stimulation in vitro (15). The anticancer therapies given
directly into tumors may be more effective than given systemically,
because local therapies could overcome natural suppressive factors
in the tumor micro-environment, and induce systemic antitumor
immunity (39). For solid tumors,
especially breast cancer, direct intratumoral injection is safe and
effective (40). In the 4T1 mouse
model of breast cancer, intratumoral administration of SZU101
generated systemic antitumor immunity and suppressed both injected
and distant tumors. It implied that intratumoral immune activation
could induce local and systemic antitumor immunity by SZU101. Our
findings in the present study provide an effective breast cancer
immunotherapy by targeting tumor microenvironment.
Acknowledgments
We thank Professor Dennis Carson, University of
California at San Diego, USA, for excellent technical guidance. We
also thank the National Natural Science Foundation of China (grant
81202396 and 81273374), the Science Foundation of Shenzhen (grant
JCYJ20130326112757843 and JCYJ20130326110057374) and China
Postdoctoral Science Foundation (grant 2013M542200) for providing
financial support of the present study.
References
|
1
|
Barton GM and Medzhitov R: Toll-like
receptors and their ligands. Curr Top Microbiol Immunol. 270:81–92.
2002.PubMed/NCBI
|
|
2
|
Iwasaki A and Medzhitov R: Toll-like
receptor control of the adaptive immune responses. Nat Immunol.
5:987–995. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gibson SJ, Lindh JM, Riter TR, Gleason RM,
Rogers LM, Fuller AE, Oesterich JL, Gorden KB, Qiu X, McKane SW, et
al: Plasmacytoid dendritic cells produce cytokines and mature in
response to the TLR7 agonists, imiquimod and resiquimod. Cell
Immunol. 218:74–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Duin D, Medzhitov R and Shaw AC:
Triggering TLR signaling in vaccination. Trends Immunol. 27:49–55.
2006. View Article : Google Scholar
|
|
5
|
Paulos CM, Kaiser A, Wrzesinski C,
Hinrichs CS, Cassard L, Boni A, Muranski P, Sanchez-Perez L, Palmer
DC, Yu Z, et al: Toll-like receptors in tumor immunotherapy. Clin
Cancer Res. 13:5280–5289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adams S: Toll-like receptor agonists in
cancer therapy. Immunotherapy. 1:949–964. 2009. View Article : Google Scholar
|
|
7
|
Lakshminarayanan V, Thompson P, Wolfert
MA, Buskas T, Bradley JM, Pathangey LB, Madsen CS, Cohen PA,
Gendler SJ and Boons GJ: Immune recognition of tumor-associated
mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated
MUC1 tripartite vaccine. Proc Natl Acad Sci USA. 109:261–266. 2012.
View Article : Google Scholar :
|
|
8
|
Hurwitz AA and Watkins SK: Immune
suppression in the tumor microenvironment: A role for dendritic
cell-mediated tolerization of T cells. Cancer Immunol Immunother.
61:289–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng C, Qu QX, Shen Y, Lv YT, Zhu YB,
Zhang XG and Huang JA: Overexpression of B7-H4 in tumor infiltrated
dendritic cells. J Immunoassay Immunochem. 32:353–364. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jochems C and Schlom J: Tumor-infiltrating
immune cells and prognosis: The potential link between conventional
cancer therapy and immunity. Exp Biol Med (Maywood). 236:567–579.
2011. View Article : Google Scholar
|
|
11
|
Aranda F, Vacchelli E, Obrist F, Eggermont
A, Galon J, Sautès-Fridman C, Cremer I, Henrik Ter, Meulen J,
Zitvogel L, Kroemer G, et al: Trial Watch: Toll-like receptor
agonists in oncological indications. OncoImmunology. 3:e291792014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hemmi H, Kaisho T, Takeuchi O, Sato S,
Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K and Akira S:
Small anti-viral compounds activate immune cells via the TLR7
MyD88-dependent signaling pathway. Nat Immunol. 3:196–200. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee J, Chuang TH, Redecke V, She L, Pitha
PM, Carson DA, Raz E and Cottam HB: Molecular basis for the
immunostimulatory activity of guanine nucleoside analogs:
Activation of Toll-like receptor 7. Proc Natl Acad Sci USA.
100:6646–6651. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stary G, Bangert C, Tauber M, Strohal R,
Kopp T and Stingl G: Tumoricidal activity of TLR7/8-activated
inflammatory dendritic cells. J Exp Med. 204:1441–1451. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu J, He S, Du J, Wang Z, Li W, Chen X,
Jiang W, Zheng D and Jin G: Local administration of a novel
Toll-like receptor 7 agonist in combination with doxorubicin
induces durable tumouricidal effects in a murine model of T cell
lymphoma. J Hematol Oncol. 8:212015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zamarin D, Holmgaard RB, Subudhi SK, Park
JS, Mansour M, Palese P, Merghoub T, Wolchok JD and Allison JP:
Localized oncolytic virotherapy overcomes systemic tumor resistance
to immune checkpoint blockade immunotherapy. Sci Transl Med.
6:226ra322014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stewart TJ and Smyth MJ: Improving cancer
immunotherapy by targeting tumor-induced immune suppression. Cancer
Metastasis Rev. 30:125–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanzler H, Barrat FJ, Hessel EM and
Coffman RL: Therapeutic targeting of innate immunity with Toll-like
receptor agonists and antagonists. Nat Med. 13:552–559. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stojanovic A and Cerwenka A: Natural
killer cells and solid tumors. J Innate Immun. 3:355–364. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whiteside TL: Immune suppression in
cancer: Effects on immune cells, mechanisms and future therapeutic
intervention. Semin Cancer Biol. 16:3–15. 2006. View Article : Google Scholar
|
|
21
|
Marcus A, Gowen BG, Thompson TW, Iannello
A, Ardolino M, Deng W, Wang L, Shifrin N and Raulet DH: Recognition
of tumors by the innate immune system and natural killer cells. Adv
Immunol. 122:91–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho D1, Shook DR, Shimasaki N, Chang YH,
Fujisaki H and Campana D: Cytotoxicity of activated natural killer
cells against pediatric solid tumors. Clin Cancer Res.
16:3901–3909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moreno M, Mol BM, von Mensdorff-Pouilly S,
Verheijen RH, von Blomberg BM, van den Eertwegh AJ, Scheper RJ and
Bontkes HJ: Toll-like receptor agonists and invariant natural
killer T-cells enhance antibody-dependent cell-mediated
cytotoxicity (ADCC). Cancer Lett. 272:70–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lauzon NM, Mian F, MacKenzie R and Ashkar
AA: The direct effects of Toll-like receptor ligands on human NK
cell cytokine production and cytotoxicity. Cell Immunol.
241:102–112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vivier E, Raulet DH, Moretta A, Caligiuri
MA, Zitvogel L, Lanier LL, Yokoyama WM and Ugolini S: Innate or
adaptive immunity? The example of natural killer cells. Science.
331:44–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chanmee T, Ontong P, Konno K and Itano N:
Tumor-associated macrophages as major players in the tumor
microenvironment. Cancers (Basel). 6:1670–1690. 2014. View Article : Google Scholar
|
|
27
|
Mosser DM and Edwards JP: Exploring the
full spectrum of macrophage activation. Nat Rev Immunol. 8:958–969.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ostuni R, Kratochvill F, Murray PJ and
Natoli G: Macrophages and cancer: From mechanisms to therapeutic
implications. Trends Immunol. 36:229–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lopez-Castejón G, Baroja-Mazo A and
Pelegrín P: Novel macrophage polarization model: From gene
expression to identification of new anti-inflammatory molecules.
Cell Mol Life Sci. 68:3095–3107. 2011. View Article : Google Scholar
|
|
30
|
Ullrich E, Ménard C, Flament C, Terme M,
Mignot G, Bonmort M, Plumas J, Chaperot L, Chaput N and Zitvogel L:
Dendritic cells and innate defense against tumor cells. Cytokine
Growth Factor Rev. 19:79–92. 2008. View Article : Google Scholar
|
|
31
|
Singh M, Khong H, Dai Z, Huang XF, Wargo
JA, Cooper ZA, Vasilakos JP, Hwu P and Overwijk WW: Effective
innate and adaptive antimelanoma immunity through localized TLR7/8
activation. J Immunol. 193:4722–4731. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mao Y, Keller ET, Garfield DH, Shen K and
Wang J: Stromal cells in tumor microenvironment and breast cancer.
Cancer Metastasis Rev. 32:303–315. 2013. View Article : Google Scholar
|
|
33
|
Diederichsen AC, Hjelmborg J, Christensen
PB, Zeuthen J and Fenger C: Prognostic value of the
CD4+/CD8+ ratio of tumour infiltrating
lymphocytes in colorectal cancer and HLA-DR expression on tumour
cells. Cancer Immunol Immunother. 52:423–428. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Broomfield SA, van der Most RG, Prosser
AC, Mahendran S, Tovey MG, Smyth MJ, Robinson BW and Currie AJ:
Locally administered TLR7 agonists drive systemic antitumor immune
responses that are enhanced by anti-CD40 immunotherapy. J Immunol.
182:5217–5224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hiraoka K, Miyamoto M, Cho Y, Suzuoki M,
Oshikiri T, Nakakubo Y, Itoh T, Ohbuchi T, Kondo S and Katoh H:
Concurrent infiltration by CD8+ T cells and
CD4+ T cells is a favourable prognostic factor in
non-small-cell lung carcinoma. Br J Cancer. 94:275–280. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Reissfelder C, Stamova S, Gossmann C,
Braun M, Bonertz A, Walliczek U, Grimm M, Rahbari NN, Koch M,
Saadati M, et al: Tumor-specific cytotoxic T lymphocyte activity
determines colorectal cancer patient prognosis. J Clin Invest.
125:739–751. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yamaguchi T and Sakaguchi S: Regulatory T
cells in immune surveillance and treatment of cancer. Semin Cancer
Biol. 16:115–123. 2006. View Article : Google Scholar
|
|
38
|
Wang C, Zhou Q, Wang X, Wu X, Chen X, Li
J, Zhu Z, Liu B and Su L: The TLR7 agonist induces tumor regression
both by promoting CD4+ T cells proliferation and by
reversing T regulatory cell-mediated suppression via dendritic
cells. Oncotarget. 6:1779–1789. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nelson D, Fisher S and Robinson B: The
'Trojan Horse' approach to tumor immunotherapy: Targeting the tumor
microenvironment. J Immunol Res. 2014:7890692014. View Article : Google Scholar
|
|
40
|
Van der Jeught K, Bialkowski L,
Daszkiewicz L, Broos K, Goyvaerts C, Renmans D, Van Lint S, Heirman
C, Thielemans K and Breckpot K: Targeting the tumor
microenvironment to enhance antitumor immune responses. Oncotarget.
6:1359–1381. 2015. View Article : Google Scholar : PubMed/NCBI
|