Introduction
Laparoscopic technology has been widely adopted in
colorectal surgery and CO2 is commonly used to create
laparoscopic pneumoperitoneum. However, the effect of
CO2 pneumoperitoneum on tumor proliferation and
metastasis is still controversial. Various researchers believed
that the therapeutic effect of laparoscopic colon cancer surgery
under CO2 pneumoperitoneum was equivalent to that of
conventional laparotomy, increasing neither the recurrence rate nor
the local or remote metastasis rate (1). However, a majority of them considered
that CO2 pneumoperitoneum could promote colon cancer
cell proliferation or metastasis under persistent pressure for a
certain time period (2–4). Intraperitoneal hyperthermia
chemoperfusion (IHCP) was reported to effectively eliminate tumor
cells and small tumor metastasis in the peritoneal cavity, and
prevent tumor peritoneal dissemination (5,6), which
has been utilized as adjuvant therapy after open surgeries on
gastric, colorectal and ovarian cancer. However, it is still an
open question how to reduce the adverse effect of CO2
pneumoperitoneum on the therapeutic effect of colon cancer surgery,
and how to utilize IHCP as combined therapy. It has been speculated
that the therapeutic effect may be improved by introducing heated
CO2 into the abdomen simulating IHCP at 43°C before
laparoscopic therapy. In the present study, the in vitro
assay was used to investigate the effect of CO2
pneumoperitoneum with hyperthermia on colon cancer cell
proliferation and metastasis.
Materials and methods
Cell culture
Colon cell line SW-480 was provided by Shanghai Cell
Bank of Chinese Academy of Sciences (Shanghai, China) and was
cultured with L-15 medium (pH 7.35) containing 10% calf serum in an
incubator at 37°C supplemented with 5% CO2, 20%
O2 and 75% N2. The culture medium was
refreshed every other day, and the cells at logarithmic growth
phase were used for the experiment.
Materials and instruments
L-15 culture medium was purchased from Gibco (Thermo
Fisher Scientific, San Jose, CA, USA), and calf serum was obtained
from Hangzhou Tianhang Biological Technology Co., Ltd. (Hangzhou,
China). The following reagents or kits were used: CCK-8
cytotoxicity analysis kit, Annexin V-FITC apoptosis detection kit
(both from Dojindo Laboratories, Japan), KGI cell DNA content
detection kit, TRIzol reagent (Invitrogen, USA), RNA extraction
kit-RNAiso Plus (Takara, Japan), First Strand cDNA Synthesis kit
and Maxima SYBR-Green/ROX qPCR Master mix (both from Thermo Fisher
Scientific). The first antibodies were procured from Abcam (USA),
including mouse monoclonal antibody against HSP-70 or HIF-1α and
rabbit monoclonal antibody against Matrix metalloproteinase-9
(MMP-9) or caspase-3. The secondary antibodies were procured from
Arigo, including goat anti-rabbit IgG and goat anti-mouse IgG.
Polymerase chain reaction (PCR) primers were designed and
synthesized by Sangon Biotech (Shanghai, China). The equipment
included the ELISA reader (Thermo, USA), flow cytometry (BD
FACSCalibur; USA), western blot electrophoresis (Bio-Rad, USA) and
fluorescent-PCR machine (Applied Biosystems, USA).
Cell treatment and experimental
grouping
The disposable 2-L urine collection bag was utilized
to simulate the pneumoperitoneum. A small cut was made on the
lateral part of the urine collection bag, through which a balanced
plate was inserted and the Petri dish or 96-well plate was
attached. The cut was then sealed using the sealer. One port of the
urine collection bag was connected with 100% CO2, while
the other port was connected to blood-pressure meter to monitor the
pressure of CO2 with the preset of 14 mmHg. The
temperature was set at 43 and 37°C, using a cell culture incubator
for 2 h. The cells were divided into the control, the
CO2 pneumoperitoneum and the hyperthermo-CO2
pneumoperitoneum group, and then put back into the normal
incubator.
Proliferation and morphology of
cells
The cells were seeded onto the six-well plate at a
density of 5×105/well in 2 ml. The cells from each group
were observed and photographed under the microscope at 12, 24, 36,
48, 60 and 72 h after the treatment. The experiment was repeated
three times.
Cell proliferation inhibition detection
using the WST-8 test
The cells of 1×104/well at logarithmic
growth phase were seeded onto the 96-well plate and cultured for 24
h. Then the cells from each group were separately treated with
continued culturing for 12, 24, 36, 48, 60 and 72 h. CCK-8 (10
µl) was added to each well for 4 h in the absence of light.
The optical density (OD) value at 450 nm was measured to represent
the number of viable cells after subtracting the OD value from the
OD value of phosphate-buffered saline (PBS). The triplicate wells
were used for average calculation, and the cell proliferation
inhibition was calculated to plot the curve of proliferation
inhibition, using the formula: Cell proliferation inhibition rate =
1 - (OD of treated group - OD of background)/(OD of control group -
OD of background) × 100%.
Cell apoptosis and necrosis detection
using fluorescence-activated cell sorting analysis
The cells were seeded onto the six-well plate at a
density of 5×105/well in 2 ml and cultured for 24 h.
Then, the cells were separately treated and collected after another
12 h of normal culture. The cells were resuspended in 1X binding
buffer after washing twice with cold PBS, and adjusted to the
density of 1×106/ml. Cell suspensions (100 µl)
were transferred to a 5-ml tube for fluorescence-activated cell
sorting (FACS). Annexin V-FITC and propidium iodide (PI), 5
µl each, were added into the cell suspensions and incubated
for 15 min at room temperature in the absence of light. The cell
mixture was incubated on ice after adding 400 µl of 1X
binding buffer, and FACS analysis was performed within 1 h.
FITC−/PI− was defined as normal cells,
FITC+/PI− was defined as apoptotic cells at
an early stage, FITC+/PI+ was defined as
apoptotic cells at a late stage, and
FITC−/PI+ was defined as necrotic cells. The
experiment was repeated three times. The apoptotic index (AI) and
necrotic rate were calculated using the averaged measurements from
the experiments. AI = (number of apoptotic cells at late stage +
number of apoptotic cells at early stage)/total number of cells;
necrotic rate = number of necrotic cells/total number of cells.
Cell cycle detection using FACS
Cells (1×106/ml) were collected and
washed twice with ice-cold PBS. Then the cells were fixed in 70%
precooled ethanol overnight. The fixation buffer was discarded,
RNAase (0.1 mg/ml) was added into the cells after washing twice
with PBS, and the cells were incubated at 37°C for 30 min. Then, 1
ml of PI (50 mg/l) was added and incubated for 30 min before FACS
analysis. The experiments were repeated three times.
Extracellular fluid temperature and pH
measurements
The temperature and pH of the culture medium from
each group were measured using a thermometer and a pH probe.
Cell scratch test
We used the scratch test to detect the migration of
the cells, since SW-480 cells showed adherent growth. The cells at
logarithmic growth phase were seeded onto the six-well plate at a
density of 5×105/well in 2 ml and cultured for 48 h. The
normal culture was continued for 24 h after the treatment of each
group. A straight line was scratched onto the bottom of Petri dish
using a syringe needle, and the cell proliferation and crossing
line were observed under the microscope at 24, 36, 48, 60 and 72
h.
Expression of protein detection using
western blotting
The cells were continuously cultured for 2 or 12 h
after the treatment, and 1×107 cells were collected.
Radioimmunoprecipitation assay lysis buffer (150 µl) was
added, and the supernatants were used for total protein
determination; the protein concentration ranged between 1.5 and 2.5
mg/ml. The supernatants were aliquoted into 20 µl ×2 and
stored at −80°C. Sodium dodecyl sulfate (SDS)-polyacrylamide
separating (10%) and concentrating gels (6%) were prepared; 80
µg proteins from each group were mixed with 5X SDS loading
buffer and subjected to SDS-polyacrylamide gel electrophoresis
(PAGE) after denaturing at 100°C for 10 min. The PAGE was stopped
according to the migration of the dye and protein markers, and the
proteins were then transferred to the polyvinylidene fluoride
(PVDF) membrane. The first antibody was incubated with PVDF
membrane overnight after blocking with skimmed milk solution for 1
h, and the secondary antibody was incubated for 1 h at room
temperature before enhanced chemiluminescence developing and
photographing. The molecular weight of the targeted protein was
estimated using protein markers, and β-actin was adopted as the
internal control to evaluate the total amount of protein-loaded
onto each lane. The images were analyzed using ImageJ software; the
amount of the targeted protein = relative gray scale x area
(mm2). The expression levels of HSP-70, caspase-3,
hypoxia-inducible factor-1α (HIF-lα) and MMP-9 were separately
calculated for comparison.
Fluorescence quantitative PCR
The relative mRNA amounts of HSP-70, caspase-3,
HIF-lα and MMP-9 were detected using fluorescence quantitative PCR.
The cells were collected after each treatment, the total RNA was
extracted using TRIzol reagent and cDNA was synthesized using a
First Strand cDNA Synthesis kit (MD, USA). The primers were
designed and synthesized by Sango Biotech as follows: HSP-70
forward, TA CTGTGGACCTGCCAATCG and reverse, TAGCATCATTC CGCTCCTTC;
HIF-lα forward, GCAGCAACGACACAGA AACT and reverse,
AGCGGTGGGTAATGGAGAC; MMP-9 forward, CCAACTACGACACCGACGAC and
reverse TGGA AGATGAATGGAAACTGG; caspase-3 forward, AGATGG
TTTGAGCCTGAGCA and reverse, CAGTGCGTATGGAG AAATGG; β-actin forward,
GATGCAGAAGGAGATCAC TG and reverse, TAGTCCGCCTAGAAGCATTTG.
The specific primers and Maxima SYBR-Green/ROX qPCR
Master mix were used for qPCR, with the reaction conditions as
follows: 50°C pretreated for 2 min, 95°C pre-denaturing for 10 min
and 95°C denaturing for 15 sec, and 60°C annealing, and extension
for 60 sec for a total of 40 cycles. Triplicate wells were utilized
and β-actin was used as the internal control. Quantitation was
represented by cycle-threshold value (ct value). Relative mRNA
value = 2−Δct (ΔCt = Cttarget gene -
Ctβ-actin). The differences between the treated and the
control group were analyzed.
Statistical analysis
The data are presented as mean ± SD, and the
differences between the two groups were analyzed using
χ2 test. The differences among groups were analyzed
using one-way analysis of variance test. SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA) was used in the present study, and
P<0.05 was considered to indicate a statistically significant
result.
Results
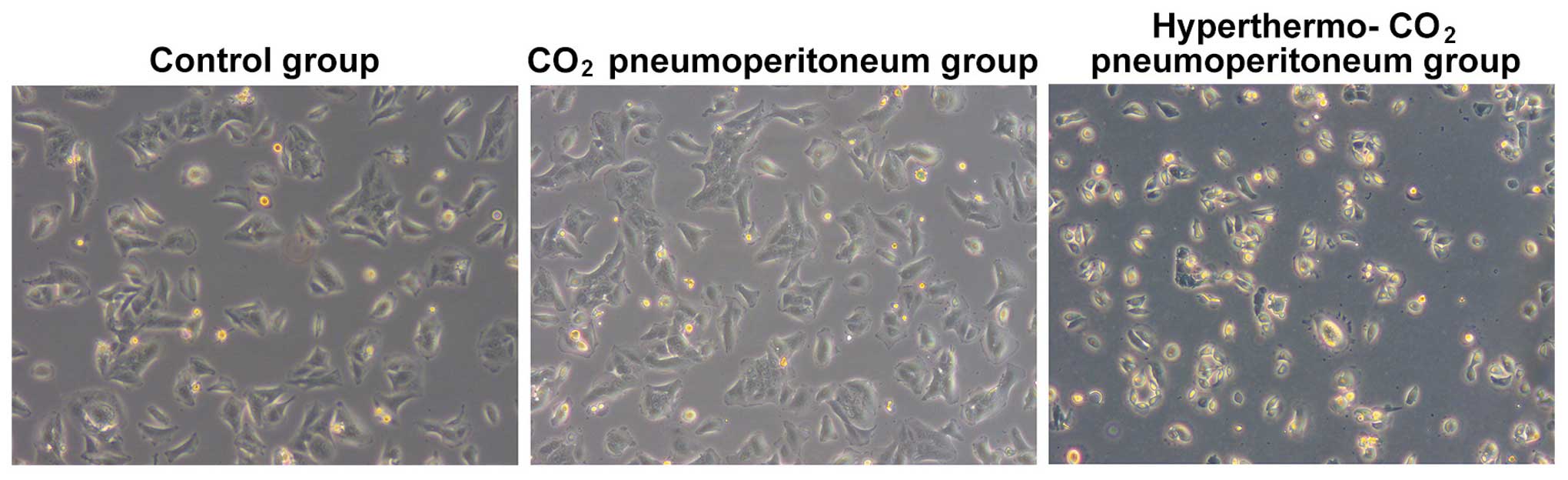
Dynamic observation under a microscope on
the cell proliferation and morphology
The cells from the control and CO2
pneumoperitoneum group were attached to the wall of the Petri dish
with rod-, spindle-, leave- or branch-like morphology; all were in
good condition with a rapid growth rate, which reached 100%
confluence within 3 or 4 days, without obvious dead cells. However,
the majority of the cells from the hyper-thermo-CO2
pneumoperitoneum group started to shrink after 12 h. The cells
presented with triangular or round morphology without sparapodium,
the refraction of the cells increased, and the cells were detached
from the wall of the Petri dish and suspended into the culture
medium. The cell death and cell debris could be observed after 24
h. The total cell number was reduced and normal morphology loss
aggravated with time. The dead cells and cell debris filled the
whole Petri dish after 48 h (Fig.
1). The cell proliferation slowed down or arrested, and the
cells recovered after 7–10 days.
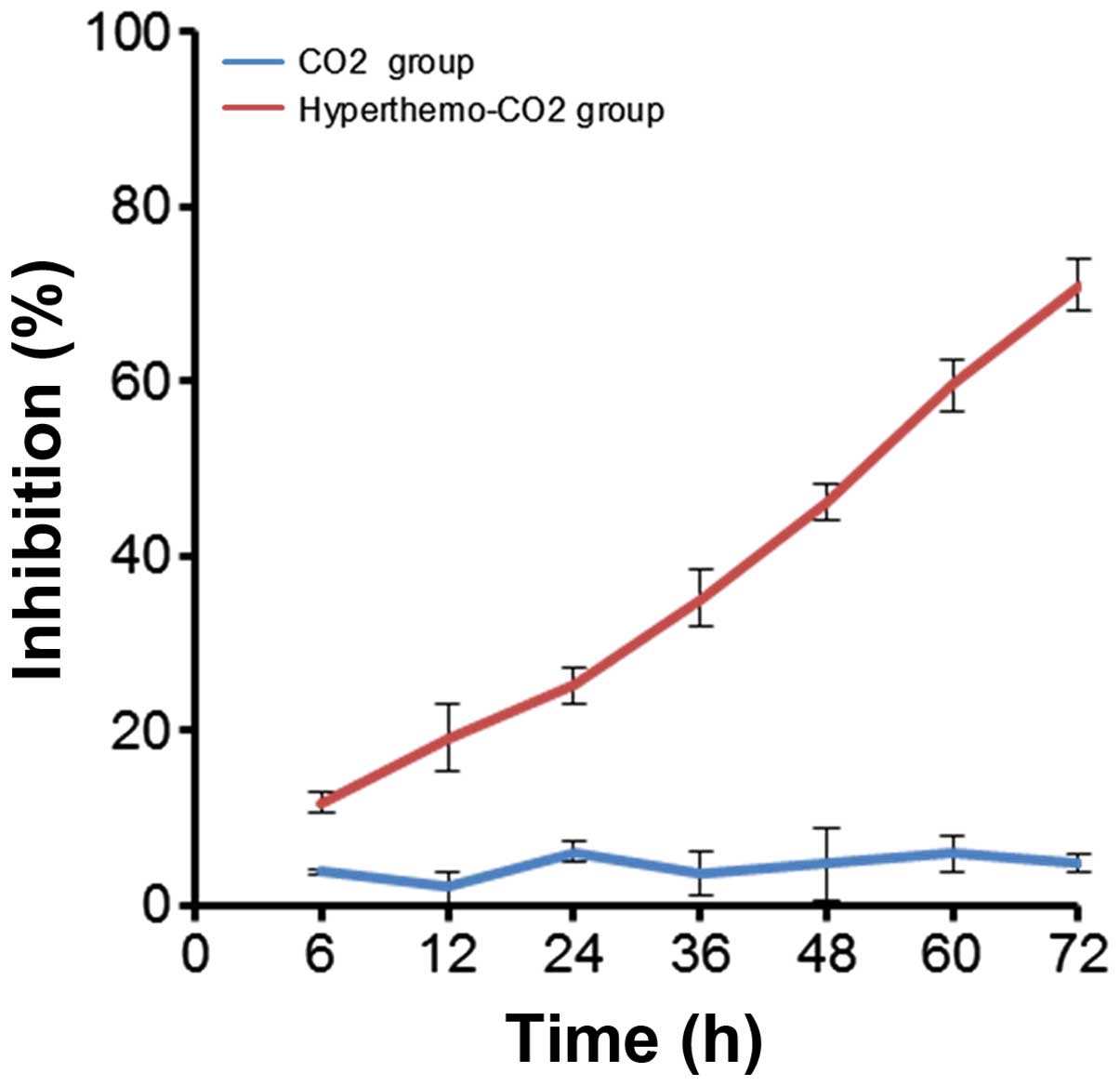
Cell proliferation inhibition detection
using WST-8 test
The results of WST-8 test for cell proliferation
inhibition detection of each group are shown in Fig. 2. The inhibition rate was 2.00±0.62,
6.33±1.53, 4.33±1.05 and 5.00±1.14% in the CO2 group
after treatment for 12, 24, 48 and 72 h, respectively; whereas, it
was 19.33±3.76, 46.33±4.86, 60.67±5.37 and 83.00±6.64% in the
hyperthermo-CO2 group. The difference between the two
groups could be observed at each time point with statistical
significance (P<0.05), indicating that CO2 had no
obvious effect on cell proliferation, while the
hyperthermo-CO2 had a significant inhibitory effect.
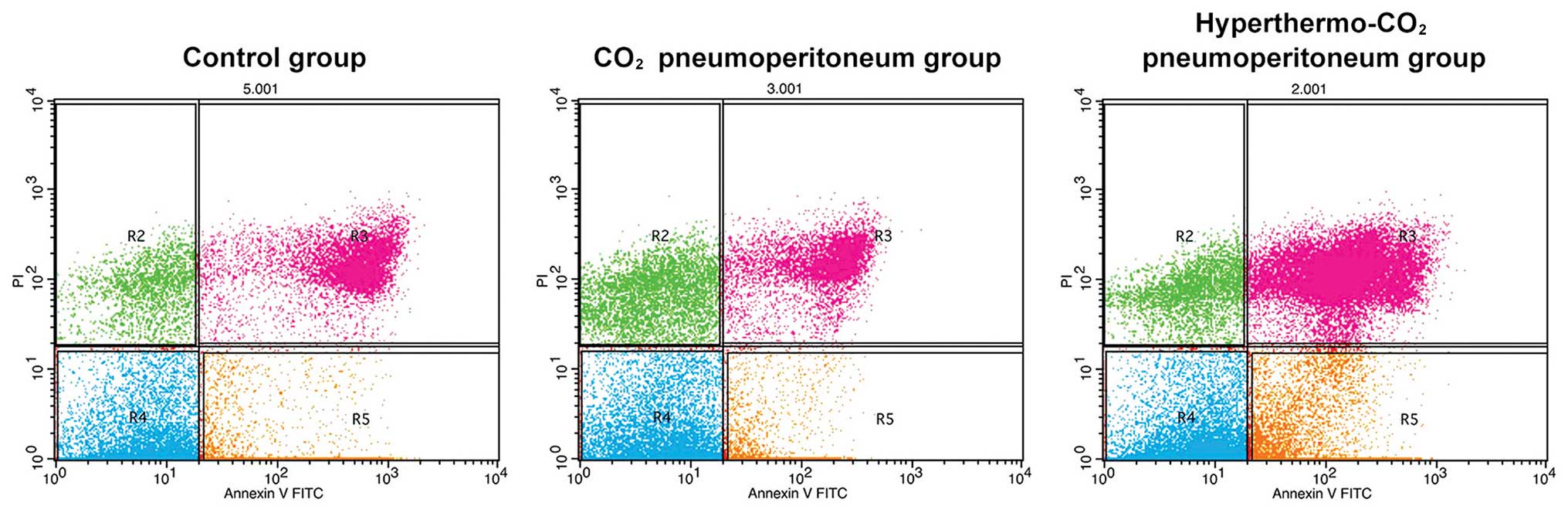
Cell apoptosis detection using FACS
analysis
The apoptosis and necrosis of cells after treatment
were analyzed using FACS with Annexin V/PI staining. The formula
used for apoptosis calculation was as follows: Apoptosis rate (%) =
R3 + R5. The apoptosis and necrosis rates were 11.37±0.87 and
5.32±0.46% in the control group, 13.26±0.95 and 5.76±0.58% in the
CO2 group and 45.20±3.11 and 32.50±5.12% in the
hyperthermo-CO2 group, respectively (Fig. 3). There was no significant
difference between the control group and the CO2 group
(P>0.05), while the apoptosis and necrosis rates in the
hyperthermo-CO2 group significantly increased
(P<0.05), indicating that hyperthermo-CO2 could
induce apoptosis and promote necrosis of cancer cells.
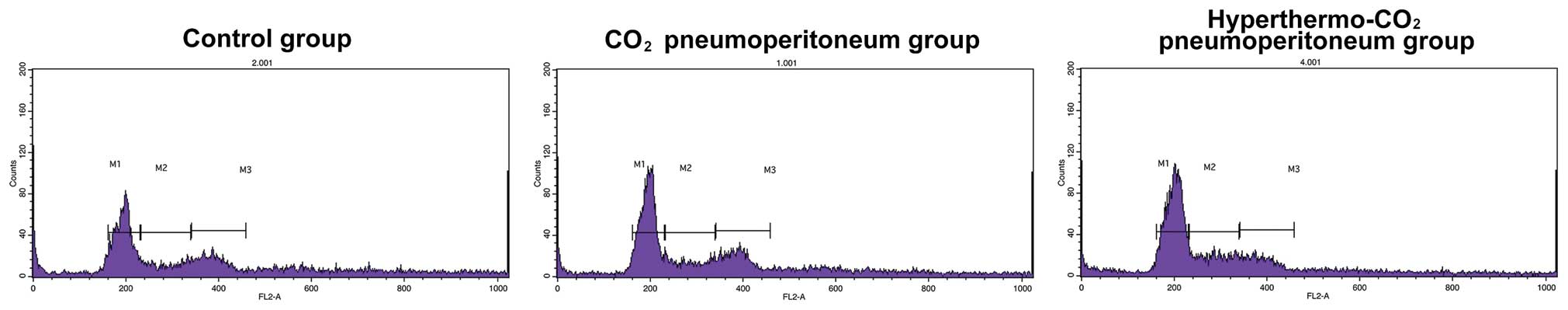
Cell cycle analysis using FACS
The cell cycle was analyzed using FACS after
treatment for 12 h (Fig. 4). It
showed G0/G1 and S phase of 35.77±3.13%, and S phase of 54.17±1.64%
in the control, 39.37±1.12 and 50.47±1.96% in the CO2,
and 65.53±1.75 and 19.57±1.06% in the hyperthermo-CO2
group, respectively. There was no significant difference between
the control and the CO2 group (P=0.662), while the
number of cells at G0/G1 phase increased and the number of cells at
S phase decreased in the hyperthermo-CO2 group
(P<0.05), indicating that hyperthermo-CO2 could
arrest the cell cycle.
Extracellular fluid temperature and pH
measurements
The pH value of the culture medium from the
CO2 group decreased from 7.35 to 6.12 after 30-min
treatment; the temperature remained unchanged. The pH value of the
culture medium from the hyperthermo-CO2 group decreased
from 7.34 to 6.20, while the temperature increased to 43°C within 5
min. The pH value of both the groups gradually returned to 7.30
after 2 h, indicating that hyperthermo-CO2 treatment may
create a transient hyperthermic, acidic and hypoxic
microenvironment while the CO2 treatment only created a
transient acidic and hypoxic microenvironment.
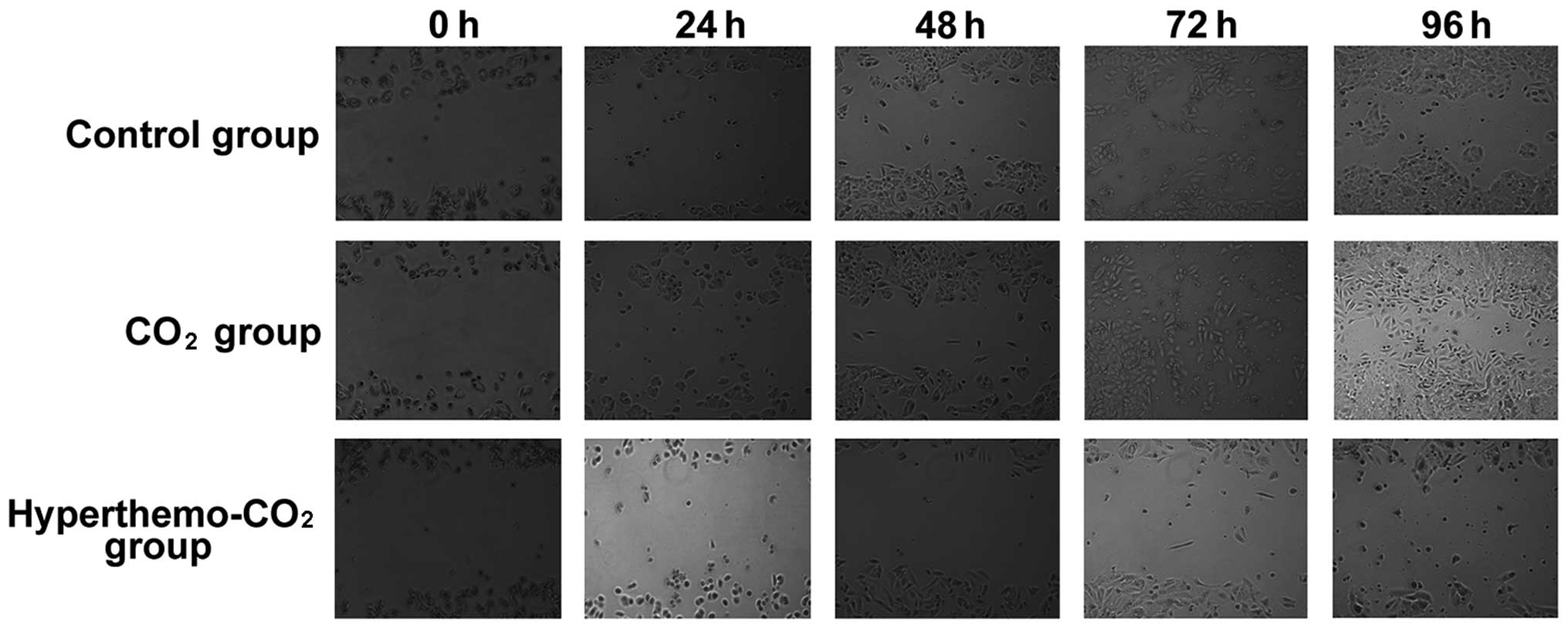
Effect on cell migration
The migration ability of cells was tested using
scratch assay (Fig. 5). The scratch
gap was large with clear parallel boundaries in the three groups
after scratching. The cells started to migrate into the gap after
48 h in the control group, the gap narrowed down after 72 h, and
disappeared with migration of many cells into the gap. In the
CO2 group, similar observations were found. However, in
the hyperthermo-CO2 group, the gap remained large after
72 and 96 h with shrinking of the surrounding cells, apoptosis, and
necrosis; the cell debris could be observed in the gap, indicating
that the migration ability of cells in the
hyperthermo-CO2 group decreased.
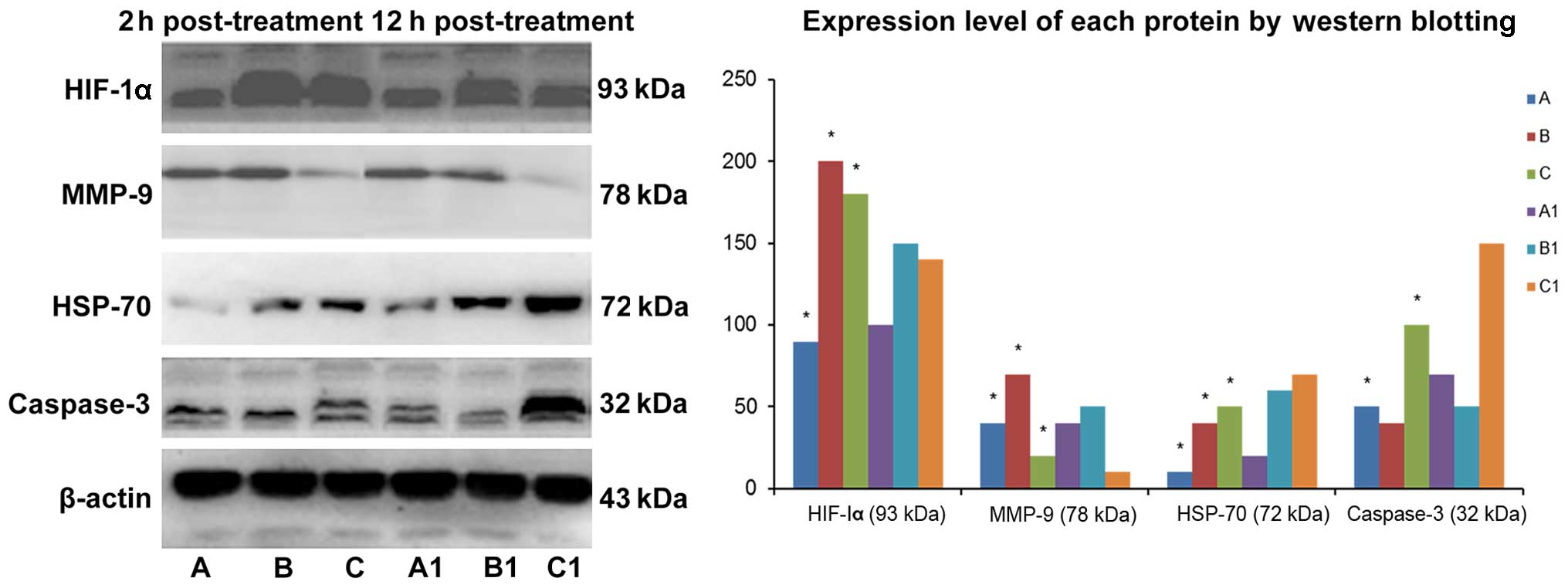
Protein expression detection using
western blotting
The results of western blotting are shown in
Fig. 6. Compared with the control
group, the expression level of caspase-3 protein remained
unchanged, and the expression level of HSP-70, HIF-1α and MMP-9
proteins increased in the CO2 group. The expression
level of caspase-3, HSP-70 and HIF-1α increased and the expression
level of MMP-9 protein decreased in the hyperthermo-CO2
group. The expression level of HSP-70 and HIF-1α decreased whereas
the expression level of caspase-3 increased after treatment for 12
h in the hyperthermo-CO2 group, indicating that
CO2 pneumoperitoneum could enhance cell migration.
Hyperthermo-CO2 pneumoperitoneum could promote cell
apoptosis and inhibit cell migration, and the effect may be reduced
with time.
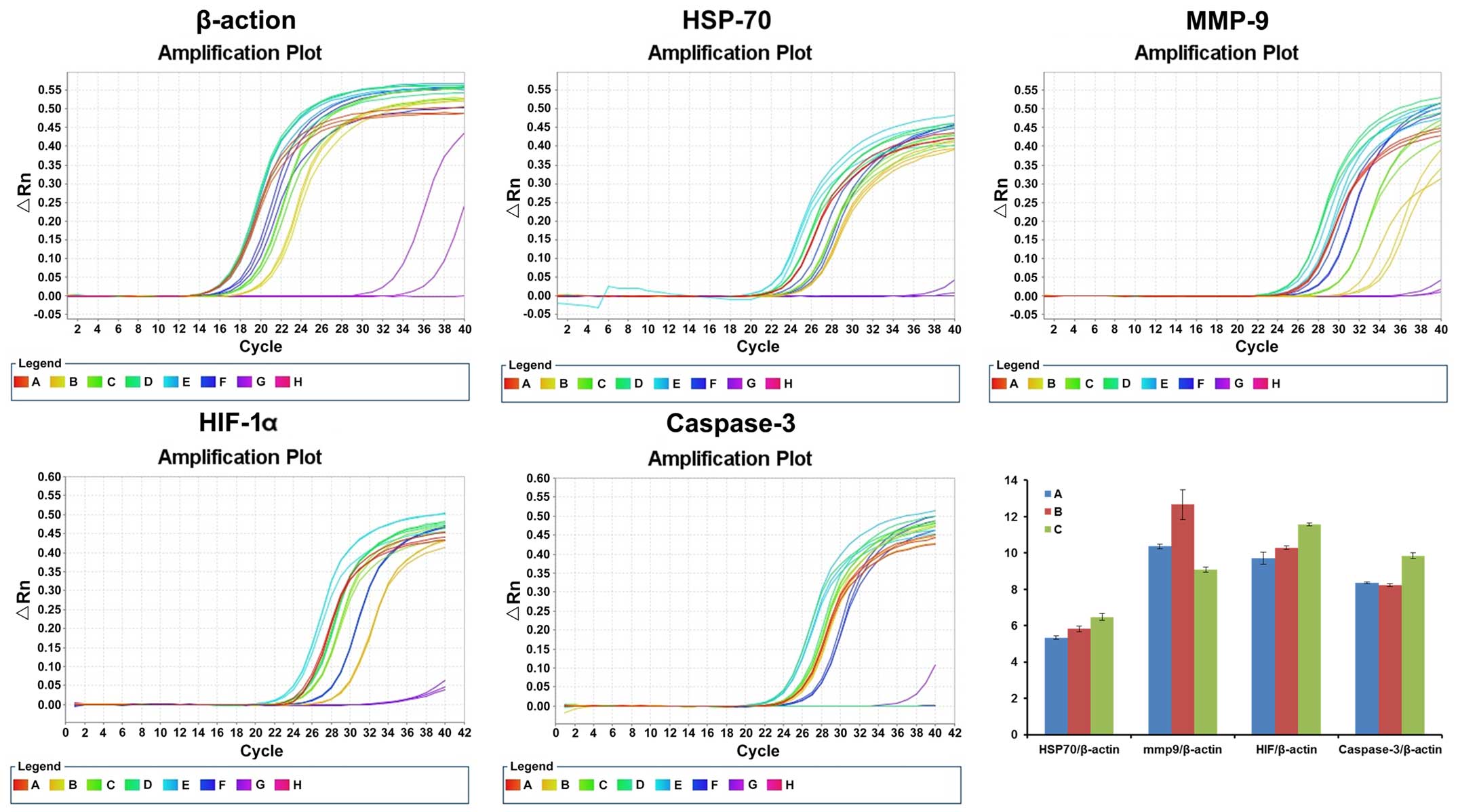
mRNA level detection using RT-PCR
RT-PCR result of each targeted gene 12 h after
treatment is shown in Fig. 7. The
relative expression of HSP-70/β-actin in the control,
CO2 and hyperthermo-CO2 groups was 5.34±0.09,
5.82±0.15 and 6.48±0.18, respectively. The relative expression of
MMP-9/β-actin in the control, CO2 and
hyperthermo-CO2 groups was 10.36±0.12, 12.66±0.83 and
9.09±0.14, respectively. The relative expression of HIF-1α/β-actin
in the control, CO2, and hyperthermo-CO2
groups was 9.72±0.33, 10.29±0.09 and 11.56±0.09, respectively. The
relative expression of caspase-3/β-actin in the control,
CO2 and hyperthermo-CO2 groups was 8.35±0.06,
8.23±0.08 and 9.84±0.16, respectively. Compared with the control
group, the expression level of caspase-3 mRNA remained unchanged
(P=0.102), and the expression level of HSP-70, HIF-1α and MMP-9
mRNA increased in the CO2 group (P<0.05). The mRNA
level of caspase-3, HSP-70 and HIF-1α increased, and MMP-9 mRNA
decreased in the hyperthermo-CO2 group (P<0.05). The
findings of the present study indicated that CO2 could
increase the expression of HIF-1α and MMP-9, which promoted the
migration of cells; whereas, the hyperthermo-CO2
increased HIF-1α, HSP-70 and caspase-3 expression, but decreased
MMP-9 expression, which promoted cell apoptosis and inhibited cell
migration.
Discussion
Laparoscopic technology has rapidly developed since
the first adoption in colorectal surgery, and is widely used in
colorectal cancer surgeries. CO2 is commonly used to
create pneumoperitoneum, which has been a concern for some surgeons
in that CO2 pneumoperitoneum may be associated with
tumor cell migration and infiltration. The potential mechanisms may
include the velocity and pressure created by CO2
pneumoperitoneum-induced tumor cell detachment and spreading
(7), tumor cells seeded at the
puncture site of casing pipes (8),
aerosol dissemination of tumor cells by an ultrasonic knife,
peritoneal acidic hypoxic microenvironment caused by CO2
pneumoperitoneum (9), and cellular
immunity alteration (10). Other
researchers believed that CO2 pneumoperitoneum had no
obvious effect on tumor invasion and metastasis (1,11). It
is, however, necessary to avoid any possibility of tumor invasion
and metastasis caused by CO2 pneumoperitoneum.
Hyperthermia is a novel therapeutic method that increases
temperature systematically or locally to the treatment temperature
(42–45°C) and sustains it for a certain period to eliminate tumor
cells. The major mechanism is to impair DNA repair system, denature
protein, inhibit oxidative metabolism, alter microenvironment, and
activate lysosomal enzymes, which eventually leads to tumor cell
necrosis or apoptosis (12). In
addition, the hyperthermia alters the signaling transduction
pathways or networks of cells, leading to impaired cell cycle and
DNA replication checkpoint and activation of transcription factors
responsible for tumor cell apoptosis (13–16).
CO2 gas is a carrier with good heat conduction and
dispersion, which could conduct heat rapidly within the abdominal
cavity. In the present study, CO2 was heated to 43°C,
which is the hyperthermia temperature, and the pressure of 14 mmHg
was maintained for 2 h, which also simulates a laparoscopic
operation. This setting may be able to inhibit tumor cell migration
and eliminate the aerosol disseminated tumor cells in the first
place during the laparoscopic operations, which was expected to
prevent or reduce the invasion and metastasis of tumor cells by
CO2 pneumoperitoneum.
The present study showed that CO2
pneumoperitoneum had no obvious effect on cell morphology,
proliferation or necrosis; however, the hyperthermo-CO2
pneumoperitoneum led to cell shrinking, reduced proliferation or
growth arrest, apoptosis or necrosis. The proliferation inhibition
rate and the apoptosis rate were 5% and 3.26±0.95% by
CO2 pneumoperitoneum, respectively; and they were
>40% and 45.20±3.11% by hyperthermo-CO2
pneumoperitoneum, respectively. There was no effect of
CO2 pneumoperitoneum on the cell cycle, while the cell
cycle was arrested by hyperthermo-CO2 pneumoperitoneum.
These findings indicated that CO2 pneumoperitoneum may
not affect cell growth, proliferation and apoptosis; whereas,
hyperthermo-CO2 pneumoperitoneum was able to inhibit
cell growth and proliferation and promote cell apoptosis. Heat
shock protein (HSP) is a heat-induced adaptive protein. HSP could
be produced when the cells were exposed to external stimuli such as
heat, radiation and chemotherapy. HSP-70 is a member of the HSP
family, which is involved in protein synthesis, processing, folding
and transport, and related to the occurrence, development,
immunity, drug resistance and prognosis of the tumor (17). The hyperthermo-CO2
pneumoperitoneum was shown to upregulate the expression of HSP-70
mRNA and protein in the present study, and the increased level of
HSP-70 promoted tumor cell apoptosis and inhibited tumor cell
metastasis. Caspase family plays an important role in cell
apoptosis. Caspase-3 is the key member and plays multiple roles in
the signaling pathways of apoptosis. Caspase-3 exists in cytoplasm
as zymogen and is activated in the early stage of apoptosis to
degrade the substrates in cytoplasm and nuclei, eventually leading
to apoptosis (18). Western
blotting and RT-PCR showed that caspase-3 gene and protein both
increased after hyperthermo-CO2 treatment in the present
study. The hyperthermo-CO2 pneumoperitoneum in the
present study may be able to enhance caspase-3 activation in tumor
cells through heat damage, acidosis and hypoxia, and finally
initiate apoptosis. In contrast, CO2 treatment had no
obvious effect on caspase-3 at either gene or protein level,
implying that CO2 treatment may not have a significant
effect on tumor cell apoptosis.
The migration ability of tumor cells is one of the
important factors for metastasis. Our findings of cell scratch
assay indicated that CO2 pneumoperitoneum could increase
the migration of tumor cells while the hyperthermo-CO2
pneumoperitoneum inhibited migration. Hypoxia-inducible factor-1α
(HIF-lα) was reported to regulate the transcription of a number of
regulatory factors to elicit adaptive responses to hypoxia,
maintaining high energy metabolism of tumor cells, inducing
angiogenesis, promoting tumor invasion and metastasis, and
resulting in drug resistance (19).
Both CO2 pneumoperitoneum and hyperthermo-CO2
pneumoperitoneum could create hypoxia microenvironment and increase
HIF-lα expression at both gene and protein levels, which was
confirmed in the present study by western blotting and RT-PCR.
CO2 pneumoperitoneum was shown to promote tumor cell
migration; however, hyperthermo-CO2 pneumoperitoneum
could directly adversely affect tumor cells by heat damage. Matrix
metalloproteinase-9 (MMP-9) is a type of zinc ion-dependent
endopeptidase degrading extracellular matrix (ECM) and playing a
key role in tumor invasion and metastasis. MMP-9 is considered a
marker of tumor invasion and metastasis (20). In the present study, western
blotting and RT-PCR showed that the expression of MMP-9 increased
by CO2 pneumoperitoneum; however, it decreased by
hyperthermo-CO2 pneumoperitoneum, indicating that
CO2 pneumoperitoneum could promote invasion and
migration of tumor cells by upregulating MMP-9 while
hyperthermo-CO2 pneumoperitoneum inhibited invasion and
migration of tumor cells by downregulating MMP-9.
Hence, CO2 pneumoperitoneum may not have
an obvious effect on the proliferation and apoptosis of colon
cancer cells, but could enhance the migration ability by
upregulating HIF-lα and MMP-9 expression by creating acidic hypoxic
microenvironment. However, hyperthermo-CO2
pneumoperitoneum could inhibit growth, proliferation and migration
of colon cancer cells by upregulating HSP-70, HIF-lα and caspase-3
expression and downregulating MMP-9 expression by creating
hyperthermic acidic hypoxic microenvironment.
Hyperthermo-CO2 pneumoperitoneum could convert the
CO2-induced promotion effect on cell migration. Since
the present study was an in vitro assay based on a tumor
cell line, the effect of hyperthermo-CO2
pneumoperitoneum on normal peritoneal cells should be investigated.
Animal as well as clinical studies are also needed to further
confirm the findings of the present study.
Acknowledgments
The present study was supported by funding from the
Shanghai Municipal Commission of Health and Family Planning
(20134260).
References
|
1
|
Bloomston M, Kaufman H, Winston J, Arnold
M and Martin E: Surgical management of colorectal cancer in the
laparoscopic era: A review of prospective randomized trials. J Natl
Compr Canc Netw. 3:517–524. 2005.PubMed/NCBI
|
|
2
|
Bing F and Hong Z: The effect of
CO2 pneumoperitoneum on the growth and metastasis of
malignant tumors of the rectum. J Exp Sur. 22:10212005.
|
|
3
|
Jingli C, Rong C and Rubai X: Influence of
colorectal laparoscopic surgery on dissemination and seeding of
tumor cells. Surg Endosc. 20:1759–1761. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hohenberger W, Schneider C, Reymond MA,
Scheidbach H and Köckerling F: Laparoscopic resection of colorectal
malignancy - an oncological risk? Zentralbl Chir. 122:1127–1133.
1997.In German.
|
|
5
|
Al-Shammaa HA, Li Y and Yonemura Y:
Current status and future strategies of cytoreductive surgery plus
intraperitoneal hyperthermic chemotherapy for peritoneal
carcinomatosis. World J Gastroenterol. 14:1159–1166. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhenggang Z: Comprehensive treatment and
issues related to gastric cancer recurrence. Chin J Sur.
25:181–183. 2005.
|
|
7
|
Gutt CN, Kim ZG, Hollander D, Bruttel T
and Lorenz M: CO2 environment influences the growth of
cultured human cancer cells dependent on insufflation pressure.
Surg Endosc. 15:314–318. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ceccarelli G, Casciola L, Nati S, Bartoli
A, Spaziani A, Stefanoni M, Conti D, Fettucciari V, Di Zitti L,
Valeri R, et al: Neoplastic residues in the trocar tract in
oncologic laparoscopic surgery. Minerva Chir. 59:243–248. 2004.In
Italian. PubMed/NCBI
|
|
9
|
Wittich P, Steyerberg EW, Simons SH,
Marquet RL and Bonjer HJ: Intraperitoneal tumor growth is
influenced by pressure of carbon dioxide pneumoperitoneum. Surg
Endosc. 14:817–819. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Novitsky YW, Czerniach DR, Kaban GK,
Bergner A, Gallagher KA, Perugini RA and Litwin DE: Immunologic
effects of hand-assisted surgery on peritoneal macrophages:
Comparison to open and standard laparoscopic approaches. Surgery.
139:39–45. 2006. View Article : Google Scholar
|
|
11
|
Hao YX, Zhong H, Zhang C, Zeng DZ, Shi Y,
Tang B and Yu PW: Effects of simulated carbon dioxide and helium
peumoperitoneum on proliferation and apoptosis of gastric cancer
cells. World J Gastroenterol. 14:2241–2245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van der Speeten K, Stuart OA and
Sugarbaker PH: Using pharmacologic data to plan clinical treatments
for patients with peritoneal surface malignancy. Curr Drug Discov
Technol. 6:72–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang XL, Hu AB, Cui SZ and Wei HB:
Thermotherapy enhances oxaliplatin-induced cytotoxicity in human
colon carcinoma cells. World J Gastroenterol. 18:646–653. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tabuchi Y, Wada S, Furusawa Y, Ohtsuka K
and Kondo T: Gene networks related to the cell death elicited by
hyperthermia in human oral squamous cell carcinoma HSC-3 cells. Int
J Mol Med. 29:380–386. 2012.
|
|
15
|
Jung HJ and Seo YR: Protective effects of
thioredoxin-mediated p53 activation in response to mild
hyperthermia. Oncol Rep. 27:650–656. 2012.
|
|
16
|
Sturm I, Rau B, Schlag PM, Wust P,
Hildebrandt B, Riess H, Hauptmann S, Dörken B and Daniel PT:
Genetic dissection of apoptosis and cell cycle control in response
of colorectal cancer treated with preoperative radiochemotherapy.
BMC Cancer. 6:1242006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ito A, Shinkai M, Honda H, Yoshikawa K,
Saga S, Wakabayashi T, Yoshida J and Kobayashi T: Heat shock
protein 70 expression induces antitumor immunity during
intracellular hyperthermia using magnetite nanoparticles. Cancer
Immunol Immunother. 52:80–88. 2003.PubMed/NCBI
|
|
18
|
Depraetere V and Golstein P: Dismantling
in cell death: Molecular mechanisms and relationship to caspase
activation. Scand J Immunol. 47:523–531. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu H, Feng Y, Zhang J, Zhou X, Hao B,
Zhang G and Shi R: Inhibition of hypoxia inducible factor 1α
expression suppresses the progression of esophageal squamous cell
carcinoma. Cancer Biol Ther. 11:981–987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu D, Guo H, Li Y, Xu X, Yang K and Bai
Y: Association between polymorphisms in the promoter regions of
matrix metalloproteinases (MMPs) and risk of cancer metastasis: A
meta-analysis. PLoS One. 7:e312512012. View Article : Google Scholar : PubMed/NCBI
|