Introduction
The burden of cancer has shifted from more developed
countries to less developed countries, and gastric cancer accounts
for 57% of cancer cases and 65% of cancer-related deaths worldwide.
Gastric cancer, which has the highest incidence rate in Eastern
Asia (particularly in Japan, Korea and China), is the third most
commonly diagnosed cancer type and the leading cause of
cancer-related death in less developed countries (1). Although many chemotherapeutic
compounds have been investigated to improve the quality of life and
prolong the survival of patients with gastric cancer, progress in
the treatment of gastric cancer is unsatisfactory.
Oleanolic acid (OA) is a ubiquitous pentacyclic
triterpenoid compound, which is abundant in dietary and medicinal
plants. It has been isolated from more than 1,600 plant species in
nature. It is considered to be a basic molecule for chemical
modifications due to its pharmacological activities, availability,
and low production costs. Several portions of OA, such as the C-3
hydroxy, the C-12-C-13 double bond and the C-28 carboxylic acid,
have led to a series of new synthetic derivatives. It also serves
as an aglycone of triterpenoid saponins that is linked with sugar
chains to form glycosides. Both OA and its derivatives possess
several biological activities, including hepatoprotective effects,
antioxidant, anti-inflammatory, antiviral and anticancer activities
(2–4). Many OA derivatives have shown their
chemopreventive and chemotherapeutic functions among a series of
cancer types, such as acute myeloid leukemia, breast cancer and
prostate cancer (5–7). However, reports concerning the
anticancer effects and mechanism of OA derivatives on gastric
cancer are sparse.
In the present study, SZC017, a derivative of OA,
was newly synthesized and evaluated in regards to its anticancer
activity against gastric cancer cells. Furthermore, we aimed to
ascertain whether the inhibitory effect of SZC017 on cell viability
was mediated by inducing apoptosis and cell cycle arrest in MGC-803
and SGC-7901 cells, or mechanistically mediated by inhibiting
Akt/NF-κB/topoisomerase signaling. Therefore, our present study
provides enhanced knowledge of the anticancer activity of SZC017
against gastric cancer cells.
Materials and methods
Chemicals and apparatus
All reagents and chemicals were obtained from
standard commercial sources and used without further purification.
Silica gel for column chromatography was purchased from Qingdao
Haiyang Chemical Co. Ltd. NMR spectra were recorded on a Bruker
DRX-400 with TMS as a reference. Electrospray ionization (ESI) mass
spectra were recorded using an LC/Q-TOF MS spectrometer. Melting
points were measured using X-4 Digital Micro Melting Point
apparatus. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT), penicillin and streptomycin were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's modified Eagle's
medium (DMEM), trypsin-EDTA, and fetal bovine serum (FBS) were
purchased from Gibco-BRL (Gaithersburg, MD, USA). The Annexin
V-FITC apoptosis detection kit was purchased from Nanjing Jiancheng
Bioengineering Institute (Nanjing, Jiangsu, China). Cell cycle and
apoptosis analysis and the nuclear and cytoplasmic extraction kits
were obtained from Beyotime Institute of Biotechnology (Haimen,
Jiangsu, China). Antibodies to β-actin, histone H3, cleaved-PARP,
procaspase-3, procaspase-9, Bax, Bcl-2, Akt, p-Akt, DNA
topoisomerase I (Top-I, rabbit polyclonal) and DNA topoisomerase
IIα (Top-IIα, rabbit monoclonal) were purchased from Proteintech
(Chicago, IL, USA). The primary antibodies against p-p65 (Ser536),
p-IκBα (Ser32/Ser36) and the secondary antibody HRP goat
anti-rabbit IgG were obtained from Abbkine (Redlands, CA, USA).
Synthesis of 3-oxo-olean-12-en-28-oic
acid (3-oxo-OA) (Fig. 1A)
OA 2.0 g (4.4 mmol) was dissolved in a 100-ml mixed
solvent of dichloromethane and acetone (volume ratio of 1:1). The
solution was cooled to 0°C, and 2.0 ml Jones reagent was added
dropwise within 30 min. The reaction mixture was stirred for 30 min
and 2 ml isopropanol was added. After 10 min, the mixture was
filtered, and the filtrate was evaporated under reduced pressure to
yield a solid residue, which was dissolved in 50 ml ethyl acetate,
and then washed with saturated brine (50 ml ×3). The organic phase
was dried with anhydrous sodium sulfate and concentrated in
vacuo. The crude product was purified by silica gel column
chromatography eluting with petroleum ether/ethyl acetate (20:1,
v:v) to afford 3-oxo-OA as a white solid (1.97 g, 98.2% yield). mp.
181–182°C. Positive ESI-TOF-MS: m/z 499.2864
[M+2Na-H]+. 1H NMR (CDCl3, 400
MHz): 0.81, 0.91, 0.93, 1.03, 1.05, 1.08, 1.15 (each s, 3H,
7×CH3), 5.30 (1H, t, J=3.3 Hz, H-12), 2.84 (1H,
dd, J = 13.6, 3.8 Hz, H-18), 2.33–2.55 (2H, m, H-2).
Synthesis of
2-(piperidine-1-methyl)-3-oxo-olean-12-en-28-oic acid (SZC017)
(Fig. 1B)
Piperidine hydrochloride 1.21 g (10.0 mmol),
SnCl2 0.38 g (2.0 mmol) and paraformaldehyde 0.3 g were
added to a solution of 3-oxo-OA (0.91 g, 2.0 mmol) in 40 ml
ethanol. The reaction mixture was heated at reflux for 20 h and
then filtrated. The filtrate was concentrated to dryness under
reduced pressure. The residue was dissolved in 200 ml ethyl
acetate, and then washed with saturated brine (50 ml ×3). The
organic layer was dried with anhydrous sodium sulfate and
concentrated in vacuo. The crude product was purified by
silica gel column chromatography eluting with
chloroform/ethanol/water (15:1:0.1, v:v:v) to give compound SZC017
as a pale yellow solid (0.18 g, 16.3% yield). mp 198–200°C. MS
(TOF-ES positive) m/z: 552.5 [M+H]+.
1H NMR (CDCl3, 400 MHz): 0.82, 0.90, 0.94,
1.09, 1.10, 1.12, 1.38 (each s, 3H, 7× CH3), 1.86 [m,
4H, N(CH2CH2)2CH2],
2.78 (m, 1H, -NCH2-), 2.84 (m, 1H, H-18), 2.86 [m, 2H,
N(CH2CH2)2CH2], 3.24
(m, 1H, -NCH2-), 3.59 [m, 2H,
N(CH2CH2)2CH2], 3.85
(m, 1H, H-2),5.26 (t, 1H, J=3.3 Hz, H-12).
Cell culture
Human gastric cancer cell lines MGC-803 and SGC-7901
were purchased from the Institute of Biochemistry Cell Biology
(Shanghai, China). Cells were cultured in high-glucose DMEM
containing 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin. Cells were maintained at 37°C in a humidified
incubator of 5% CO2.
MTT assay
Cells were seeded into 96-well plates and then
cultured for 24 h. After being exposed to SZC017 with different
concentrations for the indicated time, 15 µl MTT stock
solution (5 mg/ml) was added into each well. After an additional
4-h incubation, 100 µl SDS-isobutanol-HCl solution (10% SDS,
5% isobutanol and 12 mM HCl) was added into each well. After an
incubation at 37°C overnight, light absorption was detected at 570
nm using a microplate reader (Multiskan MK3; Shaanxi Pioneer
Biotech Co., Ltd., Xi'an, China).
Cell apoptosis analysis
To determine whether apoptosis is involved in the
inhibition of cell viability by SZC017 in the MGC-803 and SGC-7901
cells, the Annexin V-FITC apoptosis detection kit was performed.
According to the manufacturer's instructions, the cells
(5×105 cells/ml) were seeded into 6-well plates with a
further incubation overnight, and treated with different
concentrations of SZC017 for 24 h. Subsequently, the cells were
collected and stained with Annexin V-FITC and propidium iodide (PI)
for 30 min in the dark at room temperature. Finally, the samples
were analyzed using FACScan flow cytometry (BD FACSAria II; BD
Biosciences, Franklin Lakes, NJ, USA).
Cell cycle analysis
To evaluate the cell cycle distribution after
exposure to SZC017, the cells were treated with different
concentrations of SZC017 for 24 h. After the treatment, the cells
were collected and fixed with 70% cold ethanol overnight at 4°C.
According to the manufacturer's instructions, PI staining reagent
(50 mg/ml PI and 1 mg/ml RNAse in 1 ml of sodium citrate buffer, pH
7.4) was prepared, and the samples were then suspended with the
reagent in the dark at 37°C for 30 min. The cell cycle distribution
was detected using FACScan flow cytometry (BD FACSAria II; BD
Biosciences), and the data were analyzed using the multicycle
program from Phoenix Flow Systems (San Diego, CA, USA).
Transmission electron microscopy
(TEM)
Cells were seeded into 6-well plates and then
treated with SZC017 after a further 24-h incubation. After an
additional 24-h incubation, the cells were collected and prefixed
with 2.5% glutaraldehyde overnight at 4°C. The cells were next
post-fixed, dehydrated, embedded, sectioned, and stained as
previously described (8). Finally,
the electron micrographs were recorded using a transmission
electron microscope (JEM-2000EX; Jeol Co., Ltd., Akishima,
Japan).
Western blotting
Whole-cell lysates were prepared in an ice-cold
lysis buffer containing 150 mM NaCl, 20 mM Tris-Cl (pH 7.5), 1%
Triton X-100, 1 mM PMSF, 1 mM Na3VO4, 25 mM
NaF, 1% aprotinin and 10 µg/ml leupeptin. Cytoplasmic and
nuclear extracts were prepared using nuclear and cytoplasmic
extraction kit according to the manufacturer's instructions. The
extracts were fractionated by 10 or 12% SDS-polyacrylamide gel and
then electrically transferred onto a polyvinylidene difluoride
(PVDF) membrane. After blotting with TBST buffer [500 mM NaCl, 20
mM Tris-HCl (pH 7.4), and 0.1% Tween-20] containing 5% non-fat dry
milk, the PVDF membrane was probed with the primary antibody
(1:1,000) diluted in TBST buffer overnight at 4°C and then cultured
with the secondary antibody (1:1,000) diluted in TBST for 1 h at
room temperature. Membranes were then visualized using enhanced
chemiluminescence reagent with LabWorks software (UVP, Upland, CA,
USA).
Statistical analysis
Results are expressed as the mean ± standard
deviation (SD) of three replicates. The statistical significance
between the control and the treatment groups was calculated using
one-way ANOVA test and Tukey's multiple-comparison test. SPSS 17.0
software was used to analyze the data. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of SZC017 on the cell viability of
MGC-803 and SGC-7901 cells
To determine the effect of SZC017 on the cell
viability of gastric cancer cells, gastric cancer cells were
treated with different concentrations of SZC017, and cell viability
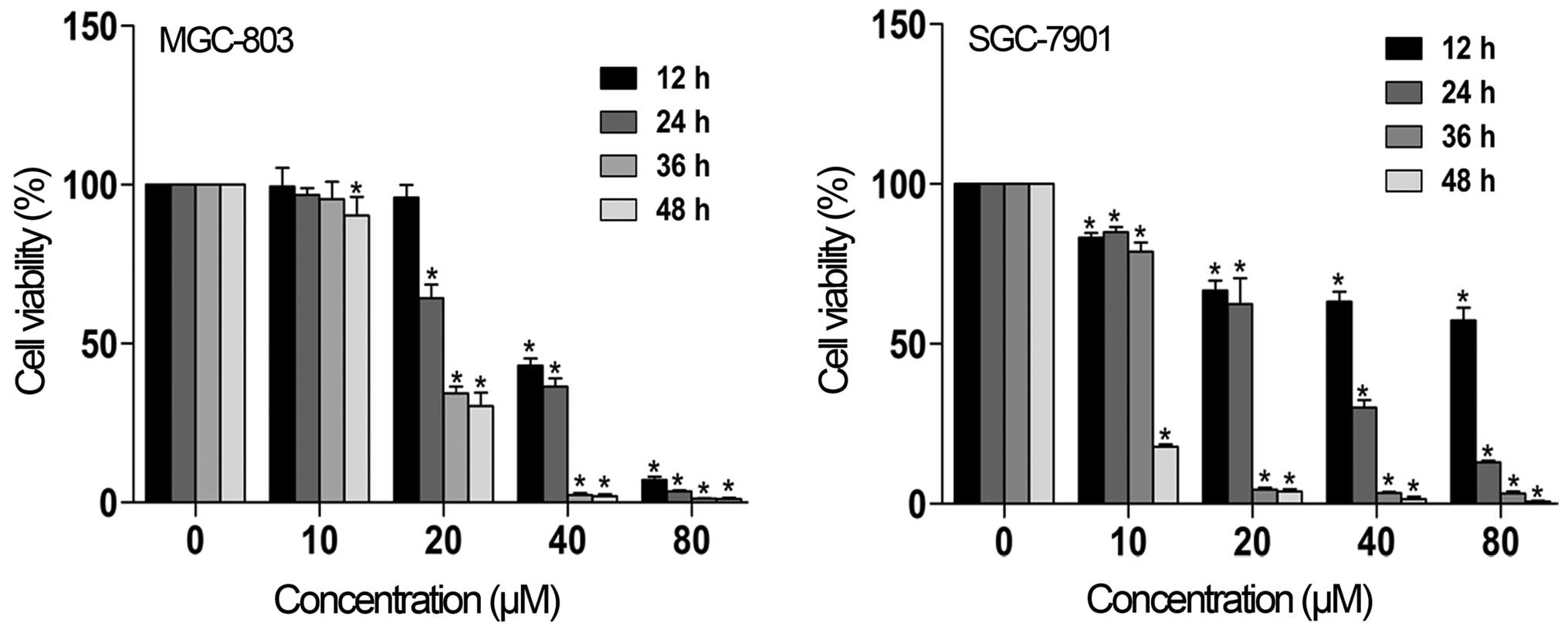
was determined by MTT assay. As shown in Fig. 2, SZC017 decreased the cell viability
of the MGC-803 and SGC-7901 cell lines in a time- and
concentration-dependent manner. The IC50 value after 24
h of SZC017 treatment was 28.46 µM for MGC-803 cells and
26.05 µM for SGC-7901 cells. However, insignificant
reductions in cell viability were observed after treatment with 10
µM SZC017 for 12, 24 and 36 h in the MGC-803 cells, as
compared with the SGC-7901 cells. These findings indicate that
SZC017 may be a potential compound against gastric cancer
cells.
SZC017 induces apoptosis in the gastric
cancer cells
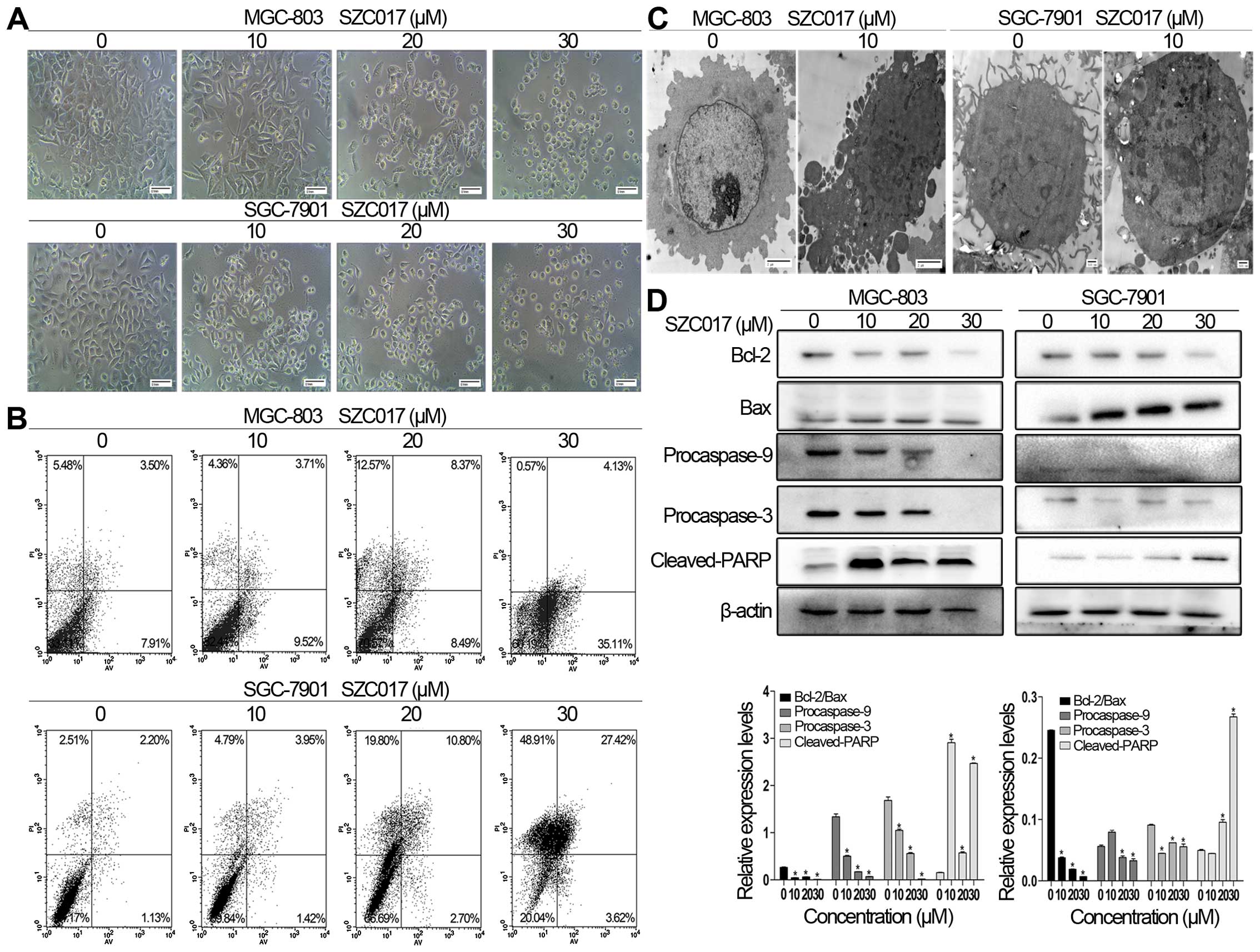
Morphological changes of apoptosis, including cell
shrinkage and fragmentation, were observed in both the MGC-803 and
SGC-7901 cells after treatment with SZC017 (Fig. 3A). Whether the inhibition of cell
viability of the gastric cancer cells by SZC017 was due to the
induction of apoptosis was confirmed by flow cytometry. Our results
showed that SZC017 induced apoptosis in the gastric cancer cells in
a concentration-dependent manner. After treatment with different
concentrations of SZC017 for 24 h, the total apoptotic ratio was
increased from 11.41 to 39.24% in the MGC-803 cells and from 3.33
to 31.04% in the SGC-7901 cells (Fig.
3B). Early apoptosis was induced by SZC017 in the MGC-803
cells, whereas late apoptosis was more obviously induced in the
SGC-7901 cells (Fig. 3B). SZC017
treatment resulted in typical morphological changes in apoptosis,
such as chromatin condensation, nuclear fragmentation and
apoptosome formation (Fig. 3C). To
determine whether intrinsic apoptosis is involved in SZC017-induced
apoptosis in both cell lines, we next evaluated the expression of
various intrinsic apoptosis-related proteins. The results showed
that SZC017 induced intrinsic apoptosis in both cell lines. SZC017
significantly increased the expression of cleaved-PARP, which is an
executioner and a hallmark of apoptosis (Fig. 3D) (9). As expected, the ratio of Bcl-2/Bax,
which determines the susceptibility to apoptosis by regulating
mitochondrial functions (10), was
reduced by SZC017 (Fig. 3D). The
levels of procaspase-9 and procaspase-3 were also suppressed by
SZC017 in both cell lines. Taken together, our findings indicate
that SZC017 induced intrinsic apoptosis in the MGC-803 and SGC-7901
cells.
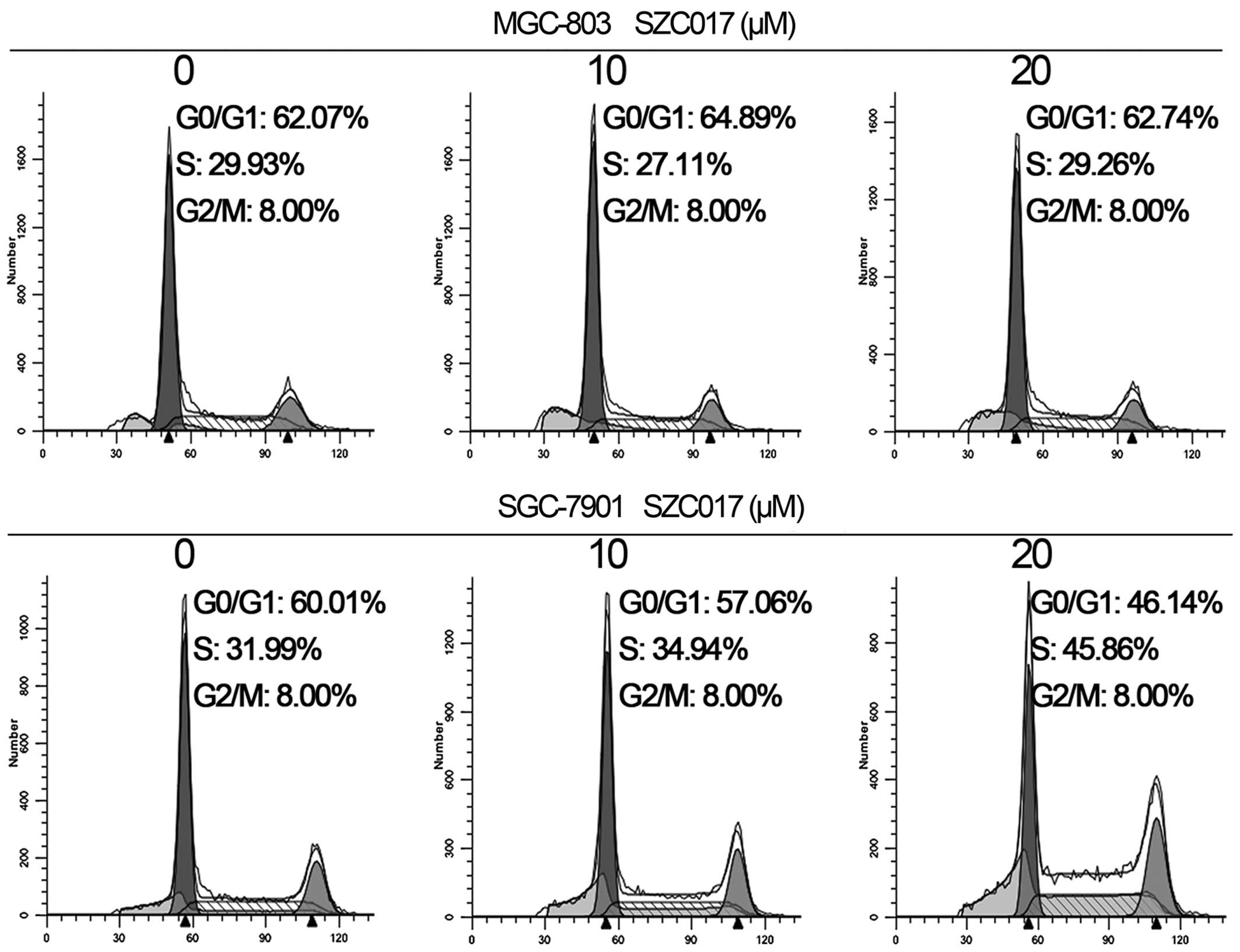
Effect of SZC017 on cell cycle
distribution of gastric cancer cells
Cell cycle arrest is an important mechanism that
inhibits cancer cell growth (11).
Our results revealed that SZC017 treatment caused an accumulation
of SGC-7901 cells in the S phase. The percentage of cells in the S
phase was increased from 31.99 in the control group to 45.86% in
the 20 µM SZC017 group (Fig.
4). However, no cell cycle arrest was observed in the MGC-803
cells after treatment with SZC017. Our findings suggest that SZC017
induced S phase arrest of the SGC-7901 cells, but had no effect on
cell cycle distribution of the MGC-803 cells.
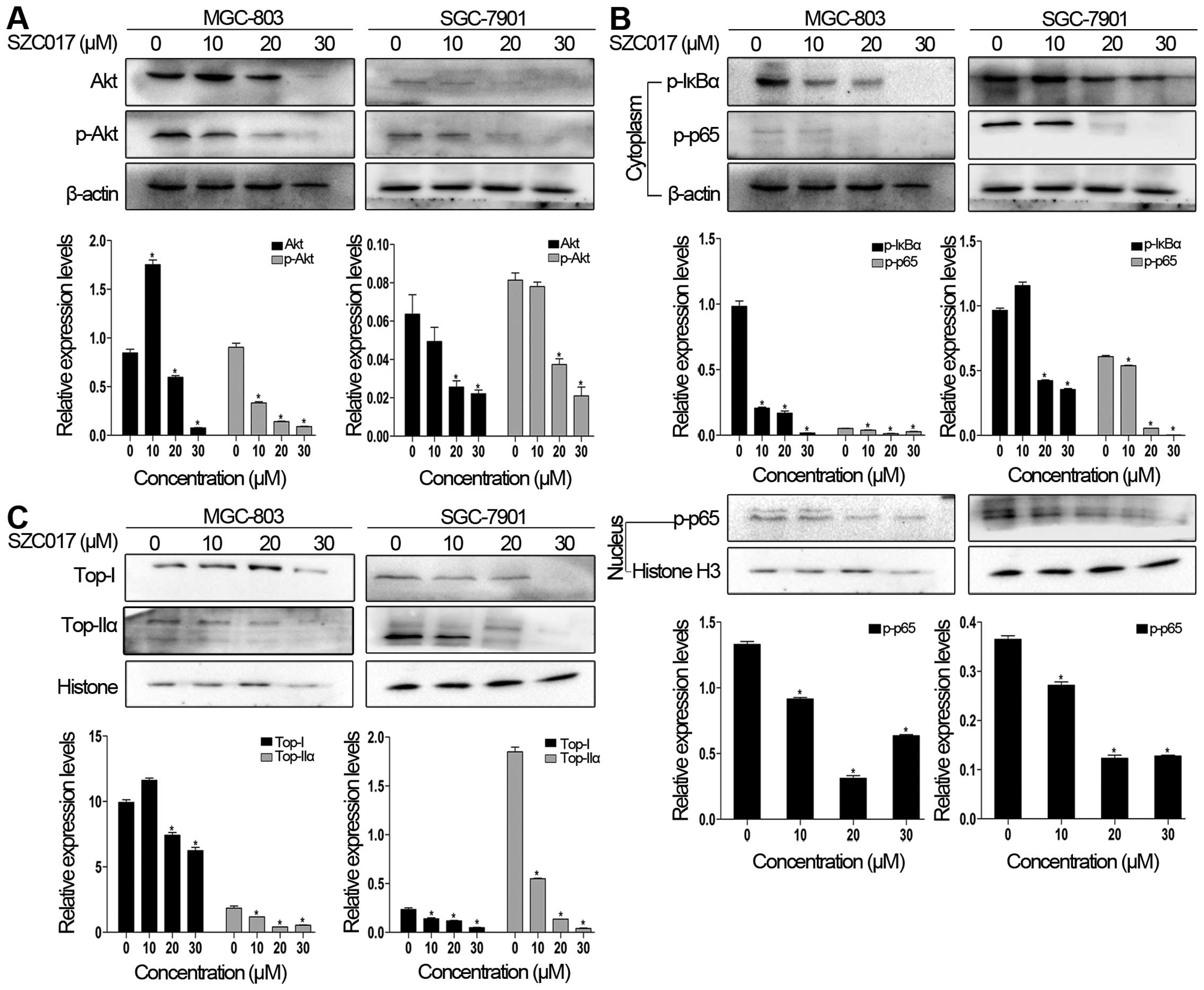
SZC017 inhibits Akt, NF-κB and
topoisomerase signaling proteins
Chemotherapeutic compounds can induce cancer cell
apoptosis via inhibition of the Akt signaling pathway (12,13).
Therefore, we first evaluated the levels of Akt and p-Akt of
SZC017-treated gastric cancer cells. Fig. 5A clearly shows that the levels of
Akt and p-Akt were suppressed by SZC017 in both cell lines. We next
elucidated the effect of SZC017 on NF-κB signaling proteins in the
gastric cancer cells. The results showed that the expression of
p-IκBα in the cytoplasm, which is a key inhibitory protein in
modulating NF-κB function (14),
was reduced by SZC017 (Fig. 5B).
Furthermore, a decrease in p-p65 in the cytoplasm and nucleus was
also observed in both cell lines (Fig.
5B). DNA topoisomerase I (Top-I) and topoisomerase IIα
(Top-IIα) are effective targets for chemotherapy which are tightly
connected with the NF-κB pathway (15,16).
As expected, SZC017 decreased the expression of both Top-I and
Top-IIα in the nucleus in both cell lines, respectively (Fig. 5C). Taken together, our findings
indicate that SZC017 is an effective compound against gastric
cancer cells via targeting Akt/NF-κB signaling and Top-I and
-IIα.
Discussion
The present study demonstrated that SZC017, a novel
derivative of OA, exhibited an anticancer effect against gastric
cancer cells via induction of apoptosis, which was mainly mediated
by inhibiting Akt/NF-κB signaling and the targeting of Top-I and
-IIα. SZC017-induced apoptosis of gastric cancer cells occurred via
the intrinsic pathway, as manifested by a decreased expression of
procaspase-9, procaspase-3 and the ratio of Bcl-2/Bax, and a
increased expression of cleaved-PARP.
Dysregulated apoptosis and DNA damage response are
two main characteristics of cancer cells and are the main causes of
cancer therapy chemoresistance. Therefore, induction of apoptosis
and cell cycle arrest are currently two essential mechanisms of
anticancer drugs (17,18). Apoptosis induction is a critical
mechanism that decreases cell viability in gastric cancer cells, as
evidenced by the presence of chromatin condensation, nuclear
fragmentation and apoptosome formation (19), an increase in cleaved-PARP, an
executioner of apoptosis (9), and
flow cytometry analysis (Fig. 3).
Many derivatives of OA induce cancer cell apoptosis via triggering
intrinsic apoptosis pathway (7,20–22),
which is characterized by the release of cytochrome c from
the mitochondria through a decrease in the ratio of Bcl-2/Bax,
interacting with apoptotic protease-activating factor 1, activating
procaspase-9, and finally activating procaspase-3 (18,19).
Similarly, our results supported the above associated theory. Thus,
the action of SZC017 was carried out by targeting the mitochondria
and thereby leading to intrinsic apoptosis. Cell cycle arrest is
considered to be another important mechanism for inhibiting cell
viability (11). Interestingly, S
phase arrest was observed after SZC017 treatment in SGC-7901 cells,
whereas no effect on cell cycle distribution was presented in the
MGC-803 cells suggesting that there are perhaps different
mechanisms in regulating the cell cycle in these two cell
lines.
Akt/NF-κB signaling is a major anti-apoptosis
pathway for controlling cell survival and growth, and is frequently
hyperactivated in cancer cells (23). OA and its several derivatives induce
cancer cell apoptosis and show anticancer activity via inhibition
of Akt and p-Akt (6,13,24,25).
Activated p-Akt promotes cancer cell survival via inactivation of
downstream molecules such as procaspase-9 and Bad (26). During our observations, both Akt and
p-Akt were significantly suppressed by SZC017 in the MGC-803 and
SGC-7901 cell lines, and procaspase-9 was also inhibited suggesting
that targeting the Akt pathway may be considered to be an effective
mechanism in gastric cancer cells in response to SZC017
treatment.
NF-κB is a downstream molecule of Akt that can
activate the NF-κB pathway through phosphorylation of IκBα. NF-κB
can regulate several biological activities, including inflammation
and apoptosis, and the NF-κB pathway has been a pharmacological
therapeutic and preventive target (14,27).
NF-κB complexes are usually located in the cytoplasm due to its
connection with inhibitor protein IκBα. In the classical pathway,
stimulation induces IKK activation leading to phosphorylation of
IκBα which subsequently is ubiquitinated and degradated. After
posttranslational modifications, the NF-κB dimer then translocates
into the nucleus and binds to κB sites to modulate specific gene
expression (14,28,29).
There are two IκBα-related mechanisms for inhibiting the NF-κB
pathway. Bortezomib, a clinical proteasome inhibitor, inhibits the
NF-κB pathway in multiple myeloma cells via inhibition of
proteasomes, stabilizing IκBα, and thus suppressing expression of
p-p65 in the cytoplasm and its nuclear translocation (30,31).
Different from bortezomib, DETT, an anti-leishmanial thiadiazine
agent, induces multiple myeloma cell apoptosis via suppression of
p-p65 expression in the cytoplasm and its nuclear translocation, in
addition to suppression of the phosphorylation of IκBα in the
cytoplasm (32). Similar to DETT
activity, inhibition of p-IκBα prevents IκBα from degradation by
proteasomes which is considered to be a critical step in
suppressing the NF-κB pathway in gastric cancer cells after
treatment with SZC017. Yet the effect of SZC017 on total IκBα
requires further investigation. The inhibition of the NF-κB pathway
by bortezomib is implemented through the stabilization of p-IκBα
from degradation by the proteasome, while SZC017 and DETT decrease
p-IκBα and thus prevent IκBα from degradation. Although the effects
of SZC017, DETT and bortezomib on the NF-κB pathway are distinct in
terms of p-IκBα, the final effect is the same. Phosphorylation of
p65 at Ser536 facilitates p65 nuclear translocation and DNA
binding, which in turn modulates downstream gene expression
(33). p-p65-dependent NF-κB
pathway activation is considered to play a critical role in cancer
cell survival (32). To determine
whether SZC017 inhibits p-p65 activity, we evaluated the expression
of p-p65 in both the cytoplasm and nucleus, respectively. Our
findings indicated that SZC017 first suppressed p-IκBα, and then
inhibited p-p65 expression in the cytoplasm and nucleus in gastric
cancer cells and thereby led to the inhibition of the NF-κB
pathway. Nuclear translocation of NF-κB complexes is an important
aspect of NF-κB activation. However, additional posttranslational
modifications of NF-κB itself is also critical in regulating the
downstream gene expression (34,35).
Taken together, the suppression of p-IκBα and p-p65 in the
cytoplasm and nucleus contributes to inhibition of the NF-κB
pathway in SZC017-treated gastric cancer cells.
Recent research demonstrates that
topoisomerase-targeting drugs activate the NF-κB pathway, and thus
lead to chemoresistance. In patients with colorectal cancer, CPT-11
(irinotecan), a topoisomerase inhibitor, can induce chemoresistance
in malignant cells via activation of the NF-κB pathway (36–38).
DNA topoisomerases are molecules that modulate chromosome
superstructure and integrity via cutting, shuffling DNA strands,
removing DNA supercoils, and disentangling snarled DNA segments
(39). DNA topoisomerase I (Top-I)
and topoisomerase IIα (Top-IIα) are effective targets for
chemotherapy (15,16,40).
Our results indicate that Top-I and Top-IIα are effective targets
in SZC017 against gastric cancer cells. Interestingly, different
from CPT-11, SZC017 suppressed the level of Top-I and Top-IIα, and
inhibited the NF-κB pathway in a p-IκBα and p-p65-dependent manner.
Therefore, our findings suggest that SZC017 may be an effective
anticancer agent in terms of its inhibitiory function of both the
Akt/NF-κB pathway and topoisomerases (Top-I and Top-IIα).
In the present study, we demonstrated that SZC017, a
novel derivative of OA, possessed a potential anticancer effect
against gastric cancer cells via induction of intrinsic apoptosis,
inhibition of Akt/NF-κB signaling, and targeting of Top-I and -IIα
proteins. Therefore, our data revealed a potential chemotherapeutic
agent for inducing gastric cancer cell death. However, further
research on the effect of SZC017 on other cancer types warrants
investigation.
Acknowledgments
The present study was supported by the Natural
Science Foundation of China (no. 30772601) and the University
Innovation Team Project Foundation of the Education Department of
Liaoning (no. LT2013019).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liby KT, Yore MM and Sporn MB:
Triterpenoids and rexinoids as multifunctional agents for the
prevention and treatment of cancer. Nat Rev Cancer. 7:357–369.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pollier J and Goossens A: Oleanolic acid.
Phytochemistry. 77:10–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shanmugam MK, Dai X, Kumar AP, Tan BK,
Sethi G and Bishayee A: Oleanolic acid and its synthetic
derivatives for the prevention and therapy of cancer: Preclinical
and clinical evidence. Cancer Lett. 346:206–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bishayee A, Mandal A, Thoppil RJ, Darvesh
AS and Bhatia D: Chemopreventive effect of a novel oleanane
triterpenoid in a chemically induced rodent model of breast cancer.
Int J Cancer. 133:1054–1063. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deeb D, Gao X, Liu Y, Jiang D, Divine GW,
Arbab AS, Dulchavsky SA and Gautam SC: Synthetic triterpenoid CDDO
prevents the progression and metastasis of prostate cancer in TRAMP
mice by inhibiting survival signaling. Carcinogenesis. 32:757–764.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Konopleva M, Contractor R, Kurinna SM,
Chen W, Andreeff M and Ruvolo PP: The novel triterpenoid CDDO-Me
suppresses MAPK pathways and promotes p38 activation in acute
myeloid leukemia cells. Leukemia. 19:1350–1354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shao Y, Gao Z, Marks PA and Jiang X:
Apoptotic and autophagic cell death induced by histone deacetylase
inhibitors. Proc Natl Acad Sci USA. 101:18030–18035. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boucher D, Blais V and Denault JB:
Caspase-7 uses an exosite to promote poly(ADP ribose) polymerase 1
proteolysis. Proc Natl Acad Sci USA. 109:5669–5674. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cory S and Adams JM: Killing cancer cells
by flipping the Bcl-2/Bax switch. Cancer Cell. 8:5–6. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiu P, Guan H, Dong P, Li S, Ho CT, Pan
MH, McClements DJ and Xiao H: The p53-, Bax-and p21-dependent
inhibition of colon cancer cell growth by 5-hydroxy
polymethoxyflavones. Mol Nutr Food Res. 55:613–622. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim W, Yang HJ, Youn H, Yun YJ, Seong KM
and Youn B: Myricetin inhibits Akt survival signaling and induces
Bad-mediated apoptosis in a low dose ultraviolet (UV)-B-irradiated
HaCaT human immortalized keratinocytes. J Radiat Res (Tokyo).
51:285–296. 2010. View Article : Google Scholar
|
|
13
|
Wang X, Bai H, Zhang X, Liu J, Cao P, Liao
N, Zhang W, Wang Z and Hai C: Inhibitory effect of oleanolic acid
on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest
and mitochondrial-dependent apoptosis. Carcinogenesis.
34:1323–1330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gilmore TD and Herscovitch M: Inhibitors
of NF-kappaB signaling: 785 and counting. Oncogene. 25:6887–6899.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li TK and Liu LF: Tumor cell death induced
by topoisomerase-targeting drugs. Annu Rev Pharmacol Toxicol.
41:53–77. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nitiss JL: DNA topoisomerase II and its
growing repertoire of biological functions. Nat Rev Cancer.
9:327–337. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Curtin NJ: Inhibiting the DNA damage
response as a therapeutic manoeuvre in cancer. Br J Pharmacol.
169:1745–1765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghavami S, Hashemi M, Ande SR, Yeganeh B,
Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, et
al: Apoptosis and cancer: Mutations within caspase genes. J Med
Genet. 46:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Call JA, Eckhardt SG and Camidge DR:
Targeted manipulation of apoptosis in cancer treatment. Lancet
Oncol. 9:1002–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ravanan P, Sano R, Talwar P, Ogasawara S,
Matsuzawa S, Cuddy M, Singh SK, Rao GS, Kondaiah P and Reed JC:
Synthetic triterpenoid cyano enone of methyl boswellate activates
intrinsic, extrinsic, and endoplasmic reticulum stress cell death
pathways in tumor cell lines. Mol Cancer Ther. 10:1635–1643. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hyer ML, Shi R, Krajewska M, Meyer C,
Lebedeva IV, Fisher PB and Reed JC: Apoptotic activity and
mechanism of 2-cyano-3, 12-dioxoolean-1,9-dien-28-oic-acid and
related synthetic triterpenoids in prostate cancer. Cancer Res.
68:2927–2933. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inoue S, Snowden RT, Dyer MJ and Cohen GM:
CDDO induces apoptosis via the intrinsic pathway in lymphoid cells.
Leukemia. 18:948–952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Altomare DA and Testa JR: Perturbations of
the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ling X, Konopleva M, Zeng Z, Ruvolo V,
Stephens LC, Schober W, McQueen T, Dietrich M, Madden TL and
Andreeff M: The novel triterpenoid C-28 methyl ester of 2-cyano-3,
12-dioxoolen-1, 9-dien-28-oic acid inhibits metastatic murine
breast tumor growth through inactivation of STAT3 signaling. Cancer
Res. 67:4210–4218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dai S, Zheng Y, Chen B, Gao M, Zhang Y,
Zhang L, Gong W and He F: Two Gln187 mutants of human soluble APRIL
inhibit proliferation of lung carcinoma A549 cells. Acta Biochim
Pol. 56:703–710. 2009.PubMed/NCBI
|
|
26
|
Datta SR, Dudek H, Tao X, Masters S, Fu H,
Gotoh Y and Greenberg ME: Akt phosphorylation of BAD couples
survival signals to the cell-intrinsic death machinery. Cell.
91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahmad A, Biersack B, Li Y, Kong D, Bao B,
Schobert R, Padhye SB and Sarkar FH: Targeted regulation of
PI3K/Akt/mTOR/NF-κB signaling by indole compounds and their
derivatives: Mechanistic details and biological implications for
cancer therapy. Anticancer Agents Med Chem. 13:1002–1013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tully JE, Nolin JD, Guala AS, Hoffman SM,
Roberson EC, Lahue KG, van der Velden J, Anathy V, Blackwell TS and
Janssen-Heininger YM: Cooperation between classical and alternative
NF-κB pathways regulates proinflammatory responses in epithelial
cells. Am J Respir Cell Mol Biol. 47:497–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Manna S, Singha B, Phyo SA, Gatla HR,
Chang TP, Sanacora S, Ramaswami S and Vancurova I: Proteasome
inhibition by bortezomib increases IL-8 expression in
androgen-independent prostate cancer cells: the role of IKKalpha. J
Immunol. 191:2837–2846. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murray RZ and Norbury C: Proteasome
inhibitors as anti-cancer agents. Anticancer Drugs. 11:407–417.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen G, Han K, Xu X, Du X, Zhang Z, Tang
J, Shi M, Wang M, Li J, Cao B, et al: An anti-leishmanial
thiadiazine agent induces multiple myeloma cell apoptosis by
suppressing the nuclear factor kappaB signalling pathway. Br J
Cancer. 110:63–70. 2014. View Article : Google Scholar :
|
|
33
|
Buss H, Dörrie A, Schmitz ML, Hoffmann E,
Resch K and Kracht M: Constitutive and interleukin-1-inducible
phosphorylation of p65 NF-κB at serine 536 is mediated by multiple
protein kinases including IκB kinase (IKK)-α, IKKβ, IKKε, TRAF
family member-associated (TANK)-binding kinase 1 (TBK1), and an
unknown kinase and couples p65 to TATA-binding protein-associated
factor II31-mediated interleukin-8 transcription. J Biol Chem.
279:55633–55643. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deng WG, Zhu Y and Wu KK: Up-regulation of
p300 binding and p50 acetylation in tumor necrosis
factor-alpha-induced cyclooxy-genase-2 promoter activation. J Biol
Chem. 278:4770–4777. 2003. View Article : Google Scholar
|
|
35
|
Zhong H, SuYang H, Erdjument-Bromage H,
Tempst P and Ghosh S: The transcriptional activity of NF-kappaB is
regulated by the IkappaB-associated PKAc subunit through a cyclic
AMP-independent mechanism. Cell. 89:413–424. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lagadec P, Griessinger E, Nawrot MP,
Fenouille N, Colosetti P, Imbert V, Mari M, Hofman P, Czerucka D,
Rousseau D, et al: Pharmacological targeting of NF-kappaB
potentiates the effect of the topoisomerase inhibitor CPT-11 on
colon cancer cells. Br J Cancer. 98:335–344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nakanishi C and Toi M: Nuclear
factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat
Rev Cancer. 5:297–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu Y and Villalona-Calero MA: Irinotecan:
Mechanisms of tumor resistance and novel strategies for modulating
its activity. Ann Oncol. 13:1841–1851. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vos SM, Tretter EM, Schmidt BH and Berger
JM: All tangled up: How cells direct, manage and exploit
topoisomerase function. Nat Rev Mol Cell Biol. 12:827–841. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pommier Y: Topoisomerase I inhibitors:
Camptothecins and beyond. Nat Rev Cancer. 6:789–802. 2006.
View Article : Google Scholar : PubMed/NCBI
|