Introduction
Gastric cancer is the second-leading cause of
cancer-related mortality worldwide, and the prognosis of advanced
gastric cancer remains poor (1).
Despite advances in the treatment of gastric cancer, no standard
palliative chemotherapy has been accepted for patients with
metastatic gastric cancer (2,3). Thus,
there is a strong demand for new treatment options to address
advanced stages of gastric cancer.
Galectins are classified according to their
carbohydrate-recognition domain (CRD) and are subdivided into three
groups: prototype galectins (galectins-1, -2, -7, -10, -13, and
-14) and the chimera-type galectin (galectin-3) have a single CRD,
while tandem-repeat-type galectins (galectins-4, -8, -9, and -12)
have two CRDs joined by a flexible peptide linker (4–6).
Galectin-9 (Gal-9) is a tandem-repeat-type galectin that is known
for its key roles in eosinophil chemoattraction and activation
(7–9). Similar to other galectins, Gal-9
regulates various cellular functions, such as aggregation, adhesion
and apoptosis (10,11). In recent studies, Gal-9 has been
shown to induce apoptosis and thereby suppress cell proliferation
and tumor growth in various hematologic malignancies, such as human
melanoma (12,13) and chronic myelogenous leukemia
(14). Additionally, our recent
studies revealed the antitumor effects of recombinant Gal-9 in
various solid malignancies, such as hepatocellular carcinoma
(15) and cholangiocarcinoma
(16).
However, it is unclear whether Gal-9 suppresses
gastric cancer cell proliferation. The aim of this study was to
determine the effectiveness of Gal-9 against gastric cancer cell
proliferation. Possible mechanisms associated with the antitumor
effect of Gal-9 were also explored, including the activation of
receptor tyrosine kinases, angiogenesis and microRNAs (miRNAs).
Materials and methods
Reagents and chemicals
Recombinant stable and mutant forms of human Gal-9
lacking the entire linker region were expressed and purified as
described in our previous studies (17). Lactose, sucrose, and fetal bovine
serum (FBS) were purchased from Wako Chemicals (Osaka, Japan). The
Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Laboratories
(Kumamoto, Japan), and all other chemicals were obtained from Sigma
Chemical (Tokyo, Japan).
Cell lines and culture
The human gastric cancer cell lines MKN1, MKN7,
MKN45, and MKN74 were obtained from the Japanese Cancer Research
Resources Bank (Tokyo, Japan). The cells were cultured in RPMI-1640
(Gibco Invitrogen, Carlsbad, CA, USA) supplemented with 10%
heat-inactivated FBS and penicillin-streptomycin (100 µg/ml;
Invitrogen) in a humidified atmosphere with 5% CO2 at
37°C.
Cell proliferation assay
We performed cell proliferation assays using a CCK-8
according to the manufacturer's instructions. Samples of each cell
line (1×104 cells/well) were seeded into a 96-well plate
and cultured in 100 µl of RPMI-1640 supplemented with 10%
FBS. Twenty-four hours after seeding, the cells were treated with
0.01, 0.03, 0.1 or 0.3 µmol/l Gal-9 in the culture medium
and cultured for an additional 48 h. To inhibit the galactoside
binding of Gal-9, 30 mM lactose was added. Sucrose was added as a
control. CCK-8 reagent (10 µl) was then added to each well,
and the 96-well plate was incubated at 37°C for 3 h. The absorbance
of each well was measured at 450 nm using an auto-microplate
reader.
Enzyme-linked immunosorbent assay
(ELISA)
Cell apoptosis assays were conducted by measuring
the amounts of caspase-cleaved keratin-18 (CCK-18) using an M30
Apoptosense ELISA kit (Previva, Bromma, Sweden) (18). Samples of each cell type
(5×103 cells/well) were seeded into a 96-well plate and
cultured in 100 µl of culture medium for 24 h. The seeded
cells were then treated with 0.3 µmol/l Gal-9. The remaining
steps of the assay were carried out according to the manufacturer's
instructions. The amounts of antigen in the controls and samples
were calculated by interpolation from a standard curve.
Cell and tissue lysates
The preparation of lysates was conducted according
to the methods previously described by us (19). All the steps were carried out at
4°C. Protein concentrations were measured using a dye-binding
protein assay based on the Bradford method (20).
Antibody arrays of phosphorylated
receptor tyrosine kinases (phospho-RTKs)
Human phospho-RTKs were assayed using human
phospho-RTK array kits (R&D Systems) according to the
manufacturer's instructions. Briefly, phospho-RTK array membranes
were blocked with 5% BSA/TBS (0.01 M Tris-HCl, pH 7.6) for 1 h and
incubated with 2 ml of cell line lysates after normalization so
that the amounts of proteins were equal. After 3 washes for 10 min
each with TBS plus 0.1% v/v Tween-20 and 2 washes for 10 min with
TBS alone to remove unbound materials, the membranes were incubated
with anti-phosphotyrosine-HRP antibody for 2 h at room temperature.
Unbound HRP-conjugated antibody was washed out with 0.1% Tween-20
in TBS. Finally, each array membrane was exposed to X-ray film
using a chemiluminescence detection system (Perkin-Elmer Co.).
Angiogenic profile analysis using an
antibody array
A RayBio Human Angiogenesis Antibody Array
(RayBiotech, Inc.) was used according to the manufacturer's
protocol. This method is a dot-based assay enabling the detection
and comparison of 20 angiogenesis-specific cytokines. Each array
membrane was exposed to X-ray film using a chemiluminescence
detection system (Perkin-Elmer Co.).
Analysis of a miRNA microarray
Samples of each cancer cell line were processed for
total RNA extraction with an miRNeasy Mini kit (Qiagen, Venlo, The
Netherlands) according to the manufacturer's instructions. Using an
Agilent 2100 Bioanalyzer (Agilent Technologies), the RNA samples
typically had A260/280 ratios between 1.9 and 2.1.
After measuring RNA levels with an RNA 6000 Nano kit
(Agilent Technologies), the samples were labeled using a miRCURY
Hy3/Hy5 Power labeling kit and were hybridized to a human miRNA
Oligo chip 10, version 19.0 (Toray Industries, Tokyo, Japan).
Scanning was conducted with a 3D-Gene Scanner 3000 (Toray
Industries). 3D-Gene Extraction version 1.2 software (Toray
Industries) was used to read the raw intensities of the images. To
determine changes in miRNA expression between the Gal-9-treated and
control samples, the raw data were analyzed via GeneSpring GX
version 10.0 (Agilent Technologies). The samples were first
normalized relative to 28S RNA and baseline-corrected to the median
of all the samples.
Replicate data were consolidated into two groups,
i.e., those from the galectin-9-treated cells and those from the
control cells, and were organized using the hierarchical clustering
and ANOVA functions in GeneSpring software. Hierarchical clustering
was performed using the clustering function (condition tree) and
the Euclidean correlation as a distance metric. A two-way ANOVA was
conducted, and asymptotic p-values (<0.05) were determined with
no error corrections to identify the miRNAs that varied most
prominently across the different groups. Only changes >50% for
at least one of the time-points for each sample were considered
significant. All the analyzed data were globally normalized. The
statistical significance of differentially expressed miRNAs was
analyzed using Student's t-test.
Statistical analysis
All analyses were conducted using JMP8.0 (SAS
Institute, Cary, NC, USA). Paired analyses between the groups were
conducted using Student's t-test. A p<0.05 was considered to
indicate a significant difference between groups.
Results
Gal-9 suppresses the proliferation of
human gastric cancer cells
To evaluate the effect of Gal-9 on the in
vitro growth of human gastric cancer cells, we examined the
effect of Gal-9 on the proliferation of 4 gastric cancer cell
lines, MKN1, MKN7, MKN45 and MKN74. Cells were grown in 10% FBS and
treated with 0.01, 0.03, 0.1 or 0.3 µmol/l Gal-9 or without
Gal-9 as a control. The cell proliferation assay was conducted 48 h
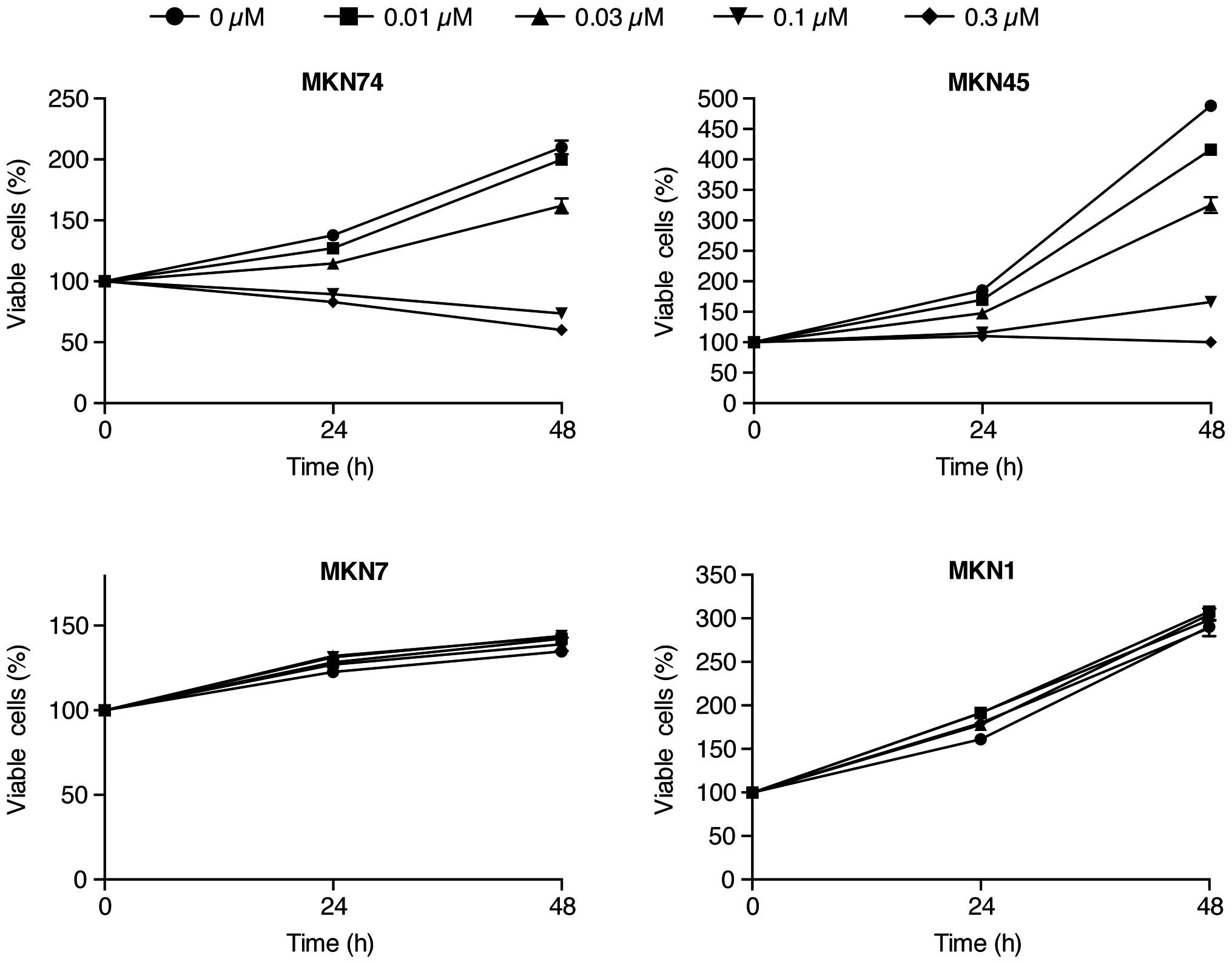
after the addition of the agents. As depicted in Fig. 1, treatment with Gal-9 led to a
strong, dose-dependent inhibition of cell proliferation in the
MKN74 and MKN45 cells, which are Gal-9-sensitive gastric cancer
cell lines (Fig. 1). However, no
anti-proliferative effects of Gal-9 were detected in the MKN7 and
MKN1 cells (Fig. 1).
The anti-proliferative effect of Gal-9 is
inhibited by lactose in Gal-9-sensitive gastric cancer cells
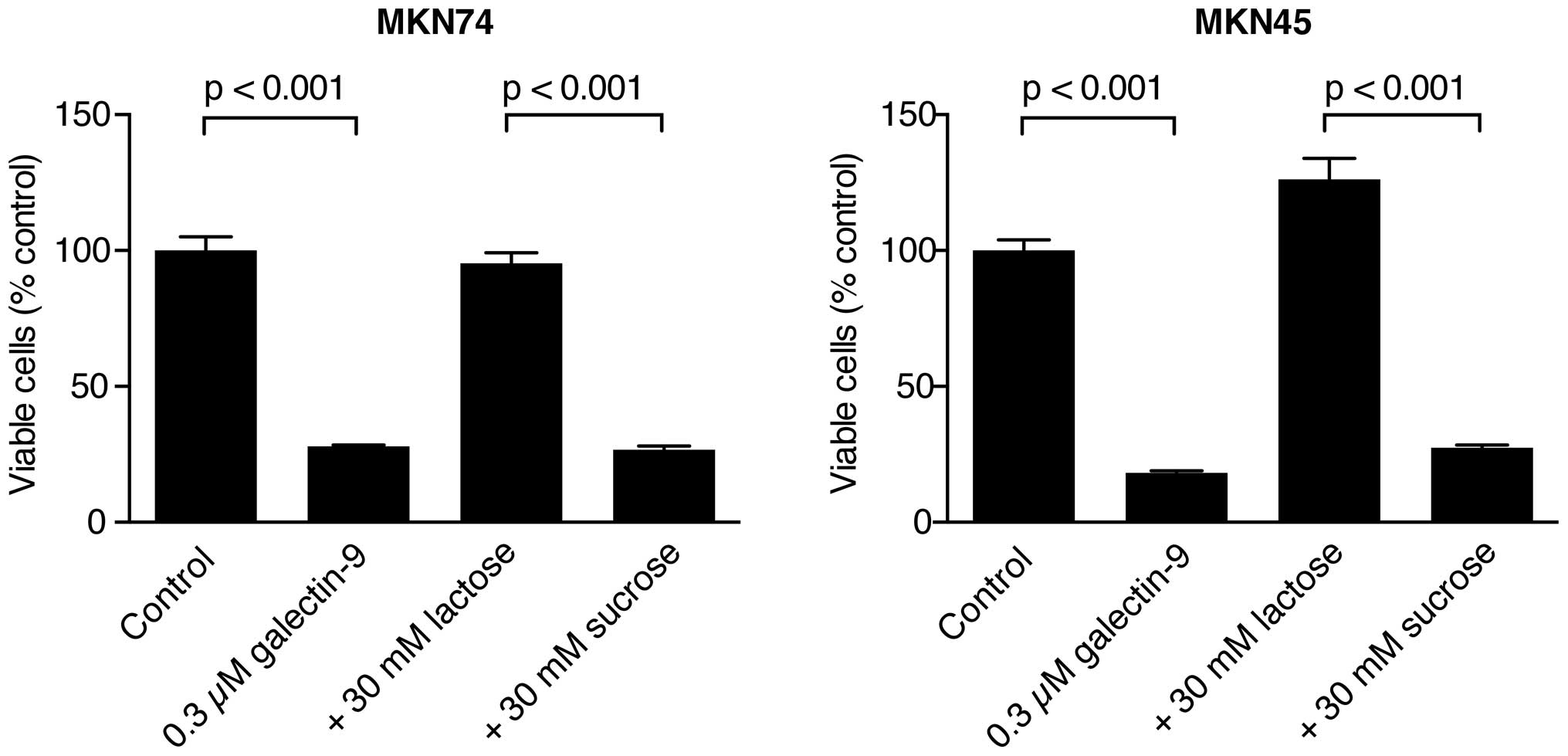
The growth of the MKN74 and MKN45 cells was
inhibited by Gal-9 with 30 mM sucrose but not with 30 mM lactose
(Fig. 2). These data suggest that
the binding of β-galactoside is essential for Gal-9 to inhibit the
proliferation of the MKN74 and MKN45 cells, which are
Gal-9-sensitive gastric cancer cells.
Gal-9 induces apoptosis in
Gal-9-sensitive gastric cancer cells (MKN74 cells) but not
Gal-9-resistant cells (MKN7 cells)
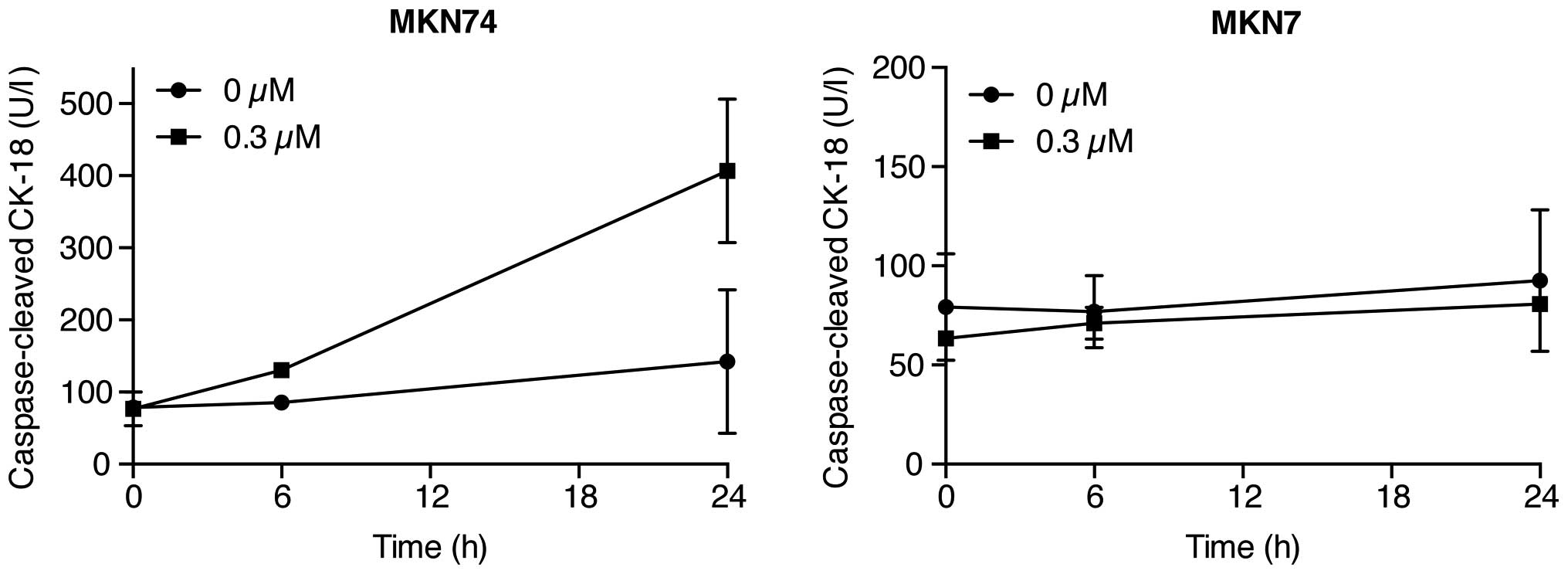
To determine the mechanism(s) of the Gal-9
anti-proliferative effects, the level of caspase-cleaved
cytokeratin 18 (CCK18), which is specifically produced during
apoptosis, was measured using ELISAs in gastric cancer cells
treated with 0.3 µM Gal-9. Remarkably, Gal-9 increased the
levels of CCK18 in Gal-9-sensitive cells (MKN74 cells) but not in
Gal-9-resistant cells (MKN7 cells) (Fig. 3). This result suggests that
apoptosis is involved in Gal-9-induced cytostasis in the
Gal-9-sensitive cells.
Differences in phospho-RTKs in
Gal-9-sensitive versus Gal-9-resistant gastric cancer cells treated
with and without Gal-9
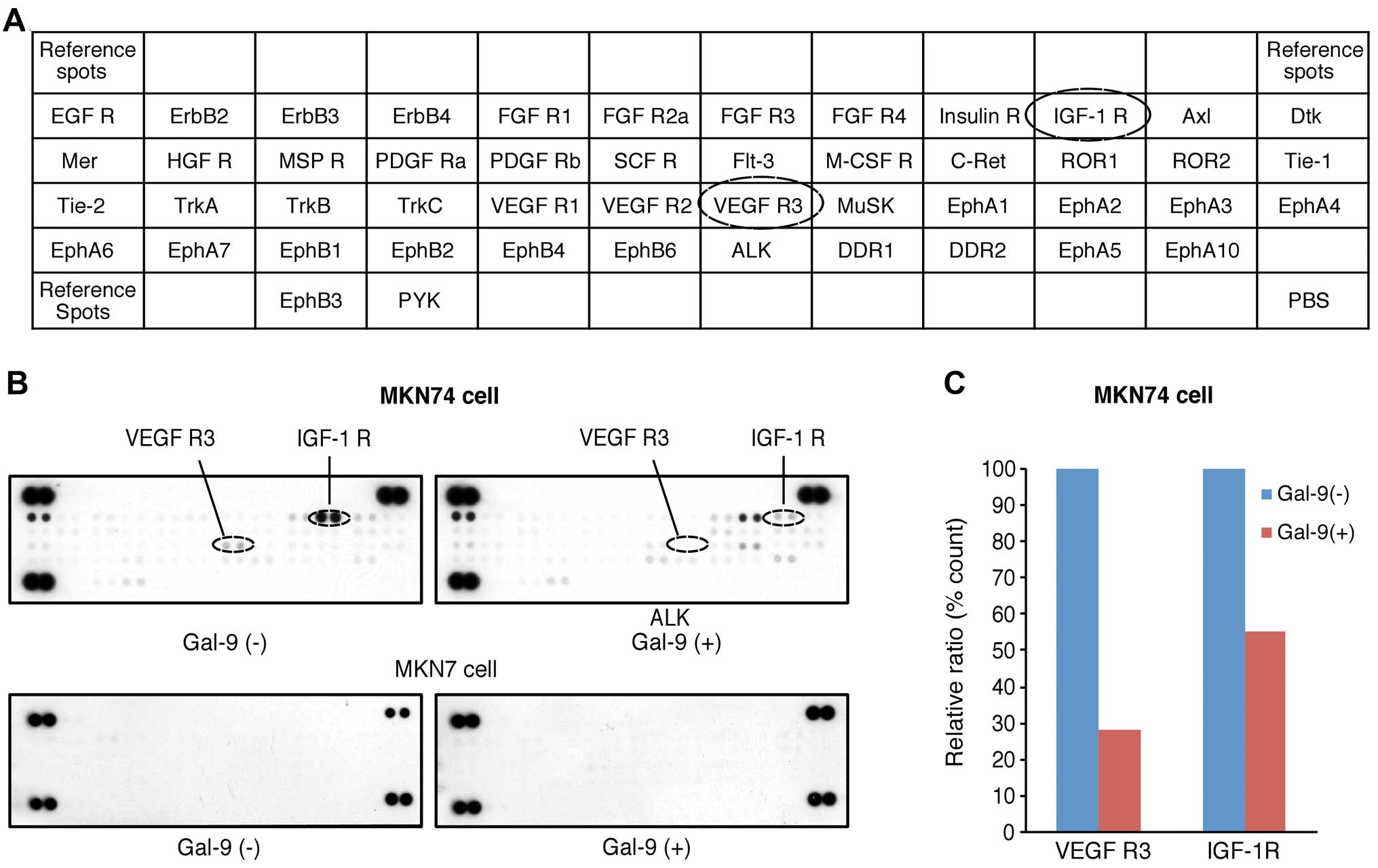
We next used a phospho-RTK array system to identify
the key receptor tyrosine kinases (RTKs) associated with the
anti-proliferative effects of Gal-9. Using this antibody array
(Fig. 4A), we simultaneously
screened the expression levels of 42 different RTKs activated in
MKN74 and MKN7 cells with or without 0.3 µM Gal-9. Gal-9
reduced the expression levels of vascular endothelial growth factor
receptor-3 (VEGFR-3) and phosphorylated insulin-like-growth
factor-1 receptor (IGF-1R) in the MKN74 cells (Fig. 4B). In contrast, no activated RTKs
were upregulated in the Gal-9-resistant MKN1 cells.
Densitometric analyses revealed that the ratios of
the p-VEGFR-3 and p-IGF-1R spots of the Gal-9-treated cells to
those of the untreated cells were 28.2 and 55.1%, respectively
(Fig. 4C).
Effects of Gal-9 on angiogenesis in
Gal-9-sensitive versus Gal-9-resistant gastric cancer cells
To examine the relationship between angiogenesis and
Gal-9, an angiogenesis antibody array analysis was conducted
regarding the anti-tumor effects of Gal-9 (Fig. 5A). Using the antibody array, we
simultaneously screened the expression levels of 20 different
angiogenesis-related proteins in MKN7 and MKN74 cells with or
without Gal-9. The expression levels of interleukin-8 (IL-8),
tissue inhibitor of metalloproteinase-1 (TIMP-1), TIMP-2,
growth-related oncogene (GRO) and regulated-on-activation
normal-T-cell-expressed and secreted (RANTES) were induced by Gal-9
treatment in the MKN74 cells as detected by the protein array
(Fig. 5B). In the Gal-9-resistant
MKN1 cells, no altered expression of angiogenesis molecules was
detected with Gal-9 treatment.
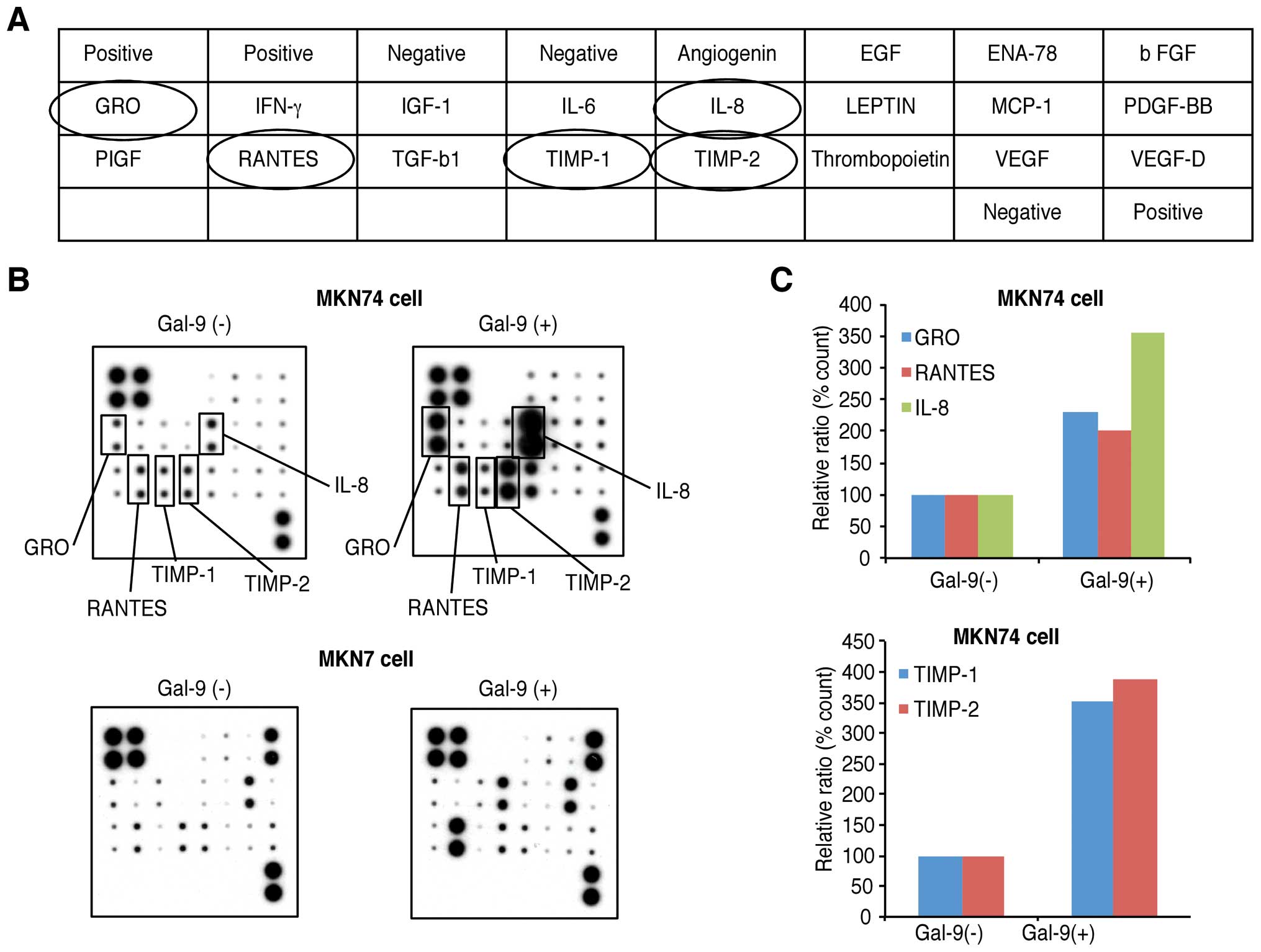 | Figure 5Effects of galectin-9 (Gal-9) on
angiogenesis in the MKN74 and MKN7 cells. (A) Template depicting
the location of antibodies for angiogenesis-related proteins
spotted onto a human angiogenesis array. (B) Representative
expression levels of various antibodies for angiogenesis-related
proteins in the MKN7 and MKN74 cells treated with or without Gal-9.
Increased expression levels of IL-8, TIMP-1, TIMP-2, GRO, and
RANTES were detected in the MKN74 cells treated with Gal-9. (C)
Densitometry analysis indicated that the ratios of the IL-8,
TIMP-1, TIMP-2, GRO, and RANTES spots of the Gal-9-treated cells to
those of the untreated cells were 356.1, 355.3, 389.36, 231.1 and
201.0%, respectively. |
Densitometric analyses indicated that the ratios of
the IL-8, TIMP-1, TIMP-2, GRO, and RANTES spots of the
Gal-9-treated cells to those of the untreated cells were 356.1,
355.3, 389.36, 231.1 and 201.0%, respectively (Fig. 5C).
miRNA profiles of the cell lines treated
in vitro with or without Gal-9
Using a custom microarray platform, we analyzed the
in vitro expression levels of 2,555 human miRNA probes in
gastric cancer cell lines that were treated with or without Gal-9.
As presented in Table I, when the
expression levels of miRNAs were measured in the MKN74 cells
treated in vitro with or without 0.3 µmol/l Gal-9,
153 miRNAs were significantly upregulated 24 h after the Gal-9
treatment, whereas 18 miRNAs were downregulated.
 | Table IChanges in expression compared with
untreated cells and chromosomal locations of miRNAs in MKN74 cells
treated with galectin-9. |
Table I
Changes in expression compared with
untreated cells and chromosomal locations of miRNAs in MKN74 cells
treated with galectin-9.
| miRNA | Fold
(treated/control) means ± SD | P-value | Chromosomal
localization |
|---|
| Upregulated |
| hsa-miR-204-3p | 12.41±3.011 | 0.00023 | 9q21.12 |
| hsa-miR-4688 | 11.27±2.983 | 0.00032 | 11 |
|
hsa-miR-4731-5p | 10.05±4.236 | 0.00091 | 17 |
| hsa-miR-4459 | 8.36±2.17 | 0.00016 | 5 |
| hsa-miR-4730 | 7.78±2.432 | 0.00406 | 17 |
| hsa-miR-4294 | 7.54±3.654 | 0.00164 | 10 |
| hsa-miR-3196 | 7.39±2.829 | 0.00191 | 20 |
| hsa-miR-3648 | 7.32±1.06 | 0.00049 | 21 |
|
hsa-miR-4687-3p | 6.74±1.938 | 0.00011 | 11 |
| hsa-miR-3197 | 6.46±1.153 | 0.00408 | 21 |
| hsa-miR-4530 | 6.35±0.738 | 0.00058 | 19 |
| hsa-miR-638 | 6.33±1.676 | 0.0023 | 19p13.2 |
| hsa-miR-1908 | 6.31±1.595 | 0.00116 | 11 |
| hsa-miR-4442 | 6.28±1.781 | 0.00281 | 3 |
| hsa-miR-4488 | 6.19±1.839 | 0.00057 | 11 |
| hsa-miR-6125 | 6.16±0.699 | 0.00048 | 12 |
|
hsa-miR-4787-5p | 5.98±1.567 | 0.00031 | 3 |
| hsa-miR-6088 | 5.79±1.124 | 0.00091 | 19 |
| hsa-miR-4466 | 5.78±1.562 | 0.00633 | 6 |
| hsa-miR-3621 | 5.6±1.271 | 0.0008 | 9 |
|
hsa-miR-1237-5p | 5.6±1.902 | 0.00142 | 11 |
|
hsa-miR-3940-5p | 5.54±1.068 | 0.00106 | 19 |
|
hsa-miR-1227-5p | 5.52±1.785 | 0.01023 | 19 |
| hsa-miR-4792 | 5.49±1.065 | 0.0012 | 3 |
| hsa-miR-4508 | 5.41±0.595 | 0.00019 | 15 |
| hsa-miR-3665 | 5.4±1.682 | 0.00066 | 13 |
| hsa-miR-2861 | 5.38±1.946 | 0.00057 | 9 |
| hsa-miR-6126 | 5.36±1.517 | 0.00735 | 16 |
| hsa-miR-5787 | 5.29±0.798 | 0.00049 | 3 |
| hsa-miR-3656 | 5.15±1.083 | 0.00053 | 11 |
| hsa-miR-6075 | 5.14±1.757 | 0.00036 | 5 |
| hsa-miR-1246 | 5.08±1.896 | 0.03458 | 2q31.1 |
|
hsa-miR-1229-5p | 4.92±1.854 | 0.00022 | 5 |
|
hsa-miR-4667-5p | 4.84±0.891 | 0.00294 | 9 |
|
hsa-miR-4756-5p | 4.68±4.77 | 0.00762 | 20 |
| hsa-miR-6132 | 4.63±0.546 | 0.00712 | 7 |
| hsa-miR-1268a | 4.63±0.402 | 0.00071 | 15q11.2 |
| hsa-miR-6085 | 4.28±1.456 | 0.00273 | 15 |
| hsa-miR-4484 | 4.17±1.354 | 0.01331 | 10 |
| hsa-miR-652-5p | 4.15±0.76 | 0.001 | Xq23 |
|
hsa-miR-4800-5p | 4.1±0.03 | 0.02576 | 4 |
| hsa-miR-6131 | 4.08±0.446 | 0.02093 | 5 |
|
hsa-miR-6724-5p | 4.07±1.875 | 0.0041 | 21 |
|
hsa-miR-4745-5p | 4.04±0.883 | 0.01705 | 19 |
|
hsa-miR-642a-3p | 4±0.901 | 0.0114 | 19q13.32 |
| hsa-miR-1973 | 3.97±0.422 | 0.00364 | 4 |
| hsa-miR-663a | 3.88±0.336 | 0.00185 | 20p11.1 |
|
hsa-miR-4749-5p | 3.87±0.722 | 0.00053 | 19 |
| hsa-miR-4741 | 3.8±0.168 | 0.00361 | 18 |
| hsa-miR-1469 | 3.8±0.652 | 0.00034 | 15q26.2 |
|
hsa-miR-1228-5p | 3.8±0.564 | 0.00047 | 12 |
| hsa-miR-4463 | 3.78±0.131 | 0.00074 | 6 |
| hsa-miR-4505 | 3.73±0.418 | 0.00081 | 14 |
|
hsa-miR-1915-3p | 3.72±1.571 | 0.00219 | 10p12.31 |
|
hsa-miR-4763-3p | 3.7±0.411 | 0.00186 | 22 |
| hsa-miR-491-5p | 3.69±0.866 | 0.00014 | 9p21.3 |
| hsa-miR-4651 | 3.66±0.884 | 0.00136 | 7 |
| hsa-miR-4435 | 3.62±1.308 | 0.0397 | 2 |
| hsa-miR-1275 | 3.62±1.031 | 0.00237 | 6 |
| hsa-miR-3180 | 3.53±1.989 | 0.00998 | 16 |
| hsa-miR-31-5p | 3.44±1.15 | 0.00019 | 9p21.3 |
|
hsa-miR-1909-3p | 3.34±0.825 | 0.00498 | 19p13.3 |
|
hsa-miR-3620-5p | 3.3±0.472 | 0.00182 | 1 |
| hsa-miR-149-3p | 3.21±0.307 | 0.00355 | 2q37.3 |
|
hsa-miR-4725-3p | 3.18±0.711 | 0.00036 | 17 |
| hsa-miR-132-3p | 3.17±2.048 | 0.00242 | 17p13.3 |
| hsa-miR-1587 | 3.13±0.557 | 0.0024 | Xp11.4 |
| hsa-miR-4327 | 3.12±0.843 | 0.0008 | 21 |
|
hsa-miR-4726-5p | 3.12±0.454 | 0.00084 | 17 |
|
hsa-miR-1247-3p | 3.11±1.069 | 0.02297 | 14q32.31 |
| hsa-miR-486-3p | 3.1±0.267 | 0.01475 | 8p11.21 |
| hsa-miR-1268b | 3.1±0.561 | 0.00049 | 17 |
| hsa-miR-4497 | 3.08±0.395 | 0.00092 | 12 |
|
hsa-miR-642b-3p | 3.06±0.565 | 0.00667 | 19 |
| hsa-miR-937-5p | 3.06±0.337 | 0.005 | 8q24.3 |
|
hsa-miR-4728-5p | 3.03±1.161 | 0.04874 | 17 |
|
hsa-miR-365a-5p | 3.03±0.216 | 0.00296 | 16p13.12 |
|
hsa-miR-3180-3p | 3.01±0.606 | 0.00032 | 16 |
| hsa-miR-675-5p | 2.97±1.027 | 0.02744 | 11p15.5 |
| hsa-miR-4656 | 2.89±0.275 | 0.00358 | 7 |
| hsa-miR-4270 | 2.83±0.615 | 0.01617 | 3 |
|
hsa-miR-5001-5p | 2.83±0.452 | 0.01792 | 2 |
| hsa-miR-4257 | 2.81±0.263 | 0.00051 | 1 |
| hsa-miR-4450 | 2.79±1.073 | 0.01547 | 4 |
| hsa-miR-4507 | 2.79±0.356 | 0.01068 | 14 |
| hsa-miR-3937 | 2.77±1.063 | 0.00578 | X |
|
hsa-miR-513a-5p | 2.74±0.442 | 0.023 | Xq27.3 |
| hsa-miR-1202 | 2.7±0.082 | 0.00084 | 6 |
| hsa-miR-760 | 2.69±0.634 | 0.0243 | 1p22.1 |
|
hsa-miR-4638-3p | 2.67±0.394 | 0.01986 | 5 |
|
hsa-miR-5585-3p | 2.67±1.341 | 0.00679 | 1p35.2 |
| hsa-miR-671-5p | 2.66±0.892 | 0.00623 | 7q36.1 |
|
hsa-miR-4655-5p | 2.65±0.38 | 0.00198 | 7 |
| hsa-miR-212-3p | 2.64±0.448 | 0.02006 | 17p13.3 |
| hsa-miR-4299 | 2.56±1.727 | 0.01568 | 11 |
| hsa-miR-4706 | 2.54±0.781 | 0.00115 | 14 |
| hsa-miR-132-5p | 2.52±0.469 | 0.01826 | 17p13.3 |
|
hsa-miR-4722-5p | 2.49±0.116 | 0.01955 | 16 |
| hsa-miR-3135b | 2.48±0.096 | 0.00061 | 6 |
|
hsa-miR-4732-5p | 2.48±0.216 | 0.00737 | 17 |
|
hsa-miR-4758-5p | 2.46±0.487 | 0.0002 | 20 |
| hsa-miR-2392 | 2.4±0.749 | 0.01092 | 14 |
| hsa-miR-4449 | 2.37±0.582 | 0.04587 | 4 |
| hsa-miR-29a-3p | 2.36±0.685 | 0.00438 | 7q32.3 |
|
hsa-miR-6722-3p | 2.34±0.282 | 0.00175 | 9 |
| hsa-miR-3714 | 2.31±0.186 | 0.03306 | 3 |
| hsa-miR-744-5p | 2.3±1.104 | 0.02676 | 17p12 |
|
hsa-miR-5006-5p | 2.23±0.694 | 0.01224 | 13 |
| hsa-miR-3141 | 2.22±0.677 | 0.00299 | 5 |
|
hsa-miR-1185-1-3p | 2.21±0.698 | 0.03128 | 14 |
|
hsa-miR-4436b-5p | 2.19±0.712 | 0.01295 | 2 |
| hsa-miR-4417 | 2.14±0.536 | 0.00643 | 1 |
|
hsa-miR-5196-5p | 2.13±0.42 | 0.00448 | 19 |
| hsa-miR-296-5p | 2.12±0.383 | 0.00725 | 20q13.32 |
|
hsa-miR-6722-5p | 2.11±0.255 | 0.01874 | 9 |
| hsa-miR-614 | 2.09±0.597 | 0.00179 | 12p13.1 |
|
hsa-miR-3678-3p | 2.08±0.805 | 0.04442 | 17 |
|
hsa-miR-1914-3p | 2.03±0.474 | 0.02587 | 20q13.33 |
|
hsa-miR-4640-5p | 2.03±0.371 | 0.03783 | 6 |
| hsa-miR-4498 | 2.03±0.522 | 0.04462 | 12 |
| hsa-miR-194-5p | 1.97±0.588 | 0.00849 | 1q41 |
|
hsa-miR-29b-1-5p | 1.97±0.34 | 0.00444 | 7q32.3 |
|
hsa-miR-4695-5p | 1.95±0.352 | 0.00097 | 1 |
|
hsa-miR-4707-5p | 1.93±0.2 | 0.04286 | 14 |
|
hsa-miR-4690-5p | 1.92±0.098 | 0.01814 | 11 |
| hsa-miR-92b-5p | 1.91±0.499 | 0.00537 | 1q22 |
| hsa-miR-4465 | 1.9±0.148 | 0.00753 | 6 |
|
hsa-miR-3679-3p | 1.88±0.185 | 0.00101 | 2 |
| hsa-miR-711 | 1.87±0.536 | 0.0124 | 3 |
| hsa-miR-3185 | 1.84±0.161 | 0.01036 | 17 |
| hsa-miR-192-5p | 1.83±0.464 | 0.02096 | 11q13.1 |
| hsa-miR-370 | 1.82±0.547 | 0.02131 | 14q32.2 |
| hsa-miR-658 | 1.81±0.577 | 0.03598 | 22q13.1 |
| hsa-miR-222-3p | 1.76±0.614 | 0.01237 | Xp11.3 |
| hsa-miR-3195 | 1.71±0.131 | 0.02378 | 20 |
| hsa-miR-557 | 1.67±0.415 | 0.01678 | 1q24.2 |
| hsa-miR-221-3p | 1.59±0.406 | 0.03976 | Xp11.3 |
| hsa-miR-4634 | 1.54±0.367 | 0.04868 | 5 |
|
hsa-miR-1225-5p | 1.52±0.189 | 0.03676 | 16p13.3 |
| hsa-miR-3609 | 1.52±0.002 | 0.0084 | 7 |
|
hsa-miR-30c-1-3p | 1.48±0.065 | 0.00171 | 1p34.2 |
|
hsa-miR-6515-3p | 1.45±0.106 | 0.0161 | 19 |
| hsa-miR-21-3p | 1.44±0.343 | 0.02759 | 17q23.1 |
|
hsa-miR-3144-5p | 1.44±0.124 | 0.00824 | 6 |
| hsa-miR-22-3p | 1.41±0.028 | 0.01301 | 17p13.3 |
| hsa-miR-622 | 1.38±0.072 | 0.01576 | 13q31.3 |
|
hsa-miR-4650-3p | 1.38±0.096 | 0.00266 | 7q11.21 |
| hsa-miR-877-5p | 1.38±0.156 | 0.02297 | 6p21.33 |
| hsa-miR-660-5p | 1.3±0.247 | 0.03583 | Xp11.23 |
| hsa-miR-3907 | 1.25±0.128 | 0.01567 | 7 |
| hsa-miR-3161 | 1.24±0.009 | 0.00212 | 11 |
|
hsa-miR-4714-3p | 1.24±0.04 | 0.04315 | 15 |
| hsa-miR-532-5p | 1.22±0.135 | 0.01241 | Xp11.23 |
| Downregulated |
| hsa-miR-30d-3p | 0.36±0.137 | 0.01586 | 8q24.22 |
| hsa-miR-4785 | 0.44±0.144 | 0.04552 | 2 |
| hsa-miR-4521 | 0.46±0.185 | 0.02384 | 17 |
|
hsa-miR-4722-3p | 0.5±0.171 | 0.03034 | 16 |
| hsa-miR-4683 | 0.56±0.089 | 0.01651 | 10 |
|
hsa-miR-200b-5p | 0.58±0.137 | 0.01679 | 1p36.33 |
| hsa-miR-345-5p | 0.6±0.19 | 0.04389 | 14q32.2 |
| hsa-miR-590-5p | 0.6±0.077 | 0.00326 | 7q11.23 |
|
hsa-miR-103a-2-5p | 0.61±0.116 | 0.0355 | 20p13 |
| hsa-miR-761 | 0.65±0.017 | 0.0427 | 1 |
| hsa-miR-5692c | 0.65±0.132 | 0.01502 | 5 |
| hsa-miR-299-3p | 0.68±0.02 | 0.03763 | 14q32.31 |
| hsa-miR-126-5p | 0.69±0.079 | 0.00758 | 9q34.3 |
| hsa-miR-4301 | 0.7±0.152 | 0.03201 | 11 |
| hsa-miR-4522 | 0.72±0.161 | 0.03506 | 17 |
| hsa-miR-627 | 0.74±0.034 | 0.00686 | 15q15.1 |
| hsa-miR-1323 | 0.75±0.066 | 0.04283 | 19q13.42 |
|
hsa-miR-2681-5p | 0.76±0.055 | 0.03387 | 13 |
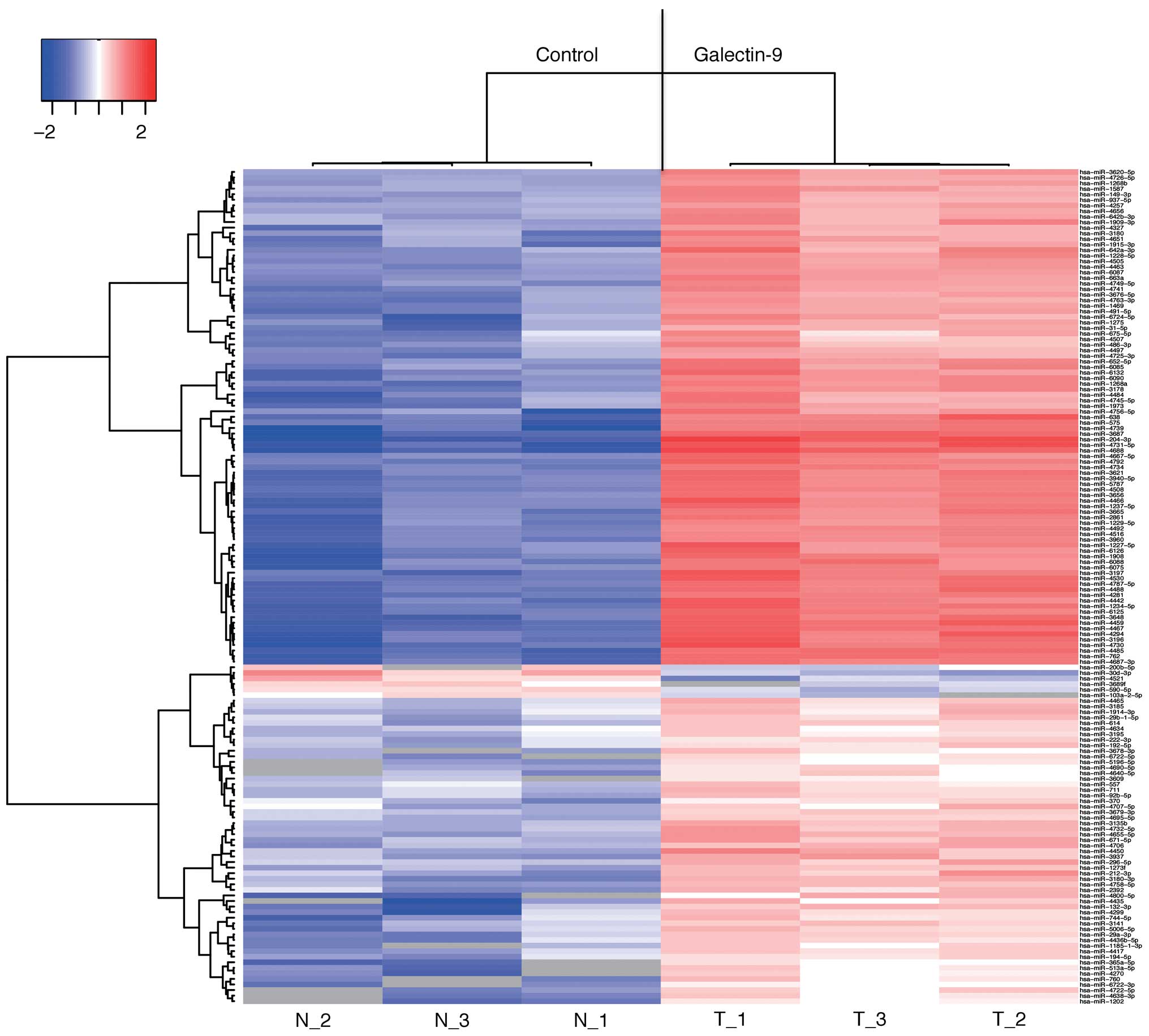
An unsupervised hierarchical clustering analysis and
a Pearson's correlation analysis indicated that MKN74 cells treated
in vitro with Gal-9 clustered together and separately from
the untreated MKN74 cells (Fig.
6).
Discussion
Gastric cancer is a very common disease worldwide
and is the second most frequent cause of cancer-related deaths
(21,22). Although the overall incidence and
mortality have dramatically declined over the past few decades,
gastric cancer remains a major health problem (23). More than half of gastric cancer
patients have lymph node metastasis when initially diagnosed or
after surgical resection, which results in a poor prognosis
(24–26). Patients with advanced stages of
gastric cancer are treated with chemotherapy and radiation, either
singly or in combination. However, none of these therapies is fully
curative. Therefore, new therapeutic strategies are urgently
required for the treatment of advanced gastric cancer. In recent
studies, higher levels of Gal-9 expression were observed in
patients without lymph-vascular invasion, lymph node metastases or
distant metastases, and Gal-9 expression is closely associated with
better survival rates in gastric cancer (27). Additionally, the downregulation of
Gal-9 mRNA levels was observed in gastric cancer tissues;
therefore, the loss of Gal-9 expression may be involved in the
progression of gastric cancers (28). In the present study, we found that
Gal-9 inhibited the proliferation of gastric cancer cell lines. In
addition, we identified miRNAs associated with the antitumor effect
of Gal-9 in gastric cancer.
Recombinant Gal-9 inhibits proliferation in various
cancers such as melanoma (13) and
chronic myelogenous leukemia (14)
by inducing apoptosis. Our present results revealed that Gal-9
suppresses the proliferation of human gastric cancer cell lines
in vitro. Gal-9 strongly inhibited cell proliferation in a
dose-dependent manner in MKN45 and MKN74 cells but not in MKN1 and
MKN7 cells. Notably, the histological type of the MKN74 and MKN7
cell lines is intestinal, while the MKN45 cells are a diffuse type,
and the MKN1 cells are an adenosquamous carcinoma (29). Furthermore, the MKN7 and MKN1 cells
were established from metastatic foci in the lymph nodes (29,30),
whereas the MKN74 and MKN45 cells were derived from liver
metastases (29,30). Accordingly, these data suggest that
the liver metastatic type of gastric cancer cells (MKN74 and MKN45
cells) are more sensitive to Gal-9 treatment compared with the
lymph node metastatic type of cells (MKN7 and MKN1 cells),
regardless of their respective histological differentiation. These
results suggest that Gal-9 may be a potential targeting moiety
useful in distant metastasis in the advanced stages of gastric
cancer.
The cleavage of cytokeratin by caspase (CCK18)
occurs as an early event during apoptosis following the activation
of apoptosis executioners, particularly effector caspases. However,
cytokeratin remains intact during other types of cell death, such
as autophagy or necrosis (31). In
the present study, Gal-9 increased the levels of CCK18 in the MKN74
cells, which are sensitive to Gal-9, but not the MKN1 cells, which
are resistant to Gal-9. These data indicate that Gal-9 suppresses
the proliferation of Gal-9-sensitive gastric cancer cells by
inducing apoptosis.
Since the discovery of these proteins, RTKs have
been investigated as key regulators of the proliferation,
differentiation, and metastasis of gastric cancer cells (32). Our present study used phospho-RTK
arrays to demonstrate that Gal-9 treatment reduced the expression
levels of IGF-1R and VEGFR-3 in Gal-9-sensitive cells (MKN74) when
compared with those in Gal-9-resistant cells (MKN7). IGF-1R is
involved in cell proliferation and the prevention of apoptosis
(32,33). Galamb et al found via
immunohistochemistry that IGF-1R was overexpressed in gastric
cancer tissues when compared with normal mucosa (34). Additionally, the mRNA expression
levels tended to be higher in cancer tissues than in normal mucosa
(35). The overexpression of IGF-1R
is associated with a poor response to chemotherapy and poor
outcomes in stage I–IV gastric cancers (36). Gal-9 may reduce the expression
levels of phospho-IGF-1R in gastric cancer and thereby improve
chemosensitivity and the disease prognosis.
Galectins are important regulators of tumor
progression that influence tumor cell transformation, immune escape
and angiogenesis (4–6). Using angiogenesis-related protein
arrays, we observed that IL-8, GRO, TIMP-1, TIMP-2 and RANTES were
enhanced in Gal-9-sensitive gastric cancer cells. The enhanced
expression of IL-8, in particular, may be associated with a poor
prognosis, as determined by stage and histology, and indicate a
more aggressive gastric cancer (37–39).
These data suggest that Gal-9-sensitive gastric cancer cells immune
to the antitumor effect of Gal-9 may produce angiogenesis-related
proteins and thereby regulate angiogenesis in the in vivo
microenvironment of gastric cancer tissues.
MicroRNAs, small non-coding RNA sequences, have been
shown to regulate the development and progression of various
cancers (40). To identify the
miRNAs associated with the antitumor effects of Gal-9, we used
miRNA expression arrays to determine variations in the miRNA
profiles of the MKN74 cell line in cultures treated with or without
Gal-9. The cluster-based analysis clearly demonstrated that Gal-9
treatment affected the expression of numerous miRNAs. In the
analysis, we selected sets of miRNAs with significantly altered
expression levels with or without Gal-9 treatment. These altered
miRNAs may provide clues to the molecular basis for the anticancer
effects of Gal-9 in gastric cancer. Our data revealed that
miR-204-3p and miR-1246 were upregulated in MKN74 cells treated
with Gal-9. miR-204 targets Bcl-2, brain-derived neurotrophic
factor (BDNF), SIRT1 and IGFBP5 expression and leads to apoptosis
and cell cycle arrest in gastric cancer, neuroblastoma, ovarian
cancer and papillary thyroid carcinoma. Furthermore, miR-204-3p has
also been reported to act on fibronectin, inhibit the proliferation
of hepatocellular carcinoma (HCC) endothelial cells and promote
apoptosis (41). In contrast,
miR-1246 has been reported to be a target of the p53 family, to
inhibit Down syndrome-associated DYRK1A, and consequently activate
NFTA1c and induce apoptosis (39).
Our previous studies have shown that Gal-9 treatment upregulated
miR-1246 in hepatocellular carcinoma (15) and cholangiocarcinoma (16), suggesting that miR-1246 may be
associated with the anti-proliferative effects of Gal-9 in various
cancer cells.
In conclusion, our results revealed that Gal-9
suppresses human gastric cancer cell proliferation, possibly by
inducing apoptosis, regulating RTK pathways and
angiogenesis-related molecules, and altering miRNA expression
profiles. These findings suggest that Gal-9 may be a novel
therapeutic target in the treatment of gastric cancer.
Acknowledgments
We thank Ms. Ryoko Unose, Ms. Kana Ogawa, Ms. Kayo
Hirose, Ms. Miwako Watanabe, Ms. Kayo Endo, and Ms. Noriko Murao
for providing technical assistance.
Abbreviations:
|
Gal-9
|
galectin-9
|
|
CRDs
|
carbohydrate-recognition domains
|
|
miRNAs
|
microRNAs
|
|
CCK-8
|
Cell Counting Kit-8
|
|
phospho-RTKs
|
phosphorylated receptor tyrosine
kinases
|
|
VEGR-3
|
vascular endothelial growth factor
receptor-3
|
|
IGF-1R
|
insulin-like growth factor-1
receptor
|
References
|
1
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lordick F and Siewert JR: Recent advances
in multimodal treatment for gastric cancer: A review. Gastric
Cancer. 8:78–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bilici A: Treatment options in patients
with metastatic gastric cancer: Current status and future
perspectives. World J Gastroenterol. 20:3905–3915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thijssen VL, Heusschen R, Caers J and
Griffioen AW: Galectin expression in cancer diagnosis and
prognosis: A systematic review. Biochim Biophys Acta. 1855:235–247.
2015.PubMed/NCBI
|
|
5
|
Wiersma VR, de Bruyn M, Helfrich W and
Bremer E: Therapeutic potential of galectin-9 in human disease. Med
Res Rev. 33(Suppl 1): E102–E126. 2013. View Article : Google Scholar
|
|
6
|
Fujihara S, Mori H, Kobara H, Rafiq K,
Niki T, Hirashima M and Masaki T: Galectin-9 in cancer therapy.
Recent Pat Endocr Metab Immune Drug Discov. 7:130–137. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirashima M: Ecalectin/galectin-9, a novel
eosinophil chemo-attractant: Its function and production. Int Arch
Allergy Immunol. 122(Suppl 1): 6–9. 2000. View Article : Google Scholar
|
|
8
|
Matsumoto R, Matsumoto H, Seki M, Hata M,
Asano Y, Kanegasaki S, Stevens RL and Hirashima M: Human ecalectin,
a variant of human galectin-9, is a novel eosinophil
chemoattractant produced by T lymphocytes. J Biol Chem.
273:16976–16984. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsushita N, Nishi N, Seki M, Matsumoto
R, Kuwabara I, Liu FT, Hata Y, Nakamura T and Hirashima M:
Requirement of divalent galactoside-binding activity of
ecalectin/galectin-9 for eosinophil chemoattraction. J Biol Chem.
275:8355–8360. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saita N, Goto E, Yamamoto T, Cho I,
Tsumori K, Kohrogi H, Maruo K, Ono T, Takeya M, Kashio Y, et al:
Association of galectin-9 with eosinophil apoptosis. Int Arch
Allergy Immunol. 128:42–50. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asakura H, Kashio Y, Nakamura K, Seki M,
Dai S, Shirato Y, Abedin MJ, Yoshida N, Nishi N, Imaizumi T, et al:
Selective eosinophil adhesion to fibroblast via IFN-gamma-induced
galectin-9. J Immunol. 169:5912–5918. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kageshita T, Kashio Y, Yamauchi A, Seki M,
Abedin MJ, Nishi N, Shoji H, Nakamura T, Ono T and Hirashima M:
Possible role of galectin-9 in cell aggregation and apoptosis of
human melanoma cell lines and its clinical significance. Int J
Cancer. 99:809–816. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi T, Kuroda J, Ashihara E, Oomizu
S, Terui Y, Taniyama A, Adachi S, Takagi T, Yamamoto M, Sasaki N,
et al: Galectin-9 exhibits anti-myeloma activity through JNK and
p38 MAP kinase pathways. Leukemia. 24:843–850. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuroda J, Yamamoto M, Nagoshi H, Kobayashi
T, Sasaki N, Shimura Y, Horiike S, Kimura S, Yamauchi A, Hirashima
M, et al: Targeting activating transcription factor 3 by galectin-9
induces apoptosis and overcomes various types of treatment
resistance in chronic myelogenous leukemia. Mol Cancer Res.
8:994–1001. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujita K, Iwama H, Sakamoto T, Okura R,
Kobayashi K, Takano J, Katsura A, Tatsuta M, Maeda E, Mimura S, et
al: Galectin-9 suppresses the growth of hepatocellular carcinoma
via apoptosis in vitro and in vivo. Int J Oncol. 46:2419–2430.
2015.PubMed/NCBI
|
|
16
|
Kobayashi K, Morishita A, Iwama H, Fujita
K, Okura R, Fujihara S, Yamashita T, Fujimori T, Kato K, Kamada H,
et al: Galectin-9 suppresses cholangiocarcinoma cell proliferation
by inducing apoptosis but not cell cycle arrest. Oncol Rep.
34:1761–1770. 2015.PubMed/NCBI
|
|
17
|
Nishi N, Itoh A, Fujiyama A, Yoshida N,
Araya S, Hirashima M, Shoji H and Nakamura T: Development of highly
stable galectins: Truncation of the linker peptide confers
protease-resistance on tandem-repeat type galectins. FEBS Lett.
579:2058–2064. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schutte B, Henfling M, Kölgen W, Bouman M,
Meex S, Leers MP, Nap M, Björklund V, Björklund P, Björklund B, et
al: Keratin 8/18 breakdown and reorganization during apoptosis. Exp
Cell Res. 297:11–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Masaki T, Tokuda M, Yoshida S, Nakai S,
Morishita A, Uchida N, Funaki T, Kita Y, Funakoshi F, Nonomura T,
et al: Comparison study of the expressions of myristoylated
alanine-rich C kinase substrate in hepatocellular carcinoma, liver
cirrhosis, chronic hepatitis, and normal liver. Int J Oncol.
26:661–671. 2005.PubMed/NCBI
|
|
20
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abe N, Watanabe T, Suzuki K, Machida H,
Toda H, Nakaya Y, Masaki T, Mori T, Sugiyama M and Atomi Y: Risk
factors predictive of lymph node metastasis in depressed early
gastric cancer. Am J Surg. 183:168–172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamaguchi T, Sano T, Katai H, Sasako M and
Maruyama K: Node-positive mucosal gastric cancer: A follow-up
study. Jpn J Clin Oncol. 31:153–156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Manzoni G, Verlato G, di Leo A,
Guglielmi A, Laterza E, Ricci F and Cordiano C: Perigastric lymph
node metastases in gastric cancer: Comparison of different staging
systems. Gastric Cancer. 2:201–205. 1999. View Article : Google Scholar
|
|
27
|
Jiang J, Jin MS, Kong F, Cao D, Ma HX, Jia
Z, Wang YP, Suo J and Cao X: Decreased galectin-9 and increased
Tim-3 expression are related to poor prognosis in gastric cancer.
PLoS One. 8:e817992013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang J, Zhu L, Cai Y, Suo J and Jin J:
Role of downregulation of galectin-9 in the tumorigenesis of
gastric cancer. Int J Oncol. 45:1313–1320. 2014.PubMed/NCBI
|
|
29
|
Yokozaki H: Molecular characteristics of
eight gastric cancer cell lines established in Japan. Pathol Int.
50:767–777. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Motoyama T, Hojo H and Watanabe H:
Comparison of seven cell lines derived from human gastric
carcinomas. Acta Pathol Jpn. 36:65–83. 1986.PubMed/NCBI
|
|
31
|
Kramer G, Erdal H, Mertens HJ, Nap M,
Mauermann J, Steiner G, Marberger M, Bivén K, Shoshan MC and Linder
S: Differentiation between cell death modes using measurements of
different soluble forms of extracellular cytokeratin 18. Cancer
Res. 64:1751–1756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Morishita A, Gong J and Masaki T:
Targeting receptor tyrosine kinases in gastric cancer. World J
Gastroenterol. 20:4536–4545. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hilmi C, Larribere L, Giuliano S, Bille K,
Ortonne JP, Ballotti R and Bertolotto C: IGF1 promotes resistance
to apoptosis in melanoma cells through an increased expression of
BCL2, BCL-X(L), and survivin. J Invest Dermatol. 128:1499–1505.
2008. View Article : Google Scholar
|
|
34
|
Numata K, Oshima T, Sakamaki K, Yoshihara
K, Aoyama T, Hayashi T, Yamada T, Sato T, Cho H, Shiozawa M, et al:
Clinical significance of IGF1R gene expression in patients with
stage II/III gastric cancer who receive curative surgery and
adjuvant chemotherapy with S-1. J Cancer Res Clin Oncol. Sep
3–2015.(Epub ahead of print). http://dx.doi.org/10.1007/s00432-015-2039-6.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Galamb O, Sipos F, Molnar B, Szoke D,
Spisak S and Tulassay Z: Evaluation of malignant and benign gastric
biopsy specimens by mRNA expression profile and multivariate
statistical methods. Cytometry B Clin Cytom. 72:299–309. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ge J and Chen Z, Wu S, Chen J, Li X, Li J,
Yin J and Chen Z: Expression levels of insulin-like growth factor-1
and multidrug resistance-associated protein-1 indicate poor
prognosis in patients with gastric cancer. Digestion. 80:148–158.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yamada S, Kato S, Matsuhisa T,
Makonkawkeyoon L, Yoshida M, Chakrabandhu T, Lertprasertsuk N,
Suttharat P, Chakrabandhu B, Nishiumi S, et al: Predominant mucosal
IL-8 mRNA expression in non-cagA Thais is risk for gastric cancer.
World J Gastroenterol. 19:2941–2949. 2013.PubMed/NCBI
|
|
38
|
Lee KH, Bae SH, Lee JL, Hyun MS, Kim SH,
Song SK and Kim HS: Relationship between urokinase-type plasminogen
receptor, interleukin-8 gene expression and clinicopathological
features in gastric cancer. Oncology. 66:210–217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee KE, Khoi PN, Xia Y, Park JS, Joo YE,
Kim KK, Choi SY and Jung YD: Helicobacter pylori and interleukin-8
in gastric cancer. World J Gastroenterol. 19:8192–8202. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Morishita A and Masaki T: miRNA in
hepatocellular carcinoma. Hepatol Res. 45:128–141. 2015. View Article : Google Scholar
|
|
41
|
Cui ZH, Shen SQ, Chen ZB and Hu C: Growth
inhibition of hepatocellular carcinoma tumor endothelial cells by
miR-204-3p and underlying mechanism. World J Gastroenterol.
20:5493–5504. 2014. View Article : Google Scholar : PubMed/NCBI
|