Introduction
According to the 2014 cancer statistics from the
American Cancer Society, colon cancer is the third cause of human
cancer incidence and mortality. A total of 96,830 new colon cancer
cases and 50,310 deaths are projected to occur in the US in 2014
(1), and these numbers are still
indicating an increasing trend each year. Treatment of colon cancer
is primary surgery, supplemented by radiotherapy and chemotherapy,
while surgery is difficult to completely remove tumor cells,
remaining cells may cause tumor recurrence and metastasis. Besides,
due to their severe side-effects and drug resistance, radiotherapy
and chemotherapy are greatly limited in clinic. Currently, gene
therapy is the focus of cancer treatment, and many research efforts
have already been made. However, the effects of gene therapy are
still unsatisfactory (2,3). The mechanisms of tumorigenesis are
complex, and specific target genes for therapy are difficult to
identify. A common belief is that apoptosis is an important
mechanism of tumorigenesis. Inducing tumor cell apoptosis
effectively is the key of successful cancer treatment (4). Studies have proved that
Bcl-2-associated athanogene 1 (Bag-1) one of most important
anti-apoptotic genes, its mutation is an important molecular
biological basis of colon cancer, as well as a key part in
development and progression of colon cancer. Bag-1 protein is a
multifunctional binding protein, which can enhance the
anti-apoptotic ability of Bcl-2 family members (5). In our previous studies, we found that
Bag-1 expression was significantly higher in colon cancer tissues
than normal colon tissue, both in colon cancer cells cytoplasm and
nucleus. In addition, Bag-1 expression level is proportional to the
level of colon cancer malignancy, which indicates Bag-1 plays an
important role in the occurrence and development of colon cancer
(6). Studies have also shown that
knockout Bag-1 gene can inhibit transcriptional activity of NF-κB
in colon cancer cell lines (7).
Therefore, Bag-1 gene as a target for cancer therapy, specifically
blocking its expression, is expected to be an effective in colon
cancer gene therapy.
Gene therapy is limited by the gene delivery system.
It is generally known that virus gene vectors cannot be repeatedly
applied in the body, and serious potential unknown effects exist.
On the contrary, non-virus gene vectors are safer and have no known
serious side effect, with low toxicity, low immune response and
active surface modification characteristics. While, low target
specificity and poor transfection efficiency are apparent defects
of common non virus gene vectors that limits their applications on
clinic. Nanoparticles are new non-viral vectors, which have
received great attention in recent years. Nanoparticles are solid
colloidal particles with diameter range from 10 to 500 nm, which
can enhance gene resistance ability to nuclease, prevent them from
degradation, increase their stability in cells and realize
controlled-release, extending their effective duration time in
vivo (8,9). Nanoparticles are non-toxic,
non-immunogenic and biodegradable (10). In addition, nanoparticles have
tissue penetration ability, which can easily absorbed by cells
(11). The above make nanoparticles
unique and superior gene therapy vectors (12,13).
The present study applied magnetic gold nanoparticles as gene
vectors for loading the recombinant plasmid which was inserted by
Bag-1 RNAi gene to transfect into colon cancer cells. In this way,
we detected and analyzed the effects of silencing Bag-1 gene for
colon cancer gene therapy.
Materials and methods
Optimal ratio of nanoparticles and Bag-1
RNAi recombinant plasmid
Seven aliquot magnetic gold nanoparticles were mixed
with plasmid solution respectively, according to mass ratio of 1:
1,2: 1,3: 1,4 : 1, 5: 1, 10: 1, 15: 1, and incubated for 20 min.
Electrophoretic mobility shift assays analyzed the combine ratio of
each group, then selecting the optimal ratio.
Preparation of nanoparticle/Bag-1 RNAi
recombinant plasmid complex
We placed the required magnetic gold nanoparticles
to 1.5 ml EP tube, added quantitative serum-free F-12 medium for 5
min; according to optimal combine ratio of magnetic gold
nanoparticles and plasmid, corresponding amount of quantitative
serum-free F-12 medium was used to dilute the plasmids, mixed them
lightly in the EP tube, then incubated at room temperature for 20
min. Magnetic gold nanoparticle/Bag-1 RNAi recombinant plasmid
complex was prepared.
Cell culture and siRNA transfection
Human colon cancer cell lines (LoVo) were obtained
from Cell Resource Center of Shanghai Institutes for Biological
Sciences. All cells were routinely cultured in F12 medium
supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin at 37°C in humidified atmosphere of 5%
CO2 in air. For transfection, cells were divided into 4
groups (Nano-Plasmid, Nano and Plasmid transfections, and control
group) and transfected with the nanoparticle plasmid complex,
magnetic gold nanoparticles, Bag-1 RNAi recombinant plasmid and
serum-free F-12 medium, respectively, according to the
manufacturer's instructions of magnetic gold nanoparticles (GodMag,
Xi'an, China). Transfection was efficiency assessed by
phase-contrast microscope and fluorescence microscopy at 24, 48 and
72 h after transfection. Cell apoptosis and transfection efficiency
were assessed by flow cytometry (FCM) at highest transfection
efficiency, time detected by phase-contrast microscope and
fluorescence microscopy.
Cell viability assay
Cell viability was measured by MTT assay (MTT;
Dojindo, Kumamoto, Japan). In brief, LoVo cells
(5×103/well) were seeded into 96-well plates and were
transfected with magnetic gold nanoparticles, the nanopartilce
plasmid complex, serum-free F-12 medium (as control group) and
Lipofectamine 2000 transfection reagent, respectively. After
transfection for 24, 48 and 72 h, MTT reagents were added and were
incubated with the cells for 1 h. Then, the absorbance was detected
at 450 nm according to the manufacturer's instruction. Cell
viability = (the average OD value of experimental group/the average
OD value of control group) × 100%. The experiments were performed
in triplicate (14).
Cell apoptosis assay
Apoptosis was assayed using the Annexin V-PE/7AAD
apoptosis detection kit (Invitrogen, Carlsbad, CA, USA) according
to the manufacturer's instructions. Briefly, the cells were
harvested, washed twice with phosphate-buffered saline (PBS),
centrifuged at 1,000 × g for 5 min and resuspended in 195 µl
Annexin V-PE binding buffer. Then, 5 µl Annexin V-PE was
added gently at room temperature. After staining for 10 min in the
dark, the cells were centrifuged at 1,000 × g for 5 min, and then
gently resuspended in 190 µl of Annexin V-PE binding buffer
and 10 µl of 7AAD staining solution was added to the cells,
gently mixed and kept on ice in the dark. The cells were analyzed
by FCM (15).
RT-PCR
Total RNA was extracted from cultured cells using
the TRIzol reagent (Invitrogen) according to the manufacturer's
protocol. Τotal RNA (2 µg) was then subjected to
PrimeScript™ reverse transcription (PrimeScript™ RT
reagent kit with gDNA Eraser), followed by RT-PCR
(SYBR® Premix Ex Taq) (both from Takara)
according to the manufacturer's instructions. The primers for
RT-PCR were as follows: Bag-1 forward,
5′-GGTTCAGGCATTCCTAGCCGAGTG-3′ and reverse,
5′-GTGGCGCCATTCTTCAGGGCA-3′; GAPDH sense,
5′-TGCACCACCAACTGCTTAGC-3′ and antisense, 5′-GGC
ATGGACTGTGGTCATGAG-3′. GAPDH was used as an internal control. The
expression levels of the relative genes were calculated using
control GAPDH mRNA and the 2−ΔΔCt method.
Western blotting
Total protein extracts were prepared using RIPA
lysis buffer (Beyotime, Jiangsu, China) according to the
manufacturer's instructions. The protein concentration of the
lysates was evaluated using a BCA protein assay kit (Beyotime).
Proteins (20 µg) were separated by SDS-PAGE and were
transferred onto poly(vinylidene difluoride) membranes (Millipore,
Boston, MA, USA). The membrane was blocked for 120 min at room
temperature with TBS blocking buffer. Blots were then probed with
appropriate primary antibodies (anti-Bag-1, anti-β-catenin,
anti-c-myc and anti-β-actin) (Proteintech, Wuhan, China) overnight
at 4°C. The membranes were washed with TBST, and were then
incubated in horseradish peroxidase-conjugated secondary antibody
(Thermo Fisher Scientific, Waltham, MA, USA) for 2 h at room
temperature. After membranes were washed with TBST, the proteins
were finally visualized by fluorography using an enhanced
chemiluminescence system.
Statistical analysis
Results are expressed as mean ± SD. All statistical
analyses were carried out using SPSS 16.0. Differences between
treatment conditions were assessed for statistical significance
using one-way ANOVA, followed by the LSD or Dunnett's t-test
method. P<0.05 was considered to indicate a statistically
significant result.
Results
Optimal ratio of nanoparticles and Bag-1
RNAi recombinant plasmid
Laser light scattering particle size analyzer showed
that particle size of magnetic gold nanoparticles were (99.65±2.83)
nm, zeta potential was (39.12±2.01) mV. Magnetic gold nanoparticles
with positive charge surfaces can attract DNA to form a
nanoparticle/plasmid complex. The magnetic gold nanoparticles mixed
with plasmid according to mass ratio of 1: 1,2: 1,3: 1,4 : 1, 5: 1,
10: 1, 15: 1, respectively, underwent agarose gel electrophoresis
(Fig. 1). It is clearly
demonstrated that when the mass ratio reached 4, plasmid DNA
starting to remain in wells due to DNA encapsulation and the
compression by magnetic gold nanoparticles. The mass ratio of 4:1
was the optimal ratio to prepare the nanoparticle Bag-1 RNAi
recombinant plasmid complex for further study.
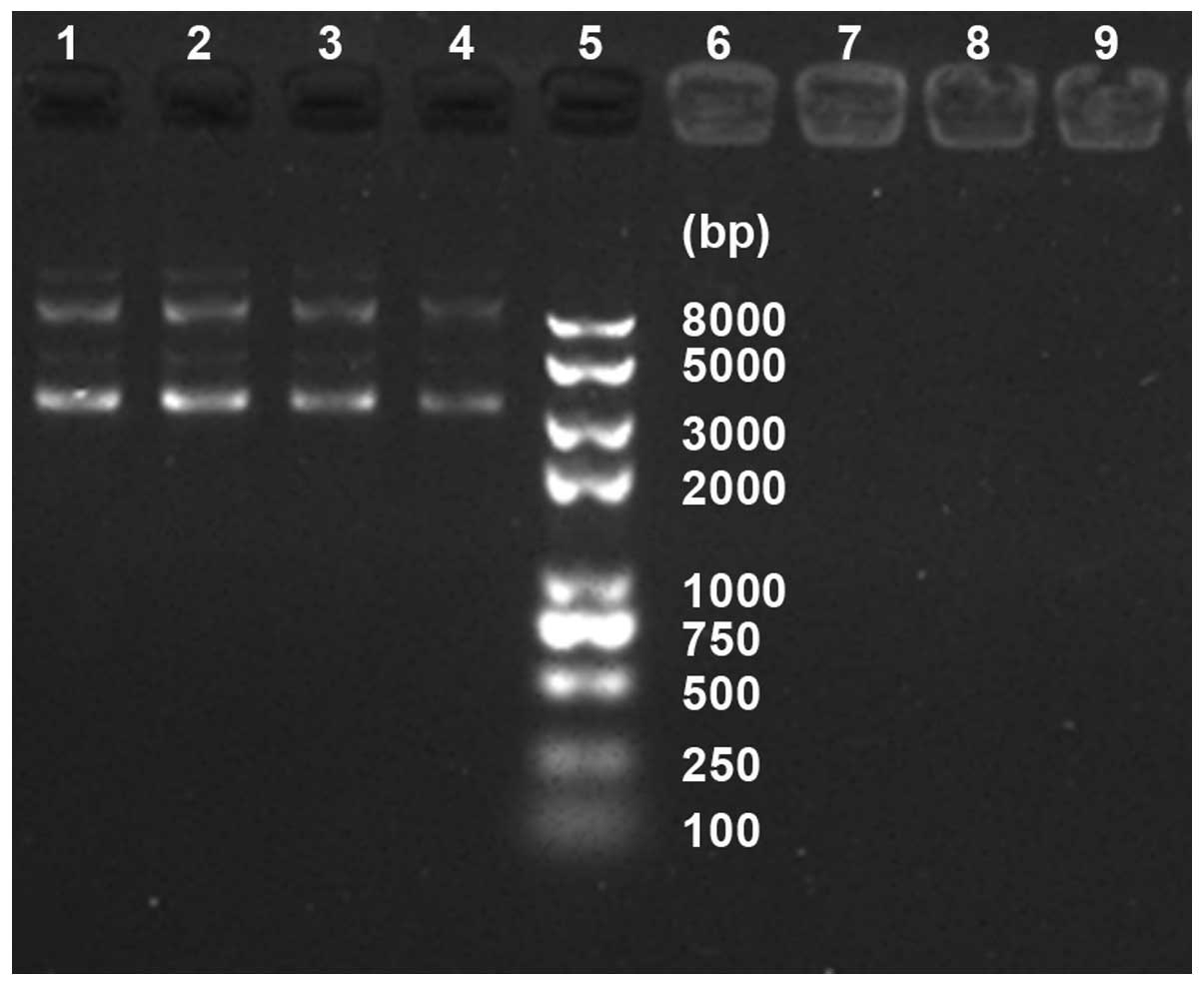 | Figure 1Optimal ratio of nanoparticles and
Bag-1 RNAi recombinant plasmid: Lane 5, DNA maker; lane 1, naked
plasmid, and the lanes from 2 to 4 and 6 to 9, respectively, the
mass ratio of 1: 1, 2: 1, 3: 1 and 4: 1, 5: 1, 10: 1, 15: 1. |
Cell culture and transfection
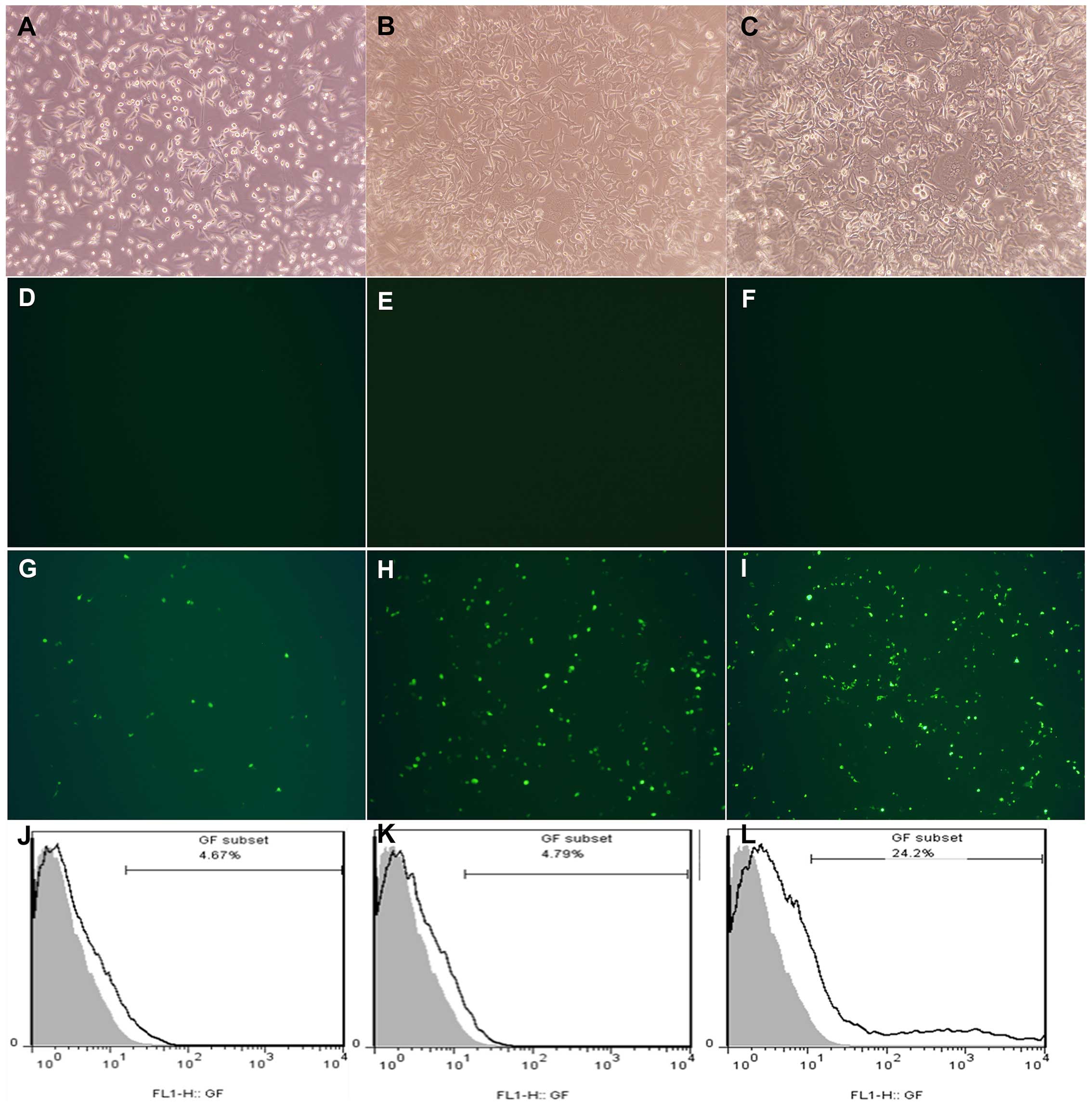
After grouping and transfecting respectively, Nano
and Plasmid transfections, and control group did not express green
fluorescent protein (GFP) under a fluorescence microscope (Fig. 2D–F), while in Nano-Plasmid
transfection group, the expression of GFP was observed in 24 h
after transfection. Besides, the expression was increased in time
and almost reach 72 h after transfection (Fig. 2G–I). Thus, we took 72 h transfection
cells of each group to detect the transfection efficiency by FCM,
and considered the control group cells as zero for control, the
transfection efficiency of Nano and Plasmid transfection groups
were 4.67 and 4.79%, respectively. Nano-Plasmid transfection group
was ~24.2% (Fig. 2J–L).
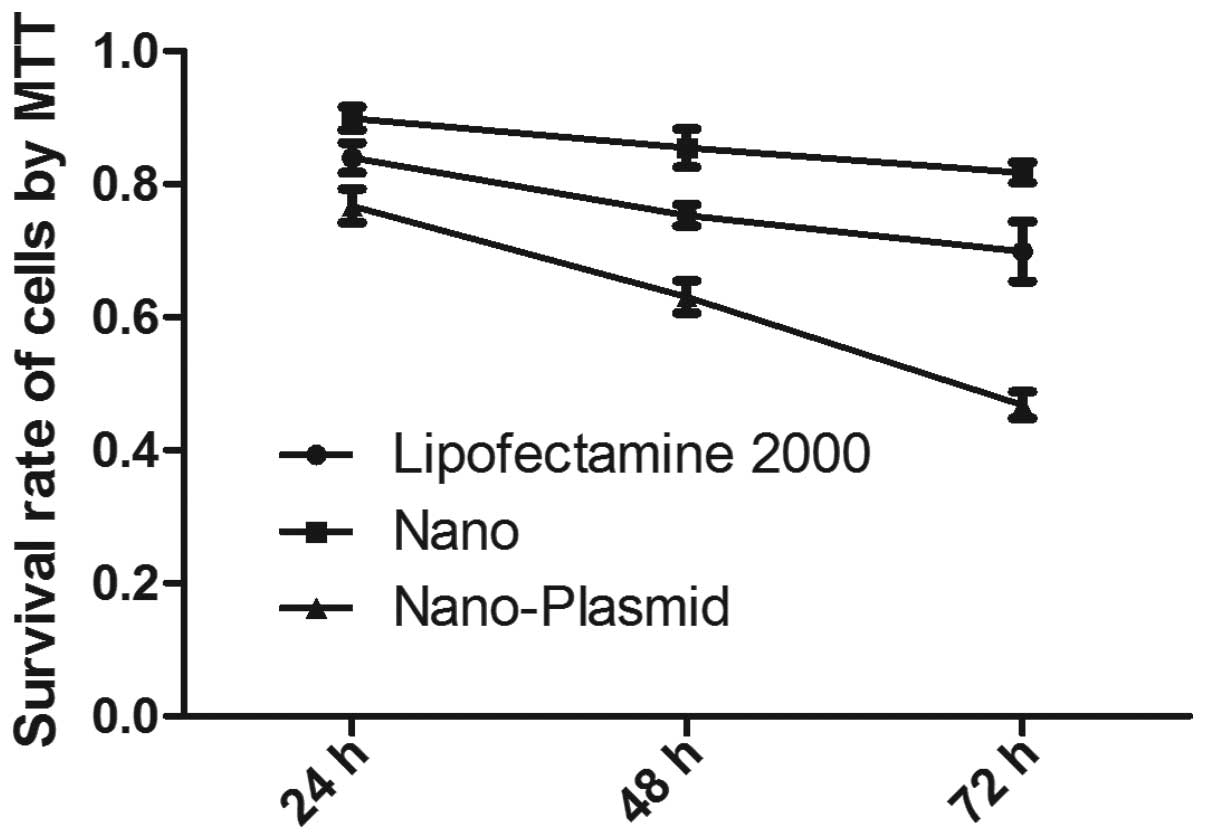
Cell viability assay
Comparing to the control group, MTT results showed
that regardless of Nano and Nano-Plasmid transfections, or
Lipofectamine 2000 transfection groups, LoVo cell viability tended
to decrease as time prolonged. Cell viability of Nano-Plasmid
transfection group was lower than the other groups at the same
culture period (P<0.05). Cell activity of Nano transfection
group was higher than Lipofectamine 2000 transfection reagent group
(P<0.05) (Fig. 3). These results
suggested that magnetic gold nanoparticles were less cytotoxic than
Lipofectamine 2000 transfection reagent and cell viability of
Nano-Plasmid transfection group was lower than Nano transfection
group indicating exogenous gene was successfully transfected into
cells, and showed increased cell apoptosis.
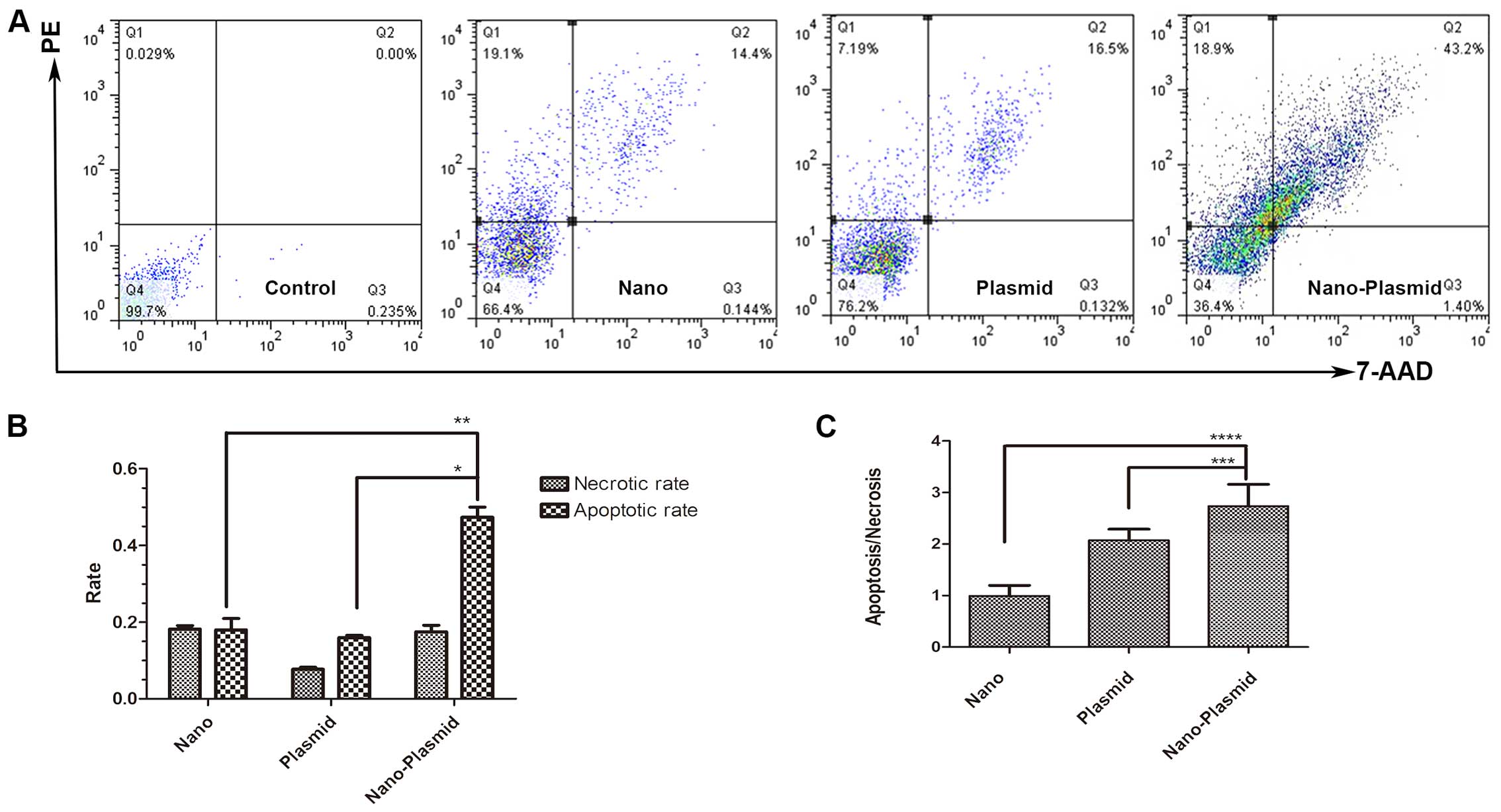
Cell apoptosis assay
The LoVo cells as control, cell apoptosis of Nano
transfection, RNAi plasmid and Nano-Plasmid transfection group was
(17.95%±0.024), (15.97%±0.0046) and (47.32%±0.021), respectively.
Apoptotic cells were mostly early apoptotic cells. The apoptosis
rate of Nano-Plasmid transfection group was markedly increased
compared to the other groups (P<0.05) (Fig. 4A and B). Apoptotic/necrotic ratio of
Nano transfection, Plasmid transfection and Nano-Plasmid
transfection groups was (0.994±0.165), (2.075±0.172) and
(2.737±0.344), respectively. The difference between Nano-Plasmid
transfection group compared to the others was statistically
significantly different (P<0.05) (Fig. 4C).
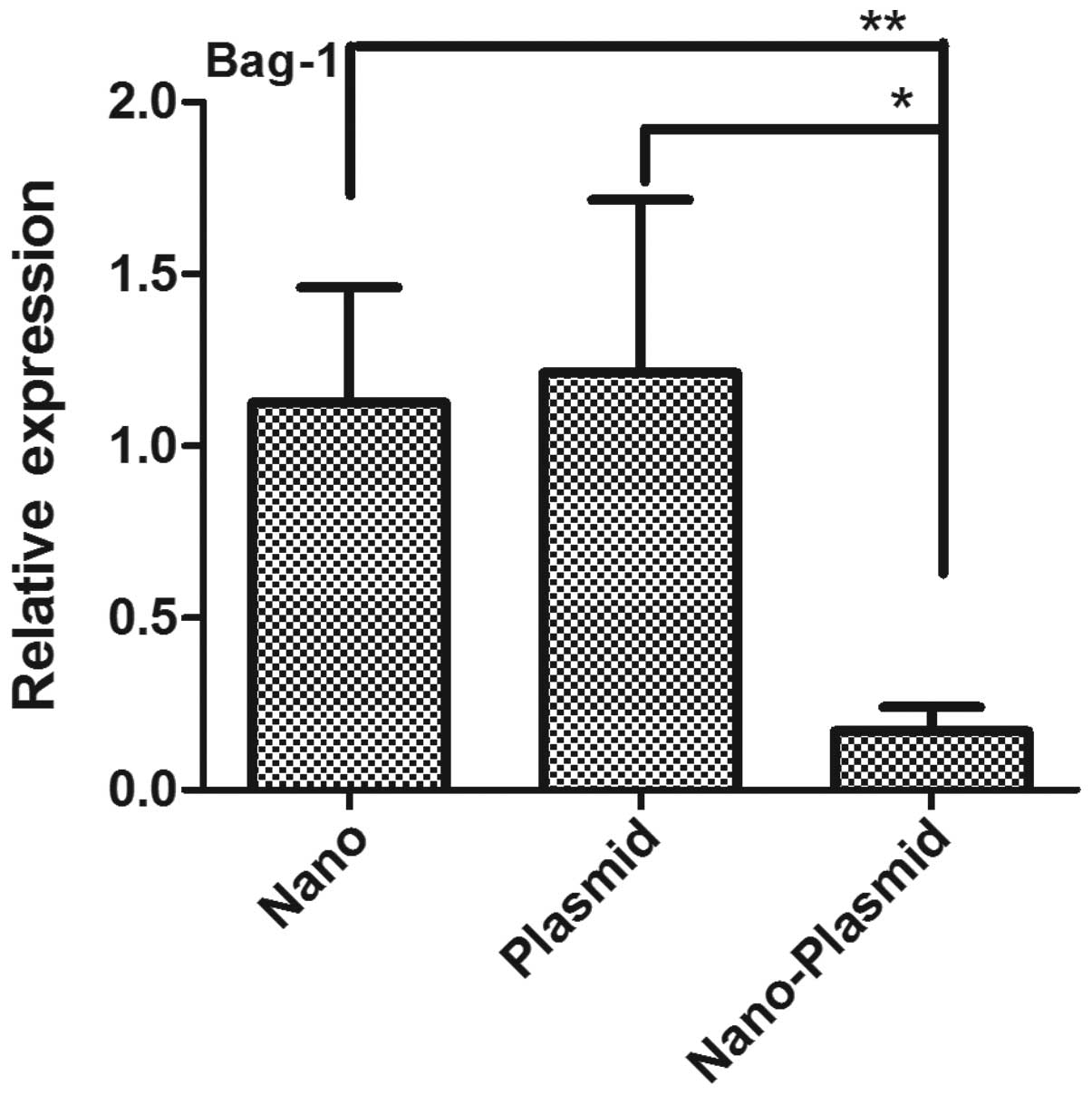
RT-PCR
The results of Bag-1 mRNA expression detected by
RT-PCR showed that CT value of Bag-1 in Nano-Plasmid transfection
group was larger than the other three groups, and delayed plateau
period. While CT value of Nano transfection, RNAi plasmid and
control group was smaller and the plateau period started earlier.
There were no significant differences among these groups. Comparing
to control group, relative amounts of Bag-1 gene expression of Nano
and Plasmid transfections, and Nano-Plasmid transfection groups
were (1.126±0.334), (1.212±0.503) and (0.170±0.071), respectively.
The expression of Nano-Plasmid transfection group was significantly
lower than the others (P<0.05) (Fig.
5).
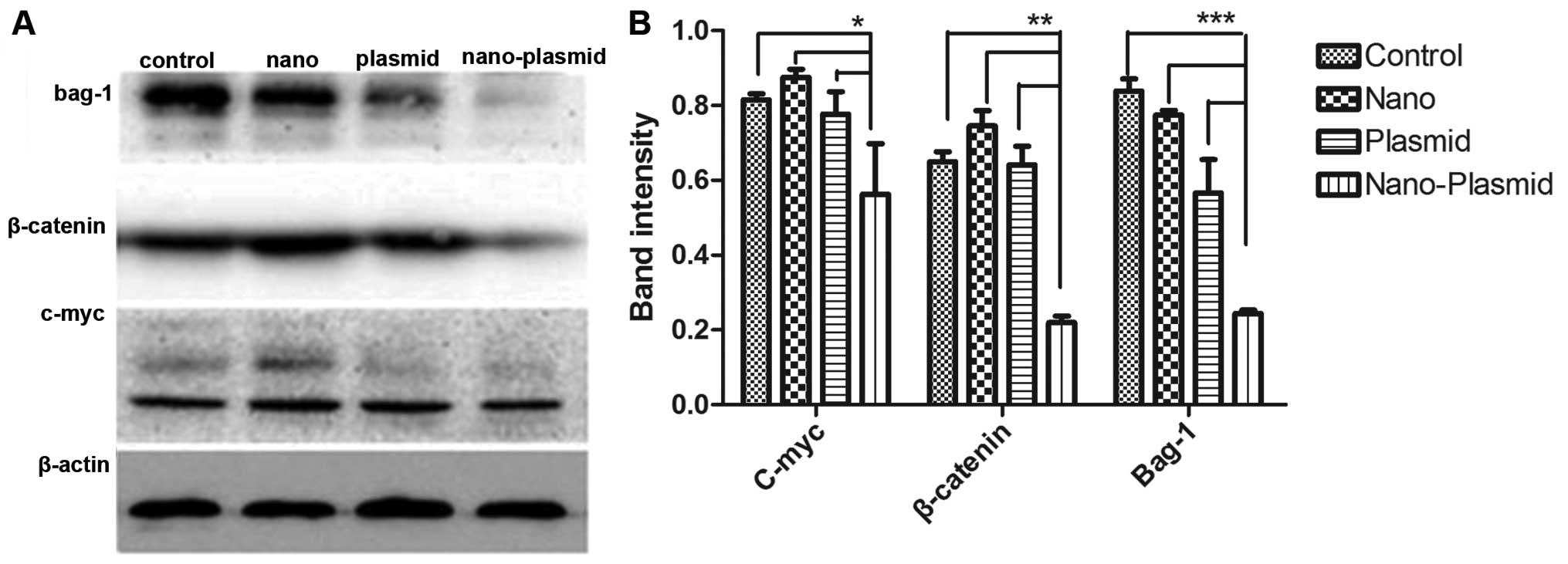
Western blotting
Western blot results showed that the image intensity
of bands of Nano-Plasmid transfection group were lower than bands
of other groups (Fig. 6A). In the
Nano-Plasmid transfection group, Bag-1 protein expression
(P<0.05), c-myc protein expression (P<0.05), and β-catenin
protein expression (P<0.05) decreased compared to the other
three groups. Whereas, among Nano transfection, RNAi plasmid and
control group, the differences of Bag-1, c-myc and β-actin protein
expression showed no statistical significance (Fig. 6B).
Discussion
It is known that the initiation, development,
invasion and metastasis of colon cancer are controlled by many
different genes and various signal transduction pathways and
involved in many important biological processes. Bag-1 which is
only slightly expressed in normal tissues, is often expressed in
breast, ovarian, oral cancer, intestinal and prostate cancer,
glioma, and particularly in colon cancer (16–21).
In the study of Li et al (22), patients with low expression in lung
cancer had significantly longer survival period than patients with
higher Bag-1 expression via RT-PCR detection which demonstrates
that Bag-1 expression may be associated with poor prognosis. Bai
et al (23) reported that
Bag-1 can promote metastasis of colon cancer. Bag-1 is a positive
regulator of Bcl-2 which is an anti-apoptotic gene. Bag-1 is not a
Bcl-2 family member, but their promoter regions show high homology
with Bcl-2 that makes it possible for them to form complex to
promote anti-apoptotic function (5). Besides, Bag-1 can combine, for
example, with estrogen, androgen and glucocorticoid receptor,
realizing a variety of ways of inhibiting apoptosis (24–26).
Combining with heat-shock cognate 70 (HSC70) and inducible
heat-shock protein 70 (HSP70), Bag-1 can play a variety of
physiological and pathological functions (27,28).
In the present study, recombinant RNAi plasmid was transfected into
LoVo cells via the magnetic gold nanoparticle gene delivery system
and expressed successfully in cells. In transfected LoVo cells,
Bag-1 gene expression were significantly inhibited and apoptosis
rate increased as the degree of Bag-1 gene suppression increased,
which further confirms Bag-1 gene is an anti-apoptotic gene and has
close relations with colon cancer development and prognosis.
Discoveries of oncogenes and tumor suppressor genes,
and elucidation of mechanisms of cell signaling pathway, have
greatly enriched knowledge of the mechanisms of carcinogenesis.
Among these mechanisms, imbalance of wnt/β-catenin signaling
pathway has been proven to have a significant correlation to a
variety of tumors, including, cervical, breast, stomach and liver
cancer, melanoma, and glioma, are found in wnt/β-catenin signaling
pathway disorders (29–34). When wnt/β-catenin signaling pathway
abnormality occurs, intracellular β-catenin protein will
accumulate, then free-β-catenin can enter the nucleus and activate
expression of backward genes, leading to tumorigenesis (35,36).
In additional studies, we found that with Bag-1 gene expression
suppressed expression of β-catenin protein and its downstream
protein c-myc protein decrease, and speculate the mechanism of
Bag-1 gene causing colon cancer may be related to the activation of
β-catenin, that causing wnt/β-catenin signaling pathway
abnormality, then leading to overexpression of its downstream gene
myc. Using small interfering RNA technology to silence Bag-1 gene,
β-catenin activation can be reduced, therefore, making reduction of
myc overexpression having a role in treating colon cancer. Besides,
as known effective gene transfection and stable gene expression are
the basis of successful gene therapy. The ideal gene delivery
system should possess high gene delivery efficiency, low
cytotoxicity, no physiological effects on normal cells as well as
ease of use and reproducible properties (37–39).
Nano-viral gene vectors have more advantages than viral gene
vectors (12,38). In the present study, we selected
magnetic gold nanoparticles as gene vectors, which are relatively
new in gene delivery systems. Magnetic nanoparticles can absorb
nucleic acid to form nanoparticle nucleic acid complexes, in this
way, DNA molecules can be protected from nuclease degradation.
After coupling specific target molecules, targeted delivery is
possible. Besides, DNA can achieve controlled release, and extend
duration releasing time (10,40,41).
In this experiment, recombinant plasmids were successfully loaded,
released and protected by magnetic gold nanoparticles. After
successfully transfecting into LoVo cells, recombinant plasmids
were expressed in cells with lower cytotoxicity. Magnetic gold
nanoparticle gene vectors have huge potential as a gene delivery
system, and mediating siRNA silencing Bag-1 is an effective gene
therapy method for colon cancer.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiu K, Yu B, Huang H, Zhang P, Huang J,
Zou S, Chen Y, Ji L and Chao H: A dendritic nano-sized hexanuclear
ruthenium(II) complex as a one- and two-photon luminescent tracking
non-viral gene vector. Sci Rep. 5:107072015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Şalva E, Turan SO, Ekentok C and Akbuğa J:
Generation of stable cell line by using chitosan as gene delivery
system. Cytotechnology. Jul 2–2015.Epub ahead of print. View Article : Google Scholar
|
|
4
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van der Zee JA, Ten Hagen TL, Hop WC, van
Dekken H, Dicheva BM, Seynhaeve AL, Koning GA, Eggermont AM and van
Eijck CH: Bcl-2 associated anthanogen-1 (Bag-1) expression and
prognostic value in pancreatic head and periampullary cancer. Eur J
Cancer. 49:323–328. 2013. View Article : Google Scholar
|
|
6
|
Sun N, Meng Q and Tian A: Expressions of
the anti-apoptotic genes Bag-1 and Bcl-2 in colon cancer and their
relationship. Am J Surg. 200:341–345. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clemo NK, Collard TJ, Southern SL, Edwards
KD, Moorghen M, Packham G, Hague A, Paraskeva C and Williams AC:
BAG-1 is up-regulated in colorectal tumour progression and promotes
colorectal tumour cell survival through increased NF-kappaB
activity. Carcinogenesis. 29:849–857. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kievit FM and Zhang M: Surface engineering
of iron oxide nanoparticles for targeted cancer therapy. Acc Chem
Res. 44:853–862. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu M, Wang Z, Zong S, Chen H, Zhu D,
Zhong Y and Cui Y: Remote-controlled DNA release from
Fe3O4 @Au nanoparticles using an alternating
electromagnetic field. J Biomed Nanotechnol. 11:979–987. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raji MA, Amara M, Amoabediny G, Tajik P,
Barin A, Magierowski S and Ghafar-Zadeh E: Cytotoxicity of
synthesized iron oxide nanoparticles: Toward novel biomarkers of
colon cancer. Conf Proc IEEE Eng Med Biol Soc. 2014:6179–6182.
2014.
|
|
11
|
Wan Q, Xie L, Gao L, Wang Z, Nan X, Lei H,
Long X, Chen ZY, He CY, Liu G, et al: Self-assembled magnetic
theranostic nanoparticles for highly sensitive MRI of minicircle
DNA delivery. Nanoscale. 5:744–752. 2013. View Article : Google Scholar
|
|
12
|
Sun NF, Liu ZA, Huang WB, Tian AL and Hu
SY: The research of nanoparticles as gene vector for tumor gene
therapy. Crit Rev Oncol Hematol. 89:352–357. 2014. View Article : Google Scholar
|
|
13
|
Singh D, McMillan JM, Kabanov AV,
Sokolsky-Papkov M and Gendelman HE: Bench-to-bedside translation of
magnetic nanoparticles. Nanomedicine. 9:501–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ben Q, An W, Fei J, Xu M, Li G, Li Z and
Yuan Y: Downregulation of L1CAM inhibits proliferation, invasion
and arrests cell cycle progression in pancreatic cancer cells in
vitro. Exp Ther Med. 7:785–790. 2014.PubMed/NCBI
|
|
15
|
Zhou Y, Xiong M, Niu J, Sun Q, Su W, Zen
K, Dai C and Yang J: Secreted fibroblast-derived miR-34a induces
tubular cell apoptosis in fibrotic kidney. J Cell Sci.
127:4494–4506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maddalo D, Neeb A, Jehle K, Schmitz K,
Muhle-Goll C, Shatkina L, Walther TV, Bruchmann A, Gopal SM, Wenzel
W, et al: A peptidic unconjugated GRP78/BiP ligand modulates the
unfolded protein response and induces prostate cancer cell death.
PLoS One. 7:e456902012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roth W, Grimmel C, Rieger L, Strik H,
Takayama S, Krajewski S, Meyermann R, Dichgans J, Reed JC and
Weller M: Bag-1 and Bcl-2 gene transfer in malignant glioma:
Modulation of cell cycle regulation and apoptosis. Brain Pathol.
10:223–234. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wood J, Lee SS and Hague A: Bag-1 proteins
in oral squamous cell carcinoma. Oral Oncol. 45:94–102. 2009.
View Article : Google Scholar
|
|
19
|
Aust S, Pils S, Polterauer S, Horvat R,
Cacsire Castillo-Tong D, Pils D, Dudek G, Schmid B, Speiser P,
Reinthaller A, et al: Expression of Bcl-2 and the antiapoptotic BAG
family proteins in ovarian cancer. Appl Immunohistochem Mol
Morphol. 21:518–524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu H, Lu S, Gu L, Gao Y, Wang T, Zhao J,
Rao J, Chen J, Hao X and Tang SC: Modulation of BAG-1 expression
alters the sensitivity of breast cancer cells to tamoxifen. Cell
Physiol Biochem. 33:365–374. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mashukova A, Kozhekbaeva Z, Forteza R,
Dulam V, Figueroa Y, Warren R and Salas PJ: The BAG-1 isoform
BAG-1M regulates keratin-associated Hsp70 chaperoning of aPKC in
intestinal cells during activation of inflammatory signaling. J
Cell Sci. 127:3568–3577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li P, Wang YD, Cheng J, Chen JC and Ha MW:
Association between polymorphisms of BAG-1 and XPD and chemotherapy
sensitivity in advanced non-small-cell lung cancer patients treated
with vinorelbine combined cisplatin regimen. Tumour Biol. Jun
30–2015.Epub ahead of print.
|
|
23
|
Bai YX, Yi JL, Li JF and Sui H:
Clinicopathologic significance of BAG1 and TIMP3 expression in
colon carcinoma. World J Gastroenterol. 13:3883–3885. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Knee DA, Froesch BA, Nuber U, Takayama S
and Reed JC: Structure-function analysis of Bag1 proteins. Effects
on androgen receptor transcriptional activity. J Biol Chem.
276:12718–12724. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmidt U, Holsboer F and Rein T: Role of
the hsp70 cochaperone BAG1 in glucocorticoid receptor function and
stress-related diseases. Proc Natl Acad Sci USA. 105:E101author
reply E102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brendel A, Felzen V, Morawe T, Manthey D
and Behl C: Differential regulation of apoptosis-associated genes
by estrogen receptor alpha in human neuroblastoma cells. Restor
Neurol Neurosci. 31:199–211. 2013.
|
|
27
|
Brive L, Takayama S, Briknarová K, Homma
S, Ishida SK, Reed JC and Ely KR: The carboxyl-terminal lobe of
Hsc70 ATPase domain is sufficient for binding to BAG1. Biochem
Biophys Res Commun. 289:1099–1105. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rauch JN and Gestwicki JE: Binding of
human nucleotide exchange factors to heat shock protein 70 (Hsp70)
generates functionally distinct complexes in vitro. J Biol Chem.
289:1402–1414. 2014. View Article : Google Scholar :
|
|
29
|
Jang GB, Kim JY, Cho SD, Park KS, Jung JY,
Lee HY, Hong IS and Nam JS: Blockade of Wnt/β-catenin signaling
suppresses breast cancer metastasis by inhibiting CSC-like
phenotype. Sci Rep. 5:124652015. View Article : Google Scholar
|
|
30
|
Li F, Wang T and Tang S: SOX14 promotes
proliferation and invasion of cervical cancer cells through
Wnt/β-catenin pathway. Int J Clin Exp Pathol. 8:1698–1704.
2015.
|
|
31
|
Akaboshi S, Watanabe S, Hino Y, Sekita Y,
Xi Y, Araki K, Yamamura K, Oshima M, Ito T, Baba H, et al: HMGA1 is
induced by Wnt/beta-catenin pathway and maintains cell
proliferation in gastric cancer. Am J Pathol. 175:1675–1685. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, He L, Du Y, Zhu P, Huang G, Luo J,
Yan X, Ye B, Li C, Xia P, et al: The long noncoding RNA lncTCF7
promotes self-renewal of human liver cancer stem cells through
activation of Wnt signaling. Cell Stem Cell. 16:413–425. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Atkinson JM, Rank KB, Zeng Y, Capen A,
Yadav V, Manro JR, Engler TA and Chedid M: Activating the
Wnt/β-Catenin pathway for the treatment of melanoma - Application
of LY2090314, a novel selective inhibitor of glycogen synthase
kinase-3. PLoS One. 10:e01250282015. View Article : Google Scholar
|
|
34
|
Hu T, Xie N, Qin C, Wang J and You Y:
Glucose-regulated protein 94 is a novel glioma biomarker and
promotes the aggressiveness of glioma via Wnt/β-catenin signaling
pathway. Tumour Biol. Jun 25–2015.Epub ahead of print. View Article : Google Scholar
|
|
35
|
Cai J, Maitra A, Anders RA, Taketo MM and
Pan D: β-Catenin destruction complex-independent regulation of
Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev.
29:1493–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mir R, Pradhan SJ, Patil P, Mulherkar R
and Galande S: Wnt/β-catenin signaling regulated SATB1 promotes
colorectal cancer tumorigenesis and progression. Oncogene. Jul
13–2015.Epub ahead of print. View Article : Google Scholar
|
|
37
|
Zhao R, Peng X, Chu H and Song W:
Phosphorylatable short peptide conjugated chitosan mediated gene
therapy for repair of articular cartilage defect in rabbits.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 28:1346–1352. 2014.In
Chinese.
|
|
38
|
Son S, Namgung R, Kim J, Singha K and Kim
WJ: Bioreducible polymers for gene silencing and delivery. Acc Chem
Res. 45:1100–1112. 2012. View Article : Google Scholar
|
|
39
|
Whitehouse A: Herpesvirus saimiri: A
potential gene delivery vector (Review). Int J Mol Med. 11:139–148.
2003.PubMed/NCBI
|
|
40
|
Vijayanathan V, Thomas T and Thomas TJ:
DNA nanoparticles and development of DNA delivery vehicles for gene
therapy. Biochemistry. 41:14085–14094. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jingting C, Huining L and Yi Z:
Preparation and characterization of magnetic nanoparticles
containing Fe3O4-dextran-anti-β-human
chorionic gonadotropin, a new generation choriocarcinomaspecific
gene vector. Int J Nanomed. 6:285–294. 2011.
|